Abstract
Sleep-wake and fasting-feeding are tightly coupled behavioral states that require coordination between several brain regions. The mammalian lateral hypothalamus (LH) is a functionally and anatomically complex brain region harboring heterogeneous cell populations that regulate sleep, feeding, and energy metabolism. Significant attempts were made to understand the cellular and circuit bases of LH actions. Rapid advancements in genetic and electrophysiological manipulation help to understand the role of discrete LH cell populations. The opposing action of LH orexin/hypocretin and melanin-concentrating hormone (MCH) neurons on metabolic sensing and sleep-wake regulation make them the candidate to explore in detail. This review surveys the molecular, genetic, and neuronal components of orexin and MCH signaling in the regulation of sleep and metabolism.
Keywords: sleep, metabolism, orexin, MCH, feeding, wake, lateral hypothalamus
Introduction
Living beings on Earth maintain internal stability despite enormous environmental challenges. This process of maintenance of physiological stability is called homeostasis. In the mammalian body, homeostasis applies to the processes that regulate critical physiological parameters such as blood pressure, heart rate, plasma glucose, body temperature, feeding, and sleep. Feeding and sleep are mutually exclusive behaviors requiring distinct but interdependent homeostatic needs. To survive, organisms require strong coordination of these behaviors to achieve their respective homeostatic conditions. For example, wakefulness is required for foraging and food consumption. The mammalian hypothalamus crucially regulates these homeostatic functions.
The hypothalamus is one of the most complex and heterogeneous brain structures involved in the regulation of numerous homeostatic functions by integrating peripheral and central signals of circadian rhythms, sleep pressure, and energy metabolism. This diverse region of the brain is subdivided into 11 anatomically distinct nuclei having 34 neuronal and 11 non-neuronal cell types, cumulative actions of these cells regulate the sleep and metabolic processes (Chen et al., 2017; Rossi et al., 2019; Jha et al., 2022; Bear et al., 2023). The complex neuronal network made up of the projections from these nuclei to the entire brain, including intrahypothalamic connections regulates behavior and physiology. The metabolic aberrations in disturbed sleep conditions and the prevalence of sleep abnormalities in metabolic syndrome indicate the involvement of proximal hypothalamic neuronal circuitry regulating sleep and metabolism. The mechanistic understanding of these networks would be essential and bring wide-ranging clinical significance.
Hypothalamic regulation of sleep and metabolism
The interactive action of arousal and sleep-promoting areas of the mammalian brain involves the regulation of the sleep-wake cycle (Saper and Fuller, 2017). Wake-sleep transition is the manifestation of inhibition of sleep or wake-promoting areas in the brainstem and hypothalamus (Saper, 2006). The pathway that stimulates and maintains wakefulness consists of glutamatergic inputs from parabrachial and pedunculopontine tegmental nuclei (PPT) to the basal forebrain (BF), and GABAergic and cholinergic neurons in the BF that innervates the cerebral cortex (Saper and Fuller, 2017). Further, GABAergic neurons in the lateral hypothalamus (LH) promote wakefulness by inhibiting sleep-promoting neurons in the thalamus and preoptic area (Herrera et al., 2016; Venner et al., 2016). Wake-promoting orexinergic neurons in the LH that mainly use glutamate to transmit their signals are spatially intermingled with sleep-promoting melanin-concentrating-hormone (MCH)-expressing cells (Rosin et al., 2003). The MCH-expressing cells are primarily GABAergic and found in LH and Zona Incerta (ZI) (Adamantidis and de Lecea, 2008b; Rolls et al., 2010). The projections of both orexin and MCH-expressing neurons to the cortex, hippocampus, amygdala, nucleus accumbens (NAc), hypothalamus, thalamus, ventral tegmental area (VTA), locus coeruleus (LC), and raphe nucleus indicating their intra- and extrahypothalamic functions (Concetti and Burdakov, 2021). Wake promotion inhibits sleep-promoting circuitries lie mainly in the hypothalamic ventrolateral preoptic (VLPO) and median preoptic (MnPO) areas. Sleep-active GABAergic neurons of preoptic areas and brainstem project to the wake-promoting area and inhibiting them in regulated manners (Saper and Fuller, 2017). This mutual inhibition regulates the sleep-wake transition.
Another critical role of the hypothalamus is to maintain energy homeostasis by regulating food intake. Like sleep, energy metabolism is also regulated by mutual inhibitory circuitries. The arcuate nucleus (ARC) harbors appetite-promoting Neuropeptide Y (NPY) and Agouti-related protein (AgRP) neurons that mutually inhibit the appetite-suppressing pro-opiomelanocortin (POMC) and amphetamine-related transcript (CART) neurons. These sets of neurons act as sensors of satiety-promoting leptin and appetite-stimulating ghrelin hormones. Leptins inhibit NPY/AgRP neurons and activate POMC/CART whereas ghrelin activates NPY/AgRP and inhibit POMC/CART neurons. The ARC integrates these peripheral signals and transmits them to other hypothalamic areas such as the dorsomedial nucleus (DMH), the paraventricular nucleus (PVH), and the LH (Milbank and Lopez, 2019). The orexin neurons in LH sense peripheral hormonal signals and levels of metabolites like glucose and amino acids. This is evident from the anatomical connection of orexin neurons to the other metabolic nuclei of the hypothalamus. Further, electrophysiological studies reveal that wake-promoting orexin neurons functionally regulate the NPY, POMC, and glucose-responsive neurons in the ARC and ventromedial nucleus of the hypothalamus (VMH) (Muroya et al., 2004). Interestingly, sleep-promoting neurons also regulate metabolism as fasting increases the expression of MCH levels, and activation of MCH neurons reduces energy expenditures (Pissios et al., 2006).
The LH is the heterogeneous structure located in the posterior hypothalamus and its diverse cell populations have been implicated in the regulation of an array of fundamental physiological processes that includes sleep, feeding, and energy metabolism (Stuber and Wise, 2016). The LH harbors heterogeneous neuronal subtypes including orexin, MCH, GABA, glutamatergic, galanin, neurotensin-releasing (Nts), leptin-receptor (LepRb) expressing neurons, and substance P-releasing neurons (Mickelsen et al., 2019; Rossi et al., 2019). In this review, we discuss the LH’s orexin and MCH neuronal circuitries that regulate energy metabolism and sleep.
LHMCH neurons
Melanin-concentrating hormone neurons are abundant in the LH, though few cells are located within the ZI. The LHMCH neurons were reported to send extensive projections to different brain areas (Skofitsch et al., 1985; Bittencourt et al., 1992; Risold et al., 1997; Murray et al., 2000; Bittencourt, 2011), including structures involved in regulating sleep-wake cycle, feeding behavior, body weight and energy balance (Qu et al., 1996; Shimada et al., 1998; Verret et al., 2003; Jego et al., 2013; Konadhode et al., 2013; Yoon and Lee, 2013). Besides MCH peptide, LHMCH neurons co-express many other neurotransmitters and neuropeptides including CART (Broberger, 1999; Elias et al., 2001), nesfatin-1 (Fort et al., 2008), neuropeptide-EI and neuropeptide-GE (Nahon et al., 1989; Bittencourt, 2011). To date, the molecular phenotypes of LHMCH neurons is still a matter of debate to decipher whether these neurons co-release GABA, glutamate, or both. Indeed, previous studies have demonstrated that LHMCH neurons express glutamic acid decarboxylase (GAD) 67 and GAD65 a key enzyme in GABA synthesis (Elias et al., 2001; Harthoorn et al., 2005; Shin et al., 2007). In addition, the results from the immunohistochemical study have reported that LHMCH terminals express the vesicular GABA transporter (VGAT) that plays an essential role in carrying GABA from the neuronal cytoplasm into the synaptic cleft (Del Cid-Pellitero and Jones, 2012). In contrast, consecutive work showed that LHMCH neurons do not overlap with VGAT-positive GABAergic neurons and were not able to express VGAT (Chee et al., 2015; Jennings et al., 2015; Mickelsen et al., 2017). Furthermore, Jego et al. (2013) confirmed that LHMCH neurons release the inhibitory neurotransmitter GABA. Collectively, these findings hint that LHMCH neurons may perhaps synthesize and release GABA. Paradoxically, other studies revealed that LHMCH neurons are not exclusively GABAergic, but they also express the vesicular glutamate transporter 2 (VGLUT2) and presumably might produce glutamate (Abrahamson et al., 2001; Chee et al., 2015). More recently, by using molecular profiling including RNAscope combined with the immunohistochemical approach Schneeberger et al. (2018) highlighted that 97% of LHMCH neurons express VGLUT2 but not VGAT suggesting that the vast majority of LHMCH neurons are glutamatergic.
LHMCH neurons and energy metabolism
The role of LHMCH neurons in the regulation of energy balance and metabolism has been studied vastly (Pissios et al., 2006; Al-Massadi et al., 2021; Lord et al., 2021). Earlier studies combing electrical stimulation and lesion approaches distinguished LH as a feeding center (Anand and Brobeck, 1951; Delgado and Anand, 1953; Teitelbaum and Stellar, 1954). More precisely, it was shown that LHMCH neurons were directly involved in the modulation of energy balance and glucose homeostasis by controlling feeding behavior, adipose tissue thermogenesis, and locomotor activity. Under fasting conditions, MCH mRNA expression increased in lean mice as well as in leptin-deficient (ob/ob) obese mice (Qu et al., 1996). Acute intracerebroventricular (ICV) administration of MCH in rodents induced short-term but robust increase in food intake (Qu et al., 1996; Sahu, 1998; Della-Zuana et al., 2002) and chronic infusion enhances food consumption and body weight associated with the substantial increase in energy storage and reduction in energy expenditure (Della-Zuana et al., 2002; Gomori et al., 2003; Ito et al., 2003; Glick et al., 2009). Further insights supporting the critical role of LHMCH neurons in regulation of energy homeostasis have emerged from genetic studies. Transgenic mice that overexpress MCH showed sustained hyperphagia, and mild weight gain associated with impaired glucose tolerance and insulin resistance (Ludwig et al., 2001). In contrast, targeted deletion of the Mch gene and MCH neurons-ablation exhibit leanness and weight loss due to hypophagia and increased energy expenditure in mice (Shimada et al., 1998; Kokkotou et al., 2005; Alon and Friedman, 2006; Izawa et al., 2022). On the same line, Jeon et al. (2006) confirmed the leaned phenotypes in aged mice lacking the Mch gene, but also reported better glucose tolerance and insulin sensitivity in these animals. Moreover, mice lacking MCH receptor 1 (MCH-R1) present normal body weight, and lean phenotype with decreased fat mass because of their hyperactivity. Intriguingly, MCH-R1 deficient mice are hyperphagic when fed on a regular chow diet and substantially resistant to high-fat diet induced obesity (Marsh et al., 2002). Furthermore, the hyperphagic phenotype persisted in ob/ob mice lacking the Mch gene, however, they exhibit a remarkable reduction in body fat due to increased energy expenditure. Regarding glucose homeostasis, the disruption of the Mch gene in ob/ob mice has improved glucose tolerance but hyperinsulinemia remained (Segal-Lieberman et al., 2003). It is noteworthy that insulin increases the activity of LHMCH neurons through phosphatidylinositol 3-kinase signaling. Thus, it has been revealed that specific deletion of insulin receptors in LHMCH neurons does not affect energy balance and glucose homeostasis in mice fed on a regular chow diet whereas it improved peripheral glucose metabolism by enhancing hepatic insulin sensitivity and suppressing the production of hepatic glucose in mice exposed to high-fat diet (Hausen et al., 2016). In aggregate, the abovementioned data (summarized in Table 1) indicate that LHMCH neurons are fundamental in regulating energy expenditure and glucose homeostasis, therefore they might be an attractive target for innovative and efficient treatment for obesity and its comorbidities. The mechanistic signaling of LHMCH neurons implicated in regulating energy expenditure and glucose metabolism is not clearly understood yet. Recently, Izawa et al. (2022) suggested that LHMCH neurons might regulate brown adipose tissue (BAT) activity and energy expenditure by sending projections to the medullary raphe nucleus to inhibit sympathetic inputs in BAT. Besides the traditional synaptic transmission, LHMCH neurons convey its orexigenic effects through a complementary pathway involving the cerebral spinal fluid (CSF). It has been shown that LHMCH neurons modulate CSF flow by regulating the frequency of ciliated ependymal cells in the third ventricle (Conductier et al., 2013). Additionally, chemogenetic activation of LHMCH neurons triggers the release of MCH peptide into the CSF which in turn promotes an increment in food intake whereas the limitation of the bioavailability of MCH present in the CSF significantly reduced feeding (Noble et al., 2018). Importantly, recent outcomes unveiled that LHMCH neurons expressing the vascular endothelial growth factor A (VEGFA) regulate the permeability of the median eminence (ME) microvascular plexus and, thus, modulate leptin action in the arcuate nucleus (ARC) to control food intake through VEGFA-dependent mechanism (Jiang et al., 2020). Together, these data propose the functional interaction between LHMCH neurons and ME barrier components in sensing and processing circulating metabolic signals fundamental to regulating energy homeostasis and metabolism.
TABLE 1.
Summary of studies that investigated the role of MCH system in food intake and metabolism.
| Experiment | Species | Food intake | Plasma glucose level | Plasma insulin level | Plasma leptin level | References |
| Acute ICV infusion of MCH | Rats (long-Evans) | Increased (regular diet) | – | – | – |
Qu et al., 1996
Sahu, 1998 Della-Zuana et al., 2002 |
| Rats (Sprague-Dawley) | Increased (regular diet) | – | – | – | ||
| Rats (Wistar; Sprague-Dawley) | Increased (regular diet) | – | – | – | ||
| Chronic ICV infusion of MCH | Rats (Wistar; Sprague-Dawley) | Increased (regular diet) | – | – | – |
Della-Zuana et al., 2002 Gomori et al., 2003; Glick et al., 2009 Gomori et al., 2003; Ito et al., 2003 |
| Mice (C57BL/6J) | Slight increase (regular diet) | No significant changes (regular diet) | No significant changes (regular diet) | Increased (regular diet) | ||
| Mice (C57BL/6J) | Increased (moderate high fat diet) | Increased (moderate high fat diet) | Increased (moderate high fat diet) | Increased (moderate high fat diet) | ||
| Genetic overexpression of MCH | Transgenic mice (FVB MCH-OE) | Increased (high fat diet) | Increased (High fat diet) | Increased (high fat diet) | Increased (high fat diet) | Ludwig et al., 2001 |
| Mch gene knockout | Transgenic mice (Mch–/–) | Decreased (regular diet) | No significant changes (regular diet) | No significant changes (regular diet) | Decreased (regular diet) |
Shimada et al., 1998
Segal-Lieberman et al., 2003 Kokkotou et al., 2005 Jeon et al., 2006 |
| Transgenic mice (Mch–/–/ob/ob) | Hyperphagic (regular diet) | Decreased (regular diet) | Increased (regular diet) | – | ||
| Transgenic mice (Mch–/–/C57BL/6J) | No significant changes (regular diet and high fat diet) | No significant changes (regular diet) Decreased (high fat diet) |
No significant changes (regular diet) Decreased (high fat diet) |
No significant changes (regular diet) Decreased (high fat diet) |
||
| Transgenic mice (Mch–/–/129) | Increased (regular diet and high fat diet) | No significant changes (regular diet) Decreased (high fat diet) |
No significant changes (regular diet and high fat diet) | No significant changes (regular diet) Decreased (high fat diet) |
||
| Transgenic mice (Aged Mch–/–) | No significant changes (regular diet) | Decreased (regular diet) | Decreased (regular diet) | – | ||
| MCH neurons ablation | Transgenic mice (MCH/ataxin-3) | Decreased (regular diet) | Decreased (regular diet) | Decreased (regular diet) | Decreased (regular diet) |
Alon and Friedman, 2006
Izawa et al., 2022 |
| Transgenic mice (MCH/ataxin-3/ob/ob) | – | Decreased (regular diet) | No significant changes (regular diet) | No significant changes (regular diet) | ||
| Transgenic mice (MCH-tTA; TetO-DTA) | No significant changes (regular diet) | – | – | – | ||
| MCH1-receptors deletion | Transgenic mice (Mch1r–/–) | Increased (regular diet) Decreased (high fat diet) |
No significant changes (regular diet) | No significant changes (regular diet) | Decreased (regular diet) | Marsh et al., 2002 |
| Insulin receptors inactivation on MCH neurons | Transgenic mice IRΔ MCH mice | No significant changes (regular diet and high fat diet) | No significant changes (regular diet) Improved pyruvate tolerance (high fat diet) |
No significant changes (regular diet) Improved insulin sensitivity (high fat diet) |
No significant changes (regular diet and high fat diet) | Hausen et al., 2016 |
| Chemoactivation of MCH neurons | Rats (Sprague-Dawley) | Increased (regular diet) | – | – | – | Noble et al., 2018 |
LHMCH neurons and sleep-wake cycle
There is numerous evidence that establishes the role of LHMCH neurons in sleep-wake regulation. Based upon the earlier neuroanatomical experiments where c-Fos was used as a marker of neuronal activity, Verret et al. (2003) noticed that a large majority of LHMCH neurons were active during rapid eye-movement (REM) sleep rebound that followed 72 h of selective REM sleep deprivation. In addition, they found that ICV infusions of MCH peptide significantly increased the number of REM bouts (up to 200%) without affecting their duration and provoked a modest prolongation in the time spent in non-rapid eye-movement (NREM) sleep (up to 70%) in a dose-dependent manner (Verret et al., 2003). Subsequent histological studies supported the previous findings and reinforced the hypothesis implying LHMCH neurons in promoting sleep (Modirrousta et al., 2005; Hanriot et al., 2007; Kitka et al., 2011). Similarly, other studies explore the effects of microinjections of MCH into different brain areas involved in sleep-wake regulation. Of note, targeted injections of MCH into wake-promoting nuclei including the dorsal raphe nucleus (DRN) in the rat and cat (Lagos et al., 2009; Devera et al., 2015), median raphe nucleus (MnR) (Pascovich et al., 2020, 2021), LC (Monti et al., 2015), BF (Lagos et al., 2012), and nucleus pontis oralis (NPO) of the cat (Torterolo et al., 2009) produced a dose-dependent increase in REM sleep. In contrast, the local infusions of MCH into the sublaterodorsal tegmental nucleus (SLD), recognized as the key structure involved in REM sleep generation, significantly impeded REM sleep in rats because of decreasing the time spent in REM sleep and the number of REM bouts (Monti et al., 2016). Moreover, microinjections of MCH peptide directly into the VLPO, one of the major NREM-promoting regions, increased the time spent in NREM sleep without affecting REM sleep (Benedetto et al., 2013). Curiously, subcutaneous administration of MCH-R1 antagonists decreased the time spent in sleep stages and prolonged the onset latency of both NREM and REM sleep (Ahnaou et al., 2008), however, the oral supply of MCH-R1 antagonist has no effects on sleep-wake pattern (Able et al., 2009). In agreement with the pharmacological studies, MCH-R1 knockout mice exhibit a significant decrease in NREM sleep through the light-dark cycle, along with this an enhancement in wakefulness and reduction of REM sleep was detected in these transgenic mice when they were exposed to a restraint stress procedure, followed by a homeostatic rebound sleep (Ahnaou et al., 2011). Moreover, targeted deletion of the Mch gene in mice increased wakefulness and reduced time spent in NREM and REM sleep compared to wild-type animals. Under fasting conditions, these transgenic mice displayed a massive reduction in REM sleep and remarkable hyperactivity correlated with their lean phenotype (Willie et al., 2008). These behavioral responses in mice lacking the Mch gene drew the attention of researchers to investigate MCH-dependent mechanisms underlying sleep-wake regulation in response to changes in energy homeostasis (Willie et al., 2001; Arrigoni et al., 2019). Inconsistent with the previous reports which depicted the integral role of the MCH system in REM sleep regulation, Adamantidis et al. (2008) showed that MCH-R1 knockout mice present an unexpected increase in the REM sleep during the natural sleep-wake cycle and after total sleep deprivation. This differing outcome might be related to compensatory mechanisms established to counterbalance the MCH-R1 disruption caused by the targeted gene deletion approach. Consistent with these crucial findings, in vivo electrophysiology recordings combined with juxtacellular labeling of neurons in head-fixed rats revealed that LHMCH neurons were quiet during wakefulness, occasionally firing during NREM sleep and discharging at their maximum rate during REM sleep (Hassani et al., 2009). Subsequently, both deep-brain calcium imaging and fiber photometry studies performed in freely behaving mice have also confirmed that LHMCH neurons displayed a robust activity during REM sleep as well as during the transition from NREM to REM sleep, whereas there were less active in wakefulness and during the transition from REM sleep to wakefulness (Blanco-Centurion et al., 2019; Izawa et al., 2019). Despite this outstanding experimental evidence, no consensus has been reached yet to decipher the specific role of LHMCH neurons in the regulation of REM and NREM sleep. To clarify this point, a collection of optogenetic or chemogenetic experiments were deployed to scrutinize the defined role of LHMCH neurons in the neurobiological mechanisms of sleep-wake behavior. For instance, acute optogenetic activation of LHMCH neurons at 20 Hz during NREM sleep enhanced transitions from NREM to REM sleep while the duration of REM sleep episodes was significantly extended when the optogenetic stimulation of LHMCH neurons occurred at the onset of REM sleep (Jego et al., 2013). Another group of researchers showed that optogenetic stimulation of LHMCH neurons at 10 Hz for 3 h facilitated the transition from NREM to REM sleep, resulting in a significant increase in the time spent in REM sleep and a concomitant decrease in NREM sleep time (Tsunematsu et al., 2014). Surprisingly, chronic optogenetic activation of MCH neurons (ZI and LH) induced a robust increase in the total time in NREM and REM sleep during the night period and notably increased electroencephalogram (EEG) delta power (0.5–4 Hz), an electrophysiological indicator of sleep intensity. For note, Konadhode et al. (2013) and Blanco-Centurion et al. (2016) found that only REM sleep time extended upon optogenetic stimulation during the daytime in nocturnal rodents. Presumably, these divergent outcomes reported in the abovementioned optogenetic studies might be due to differences in genetic strategies implemented to selectively target MCH neurons as well as to differences in the light pulse stimulation paradigms applied to manipulate the activity of these neurons. In addition, chemogenetic activation of LHMCH neurons increased the number of REM bouts during the light period, which was doubled during the dark period, whereas the duration of bouts did not change (Vetrivelan et al., 2016). In this study, the authors demonstrated that LHMCH neurons play a crucial role in promoting REM sleep and facilitating transitions from NREM to REM sleep. Nevertheless, the role of LHMCH neurons in the spontaneous REM sleep generation is still perplexing since several experiments have yielded inconsistent outcomes. For instance, selective ablation of MCH neurons using a genetically targeted diphtheria toxin approach significantly increased the number of REM bouts and shortened the mean bout duration of wake during the light period (Vetrivelan et al., 2016). In another study, Tet-Off system mice were used to specifically disrupt MCH neurons in a reversible and controlled manner. Paradoxically, the results obtained from this study showed that MCH neurons ablation did not affect the total time in REM sleep and the mean episode duration of REM sleep (Tsunematsu et al., 2014). Moreover, transgenic mice with Ataxin 3-mediated ablation of MCH neurons unexpectedly displayed an increase in the REM sleep amounts during the light period (Varin et al., 2016). Conversely, acute optogenetic silencing of MCH neurons, while mice were in REM sleep, did not change REM sleep episode duration (Jego et al., 2013). In addition, chemogenetic inhibition of MCH neurons increased the NREM sleep amounts and notably extended the mean bout duration of NREM sleep without affecting REM sleep duration, suggesting that active MCH neurons hinder the generation of NREM sleep to facilitate the entry into REM sleep (Varin et al., 2018).
Collectively, evidence from pharmacological, electrophysiology, genetic, chemogenetic, and optogenetic studies (summarized in Table 2) revealed that the MCH system plays a critical role in the regulation of REM sleep, whereas further investigations are required to unravel their role in NREM sleep modulation.
TABLE 2.
Summary of studies that investigated the role of MCH system in sleep-wake regulation.
| Experiment | Species | Effect on wakefulness | Effect on NREM sleep | Effect on REM sleep | References | |
| Acute ICV infusion of MCH | Rats (Sprague-Dawley) | Decreased in wake amounts | Increased in NREM amounts | Increased in REM amounts Increased in the number of REM bouts No change in the duration of REM bouts |
Verret et al., 2003 | |
| Microinjection of MCH | DRN | Rats (Wistar) | Decreased in wake amounts | Moderate increase in the NREM amounts | Increased in REM amounts Increased in the number of REM bouts No change in the duration of REM bouts |
Lagos et al., 2009 |
| MnR | Rats (Wistar) | Decreased in wake amounts | No significant change in NREM amounts | Increased in REM amounts Increased in the number of REM bouts No change in the duration of REM bouts |
Pascovich et al., 2021 | |
| LC | Rats (Wistar) | No significant change in wake amounts | No significant change in NREM amounts | Increased in REM amounts Increased in the number of REM bouts No change in the duration of REM bouts |
Monti et al., 2015 | |
| BF | Rats (Wistar) | Decreased in wake amounts during the first 2-h post-injection | No significant change in NREM amounts | Increased in REM amounts during the first 2-h post-injection Increased in the number of REM bouts No change in the duration of REM bouts |
Lagos et al., 2012 | |
| NPO | Cats | Decreased in wake amounts during the first hour post-injection | No significant change in NREM amounts | Increased in REM amounts during the first hour post-injection No change in the number of REM bouts No change in the duration of REM bouts Decreased in the latency to REM |
Torterolo et al., 2009 | |
| SLD | Rats (Wistar) | No significant changes in wake amounts | No significant change in NREM amounts | Decreased in REM amounts during the first and the second 2-h post-injection Decreased in the number of REM bouts No change in the duration of REM bouts Increased in the latency to REM |
Monti et al., 2016 | |
| VLPO | Rats (Wistar) | Decreased in wake amounts during 4–5 h block post-injection Decreased in the duration of Wake bouts |
Increased in NREM amounts | No change in REM amounts | Benedetto et al., 2013 | |
| Pharmacological blockade of MCH-R1 | Subcutaneous administration | Rats (Sprague Dawley) | Increased in wake amounts Increased in the number of wake bouts Moderate increase in the duration of Wake bouts at the higher dose |
Decreased in NREM amounts Decreased in the duration of NREM bouts |
Decreased in REM amounts Decreased in the number of REM bouts Decreased in the duration of REM bouts at the higher dose |
Ahnaou et al., 2008 |
| Oral administration | No change in wake parameters | No change in NREM parameters | No change in REM parameters | Able et al., 2009 | ||
| MCH1-receptors deletion | Transgenic mice (Mch1r–/–) | No change in wake parameters |
No change in NREM parameters |
Increased in REM amounts during light period Increased in the number of REM bouts during the light period |
Adamantidis et al., 2008 | |
| Increased in wake amounts Increased in the duration of wake bouts No change in the number of wake bouts Increased in wake amounts under restraint stress condition |
Decreased in NREM amounts Decreased in the duration of NREM bouts No change in the number of NREM bouts Decreased in NREM amounts under restraint stress condition |
No change in REM parameters Decreased in REM amounts under restraint stress condition |
Ahnaou et al., 2011 | |||
| Mch gene knockout | Transgenic mice (Mch–/–) | Increased in wake amounts Increased in the duration of wake bouts Increased in wake amounts under fasting condition during both light and dark phase Increased in the duration of wake bouts during both light and dark phase |
Decreased in NREM amounts Decreased in NREM amounts under fasting condition during both light and dark phase |
Decreased in REM amounts Massive decrease in REM amounts under fasting condition during both light and dark phase Decreased in the duration of REM bouts under fasting condition during both light and dark phase |
Willie et al., 2008 | |
|
In vivo electrophysiology (Unit recordings of MCHergic neurons) |
Rats (long-Evans) | MCHergic neurons not firing | MCHergic neurons fired occasionally | MCHergic neurons fired maximally | Hassani et al., 2009 | |
| Optogenetic manipulation of MCH neurons | Acute activation of MCH neurons at the onset of NREM | Transgenic mice (Pmch-Cre) | – | No change in the duration of NREM bouts Increased in the transition from NREM-to-REM |
– | Jego et al., 2013 |
| Acute activation of MCH neurons at the onset of REM | – | Increased in the duration of REM bouts | ||||
| Inhibition of MCH neurons at the onset of REM | No changes | Decreased in the frequency and amplitude of REM theta power | ||||
| Acute activation of MCH neurons |
MCH-tTA; TetO ChR2 bigenic mice |
No change in wake amounts Increased in the number of wake bouts |
Decreased in NREM amounts Decreased in the duration of NREM bouts Increased in the number of NREM bouts Increased in the transition from NREM-to-REM |
Increased in REM amounts Increased in the number of REM bouts |
Tsunematsu et al., 2014 | |
| Acute inhibition of MCH neurons |
MCH-tTA; TetO ArchT bigenic mice |
No changes | No changes | No changes | ||
| Chronic activation of MCH neurons | C57BL/6J mice | Decreased in wake amounts during dark phase Decreased in the duration of wake bouts |
Increased in NREM amounts during dark phase No change in the duration of NREM bouts Increased in NREM delta power |
Increased in REM amounts during dark phase No change in the duration of REM bouts No change in REM theta power |
Konadhode et al., 2013 | |
| Rats (long-evans) | Deceased in wake amounts during dark phase Deceased in the number of wake long bouts (> 32 min) during both day and night phases |
Increased in NREM amounts during dark phase Increased in the number of NREM short bouts during both day and night phases Increased in NREM delta power during day phase |
Increased in REM amounts during both dark and day phases Increased in the number of REM short bouts during both day and night phases Increased in REM theta power during both night and day phases |
Blanco-Centurion et al., 2016 | ||
| Pharmacogenetic manipulation of MCH neurons | Chemoactivation (0.3 mg/Kg CNO) | Transgenic mice (MCH-Cre) | No changes | No changes | Increased in REM amounts during both day and night phases Increased in the number of REM bouts during both day and night phases No change in the duration of REM bouts |
Vetrivelan et al., 2016 |
| Chemoactivation (0.5 mg/Kg CNO) | Transgenic mice (Pmch-Cre) | – | Deceased in NREM amounts Decreased in the duration of NREM bouts |
Increased in REM amounts Increased in the duration of REM bouts |
Varin et al., 2018 | |
| Chemoinhibition (5 mg/Kg CNO) |
– | Increased in NREM amounts Increased in the duration of NREM bouts |
Deceased in REM amounts No change in the duration of REM bouts |
|||
| MCH neurons ablation | Transgenic mice (MCH-Cre/ + ; iDTR) | Decreased in the duration of wake bouts during the day phase | No changes | Increased in REM amounts Increased in the number of REM bouts during the day phase |
Vetrivelan et al., 2016 | |
|
MCH-tTA; TetO DTA bigenic mice |
Increased in wake amounts during both light and dark phases No change in the duration of wake bouts during both light and dark phases |
Decreased in NREM amounts during both light and dark phases Decreased in the duration of NREM bouts during the dark phase No change on EEG power during NREM |
No change in REM amounts in both light and dark phases No change on EEG power during REM |
Tsunematsu et al., 2014 | ||
| Transgenic mice (MCH/ataxin-3) | No change in wake amounts | No change in NREM amounts | Increase in REM amounts only during the light phase | Varin et al., 2016 | ||
| Deep brain imaging | Transgenic mice (MCH-Cre) | – | The activity of MCH neurons began to increase during the transition from NREM to REM | Dynamic activation of MCH neurons during REM sleep and exploratory behavior | Blanco-Centurion et al., 2019 | |
| Fiber photometry | Transgenic mice (MCH-Cre) | Moderate increase in the activity of MCH neurons | The activity of MCH neurons significantly increased during transitions from NREM to REM and from NREM to Wake | Increased in the activity of MCH neurons The activity of MCH neurons deceased during the transition from REM to Wake |
Izawa et al., 2019 | |
LHOrexin neurons
Orexin neurons are exclusively localized in LH and the adjacent perifornical area (PFH) and send widespread projections throughout the central nervous system (CNS) (Peyron et al., 1998; Nambu et al., 1999) implicating in the regulation of various behavioral and physiological processes predominantly associated with feeding behavior, energy homeostasis, sleep-wake cycle, and reward system (Willie et al., 2001; Yamanaka et al., 2002, 2003; Harris et al., 2005). Previous studies demonstrated that orexin neurons produce two excitatory neuropeptides orexin-A and orexin-B (also known as hypocretin 1 and hypocretin 2) (de Lecea et al., 1998; Sakurai et al., 1998) and also co-release glutamate (Torrealba et al., 2003; Henny et al., 2010) as well as the inhibitory neuropeptide dynorphin (Chou et al., 2001) and the inhibitory neurotransmitter GABA (Harthoorn et al., 2005). Additionally, orexin-A and orexin-B depolarize the post-synaptic target membrane resulting in increased neuronal excitability by acting selectively on two G protein-coupled receptors (GPCR) named orexin receptor type 1 (OX1R) and orexin receptor type 2 (OX2R). Interestingly, orexin-A binds to OX1R and OX2R, however, orexin-B binds specifically to OX2R (Sakurai et al., 1998; Ammoun et al., 2003; Scammell and Winrow, 2011). Pieces of evidence from subsequent experiments revealed that OX1R couples exclusively to the Gq/11 subclass of GPCR, whereas OX2R couples to Gi/o and Gq subclass of GPCR (Sakurai et al., 1998; van den Pol et al., 1998), mediating orexinergic signaling through the activation of Na+/Ca2+ exchanger (Eriksson et al., 2001; Yang and Ferguson, 2002; Burdakov et al., 2003), or through the decrease of potassium conductance (Ivanov and Aston-Jones, 2000; Bisetti et al., 2006). Additionally, orexin signaling pathways involved other intracellular mechanisms including the activation of phospholipase D/phosphatidic acid (Johansson et al., 2008), phospholipase A/arachidonic acid (Turunen et al., 2012), and mitogen-activated protein kinase cascade (Ramanjaneya et al., 2009; Wen et al., 2015). It is noteworthy to highlight that the activation of orexin neurons triggers excitatory post-synaptic responses whereas the stimulation of MCH neurons engenders inhibitory post-synaptic effects (Sakurai, 2007; Adamantidis and de Lecea, 2008a).
LHOrexin neurons and energy metabolism
A myriad of investigations in the field of molecular, cellular, and behavioral neuroscience provide evidence suggesting the implication of orexin neurons in the regulation of feeding behavior and energy homeostasis. Several studies established that orexin system dysfunction has been implicated in serious neurological disorders including narcolepsy (Lin et al., 1999; Thannickal et al., 2000), addiction (Georgescu et al., 2003; Boutrel et al., 2005; Espana et al., 2011), depression (Taheri et al., 2001; Allard et al., 2004; Brundin et al., 2007; Rotter et al., 2011), anxiety (Suzuki et al., 2005; Li et al., 2010; Avolio et al., 2011; Lungwitz et al., 2012), post-traumatic disorder (Strawn et al., 2010), schizophrenia (Nishino et al., 2002; Dalal et al., 2003; Meerabux et al., 2005; Fukunaka et al., 2007), and severe eating behaviors and metabolic impairments such as anorexia nervosa (Bronsky et al., 2011; Janas-Kozik et al., 2011), hyperphagia and eventually obesity in Prader-Willi syndrome (Nevsimalova et al., 2005; Fronczek et al., 2009).
Several studies have reported that prepro-orexin mRNA level and also the activity of orexin neurons was significantly increased during fasting (Sakurai et al., 1998; Lopez et al., 2000; Diano et al., 2003; Horvath and Gao, 2005). Further, ICV microinjection experiments of orexins have confirmed the potential role of orexins in feeding behavior and energy homeostasis. In fact, acute ICV administration of orexin-A in freely fed rats enhanced food consumption in a dose-dependent manner during the light phase (Sakurai et al., 1998; Edwards et al., 1999; Haynes et al., 1999; Dube et al., 2000; Jain et al., 2000; Yamanaka et al., 2000; Lopez et al., 2002). In the same line with these previous outcomes, microinjections of orexin-A directly into different hypothalamic nuclei such as paraventricular nucleus (PVN), dorsomedial nucleus (DMN), LH, and PFH significantly increased food intake, however, no effect was detected after performing microinjections into ARC, ventromedial nucleus (VMN), preoptic area (POA), central nucleus of the amygdala (CeA) and nucleus of the tractus solitaries (NTS). Similar experiments showed that orexin-B failed to stimulate feeding behavior after infusing it into the aforementioned brain areas (Dube et al., 1999; Thorpe et al., 2003). However, Sweet et al. (1999) reported that orexin-B stimulated feeding only after ICV administration. Subsequent pharmacological studies established that the blockade of OX1R by a selective antagonist (SB-334867-A) provokes a robust reduction in food intake in both fed and fasted rats (Haynes et al., 2000; Rodgers et al., 2001; White et al., 2005). Recently, Jin et al. (2020) reported that infusion of orexin-A into the CeA robustly enhanced palatable high-fat diet consumption suggesting a possible role of the orexin system in the regulation of hedonic feeding. Surprisingly, microinjection of orexin-A into the VLPO significantly increased spontaneous physical activity and non-exercise thermogenesis without affecting food consumption resulting in body weight loss, while blockade of OX1R and OX2R abolished the aforesaid effects of orexin-A and presumably lead to body weight gain (Mavanji et al., 2015; Coborn et al., 2017). These pharmaceutical and behavioral studies endeavoring to elucidate the role of the orexin system in feeding behavior and energy homeostasis are also supported by implementing genetic approaches. Mice lacking orexin neurons (orexin/ataxin-3 transgenic mice) or orexin gene (prepro-orexin knockout mice) exhibit a significant reduction in food intake, water intake, locomotor activity, energy expenditure, and unexpectedly late-onset obesity despite their hypophagic phenotype (Hara et al., 2001, 2005; Fujiki et al., 2006; Zhang et al., 2007). Likewise, selective ablation of orexin neurons using diphtheria toxin fragment A reduced food intake and water intake in orexin-Cre mice even though their body weight was significantly higher compared with control mice (Inutsuka et al., 2014). In agreement with these findings, chemogenetic activation of orexin neurons leads to a robust increase in food intake, water intake, spontaneous physical activity, and the respiratory exchange ratio (Inutsuka et al., 2014). Conversely, another study showed that pharmacogenetic activation of orexin neurons produced a strong enhancement in spontaneous physical activity concomitant with an increase in energy expenditure and unpredictably without inducing any changes in food intake and water intake (Zink et al., 2018). Collectively, these findings (summarized in Table 3) suggest that orexins and their receptors might be considered a promising therapeutic target for the treatment of eating disorders and energy metabolism disturbances including obesity, diabetes, and cardiovascular diseases.
TABLE 3.
Summary of studies that investigated the role of orexin system in food intake and metabolism.
| Experiment | Species | Effect on food intake | References | ||
| Acute ICV infusion of orexins | Orexin-A | Freely fed rats (Wistar and Sprague-Dawley) | Increased food intake during the light phase | Sakurai et al., 1998; Edwards et al., 1999; Sweet et al., 1999; Dube et al., 2000; Jain et al., 2000; Yamanaka et al., 2000; Lopez et al., 2002 | |
| Freely fed rats (Wistar and Sprague-Dawley) |
Increased food intake during the early light phase (first 4-h post-infusion) Failed to stimulate feeding when given prior the onset of darkness Increased feeding when given 6h into the dark phase |
Haynes et al., 1999 | |||
| Freely fasted (18 h) rats (Wistar and Sprague-Dawley) | Increased food intake during the first 4-h post-infusion and reduced during the next 20-h | ||||
| Orexin-B | Freely fed rats (Wistar and Sprague-Dawley) | Increased food intake during the light phase |
Sakurai et al., 1998; Edwards et al., 1999; Jain et al., 2000 | ||
| Failed to stimulate feeding during the early light phase | Haynes et al., 1999 | ||||
| Chronic ICV infusion of orexins | Orexin-A | Freely fed rats (Wistar and Sprague-Dawley) |
Increased food intake during the light phase Decreased food intake during the dark phase | Haynes et al., 1999 | |
| orexin-B | Failed to stimulate feeding during the early light phase | ||||
| Microinjection of orexins | PVN | Orexin-A | Rats (Sprague-Dawley) | Increased food intake |
Dube et al., 1999 |
| DMN | |||||
| LH | Orexin-A | Rats (Sprague-Dawley) | Increased food intake |
Dube et al., 1999; Sweet et al., 1999; Thorpe et al., 2003 | |
| PFH | Orexin-A | Rats (Sprague-Dawley) | Increased food intake |
Dube et al., 1999; Sweet et al., 1999 | |
| ARC | Orexin-A | Rats (Sprague-Dawley) | Failed to stimulate feeding |
Dube et al., 1999 | |
| VMN | |||||
| POA | |||||
| CeA | |||||
| NTS | |||||
| VLPO | Orexin-A | Rats (Sprague-Dawley) | Failed to stimulate feeding Increased spontaneous physical activity Increased non-exercise activity thermogenesis Stimulate body weight loss |
Mavanji et al., 2015; Coborn et al., 2017 | |
| PVN | Orexin-B | Rats (Sprague-Dawley) | Failed to stimulate feeding | Dube et al., 1999; Sweet et al., 1999 | |
| DMN | |||||
| LH | |||||
| PFH | |||||
| ARC | |||||
| VMN | |||||
| POA | |||||
| CeA | |||||
| Pharmacological blockade of OX-Rs | OX1R antagonist (intraperitoneal administration) |
Freely fed rats (Sprague-Dawley and Lister hooded) | SB-334867-A given during the light phase decreased orexin-A-induced feeding | Haynes et al., 2000; Rodgers et al., 2001 | |
| Freely fasted (18 h) rats (Sprague-Dawley) | SB-334867-A given during the light phase reduced food intake during the first 4-h after overnight fasting | ||||
| Freely fed rats (Sprague-Dawley) | SB-334867-A given during early dark phase reduced food intake during the next 24-h post-injection SB-334867-A given for 3 days during early dark phase reduced food intake over 24-h on days one and three |
||||
| Freely fed rats (Osborne-Mendel and S5B/PI) | SB-334867-A given during early dark phase reduced food intake in both strain fed at high-fat diet but only in Osborne-Mendel fed at low-fat diet SB-334867-A given early dark phase decreased body weight only in the Osborne-Mendel but not in the S5B/PI |
White et al., 2005 | |||
| OX2R antagonist (in VLPO) | Freely fed rats (Sprague-Dawley) | JNJ-10397049 given during early light phase reduced spontaneous physical activity | Mavanji et al., 2015 | ||
| OX1R and OX2R antagonist (in VLPO) | Freely fed rats (Sprague-Dawley) | TCS-1102 (selective dual orexin receptors antagonist) given during early light phase decreased the effect of orexin-A on spontaneous physical activity and energy expenditure | Coborn et al., 2017 | ||
| Orexin neurons ablation | Transgenic mice (orexin/ataxin-3) | Decreased in food intake Decreased in water intake Decreased in locomotor activity Decreased energy expenditure Mice showed late-onset obesity Increased in the leptin level in females |
Hara et al., 2001; Hara et al., 2005; Fujiki et al., 2006; Zhang et al., 2007 | ||
| Transgenic mice (orexin-Cre) selective ablation of orexin neurons using diphtheria toxin fragment A |
Decreased food intake Decreased water intake Increased body weight Decreased blood glucose level No change in locomotion |
Inutsuka et al., 2014 | |||
| Orexin gene knockout | Transgenic mice (prepro-orexin knockout mice with C57/BL6J background) | Male mice showed a mild tendency to late-onset obesity | Hara et al., 2005 | ||
| Transgenic mice (prepro-orexin knockout mice with mixed genetic background C57/BL6J and 129SvEv) | Female mice showed more prominent late-onset obesity Increased in the leptin level in females |
Fujiki et al., 2006 | |||
| Chemoactivation of orexin neurons | Transgenic mice (orexin-Cre) |
Increased food intake Increased water intake Increased locomotor activity Increased the respiratory exchange ratio Increased blood glucose independently from food intake |
Inutsuka et al., 2014 | ||
| Transgenic mice (orexin-Cre) |
Increased spontaneous physical activity No change in food intake No change in water intake Increased energy expenditure especially in mice fed at high-fat diet |
Zink et al., 2018 | |||
LHOrexin neurons and sleep-wake cycle
The orexin neurons are wake-active neurons that fire during the wake period and the extracellular level of orexin peak during wakefulness (Kiyashchenko et al., 2002; Lee et al., 2005) and remain silent during NREM and REM sleep with the exception of burst discharge in phasic REM (Mileykovskiy et al., 2005). It has been shown that ICV injection of orexin induces long periods of wakefulness and suppresses the NREM period (Mieda et al., 2011). Both chemogenetic and optogenetic stimulation of orexin neurons produce wakefulness and strongly suppress REM sleep (Adamantidis et al., 2007; Sasaki et al., 2011). It is argued that the most essential role of orexin is to maintain wakefulness (summarized in Table 4). For example, selective loss of orexin neurons in humans causes narcolepsy (Blouin et al., 2005). The deletion of OX2R produces a phenotype like narcolepsy and restoration of OX2R in double knock-out mice rescues normal sleep-wake phenotype in the mice (Willie et al., 2003; Mochizuki et al., 2011). These experiments suggest that OX2R signaling is crucial for controlling sleep-wake.
TABLE 4.
Summary of studies that investigated the role of orexin system in sleep-wake regulation.
| Experiment | Species | Effect on arousal | References | |
| Acute ICV infusion of orexins | Orexin-A | Wild type mice (C57BL/6J), Rats (Sprague-Dawley and hooded lister) |
Increased in wake amounts Decreased in NREM and REM amounts cycle Increased in locomotor activity |
Hagan et al., 1999; Piper et al., 2000; Espana et al., 2002; Mieda et al., 2011 |
| Orexin gene knockout | Transgenic mice (orexin -/- with C57BL/6J-129/SvEv mixed background) | Increased in the number of NREM and REM bouts during the dark phase Decreased in the duration of NREM and REM bouts during the dark phase Decreased in REM latency during the dark phase Decreased in the duration of wake bouts during the dark phase Alterations in the circadian frequencies of REM episodes Increased fragmentation of the sleep-wake cycle Hypersomnia |
Chemelli et al., 1999; Willie et al., 2003; Mochizuki et al., 2004 | |
| Orexin neurons ablation | Transgenic mice (orexin/ataxin-3) | Increased in REM amount during the dark phase Increased in the duration of REM bouts during the dark phase Decreased in the duration of wake bouts during the dark phase Increased fragmentation of the sleep-wake cycle |
Hara et al., 2001; Zhang et al., 2007 | |
| OX2-receptor deletion | Transgenic mice (OX2R–/–) OX2R Transcription-Disrupted mice |
Increased fragmentation of the sleep-wake cycle Decreased in the duration of wake during the dark phase Decreased in the duration of NREM during the dark phase Decreased in REM latency during the dark phase |
Willie et al., 2003; Mochizuki et al., 2011 | |
| Optogenetic stimulation of orexin neurons | Transgenic mice (Hcrt:EGFP) injected with lentivirus Hcrt:ChR2-mCherry | Increased the transition to wake from NREM or REM 5–30 Hz light pulse trains decreased wake latency Strong reduction of REM duration |
Adamantidis et al., 2007 | |
| Chemoactivation of orexin neurons | Transgenic mice (orexin-Cre) | Increased in wake amounts during the light phase Decreased in NREM amounts during the light phase Decreased in REM amounts during the light phase Modest increase in wake amounts during the dark phase Increased in the latency from wake to REM during the dark phase |
Sasaki et al., 2011 | |
There are conflicting results reported regarding histamine as a signaling element in orexin actions. Carter et al. (2009) have shown that optogenetic stimulation of orexin neurons promotes arousal in mice lacking central histamine. Contrary to this, central administration of orexin induces wakefulness in wild-type animals but not in histamine receptor 1 knock-out mice. Orexin neurons also influence sleep as it reduces the NREM and REM episodes. The central orexin signaling results in reduced REM sleep duration (Williams et al., 2008; Mieda et al., 2011; Sasaki et al., 2011). This effect is possibly mediated by the activation of both OX1R and OX2R (Mieda et al., 2011). Narcoleptic patients show short latency of REM sleep and random nap often include bouts of REM sleep (Dantz et al., 1994; Andlauer et al., 2013). Like narcoleptic individuals, mice lacking orexin signaling are also unable to suppress REM sleep bouts during the active period, indicating the role of orexin signaling during the active period to suppress REM sleep and meet temporal needs (Arrigoni et al., 2019).
MCH and orexin neuronal circuitries regulating sleep and metabolism
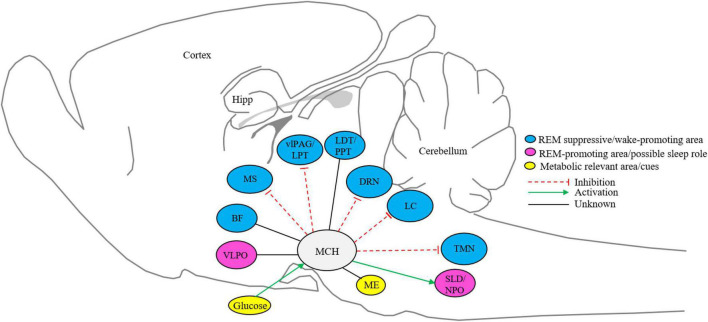
The MCH neurons in LH and ZI project to the nuclei that involve in promoting sleep and arousal (Monti et al., 2013). These projections positively modulate sleep, especially REM sleep (Torterolo et al., 2011). The LC, DRN, and regions of the ventrolateral periaqueductal gray matter and lateral pontine tegmentum (vlPAG/LPT) that are implicated in REM sleep regulation receive dense MCH projections (Torterolo et al., 2009; Costa et al., 2018). The activation of the MCH terminal in vlPAG/LPT tends to increase the duration of REM sleep (Kroeger et al., 2019). Overall, MCH neurons promote sleep by inhibiting wake-promoting areas like the medial septum (MS) and TMN (Jego et al., 2013; Figure 1). The SLD within the dorsolateral pons and NPO in the subcoeruleus (anatomical equivalent of the SLD in cats) are characterized as REM promoting area (Torterolo et al., 2009; Luppi et al., 2013). It is conceivable that MCH neurons directly activate SLD or NPO, likely through the release of glutamate (Torterolo et al., 2009, 2013; Monti et al., 2016). Further, it is considered that MCH may interact with REM-promoting cholinergic neurons within LDT and PPT based on the identification of MCH axons in these areas (Costa et al., 2018), however, there are no functional data supporting this circuit (Figure 1).
FIGURE 1.
Schematic representation of MCH system. MCH neurons in the lateral hypothalamus and zona incerta project to metabolic relevant and sleep-wake controlling nuclei (Burdakov et al., 2005; Torterolo et al., 2009; Lagos et al., 2012; Benedetto et al., 2013; Jego et al., 2013; Monti et al., 2016; Costa et al., 2018; Kroeger et al., 2019; Jiang et al., 2020). BF, basal Forebrain; DRN, dorsal raphe nucleus; Hipp, hippocampus; ME, median eminence; PPT, pedunculopontine tegmentum; LDT, laterodorsal tegmentum; LC, locus coeruleus; TMN, tuberomammillary nucleus; vLPAG/LPT, ventrolateral periaqueductal gray matter and lateral pontine tegmentum; VLPO, ventrolateral preoptic area; MS, medial septum; SLD/NPO, sublaterodorsal tegmental nucleus and nucleus pontis oralis; MCH, melanin-concentrating hormone neurons. Sleep and metabolic-relevant nuclei are color-coded and excitatory and inhibitory inputs are arrow represented.
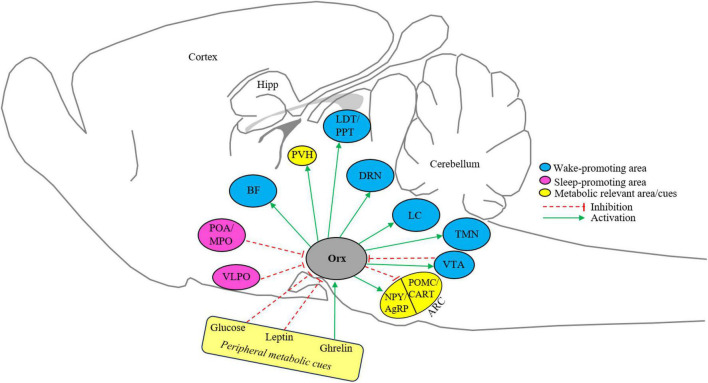
Orexin neurons are found only in LH and PFH and similarly project in the CNS like MCH neurons, however, having the opposite effect on the modulation of sleep-wake and metabolism (Figure 2). Orexin neurons project to wake-associated neurons in BF, LC, TMN, VTA, and DRN, in vitro electrophysiology studies have shown that orexin activates neurons in all these regions (Peyron et al., 1998; Horvath et al., 1999b; Brown et al., 2001; Eggermann et al., 2001; Eriksson et al., 2001; Baimel et al., 2017). So far there is no explicit explanation of the action of orexin neurons on these wake-associated areas, however, a general apprehension is that orexin neurons co-release orexin, dynorphin, and glutamate to likely activate target neurons (Arrigoni et al., 2019). The neurons from sleep-promoting areas like MPO, POA, and VLPO project to orexin neurons in LH, these areas harbor GABAergic neurons that are active during the NREM and/or REM sleep episodes and promote NREM sleep (Yoshida et al., 2006; Benedetto et al., 2012; Saito et al., 2013, 2018a; Alam et al., 2014; Chung et al., 2017). It has been shown that GABA release in LH is higher during the sleep period and blocking GABAergic signaling during the sleeping period activates orexin neurons (Nitz and Siegel, 1996; Alam et al., 2005). Moreover, GABAergic input to LH also reached from VTA as activation of GABAergic neuronal terminals in the LH promoted NREM sleep by inhibiting orexin neurons (Chowdhury et al., 2019). A recent study shows that orexin neurons indirectly target and inhibit sleep-promoting VLPO neurons to promote arousal (De Luca et al., 2022). These findings indicate that sleep-active GABAergic input from preoptic areas inhibits orexin neurons. This GABAergic inhibitions and orexin-mediated activation of wake-associated neurons and inhibition of sleep-associated neurons could be a possible mechanism by which orexin neurons regulate sleep and arousal.
FIGURE 2.
Schematic representation of the orexin system. Orexin neurons in the lateral hypothalamus project and receive projection from metabolic-relevant and sleep-wake-controlling nuclei (de Lecea et al., 1998; Peyron et al., 1998; Date et al., 1999; Horvath et al., 1999b; Brown et al., 2001; Eggermann et al., 2001; Eriksson et al., 2001; Funahashi et al., 2003; Yamanaka et al., 2003; Burdakov et al., 2006; Yoshida et al., 2006; Benedetto et al., 2012; Saito et al., 2013, 2018b; Alam et al., 2014; Baimel et al., 2017; Chung et al., 2017; Chowdhury et al., 2019). BF, basal forebrain; DRN, dorsal raphe nucleus; Hipp, hippocampus; Orx, orexin; POA/MPO, preoptic area/medial preoptic area; LDT, laterodorsal tegmentum; PPT, pedunculopontine tegmentum; PVH, Paraventricular nucleus of the hypothalamus; LC, locus coeruleus; TMN, tuberomammillary nucleus; VLPO, ventrolateral preoptic area; VTA, ventral tegmental area; ARC, arcuate nucleus; NPY, Neuropeptide Y; AgRP, agouti-related protein; POMC, pro-opiomelanocortin; CART, amphetamine-related transcript. Sleep and metabolic-relevant nuclei are color-coded and excitatory and inhibitory inputs are arrow represented.
The LH neurons modulate the metabolism by regulating the feeding. The connectivity of LH to ARC may adjust the food intake depending on the energy needs of the animals. The orexin neurons project to ARC that harbor NPY and POMC neurons expressing orexin and leptin receptors (de Lecea et al., 1998; Date et al., 1999; Funahashi et al., 2003). Orexin induces feeding by activating NPY and inhibiting POMC neurons (Dube et al., 2000; Jain et al., 2000; Guan et al., 2001; Ma et al., 2007; Figure 2). The feeding circuity of orexin may extend to PVH as orexin neurons project to PVH and ICV injection of orexin activates ARC (Date et al., 1999; Edwards et al., 1999). However, it is not explicitly known how orexin acts on PVH and whether orexin regulates feeding through PVH. Orexin neurons also act as metabolic sensors as they respond to peripheral metabolic cues. Extracellular glucose inhibits orexin neurons (Burdakov et al., 2006). In addition to that direct sensing of extracellular glucose levels, orexin neurons sense the other peripheral indicators of energy status such as the satiety hormone leptin and hunger hormone ghrelin. Leptin inhibits orexin neurons whereas ghrelin activates the same (Yamanaka et al., 2003). Thus, negative energy balance activates orexin neurons and hence hunger keeps animals awake. Contrary to this, MCH neurons promote positive energy balance. The MCH neurons are activated by high glucose levels and physiological shifts in glucose have the opposite effects on the electrical activity of orexin neurons (Burdakov et al., 2005, 2006). This differential glucose-sensing ability of orexin and MCH neurons suggests that hyperglycemia may reduce feeding by hyperpolarization of excitatory (orexin neurons) and depolarization of inhibitory (MCH neurons) input to ARC neurons. The direct projection of MCH neurons to ARC is not known, however, a recent study suggests that MCH neurons project to the median eminence (ME), and its activation enhances leptin action in the ARC (Jiang et al., 2020).
Orexin and MCH neurons act as sensors of metabolic changes and arousal
In mammals, maintaining the balance between energy intake and energy expenditure is crucial for survival. However, energy homeostasis imbalance underlies serious metabolic disturbances and diseases such as obesity, diabetes, hyperlipidemia, hypertension, cardiovascular diseases, and cancers (Crowley et al., 2002; Calle et al., 2003; Kim et al., 2016; Tanaka and Itoh, 2019).
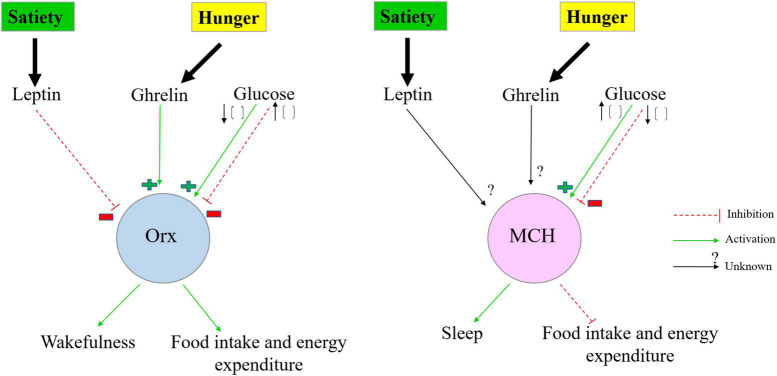
A panoply of experimental evidence revealed that energy homeostasis is regulated via a complex and widespread neuronal circuit located mainly in the brainstem and hypothalamus (Schwartz et al., 2000; Myers and Olson, 2012; Morton et al., 2014; Roh et al., 2016). Distinct neuronal populations within particular nuclei of the brainstem and the hypothalamus sense variations in the energy status of the body by integrating and responding to multiple peripheral (glucose, insulin, leptin, ghrelin, glucagon-like peptide 1) and central [GABA, NPY, AgRP, α-melanocyte-stimulating hormone (α-MSH), serotonin] metabolic signals to maintain energy homeostasis by coordinating energy intake with energy expenditure over time (Burdakov et al., 2005; Schwartz and Porte, 2005; Williams et al., 2008; Morton et al., 2014; Gautron et al., 2015; Roh et al., 2016; Timper and Bruning, 2017; Mavanji et al., 2022). In this context, it is of interest to highlight the crucial role of orexin and MCH systems in regulating energy balance in response to fasting. In fact, the activation of orexin neurons promotes food foraging and increases energy expenditure, whereas the activation of MCH neurons enhances food intake and decreases energy expenditure leading to an increase in energy storage (Figure 3). Both orexin and MCH neurons are activated by fasting (Qu et al., 1996; Sakurai et al., 1998; Lopez et al., 2000; Yamamoto et al., 2000; Diano et al., 2003; Horvath and Gao, 2005; Mogi et al., 2005; Buczek et al., 2020). Interestingly, it was recently revealed that MCH neurons are activated during the early phase of fasting (12 h of fasting), however, orexin neurons exhibit a delayed activation during food deprivation (24 h of fasting). This alternate activation of MCH and orexin neurons play a potential role in coordinating foraging behaviors and energy storage to adjust energy homeostasis during prolonged fasting (Linehan and Hirasawa, 2022). Taken together, these findings insinuate that orexin and MCH neurons are capable of sensing and integrating circulating metabolic signals that convey precise information regarding the status of energy stores, leading to dynamic coordination between energy intake and energy expenditure to restore energy balance.
FIGURE 3.
Simplified schematic representation of the orexin and MCH systems as a sensor of metabolic changes and arousal. In negative energy balance, low extracellular glucose concentration and high circulating level of ghrelin activate the orexin system but inhibit MCH neurons leading to an increase in orexin release and a decrease in MCH release to promote wakefulness, activity, foraging, and food intake. By contrast, in positive energy balance, high extracellular glucose concentration activates MCH neurons but suppresses orexin neurons which are also inhibited by the circulating level of leptin promoting sleep and decreasing energy expenditure. Orx, orexin neurons; MCH, melanin-concentrating hormone neurons.
Pioneering studies reported that orexin-producing neurons are involved in sensing glucose, ghrelin, and leptin levels and eventually promoting arousal (Figure 2). Indeed, electrophysiological evidence revealed that increasing glucose levels induced a striking hyperpolarization and cessation of both spontaneous and evoked action potentials in isolated orexin neurons (Yamanaka et al., 2003; Burdakov et al., 2005; Sheng et al., 2014). Furthermore, the blockade of glycolytic metabolism of glucose by selective inhibitors of glucokinase failed to change the effects of glucose on the action potentials of orexin neurons. These results indicate that orexin neurons are capable to sense trends in glucose levels independently of glucose metabolism (Gonzalez et al., 2008, 2009). Actually, glucose inhibits orexin neurons by acting at the extracellular tandem-pore K + (K2P) channels to induce membrane hyperpolarization and decrease the firing rate of orexin neurons (Burdakov et al., 2006). Here, it is worthwhile to highlight that glucose inhibited orexin neurons only when their intracellular energy levels are low, but paradoxically glucose failed to block orexin neurons when the intracellular levels of lactate, pyruvate, and ATP are high. These results reveal an unexpected glucose-sensing mechanism in orexin neurons that is tightly modulated by the cellular energy status (Venner et al., 2011). Strikingly, recent experimental findings showed for the first time an unexpected complex relationship between orexin neuron activity and blood glucose changes in living organisms. In fact, orexin neurons activity vs. blood glucose variability exhibited a non-canonical temporal profile instead of the expected linear pattern. Basically, orexin neurons track blood glucose concentration at the temporal resolution of minutes and promptly convey its changes into targeted brain regions to trigger adaptive behavior strategies in order to optimize energy balance (Viskaitis et al., 2022).
In addition to sensing peripheral glucose changes, orexin neurons are also involved in detecting and processing signals from other circulating factors such as leptin. Leptin, a product of ob gene, is an anorexigenic hormone predominantly released by adipose tissues (Zhang et al., 1994) and plays a critical role in regulating satiety, blood glucose levels, and energy homeostasis by acting on defined target neurons of the CNS (Schwartz et al., 1996). Previous findings demonstrated that ICV administration of leptin prevents an increase of prepro-orexin mRNA and orexin receptor 1 mRNA in fasted rats, suggesting that leptin has inhibitory feedback on the regulation of orexin gene expression (Lopez et al., 2000). Moreover, Zhu et al. (2002) confirmed orexin neurons induce feeding behavior through both leptin-sensitive and leptin-insensitive pathways. In this sense, we can speculate that leptin might regulate the activity of orexin neurons via complex circuit mechanisms. Indeed, earlier reports yielded conflicting results concerning the expression of leptin receptors (LepRb) on orexin neurons. Findings from immunohistochemistry studies performed in rodent and monkey brains demonstrated that orexin neurons in the LH possess LepRb and thus supporting the hypothesis that leptin might act directly upon these neurons to reduce food seeking and regulate energy balance (Hakansson et al., 1999; Horvath et al., 1999a; Iqbal et al., 2001). Subsequent investigation revealed that bath application of leptin onto isolated orexin neurons provoked hyperpolarization of the membrane potential and suppressed the action potential firing in these cells, resulting in inhibition of orexin neurons (Yamanaka et al., 2003). However, using transgenic LepRbEGFP mice where enhanced green fluorescence protein (EGFP) expression is under the control of the LepRb promotor to scrutinize the possible colocalization of EGFP with orexin neurons, displayed that LepRb-expressing neurons represent a distinct population from orexin neurons in the LH (Leinninger et al., 2009; Louis et al., 2010; Laque et al., 2013). In general support of these results, further experimental works were performed using electrophysiology recordings in brain slices, knock-in mice lines and single-cell expression profiling approaches to elucidate that orexin neurons do not express LepRb and are only indirectly regulated by leptin (Leinninger et al., 2011; Goforth et al., 2014; Sheng et al., 2014; Mickelsen et al., 2017). Several studies have shown that LepRb-expressing neurons lie in synaptic contact with orexin neurons within the LH and the majority of these LepRb neurons contain neurotensin (LepRbNts) (Louis et al., 2010; Leinninger et al., 2011). In addition, pharmacogenetic activation of LepRbNts in hypothalamic slices hyperpolarized membrane potential and reduced action potential firing in orexin neurons. Likewise, the selective genetic deletion of LepRb from LH LepRbNts neurons abolishes leptin-induced inhibition of orexin neurons (Leinninger et al., 2011; Goforth et al., 2014). Together these data suggest that leptin inhibits indirectly the activity of orexin neurons by acting on LepRbNts cells within the LH. Here it is worthwhile to emphasize that LepRbNts also co-release the inhibitory neuropeptide galanin (Laque et al., 2013) which plays an important role in the regulation of orexin neurons by leptin whereas Nts has a tendency to stimulate these cells indicating that this peptide is not implicated in leptin-induced inhibition of orexin neurons (Goforth et al., 2014). It was also reported that leptin failed to significantly enhance GABAA-mediated inhibitory synaptic transmission in orexin neurons and the blockade of GABA receptors could not prevent leptin inhibition of orexin neurons (Goforth et al., 2014). In aggregate, leptin indirectly inhibits orexin neurons by activating LepRbNts neurons through the release of galanin and via GABA-independent mechanisms including the presynaptic inhibition of glutamate inputs onto orexin neurons and the post-synaptic opening of ATP-sensitive potassium KATP channels.
In addition to glucose and leptin, the orexin system is also involved in sensing other circulating factors and hormones such as ghrelin to coordinate behaviors with metabolic needs. Ghrelin is a gastrointestinal hormone released predominantly from the stomach during periods of energy deficit to enhance appetite and food intake (Ariyasu et al., 2001; Nakazato et al., 2001; Lawrence et al., 2002; Olszewski et al., 2003). Importantly, ghrelin is also produced in the brain by a distinct hypothalamic neuronal population adjacent to the third ventricle between the DMH, the VMH, and the ARC. These neurons send wide projections into several hypothalamic nuclei including the ARC and LH to synapse, respectively, with NPY and orexin neurons (Cowley et al., 2003; Toshinai et al., 2003). For note, ghrelin mediates its effects by binding to growth hormone secretagogue receptors, a subtype of the GPCR family highly expressed in the brain as well as in peripheral tissues including stomach, intestine, pancreas, liver, heart, and skeletal muscles (Kojima et al., 1999; Papotti et al., 2000; Gnanapavan et al., 2002; Sun et al., 2004; Yin et al., 2014). Hence, ghrelin can participate in regulating multiple biological processes comprising glucose metabolism (Broglio et al., 2001; Saad et al., 2002; Dezaki et al., 2004; Verhulst and Depoortere, 2012), energy homeostasis (Ravussin et al., 2001; Druce et al., 2005; Malik et al., 2008; Lin et al., 2011), cardiovascular functions (Mao et al., 2012, 2013; Cao et al., 2013; Khazaei and Tahergorabi, 2013), reproduction (Comninos et al., 2014), cell proliferation (Costa et al., 2011; Delhanty et al., 2014; Miao et al., 2019), inflammation and immune system (Lee et al., 2010; Baatar et al., 2011; Wei et al., 2015; Azizzadeh et al., 2017; Santos et al., 2017), learning and memory performance (Carlini et al., 2002; Diano et al., 2006; Kanoski et al., 2013), sleep-wake cycle and (Tolle et al., 2002; Weikel et al., 2003; Szentirmai et al., 2007a,b), and other circadian rhythms (Yannielli et al., 2007; Wang et al., 2018; Qian et al., 2019). Here it is worthwhile to report that ghrelin regulates feeding behaviors and energy homeostasis by interacting with distinct neuronal populations within the CNS including orexin neurons (Olszewski et al., 2003; Toshinai et al., 2003, 2006). In contrast to leptin and glucose, it has been reported that ghrelin stimulates orexin neurons (Yamanaka et al., 2003; Goforth et al., 2014; Sheng et al., 2014). Previous studies have shown that peripheral or central administration of ghrelin robustly increased food intake and induced Fos expression in orexin-immunoreactive neurons but not in MCH-containing neurons (Nakazato et al., 2001; Lawrence et al., 2002; Tolle et al., 2002; Toshinai et al., 2003). Moreover, electrophysiological evidence showed that ghrelin directly activates isolated orexin neurons by inducing membrane depolarization and increasing the action potential firing in these cells (Yamanaka et al., 2003; Sheng et al., 2014). During periods of starvation, elevated circulating levels of ghrelin enhanced the sensitivity of orexin neurons to glucose changes, and thus contribute to maintaining energy homeostasis (Sheng et al., 2014).
In contrast to orexin neurons which are inhibited by glucose, MCH neurons are activated by glucose. A rise in the extracellular glucose levels directly enhanced the excitability of MCH neurons by inducing membrane depolarization of MCH neurons accompanied by an increase in its resistance (Burdakov et al., 2005). Subsequent investigations revealed that glucose sensing by MCH neurons implicates KATP channels and is modulated by a mitochondrial protein UCP2 that decreases ATP production (Krauss et al., 2003; Kong et al., 2010). Additionally, the action of leptin and ghrelin on MCH neurons is yet to be precisely delineated (Figure 3).
Perspective
Based on the currently available data, it emerges as orexin and MCH system in LH mediating its opposing action on sleep-wake and energy metabolism by utilizing multiple neuronal circuits and peripheral cues. The behavioral strategy required to regulate arousal with respect to hunger and satiety with respect to sleep is under the control of orexin and MCH neurons and their extended circuitries. The MCH neurons promote sleep whereas orexin promotes wakefulness, whereas both these neurons promote feeding by interacting with ARC neurons. However, the feeding preference is different as orexin neurons motivate palatable food consumption, whereas MCH neurons motivate caloric food consumption. Interestingly, hunger and hypoglycemia activate orexin neurons that induce arousal required for foraging and food consumption. On the other hand, MCH neurons sense the rise in glucose levels and promote inactivity and sleep. This indicates interconnectivity of LH to ARC is crucial in maintaining sleep and energy homeostasis and effective to deal with challenges such as starvation and sleep disruption. Sleep disruption influences metabolic processes (Donga et al., 2010; Jha et al., 2016). Sleep deprivation increases ghrelin and decreases leptin levels (Schmid et al., 2008; Mosavat et al., 2021), which activates the orexin system. Thus, these arousing cues promote consummatory behavior, inhibition of sleep, and energy conservation. The inhibition of this signaling in recovery sleep may stabilize it by maintaining the sleep-wake cycle. How the MCH system senses these metabolic cues are not clear yet, however, the MCH system may respond to it by stabilizing sleep and decreasing the energy expenditure by interacting with the orexin neuron activity and brain circuits involved in sleep and metabolism. Moreover, metabolic abnormalities also disrupt sleep (Ogilvie and Patel, 2017). Disruption of the sleep-wake cycle in obesity and other metabolic conditions is not studied at the mechanistic level. There are possibilities that the peripheral metabolic cues may directly or indirectly interact with the orexin/MCH system to alter the sleep phenotype in metabolic disorders. Metabolic disruption may influence LH’s neuronal systems as it has been shown that obesity shifts the activity and transcriptional profile of LHA glutamatergic neurons (Rossi et al., 2019). By knowing these co-localized and interacting neural systems that govern the distinct and interdependent behavioral programs—sleep and feeding, it would be enticing to dissect the neuronal bases of the interaction of both these behaviors.
Conclusion
Sleep and the metabolic system are bidirectionally linked to maintaining homeostasis in challenging environments. In this review, we summarized how molecular and cellular components of MCH and orexin signaling maintain this bidirectionality by integration of sleep-wake and energy metabolism. Both these classes of neurons sense the metabolic signals and regulate the sleep-wake states. A substantial chunk of work has been done to understand how orexin and MCH neurons in LH coordinate the metabolism and behavioral states. Future works on how sleep and metabolism influence each other, and the mechanistic explanation of their interaction would be helpful to assign the target for therapeutic intervention for metabolic and arousal-related disorders.
Author contributions
Both authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Able S. L., Ivarsson M., Fish R. L., Clarke T. L., McCourt C., Duckworth J. M., et al. (2009). Localisation of melanin-concentrating hormone receptor 1 in rat brain and evidence that sleep parameters are not altered despite high central receptor occupancy. Eur. J. Pharmacol. 616 101–106. 10.1016/j.ejphar.2009.06.009 [DOI] [PubMed] [Google Scholar]
- Abrahamson E. E., Leak R. K., Moore R. Y. (2001). The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport 12 435–440. [DOI] [PubMed] [Google Scholar]
- Adamantidis A. R., Zhang F., Aravanis A. M., Deisseroth K., de Lecea L. (2007). Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450 420–424. 10.1038/nature06310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis A., de Lecea L. (2008a). Physiological arousal: A role for hypothalamic systems. Cell Mol. Life Sci. 65 1475–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis A., de Lecea L. (2008b). Sleep and metabolism: Shared circuits, new connections. Trends Endocrinol. Metab. 19 362–370. 10.1016/j.tem.2008.08.007 [DOI] [PubMed] [Google Scholar]
- Adamantidis A., Salvert D., Goutagny R., Lakaye B., Gervasoni D., Grisar T., et al. (2008). Sleep architecture of the melanin-concentrating hormone receptor 1-knockout mice. Eur. J. Neurosci. 27 1793–1800. 10.1111/j.1460-9568.2008.06129.x [DOI] [PubMed] [Google Scholar]
- Ahnaou A., Dautzenberg F. M., Huysmans H., Steckler T., Drinkenburg W. H. (2011). Contribution of melanin-concentrating hormone (MCH1) receptor to thermoregulation and sleep stabilization: Evidence from MCH1 (-/-) mice. Behav. Brain Res. 218 42–50. 10.1016/j.bbr.2010.11.019 [DOI] [PubMed] [Google Scholar]
- Ahnaou A., Drinkenburg W. H., Bouwknecht J. A., Alcazar J., Steckler T., Dautzenberg F. M. (2008). Blocking melanin-concentrating hormone MCH1 receptor affects rat sleep-wake architecture. Eur. J. Pharmacol. 579 177–188. 10.1016/j.ejphar.2007.10.017 [DOI] [PubMed] [Google Scholar]
- Alam M. A., Kumar S., McGinty D., Alam M. N., Szymusiak R. (2014). Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery sleep. J. Neurophysiol. 111 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M. N., Kumar S., Bashir T., Suntsova N., Methippara M. M., Szymusiak R., et al. (2005). GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J. Physiol. 563 569–582. 10.1113/jphysiol.2004.076927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard J. S., Tizabi Y., Shaffery J. P., Trouth C. O., Manaye K. (2004). Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides 38 311–315. 10.1016/j.npep.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Al-Massadi O., Dieguez C., Schneeberger M., Lopez M., Schwaninger M., Prevot V., et al. (2021). Multifaceted actions of melanin-concentrating hormone on mammalian energy homeostasis. Nat. Rev. Endocrinol. 17 745–755. 10.1038/s41574-021-00559-1 [DOI] [PubMed] [Google Scholar]
- Alon T., Friedman J. M. (2006). Late-onset leanness in mice with targeted ablation of melanin concentrating hormone neurons. J. Neurosci. 26 389–397. 10.1523/JNEUROSCI.1203-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammoun S., Holmqvist T., Shariatmadari R., Oonk H. B., Detheux M., Parmentier M., et al. (2003). Distinct recognition of OX1 and OX2 receptors by orexin peptides. J. Pharmacol. Exp. Ther. 305 507–514. [DOI] [PubMed] [Google Scholar]
- Anand B. K., Brobeck J. R. (1951). Localization of a “feeding center” in the hypothalamus of the rat. Proc. Soc. Exp. Biol. Med. 77 323–324. [DOI] [PubMed] [Google Scholar]
- Andlauer O., Moore H., Jouhier L., Drake C., Peppard P. E., Han F., et al. (2013). Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol. 70 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyasu H., Takaya K., Tagami T., Ogawa Y., Hosoda K., Akamizu T., et al. (2001). Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 86 4753–4758. 10.1210/jcem.86.10.7885 [DOI] [PubMed] [Google Scholar]
- Arrigoni E., Chee M. J. S., Fuller P. M. (2019). To eat or to sleep: That is a lateral hypothalamic question. Neuropharmacology 154 34–49. [DOI] [PubMed] [Google Scholar]
- Avolio E., Alo R., Carelli A., Canonaco M. (2011). Amygdalar orexinergic-GABAergic interactions regulate anxiety behaviors of the Syrian golden hamster. Behav. Brain Res. 218 288–295. 10.1016/j.bbr.2010.11.014 [DOI] [PubMed] [Google Scholar]
- Azizzadeh F., Mahmoodi J., Sadigh-Eteghad S., Farajdokht F., Mohaddes G. (2017). Ghrelin exerts analgesic effects through modulation of IL-10 and TGF-beta levels in a rat model of inflammatory pain. Iran. Biomed. J. 21 114–119. 10.18869/acadpub.ibj.21.2.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baatar D., Patel K., Taub D. D. (2011). The effects of ghrelin on inflammation and the immune system. Mol. Cell Endocrinol. 340 44–58. [DOI] [PubMed] [Google Scholar]
- Baimel C., Lau B. K., Qiao M., Borgland S. L. (2017). Projection-target-defined effects of orexin and dynorphin on VTA dopamine neurons. Cell Rep. 18 1346–1355. 10.1016/j.celrep.2017.01.030 [DOI] [PubMed] [Google Scholar]
- Bear M. H., Reddy V., Bollu P. C. (2023). Neuroanatomy, hypothalamus. Treasure Island, FL: StatPearls. [PubMed] [Google Scholar]
- Benedetto L., Chase M. H., Torterolo P. (2012). GABAergic processes within the median preoptic nucleus promote NREM sleep. Behav. Brain Res. 232 60–65. [DOI] [PubMed] [Google Scholar]
- Benedetto L., Rodriguez-Servetti Z., Lagos P., D’Almeida V., Monti J. M., Torterolo P. (2013). Microinjection of melanin concentrating hormone into the lateral preoptic area promotes non-REM sleep in the rat. Peptides 39 11–15. 10.1016/j.peptides.2012.10.005 [DOI] [PubMed] [Google Scholar]
- Bisetti A., Cvetkovic V., Serafin M., Bayer L., Machard D., Jones B. E., et al. (2006). Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience 142 999–1004. 10.1016/j.neuroscience.2006.07.018 [DOI] [PubMed] [Google Scholar]
- Bittencourt J. C. (2011). Anatomical organization of the melanin-concentrating hormone peptide family in the mammalian brain. Gen. Comp. Endocrinol. 172 185–197. 10.1016/j.ygcen.2011.03.028 [DOI] [PubMed] [Google Scholar]
- Bittencourt J. C., Presse F., Arias C., Peto C., Vaughan J., Nahon J. L., et al. (1992). The melanin-concentrating hormone system of the rat brain: An immuno- and hybridization histochemical characterization. J. Comp. Neurol. 319 218–245. 10.1002/cne.903190204 [DOI] [PubMed] [Google Scholar]
- Blanco-Centurion C., Liu M., Konadhode R. P., Zhang X., Pelluru D., van den Pol A. N., et al. (2016). Optogenetic activation of melanin-concentrating hormone neurons increases non-rapid eye movement and rapid eye movement sleep during the night in rats. Eur. J. Neurosci. 44 2846–2857. 10.1111/ejn.13410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Centurion C., Luo S., Spergel D. J., Vidal-Ortiz A., Oprisan S. A., Van den Pol A. N., et al. (2019). Dynamic network activation of hypothalamic MCH neurons in REM sleep and exploratory behavior. J. Neurosci. 39 4986–4998. 10.1523/JNEUROSCI.0305-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin A. M., Thannickal T. C., Worley P. F., Baraban J. M., Reti I. M., Siegel J. M. (2005). Narp immunostaining of human hypocretin (orexin) neurons: Loss in narcolepsy. Neurology 65 1189–1192. 10.1212/01.wnl.0000175219.01544.c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B., Kenny P. J., Specio S. E., Martin-Fardon R., Markou A., Koob G. F., et al. (2005). Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc. Natl. Acad. Sci. U. S. A. 102 19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C. (1999). Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: Histochemical relationship to thyrotropin-releasing hormone, melanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res. 848 101–113. 10.1016/s0006-8993(99)01977-0 [DOI] [PubMed] [Google Scholar]
- Broglio F., Arvat E., Benso A., Gottero C., Muccioli G., Papotti M., et al. (2001). Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J. Clin. Endocrinol. Metab. 86 5083–5086. 10.1210/jcem.86.10.8098 [DOI] [PubMed] [Google Scholar]
- Bronsky J., Nedvidkova J., Krasnicanova H., Vesela M., Schmidtova J., Koutek J., et al. (2011). Changes of orexin A plasma levels in girls with anorexia nervosa during eight weeks of realimentation. Int. J. Eat Disord. 44 547–552. 10.1002/eat.20857 [DOI] [PubMed] [Google Scholar]
- Brown R. E., Sergeeva O., Eriksson K. S., Haas H. L. (2001). Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology 40 457–459. [DOI] [PubMed] [Google Scholar]
- Brundin L., Bjorkqvist M., Petersen A., Traskman-Bendz L. (2007). Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur. Neuropsychopharmacol. 17 573–579. [DOI] [PubMed] [Google Scholar]
- Buczek L., Migliaccio J., Petrovich G. D. (2020). Hedonic eating: Sex differences and characterization of orexin activation and signaling. Neuroscience 436 34–45. 10.1016/j.neuroscience.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D., Gerasimenko O., Verkhratsky A. (2005). Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J. Neurosci. 25 2429–2433. 10.1523/JNEUROSCI.4925-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D., Jensen L. T., Alexopoulos H., Williams R. H., Fearon I. M., O’Kelly I., et al. (2006). Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron 50 711–722. [DOI] [PubMed] [Google Scholar]
- Burdakov D., Liss B., Ashcroft F. M. (2003). Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium–calcium exchanger. J. Neurosci. 23 4951–4957. 10.1523/JNEUROSCI.23-12-04951.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle E. E., Rodriguez C., Walker-Thurmond K., Thun M. J. (2003). Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 348 1625–1638. 10.1056/NEJMoa021423 [DOI] [PubMed] [Google Scholar]
- Cao Y., Tang J., Yang T., Ma H., Yi D., Gu C., et al. (2013). Cardioprotective effect of ghrelin in cardiopulmonary bypass involves a reduction in inflammatory response. PLoS One 8:e55021. 10.1371/journal.pone.0055021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini V. P., Monzon M. E., Varas M. M., Cragnolini A. B., Schioth H. B., Scimonelli T. N., et al. (2002). Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem. Biophys. Res. Commun. 299 739–743. [DOI] [PubMed] [Google Scholar]
- Carter M. E., Adamantidis A., Ohtsu H., Deisseroth K., de Lecea L. (2009). Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J. Neurosci. 29 10939–10949. 10.1523/JNEUROSCI.1205-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. J., Arrigoni E., Maratos-Flier E. (2015). Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J. Neurosci. 35 3644–3651. 10.1523/JNEUROSCI.4187-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli R. M., Willie J. T., Sinton C. M., Elmquist J. K., Scammell T., Lee C., et al. (1999). Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 98 437–451. [DOI] [PubMed] [Google Scholar]
- Chen R., Wu X., Jiang L., Zhang Y. (2017). Single-cell RNA-seq reveals hypothalamic cell diversity. Cell Rep. 18 3227–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T. C., Lee C. E., Lu J., Elmquist J. K., Hara J., Willie J. T., et al. (2001). Orexin (hypocretin) neurons contain dynorphin. J. Neurosci. 21:RC168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S., Matsubara T., Miyazaki T., Ono D., Fukatsu N., Abe M., et al. (2019). GABA neurons in the ventral tegmental area regulate non-rapid eye movement sleep in mice. Elife 8:e44928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Weber F., Zhong P., Tan C. L., Nguyen T. N., Beier K. T., et al. (2017). Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 545 477–481. 10.1038/nature22350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coborn J. E., DePorter D. P., Mavanji V., Sinton C. M., Kotz C. M., Billington C. J., et al. (2017). Role of orexin-A in the ventrolateral preoptic area on components of total energy expenditure. Int. J. Obes. 41 1256–1262. 10.1038/ijo.2017.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comninos A. N., Jayasena C. N., Dhillo W. S. (2014). The relationship between gut and adipose hormones, and reproduction. Hum. Reprod. Update 20 153–174. [DOI] [PubMed] [Google Scholar]
- Concetti C., Burdakov D. (2021). Orexin/hypocretin and MCH neurons: Cognitive and motor roles beyond arousal. Front. Neurosci. 15:639313. 10.3389/fnins.2021.639313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conductier G., Martin A. O., Risold P. Y., Jego S., Lavoie R., Lafont C., et al. (2013). Control of ventricular ciliary beating by the melanin concentrating hormone-expressing neurons of the lateral hypothalamus: A functional imaging survey. Front. Endocrinol. 4:182. 10.3389/fendo.2013.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A., Castro-Zaballa S., Lagos P., Chase M. H., Torterolo P. (2018). Distribution of MCH-containing fibers in the feline brainstem: Relevance for REM sleep regulation. Peptides 104 50–61. 10.1016/j.peptides.2018.04.009 [DOI] [PubMed] [Google Scholar]
- Costa J. L., Naot D., Lin J. M., Watson M., Callon K. E., Reid I. R., et al. (2011). Ghrelin is an osteoblast mitogen and increases osteoclastic bone resorption In Vitro. Int. J. Pept. 2011:605193. 10.1155/2011/605193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley M. A., Smith R. G., Diano S., Tschop M., Pronchuk N., Grove K. L., et al. (2003). The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37 649–661. 10.1016/s0896-6273(03)00063-1 [DOI] [PubMed] [Google Scholar]
- Crowley V. E., Yeo G. S., O’Rahilly S. (2002). Obesity therapy: Altering the energy intake-and-expenditure balance sheet. Nat. Rev. Drug Discov. 1 276–286. 10.1038/nrd770 [DOI] [PubMed] [Google Scholar]
- Dalal M. A., Schuld A., Pollmacher T. (2003). Lower CSF orexin A (hypocretin-1) levels in patients with schizophrenia treated with haloperidol compared to unmedicated subjects. Mol. Psychiatry 8 836–837. 10.1038/sj.mp.4001363 [DOI] [PubMed] [Google Scholar]
- Dantz B., Edgar D. M., Dement W. C. (1994). Circadian rhythms in narcolepsy: Studies on a 90 minute day. Electroencephalogr. Clin. Neurophysiol. 90 24–35. 10.1016/0013-4694(94)90110-4 [DOI] [PubMed] [Google Scholar]
- Date Y., Ueta Y., Yamashita H., Yamaguchi H., Matsukura S., Kangawa K., et al. (1999). Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc. Natl. Acad. Sci. U. S. A. 96 748–753. 10.1073/pnas.96.2.748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L., Kilduff T. S., Peyron C., Gao X., Foye P. E., Danielson P. E., et al. (1998). The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U. S. A. 95 322–327. 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca R., Nardone S., Grace K. P., Venner A., Cristofolini M., Bandaru S. S., et al. (2022). Orexin neurons inhibit sleep to promote arousal. Nat. Commun. 13:4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cid-Pellitero E., Jones B. E. (2012). Immunohistochemical evidence for synaptic release of GABA from melanin-concentrating hormone containing varicosities in the locus coeruleus. Neuroscience 223 269–276. 10.1016/j.neuroscience.2012.07.072 [DOI] [PubMed] [Google Scholar]
- Delgado J. M., Anand B. K. (1953). Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am. J. Physiol. 172 162–168. [DOI] [PubMed] [Google Scholar]
- Delhanty P. J., van der Eerden B. C., van Leeuwen J. P. (2014). Ghrelin and bone. Biofactors 40 41–48. [DOI] [PubMed] [Google Scholar]
- Della-Zuana O., Presse F., Ortola C., Duhault J., Nahon J. L., Levens N. (2002). Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague-Dawley rats. Int. J. Obes. Relat. Metab. Disord. 26 1289–1295. 10.1038/sj.ijo.0802079 [DOI] [PubMed] [Google Scholar]
- Devera A., Pascovich C., Lagos P., Falconi A., Sampogna S., Chase M. H., et al. (2015). Melanin-concentrating hormone (MCH) modulates the activity of dorsal raphe neurons. Brain Res. 1598 114–128. [DOI] [PubMed] [Google Scholar]
- Dezaki K., Hosoda H., Kakei M., Hashiguchi S., Watanabe M., Kangawa K., et al. (2004). Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: Implication in the glycemic control in rodents. Diabetes 53 3142–3151. 10.2337/diabetes.53.12.3142 [DOI] [PubMed] [Google Scholar]
- Diano S., Farr S. A., Benoit S. C., McNay E. C., da Silva I., Horvath B., et al. (2006). Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci. 9 381–388. 10.1038/nn1656 [DOI] [PubMed] [Google Scholar]
- Diano S., Horvath B., Urbanski H. F., Sotonyi P., Horvath T. L. (2003). Fasting activates the nonhuman primate hypocretin (orexin) system and its postsynaptic targets. Endocrinology 144 3774–3778. 10.1210/en.2003-0274 [DOI] [PubMed] [Google Scholar]
- Donga E., van Dijk M., van Dijk J. G., Biermasz N. R., Lammers G. J., van Kralingen K. W., et al. (2010). A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J. Clin. Endocrinol. Metab. 95 2963–2968. 10.1210/jc.2009-2430 [DOI] [PubMed] [Google Scholar]
- Druce M. R., Wren A. M., Park A. J., Milton J. E., Patterson M., Frost G., et al. (2005). Ghrelin increases food intake in obese as well as lean subjects. Int. J. Obes. 29 1130–1136. [DOI] [PubMed] [Google Scholar]
- Dube M. G., Horvath T. L., Kalra P. S., Kalra S. P. (2000). Evidence of NPY Y5 receptor involvement in food intake elicited by orexin A in sated rats. Peptides 21 1557–1560. 10.1016/s0196-9781(00)00311-9 [DOI] [PubMed] [Google Scholar]
- Dube M. G., Kalra S. P., Kalra P. S. (1999). Food intake elicited by central administration of orexins/hypocretins: Identification of hypothalamic sites of action. Brain Res. 842 473–477. 10.1016/s0006-8993(99)01824-7 [DOI] [PubMed] [Google Scholar]
- Edwards C. M., Abusnana S., Sunter D., Murphy K. G., Ghatei M. A., Bloom S. R. (1999). The effect of the orexins on food intake: Comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J. Endocrinol. 160 R7–R12. 10.1677/joe.0.160r007 [DOI] [PubMed] [Google Scholar]
- Eggermann E., Serafin M., Bayer L., Machard D., Saint-Mleux B., Jones B. E., et al. (2001). Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience 108 177–181. 10.1016/s0306-4522(01)00512-7 [DOI] [PubMed] [Google Scholar]
- Elias C. F., Lee C. E., Kelly J. F., Ahima R. S., Kuhar M., Saper C. B., et al. (2001). Characterization of CART neurons in the rat and human hypothalamus. J. Comp. Neurol. 432 1–19. [DOI] [PubMed] [Google Scholar]
- Eriksson K. S., Sergeeva O., Brown R. E., Haas H. L. (2001). Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J. Neurosci. 21 9273–9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana R. A., Melchior J. R., Roberts D. C., Jones S. R. (2011). Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology 214 415–426. 10.1007/s00213-010-2048-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana R. A., Plahn S., Berridge C. W. (2002). Circadian-dependent and circadian-independent behavioral actions of hypocretin/orexin. Brain Res. 943 224–236. 10.1016/s0006-8993(02)02653-7 [DOI] [PubMed] [Google Scholar]
- Fort P., Salvert D., Hanriot L., Jego S., Shimizu H., Hashimoto K., et al. (2008). The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience 155 174–181. [DOI] [PubMed] [Google Scholar]
- Fronczek R., Baumann C. R., Lammers G. J., Bassetti C. L., Overeem S. (2009). Hypocretin/orexin disturbances in neurological disorders. Sleep Med. Rev. 13 9–22. [DOI] [PubMed] [Google Scholar]
- Fujiki N., Yoshida Y., Zhang S., Sakurai T., Yanagisawa M., Nishino S. (2006). Sex difference in body weight gain and leptin signaling in hypocretin/orexin deficient mouse models. Peptides 27 2326–2331. 10.1016/j.peptides.2006.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaka Y., Shinkai T., Hwang R., Hori H., Utsunomiya K., Sakata S., et al. (2007). The orexin 1 receptor (HCRTR1) gene as a susceptibility gene contributing to polydipsia-hyponatremia in schizophrenia. Neuromol. Med. 9 292–297. 10.1007/s12017-007-8001-2 [DOI] [PubMed] [Google Scholar]
- Funahashi H., Yamada S., Kageyama H., Takenoya F., Guan J. L., Shioda S. (2003). Co-existence of leptin- and orexin-receptors in feeding-regulating neurons in the hypothalamic arcuate nucleus-a triple labeling study. Peptides 24 687–694. 10.1016/s0196-9781(03)00130-x [DOI] [PubMed] [Google Scholar]
- Gautron L., Elmquist J. K., Williams K. W. (2015). Neural control of energy balance: Translating circuits to therapies. Cell 161 133–145. 10.1016/j.cell.2015.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D., Zachariou V., Barrot M., Mieda M., Willie J. T., Eisch A. J., et al. (2003). Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J. Neurosci. 23 3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick M., Segal-Lieberman G., Cohen R., Kronfeld-Schor N. (2009). Chronic MCH infusion causes a decrease in energy expenditure and body temperature, and an increase in serum IGF-1 levels in mice. Endocrine 36 479–485. 10.1007/s12020-009-9252-5 [DOI] [PubMed] [Google Scholar]
- Gnanapavan S., Kola B., Bustin S. A., Morris D. G., McGee P., Fairclough P., et al. (2002). The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 87:2988. 10.1210/jcem.87.6.8739 [DOI] [PubMed] [Google Scholar]
- Goforth P. B., Leinninger G. M., Patterson C. M., Satin L. S., Myers M. G., Jr. (2014). Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA-independent mechanisms. J. Neurosci. 34 11405–11415. 10.1523/JNEUROSCI.5167-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomori A., Ishihara A., Ito M., Mashiko S., Matsushita H., Yumoto M., et al. (2003). Chronic intracerebroventricular infusion of MCH causes obesity in mice. Melanin-concentrating hormone. Am. J. Physiol. Endocrinol. Metab. 284 E583–E588. [DOI] [PubMed] [Google Scholar]
- Gonzalez J. A., Jensen L. T., Fugger L., Burdakov D. (2008). Metabolism-independent sugar sensing in central orexin neurons. Diabetes 57 2569–2576. 10.2337/db08-0548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J. A., Reimann F., Burdakov D. (2009). Dissociation between sensing and metabolism of glucose in sugar sensing neurones. J. Physiol. 587 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J. L., Saotome T., Wang Q. P., Funahashi H., Hori T., Tanaka S., et al. (2001). Orexinergic innervation of POMC-containing neurons in the rat arcuate nucleus. Neuroreport 12 547–551. 10.1097/00001756-200103050-00023 [DOI] [PubMed] [Google Scholar]
- Hagan J. J., Leslie R. A., Patel S., Evans M. L., Wattam T. A., Holmes S., et al. (1999). Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc. Natl. Acad. Sci. U. S. A. 96 10911–10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson M., de Lecea L., Sutcliffe J. G., Yanagisawa M., Meister B. (1999). Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. J. Neuroendocrinol. 11 653–663. 10.1046/j.1365-2826.1999.00378.x [DOI] [PubMed] [Google Scholar]
- Hanriot L., Camargo N., Courau A. C., Leger L., Luppi P. H., Peyron C. (2007). Characterization of the melanin-concentrating hormone neurons activated during paradoxical sleep hypersomnia in rats. J. Comp. Neurol. 505 147–157. [DOI] [PubMed] [Google Scholar]
- Hara J., Beuckmann C. T., Nambu T., Willie J. T., Chemelli R. M., Sinton C. M., et al. (2001). Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30 345–354. [DOI] [PubMed] [Google Scholar]
- Hara J., Yanagisawa M., Sakurai T. (2005). Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci. Lett. 380 239–242. 10.1016/j.neulet.2005.01.046 [DOI] [PubMed] [Google Scholar]
- Harris G. C., Wimmer M., Aston-Jones G. (2005). A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437 556–559. [DOI] [PubMed] [Google Scholar]
- Harthoorn L. F., Sane A., Nethe M., Van Heerikhuize J. J. (2005). Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons. Cell Mol. Neurobiol. 25 1209–1223. 10.1007/s10571-005-8184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani O. K., Lee M. G., Jones B. E. (2009). Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc. Natl. Acad. Sci. U. S. A. 106 2418–2422. 10.1073/pnas.0811400106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausen A. C., Ruud J., Jiang H., Hess S., Varbanov H., Kloppenburg P., et al. (2016). Insulin-dependent activation of MCH neurons impairs locomotor activity and insulin sensitivity in obesity. Cell Rep. 17 2512–2521. 10.1016/j.celrep.2016.11.030 [DOI] [PubMed] [Google Scholar]
- Haynes A. C., Jackson B., Chapman H., Tadayyon M., Johns A., Porter R. A., et al. (2000). A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul. Pept. 96 45–51. [DOI] [PubMed] [Google Scholar]
- Haynes A. C., Jackson B., Overend P., Buckingham R. E., Wilson S., Tadayyon M., et al. (1999). Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides 20 1099–1105. 10.1016/s0196-9781(99)00105-9 [DOI] [PubMed] [Google Scholar]
- Henny P., Brischoux F., Mainville L., Stroh T., Jones B. E. (2010). Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience 169 1150–1157. 10.1016/j.neuroscience.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C. G., Cadavieco M. C., Jego S., Ponomarenko A., Korotkova T., Adamantidis A. (2016). Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat. Neurosci. 19 290–298. 10.1038/nn.4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath T. L., Gao X. B. (2005). Input organization and plasticity of hypocretin neurons: Possible clues to obesity’s association with insomnia. Cell Metab. 1 279–286. 10.1016/j.cmet.2005.03.003 [DOI] [PubMed] [Google Scholar]
- Horvath T. L., Diano S., van den Pol A. N. (1999a). Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: A novel circuit implicated in metabolic and endocrine regulations. J. Neurosci. 19 1072–1087. 10.1523/JNEUROSCI.19-03-01072.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath T. L., Peyron C., Diano S., Ivanov A., Aston-Jones G., Kilduff T. S., et al. (1999b). Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J. Comp. Neurol. 415 145–159. [PubMed] [Google Scholar]
- Inutsuka A., Inui A., Tabuchi S., Tsunematsu T., Lazarus M., Yamanaka A. (2014). Concurrent and robust regulation of feeding behaviors and metabolism by orexin neurons. Neuropharmacology 85 451–460. 10.1016/j.neuropharm.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Iqbal J., Pompolo S., Murakami T., Grouzmann E., Sakurai T., Meister B., et al. (2001). Immunohistochemical characterization of localization of long-form leptin receptor (OB-Rb) in neurochemically defined cells in the ovine hypothalamus. Brain Res. 920 55–64. 10.1016/s0006-8993(01)02932-8 [DOI] [PubMed] [Google Scholar]
- Ito M., Gomori A., Ishihara A., Oda Z., Mashiko S., Matsushita H., et al. (2003). Characterization of MCH-mediated obesity in mice. Am. J. Physiol. Endocrinol. Metab. 284 E940–E945. [DOI] [PubMed] [Google Scholar]
- Ivanov A., Aston-Jones G. (2000). Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport 11 1755–1758. 10.1097/00001756-200006050-00031 [DOI] [PubMed] [Google Scholar]
- Izawa S., Chowdhury S., Miyazaki T., Mukai Y., Ono D., Inoue R., et al. (2019). REM sleep-active MCH neurons are involved in forgetting hippocampus-dependent memories. Science 365 1308–1313. 10.1126/science.aax9238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Yoneshiro T., Kondoh K., Nakagiri S., Okamatsu-Ogura Y., Terao A., et al. (2022). Melanin-concentrating hormone-producing neurons in the hypothalamus regulate brown adipose tissue and thus contribute to energy expenditure. J. Physiol. 600 815–827. 10.1113/JP281241 [DOI] [PubMed] [Google Scholar]
- Jain M. R., Horvath T. L., Kalra P. S., Kalra S. P. (2000). Evidence that NPY Y1 receptors are involved in stimulation of feeding by orexins (hypocretins) in sated rats. Regul. Pept. 87 19–24. [DOI] [PubMed] [Google Scholar]
- Janas-Kozik M., Stachowicz M., Krupka-Matuszczyk I., Szymszal J., Krysta K., Janas A., et al. (2011). Plasma levels of leptin and orexin A in the restrictive type of anorexia nervosa. Regul. Pept. 168 5–9. 10.1016/j.regpep.2011.02.005 [DOI] [PubMed] [Google Scholar]
- Jego S., Glasgow S. D., Herrera C. G., Ekstrand M., Reed S. J., Boyce R., et al. (2013). Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat. Neurosci. 16 1637–1643. 10.1038/nn.3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings J. H., Ung R. L., Resendez S. L., Stamatakis A. M., Taylor J. G., Huang J., et al. (2015). Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160 516–527. 10.1016/j.cell.2014.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J. Y., Bradley R. L., Kokkotou E. G., Marino F. E., Wang X., Pissios P., et al. (2006). MCH-/- mice are resistant to aging-associated increases in body weight and insulin resistance. Diabetes 55 428–434. [DOI] [PubMed] [Google Scholar]
- Jha P. K., Foppen E., Kalsbeek A., Challet E. (2016). Sleep restriction acutely impairs glucose tolerance in rats. Physiol. Rep. 4:e12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha P. K., Valekunja U. K., Ray S., Nollet M., Reddy A. B. (2022). Single-cell transcriptomics and cell-specific proteomics reveals molecular signatures of sleep. Commun. Biol. 5:846. 10.1038/s42003-022-03800-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Gallet S., Klemm P., Scholl P., Folz-Donahue K., Altmuller J., et al. (2020). MCH neurons regulate permeability of the median eminence barrier. Neuron 107 306–319.e309. 10.1016/j.neuron.2020.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T., Jiang Z., Luan X., Qu Z., Guo F., Gao S., et al. (2020). Exogenous orexin-A microinjected into central nucleus of the amygdala modulates feeding and gastric motility in rats. Front. Neurosci. 14:274. 10.3389/fnins.2020.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L., Ekholm M. E., Kukkonen J. P. (2008). Multiple phospholipase activation by OX(1) orexin/hypocretin receptors. Cell Mol. Life Sci. 65 1948–1956. 10.1007/s00018-008-8206-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski S. E., Fortin S. M., Ricks K. M., Grill H. J. (2013). Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol. Psychiatry 73 915–923. 10.1016/j.biopsych.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaei M., Tahergorabi Z. (2013). Systemic ghrelin administration alters serum biomarkers of angiogenesis in diet-induced obese mice. Int. J. Pept. 2013:249565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Despres J. P., Koh K. K. (2016). Obesity and cardiovascular disease: Friend or foe? Eur. Heart J. 37 3560–3568. [DOI] [PubMed] [Google Scholar]
- Kitka T., Adori C., Katai Z., Vas S., Molnar E., Papp R. S., et al. (2011). Association between the activation of MCH and orexin immunorective neurons and REM sleep architecture during REM rebound after a three day long REM deprivation. Neurochem. Int. 59 686–694. 10.1016/j.neuint.2011.06.015 [DOI] [PubMed] [Google Scholar]
- Kiyashchenko L. I., Mileykovskiy B. Y., Maidment N., Lam H. A., Wu M. F., John J., et al. (2002). Release of hypocretin (orexin) during waking and sleep states. J. Neurosci. 22 5282–5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. (1999). Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402 656–660. [DOI] [PubMed] [Google Scholar]
- Kokkotou E., Jeon J. Y., Wang X., Marino F. E., Carlson M., Trombly D. J., et al. (2005). Mice with MCH ablation resist diet-induced obesity through strain-specific mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289 R117–R124. 10.1152/ajpregu.00861.2004 [DOI] [PubMed] [Google Scholar]
- Konadhode R. R., Pelluru D., Blanco-Centurion C., Zayachkivsky A., Liu M., Uhde T., et al. (2013). Optogenetic stimulation of MCH neurons increases sleep. J. Neurosci. 33 10257–10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D., Vong L., Parton L. E., Ye C., Tong Q., Hu X., et al. (2010). Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab. 12 545–552. 10.1016/j.cmet.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S., Zhang C. Y., Scorrano L., Dalgaard L. T., St-Pierre J., Grey S. T., et al. (2003). Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J. Clin. Invest. 112 1831–1842. 10.1172/JCI19774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger D., Bandaru S. S., Madara J. C., Vetrivelan R. (2019). Ventrolateral periaqueductal gray mediates rapid eye movement sleep regulation by melanin-concentrating hormone neurons. Neuroscience 406 314–324. 10.1016/j.neuroscience.2019.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos P., Monti J. M., Jantos H., Torterolo P. (2012). Microinjection of the melanin-concentrating hormone into the lateral basal forebrain increases REM sleep and reduces wakefulness in the rat. Life Sci. 90 895–899. 10.1016/j.lfs.2012.04.019 [DOI] [PubMed] [Google Scholar]
- Lagos P., Torterolo P., Jantos H., Chase M. H., Monti J. M. (2009). Effects on sleep of melanin-concentrating hormone (MCH) microinjections into the dorsal raphe nucleus. Brain Res. 1265 103–110. [DOI] [PubMed] [Google Scholar]
- Laque A., Zhang Y., Gettys S., Nguyen T. A., Bui K., Morrison C. D., et al. (2013). Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. Am. J. Physiol. Endocrinol. Metab. 304 E999–E1011. 10.1152/ajpendo.00643.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. B., Snape A. C., Baudoin F. M., Luckman S. M. (2002). Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 143 155–162. 10.1210/endo.143.1.8561 [DOI] [PubMed] [Google Scholar]
- Lee J., Lim E., Kim Y., Li E., Park S. (2010). Ghrelin attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. J. Endocrinol. 205 263–270. 10.1677/JOE-10-0040 [DOI] [PubMed] [Google Scholar]
- Lee M. G., Hassani O. K., Jones B. E. (2005). Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J. Neurosci. 25 6716–6720. 10.1523/JNEUROSCI.1887-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger G. M., Jo Y. H., Leshan R. L., Louis G. W., Yang H., Barrera J. G., et al. (2009). Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 10 89–98. 10.1016/j.cmet.2009.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger G. M., Opland D. M., Jo Y. H., Faouzi M., Christensen L., Cappellucci L. A., et al. (2011). Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 14 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li S., Wei C., Wang H., Sui N., Kirouac G. J. (2010). Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology 212 251–265. 10.1007/s00213-010-1948-y [DOI] [PubMed] [Google Scholar]
- Lin L., Faraco J., Li R., Kadotani H., Rogers W., Lin X., et al. (1999). The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98 365–376. [DOI] [PubMed] [Google Scholar]
- Lin L., Saha P. K., Ma X., Henshaw I. O., Shao L., Chang B. H., et al. (2011). Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell 10 996–1010. 10.1111/j.1474-9726.2011.00740.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan V., Hirasawa M. (2022). Short-term fasting induces alternate activation of orexin and melanin-concentrating hormone neurons in rats. Neuroscience 491 156–165. 10.1016/j.neuroscience.2022.04.006 [DOI] [PubMed] [Google Scholar]
- Lopez M., Seoane L. M., Garcia Mdel C., Dieguez C., Senaris R. (2002). Neuropeptide Y, but not agouti-related peptide or melanin-concentrating hormone, is a target peptide for orexin-A feeding actions in the rat hypothalamus. Neuroendocrinology 75 34–44. [DOI] [PubMed] [Google Scholar]
- Lopez M., Seoane L., Garcia M. C., Lago F., Casanueva F. F., Senaris R., et al. (2000). Leptin regulation of prepro-orexin and orexin receptor mRNA levels in the hypothalamus. Biochem. Biophys. Res. Commun. 269 41–45. [DOI] [PubMed] [Google Scholar]
- Lord M. N., Subramanian K., Kanoski S. E., Noble E. E. (2021). Melanin-concentrating hormone and food intake control: Sites of action, peptide interactions, and appetition. Peptides 137:170476. 10.1016/j.peptides.2020.170476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis G. W., Leinninger G. M., Rhodes C. J., Myers M. G., Jr. (2010). Direct innervation and modulation of orexin neurons by lateral hypothalamic LepRb neurons. J. Neurosci. 30 11278–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig D. S., Tritos N. A., Mastaitis J. W., Kulkarni R., Kokkotou E., Elmquist J., et al. (2001). Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J. Clin. Invest. 107 379–386. 10.1172/JCI10660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungwitz E. A., Molosh A., Johnson P. L., Harvey B. P., Dirks R. C., Dietrich A., et al. (2012). Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol. Behav. 107 726–732. 10.1016/j.physbeh.2012.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi P. H., Clement O., Fort P. (2013). Paradoxical (REM) sleep genesis by the brainstem is under hypothalamic control. Curr. Opin. Neurobiol. 23 786–792. 10.1016/j.conb.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Ma X., Zubcevic L., Bruning J. C., Ashcroft F. M., Burdakov D. (2007). Electrical inhibition of identified anorexigenic POMC neurons by orexin/hypocretin. J. Neurosci. 27 1529–1533. 10.1523/JNEUROSCI.3583-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S., McGlone F., Bedrossian D., Dagher A. (2008). Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 7 400–409. 10.1016/j.cmet.2008.03.007 [DOI] [PubMed] [Google Scholar]
- Mao Y., Tokudome T., Otani K., Kishimoto I., Miyazato M., Kangawa K. (2013). Excessive sympathoactivation and deteriorated heart function after myocardial infarction in male ghrelin knockout mice. Endocrinology 154 1854–1863. 10.1210/en.2012-2132 [DOI] [PubMed] [Google Scholar]
- Mao Y., Tokudome T., Otani K., Kishimoto I., Nakanishi M., Hosoda H., et al. (2012). Ghrelin prevents incidence of malignant arrhythmia after acute myocardial infarction through vagal afferent nerves. Endocrinology 153 3426–3434. 10.1210/en.2012-1065 [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Weingarth D. T., Novi D. E., Chen H. Y., Trumbauer M. E., Chen A. S., et al. (2002). Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc. Natl. Acad. Sci. U. S. A. 99 3240–3245. 10.1073/pnas.052706899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavanji V., Perez-Leighton C. E., Kotz C. M., Billington C. J., Parthasarathy S., Sinton C. M., et al. (2015). Promotion of wakefulness and energy expenditure by orexin-A in the ventrolateral preoptic area. Sleep 38 1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavanji V., Pomonis B., Kotz C. M. (2022). Orexin, serotonin, and energy balance. Wires Mech. Dis. 14:e1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerabux J., Iwayama Y., Sakurai T., Ohba H., Toyota T., Yamada K., et al. (2005). Association of an orexin 1 receptor 408Val variant with polydipsia-hyponatremia in schizophrenic subjects. Biol. Psychiatry 58 401–407. 10.1016/j.biopsych.2005.04.015 [DOI] [PubMed] [Google Scholar]
- Miao H., Pan H., Wang L., Yang H., Zhu H., Gong F. (2019). Ghrelin promotes proliferation and inhibits differentiation of 3T3-L1 and human primary preadipocytes. Front. Physiol. 10:1296. 10.3389/fphys.2019.01296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen L. E., Bolisetty M., Chimileski B. R., Fujita A., Beltrami E. J., Costanzo J. T., et al. (2019). Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci. 22 642–656. 10.1038/s41593-019-0349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen L. E., Kolling F. W. T., Chimileski B. R., Fujita A., Norris C., Chen K., et al. (2017). Neurochemical heterogeneity among lateral hypothalamic hypocretin/orexin and melanin-concentrating hormone neurons identified through single-cell gene expression analysis. eNeuro 4 ENEURO.0013–ENEURO.17. 10.1523/ENEURO.0013-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M., Hasegawa E., Kisanuki Y. Y., Sinton C. M., Yanagisawa M., Sakurai T. (2011). Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J. Neurosci. 31 6518–6526. 10.1523/JNEUROSCI.6506-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbank E., Lopez M. (2019). Orexins/hypocretins: Key regulators of energy homeostasis. Front. Endocrinol. 10:830. 10.3389/fendo.2019.00830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy B. Y., Kiyashchenko L. I., Siegel J. M. (2005). Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T., Arrigoni E., Marcus J. N., Clark E. L., Yamamoto M., Honer M., et al. (2011). Orexin receptor 2 expression in the posterior hypothalamus rescues sleepiness in narcoleptic mice. Proc. Natl. Acad. Sci. U. S. A. 108 4471–4476. 10.1073/pnas.1012456108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T., Crocker A., McCormack S., Yanagisawa M., Sakurai T., Scammell T. E. (2004). Behavioral state instability in orexin knock-out mice. J. Neurosci. 24 6291–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modirrousta M., Mainville L., Jones B. E. (2005). Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur. J. Neurosci. 21 2807–2816. 10.1111/j.1460-9568.2005.04104.x [DOI] [PubMed] [Google Scholar]
- Mogi K., Funabashi T., Mitsushima D., Hagiwara H., Kimura F. (2005). Sex difference in the response of melanin-concentrating hormone neurons in the lateral hypothalamic area to glucose, as revealed by the expression of phosphorylated cyclic adenosine 3’,5’-monophosphate response element-binding protein. Endocrinology 146 3325–3333. 10.1210/en.2005-0078 [DOI] [PubMed] [Google Scholar]
- Monti J. M., Lagos P., Jantos H., Torterolo P. (2015). Increased REM sleep after intra-locus coeruleus nucleus microinjection of melanin-concentrating hormone (MCH) in the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry 56 185–188. 10.1016/j.pnpbp.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Monti J. M., Torterolo P., Lagos P. (2013). Melanin-concentrating hormone control of sleep-wake behavior. Sleep Med. Rev. 17 293–298. [DOI] [PubMed] [Google Scholar]
- Monti J. M., Torterolo P., Jantos H., Lagos P. (2016). Microinjection of the melanin-concentrating hormone into the sublaterodorsal tegmental nucleus inhibits REM sleep in the rat. Neurosci. Lett. 630 66–69. 10.1016/j.neulet.2016.07.035 [DOI] [PubMed] [Google Scholar]
- Morton G. J., Meek T. H., Schwartz M. W. (2014). Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 15 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosavat M., Mirsanjari M., Arabiat D., Smyth A., Whitehead L. (2021). The role of sleep curtailment on leptin levels in obesity and diabetes mellitus. Obes. Facts 14 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroya S., Funahashi H., Yamanaka A., Kohno D., Uramura K., Nambu T., et al. (2004). Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: Orexigenic neuronal pathways in the mediobasal hypothalamus. Eur. J. Neurosci. 19 1524–1534. 10.1111/j.1460-9568.2004.03255.x [DOI] [PubMed] [Google Scholar]
- Murray J. F., Mercer J. G., Adan R. A., Datta J. J., Aldairy C., Moar K. M., et al. (2000). The effect of leptin on luteinizing hormone release is exerted in the zona incerta and mediated by melanin-concentrating hormone. J. Neuroendocrinol. 12 1133–1139. 10.1046/j.1365-2826.2000.00577.x [DOI] [PubMed] [Google Scholar]
- Myers M. G., Jr., Olson D. P. (2012). Central nervous system control of metabolism. Nature 491 357–363. [DOI] [PubMed] [Google Scholar]
- Nahon J. L., Presse F., Bittencourt J. C., Sawchenko P. E., Vale W. (1989). The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology 125 2056–2065. 10.1210/endo-125-4-2056 [DOI] [PubMed] [Google Scholar]
- Nakazato M., Murakami N., Date Y., Kojima M., Matsuo H., Kangawa K., et al. (2001). A role for ghrelin in the central regulation of feeding. Nature 409 194–198. [DOI] [PubMed] [Google Scholar]
- Nambu T., Sakurai T., Mizukami K., Hosoya Y., Yanagisawa M., Goto K. (1999). Distribution of orexin neurons in the adult rat brain. Brain Res. 827 243–260. [DOI] [PubMed] [Google Scholar]
- Nevsimalova S., Vankova J., Stepanova I., Seemanova E., Mignot E., Nishino S. (2005). Hypocretin deficiency in Prader-Willi syndrome. Eur. J. Neurol. 12 70–72. [DOI] [PubMed] [Google Scholar]
- Nishino S., Ripley B., Mignot E., Benson K. L., Zarcone V. P. (2002). CSF hypocretin-1 levels in schizophrenics and controls: Relationship to sleep architecture. Psychiatry Res. 110 1–7. 10.1016/s0165-1781(02)00032-x [DOI] [PubMed] [Google Scholar]
- Nitz D., Siegel J. M. (1996). GABA release in posterior hypothalamus across sleep-wake cycle. Am. J. Physiol. 271 R1707–R1712. 10.1152/ajpregu.1996.271.6.R1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble E. E., Hahn J. D., Konanur V. R., Hsu T. M., Page S. J., Cortella A. M., et al. (2018). Control of feeding behavior by cerebral ventricular volume transmission of melanin-concentrating hormone. Cell Metab. 28:e57. 10.1016/j.cmet.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie R. P., Patel S. R. (2017). The epidemiology of sleep and obesity. Sleep Health 3 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski P. K., Li D., Grace M. K., Billington C. J., Kotz C. M., Levine A. S. (2003). Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides 24 597–602. 10.1016/s0196-9781(03)00105-0 [DOI] [PubMed] [Google Scholar]
- Papotti M., Ghe C., Cassoni P., Catapano F., Deghenghi R., Ghigo E., et al. (2000). Growth hormone secretagogue binding sites in peripheral human tissues. J. Clin. Endocrinol. Metab. 85 3803–3807. [DOI] [PubMed] [Google Scholar]
- Pascovich C., Lagos P., Urbanavicius J., Devera A., Rivas M., Costa A., et al. (2020). Melanin-concentrating hormone (MCH) in the median raphe nucleus: Fibers, receptors and cellular effects. Peptides 126:170249. [DOI] [PubMed] [Google Scholar]
- Pascovich C., Nino S., Mondino A., Lopez-Hill X., Urbanavicius J., Monti J., et al. (2021). Microinjection of melanin-concentrating hormone (MCH) into the median raphe nucleus promotes REM sleep in rats. Sleep Sci. 14 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C., Tighe D. K., van den Pol A. N., de Lecea L., Heller H. C., Sutcliffe J. G., et al. (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper D. C., Upton N., Smith M. I., Hunter A. J. (2000). The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur. J. Neurosci. 12 726–730. [DOI] [PubMed] [Google Scholar]
- Pissios P., Bradley R. L., Maratos-Flier E. (2006). Expanding the scales: The multiple roles of MCH in regulating energy balance and other biological functions. Endocr. Rev. 27 606–620. 10.1210/er.2006-0021 [DOI] [PubMed] [Google Scholar]
- Qian J., Morris C. J., Caputo R., Garaulet M., Scheer F. (2019). Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. Int. J. Obes. 43 1644–1649. 10.1038/s41366-018-0208-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D., Ludwig D. S., Gammeltoft S., Piper M., Pelleymounter M. A., Cullen M. J., et al. (1996). A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 380 243–247. [DOI] [PubMed] [Google Scholar]
- Ramanjaneya M., Conner A. C., Chen J., Kumar P., Brown J. E., Johren O., et al. (2009). Orexin-stimulated MAP kinase cascades are activated through multiple G-protein signalling pathways in human H295R adrenocortical cells: Diverse roles for orexins A and B. J. Endocrinol. 202 249–261. 10.1677/JOE-08-0536 [DOI] [PubMed] [Google Scholar]
- Ravussin E., Tschop M., Morales S., Bouchard C., Heiman M. L. (2001). Plasma ghrelin concentration and energy balance: Overfeeding and negative energy balance studies in twins. J. Clin. Endocrinol. Metab. 86 4547–4551. 10.1210/jcem.86.9.8003 [DOI] [PubMed] [Google Scholar]
- Risold P. Y., Thompson R. H., Swanson L. W. (1997). The structural organization of connections between hypothalamus and cerebral cortex. Brain Res. Brain Res. Rev. 24 197–254. [DOI] [PubMed] [Google Scholar]
- Rodgers R. J., Halford J. C., Nunes de Souza R. L., Canto, de Souza A. L., Piper D. C., et al. (2001). SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur. J. Neurosci. 13 1444–1452. 10.1046/j.0953-816x.2001.01518.x [DOI] [PubMed] [Google Scholar]
- Roh E., Song D. K., Kim M. S. (2016). Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 48:e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A., Schaich Borg J., de Lecea L. (2010). Sleep and metabolism: Role of hypothalamic neuronal circuitry. Best Pract. Res. Clin. Endocrinol. Metab. 24 817–828. [DOI] [PubMed] [Google Scholar]
- Rosin D. L., Weston M. C., Sevigny C. P., Stornetta R. L., Guyenet P. G. (2003). Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J. Comp. Neurol. 465 593–603. [DOI] [PubMed] [Google Scholar]
- Rossi M. A., Basiri M. L., McHenry J. A., Kosyk O., Otis J. M., van den Munkhof H. E., et al. (2019). Obesity remodels activity and transcriptional state of a lateral hypothalamic brake on feeding. Science 364 1271–1274. 10.1126/science.aax1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter A., Asemann R., Decker A., Kornhuber J., Biermann T. (2011). Orexin expression and promoter-methylation in peripheral blood of patients suffering from major depressive disorder. J. Affect. Disord. 131 186–192. 10.1016/j.jad.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Saad M. F., Bernaba B., Hwu C. M., Jinagouda S., Fahmi S., Kogosov E., et al. (2002). Insulin regulates plasma ghrelin concentration. J. Clin. Endocrinol. Metab. 87 3997–4000. [DOI] [PubMed] [Google Scholar]
- Sahu A. (1998). Leptin decreases food intake induced by melanin-concentrating hormone (MCH), galanin (GAL) and neuropeptide Y (NPY) in the rat. Endocrinology 139 4739–4742. 10.1210/endo.139.11.6432 [DOI] [PubMed] [Google Scholar]
- Saito Y. C., Maejima T., Nishitani M., Hasegawa E., Yanagawa Y., Mieda M., et al. (2018a). Monoamines inhibit GABAergic neurons in ventrolateral preoptic area that make direct synaptic connections to hypothalamic arousal neurons. J. Neurosci. 38 6366–6378. 10.1523/JNEUROSCI.2835-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y. C., Tsujino N., Abe M., Yamazaki M., Sakimura K., Sakurai T. (2018b). Serotonergic input to orexin neurons plays a role in maintaining wakefulness and REM sleep architecture. Front. Neurosci. 12:892. 10.3389/fnins.2018.00892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y. C., Tsujino N., Hasegawa E., Akashi K., Abe M., Mieda M., et al. (2013). GABAergic neurons in the preoptic area send direct inhibitory projections to orexin neurons. Front. Neural Circuits 7:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. (2007). The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat. Rev. Neurosci. 8 171–181. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R. M., Tanaka H., et al. (1998). Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:696. [DOI] [PubMed] [Google Scholar]
- Santos V. V., Stark R., Rial D., Silva H. B., Bayliss J. A., Lemus M. B., et al. (2017). Acyl ghrelin improves cognition, synaptic plasticity deficits and neuroinflammation following amyloid beta (Abeta1-40) administration in mice. J. Neuroendocrinol. 29 1–11. 10.1111/jne.12476 [DOI] [PubMed] [Google Scholar]
- Saper C. B. (2006). Staying awake for dinner: Hypothalamic integration of sleep, feeding, and circadian rhythms. Prog. Brain Res. 153 243–252. 10.1016/S0079-6123(06)53014-6 [DOI] [PubMed] [Google Scholar]
- Saper C. B., Fuller P. M. (2017). Wake-sleep circuitry: An overview. Curr. Opin. Neurobiol. 44 186–192. 10.1016/j.conb.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Suzuki M., Mieda M., Tsujino N., Roth B., Sakurai T. (2011). Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One 6:e20360. 10.1371/journal.pone.0020360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell T. E., Winrow C. J. (2011). Orexin receptors: Pharmacology and therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 51 243–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. M., Hallschmid M., Jauch-Chara K., Born J., Schultes B. (2008). A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J. Sleep Res. 17 331–334. 10.1111/j.1365-2869.2008.00662.x [DOI] [PubMed] [Google Scholar]
- Schneeberger M., Tan K., Nectow A. R., Parolari L., Caglar C., Azevedo E., et al. (2018). Functional analysis reveals differential effects of glutamate and MCH neuropeptide in MCH neurons. Mol. Metab. 13 83–89. 10.1016/j.molmet.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. W., Porte D., Jr. (2005). Diabetes, obesity, and the brain. Science 307 375–379. [DOI] [PubMed] [Google Scholar]
- Schwartz M. W., Baskin D. G., Bukowski T. R., Kuijper J. L., Foster D., Lasser G., et al. (1996). Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes 45 531–535. 10.2337/diab.45.4.531 [DOI] [PubMed] [Google Scholar]
- Schwartz M. W., Woods S. C., Porte D., Jr., Seeley R. J., Baskin D. G. (2000). Central nervous system control of food intake. Nature 404 661–671. [DOI] [PubMed] [Google Scholar]
- Segal-Lieberman G., Bradley R. L., Kokkotou E., Carlson M., Trombly D. J., Wang X., et al. (2003). Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc. Natl. Acad. Sci. U. S. A. 100 10085–10090. 10.1073/pnas.1633636100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z., Santiago A. M., Thomas M. P., Routh V. H. (2014). Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Mol. Cell Neurosci. 62 30–41. 10.1016/j.mcn.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M., Tritos N. A., Lowell B. B., Flier J. S., Maratos-Flier E. (1998). Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 396 670–674. [DOI] [PubMed] [Google Scholar]
- Shin S. Y., Yang J. H., Lee H., Erdelyi F., Szabo G., Lee S. Y., et al. (2007). Identification of the adrenoceptor subtypes expressed on GABAergic neurons in the anterior hypothalamic area and rostral zona incerta of GAD65-eGFP transgenic mice. Neurosci. Lett. 422 153–157. 10.1016/j.neulet.2007.05.060 [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Jacobowitz D. M., Zamir N. (1985). Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res. Bull. 15 635–649. 10.1016/0361-9230(85)90213-8 [DOI] [PubMed] [Google Scholar]
- Strawn J. R., Pyne-Geithman G. J., Ekhator N. N., Horn P. S., Uhde T. W., Shutter L. A., et al. (2010). Low cerebrospinal fluid and plasma orexin-A (hypocretin-1) concentrations in combat-related posttraumatic stress disorder. Psychoneuroendocrinology 35 1001–1007. 10.1016/j.psyneuen.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Stuber G. D., Wise R. A. (2016). Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci. 19 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Wang P., Zheng H., Smith R. G. (2004). Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci. U. S. A. 101 4679–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Beuckmann C. T., Shikata K., Ogura H., Sawai T. (2005). Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res. 1044 116–121. 10.1016/j.brainres.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Sweet D. C., Levine A. S., Billington C. J., Kotz C. M. (1999). Feeding response to central orexins. Brain Res. 821 535–538. [DOI] [PubMed] [Google Scholar]
- Szentirmai E., Kapas L., Krueger J. M. (2007a). Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292 R575–R585. 10.1152/ajpregu.00448.2006 [DOI] [PubMed] [Google Scholar]
- Szentirmai E., Kapas L., Sun Y., Smith R. G., Krueger J. M. (2007b). Spontaneous sleep and homeostatic sleep regulation in ghrelin knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293 R510–R517. 10.1152/ajpregu.00155.2007 [DOI] [PubMed] [Google Scholar]
- Taheri S., Gardiner J., Hafizi S., Murphy K., Dakin C., Seal L., et al. (2001). Orexin A immunoreactivity and preproorexin mRNA in the brain of Zucker and WKY rats. Neuroreport 12 459–464. 10.1097/00001756-200103050-00008 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Itoh H. (2019). Hypertension as a metabolic disorder and the novel role of the gut. Curr. Hypertens. Rep. 21:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum P., Stellar E. (1954). Recovery from the failure to eat produced by hypothalamic lesions. Science 120 894–895. 10.1126/science.120.3126.894 [DOI] [PubMed] [Google Scholar]
- Thannickal T. C., Moore R. Y., Nienhuis R., Ramanathan L., Gulyani S., Aldrich M., et al. (2000). Reduced number of hypocretin neurons in human narcolepsy. Neuron 27 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe A. J., Mullett M. A., Wang C., Kotz C. M. (2003). Peptides that regulate food intake: Regional, metabolic, and circadian specificity of lateral hypothalamic orexin A feeding stimulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284 R1409–R1417. 10.1152/ajpregu.00344.2002 [DOI] [PubMed] [Google Scholar]
- Timper K., Bruning J. C. (2017). Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model Mech. 10 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolle V., Bassant M. H., Zizzari P., Poindessous-Jazat F., Tomasetto C., Epelbaum J., et al. (2002). Ultradian rhythmicity of ghrelin secretion in relation with GH, feeding behavior, and sleep-wake patterns in rats. Endocrinology 143 1353–1361. 10.1210/endo.143.4.8712 [DOI] [PubMed] [Google Scholar]
- Torrealba F., Yanagisawa M., Saper C. B. (2003). Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience 119 1033–1044. 10.1016/s0306-4522(03)00238-0 [DOI] [PubMed] [Google Scholar]
- Torterolo P., Lagos P., Monti J. M. (2011). Melanin-concentrating hormone: A new sleep factor? Front. Neurol. 2:14. 10.3389/fneur.2011.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P., Sampogna S., Chase M. H. (2009). MCHergic projections to the nucleus pontis oralis participate in the control of active (REM) sleep. Brain Res. 1268 76–87. 10.1016/j.brainres.2009.02.055 [DOI] [PubMed] [Google Scholar]
- Torterolo P., Sampogna S., Chase M. H. (2013). Hypocretinergic and non-hypocretinergic projections from the hypothalamus to the REM sleep executive area of the pons. Brain Res. 1491 68–77. 10.1016/j.brainres.2012.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshinai K., Date Y., Murakami N., Shimada M., Mondal M. S., Shimbara T., et al. (2003). Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 144 1506–1512. 10.1210/en.2002-220788 [DOI] [PubMed] [Google Scholar]
- Toshinai K., Yamaguchi H., Sun Y., Smith R. G., Yamanaka A., Sakurai T., et al. (2006). Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology 147 2306–2314. [DOI] [PubMed] [Google Scholar]
- Tsunematsu T., Ueno T., Tabuchi S., Inutsuka A., Tanaka K. F., Hasuwa H., et al. (2014). Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J. Neurosci. 34 6896–6909. 10.1523/JNEUROSCI.5344-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen P. M., Jantti M. H., Kukkonen J. P. (2012). OX1 orexin/hypocretin receptor signaling through arachidonic acid and endocannabinoid release. Mol. Pharmacol. 82 156–167. 10.1124/mol.112.078063 [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Gao X. B., Obrietan K., Kilduff T. S., Belousov A. B. (1998). Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J. Neurosci. 18 7962–7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varin C., Arthaud S., Salvert D., Gay N., Libourel P. A., Luppi P. H., et al. (2016). Sleep architecture and homeostasis in mice with partial ablation of melanin-concentrating hormone neurons. Behav. Brain Res. 298 100–110. 10.1016/j.bbr.2015.10.051 [DOI] [PubMed] [Google Scholar]
- Varin C., Luppi P. H., Fort P. (2018). Melanin-concentrating hormone-expressing neurons adjust slow-wave sleep dynamics to catalyze paradoxical (REM) sleep. Sleep 41:zsy068. 10.1093/sleep/zsy068 [DOI] [PubMed] [Google Scholar]
- Venner A., Anaclet C., Broadhurst R. Y., Saper C. B., Fuller P. M. (2016). A novel population of wake-promoting GABAergic neurons in the ventral lateral hypothalamus. Curr. Biol. 26 2137–2143. 10.1016/j.cub.2016.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venner A., Karnani M. M., Gonzalez J. A., Jensen L. T., Fugger L., Burdakov D. (2011). Orexin neurons as conditional glucosensors: Paradoxical regulation of sugar sensing by intracellular fuels. J. Physiol. 589 5701–5708. 10.1113/jphysiol.2011.217000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst P. J., Depoortere I. (2012). Ghrelin’s second life: From appetite stimulator to glucose regulator. World J. Gastroenterol. 18 3183–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L., Goutagny R., Fort P., Cagnon L., Salvert D., Leger L., et al. (2003). A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 4:19. 10.1186/1471-2202-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivelan R., Kong D., Ferrari L. L., Arrigoni E., Madara J. C., Bandaru S. S., et al. (2016). Melanin-concentrating hormone neurons specifically promote rapid eye movement sleep in mice. Neuroscience 336 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskaitis P., Tesmer A. L., Karnani M. M., Arnold M., Donegan D., Bracey E. F., et al. (2022). Orexin cells efficiently decode blood glucose dynamics to drive adaptive behavior. bioRxiv [Preprint]. 10.1101/2022.04.14.488310 [DOI] [Google Scholar]
- Wang Q., Yin Y., Zhang W. (2018). Ghrelin restores the disruption of the circadian clock in steatotic liver. Int. J. Mol. Sci. 19:3134. 10.3390/ijms19103134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Cao X., Zeng Q., Zhang F., Xue Q., Luo Y., et al. (2015). Ghrelin inhibits proinflammatory responses and prevents cognitive impairment in septic rats. Crit. Care Med. 43 e143–e150. 10.1097/CCM.0000000000000930 [DOI] [PubMed] [Google Scholar]
- Weikel J. C., Wichniak A., Ising M., Brunner H., Friess E., Held K., et al. (2003). Ghrelin promotes slow-wave sleep in humans. Am. J. Physiol. Endocrinol. Metab. 284 E407–E415. [DOI] [PubMed] [Google Scholar]
- Wen J., Zhao Y., Shen Y., Guo L. (2015). Effect of orexin A on apoptosis in BGC-823 gastric cancer cells via OX1R through the AKT signaling pathway. Mol. Med. Rep. 11 3439–3444. 10.3892/mmr.2015.3190 [DOI] [PubMed] [Google Scholar]
- White C. L., Ishii Y., Mendoza T., Upton N., Stasi L. P., Bray G. A., et al. (2005). Effect of a selective OX1R antagonist on food intake and body weight in two strains of rats that differ in susceptibility to dietary-induced obesity. Peptides 26 2331–2338. 10.1016/j.peptides.2005.03.042 [DOI] [PubMed] [Google Scholar]
- Williams R. H., Alexopoulos H., Jensen L. T., Fugger L., Burdakov D. (2008). Adaptive sugar sensors in hypothalamic feeding circuits. Proc. Natl. Acad. Sci. U. S. A. 105 11975–11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie J. T., Chemelli R. M., Sinton C. M., Yanagisawa M. (2001). To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu. Rev. Neurosci. 24 429–458. [DOI] [PubMed] [Google Scholar]
- Willie J. T., Chemelli R. M., Sinton C. M., Tokita S., Williams S. C., Kisanuki Y. Y., et al. (2003). Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: Molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron 38 715–730. 10.1016/s0896-6273(03)00330-1 [DOI] [PubMed] [Google Scholar]
- Willie J. T., Sinton C. M., Maratos-Flier E., Yanagisawa M. (2008). Abnormal response of melanin-concentrating hormone deficient mice to fasting: Hyperactivity and rapid eye movement sleep suppression. Neuroscience 156 819–829. 10.1016/j.neuroscience.2008.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Ueta Y., Serino R., Nomura M., Shibuya I., Yamashita H. (2000). Effects of food restriction on the hypothalamic prepro-orexin gene expression in genetically obese mice. Brain Res. Bull. 51 515–521. 10.1016/s0361-9230(99)00271-3 [DOI] [PubMed] [Google Scholar]
- Yamanaka A., Beuckmann C. T., Willie J. T., Hara J., Tsujino N., Mieda M., et al. (2003). Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38 701–713. 10.1016/s0896-6273(03)00331-3 [DOI] [PubMed] [Google Scholar]
- Yamanaka A., Kunii K., Nambu T., Tsujino N., Sakai A., Matsuzaki I., et al. (2000). Orexin-induced food intake involves neuropeptide Y pathway. Brain Res. 859 404–409. [DOI] [PubMed] [Google Scholar]
- Yamanaka A., Tsujino N., Funahashi H., Honda K., Guan J. L., Wang Q. P., et al. (2002). Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem. Biophys. Res. Commun. 290 1237–1245. [DOI] [PubMed] [Google Scholar]
- Yang B., Ferguson A. V. (2002). Orexin-A depolarizes dissociated rat area postrema neurons through activation of a nonselective cationic conductance. J. Neurosci. 22 6303–6308. 10.1523/JNEUROSCI.22-15-06303.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannielli P. C., Molyneux P. C., Harrington M. E., Golombek D. A. (2007). Ghrelin effects on the circadian system of mice. J. Neurosci. 27 2890–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Li Y., Zhang W. (2014). The growth hormone secretagogue receptor: Its intracellular signaling and regulation. Int. J. Mol. Sci. 15 4837–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y. S., Lee H. S. (2013). Projections from melanin-concentrating hormone (MCH) neurons to the dorsal raphe or the nuclear core of the locus coeruleus in the rat. Brain Res. 1490 72–82. [DOI] [PubMed] [Google Scholar]
- Yoshida K., McCormack S., Espana R. A., Crocker A., Scammell T. E. (2006). Afferents to the orexin neurons of the rat brain. J. Comp. Neurol. 494 845–861. 10.1002/cne.20859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Zeitzer J. M., Sakurai T., Nishino S., Mignot E. (2007). Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J. Physiol. 581 649–663. 10.1113/jphysiol.2007.129510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. (1994). Positional cloning of the mouse obese gene and its human homologue. Nature 372 425–432. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Yamanaka A., Kunii K., Tsujino N., Goto K., Sakurai T. (2002). Orexin-mediated feeding behavior involves both leptin-sensitive and -insensitive pathways. Physiol. Behav. 77 251–257. 10.1016/s0031-9384(02)00843-0 [DOI] [PubMed] [Google Scholar]
- Zink A. N., Bunney P. E., Holm A. A., Billington C. J., Kotz C. M. (2018). Neuromodulation of orexin neurons reduces diet-induced adiposity. Int. J. Obes. 42 737–745. 10.1038/ijo.2017.276 [DOI] [PMC free article] [PubMed] [Google Scholar]