Abstract
The expansion of the spectrum of human epidermal growth factor receptor 2 (HER2)-status to HER2-low, defined as HER2 expression of 1+ by immunohistochemistry (IHC) or 2+ by IHC without gene amplification, has made a major impact in the field of oncology. The HER2-low expression has emerged as a targetable biomarker, and anti-HER2 antibody-drug conjugate trastuzumab deruxtecan (T-DXd) has shown significant survival benefit in pre-treated metastatic HER2-low breast cancer (BC). With this recent data, the treatment algorithm for hormone-receptor-positive and triple-negative breast cancer needs to be reconsidered, as about half of these BCs are HER2-low. Although we have different therapeutic agents for hormone receptor-positive and negative HER2-low BCs, there is no consensus regarding the sequencing of these agents. In our article, we enumerate the treatment options for HER2-low BC and propose a treatment sequencing algorithm based on the current clinical evidence.
Keywords: HER2-low breast cancer, Hormone receptor-positive HER2-low, Hormone receptor negative HER2-low, Trastuzumab deruxtecan, Sacituzumab govitecan, Alpelisib, Everolimus, PARP inhibitors
Condensed abstract:
The expansion of the spectrum of human epidermal growth factor receptor 2 (HER2)-status to HER2-low has made a major impact in the field of oncology. As there is no consensus regarding the sequencing of the HER2- targeting agents in HER2-low breast cancers, we enumerate the treatment options for HER2-low BC and propose a treatment sequencing algorithm based on the current clinical evidence.
Introduction
Breast cancer (BC) is the most common malignancy in females globally, with nearly 300,000 new cases estimated in the United States (US) in 2022. It accounts for the most common cause of cancer-related deaths in women globally and the second most common cause of cancer-related mortality in the US.1,2 BC comprises several subtypes based on the expression of estrogen receptor (ER), progesterone receptor (PR), and transmembrane tyrosine kinase receptor protein- human epidermal growth factor receptor 2 (HER2), with unique biologies and response to therapies: hormone receptor-positive (HR+; ER-positive and/or PR-positive) and HER-2 negative (HER2-), HER2 + (HER2-positive, any ER, and PR expression) and triple-negative breast cancer (TNBC; ER-negative, PR-negative, and HER2-negative).3
Historically, the HER2 status of BC was defined in a binary fashion, where BC with high levels of HER2 expression and/or gene amplification was classified as HER2+, with the remaining classified as HER2-negative. However, the HER2-negative category is a spectrum that ranges from BC without HER2-expression (HER2 0) to tumors with low levels of HER2 expression, recently classified as HER2-low, defined as HER2 expression of 1+ by immunohistochemistry (IHC) or 2+ by IHC without gene amplification. Although HER2-low BC was previously identified, the therapeutic implication of this subtype was unknown. Approximately 15–20% of all BC are HER2+ by classical criteria, but about 50% of all BC are estimated to be HER2-low, 4,5 significantly expanding the pool of patients where new anti-HER2 targeted agents might be utilized.
In a large, phase III, randomized trial, the addition of the HER2-targeted antibody, trastuzumab, to adjuvant chemotherapy in HER2-low BC failed to provide any significant clinical benefit. 6 This observation slowed further clinical research in HER2-low BC with the clinical utility of HER2-targeted agents being restricted mainly to HER2+ BC. However, the phase III DESTINY-Breast 04 trial, investigating an antibody-drug conjugate (ADC) targeting HER2, trastuzumab-deruxtecan (T-DXd), in pre-treated HER2-low metastatic breast cancer, challenged this fact. T-DXd was associated with improved progression-free survival (PFS) and overall survival (OS) compared with standard chemotherapy in this trial.7 This was a paradigm shift in the management of HER2-low BC, and T-DXd expeditiously became the new standard of care in this setting, establishing HER2-low BC as a distinct targetable entity.
Although a targetable entity, the role of HER2-low in determining BC prognosis remains unclear. HER2-low BC is a heterogenous population comprising both HR+ and HR-negative subgroups, which differ in their clinical nature, tumor biology, prognosis, and response to different treatments. T-DXd has shown efficacy in HR+ and all enrolled patients (including HR-negative) with HER2-low BC in the DESTINY-Breast-04 trial and is approved for use in HER2-low BC irrespective of the HR status.7 However, the approval raises a question of the optimal sequencing strategy of T-DXd with other standard of care treatments. Our review article addresses this important question, both in HR+ and HR-negative BC.
What is HER2-low breast cancer?
HER2 expression is assessed in all breast cancers, regardless of stage, to determine the biology, aggressiveness, prognosis, and treatment of the disease. According to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) 2018 guideline updates, IHC is done first, given its simplicity and faster turnaround time. 8 It is reported as a score from 0 to 3+, based upon staining patterns of the HER2 protein on the cell membrane. While a score of 3+ is considered HER2 +, scores 0 and 1+ are considered HER2-negative. IHC score 2+ is considered equivocal and is reflex tested with in situ hybridization (ISH), a more specific test that uses genetic probes to identify the copy number of HER2 genes. If the HER2 gene is amplified based on the ISH, then this is considered HER2 +. Overall, BC with IHC scores 0, 1+, or 2+ with a negative ISH assay are considered HER2-negative. 8,9 HER2-negative BC with an IHC score of 1+ or 2+ and negative on ISH are newly categorized as “HER2-low”.10 A subset of IHC score 0 BC with incomplete and faint HER2 staining in ≤ 10% of tumor cells are recently recognized as “HER2-ultra-low” phenotype. 11 Figure 1 shows the algorithm for diagnosis of HER2-low BC.
Figure 1:
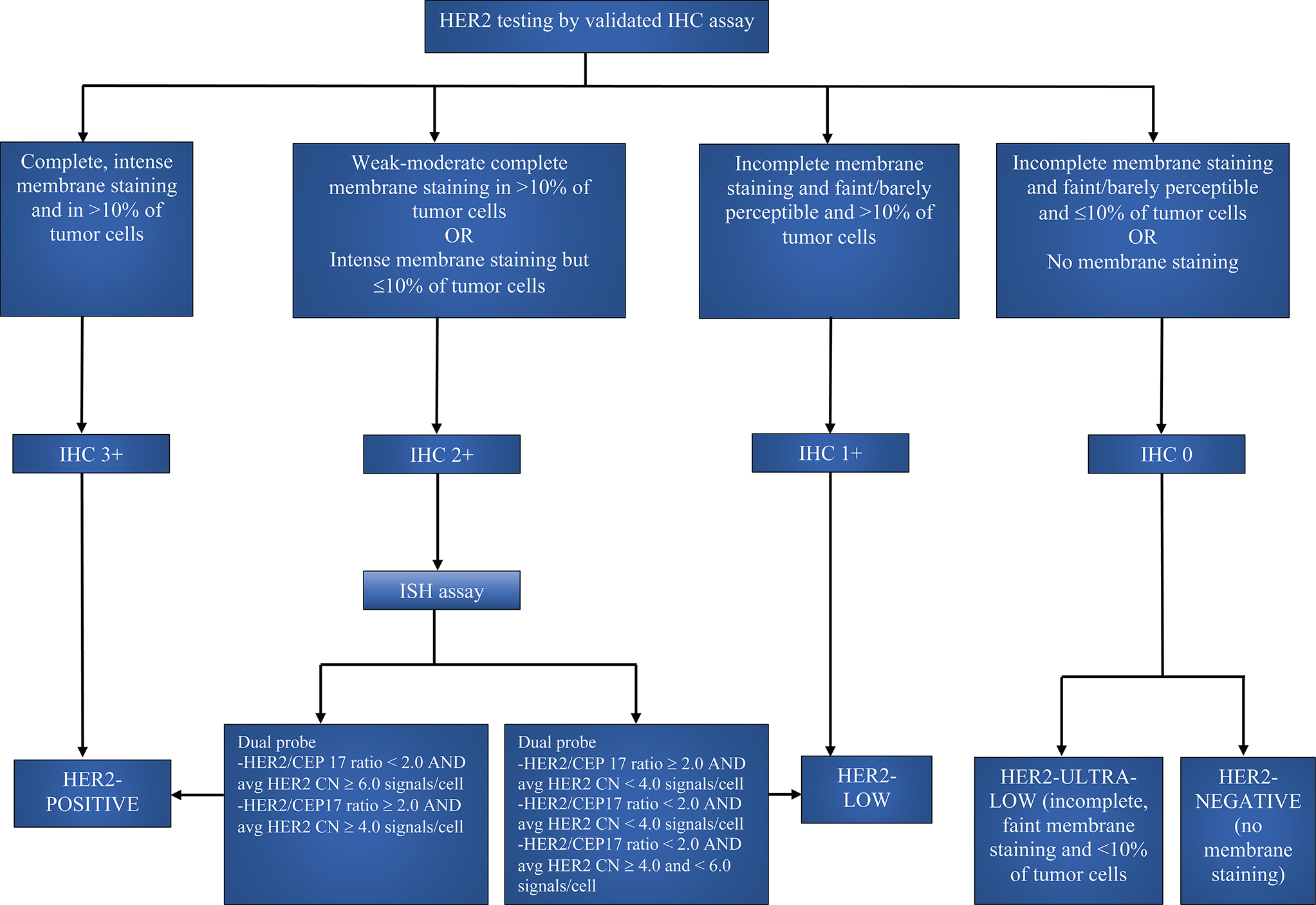
Algorithm for diagnosis of HER2-low BC according to the ASCO/CAP guidelines. HER2= Human epidermal growth factor receptor 2, IHC= Immuno histochemistry, ISH= In situ hybridization, CN= Copy number, avg= Average
There is an ongoing debate about whether HER2-low is a distinct clinical entity. Biologically, HER2-low BCs have fewer grade 3 tumors, lower proliferation rates, and less TP53 mutation.10 It is reported that the gene expression analysis of stage I-III TNBC suggests that drivers of resistance to neoadjuvant treatment differ between HER2-zero and HER2-low tumors.12 Several studies reported that HER2-low tumors have a lower pathological complete response (pCR) and better prognosis.10,13,14 HER2-low disease is more frequently seen in HR-positive BC (luminal biology) than TNBC (60 to 66% vs. 33 to 37%), and most of the HER2-low tumors are HR-positive (88%).10,15After correction for HR expression, there are only marginal differences in clinicopathological characteristics and prognosis for HER-2 low compared to HER2-zero BC. HER2-low and HER2-zero BC have similar genomic characterization and responses to different treatments, including neoadjuvant chemotherapy or CDK 4/6 inhibitors and anti-estrogen treatment.10,16–18 Indeed, HER2-low subtype is not characterized by an oncogenic driver but instead a biomarker; that is low HER2 expression which is targetable via ADCs.19 Genomic and transcriptomic analysis of HER2-low BC by Berrino et.al. revealed that HER2-low BC possesses distinct features compared to both HER2-zero and HER2-positive BC. Interestingly, the differences in the spectrum of mutations were observed in the HER2-low IHC subgroups as well. The tumor mutational burden was high in HER2-low IHC 1+ tumors compared to HER2-low IHC2+ tumors and TP53 mutation was found at higher frequency in HER2-low IHC 1+ tumors. It was found that the somatic gene mutation pattern of HER2 IHC score 1+ tumors resembled more to the HER2-zero BC and HER2 IHC score 2+ with equivocal HER2 gene copy numbers showed the most distinct mutational profile with the highest rate of ERBB2 gene mutations.20 Another study that assessed the genomic data of BC patients reported similar findings. They observed that the PIK3-Akt signaling mutations were frequently seen in HER2-low tumors compared to HER2-zero and HER2-positive tumors.14 Studies have shown that HER2-low is not biologically distinct but indeed is a dynamic entity as HER2 expression can change with treatment within the same patient, in different metastatic sites within the same patient at the same time, and in different areas within the same metastatic lesion.4,16 Therefore, a practical definition of HER2-low breast cancer is a HER2 nonamplified tumor that showed HER2-low expression on a tumor specimen in the course of the disease.
The DESTINY-Breast 04 has shown that HER2-low expression is targetable; however, growing evidence suggests that HER2-low BC is not a distinct biologic entity. Moreover, the definition of the HER2-low is dynamic and one that is constantly evolving based on the results of ongoing studies. HER2-ultra-low BC patients (HER2 IHC >0<1+ expression) are being enrolled along with the HER2-low patients in the phase III DESTINY-Breast 06 clinical trial (NCT04494425). If DESTINY-Breast 06 results are positive, then we may need to further expand our spectrum of eligible patients to include HER2-ultra-low expression as a potential targetable entity. The utility of T-DXd in advanced BC with various levels of HER2 expression (including HER2-zero) is evaluated in the phase II DAISY trial (NCT04132960).21Further findings from these trials will help us to revamp the categorization of HER2 expression and will impact the treatment strategies.
Anti-HER2 agents clinically investigated in HER2-low breast cancer
As HER2-low BC still expresses a substantial quantity of targetable HER2 expression, anti-HER2 agents have been studied in this group. Based on the mice xenograft models, trastuzumab was hypothesized to work in BC with minimal HER2 expression through the HER2 growth signaling pathway and its antibody-dependent cellular cytotoxicity.22 Similarly, pertuzumab, another HER2-directed antibody, was also found to inhibit tumor growth in xenograft models regardless of the level of HER2 expression. But despite the promising preclinical evidence, clinical trials did not show a benefit in HER2-low BC6,23,24. Table 1 (completed trials) lists the different monoclonal antibodies, such as trastuzumab, pertuzumab, and margetuximab that were tested in HER2-low BC.
Table 1:
List of completed and ongoing clinical trials that includes HER2-low breast cancer
| Trial Name/ Identifier | Class of Drug and Anti-HER2-agent | Phase, Estimated Enrollment | Study Design | Population | Intervention | 10 Outcome Measures | Key 20 Outcome Measures | Estimated 10 completion date | Results |
|---|---|---|---|---|---|---|---|---|---|
| Completed Trials | |||||||||
| NCT01275677 | ADC, Transtuzumab | Phase III, N = 3270 | Randomized, Open label, two arm | Pre-treated node-positive or high-Risk node-negative HER2-low early-stage invasive BC | Chemotherapy alone (TC or ACT) vs Chemotherapy (TC or ACT) + Trastuzumab | IDFS | BCFS, RFI, DRFI, OS, AE | Completed 31-Jul-2017 | Addition of trastuzumab to chemotherapy failed to show clinical benefit: 5-year IDFS= 89.8% vs 89.2%, HR= 0.98, p= 0.85; 5-year DRFI= 92.7% vs 93.6%, HR= 1.1, p= 0.55; OS= 94.8% vs 96.3%, HR= 1.33, p= 0.15 |
| NCT02491892 | ADC, Pertuzumab | Phase II, N = 78 | Randomized, Open label | Pre-treated metastatic HER2-low BC | Two different doses of pertuzumab (1050 mg vs loading dose of 840 mg followed by maintenance dose of 420 mg) | ORR | Time to response, OS | Completed 01-April-2005 | ORR 2.5% |
| NCT01828021 | ADC, Margetuximab | Phase II, N = 25 | Single arm, open label | Relapse refractory or advanced HER2-low BC | Margetuximab monotherapy | ORR | Response rate | Completed 14-April-2017 | Results pending |
| NCT04420598/ DEBBRAH | ADC, T-DXd | Phase II, N = 41 | Single arm, multi-cohort, open label | HER2-positive and HER2-low advanced BC with brain metastasis and LMC | Single agent T-DXd | PFS, ORR-IC, OS | ORR, TTR, clinical best response, DoR, best percentage of change, safety and tolerability of T-DXd | Completed 07-April-2022 | Results pending |
| Ongoing Clinical Trials | |||||||||
| NCT03734029/DESTINY-Breast 04 | ADC, T-DXd | Phase III, N = 557 | Randomized, open label, 2 arm | Pre-treated metastatic HER2-low BC | T-DXd vs treatment of physician's choice of chemotherapy (capecitabine or eribulin or gemcitabine or paclitaxel or nab-paclitaxel) | PFS | OS, ORR, DoR | 1-Mar-23 | Active, not recruiting. Results: T-DXd arm had improved PFS, and OS compared to physician's choice of chemotherapy (mPFS= 9.9 vs 5.1, HR= 0.5, p<0.0001; OS= 23.4 vs 16.8, HR= 0.64, p= 0.001) |
| NCT04556773/DESTINY-Breast 08 | ADC, T-DXd | Phase 1b, N = 182 | Non-randomized, open label, 5 study arms | HER2-low advanced/metastatic BC | T-DXd + capecitabine or T-DXd + durvalumab + paclitaxel or T-DXd + capivasertib or T-DXd + anastrozole or T-DXd + fulvestrant | AE, SAE | ORR, PFS, DoR, OS | 26-Jan-24 | Active, Recruiting |
| NCT04494425/DESTINY-Breast 06 | ADC, T-DXd | Phase III, N = 850 | Randomized, open label (sponsor-blind), dual arm CT | HER2-low HR+ metastatic BC with progression on ET, includes HER2-ultra-low BC patients | T-DXd vs standard of care (capecitabine or paclitaxel or nab-paclitaxel) | PFS | OS, ORR, DoR, AEs, serum concentration/ immunogenicity of T-DXd, health-related quality of life | 19-Jun-26 | Active, Recruiting |
| NCT04132960/DAISY | ADC, T-DXd | Phase II, N= 186 | Single group assignment, Open-label, 3 cohorts | Advanced HER2+, HER2-low, HER2-zero BC | Single agent T-DXd | BOR | PFS, DoR | 30-Mar-25 | Active, Not recruiting |
| NCT04553770 (TRIO-US B-12 TALENT) | ADC, T-DXd | Phase II, N = 88 | Randomized, open-label, dual arm CT | Early stage HER2-low HR+ BC | T-DXd alone vs T-DXd + Anastrozole | pCR | AEs, clinical objective response, Biomarker analyses, Molecular changes in tumour biomarkers (Ki67 expresion) | 30-Sep-25 | Active, Recruiting |
| NCT03052634 | ADC, RC48 | Phase Ib, N = 112 | Non-randomized, open-label, single arm CT | Advanced HER2+/HER2-low BC | 3 treatment arms with RC48 at doses of 1.5/2/2.5 mg/kg in HER2-positive, and 1 arm at dose of 2 mg/kg in HER2-low | RP2D | AUC, Cmax, Tmax, ORR, CBR, PFS | 1-Dec-22 | Active, Not recruiting |
| NCT03742102/ BEGONIA | ADC, T-DXd, Dato-DXd | Phase Ib/II, N = 210 | Randomized, Open-label, 5 arm CT | Advanced/ unresectable/ metastatic TNBC (with HER2-low expression for Durvalumab/T-DXd arm) | 5 treatment arms - (durvalumab + paclitaxel) vs (durvalumab + paclitaxel + capivasertib) vs (durvalumab + paclitaxel + oleclumab) vs (durvalumab + T-Dxd) vs (durvalumab + Dato-Dxd) | AE, Safety/ tolerability |
ORR, PFS, DoR, OS | 13-Feb-23 | Active, Not recruiting |
| NCT05104866/ TROPION-Breast01 | ADC, Dato-DXd | Phase III, N = 700 | Randomized, Open-label, dual arm CT | Inoperable or metastatic HR+ HER2− BC with prior 1/2 lines of systemic chemotherapy | Dato-DXd vs investigators choice of chemotherapy (capecitabine or gemcitabine or eribulin or vinorelbine) | PFS, OS | ORR, DoR, DCR | 18-Jul-25 | Active, Recruiting |
| NCT04742153 | ADC, MRG002 | Phase II, N = 66 | Non-randomized, open-label, single arm | Locally advanced or metastatic HER2-low BC | Single agent MRG002 IV infusion | ORR | PFS, 6-month/12-month PFSR, TTR, DoR, DCR, OS, Aes | 1-Feb-23 | Active, Recruiting |
|
NCT05165225/ PILHLE-001 |
Pan-ERB TKI, Pyrotinib | Phase II, N = 46 | Open label, single arm CT | Early/locally advanced HER2-low/HR+ BC | Pyrotinib + epirubicin and cyclophosphamide followed by docetaxel (neoadjuvant) | pCR | Miller-Payne grade PR, ORR, BCR, DFS, OS | 19-May-28 | Active, Recruiting |
ADC = Antibody drug conjugate; HER2= human epidermal growth factor receptor 2; CT= Clinical trial; BC= Breast cancer; TC= Docetaxel and Cyclophosphamide; ACT= Doxorubicin, Cyclophosphamide and Paclitaxel; IDFS: Invasive disease-free survival; BCFS= Breast cancer free survival; RFI= Relapse free interval; DRFI= Distant relapse free interval; OS= Overall survival; AE= Adverse events; HR= Hormone receptor; Dato-DXd= Datopotamab deruxtecan, LMC= Leptomeningeal Carcinomatosis; Pan-ERB TKI = pan-Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor; ICPI = Immune Checkpoint Inhibitors; TNBC = Triple negative Breast Cancer; T-DXd = trastuzumab Deruxtecan; RP2D = Recommended Phase II dose; pCR = pathologic Complete Response rate; SAEs = Serious Adverse Events; Cmax = Maximum Observed Plasma Concentration; AUC = Area Under Curve; Tmax = Time for Cmax, ORR = Overall Response Rate; CBR = Clinical Benefit Rate; PFS = Progression Free Survival; DoR = Duration of Response; PFSR= Progression free survival rate; ITT = Intention To Treat; DCR = Disease Control Rate; TTD = Time to Deterioration; TTR= Time to response; BOR= Best Objective Response rate
T-DXd (also known as DS-8201) is a novel ADC comprised of humanized HER2 antibody and DXd, an ultra-toxic topoisomerase I inhibitor payload which is an analog of the active metabolite of irinotecan (SN-38), linked together by self-immolating tetra peptide based cleavable linker.25 The linker makes it more stable in the plasma with low payload clearance and is preferentially cleaved near the tumor cells, thus showing a better systemic toxicity profile. In addition, the higher drug-antibody ratio (DAR) (of 8:1) elicits a greater antitumor effect. 26,27 DXd has better membrane penetrability, and it exerts a bystander-killing effect in the surrounding HER2-negative cells, thereby showing efficacy in the HER2-low or heterogeneous tumor microenvironment (TME). 28 T-DXd also shows clinical efficacy in tumors with acquired resistance to trastuzumab or T-DM1.27
T-DXd showed clinical activity in HER2-positive patient-derived xenograft models and several BC models with low levels of HER2 expression.29 In a phase Ib trial, T-DXd showed promising antitumor activity in HER2-low BC.30 This was further explored in the groundbreaking phase III randomized, multicenter clinical trial DESTINY-Breast 04 which demonstrated promising clinical effects of T-DXd compared to the physician’s choice of chemotherapy (PCC) in previously treated metastatic HER2-low BC. Out of the 557 patients, 89% (n= 494) had HR+ disease, and 11% (n= 63) had HR-negative disease. Patients with metastatic HER-2 low cancers who have received at least one line of chemotherapy were enrolled. Those with HR+ disease should have also received at least one line of hormonal therapy. The (neo)adjuvant chemotherapy was counted as one line of therapy if the recurrence occurred within six months of (neo)adjuvant chemotherapy. In the overall cohort, the median PFS was significantly prolonged in the T-DXd arm (PFS: 9.9 months (m) vs. 5.1m, hazard ratio (HR)= 0.5, p<0.001) with an acceptable safety profile. The PFS in the HR-positive and HR-negative subgroups, which received T-DXd was higher (10.1m vs. 5.4m, HR= 0.51, p<0.001) and (8.5m vs. 2.9m, HR= 0.46) compared to PCC. The median overall survival (OS) in the T-DXd arm was higher in the overall (23.4m vs. 16.8m, HR= 0.64, p= 0.001), HR-positive (23.9m vs. 17.5m, HR= 0.64, p= 0.003) and HR-negative subgroups (18.2m vs. 8.3m, HR= 0.48). Even though the HR-negative HER2-low patients are underrepresented in this trial, the PFS and OS benefits were significant in this group. The most common adverse events (AE) observed in the T-DXd arm were nausea and fatigue. Interstitial lung disease (ILD), a serious AE associated with T-DXd, was observed in 12% of the patients in this study. Grade 3 or higher AEs were more in the chemotherapy arm (67.4% vs. 62.6%). Given this encouraging results, the Food and Drug Administration (FDA) approved T-DXd for patients with unresectable or metastatic HER2-low BC in August 2022.
Since T-DXd is approved after one line of chemotherapy, here we discuss the outcomes with other contemporary treatments, both in the HR+ and HR-negative BC- sacituzumab govitecan (SG), alpelisib, everolimus, and PARP inhibitors to guide optimal sequencing strategy.
Other therapeutic agents in HER2-low breast cancer
Sacituzumab govitecan
Sacituzumab govitecan (SG) is an ADC comprising of humanized IgG1 targeting the trophoblast cell surface antigen (Trop2), which is overexpressed in most human solid epithelial cancers, conjugated with the active metabolite of irinotecan (SN-38) by a hydrolysable C2LA covalent linker.31,32 The conjugate has intermediate serum stability and is cleaved in low pH within the TME and the lysosomes, possessing a DAR of 7.6:1.31 It was initially approved for use in advanced TNBC and now approved for treatment of pre-treated endocrine-resistant HR-+ HER2-negative metastatic BC. Its role in HR + HER2-low or negative disease is being evaluated.33
Efficacy in TNBC/ HR-negative HER-2 low BC
In a phase I/II IMMU-132-01 basket study, SG was found to have a durable objective response rate (ORR) of 33% and median PFS and OS of 5.5m and 13m, respectively in patients with heavily pre-treated metastatic TNBC. 34 Later, in the randomized, phase III ASCENT trial, SG was found to have a significant PFS and OS benefit compared to single-agent PCC among patients with metastatic TNBC.35 Based on this data, SG received accelerated initial approval by the FDA in April 2020, followed by regular approval in April 2021.
In the ASCENT trial, patients who received two or more standard chemotherapy regimens were enrolled (including taxanes). The median PFS and OS in patients without brain metastasis were higher in the SG arm compared to the chemotherapy arm (PFS: 5.6m vs. 1.7m, HR= 0.41, p <0.001, OS: 12.1m vs. 6.7m, HR= 0.48, p<0.001). The ORR was 35% with SG and 5% with chemotherapy.35 In the post hoc subgroup analysis of the ASCENT trial, HER2-low had good outcomes. The mPFS of HER2 IHC 0 and HER2-low cancers were prolonged compared to the PCC (4.3m vs. 1.6m, HR= 0.38, p<0.001; 6.2m vs. 2.9m, HR= 0.44, p= 0.002 respectively). The mOS was also longer in the HER2 IHC 0 and HER2-low arm with SG compared to the PCC (11.3m vs. 5.9m, HR= 0.51, p<0.001; 14m vs. 8.7m, HR= 0.43, p<0.001 respectively). The ORR of the HER2 IHC 0 and HER2-low groups with SG compared to the PCC was 31% vs. 3% and 32% vs. 8%, respectively.36 This post hoc analysis demonstrates the uniform clinical benefit of SG compared to chemotherapy regardless of the HER2-low vs. HER2 IHC 0.
Although SG was initially approved for the management of metastatic TNBC, it is important to note that a significant proportion of TNBC cancers are HR-negative HER2-low (26.3%). Hence, this raises the question of the comparative efficacy of SG vs. T-DXd in this subgroup. The ASCENT trial, overall, enrolled a more heavily pre-treated population compared to DESTINY-Breast 04 (the population that received chemotherapy (the control arm) had ORR of 5% in ASCENT vs. around 16.7% in DESTINY-Breast04), the shorter PFS for SG in ASCENT compared to T-DXd in DESTINY Breast 04 could be somewhat justified. The lack of head-to-head comparisons of T-DXd and SG limits the appropriate comparisons.
Efficacy in HR-positive HER-2 negative/low BC
The benefit of SG in metastatic or locally advanced inoperable HR + HER2-negative BC is evaluated in phase III, randomized, TROPiCS-02 trial. The eligibility criteria included receipt of prior hormonal therapy, CDK4/6 inhibitors, and at least two lines of chemotherapy (including taxane). Those with one prior therapy for metastatic disease were allowed if the disease progressed ≤ 12 months after (neo)adjuvant therapy.37 The PFS was found to be higher in the SG arm compared to PCC (5.5m vs. 4m, HR= 0.66, p= 0.0003). 38 Similar to the ASCENT trial, neutropenia (51%) and diarrhea (10%) are the main observed AEs in the SG arm. The median OS was found to be significantly higher in the SG arm compared to PCC (14.4m vs 11.2m, HR= 0.79, p=0.020). The ORR is reported to be 21% vs. 14% favoring SG. 39 Given the results of the TROPiCS-02 trial, FDA has approved SG in HR + HER2-negative BC on February 3, 2023.40
In a post-hoc analysis of TROPiCS-02, the efficacy of SG based on the HER2 IHC status was reported. The mPFS of patients with HER2 IHC 0 and HER2-low BC with SG compared with the PCC was 5.0m vs. 3.4m (HR= 0.72, p=0.05) and 6.4m vs. 4.2m (HR= 0.58, p<0.001) respectively. The ORR was similar in the HER2 0 (16% vs. 15%) and higher with SG in the HER2-low (26% vs. 12%) group arms compared to PCC. The safety profile was also similar between the HER2 IHC 0 and HER2-low groups. Therefore, SG can be considered as an effective treatment option in the HR + HER2-negative BC patients regardless of the level of HER2 expression.33
Both the ASCENT and TROPiCS-02 trials were designed for patients classified as HER2-negative, therefore, the primary results are regardless of HER2-low vs HER2–0 expression. However, the post-hoc analyses suggest very similar benefit, so distinction by HER2-zero or HER2-low does not appear to be important for the activity of this agent.
Poly (ADP-ribose) polymerase inhibitors (PARPi)
Germline mutations in BRCA1/2 genes result in hereditary breast and ovarian cancer syndrome. PARPi have been used to treat BC in patients with germline BRCA1/2 mutations.41 In the phase III OlympiAD trial, the efficacy of olaparib, an oral PARPi has been compared with single-agent standard chemotherapy of physician’s choice in the metastatic HER2-negative BRCA mutated BC patients. The trial included 49.7% (n=150) TNBC and 50.3% (n=152) HR + patients. The mPFS was longer in the olaparib arm compared to the chemotherapy arm (7m vs. 4.2m, HR= 0.58, p<0.001). The ORR was also higher in the olaparib arm (59.9% vs. 28.8%). Olaparib was found to have better mPFS in the HR + (HR=0.82, 95% CI= 0.55–1.26) and HR-negative (HR= 0.43, 95% CI= 0.29–0.63) groups compared to standard therapy.42 Although olaparib failed to show a statistically significant OS improvement compared to chemotherapy (overall cohort: mOS 19.3m w 17.1m, HR= 0.90, p= 0.51, TNBC: HR= 0.93, 95% CI= 0.62–1.43, HR +: HR= 0.86, 95% CI= 0.55–1.36), a potentially meaningful benefit was observed in patients who had not received chemotherapy for metastatic disease (22.6% vs. 14.7%, HR= 0.51, 95% CI= 0.29–0.9, p= 0.02).43
Talazoparib is another oral PARPi approved for advanced HER2-negative BRCA mutated BC patients. In the randomized, phase III EMBRACA trial, talazoparib was compared against standard single-agent chemotherapy of physician’s choice. The mPFS (8.6m vs. 5.6m, HR= 0.54, p<0.001) and ORR (62.2% vs. 27.2%) were significantly higher in the talazoparib group. The trial included 44% (n=190) TNBC and 56% (n= 241) HR + patients. The mPFS was better in both TNBC (HR= 0.6, 95% CI= 0.41–0.87) and HR + (HR= 0.47 95% CI= 0.32–0.7) subgroups.44 Like olaparib, talazoparib failed to show significant OS benefit (mOS= 19.3m vs. 19.5m, HR=0.84, p=0.17).45
Although NCCN recommends using both olaparib and talazoparib in BRCA-mutated, HER2-negative BC, we do not have separate data for assessing the benefit in HER2-low vs. HER2 IHC 0 with these two PARPi.40
Other agents in HR-positive HER2-negative/low BC
In HR + HER2-negative BC patients with PIK3CA gene mutation, alpelisib (BYL719) has been shown to have clinical benefits and is widely used after progression on the CDK4/6 inhibitors. In the SOLAR-1 trial, the efficacy of the combination of alpelisib and fulvestrant in patients with HR + HER2-negative advanced BC who had received endocrine therapy previously was evaluated. The combination of alpelisib with fulvestrant had significantly improved PFS in the PIK3CA mutated cancer cohort compared to the placebo-fulvestrant cohort (PFS: 11m vs. 5.7m, HR= 0.65, p<0.001). The ORR was 26.6% vs. 12.8% in the alpelisib/fulvestrant group compared to the placebo/fulvestrant group. But it did not show any OS benefit (39.3m vs. 31.4m, HR= 0.86, p=0.15).46 The ongoing phase II, multicenter, BYLieve study evaluated the efficacy of alpelisib and fulvestrant in PIK3CA-mutated HR + HER2-negative advanced BC after CDK4/6 inhibitors and has shown activity with manageable toxicity. The mPFS was 7.3m (95% CI= 5.6–8.3) and the mOS was 17.3m (95% CI= 17.2–20.7).47 Although alpelisib has shown efficacy in these patients, real-world evidence suggests poor tolerance given increased side effects including hyperglycemia, rash, diabetic ketoacidosis, especially in heavily pre-treated patients, highlighting the need for improved therapies.48 49,50 However, due to a significant improvement of PFS with the addition of alpelisib to fulvestrant, this combination is still the preferred second-line treatment for HR + HER2-low breast cancer after progression on a CDK4/6 inhibitor plus an aromatase inhibitor.
Everolimus is approved in the second line and later settings in HR + HER2-negative BC patients. In the phase III, randomized, BOLERO-2 clinical trial, in patients with HR + HER2-negative BC who had recurrence or progression with previous nonsteroidal aromatase inhibitors, everolimus combined with an aromatase inhibitor exemestane improved PFS (10.9m in everolimus-exemestane vs. 4.1m in placebo-exemestane group, HR= 0.36, P<0.001) but did not show OS benefit (31m vs 26.6m, HR= 0.89, p= 0.14)51. The most common AEs were stomatitis (8%) and anemia (6%).52 Similarly to alpelisib, tolerance to everolimus is poor in the real world.53,54 With the more recent approval of more targeted therapies like alpelisib, everolimus-based therapy is generally used third-line or second-line in PI3K- wildtype cancers. There is no prospective data to help us choose between everolimus-based therapy vs. T-DXd for metastatic ER + HER2-low breast cancer. Often it is influenced by patients’ and clinicians’ preferences based on the route of administration, expected efficacy, and toxicities. The magnitude of benefit seems greater with T-DXd in the DESTINY-Breast 04 as compared to the everolimus in the BOLERO 2, however this observation is based on cross-trials comparison. Of note, all the patients on the SOLAR-1 trial and BOLERO-2 trials were chemotherapy naïve, while T-DXd was given to a group of patients who had received prior chemotherapy. Additionally, there was a differential loss to follow-up (informative censoring) on SOLAR-1 and BOLERO-2, which could lead to an overestimation of clinical response with these drugs.55 Therefore, in patients with chemotherapy-resistant HR + HER2-low metastatic BC, T-DXd could be considered over everolimus-based therapy.
Based on the results of the EMERALD trial, elacestrant (oral selective estrogen receptor degrader) was recently approved as second line and later for ER+ HER2-low metastatic BC with ESR1 mutation on January 27, 2023. The trial enrolled ER+ HER2-negative metastatic BC patients who had one-two lines of ET including CDK4/6 inhibitors and up to one line of chemotherapy. The PFS was significantly increased for patients with ESR1 mutation [3.8 months (95% CI: 2.2, 7.3) vs. 1.9 months (95% CI: 1.9, 2.1); HR= 0.55, 95% CI= 0.39–0.77, p= 0.0005].56 Efficacy data of elacestrant in HER2-low vs. HER2 0 has not been reported.
Table 2 shows the comparative efficacy of T-DXd, SG, PARPi, alpelisib, and everolimus in HER2-negative breast cancer.
Table 2.
shows the comparative efficacy of therapeutic agents in HR-positive HER2-negative breast cancer and triple-negative breast cancer across different trials
| Variables | HR-positive HER2-negative BC | TNBC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials | TROPiCS-02 | DESTINY-Breast04 | OlympiAD | EMBRACA | SOLAR-1 | BOLERO-2 | EMERALD | ASCENT trial | DESTINY-Breast04 | OlympiAd | EMBRACA |
| Study agent | Sacituzumab govitecan | Trastuzumab deruxtecan | Olaparib | Talazoparib | Alpelisib + Fulvestrant | Everolimus + Exemestane | Elacestrant | Sacituzumab govitecan | Trastuzumab deruxtecan | Olaparib | Talazoparib |
| Study population | HR+/HER2-negative, metastatic BC | Relapsed/Refractory metastatic HER2-low HR+/− BC pts | Relapsed/refractory metastatic germline BRCA mutated, HER2-negative BC pts | Locally advanced/metastatic germline BRCA mutated, HER2-negative pts | HR+ HER2-negative advanced BC | HR+ HER2-negative advanced BC | ER+HER2-negative advanced BC | Relapsed/refractory metastatic TNBC | Relapsed/Refractory metastatic HER2-low HR+/− BC pts | Relapsed/refractory metastatic germline BRCA mutated, HER2-negative BC pts | Locally advanced/metastatic germline BRCA mutated, HER2-negative pts |
| Sample size | 543 | 494 | 152 | 241 | 572 | 724 | 477 | 468 | 63 | 150 | 190 |
| Comparator arm | Single-agent chemotherapy of physician’s choice- eribulin, capecitabine, vinorelbine or gemcitabine | Single-agent chemotherapy of physician’s choice- eribulin, capecitabine, gemcitabine, paclitaxel, nab-paclitaxel | Single-agent chemotherapy of physician’s choice- eribulin, capecitabine, vinorelbine | Single-agent chemotherapy of physician’s choice- eribulin, capecitabine, vinorelbine or gemcitabine | Placebo + fulvestrant | Placebo + exemestane | Endocrine monotherapy- aromatase inhibitors or fulvestrant | Single-agent chemotherapy of physician’s choice eribulin, vinorelbine, capecitabine, gemcitabine | Single-agent chemotherapy of physician’s choice- eribulin, capecitabine, gemcitabine, paclitaxel, nab-paclitaxel | Single-agent chemotherapy of physician’s choice- eribulin, capecitabine, vinorelbine | Single-agent chemotherapy of physician’s choice- eribulin, capecitabine, vinorelbine, or gemcitabine |
| Study design | Phase 3, randomized, open label | Phase 3, randomized, open label | Phase 3, randomized, open label | Phase 3, randomized, open label | Phase 3, randomized, double blind, placebo controlled | Phase 3, randomized, double blind, placebo controlled | Phase 3, randomized, open label | Phase 3, randomized, open label | Phase 3, randomized, open label | Phase 3, randomized, open label | Phase 3, randomized, open label |
| Randomization | 1:1 | 2:1 | 2:1 | 2:1 | 1:1 | 2:1 | 1:1 | 1:1 | 2:1 | 2:1 | 2:1 |
| Inclusion criteria for treatment line | Atleast 2, not more than 4 prior lines of CT (must include a taxane), atleast one hormonal treatment and one CDK4/6 inhibitor | Relapsed/refractory to 1 or 2 CT | No more than 2 prior CT for metastatic disease, and had received (neo)adjuvant/ metastatic disease treatment with anthracycline and taxane, and atleast one endocrine therapy | No more than 3 prior CT and had received prior taxane, anthracycline or both | Received endocrine therapy prior | Refractory to prior letrozole or anastrazole, single prior CT | Received 1 or 2 lines of endocrine therapy, and CDKi 4/6 inhibitor, and ≤1 CT | Relapsed/refarctory to 2 or above CT (had to include taxane) | Relapsed/refractory to 1 or 2 CT | No more than 2 prior CT for metastatic disease, and had received (neo)adjuvant/ metastatic disease treatment with anthracycline and taxane | No more than 3 prior CT and had received prior taxane, anthracycline or both |
| Median number of previous anti-cancer regimens (range) | NA, CT: 3 (0–8) | 3 (1–9) vs 3 (1–8) | NA, CT: (0–2) | Hormonal agents: 2 (0.6) vs 2 (0–6), CT: (0–3) | NA | 3 (1->=3) | NA,Endocrine: (1–2), CT: (0–1) | 3 (1–16) vs 3 (1–12) | 3 (1–9) vs 3 (1–8) | NA, CT: (0–2) | NA, CT: (0–3) |
| Median progression free survival: months (HR, 95% CI, p-value) | 5.5 vs 4.0 (0.66, 0.53–0.83, p=0.0003) | 10.1 vs 5.4 (0.51, 0.4–0.64, p<0.001) | NA (0.82, 0.55–1.26) | NA (0.47, 0.32–0.71) | PIK3CA-mutated cohort: 11.0 vs 5.7 (0.65, 0.50–0.85, p<0.001) | Local assessment: 6.9 vs 2.8 (0.43, 0.35–0.54, p<0.001), Central assessment: 10.6 vs 4.1 (0.36, 0.27–0.47, p<0.001) | Overall: 2.8 vs 1.9 (0.70, 0.55–0.88, p=0.002) 12-month PFS: 22.3% vs 9.4% ESR1 mutated pts: 3.8 vs 1.9 (0.55, 0.39–0.77, 0.0005) 12-momth PFS: 26.8% vs 8.2% |
5.6 vs 1.7 (0.41, 0.32–0.52, p<0.001) | 8.5 vs 2.9 (0.46, 0.24–0.89) | NA (0.43, 0.29–0.63) | NA (0.6, 0.41–0.87) |
| Median overall survival: months (HR, 95% CI, p-value) | 14.4 vs 11.2 (0.79, 0.65–0.96, p= 0.02) | 23.9 vs 17.5 (0.64, 0.48–0.86, p= 0.003) | 21.8 vs 21.3 (0.86, 0.55–1.36, NS) | NA (0.82, 0.59–1.14, NS) | PIK3CA-mutated cohort: 39.3 vs 31.4 (0.86, 0.64–1.15, p= 0.15) | 31 vs 26.6 (0.89, 0.73–1.1, p= 0.14) | Interim analysis Overall: Events: 70 vs 79 (0.75, 0.54–1.04, 0.08) 12-month OS: 79.3% vs 73.3% ESR1 mutated pts: Events: 28 vs 40 (0.59, 0.36–0.96, 0.032) 12-month OS:82.6% vs 73.6% |
12.1 vs 6.7 (0.48, 0.38–0.59, p<0.001) | 18.2 vs 8.3 (0.48, 0.25–0.95) | 17.4 vs 14.9 (0.93, 0.62–1.43, NS) | NA (0.89, 0.64–1.2, NS) |
| Objective response rate | 21% vs 14% | 52.6% vs 16.3% | 53% vs 12% | 62.6% vs 27.2% (overall) | 26.6% vs 12.8% | Local assesment: 9.5% vs 0.4%, Central assessment: 7% vs 0.4% | NA | 35% vs 5% | 50% vs 16.7% | 47% vs 7% | 62.6% vs 27.2% (overall) |
| Median duration of response (months) | 8.1 vs 5.6 | 10.7 vs 6.8 | 6.4 vs 7.1 (overall) | NA | NA | NA | NA | 6.3 vs 3.6 | 8.6 vs 4.9 | 6.4 vs 7.1 (overall) | NA |
| Treatment related adverse events of any grade (most common) | Neutropenia (51%), diarrhea (10%) | Nausea (73%), fatigue (47.7%), neutropenia, anemia (33.2%) | Nausea (58%), anemia (40%), vomiting (32.2%) | Anemia (52.8%), fatigue (50.3%), nausea (48.6%) | Hyperglycemia (64.8%), diarrhea (59.5%), nausea (46.8%), rash (35.6%) | Stomatitis (56%), rash (36%), fatigue (33%) | Nausea (25%), decreased appetite/arthralgia/diarrhea (13.2%), Headache/Fatigue (11.8%) | Neutropenia (63%), diarrhea (59%), nausea (57%) | Nausea (73%), fatigue (47.7%), neutropenia, anemia (33.2%) | Nausea (58%), anemia (40%), vomiting (29.8%) | Anemia (52.8%), fatigue (50.3%), nausea (48.6%) |
TNBC: Triple negative breast cancer, BS: breast cancer, NS: Non-significant, Pts: patients, CT: chemotherapy, NA: Not available, HR+: Hormone receptor positive, HER2: Human epidermal growth factor receptor, CDK4/6 i: cyclin dependent kinase 4/6 inhibitors
HER2-low BC with brain metastasis
The number of BC patients with brain metastasis enrolled in the above trials was minimal. In DESTINY-Breast 04, separate subset analysis was not performed as the percentage of patients with brain metastasis in the T-DXd and chemotherapy arms were only 6.4% and 8.3%.7 As T-DXd has shown significant intracranial activity against stable and active HER2 + breast cancer brain metastasis, it is important to determine its activity in HER2-low BC with brain metastasis in a prospective clinical trial.57,58 The results of the phase II clinical trial DEBBRAH, which studied the efficacy of T-DXd in locally advanced/unresected/metastatic HER2-positive/HER2-low BC with untreated or treated brain metastatic or leptomeningeal carcinomatosis, are currently pending (NCT04420598). The central nervous system (CNS) activity of other therapies against HER2-low breast cancer is limited. A total of 11.5% (n=61) TNBC patients with brain metastasis were enrolled in the ASCENT trial.35 In the brain metastasis cohort, median PFS was 2.8 months with SG compared to 1.6 months with chemotherapy. However, the OS was 6.8 months with SG as compared to 7.5 months with chemotherapy.59 HER2 expression data of the brain metastasis cohort have not been presented. In TROPiCS-02, patients with active CNS metastasis (unless stable for at least 4 weeks) were excluded.37 Details of CNS metastasis in the enrolled patients are not reported in OlympiAD trial.42 In the EMBRACA trial, 15% of patients in the Talazoparib arm and 13.9% in the standard chemotherapy arm had CNS metastasis. However, to be eligible, those patients had to receive definite local CNS therapy prior to study enrollment. The mPFS and mOS in this group was also better in the Talazoparib arm compared to standard chemotherapy (mPFS: HR= 0.32, 95% CI= 0.15–0.68, mOS: HR=0.67, 95% CI= 0.37–1.2), however the details of response rates in CNS metastasis is not reported.44,45 In SOLAR-1 and BOLERO-2, patients with CNS metastasis were excluded.46,52 In BYLieve trial, only 2% of the enrolled patients had CNS metastasis and subset analysis based on CNS metastasis is not available.47 Therefore, based on the very limited data, currently we do not have any consensus regarding the sequencing of treatments in HER2-low BC patients with brain metastasis.
Proposed Sequencing of T-DXd and Rationale.
Apart from T-DXd, none of the other agents discussed have been investigated in patients with HER2-low cancers. Therefore, data from clinical trials and real-world evidence are critically needed to make recommendations on the optimal sequencing of these agents for maximum clinical benefit while maintaining the quality of life. In the absence of such data, we have utilized current evidence from the literature to propose a sequencing strategy for HR + and HR-negative HER2-low metastatic breast cancers (Figure 2a and 2b). In the DESTINY-Breast 04 trial, patients are enrolled in the T-DXd arm after receipt of one to two lines of chemotherapy, and in the TROPiCS-02 trial, patients received two to four lines of chemotherapy before enrollment in the SG arm, Therefore, we propose the utilization of T-DXd before SG in the HR-positive HER2-low BC. In HR-negative HER2-low BC, although the results from DESTINY-Breast 04 were supportive of using T-DXd, it included only 63 patients HR-negative HER2-low patients. The ASCENT trial was a large phase III trial (n= 486) that enrolled metastatic TNBC patients in the SG arm after chemotherapy. Given these findings, we propose the use of either SG or T-DXd after the first-line chemotherapy in HR-negative HER2-low BC. Even though T-DXd and SG are ADCs conjugated with the same topoisomerase I inhibitor, the targets of both drugs are different (T-DXd - HER2 and SG - Trop2). The efficacy and side effects of utilization of these ADCs in a sequence are not clearly understood. Another possible way of identifying patients who would likely benefit from these drugs is through biomarker evaluation. Therefore, we could hypothesize in a data-free zone that it might be beneficial to give T-DXd to a patient with BC with higher HER2 expression than Trop2 to maximize efficacy and minimize toxicity, however, this needs further validation in future trials. More studies are needed to analyze the real-world efficacy and AEs of sequential therapy with ADCs.
Figure 2:
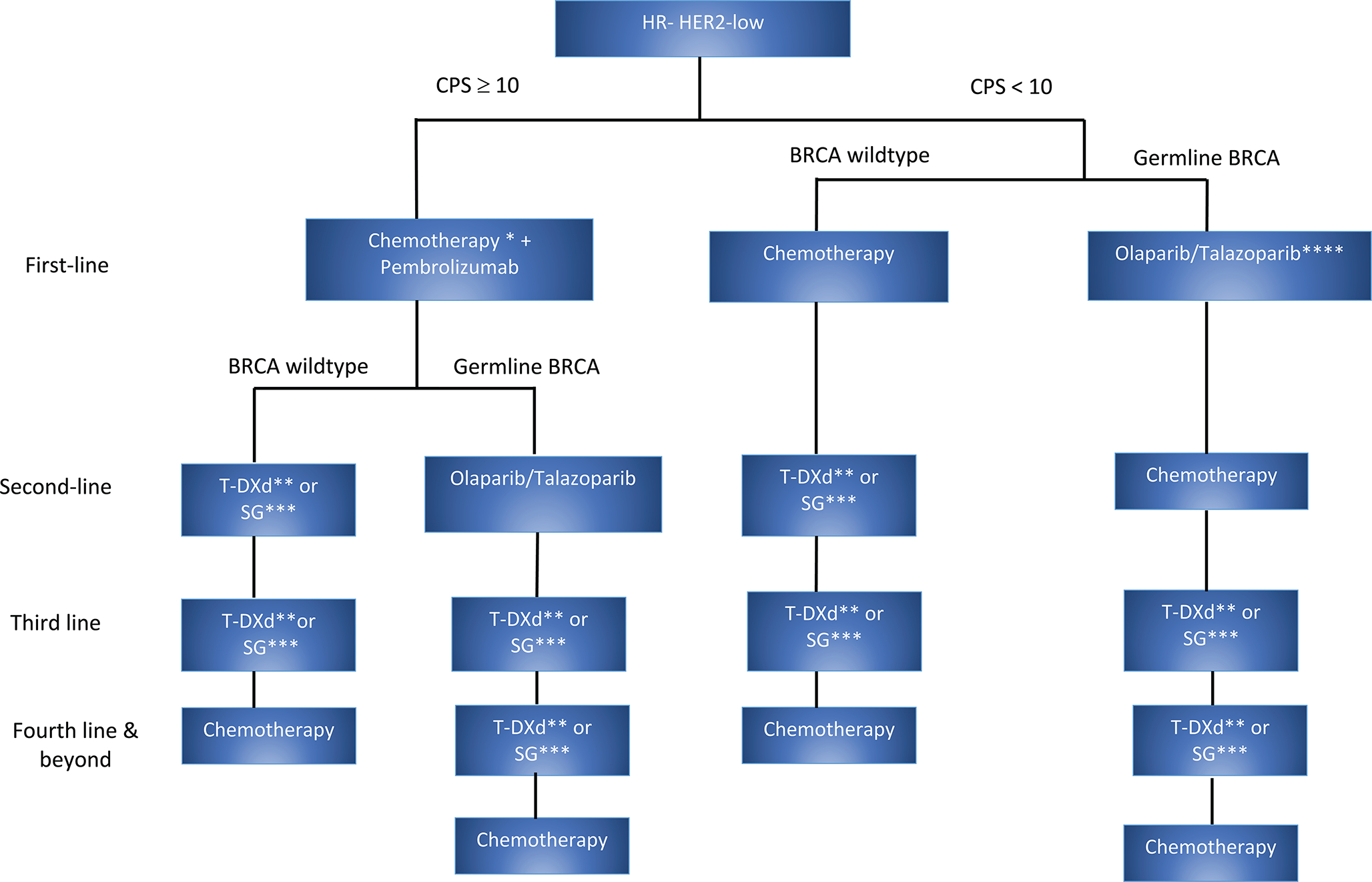
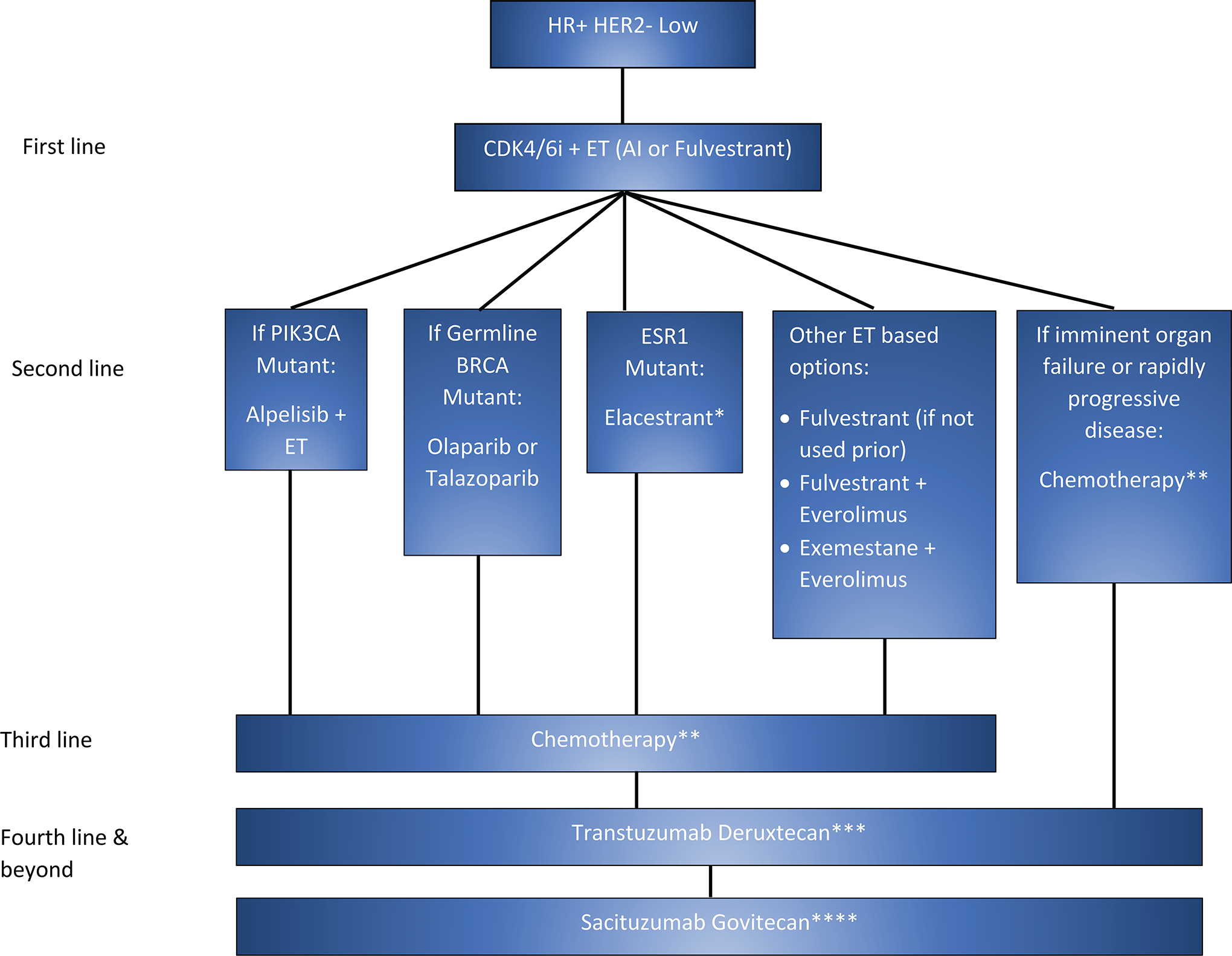
The proposed sequencing algorithm in (a) HR-negative HER2-low BC and (b) HR+ HER2-low BC
a: Sequencing Algorithm in HR-negative HER2-low BC. *Based on the keynote-355 clinical trial, in HR-negative HER2-low BC, chemotherapy + pembrolizumab can be considered as first-line treatment if CPS ≥ 10. Paclitaxel, nab-paclitaxel (if disease free survival>=12 months), and gemcitabine-carboplatin (disease free survival <=12 months) are the chemotherapy options that can be used based on the trial. **DESTINYBreast04 trial enrolled patients in the T-DXd arm after one line of chemotherapy (63 patients with HR-negative HER2-low breast cancer) and ***the ASCENT trial is a large phase III trial which enrolled patients to SG after chemotherapy. Therefore, we propose the use of either of T-DXd or SG as next-line treatment option. ****Given the significant OS benefit of PARP inhibitors if used in the first line setting based on the OlympiAD trial, we propose to use PARP inhibitors as first-line in HR-negative HER2-low patients with germline BRCA mutation. HER2= Human epidermal growth factor receptor 2, TNBC= Triple-negative breast cancer, CPS= Combined positive scoring, T-DXd= Trastuzumab deruxtecan, SG= Sacituzumab govitecan, OS= Overall survival.
b: Sequencing Algorithm in HR+ HER2-low BC. In HR-positive HER2-low BC the first-line treatment is CDK4/6 inhibitors + endocrine therapy followed by other lines of endocrine therapy. *Elacestrant is preferred when ESR1 mutation is identified through guardant 360/liquid biopsy (preferred based on FDA approval) or next generation sequencing and if the progression-free survival on CDK4/6 inhibitors is more than 12 months. When the disease is endocrine-resistant, the next line of treatment is chemotherapy (**Physician’s choice of chemotherapy based on patient’s clinical condition and preference). ***As the DESTINY-Breast04 trial enrolled patients in the T-DXd arm after one line of chemotherapy, we propose using T-DXd as the next line of treatment. ****As the TROPiCS-02 trial enrolled patients in the SG arm after two lines of chemotherapy, we propose to consider SG after T-DXd. HR+HER2= Hormone receptor-positive human epidermal growth factor receptor 2, CDK4/6 i= Cyclin-dependent kinase 4 and 6 inhibitors, ET= Endocrine therapy, T-DXd= Transtuzumab Deruxtecan, SG= Sacituzumab govitecan.
Ongoing Studies and Clinical Trials in HER2-low BC
Several ongoing trials are investigating multiple new drugs, such as, datopotamab deruxtecan (Dato-DXd, also known as DS-1062a), trastuzumab-duocarmazine (SYD985), disitamab vedotin (RC48) and novel combinations of T-DXd with immunotherapy and anti-estrogen therapy in HER2-low BC and combining HER2-targeted agent with immunotherapy (IO). Table 1 (Ongoing clinical trials) shows details of ongoing clinical trials. The preliminary analysis of phase Ib/2 BEGONIA trial (NCT03742102), which evaluates the combination of durvalumab (IO) and Dato-DXd as a first-line treatment option in advanced/metastatic TNBC shows the response in 74% of patients and responses were regardless of the PDL-1 expression. The most common AE observed was mild stomatitis (69%).60,61 The preliminary analysis of the DAISY trial shows that T-DXd has meaningful clinical activity in HER2-positive, HER2-low, and HER2-zero BC patients (mPFS= 11.1 (95% CI = 8.4- NR), 6.7 (4.6 – 8.5), 4.2 (2.1–6.9) months respectively.21
Other ADCs which are evaluated in HER2-low BC are SYD985 and RC48. 62,63 SYD985 is a novel ADC, which is composed of trastuzumab linked to DNA-alkylating duocarmycin. Despite its lower DAR (2.8:1), it is significantly more potent than T-DM1 in HER2-low patient-derived xenograft BC models. 64 In a phase I dose escalation and dose-expansion study, SYD985 showed clinical activity in heavily pre-treated patients with HER2- expressing metastatic BC, including HER2-positive T-DM1 resistant and HER2-low BC with acceptable AEs. 65 The preliminary data of the ongoing phase Ib trial utilizing RC48 in advanced HER2-positive and HER2-low BC shows that RC48 can achieve good efficacy in both HER2-positive and low patients (NCT03052634). These ongoing studies, along with real-world evidence, can inform how T-DXd can be used optimally in HER2-low BC and can investigate whether HER2-low status can be used as a biomarker for de-escalation of systemic therapies in aggressive subtypes such as TNBC.66
Future Directions and Conclusion
With the advent of promising clinical data with novel ADCs in HER2-low space, HER2-low BC has emerged as a potentially targetable entity. Data from ongoing trials with novel anti-HER2 agents will help further delineate the efficacy of these agents in HER2-low space. In the future, it is essential to investigate the relative efficacy of other approved treatments compared to T-DXd to determine the optimal sequencing strategies to get the maximum clinical benefit in HER2-low BC.
Funding:
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award numbers KL2TR001413 and UL1TR001412. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest:
Ajay Dhakal’s COI: https://coi.asco.org/share/23L-WN76/Ajay%20Dhakal
All the other authors do not have any conflict of interest
IRB approval: Does not require IRB approval as it is a review article.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209–249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2022. CA Cancer J Clin 72:7–33, 2022 [DOI] [PubMed] [Google Scholar]
- 3.Yersal O, Barutca S: Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J Clin Oncol 5:412–24, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarantino P, Hamilton E, Tolaney SM, et al. : HER2-Low Breast Cancer: Pathological and Clinical Landscape. J Clin Oncol 38:1951–1962, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Cronin KA, Harlan LC, Dodd KW, et al. : Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest 28:963–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehrenbacher L, Cecchini RS, Geyer CE Jr, et al. : NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy With or Without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and With IHC 1+ or 2. J Clin Oncol 38:444–453, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modi S, Jacot W, Yamashita T, et al. : Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 387:9–20, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolff AC, Hammond MEH, Allison KH, et al. : Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 36:2105–2122, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Wolff AC, Hammond MEH, Allison KH, et al. : Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med 142:1364–1382, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Denkert C, Seither F, Schneeweiss A, et al. : Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol 22:1151–1161, 2021 [DOI] [PubMed] [Google Scholar]
- 11.Venetis K, Crimini E, Sajjadi E, et al. : HER2 Low, Ultra-low, and Novel Complementary Biomarkers: Expanding the Spectrum of HER2 Positivity in Breast Cancer. Front Mol Biosci 9:834651, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D; Buzdar A; Candelaria R; Chen J; Clayborn A; Damodaran S; Ding Q; Garber H; Hortobagyi GN; Hunt KK; Ibrahim NK; Iheme A; Karuturi MS; Koenig K; Layman RM; Lee J; Litton JK; Mitchell M; Moscol G; Mouabbi J; Murthy RK; Oke O; Pohlmann P; Ramirez D; Ravenberg E; Saleem S; Teshome M; Valero V; White J; Williams m; Woodward WA; Yajima C; Ueno NT; Chen K; Rauch G; Huo L; Tripathy D YCLZKAMWKEHHAHASABABBCBAB: Clinical and Molecular Characteristics of HER2-low/zero Early Stage Triple-Negative Breast Cancer. San Antonio Breast Cancer Symposium 2022 [Google Scholar]
- 13.Jiang C, Perimbeti S, Deng L, et al. : Clinical outcomes of de novo metastatic HER2-low breast cancer: a National Cancer Database Analysis. NPJ Breast Cancer 8:135, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang G, Ren C, Li C, et al. : Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med 20:142, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schettini F, Chic N, Braso-Maristany F, et al. : Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 7:1, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarantino P, Jin Q, Tayob N, et al. : Prognostic and Biologic Significance of ERBB2-Low Expression in Early-Stage Breast Cancer. JAMA Oncol 8:1177–1183, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gampenrieder SP, Rinnerthaler G, Tinchon C, et al. : Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res 23:112, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hein A, Hartkopf AD, Emons J, et al. : Prognostic effect of low-level HER2 expression in patients with clinically negative HER2 status. Eur J Cancer 155:1–12, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Tarantino P, Morganti S, Curigliano G: Biologic therapy for advanced breast cancer: recent advances and future directions. Expert Opin Biol Ther 20:1009–1024, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Berrino E, Annaratone L, Bellomo SE, et al. : Integrative genomic and transcriptomic analyses illuminate the ontology of HER2-low breast carcinomas. Genome Med 14:98, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Véronique Diéras ED, Amélie Lusque, Barbara Pistilli, Thomas Bachelot, Jean-Yves Pierga, Frédéric Viret, Christelle Levy, Laura Salabert, Fanny Le Du, Florence Dalenc, Christelle Jouannaud, Laurence Venat-Bouvet, Jean-Philippe Jacquin, Xavier Durando, Thierry Petit, Céline Mahier - Aït Oukhatar, Thomas Filleron, Maria Fernanda Mosele, Magali Lacroix-Triki, Agnès Ducoulombier, Fabrice André: Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: A phase II study with biomarkers analysis (DAISY). Proceedings of the 2021 San Antonio Breast Cancer Symposium AACR; Cancer Res 82 [Google Scholar]
- 22.Ithimakin S, Day KC, Malik F, et al. : HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res 73:1635–46, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bang YJ, Giaccone G, Im SA, et al. : First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol 28:855–861, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gianni L, Llado A, Bianchi G, et al. : Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28:1131–7, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doi T, Shitara K, Naito Y, et al. : Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol 18:1512–1522, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Modi S, Saura C, Yamashita T, et al. : Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 382:610–621, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yver A, Agatsuma T, Soria JC: The art of innovation: clinical development of trastuzumab deruxtecan and redefining how antibody-drug conjugates target HER2-positive cancers. Ann Oncol 31:430–434, 2020 [DOI] [PubMed] [Google Scholar]
- 28.Staudacher AH, Brown MP: Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer 117:1736–1742, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogitani Y, Aida T, Hagihara K, et al. : DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin Cancer Res 22:5097–5108, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Modi S, Park H, Murthy RK, et al. : Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol 38:1887–1896, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starodub AN, Ocean AJ, Shah MA, et al. : First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 Conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors. Clin Cancer Res 21:3870–8, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syed YY: Sacituzumab Govitecan: First Approval. Drugs 80:1019–1025, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid JC P, Marmé F, Rugo HS, Tolaney SM, Oliveira M, Loirat D, Jhaveri K, Yoon OK, Motwani M, Wang H, Delaney RJ, Bardia A: Sacituzumab govitecan (SG) efficacy in hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2–) metastatic breast cancer (MBC) by HER2 immunohistochemistry (IHC) status in the phase III TROPiCS-02 study. Annals of Oncology 33:S88–S112, 2022 [Google Scholar]
- 34.Bardia A, Mayer IA, Vahdat LT, et al. : Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med 380:741–751, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Bardia A, Hurvitz SA, Tolaney SM, et al. : Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med 384:1529–1541, 2021 [DOI] [PubMed] [Google Scholar]
- 36.Hurvitz AB SA, Punie K, Kalinsky K, Cortes J, O’Shaughnessy J, Carey LA, Rugo HS, Yoon OK, Pan Y, Delaney RJ, Hofsess S, Hodgkins P, Phan S, Dieras V: Sacituzumab govitecan (SG) efficacy in patients with metastatic triple-negative breast cancer (mTNBC) by HER2 immunohistochemistry (IHC) status: Findings from the Phase 3 ASCENT study. Annals of Oncology 33:S194–S223, 2022 [Google Scholar]
- 37.Rugo HS, Bardia A, Tolaney SM, et al. : TROPiCS-02: A Phase III study investigating sacituzumab govitecan in the treatment of HR+/HER2− metastatic breast cancer. Future Oncol 16:705–715, 2020 [DOI] [PubMed] [Google Scholar]
- 38.Rugo HS, Bardia A, Marmé F, et al. : Primary results from TROPiCS-02: A randomized phase 3 study of sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) in patients (Pts) with hormone receptor–positive/HER2-negative (HR+/HER2-) advanced breast cancer. Journal of Clinical Oncology 40:LBA1001–LBA1001, 2022 [Google Scholar]
- 39.Rugo AB HS, Marmé F, Cortés J, Schmid P, Loirat D, Tredan O, Ciruelos EM, Dalenc F, Gomez Pardo P, Jhaveri K, Delaney RJ, Valdez T, Wang H, Verret W, Tolaney SM: Overall survival (OS) results from the phase III TROPiCS-02 study of sacituzumab govitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with HR+/HER2− metastatic breast cancer (mBC). Annals of Oncology 33:S808–S869, 2022 [Google Scholar]
- 40.Network NCC: Breast Cancer Version 4.2022. [Google Scholar]
- 41.Turk AA, Wisinski KB: PARP inhibitors in breast cancer: Bringing synthetic lethality to the bedside. Cancer 124:2498–2506, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robson M, Im SA, Senkus E, et al. : Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 377:523–533, 2017 [DOI] [PubMed] [Google Scholar]
- 43.Robson ME, Tung N, Conte P, et al. : OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol 30:558–566, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Litton JK, Rugo HS, Ettl J, et al. : Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 379:753–763, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Litton JK, Hurvitz SA, Mina LA, et al. : Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol 31:1526–1535, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andre F, Ciruelos EM, Juric D, et al. : Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol 32:208–217, 2021 [DOI] [PubMed] [Google Scholar]
- 47.Rugo HS, Lerebours F, Ciruelos E, et al. : Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol 22:489–498, 2021 [DOI] [PubMed] [Google Scholar]
- 48.Andre F, Ciruelos E, Rubovszky G, et al. : Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 380:1929–1940, 2019 [DOI] [PubMed] [Google Scholar]
- 49.Fugere T, Roy AM, Makhoul I: Alpelisib-Induced Diabetic Ketoacidosis in a Non-diabetic Patient. Cureus 13:e19295, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alaklabi S, Roy AM, Attwood K, et al. : Real world outcomes with alpelisib in metastatic hormone receptor-positive breast cancer patients: A single institution experience. Frontiers in Oncology 12, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piccart M, Hortobagyi GN, Campone M, et al. : Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2dagger. Ann Oncol 25:2357–2362, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baselga J, Campone M, Piccart M, et al. : Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520–9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma M, Duan Z, Zhao H, et al. : Real-World Patterns of Everolimus Use in Patients with Metastatic Breast Cancer. Oncologist 25:937–942, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshida-Ichikawa Y, Tanabe M, Tokuda E, et al. : Overcoming the Adverse Effects of Everolimus to Achieve Maximum Efficacy in the Treatment of Inoperable Breast Cancer: A Review of 11 Cases at Our Hospital. Case Rep Oncol 11:511–520, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosen K, Prasad V, Chen EY: Censored patients in Kaplan-Meier plots of cancer drugs: An empirical analysis of data sharing. Eur J Cancer 141:152–161, 2020 [DOI] [PubMed] [Google Scholar]
- 56.Bidard FC, Kaklamani VG, Neven P, et al. : Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J Clin Oncol 40:3246–3256, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sara Hurvitz S-BK, Wei-Pang Chung, Seock-Ah Im, Yeon Hee Park, Roberto Hegg, Min-Hwan Kim, Ling-Ming Tseng, Vanessa Petry, Chi-Feng Chung, Hiroji Iwata, Erika Hamilton, Giuseppe Curigliano, Binghe Xu, Caleb Lee, Yali Liu, Jillian Cathcart, Emarjola Bako, Sunil Verma, Javier Cortés: Trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03 [abstract]. AACR; Cancer Research 82, 2022 [Google Scholar]
- 58.Bartsch R, Berghoff AS, Furtner J, et al. : Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med 28:1840–1847, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Filipa VDRWSMTABKPABHSRKKTTLKDL: Subgroup analysis of patients with brain metastases from the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in metastatic triple-negative breast cancer. AACR; Cancer Research 81, 2021 [Google Scholar]
- 60.Schmid P JK, Wysocki PJ, Jassem J, Ma CX, Fernandes R, Huisden R, Stewart R, Vukovic R, Tablante Nunes A, Nowecki Z: Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): initial results from BEGONIA, a phase 1b/2 study. Annals of Oncology 33:S194–S223, 2022 [Google Scholar]
- 61.Schmid P, Im S-A, Armstrong A, et al. : BEGONIA: Phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—Initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). Journal of Clinical Oncology 39:1023–1023, 2021 [Google Scholar]
- 62.Eiger D, Agostinetto E, Saude-Conde R, et al. : The Exciting New Field of HER2-Low Breast Cancer Treatment. Cancers (Basel) 13, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi F, Liu Y, Zhou X, et al. : Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv 29:1335–1344, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Lee MM, Groothuis PG, Ubink R, et al. : The Preclinical Profile of the Duocarmycin-Based HER2-Targeting ADC SYD985 Predicts for Clinical Benefit in Low HER2-Expressing Breast Cancers. Mol Cancer Ther 14:692–703, 2015 [DOI] [PubMed] [Google Scholar]
- 65.Banerji U, van Herpen CML, Saura C, et al. : Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 20:1124–1135, 2019 [DOI] [PubMed] [Google Scholar]
- 66.Gupta RK, Roy AM, Gupta A, et al. : Systemic Therapy De-Escalation in Early-Stage Triple-Negative Breast Cancer: Dawn of a New Era? Cancers (Basel) 14, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]


