Abstract
Therapies targeting oncogene addiction have had a tremendous impact on tumor growth and patient outcome, but drug resistance continues to be problematic. One approach to deal with the challenge of resistance entails extending anti-cancer treatments beyond targeting cancer cells by additionally altering the tumor microenvironment. Understanding how the tumor microenvironment contributes to the evolution of diverse resistance pathways could aid in the design of sequential treatments that can elicit and take advantage of a predictable resistance trajectory. Tumor associated macrophages often support neoplastic growth and are frequently the most abundant immune cell found in tumors. Here, we used clinically relevant in vivo Braf-mutant melanoma models with fluorescent markers to track the stage-specific changes in macrophages under targeted therapy with Braf/Mek inhibitors and assessed the dynamic evolution of the macrophage population generated by therapy pressure-induced stress. During the onset of a drug-tolerant persister state, Ccr2+ monocyte-derived macrophage infiltration rose, suggesting that macrophage influx at this point could facilitate the onset of stable drug resistance that melanoma cells show after several weeks of treatment. Comparison of melanomas that develop in a Ccr2-proficient or deficient microenvironment demonstrated that lack of melanoma infiltrating Ccr2+ macrophages delayed onset of resistance and shifted melanoma cell evolution towards unstable resistance. Unstable resistance was characterized by sensitivity to targeted therapy when factors from the microenvironment were lost. Importantly, this phenotype was reversed by co-culturing melanoma cells with Ccr2+ macrophages. Overall, this study demonstrates that the development of resistance may be directed by altering the tumor microenvironment to improve treatment timing and the probability of relapse.
Introduction
Advances in our understanding of the genetic and molecular properties characteristic to melanoma have revolutionized treatment (1). Approaches leading to the identification of genetic subtypes and key driver mutations provided the rationale to enable new therapeutic strategies for melanoma treatment targeting specific molecular mechanisms of oncogenesis. BRAF-mutant cutaneous melanoma is the most common subtype, with the V600E mutation occurring in the majority of the patient cases (2). Pharmacological inhibition of oncogenic BRAF and its downstream signaling pathway is currently a first line treatment option. Intervention in the activity of this target increases the overall survival rate significantly and has made melanoma a more manageable disease (3,4). Despite the improvements made with BRAF-targeted molecular therapy, patients with advanced stage melanoma remain incurable, in most cases failing complete eradication of tumor cells with an accompanied rapid acquisition of drug resistance (5). Emergence of targeted therapy resistance is often inevitable with extended treatment and is a major problem limiting patient survival.
It has become apparent that diverse resistance phenotypes in melanoma can be caused by extensive intratumoral heterogeneity (6–8). Previous efforts to define genomic mutational profiles have laid important groundwork in understanding the development of therapeutic resistance (9–13). However, not all emergent drug-resistant cell populations can be attributed solely to genetic alterations, and non-mutational mechanisms are also important for establishing therapeutic resistance. Recent studies have unveiled mechanisms of non-genetic adaptation, often demonstrating phenotypic plasticity by gaining or relinquishing distinct drug-tolerant states under hostile circumstances (8,14,15) Single-cell transcriptomics further revealed four different drug-tolerant states in melanoma after treatment with Braf/Mek-targeting agents. These drug-tolerant states are achieved through adaptive reprogramming of tumor cells that shift their transcriptional phenotypes, rather than through selection of pre-existing cells harboring genetic alterations (16,17). Moreover, drug-tolerant states arising through non-mutational mechanisms lead to diversified therapeutic resistance paths, complicating approaches to prevent resistance (18,19).
Established melanoma tumors contain a complex microenvironment composed of many different cell types, including tumor-infiltrating immune cells. Notably, tumor associated macrophages (TAMs) are often the most abundant cell-type found in the stroma (20,21). High TAM levels is associated with poor prognosis in cancer patients, suggesting that macrophages are key determinants regulating tumor cell behavior and treatment responses (22,23). Consequently, targeting macrophages continues to be an attractive therapeutic approach, in hopes that synergies with current treatment options could reduce resistance and recurrence. Despite extensive prior work on TAM depletion as a potential therapy, clinical trial outcomes remain inconclusive with conflicting results (24).
Like tumor cells, macrophages are versatile cells which can progressively switch their properties and undergo functional compensation to adverse microenvironmental changes (25). Recent work in glioma further demonstrated that TAMs are able to engage new signaling pathways in response to long-term treatment with a macrophage-targeted therapy, thus negating improvement in response (26). Macrophages can thus act as an active component of the tumor, undergoing adaptation and altering their functional spectrum to facilitate tumor cell reprogramming towards a resistant phenotype (27). Accordingly, understanding the parallel evolution of melanoma and macrophages under therapy pressure will offer insights into developing strategies aiming to steer cancer cell evolution towards tumor cell phenotypes with less resiliency (28).
Here, we sought to determine the role of macrophages in reprogramming melanoma cells toward resistance by long-term concurrent tracking of tumor and macrophage adaptation under targeted therapy pressure. With this objective, we used Ccr2RFP/RFP mice to inhibit monocyte infiltration during treatment with targeted therapy and identified a stage-specific impact on tumor cell behavior by macrophages, in which TAMs foster paths to therapeutic resistance in both in vitro and in vivo conditions. We propose that TAMs guide treatment responses during melanoma cell reprogramming to establish stable, but likely diverse, resistance mechanisms.
Materials & Methods
Mouse models
Tyr-CreER, LSL-BrafV600E, Ptenflox/flox (TBP) mice were purchased from the Jackson Laboratories (Stock #013590). For melanoma tumorigenesis, mice (7–8 weeks of age) were treated by topical administration of 3µl of 4-Hydroxytamoxifen (4-OHT, 70% Z-isomer, Sigma) on the shaved back skin at 8–10 weeks of age. 4-OHT was prepared as a 5mM solution in 100% ethanol. After topical 4-OHT treatment, the skin region was irradiated twice with 180 mJ cm−2 UVB under anesthesia. Ccr2RFP/RFPCx3cr1GFP/GFP were purchased from the Jackson Laboratories (Stock #032127) and were maintained on C57BL/6J background. All mouse protocols and experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Cornell University. Mice were bred and maintained under pathogen-free conditions at Cornell University College of Veterinary Medicine. All genotyping was accomplished by collecting tail samples at 12 days postnatal and digesting in 0.05N NaOH at 96°C for 1 hour, followed by neutralization with 1M Tris-HCl at a pH value of 8. Genotyping PCRs were performed by using Taq polymerase, 10x PCR buffer, dNTP (G-biosciences), and using primers recommended by the Jackson Labs. For validation of leakiness in Ccr2RFP/RFP knockout animals, RFP signals in Ccr2RFP/+ heterozygous animals have been validated by flow cytometry as controls. We suggest thorough analysis of RFP expressing cells in addition to genomic PCR genotyping while using this fluorescent reporter model.
Primary murine melanoma cell isolation and transplantation
Primary murine melanoma cells were freshly isolated by physical resection of cutaneous lesions from TBP GEMMs. Tumor cells were dissociated and filtered through a 70µm cell strainer (Corning) before being placed into media. Isolated murine primary melanoma cells from TBP mice were cultured in DMEM/F12 containing 10% FBS and penicillin, streptomycin in a 5% CO2 cell culture incubator at 37°C. Primary cells within ten passages were used in this study. Around the time of experiments, TBP primary cells were not tested for mycoplasma. Eight to twelve week-old mice were used for transplantation experiments, although some were aged up to 5 months for secondary in vivo transplantation experiments. Tumor cells (1106/100µl) were subcutaneously injected into the right and left flanks. Tumor volume was measured daily (). Mice were euthanized when reaching the humane endpoint (ulceration requiring euthanasia).
Treatment of mice with targeted therapeutics
Mice received combination therapy with a dose of 30mg/kg dabrafenib and 1mg/kg trametinib (Medchem Express). Dabrafenib and trametinib were initially dissolved in dimethylsulphoxide (DMSO) and subsequently diluted into carboxymethylcellulose (CMC, 0.5% w/v), Tween 80 (0.05% v/v) in sterile water and administered to mice daily by oral gavage.
Macrophage depletion via Clodronate liposomes
Clodronate liposomes were purchased from Liposoma (The Netherlands). For depletion of peripheral blood monocytes and dermal macrophages, 200 ul of PBS (control) or clodronate-encapsulated liposomes were administered via retro-orbital injection every two days throughout the duration of targeted therapy treatment.
Tissue preparation and flow cytometry analysis
Blood was collected into EDTA-containing tubes via retro-orbital injection (under anesthesia) or cardiac puncture (from euthanized animals). Red blood cells were lysed using ACK lysis buffer (Thermo Fisher), and whole blood was stained with fluorochrome-conjugated antibodies. Tumor cells were isolated, dissociated in DMEM/F12 media containing collagenase 20mg/ml (Worthington) for 60 mins at 37°C. Digested materials were passed through 100- and 70-µm nylon cell strainers and single-cell suspensions were prepared for subsequent staining. 20µl of Accucheck counting beads (Thermo Fisher) were added to calculate absolute cell numbers. For T cell analysis, single-cell suspensions were incubated with a biotinylated anti-TCRβ antibody for 20 mins at 4°C in the dark, followed by incubation with magnetic Streptavidin Microbeads (Miltenyi Biotec) for 15 mins at 4°C in the dark. Cells were passed through LS column per the manufacturer’s instructions. Isolated TCRβ+cells were blocked from non-specific staining with anti-mouse CD16/32 and then stained with fixable viability dye and the indicated antibodies. Antibodies are listed in Supplementary Table 1. Data were acquired on BD Symphony flow cytometer. Compensation, analysis, and visualization of the flow cytometry data were performed using FlowJo™ v10.8 Software (BD Life Sciences, RRID:SCR_008520).
Tumor sphere assay
Single melanoma cell suspensions were prepared and seeded at a density of cells/mL in a final volume of 200ul into Nunclon Sphera 96-well U-shaped bottom plates (Thermo Scientific). Cells were incubated for 72h for sphere formation and followed by combination treatment of Braf/Meki. Tumor spheres were continuously exposed to dabrafenib at 500 nM and trametinib at 1nM for 10 days during the sphere forming assay. Medium was renewed every 3 days, starting with the third day of culture, by removing half of the media, and adding back the same volume of fresh medium. For each experimental condition, eight to sixteen spheroids were used. Brightfield images of spheroids were obtained daily, and spheroid diameters were measured using Leica Application Suite X Image software for analysis. For quantification, two parameters: diameter, and volume were used to determine chemosensitivity. Mean sphere volume was calculated by using the formula: assuming the spheroid’s sphericity.
Bead isolation of macrophages from tumor tissues and 3D Co-culture
Resistant tumor tissues were isolated and processed into a single-cell suspension for subsequent macrophage isolation. Single-cell suspensions were incubated with F4/80+ magnetic microbeads (Miltenyi Biotec, Cat#130-110-443) and passed through MS Columns (Miltenyi Biotec, 130-042-201) following the protocol provided in the kit. The cells were further stained with Live/Dead, CD45, CD11b, F4/80 antibodies and analyzed by flow cytometry to determine the level of purity.
A total of tumor cells were seeded in 100µl of RPMI 1640 medium (Gibco, USA) per well in Nunclon Sphera 96-well U-shaped bottom plates (Thermo Scientific). Tumor cells were incubated for 48h to promote sphere formation. Once the spheres had formed, bead-isolated macrophages were added to the culture at a ratio of 1:1 for co-cultivation. After 24h of co-culture, tumor spheroids and macrophages were treated with Braf/Meki for 10 days. Macrophage media (RPMI media containing 10% FBS, 10% L-Cell conditioned medium, 2mM L-Glutamine, and 1mM Sodium pyruvate) was refreshed every 3 days by removing half of the media and adding back an equal volume of fresh medium.
RNA isolation and quantitative real-time PCR (qRT-PCR)
The extraction of total RNA from tumor tissues was performed using Trizol Reagent (Invitrogen, USA) according to the manufacturer’s protocol. 1µg of RNA was transcribed into cDNA using the Superscript IV First-Strand Synthesis System (Thermo Fisher Scientific). Subsequently, the amplification of cDNA was carried out using real-time PCR with Luna Universal qPCR Master Mix (New England Biolabs, Cat#M3003) according to the manufacturer’s instructions. Relative mRNA expression levels were calculated using method. The sequence of primers utilized in the study are listed in Supplementary Table 2.
Tissue immunostaining
Fresh dorsal skin and tumors were embedded in OCT compound (Tissue-Tek) and snap-frozen on dry ice. Frozen fresh tissues were sectioned at 7µm and fixed in 10% neutral buffered formalin for 10min for immunofluorescence staining. Sections were serially washed before blocking with 10% normal donkey serum in PBST. Primary antibodies were diluted in blocking buffer and incubated with primary antibodies at 4°C overnight. After washing with PBST, sections were than incubated with fluorochrome conjugated secondary antibodies at room temperature for 60min. Fluoroshield mounting medium with DAPI (Abcam) was used for nuclei staining. For immunohistochemistry, dorsal skin and tumors were formalin-fixed before paraffin embedding. Paraffin-embedded tissues were cut to a 4µm thickness and deparaffinized and rehydrated. Antigen retrieval was performed using antigen unmasking solution (Citrate-based and Tris-based; Vector Laboratories) at 100°C for 20 min. Sections were stained with primary antibodies at 4°C overnight and then incubated with biotinylated secondary antibodies at room temperature for 60min. Visualization was followed by a standard avidin-biotin complex method using Vectastain ABC-AP or ABC-HRP kit (Vector Laboratories). Images were captured using a Leica DM2500 upright microscope with a DFC7000T camera. Quantification of fluorescent images was determined dividing positive stained area by DAPI-stained nuclei area using Matlab (RRID:SCR_001622).
Bulk mRNA sequencing
Macrophages were isolated from resistant tumor tissues in both control and Ccr2 knockout mice using a BD FACS Aria (BD Biosciences). Sorted macrophages were directly isolated in Trizol LS reagent and subjected to RNA extraction following the manufacturer’s instructions. The quality of mRNA was evaluated using a fragment analyzer at Cornell Biotechnology Resource Center. 50ng of total mRNA was used as input for library preparation. Poly(A) mRNA purification Module (NEB, Cat#E7490S) and the NEBNext Ultra II directional RNA library Prep kit (NEB, Cat#E7785S) were used for index library preparation, following NEB #E7760S/L, #E7765S/L version 4.0_4/21. The library indexing was performed using NEBNext Multiplex Oligos for Illumina (96 Unique Dual Index (NEB, Cat#E6440S). Libraries were sequenced on the NextSeq 2K P2 100bp kit platform (Illumina). The sequencing reads were aligned to the mouse genome using STAR, and differential expression analysis was performed using DESeq2 (29). Statistical significance of the genes was established with an adjusted P value of 0.1 according to the Benjamini-Hochberg adjustment, and a shrinked log fold change greater than 1 according to the ‘apeglm’ shrinkage method. Gene Ontology analysis was performed using the ‘clusterprofiler’ package (30). These datasets are found in Supplementary Table 3.
Statistical analysis and study design
Statistical analyses were performed using GraphPad Prism 9.0 (RRID:SCR_002798). Results were statistically analyzed using two-tailed Student’s t test when only two groups were compared. Three or more groups were performed using one-way ANOVA test with multiple comparisons to determine differences between the groups. Error bars in the graphical data indicates mean ± standard deviation (S.D). Survival curves were generated by the Kaplan-Meier method and Log-rank was used to analyze the mouse survival data. Asterisks represent statistical significance: p <0.0001 (****); p <0.001 (***); p <0.01(**); p <0.05 (*). Sample size and statistical details are included in the figure legends.
Code availability
The code used to perform the analysis found in this study is available on Code Ocean at https://codeocean.com/capsule/5097327/tree/v1.
Data availability
The bulk RNAseq data generated in this study can be found in the Gene Expression Omnibus (GEO) (RRID:SCR_005012) database. GEO accession number for data generated in this study is GSE226835. All other raw data are available upon request from the corresponding author.
Results
Melanomas resistant to Braf/Mek inhibitors are highly infiltrated by macrophages
Established melanoma tumors contain a complex microenvironment that creates a supportive niche for cancer progression and can alter tumor cell behavior to targeted therapy responses (31,32). Among the different cell types that constitute tumor tissue, macrophages are one of the most abundant components of the tumor microenvironment. To assess the role of macrophages in melanoma, we first defined macrophage presence in Tyr-CreER, LSL-BrafV600E, Ptenflox/flox (TBP) genetically engineered mice (GEMM) (33). We have previously shown that activation of TBP melanocyte stem cells by UVB recapitulates human melanoma initiation from the interfollicular epidermis and progression to malignancy (Supplementary Figure 1A, B). To assess macrophage infiltration in TBP melanomas, we compared the expression of macrophage markers CD68 and F4/80 between paired normal dorsal skin and malignant melanoma regions induced by 4-OHT administration (Figure 1A). We observed extensive macrophage abundance in melanoma compared to non-tumor skin (Figure 1B).
Figure 1. Melanomas resistant to Braf/Mek inhibitors are highly infiltrated by macrophages.
A, Scheme depicting melanoma initiation by activation of CreER from the Tyr-CreER, LSL-BrafV600E, Ptenflox/flox (TBP) mouse model. Representative image of cutaneous malignant melanoma developing in dorsal skin 12–15 weeks after topical 4-OHT treatment and subsequent UVB irradiation. B, Representative immunofluorescence images of CD68 (green) and DAPI (blue) from dorsal skin and melanoma in TBP mice. Representative immunohistochemical staining using F4/80 antibody (blue) performed on matched non-tumor dorsal skin and melanoma tissue (20 magnification, Scale bar, 100 µm). C, Scheme of syngeneic transplantation of TBP cells in C57BL/6J mice with two collection timepoints for analysis. (Growing tumor: approximately 3 weeks after transplantation when tumor volumes reached 1,000mm3. Resistant tumor: approximately 5 weeks under Braf/Meki treatment until rebound tumor reached initial size. Non-tumorous dorsal skin used for paired internal control. D, Presence of myeloid cells (upper; CD45+CD11b+) and macrophages (lower; gated further as F4/80+) assessed by flow cytometry in normal dorsal skin and transplanted TBP tumors. Representative flow cytometry plots are shown. Bar graph shows quantification of myeloid cells and macrophages respectively. Data are shown as mean ± S.D, n= 11/group (6 males and 5 females). Statistical analysis performed with two-tailed Student’s t test (**P <0.01). E, Representative immunofluorescence images of F4/80+ macrophages (green) and nuclear DAPI (blue) of normal dorsal skin, untreated growing melanoma and resistant melanoma treated with Braf/Meki from the TBP transplantation model and assessed for macrophage density (20 magnification, Scale bar, 100 µm). Quantification of F4/80+ macrophages. Data are represented as mean ± S.D. (n=3, 27 fields dorsal skin; n=4, 53 fields growing tumor; n=3, 46 fields resistant tumor). Statistical analysis performed with one-way ANOVA (*P 0.05, ****P 0.0001)
To aid in reproducibility, melanoma cells isolated from TBP animals were collected and used for syngeneic transplantation to C57BL/6J recipients (Figure 1C). Melanomas arising from transplanted cells were then assessed for macrophage presence by flow cytometry. CD45+CD11b+ myeloid cells and CD45+CD11b+Ly6G-F4/80+ macrophage percentages were 3-fold higher in melanoma tissue compared to dorsal skin. There were no significant sex-specific differences detected between male and female mice regarding the macrophage proportions within normal skin and melanomas (Figure 1D). IF staining for macrophage abundance in melanomas arising from transplanted cells appeared similar to tumors found in the TBP mouse model. Finally, we determined macrophage levels in melanomas that had achieved resistance to standard-of-care targeted therapy treatment with Braf/Mek inhibitors (Braf/Meki, dabrafenib and trametinib). We found macrophages to be significantly increased compared to established tumors during targeted therapy (Figure 1E). Since macrophages are at higher levels in targeted therapy-treated TBP melanomas compared to normal skin and non-targeted therapy-treated growing melanomas, we hypothesized that macrophages influence melanoma cell behavior when under therapy pressure.
Macrophage presence limits melanoma sensitivity to Braf/Mek inhibitors
To test this hypothesis, we first sought to deplete macrophages in TBP melanomas and assess how macrophage deficiency affects targeted therapy efficacy. Clodronate encapsulated liposomes were used as a first test, since phagocytosis of these particles leads to apoptotic cell death in monocytes and macrophages (34). TBP GEMMs were administered clodronate and control PBS liposomes every two days throughout the duration of targeted therapy treatment, which began when initial tumor volume reached ~1,000mm3 (Figure 2A). Dermal macrophages are derived from blood monocytes, comprised of two main subsets including classical (CD115+Ly6C+) and nonclassical (CD115+Ly6C-) subsets in mice (35). To determine efficacy, the monocyte sub-populations found in the circulation of tumor bearing mice following three weeks of clodronate and PBS liposome treatment were quantified. Following clodronate liposome treatment, both Ly6C+ (3.2% ± 2.6%) and Ly6C- (0.4% ± 0.4%) monocytes were found significantly depleted, in contrast to animals provided with PBS liposomes (Ly6C+17.7% ± 6.9%; Ly6C- 26.7% ± 14.1%) (Figure 2B and Supplementary Figure 2A). Depletion of macrophages in melanoma tissue was further confirmed by immunostaining with CD68, which showed substantially reduced macrophage density in clodronate liposome-treated tumors (Figure 2C). Furthermore, flow cytometry analysis identified a 3-fold decrease in F4/80+ macrophages in clodronate liposome-treated melanomas (Figure 2D and Supplementary Figure 2B).
Figure 2. Macrophage presence limits melanoma sensitivity to Braf/Mek inhibitors.
A, Scheme of experimental procedure and treatment schedule for TBP GEMMs. Topical treatment of 4-OHT and UVB exposure for melanomagenesis. Braf/Meki started when tumor size reached 1,000mm3, along with clodronate liposomes (CL) for depleting macrophages or PBS liposomes (PL) as controls (every 2 days, i.v.). Blood was collected one day prior to tumor collection. Tumors were harvested at 3 weeks of treatment. B, Flow cytometry analysis of blood monocytes gated on live/CD45+/CD11b+ cells in TBP GEMMs treated with CL and PL intravenously. Ly6Clow monocytes (left box); Ly6Chigh monocytes (right box). Histogram of Ly6C expression gated on live/CD45+/CD11b+/CD115+ cells. Blue, PL; Red, CL. Summary graphs showing the percentage of Ly6Clow and Ly6Chigh monocytes in the blood of TBP mice after 3 weeks of CL treatment. Data are shown as mean ± S.D, n= 5/group. Statistical analysis performed with two-way ANOVA (*P 0.05). C, Representative immunofluorescence images of CD68 (green) and DAPI (blue) from regressed melanoma with CL or PL treatment (20 magnification, Scale bar, 100µm). D, Representative overlay histograms of F4/80+ expression analyzed by flow cytometry in regressed tumor tissues harvested after 3 weeks of Braf/Meki treated with CL or PL. Cells gated on live/CD45+/CD11b+/Ly6G-/F4/80+. E, Average tumor volume curves in response to CL or PL upon targeted therapy. Tumor volumes were measured every day and analyzed by two-tailed Student’s t test at each timepoint. Data are shown as mean ± S.D, n= 4/group (*P 0.05, **P 0.01, ***P 0.001). F, Waterfall plots of TBP GEMMs treated with CL or PL in combination with Braf/Meki, which show percent change in final tumor volume compared to before treatment. Each bar in the waterfall plots represents a single mouse (n=4/group). Average percent change in final tumor volume between CL or PL-treated TBP tumors. Data are shown as mean ± S.D. Statistical analysis performed with two-tailed Student’s t test (*P 0.05).
To determine if macrophage depletion influences treatment response to Braf/Meki, we analyzed tumor volume regression. Mice co-treated with clodronate liposomes and targeted therapy were compared to controls starting from 2 days following the first treatment administration. The clodronate-administered group showed a significant decrease in final tumor volume (average tumor volume on day 21, 295.9mm3±62.4mm3, a 73.1% ± 5.4% reduction) compared to controls (498.7mm3 ± 68.4mm3, a 53.1% ± 10.5% endpoint volume) (Figure 2E, F). These data indicate that depletion of macrophages in melanoma tissue increases sensitivity to targeted therapy and treatment effectiveness. However, we were unable to extend clodronate liposome treatment longer than 3 weeks due to toxicity prior to the emergence of therapeutic resistance (36).
To circumvent this limitation, we employed Ccr2-RFP; Cx3cr1-GFP dual knockout/reporter mice targeting chemokine receptors found on blood monocytes (35,37) (Figure 3A). Homozygous Ccr2RFP/RFPCx3cr1GFP/GFP double knockout (dKO) animals lack function in both receptors and are unable to recruit monocytes/macrophages to tissues (38,39). Myeloid (CD45+CD11b+) populations from normal skin, clodronate liposome-treated, and double knockout animals were analyzed by flow cytometry. We observed a significantly decreased myeloid cell presence in dorsal skin from dKO animals compared to controls (Ccr2RFP/+Cx3cr1GFP/+). Myeloid cells accounted for 2.9% ± 1.1% of total live cells in the clodronate-liposome treated dorsal skin, 4.0% ± 0.8% in dKO mice, and 7.2% ± 1.8% in control mice (Figure 3B). The relative percentages of dorsal skin myeloid cells in clodronate-liposome treated and dKO mice were similar, indicating that Ccr2RFP/RFPCx3cr1GFP/GFP mice are a feasible model to assess the contribution of macrophages in therapeutic resistance. This enables long-term experiments to overcome treatment duration limitations found with clodronate liposome administration. We next assessed myeloid cell presence in melanomas from control and dKO recipients. Consistent with previous studies, myeloid presence was markedly reduced by over half in dKO mice (mean 11.9% ± 7.0%) compared to controls (mean 26.8% ± 7.0%) (Figure 3C), indicating a significant change in monocyte infiltration. Immunofluorescence staining with CD68 further confirmed a significantly decreased accumulation of macrophages in untreated melanomas from dKO recipients compared to control recipients (Figure 3D). Together, these data suggest that the majority of monocytes/macrophages found in TBP melanomas require functional Ccr2 and Cx3cr1.
Figure 3. Double Ccr2-Cx3cr1 deficiency results in inhibition of macrophage infiltration into melanomas with an improved antitumor response and delayed therapeutic resistance.
A, Scheme of Ccr2RFP/RFPCx3cr1GFP/GFP double knockout mice and experimental procedures. Modified Ccr2 and Cx3cr1 loci of Ccr2RFP and Cx3cr1GFP (RFP replaces the first 279bp; GFP replaces the first 350bp). Timeline for dorsal skin and tumor tissue harvest. Clodronate liposomes were administered every 2 days for total of 6. Growing tumor tissues were collected when the volume reached 1,000mm3. B, Presence of myeloid cells (CD45+CD11b+) in non-tumorous dorsal skin analyzed by flow cytometry from control, clodronate liposome treated, and Ccr2RFP/RFPCx3cr1GFP/GFP mice. Representative flow cytometry plots are shown. Summary graphs showing quantification of CD45+CD11b+ cells. Data are shown as mean ± S.D, (n= 9, control; n= 8, CL; n= 7, Ccr2RFP/RFPCx3cr1GFP/GFP). Statistical analysis performed with one-way ANOVA (***P 0.001, ****P 0.0001). C, Representative flow cytometry plot showing the presence of myeloid cells (CD45+CD11b+) in growing tumors analyzed from control and Ccr2RFP/RFPCx3cr1GFP/GFP mice. Representative histograms showing CD11b-expressing cells (myeloid) in Ccr2RFP/RFPCx3cr1GFP/GFP versus control animals. Data are shown as mean ± S.D (n=8, control; n=7, Ccr2RFP/RFPCx3cr1GFP/GFP). Statistical analysis performed with two-tailed Student’s t test (**P 0.01). D, Representative immunofluorescence images of CD68 (red) and DAPI (blue) from growing tumor in control and Ccr2RFP/RFPCx3cr1GFP/GFP mice (10x magnification, Scale bar, 100µm). E, Scheme of experimental treatment timeline. Mice were transplanted subcutaneously with 1106 TBP cells and allowed to progress for 30 days. Braf/Meki targeted therapy was given daily beginning at a tumor volume of 1,000mm3 and continuing over 5 weeks until rebound tumor reached endpoint (V0, initial tumor volume). F, Average tumor volume curves in response to targeted therapy from control and Ccr2RFP/RFPCx3cr1GFP/GFP mice. Tumor volumes were measured daily and analyzed by two-tailed Student’s t test at each timepoint. Data are shown as mean ± S.D, n= 8/group (***P 0.001, ****P 0.0001). G, Change in tumor volume at maximal response to Braf/Meki, comparing minimal tumor volume defined as the smallest tumor size during targeted therapy, normalized to treatment start volume V0. Data are shown as mean ± S.D. Statistical analysis performed with two-tailed Student’s t test (*P 0.05). H, Waterfall plots showing percent change in final tumor volume compared to the treatment start volume before Braf/Meki. Each bar in the waterfall plots represents a single mouse (n= 8/group). Summary graph with average percent change in final tumor volume between control and Ccr2RFP/RFPCx3cr1GFP/GFP mice. Data are shown as mean ± S.D. Statistical analysis performed with two-tailed Student’s t test (****P 0.0001). I, Kaplan-Meier survival curves comparing endpoint of control mice (blue curve) and Ccr2RFP/RFPCx3cr1GFP/GFP mice (red curve) that received Braf/Meki. Survival endpoints were defined as rebound tumors that exceeded initial pre-treatment size. Statistical significance was determined by Log-rank Mantel-Cox test (***P 0.001). Controls are Ccr2RFP/+Cx3cr1GFP/+ heterozygous mice in f-i.
Impairment in melanoma macrophage infiltration delays the emergence of Braf/Meki resistance
As reported previously, high infiltration of myeloid cells in melanoma correlates with poor prognosis (40). We also observed that clodronate-mediated macrophage depletion in our melanoma model is associated with better response and sensitivity to Braf/Meki therapy. Given this improved response, we hypothesized that macrophage depletion would also reduce the rise of resistance to targeted therapy and limit the emergence of tumor cells refractory to treatment. To address this question, we transplanted TBP cells subcutaneously to adult control and Ccr2RFP/RFPCx3cr1GFP/GFP dKO mice and began Braf/Meki treatment when melanomas reached ~1,000mm3 (Figure 3E). Mice lacking both Ccr2 and Cx3cr1 demonstrated an improved drug response, with a maximal tumor volume regression of 87.6% ± 6.6% of initial tumor volume. This result contrasts with findings in animals with functional Ccr2 and Cx3cr1, in which a maximal tumor regression of 75.9% ± 10.0% of initial tumor volume was detected. Interestingly, however, we observed that 100% of tumor-bearing mice in both groups eventually developed resistance such that tumors resumed growth despite targeted therapy, though a difference regarding tumor growth rate in rebounding tumors was detected (Figure 3, F and G). Control recipients showed an earlier tumor rebound and an enhanced tumor regrowth rate compared to dKO mice. The average rebounding tumor volume difference between control and dKO mice showed statistical significance (P < 0.005) beginning at 4 weeks of Braf/Meki. We also observed robust tumor regrowth in control mice with an average of 73.9% increase in tumor size, whereas dKO mice exhibited an average final tumor volume reduction of 44.8% (Figure 3H). We used Kaplan-Meier methodology to analyze the duration (numbers of days) for resistant melanomas to reach their initial size prior to treatment. The median value for control mice was 34 days whereas the median value in dKO mice was 43 days (p=0.0002) (Figure 3I). Taken together, we observed Ccr2RFP/RFPCx3cr1GFP/GFP recipients bearing TBP-melanomas exhibit an improved response to Braf/Meki therapy. Rapid tumor regrowth follows a prolonged plateau phase, with no apparent growth despite continuous Braf/Meki, which is shorter compared to control recipients. These findings suggest that the presence of a high level of macrophages is associated with promoting the development of therapeutic resistance in vivo.
Melanoma macrophages expand during the regressed-persistent state and are dependent on Ccr2
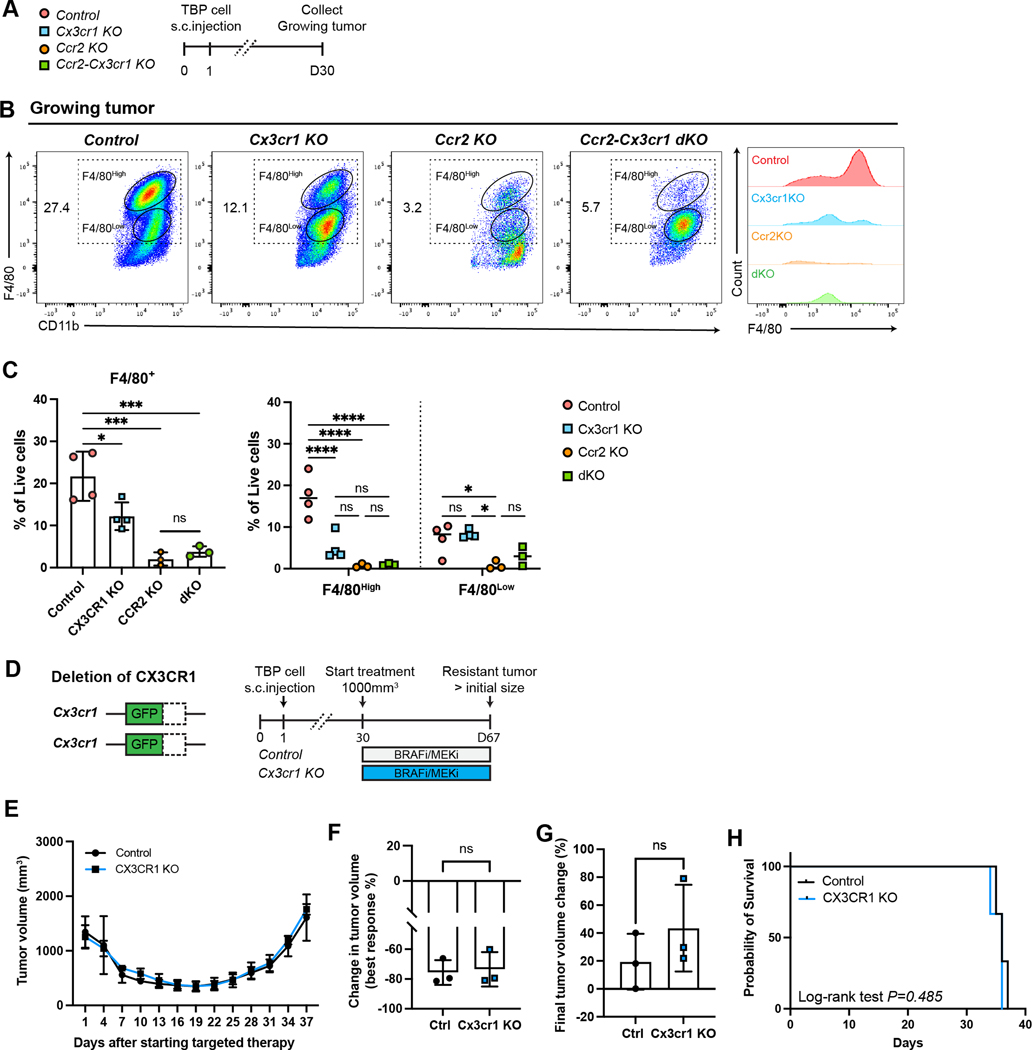
Ccr2 and Cx3cr1 are cell surface receptors that have been shown to have a functional role in macrophage migration and infiltration (41,42). To differentiate the role each chemokine receptor plays in macrophage infiltration in TBP melanoma, we transplanted TBP cells to single Ccr2RFP/RFP or Cx3cr1GFP/GFP knockout animals and compared to control animals harboring both functional receptors (Figure 4A). We collected non-Braf/Meki treated melanoma tissue and performed flow cytometry to quantify infiltration of the F4/80+ (F4/80high and F4/80low) macrophage population. Compared with the control recipients, we observed a significant reduction in the total number of F4/80+ cells among Ccr2RFP/RFP, Cx3cr1GFP/GFP single knockout and Ccr2RFP/RFPCx3cr1GFP/GFP dKO animals. Histogram analysis revealed that F4/80 expression decreased significantly in both Ccr2RFP/RFP single knockout recipients and dKO mice. The reduction observed in Ccr2RFP/RFP single knockouts and dKOs was similar and much greater than that observed in Cx3cr1GFP/GFP single knockout mice (Figure 4, B and C). These results suggest that infiltration of F4/80+ macrophages into growing TBP melanomas is primarily Ccr2 mediated. To determine the effect on therapeutic resistance acquisition due to deficiency in each chemokine receptor, we monitored tumor-bearing Ccr2RFP/RFP and Cx3cr1GFP/GFP mice under continuous targeted therapy until the re-acquisition of initial tumor volume. We hypothesized that therapeutic resistance would be dependent upon the abundance of macrophages in melanomas and thus single or dKO recipients should exhibit a longer delay to the onset of drug resistance. However, we did not observe any statistical tumor volume difference in Cx3cr1GFP/GFP mice compared with controls, indicating that lack of Cx3cr1 had no marked effect of tumor growth inhibition and acquisition of resistance (Figure 4D, E, F, G). The median value for both Cx3cr1GFP/GFP single knockout and control mice was 36 days (p=0.485) with no significant difference (Figure 4H). In contrast, Ccr2RFP/RFP melanomas showed significant tumor shrinkage (85.7% ± 6.1% reduction of tumor volume) compared with control mice (78.4% ± 6.5% reduction of tumor volume) during the Braf/Meki-induced regression phase. Primary tumor growth before Braf/Meki treatment showed no difference between control and Ccr2RFP/RFP mice (Supplementary Figure 3A). Additionally, tumor growth following the onset of Braf/Meki resistance showed a final average tumor volume of 2092.4mm3 ± 682.7mm3 in control recipients, whereas in Ccr2RFP/RFP mice a final average tumor volume of 562.2mm3 ± 303.2mm3 resulted over the same period (Figure 5A, B, C). After 37 days of targeted therapy, Ccr2RFP/RFP mice showed considerable tumor growth inhibition with an average of 35.5% reduction in tumor size, in contrast to control recipients that demonstrated an average increase of 104.5% (Figure 5D). We also observed a delayed onset of therapeutic resistance and prolonged survival time in Ccr2RFP/RFP recipients (median value 39 days) compared with controls (median value 32 days, p=0.0013) (Figure 5E). Finally, comparing melanoma macrophage abundance in control, dKO, and single knockout tumors, we detected fewer Ccr2+ cells in resistant tumor tissue from Ccr2RFP/RFP and Ccr2RFP/RFPCx3cr1GFP/GFP mice, which contrasted to the high accumulation found in control and Cx3cr1GFP/GFP melanomas (Figure 5F, G). Collectively, these findings suggest that macrophage infiltration into TBP melanomas is mainly dependent on functional Ccr2 and that Ccr2+ cells are critical for the acquisition of therapeutic resistance.
Figure 4. Macrophage infiltration into melanoma is primarily dependent on Ccr2.
A, Scheme of experimental procedures and tumor harvest timeline. Mice were transplanted subcutaneously with TBP cells and allowed to progress for 30 days in control, Ccr2RFP/RFP, Cx3cr1GFP/GFP, and Ccr2RFP/RFPCx3cr1GFP/GFP mice. Tumors were analyzed at two different timepoints; growing tumors (tumor volume 1,000mm3) and resistant tumors (approximately 5 weeks under treatment of Braf/Meki). B, Representative flow cytometry plot showing infiltrated macrophages (F4/80+ cells) gated on live/CD45+/CD11b+/Ly6g- cells in growing melanomas. Offset histogram showing F4/80 expression on growing tumors. C, Bar graph showing the frequencies of macrophages (F4/80+) of total live cells and dot plot showing the frequencies of two subsets of macrophages (F4/80high and F4/80low) among both total live cells in growing melanomas. Data are shown as mean ± S.D. (n=4, control; n=4, Cx3cr1GFP/GFP; n=3, Ccr2RFP/RFP; and n=3, Ccr2RFP/RFPCx3cr1GFP/GFP mice). Statistical analysis performed with one-way ANOVA (*P 0.05, ***<P 0.001). D, Scheme of Cx3cr1GFP/GFP mice and experimental procedures. Control and Cx3cr1GFP/GFP mice were transplanted with TBP cells subcutaneously and treated with Braf/Meki daily. Tumor tissues were harvested when rebound tumors reached endpoint (day 67). E, Average tumor volume curves in response to targeted therapy from control and Cx3cr1GFP/GFP mice. Tumor volumes were measured and analyzed by two-tailed Student’s t test at each timepoint. Data are shown as mean ± S.D, n= 3/group (ns, not significant). F, Change in tumor volume at maximal response to Braf/Meki, comparing predefined minimal tumor volume (the smallest tumor size during targeted therapy) to the treatment start volume V0, to assess tumor volume reducing effects. Data are shown as mean ± S.D. Statistical analysis performed with two-tailed Student’s t test (ns, not significant). G, Average percent changes in final tumor volume comparing control and Cx3cr1GFP/GFP mice. Data are represented as mean ± S.D. Statistical analysis performed with two-tailed Student’s t test (ns, not significant). H, Kaplan-Meier survival curves comparing endpoint of control mice (black curve) and Cx3cr1GFP/GFP mice (blue curve) that received Braf/Meki. Survival endpoints were defined as the time at which rebound tumors exceeded pre-treatment size. Statistical significance was determined by Log-rank Mantel-Cox test (ns, not significant). Controls are Ccr2RFP/+Cx3cr1GFP/+ heterozygous mice in b-h.
Figure 5. Ccr2 deficiency results in improved antitumor efficacy and delays resistance to targeted therapy.
A, Scheme of Ccr2RFP/RFP mice and experimental procedures. Control and Ccr2RFP/RFP mice were transplanted with TBP cells subcutaneously and treated with Braf/Meki daily. Tumor tissues were harvested when rebound tumors reached endpoint (day 67). B, Average tumor volume curves in response to Braf/Meki from control and Ccr2RFP/RFP mice. Tumor volumes were analyzed by two-tailed Student’s t test at each time. Data are shown as mean ± S.D, n= 7/group (***P 0.001, ****P 0.0001). C, Change in tumor volume at maximal response to Braf/Meki, comparing predefined minimal tumor volume (the smallest tumor size during targeted therapy) to the treatment start volume V0. Data are shown as mean ± S.D. Statistical analysis performed with two-tailed Student’s t test (*P 0.05). D, Waterfall plots showing percent change in final tumor volume at day 37 normalized to pre-treatment baselines. Each bar in the waterfall plots represents a single mouse (n=7/group). Summary graph with average percent change in final volume between control and Ccr2RFP/RFP recipients. Data are represented as mean ± S.D. Statistical analysis performed with two-tailed Student’s t test (***P 0.001). E, Kaplan-Meier survival curves comparing endpoint of control mice (black curve) and Ccr2RFP/RFP mice (red curve) that received Braf/Meki. Survival endpoints were defined as rebound tumors upon exceeding pre-treatment initial size. Statistical significance was determined by Log-rank Mantel-Cox test (***P 0.001). F, Representative flow cytometry plot showing Ccr2+ macrophages gated on live/CD45+/CD11b+/Ly6g-/F4/80+ cells in resistant melanomas. Offset histogram showing Ccr2 expression on resistant tumor tissues. G, Summary bar graph showing the percentage of Ccr2+ macrophages out of total macrophages (F4/80+) in tumors. Data are shown as mean ± S.D. (n=8, control; n=4, Cx3cr1GFP/GFP; n=5, Ccr2RFP/RFP; and n=4, Ccr2RFP/RFPCx3cr1GFP/GFP mice). Statistical analysis performed with one-way ANOVA (****P 0.0001, ns not significant). Controls are Ccr2RFP/+ in b-e, Ccr2RFP/+Cx3cr1GFP/+ heterozygous mice in f-g.
We next sought to define the abundance of Ccr2+ myeloid cells in TBP tumor progression and regression. Heterozygous Ccr2RFP/+ mice were used to track the dynamics of Ccr2+ cells throughout Braf/Meki treatment. Flow cytometry analysis of tumor tissues was undertaken to quantify Ccr2+ populations at three different time points: untreated-growing melanomas; regressed-persistent tumors following 3 weeks of treatment; and Braf/Meki-resistant melanomas following 6 weeks of treatment (Figure 6A). We observed a gradual decrease in the F4/80high subset and an increase in the F4/80low subset in the regressed-persistent state. This was followed by a high accumulation of F4/80+ macrophages in resistant melanomas compared with untreated-growing or regressed-persistent tumor tissue (Figure 6, B, C). Importantly, we identified robust infiltration of Ccr2+ macrophages during the regressed-persistent state, which increased from 37.2% ± 7.8% to 64.3% ± 11.9% (Figure 6, D and E). We further analyzed Ccr2 expression by combining with Ly6C expression under three different conditions and separated into three subsets: F4/80high macrophages, F4/80low and Ly6C+ monocytes. We observed an influx of extravasated Ly6Chigh blood monocytes into regressed tumors and found that the expression of Ccr2 continuously decreased as the monocytes appeared to differentiate into macrophages (Figure 6, F, G). Multiple chemokines related to Ccr2-mediated recruitment of TAMs were screened through qRT-PCR. All chemokines (Ccl2, Ccl7, Ccl8 and Ccl12) were increased in the regressed-persistent state with Ccl8 being the most highly upregulated chemokine (Figure 6H). These observations suggest that Ccr2-mediated monocyte infiltration accounts for the accumulation of macrophages in resistant TBP melanomas. This is consistent with previous studies on human patient samples where Ccl8 was identified as one of the most upregulated ligands in acquired MAPKi-treated, resistant tumor tissues (16). Taken together, this indicates that a robust increase of Ccr2+ cells during the regressed-persistent state could support cancer cell survival and lead to the emergence of drug resistance. This underscores the importance of understanding the signaling mediated by Ccr2+ cells as a component of the molecular mechanisms of drug resistance.
Figure 6. High infiltration of Ccr2+ macrophages during the drug-tolerant regressed-persistent state.
A, Scheme of Ccr2RFP/+ heterozygous mice that enable tracking Ccr2-positive cells in tumors. Timeline for tissue harvest at three different timepoints. TBP cells transplanted to Ccr2RFP/+ recipients were collected at specified timepoints (1. untreated-growing melanoma upon reaching 1000mm3; 2. regressed-persistence tumors following 3 weeks of treatment; 3. resistant melanoma following 6 weeks of treatment). B, Representative flow cytometry plot showing infiltrated macrophages (F4/80+, F4/80high and F4/80low cells) gated on live/CD45+/CD11b+/Ly6g- cells in melanomas at specified time points. C, Summary graph showing the percentage of macrophages (F4/80+) of total CD45 positive cells in tumors at specified time points (left), and percentage of two subsets of macrophages (F4/80high and F4/80low) among total CD45 positive cells (right). Data are shown as mean ± S.D. (n=7, growing melanoma; n=8 persistence melanoma; n=13, resistant melanoma). Statistical analysis performed with one-way ANOVA (**P 0.01, ****P 0.0001). Differences between sexes was determined by two-way ANOVA (ns, not significant). D, Representative flow cytometry plot showing Ccr2+ macrophages gated on live/CD45+/CD11b+/ Ly6g-/F4/80+ cells in melanomas at specified timepoints. E, Summary graph showing the percentage of Ccr2+ macrophages out of total macrophages (F4/80+) in tumors at specified timepoints (left), and between males and females (right). Data are shown as mean ± S.D. (n=7, growing melanoma; n=8 persistence melanoma; n=13, resistant melanoma). Statistical analysis performed with one-way ANOVA (****P 0.0001). F, Representative flow cytometry plots showing macrophages and monocytes at specified timepoints: F4/80high macrophages (red; CD45+CD11b+Ly6G-Ly6C-F4/80high), F4/80low macrophages (blue; CD45+CD11b+Ly6G-Ly6C-F4/80low) and Ly6C+ monocytes (yellow; CD45+CD11b+Ly6G-Ly6ChighF4/80low-mid). Representative histograms showing Ccr2-expressing cells in three subsets (down left). G, Summary of bar graph showing the percentage of Ccr2+ cells of total myeloid cells in tumors at specified timepoints (bottom right). Data are shown as mean ± S.D. (n=10, growing melanoma; n=10 regressed-persistent melanoma; n=9, resistant melanoma). Statistical analysis performed with one-way ANOVA (****P 0.0001). H, mRNA expression of key Ccr2 ligands at three timepoints (untreated, regressed-persistent, and resistant tumor). n=6/group. β-actin expression was used as a loading control. The data is summarized from at least three independent experiments. The data are represented as mean ± S.D and significance was calculated using a two-tailed Student’s t-test (ns, not significant, *P 0.05, **P 0.01, ***P 0.001). Controls are Ccr2RFP/+ heterozygous mice in b-h.
Macrophages alter the evolution of Braf/Meki resistance phenotypes in melanoma
We found that lack of Ccr2 can delay the onset of Braf/Meki resistance and regrowth in TBP melanomas, though all tumors eventually rebound under prolonged treatment. This prompted us to assess differences in rebounded tumors from Ccr2RFP/RFP mice and determine how Ccr2 deficiency may influence the drug resistance characteristics that develop. We first collected resistant tumor tissue from control and Ccr2RFP/RFP mice and isolated melanoma cells by FACS (Figure 7A). Resistant tumor cells were treated with Braf/Meki while cultured in a 2D monolayer or as 3D tumor spheres. After 2 days, 2D cultured cells were stained with S100, a melanoma marker, in combination with Ki67. We found that despite Braf/Meki treatment, there were high numbers of Ki67+S100+ cells in cultures derived from control recipients, thus demonstrating proliferation under drug pressure and the establishment of stable resistance. In contrast, cultured cells derived from Ccr2RFP/RFP knockout recipients showed a significant decrease in the number of proliferating Ki67+S100+ melanoma cells (Supplementary Figure 4, A and B). In 3D cultures, spheroid volume was quantified over 10 days to determine sensitivity to targeted therapy in vitro. We confirmed that tumor cells from resistant-control mice maintained sphere volume and exhibited no reduction under continuous drug treatment, whereas spheroids derived from resistant-Ccr2RFP/RFP mice showed significant reduction in volume (Figure 7B, C, D). In addition, we observed an upregulation of genes involved in survival and cell cycle regulation (MKi67, CCND1, CCNE2, CCNA2 and CCNB2) in control tumor spheroids (Supplementary Figure 4C) and the ratio of Ki67 expression was higher in control tumor spheroids compared to those derived from Ccr2RFP/RFP animals (Figure 7E). These data suggests that resistant melanoma cells arising from Ccr2RFP/RFP mice reacquire sensitivity to targeted therapy in vitro. Differences in resistance phenotypes was further confirmed via secondary in vivo transplantation with resistant melanoma cells collected from both control and Ccr2RFP/RFP mice (Supplementary Figure 4D). C57BL/6J animals bearing control-derived resistant tumor cells showed maintenance of resistance and exhibited a stable resistance phenotype. In contrast, Ccr2RFP/RFP-derived resistant tumors exhibited a significant reduction in tumor volume under targeted therapy and appear to favor a reversible resistance mechanism. Consistent with this, the median survival for control-derived secondary tumors was 12 days after initiation of drug treatment and 32 days in mice bearing Ccr2RFP/RFP-derived tumors (Supplementary Figure 4, E, F).
Figure 7. Ccr2+ macrophages influence the drug resistance phenotype of TBP melanoma cells.
A, Scheme of experimental procedure and timeline of treatment schedule in FACS-sorted resistant TBP cells in 3D spheroid cultures. Resistant melanoma cells were harvested from control and Ccr2RFP/RFP mice and isolated by FACS. Sorted cells were cultured with Braf/Meki for 10 days. B, Representative boxplot showing the changes in spheroid volume from control and Ccr2RFP/RFP recipients. Spheroids grown for 3 days starting from 5,000 cells and treated with Braf/Meki over a period of 10 days. Data are shown as mean ± S.D. (n=8 spheroids/group). Statistical analysis performed with Two-way ANOVA with Sidak’s multiple comparisons (**P <0.01, ****P <0.0001). C, Representative bright field images of resistant melanoma cell-derived tumor spheroids following treatment for 10 days with Braf/Meki. Comparison of day 0 before treatment and day 10 (10 magnification, Scale bar, 100µm). D, Resistant tumor spheroid section stained with hematoxylin and eosin at 10 days under Braf/Meki from control and Ccr2RFP/RFP mice. (10 magnification, Scale bar, 100µm). E, Representative immunofluorescence images of tumor spheroids for Ki67 (proliferation) after treatment with Braf/Meki from control and Ccr2RFP/RFP recipients (10 magnification, Scale bar, 100µm). Quantification of immunofluorescence staining of Ki67+ cells within tumor spheres. Data are shown as mean ± S.D. (n=40–41 spheroids/group). F, Scheme of experimental procedure and timeline of macrophage and tumor in 3D co-cultures. Representative flow cytometry plot showing F4/80+ macrophages after magnetic bead enrichment. F4/80+ macrophages stained with Wright-Giemsa staining. G, Boxplot showing the changes in spheroid volume when co-cultured with macrophages from WT and Ccr2RFP/RFP resistant tumor tissues. Data are shown as mean ± S.D. (n=31 spheroids, WT macrophage group; n=26 spheroids, Ccr2RFP/RFP macrophage group). Statistical analysis performed with Two-way ANOVA with Sidak’s multiple comparisons (****P <0.0001). H, Representative immunofluorescence images of tumor spheroids co-cultured with either WT or Ccr2RFP/RFP macrophages for Ki67 after 10 days of treatment with Braf/Meki (left). Bar graph showing quantification of Ki67 staining in tumor spheroids. Data are shown as mean ± S.D. (n=55 spheroids, WT macrophage group; n=28 spheroids, Ccr2 KO macrophage group) Statistical analysis performed using a two-tailed Student’s t-test (****P <0.0001)
We next sought to determine whether the addition of Ccr2+ monocyte-derived macrophages ex vivo could alter the resistance phenotype of tumor cells. Therefore, we isolated macrophages from rebounded tumor tissue of control and Ccr2RFP/RFP mice using F4/80+ magnetic beads. Tumor cells derived from Ccr2RFP/RFP mice, which display unstable resistance with regained sensitivity to targeted therapy, were cultured as spheroids. Following the establishment of tumor spheroids after a 2-day incubation period, macrophages that were isolated from resistant tumors from both control and Ccr2RFP/RFP mice were added for a melanoma cell/macrophage co-culture incubation of 1 day. Subsequently, the co-cultured cells were treated with Braf/Meki for 10 days (Figure 7F). Tumor spheres co-cultured with Ccr2+ macrophages led to a statistically significant increase in spheroid volume starting on day 6 of Braf/Meki treatment as compared to Ccr2RFP/RFP macrophage co-cultured spheroids (Figure 7G). On day 10 of treatment, spheroids were collected and stained with Ki67. A significantly higher abundance of Ki67+ cells were found in tumor spheroids co-cultured with macrophages isolated from resistant control tumors (Figure 7H).
Since Ccr2+ monocyte-derived macrophages can reverse TBP cell sensitivity to treatment and promote a more resistant phenotype, we conducted bulk mRNAseq to determine their potential contributions to tumor cell resistance phenotypes. To do this, macrophages were sorted from rebound tumors collected from both control and Ccr2RFP/RFP mice. Datasets were then analyzed for changes in gene expression between melanoma macrophages from Ccr2+/RFP and Ccr2RFP/RFP recipients (Figure 8A). Principal component analysis revealed distinct differences in the transcriptional profiles (Figure 8B). We observed 256 differentially upregulated expressed genes (log2FC >1, adj. p <0.05) in control macrophages and 105 upregulated in Ccr2RFP/RFP macrophages (Figure 8C). Among the top significantly upregulated genes in control were genes normally associated with monocyte recruitment and monocyte derived macrophages (Ccr2, Plac8 and Ly6c2) (43). Furthermore, control macrophages displayed increased expression of the alternatively activated macrophage marker Chil3, which promotes tumor growth and is associated with poor prognosis in various types of solid tumors (Figure 8D) (44)(45,46). To explore the potential functional differences between these two types of macrophages, we also performed gene ontology (GO) analysis and gene set enrichment analysis (GSEA). Our analysis revealed enrichment in activities related to chemokine production in control macrophages (Figure 8E, F).
Figure 8. Gene expression profiling of macrophages (Ctrl vs. Ccr2 KO) from resistant tumors.
A, Scheme of experimental procedures for isolating macrophages via FACS (n=5 per group). B, PCA plot of bulk mRNA sequencing results from all 10 macrophage samples. C, Volcano plot showing differentially expressed genes in macrophages from Ccr2RFP/RFP resistant tumors (orange, left) and control resistant tumors (green, right) (>2 fold change, adj.p<0.05). Venn diagram of 361 genes differentially expressed in control resistant macrophages compared to Ccr2RFP/RFP resistant macrophages. D, Heatmap showing all differentially expressed genes between control resistant macrophages and Ccr2RFP/RFP resistant macrophages (left). Top 25 upregulated genes in control resistant macrophages compared to Ccr2RFP/RFP resistant macrophages. Gene list was ordered based on the adjust p-values of each gene. E, Gene Ontology analysis showing the top list of upregulated biological process in control macrophages compared to Ccr2RFP/RFP macrophages (Grey bar, normalized enrichment score (NES); red line, -log(adj.p)). F, Gene set enrichment analysis (GSEA) showing enrichment score of “regulation of chemokine production” biological process (top) and heatmap showing the upregulated genes in control macrophages that are classified in this biological process (bottom). Controls are Ccr2RFP/+ heterozygous mice.
Finally, the infiltration of other immune subsets in resistant tumor tissues of both control and Ccr2RFP/RFP mice was assessed. In Ccr2RFP/RFP mice, there was a trend towards decreased T cell infiltration and less exhausted T cells (lower amounts of PD1+Tim3+). Interestingly, we observed high infiltration of B cells and CD8+ T cells with significantly higher levels of granzyme B in Ccr2RFP/RFP melanomas. These findings are consistent with previous reports regarding elevated levels of granzyme B associated with enhanced disease-free survival in pancreatic tumors (47) (Supplementary Figure 5A-F).
Discussion
Macrophages are integral components in building unique tumor microenvironments and have risen as strong candidate targets for anti-tumor therapies (40). Multiple ongoing clinical trials (NCT03455764, NCT03101254, NCT0302330) are assessing the effect of targeting TAMs in advanced melanoma patients. So far, the most common strategies for a macrophage-based therapy are TAM depletion or inhibition of monocyte/macrophage recruitment into tissues (48,49). However, the results from these efforts remain inconclusive due to the rebound effect of hematopoietic cells, which can result in an unexpected influx of monocytes, leading to unsustainable efficacy and adverse effects (26,50–52). Thus, some macrophage targeting studies are shifting in focus towards altering and reprogramming macrophage phenotypes. To achieve this, stage-specific macrophage functional diversity needs to be defined and comprehensively cataloged with respect to macrophage ontogeny, activation state, and the associated tumor microenvironment (53,54). Here, we utilized advanced stage murine models with a mature melanoma microenvironment to capture the dynamic alterations in macrophage subsets during the therapy-induced regression phase, the drug-tolerant phase and the onset of therapy resistance.
Tissue resident macrophages (TRMs) display unique characteristics according to their lineage and origin. TRMs are often seeded into tissues during embryogenesis from progenitors in the yolk sac and fetal liver and maintain their population through self-replication. TRMs have also been shown to possess distinct functions and responses compared to bone marrow-derived macrophages. However, similar to bone marrow-derived macrophages, their fates and functions are not static and can vary under pathological conditions. All macrophages exhibit functional plasticity and can adapt to changes in their local tissue environment (55,56). This ability is reflected in the altered transcriptional states observed in macrophages under different pathological conditions and drug treatments (57). Notably, previous studies have shown that macrophages with heterogenous origins exhibit functions in tumor settings that differ from homeostasis (47). Given this, we sought to define the contribution of macrophage subsets in promoting therapy resistance and elucidate how changes in macrophage presence could ultimately influence the established therapeutic resistance phenotype. Through the experiments reported here, we found that Ccr2+ macrophages are strong influencers of the phenotype and timing of drug resistance, in this model.
Initially, previous studies generated an expectation that the highest infiltration of this important Ccr2+ monocyte and macrophage population would be detected during the first week of targeted therapy, when melanoma tumors regress rapidly. It has long been acknowledged that damage-associated molecular patterns (DAMPs) released from dead cells result in the recruitment of monocytes and macrophages (58,59). Instead, the highest infiltration levels of Ccr2+ cells were found when the average tumor volume reached a minimum and continued through a drug-tolerant plateau phase. This plateau, or a minimal tumor persistence stage, can been defined as a state characterized by stabilization of tumor cell numbers and, if detectable, tumor size. Cells undergoing transcriptional and epigenetic changes during this state have been referred to as drug-tolerant persister cells, minimal residual disease cells, dormant cells, and/or cancer stem-like cells (7,8,17,60). Since the influx of new monocytes at this stage coincides with significant alterations in melanoma cell sensitivity to therapy, the most dynamic landscape of non-mutational cancer cell reprogramming appears to occur during drug-tolerant persistence, immediately prior to re-emerging as a drug-resistant tumor (61,62). Our findings provide important clues regarding drug resistance in human patients, particularly those with MAPK inhibitor resistance, as an increase in the number of macrophages has been observed in such cases (63).
Functional features of Ccr2+ monocytes/macrophages within the TME have been reported to promote tumor growth and metastasis and impede therapy responses by negatively regulating angiogenesis and T cell activity (46). Here, we add evidence that Ccr2+ monocyte-derived macrophages contribute to the shaping the processes that usher tumor cells towards specific modes of therapeutic resistance. However, we were unable to prevent eventual tumor regrowth under treatment in all four mouse strains, indicating compensatory pathways develop to confer therapy resistance despite Ccr2+ cell depletion. This highlights the need for further investigation into the signaling mechanisms that promote stable resistance pathways from a microenvironment containing Ccr2+ macrophages and unstable resistance arising from tumors in a Ccr2 null microenvironment. These mechanisms may not be singular, and a more comprehensive understanding of the complex interactions within the TME before and after treatment is crucial to create effective therapies. To begin, we profiled the non-Ccr2 macrophage population in Ccr2 knockout tumors by bulk mRNAseq, in addition to identifying changes to leukocytes in response to Ccr2 absence. These cellular and gene expression profiles will provide ample opportunities to test new hypotheses. In summary, we show that the influx of Ccr2+ monocytes during the minimal tumor persistence state is likely an important therapeutic window, pointing towards a goal to eliminate these residual tumor cells and thus improve long-term melanoma cell eradication.
Supplementary Material
Statement of significance.
Ccr2+ melanoma macrophages that are active in tumors during the drug-tolerant persister state following targeted therapy-induced regression are key contributors directing melanoma cell reprogramming toward specific therapeutic resistance trajectories.
Acknowledgements
We would like to thank Dr. Praveen Sethupathy and Dr. Robert S. Weiss for providing critical scientific comments, Dr. Lu Huang for expertise in flow cytometry, the staff at the Center for Animal Resources and Education for research animal husbandry, and the core facilities of Cornell Institute of Biotechnology for their help with flow cytometry and sequencing. The work presented here was supported by Pilot Award #816826 from the Melanoma Research Alliance and NIH R01 5R01AR075755 to A. White. Research reported in this publication was supported by the Office Of the Director, National Institutes Of Health of the National Institutions of Health under Award Number T32ODO011000 to D. Kim. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Footnotes
The authors declare no potential conflicts of interest
References
- 1.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat J-P, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang T, Dutton-Regester K, Brown KM, Hayward NK. The genomic landscape of cutaneous melanoma. Pigment Cell Melanoma Res. 2016;29:266–83. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–9. [DOI] [PubMed] [Google Scholar]
- 4.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trunzer K, Pavlick AC, Schuchter L, Gonzalez R, McArthur GA, Hutson TE, et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J Clin Oncol. 2013;31:1767–74. [DOI] [PubMed] [Google Scholar]
- 6.Müller J, Krijgsman O, Tsoi J, Robert L, Hugo W, Song C, et al. Low MITF/AXL ratio predicts early resistance to multiple targeted drugs in melanoma. Nat Commun. 2014;5:5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, et al. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell. 2013;23:811–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravindran Menon D, Das S, Krepler C, Vultur A, Rinner B, Schauer S, et al. A stress-induced early innate response causes multidrug tolerance in melanoma. Oncogene. 2015;34:4448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Allen EM, Wagle N, Sucker A, Treacy DJ, Johannessen CM, Goetz EM, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4:94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi H, Moriceau G, Kong X, Lee M-K, Lee H, Koya RC, et al. Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun. 2012;3:724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 2011;29:3085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whittaker SR, Theurillat J-P, Van Allen E, Wagle N, Hsiao J, Cowley GS, et al. A genome-scale RNA interference screen implicates NF1 loss in resistance to RAF inhibition. Cancer Discov. 2013;3:350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das Thakur M, Salangsang F, Landman AS, Sellers WR, Pryer NK, Levesque MP, et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013;494:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hugo W, Shi H, Sun L, Piva M, Song C, Kong X, et al. Non-genomic and Immune Evolution of Melanoma Acquiring MAPKi Resistance. Cell. 2015;162:1271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rambow F, Rogiers A, Marin-Bejar O, Aibar S, Femel J, Dewaele M, et al. Toward Minimal Residual Disease-Directed Therapy in Melanoma. Cell. 2018;174:843–855.e19. [DOI] [PubMed] [Google Scholar]
- 18.Marine J-C, Dawson S-J, Dawson MA. Non-genetic mechanisms of therapeutic resistance in cancer. Nat Rev Cancer. 2020;20:743–56. [DOI] [PubMed] [Google Scholar]
- 19.Hoek KS, Eichhoff OM, Schlegel NC, Döbbeling U, Kobert N, Schaerer L, et al. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res. 2008;68:650–6. [DOI] [PubMed] [Google Scholar]
- 20.Bröcker EB, Zwadlo G, Holzmann B, Macher E, Sorg C. Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. Int J Cancer. 1988;41:562–7. [DOI] [PubMed] [Google Scholar]
- 21.Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, et al. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: Possible involvement of TNFα and IL-1α. International Journal of Cancer. 2000; [PubMed] [Google Scholar]
- 22.Jensen TO, Schmidt H, Møller HJ, Høyer M, Maniecki MB, Sjoegren P, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009;27:3330–7. [DOI] [PubMed] [Google Scholar]
- 23.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–86. [DOI] [PubMed] [Google Scholar]
- 25.Ricketts TD, Prieto-Dominguez N, Gowda PS, Ubil E. Mechanisms of macrophage plasticity in the tumor environment: manipulating activation state to improve outcomes. Front Immunol. 2021;12:642285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352:aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell. 2019;35:588–602.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassetta L, Pollard JW. A timeline of tumour-associated macrophage biology. Nat Rev Cancer. 2023; [DOI] [PubMed] [Google Scholar]
- 29.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–60. [DOI] [PubMed] [Google Scholar]
- 33.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rooijen N, van Kesteren-Hendrikx E. Clodronate liposomes: perspectives in research and therapeutics. J Liposome Res. 2002;12:81–94. [DOI] [PubMed] [Google Scholar]
- 35.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Xu X, Feng X, Murphy PM. The Macrophage-depleting Agent Clodronate Promotes Durable Hematopoietic Chimerism and Donor-specific Skin Allograft Tolerance in Mice. Sci Rep. 2016;6:22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sunderkötter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–7. [DOI] [PubMed] [Google Scholar]
- 38.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu C, Edwards EW, Tacke F, Angeli V, Llodrá J, Sanchez-Schmitz G, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsou C-L, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung S-H, Hwang B-H, Shin S, Park E-H, Park S-H, Kim CW, et al. Spatiotemporal dynamics of macrophage heterogeneity and a potential function of Trem2hi macrophages in infarcted hearts. Nat Commun. 2022;13:4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang Q, Li L, Pang Y, Zhu W, Meng L. An update on Ym1 and its immunoregulatory role in diseases. Front Immunol. 2022;13:891220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Libreros S, Garcia-Areas R, Iragavarapu-Charyulu V. CHI3L1 plays a role in cancer through enhanced production of pro-inflammatory/pro-tumorigenic and angiogenic factors. Immunol Res. 2013;57:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van de Velde L-A, Allen EK, Crawford JC, Wilson TL, Guy CS, Russier M, et al. Neuroblastoma Formation Requires Unconventional CD4 T Cells and Arginase-1-Dependent Myeloid Cells. Cancer Res. 2021;81:5047–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Y, Herndon JM, Sojka DK, Kim K-W, Knolhoff BL, Zuo C, et al. Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity. 2017;47:323–338.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barceló C, Sisó P, de la Rosa I, Megino-Luque C, Navaridas R, Maiques O, et al. M-CSF as a therapeutic target in BRAFV600E melanoma resistant to BRAF inhibitors. Br J Cancer. 2022;127:1142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mok S, Koya RC, Tsui C, Xu J, Robert L, Wu L, et al. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Res. 2014;74:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonapace L, Coissieux M-M, Wyckoff J, Mertz KD, Varga Z, Junt T, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–3. [DOI] [PubMed] [Google Scholar]
- 51.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–59. [DOI] [PubMed] [Google Scholar]
- 52.Cannarile MA, Weisser M, Jacob W, Jegg A-M, Ries CH, Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17:34–40. [DOI] [PubMed] [Google Scholar]
- 54.Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541–66. [DOI] [PubMed] [Google Scholar]
- 55.Cortez-Retamozo V, Engblom C, Pittet MJ. Remote control of macrophage production by cancer. Oncoimmunology. 2013;2:e24183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pombo Antunes AR, Scheyltjens I, Lodi F, Messiaen J, Antoranz A, Duerinck J, et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci. 2021;24:595–610. [DOI] [PubMed] [Google Scholar]
- 58.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5:a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dal-Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CHY, Petri B, et al. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J Exp Med. 2015;212:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su Y, Wei W, Robert L, Xue M, Tsoi J, Garcia-Diaz A, et al. Single-cell analysis resolves the cell state transition and signaling dynamics associated with melanoma drug-induced resistance. Proc Natl Acad Sci USA. 2017;114:13679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song C, Piva M, Sun L, Hong A, Moriceau G, Kong X, et al. Recurrent Tumor Cell-Intrinsic and -Extrinsic Alterations during MAPKi-Induced Melanoma Regression and Early Adaptation. Cancer Discov. 2017;7:1248–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen S, Vagner S, Robert C. Persistent cancer cells: the deadly survivors. Cell. 2020;183:860–74. [DOI] [PubMed] [Google Scholar]
- 63.Smith MP, Sanchez-Laorden B, O’Brien K, Brunton H, Ferguson J, Young H, et al. The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFα. Cancer Discov. 2014;4:1214–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The bulk RNAseq data generated in this study can be found in the Gene Expression Omnibus (GEO) (RRID:SCR_005012) database. GEO accession number for data generated in this study is GSE226835. All other raw data are available upon request from the corresponding author.