Abstract
Objectives
This study aimed to evaluate the efficiency of therapeutic pulsed ultrasound (US) applied underwater in mild-to-moderate carpal tunnel syndrome (CTS).
Patients and methods
This randomized, placebo-controlled study included 75 patients (114 hands; 7 males, 68 females; mean age: 46.7±9.9 years; range, 24 to 64 years) diagnosed with CTS through clinical evaluation and electroneuromyography (ENMG) results between March 2012 and January 2013. Patients were randomized into three groups. Group 1 received underwater pulsed US, Group 2 received sham US, and Group 3 was the control group. All groups were given night splints. Patients were evaluated at baseline, at the end of treatment (two weeks), and 12 weeks after the treatment using clinical examination tests (Tinel, Phalen, and hand elevation test), hand grip strength, Visual Analog Scale (VAS) for pain, Pain Quality Assessment Scale (PQAS), and ENMG.
Results
In all groups, a significant improvement was detected in the clinical assessment parameters, including the pain VAS, PQAS scores, physical examination outcomes, and hand grip strength. The decrease in VAS score and PQAS was found to be superior in the pulsed US group at both two weeks after the treatment and at the 12th week after the treatment compared to the sham US and control groups (p<0.001). Improvement in ENMG parameters, such as median motor latency, median sensorial velocity, and median sensory latency, was observed only in the underwater pulsed US group (p<0.001).
Conclusion
Therapeutic underwater pulsed US is an effective, safe, and easy-to-apply treatment option in the conservative treatment of mild-to-moderate CTS.
Keywords: Carpal tunnel syndrome, physical therapy, pulsed ultrasound, splints.
Introduction
Carpal tunnel syndrome (CTS) is a well-defined clinical manifestation that develops as a result of the compression of the median nerve at the wrist in the carpal tunnel and may cause pain, numbness, tingling, and weakness in the hands. Carpal tunnel syndrome is the most common entrapment neuropathy of the upper extremity, constituting approximately 90% of all nerve compression neuropathies, and its prevalence in the general population is around 1 to 4%.[1] The prevalence of CTS is gradually increasing and affects females three times more than males, with the 45 to 64 age group having the highest incidence.[2]
The first-line treatment for mild-to-moderate CTS is conservative. Surgical treatment is preferred in cases of severe impairment, in the presence of a neurological deficit, or in cases resistant to conservative treatments. Conservative treatment includes education, modification of daily life activities, splinting, exercises, medication (e.g., nonsteroidal anti-inflammatory drugs, steroids, and gabapentin), specific manual techniques, acupuncture, physical therapy modalities, and local injections. Despite extensive and high-quality research, the superiority of conservative treatment options over each other continues to be discussed, and no successful and standard treatment protocol that has been generally accepted as a standard.[3,4]
Therapeutic ultrasound (US) is a physical therapy modality with a thermal (deep heating) and nonthermal effect formed by the conversion of a high-frequency electric current into high-frequency acoustic energy.[5] It is widely used to reduce pain and disability in musculoskeletal disorders. The efficiency of US treatment for CTS has depended on the suppression of inflammation, the removal of edema, and the stimulation of nerves.[6,7] Although US is widely used in the treatment of mild to moderate CTS cases, evidence for its efficacy is contradictory due to differences in treatment protocols (e.g., intensity, frequency, continuity, and duration) and study methodologies.[4,8-10] In therapeutic US applications as a coupling agent, gel and water have been shown to have the highest transmission capacity.[11] Underwater therapeutic US is an easy-to-apply and effective application method, particularly on small and uneven surfaces, such as the hand and wrist.[12] In this method, where the US probe does not touch the skin, there is no possible confusing effect on the treatment results due to the micro-massage caused by skin contact in the classical US method due to skin contact.
To the best of our knowledge, there are no randomized controlled studies in the literature comparing the efficacy of underwater pulsed US therapy with sham US. Therefore, this study aimed to compare the efficacy of underwater pulsed US therapy with sham US and a neutral wrist splint in mild-to-moderate CTS.
Patients and Methods
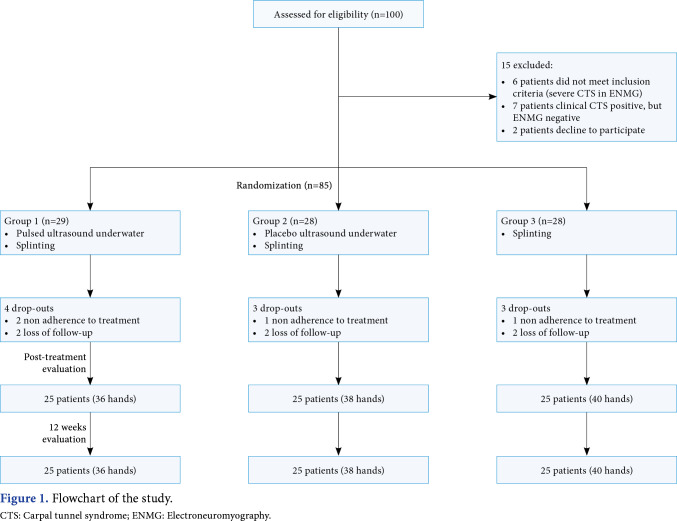
This hospital-based, three-arm, randomized, placebo-controlled study assessed 100 patients who applied to the physical medicine and rehabilitation outpatient clinics of the Konya University Meram Faculty of Medicine Hospital with complaints of pain, tingling, and numbness in their hands for eligibility between March 2012 and January 2013. Inclusion criteria for this study were as follows: being between the ages of 18 and 65 years, reporting pain and paresthesia at the hand for at least three months, having positive Phalen or Tinel’s signs, and having a diagnosis of CTS, including electrodiagnostic evidence of mild-to-moderate CTS. Exclusion criteria were as follows: patients with a body mass index >30, the presence of a secondary condition that may cause CTS (e.g., diabetes mellitus, hypothyroidism, and pregnancy), the presence of cervical radiculopathy, polyneuropathy or peripheral neuropathy of any origin other than CTS, upper motor neuron diseases, the onset of complaints following trauma, anatomic deformities of the wrist or hand, having had any treatment (including local injections, physical therapy, and splinting) for CTS within the last six months, the existence of signs of local infection or open wounds, detection of severe CTS at electroneuromyography (ENMG) or neurological deficit (thenar atrophy or muscle weakness), and having a history of surgical intervention for CTS. Six patients were excluded due to severe CTS, seven patients were excluded as no CTS was detected at ENMG, and two patients were excluded since they declined to participate. As a result, 85 patients (132 hands) who were diagnosed with mild-to-moderate CTS were included in the study.
Sequentially numbered, opaque, sealed envelopes were used for the randomization of the study. Randomization was performed by an independent investigator who was not involved in the study procedures. All 85 eligible patients were randomized into three groups: the underwater pulsed US group (Group 1), the underwater sham US group (Group 2), and the control group (Group 3). All groups underwent splinting. Evaluation of the study outcomes was performed by the same investigator. During the study, a total of 10 patients (18 hands) could not complete the treatment because of nonadherence to the treatment (n=4) and loss to follow-up (n=6). A total of 75 patients (114 hands; 7 males, 68 females; mean age: 46.7±9.9 years; range, 24 to 64 years) completed the study (Figure 1).
Figure 1. Flowchart of the study. CTS: Carpal tunnel syndrome; ENMG: Electroneuromyography.
All patients (groups) in the study were given custom-made volar splints for nighttime use with a length from the middle of the palm to the middle of the forearm and a metal support at the volar part, which ensured that the hand was kept in a neutral position and allowed for the movement of the finger metacarpophalangeal joint. In addition, they were told that they could use the splint during the day as long as their activity situation allowed. Patients were asked to wear the neutral splint continuously for 12 weeks. No medication or exercise therapy was given to the patients.
The ITO US-100® device (ITO Co. Ltd., Kawaguchi-shi, Japan) was used for US treatment. The US cap had a surface area of 5 cm2 . Patients in the therapeutic US group were administered 1.0 W/cm2 , 1 MHz of US in pulsed mode 1:4 in stabilized water by keeping the cap perpendicular to the affected hand skin at a distance of 1 to 3 cm without skin contact for 10 min per session. A total of 10 sessions of US were applied in two weeks; one session per day and five sessions per week. The same procedure was applied to the affected hand of the patients in the sham US group for the same duration and session as if the device were being operated as a placebo. No US was applied to the splint-only (control) group.
Detailed physical examination findings and sociodemographic data of all patients included in the study were recorded before treatment. All groups were evaluated with examination findings (Tinel, Phalen, and the hand elevation test), pain intensity, hand grip strength (HGS), the Visual Analog Scale (VAS), the Pain Quality Assessment Scale (PQAS), and ENMG three times: before the treatment (baseline), at the end of the treatment (two weeks), and 12 weeks after the treatment.
Outcome parameters
Tinel's test was performed by percussion on the transverse carpal ligament (over the distal wrist fold) and was considered positive in the case of paresthesia in the median nerve dermatome. In Phalen test, patients put their elbows on the table and straightened their forearms. They held their wrists at 90º flexion for 1 min, and the test was considered positive in the case of paresthesia in the median nerve distribution.[13] In the hand elevation test, both hands were elevated in the air for 2 min while the shoulders and elbows were in the free position and the test was considered positive if paresthesia developed.[13]
Visual Analog Scale was used to measure the pain intensity in wrists and hands in the last week. In this questionnaire, all the characteristics of pain (pain, numbness, tingling, and burning) felt by the patients were questioned with a single VAS. Patients evaluated their pain level by marking it on a 10-cm scale, with 0 for pain-free and 10 for severe pain. The VAS was preferred since it is easily applicable, has good testretest reliability, and is a universally accepted global scale that can adequately reflect neuropathic pain in CTS.[14]
Pain Quality Assessment Scale is a 20-item questionnaire developed by adding new items to the Neuropathic Pain Scale. As the questions are very comprehensive, this scale includes the neuropathic and nonneuropathic aspects of pain.[15] This scale includes two global items (pain intensity and unpleasantness), two spatial items (superficial pain and deep pain), and 16 quality items (hot, cold, dull, sensitivity, tenderness, sharp, itching, shooting, numbness, tingling, electrification, cramping, radiating, throbbing, aching, and heaviness). Each question is scored between 0 and 10, where 0 means "no pain/sensation" and 10 means "the most pain/sensation." The scale is used in CTS management, follow-up, and treatment effectiveness evaluation.[15] The validity and reliability of the Turkish version were demonstrated for CTS.[16]
Jamar hand dynamometer (BASELINE® Hydraulic Hand Dynamometer; Fabrication Enterprises Inc, Elmsford, NY, USA) was used for the measurement of HGS. Hand grip strength was measured in a sitting position with the patient's arm at their side, shoulder adducted, elbows at 90° flexion, forearm neutral, and wrist between 0° and 30° extension and between 0° and 15° ulnar deviation.[17] The dynamometer was deliberately squeezed at full force by the patients, and the mean scores (kilograms) of three consecutive trials were recorded.
Electrophysiologic studies of all patients were carried out in a room at 23 to 25°C, and the hand temperatures of all patients were maintained at 30 to 32°C. In the study, an EsaoteMyto-II device (Esaote, Istanbul, Türkiye) was used for ENMG. As ENMG parameters, median motor nerve distal latency (mMNDL), median motor nerve velocity, median sensory nerve distal latency (mSNDL), median sensory nerve velocity (mSNV), median nerve motor amplitude, and median sensory amplitude were examined. The antidromic method was used for the assessment of sensory conduction. An earth electrode was placed in the palm of patients for sensory examinations. Median nerve sensory velocity and mSNDL were measured by placing the active ring electrode at the second proximal interphalangeal joint, the passive ring electrode at the distal interphalangeal joint, and the stimulator at 14 cm proximal to the active electrode at the wrist level. In median motor nerve conduction analysis, the active electrode was placed on the abductor pollicis brevis muscle and the passive electrode on the first metacarpophalangeal joint. An earth electrode was placed on the forearm flexor surface. In distal stimulation, the distance between the active electrode and the stimulator was 8 cm. According to the guidelines of the American Society of Electrodiagnostic Medicine, the diagnosis of CTS is classified as mild when only sensory fibers are affected and moderate when both sensory and motor fibers are affected.[18] In the study, all electrophysiological examinations were performed by the same physician.
Statistical analysis
The G*Power version 3.1.9.4 (Heinrich-HeineUniversität Düsseldorf, Düsseldorf, Germany) was used for the power analysis of the study. The primary output of this study, the VAS value, was taken into account to calculate the sample size. As a result of the literature review, considering the study for which we chose a larger sample size (alpha=0.05, power=0.90, and effect size=0.273), it was calculated that at least 117 CTS hands should be included.[7,18,19]
Statistical analysis was performed using IBM SPSS version 20.0 (IBM Corp., Armonk, NY, USA). The Shapiro-Wilk test was used to determine whether the variables had a normal distribution. Variables were expressed as frequency (n) and percentage (%), mean ± standard deviation, or median (min-max) values according to parametric distribution. The chi-square test with Fischer exact test or the Pearson chi-square test was used to evaluate categorical data. Repeated measures analysis of variance (ANOVA) with post hoc Bonferroni correction or the Friedman test was used for repeated measurements at different times for intragroup comparisons. The Wilcoxon signed-rank test was used for two nonparametric dependent variables for intragroup measurements. The McNemar test was used for categorical variables in the intragroup comparisons. The Kruskal-Wallis or the one-way ANOVA was used to compare variables between more than two independent groups. The significance level was set at p<0.05.
Results
Figure 1 shows the flowchart of the study. No treatment-related side effects were reported by the participants or observed on medical follow-up, and no patient discontinued the study for this reason. There was no statistically significant difference between the groups regarding age, sex, body mass index, occupation, dominant hand involvement, duration of symptoms, or clinical and electrophysiological (severity of CTS) at baseline (Table 1). In addition, there was no significant difference in the number of patients who received double-handed treatment between the groups (p=0.429).
Table 1. Baseline demographic and clinical characteristics of the groups.
| Group 1 (Pulsed US + Splint) | Group 2 (Placebo US + Splint) | Group 3 (Splint) | p | |||||||||||||
| n | % | Mean±SD | Median | Min-Max | n | % | Mean±SD | Median | Min-Max | n | % | Mean±SD | Median | Min-Max | ||
| Age (year) | 48.6±9.3 | 43.4±9.8 | 47.2±10.3 | 0.064" | ||||||||||||
| Sex | 0.854e | |||||||||||||||
| Female | 23 | 23 | 22 | |||||||||||||
| Male | 2 | 2 | 3 | |||||||||||||
| Employment | 0.970b | |||||||||||||||
| Housewife | 20 | 80 | 21 | 84 | 22 | 88 | ||||||||||
| Other (officer and worker) | 5 | 20 | 4 | 16 | 4 | 16 | ||||||||||
| Body mass index (kg/m2) | 26.55±1.60 | 26.70±1.84 | 26.39±1.71 | 0.583" | ||||||||||||
| Dominant hand treated | 24 | 67 | 25 | 63 | 23 | 61 | 0.856b | |||||||||
| Symptom duration (month) | 12.17±8.75 | 13.13±8.53 | 11.00±8.27 | 0.309d | ||||||||||||
| Pain (VAS) | 5 | 3-8 | 5.5 | 3-8 | 5 | 3-7 | 0.640d | |||||||||
| CTS severity | 0.293b | |||||||||||||||
| Mild | 28 | 78 | 29 | 73 | 33 | 87 | ||||||||||
| Moderate | 8 | 22 | 11 | 27 | 5 | 14 | ||||||||||
| Tinel+ | 30 | 83 | 36 | 90 | 35 | 92 | 0.466b | |||||||||
| Phalen + | 26 | 72 | 32 | 80 | 33 | 87 | 0.293b | |||||||||
| Hand hold + | 20 | 56 | 29 | 73 | 30 | 79 | 0.080b | |||||||||
| US: Ultrasound; SD: Standard deviation; VAS: Visual Analog Scale; CTS: Carpal tunnel syndrome; a One-way ANOVA test; b Pearson’s chi-square test; c Fisher exact test; d Kruskal Wallis test. | ||||||||||||||||
In all three groups, a statistically significant improvement was detected in clinical assessment parameters, including pain VAS, PQAS scores, physical examination outcomes, and HGS at two weeks after treatment (p<0.05) and at the 12-week follow-up compared to before treatment (p<0.05, Table 2). The decrease in VAS score and PQAS was found to be superior in the underwater pulsed US group compared to the sham US and splint-only groups at two weeks after the treatment (p<0.001) and at the 12th week after the treatment (p<0.001, Table 2). Group 1 had a higher difference in HGS score than in Group 2 (p=0.011) and Group 3 (p<0.001) at 12 weeks after treatment. No significant difference was found between groups in repeated measurements of dichotomous data (Tinel, Phalen, and hand elevation test; Table 3). No superiority was found between the groups in terms of the Tinel test, the Phalen test, and the hand elevation test (Table 3).
Table 2. Comparison of the clinical parameters.
| Group 1 | Group 2 | Group 3 | Between-group analysis | ||||
| Variables | Median | Min-Max | Median | Min-Max | Median | Min-Max | p |
| VAS-pain | |||||||
| Before treatment | 5 | 3-8 | 5.5 | 3-8 | 5 | 3-7 | 0.640c |
| 2 weeks after treatment | 3 | 0-5 | 4 | 1-5 | 3 | 1-6 | 0.00Γ |
| 12 weeks after treatment | 1 | 0-2 | 2 | 1-6 | 3 | 1-4 | 0.00Γ |
| pa | <0.001 | <0.001 | <0.001 | Group 1 > Group 2 | |||
| pb (w0-w2) | <0.001 | <0.001 | <0.001 | p=0.001d | |||
| pb (w0-w12) | <0.001 | <0.001 | <0.001 | Group 1 > Group 3 | |||
| pb (w2-w12) | <0.001 | 0.002 | 0.005 | p=0.014d | |||
| The PQAS scores | |||||||
| Before treatment | 3.20 | 2.3-4.9 | 3.30 | 2.1-4.9 | 2.65 | 2-4.2 | 0.001c |
| 2 weeks after treatment | 1.63 | 0.3-3.7 | 2.38 | 1.1-3.2 | 1.56 | 0.9-3 | 0.001c |
| 12 weeks after treatment | 0.3 | 0-1.1 | 1.68 | 0.8-3.6 | 1.36 | 0.5-2.5 | 0.001c |
| pa | <0.001 | <0.001 | <0.001 | Group 1 > Group 2 | |||
| pb (w0-w2) | <0.001 | <0.001 | <0.001 | p<0.001e | |||
| pb (w0-w12) | <0.001 | <0.001 | <0.001 | Group 1 > Group 3 | |||
| pb (w2-w12) | <0.001 | <0.001 | 0.03 | p<0.001e | |||
| Hand grip strength | |||||||
| Before treatment | 20.5 | 12.2-38.9 | 20.35 | 10.0-38.7 | 20.0 | 9.0-38.7 | 0.785c |
| 2 weeks after treatment | 21.0 | 13.9-40 | 21.3 | 11.7-38.9 | 21.05 | 10.5-39 | 0.842c |
| 12 weeks after treatment | 22.0 | 15.1-40.2 | 21.2 | 12.0-38.8 | 20.45 | 10.7-38.5 | 0.371c |
| pa | <0.001 | <0.001 | 0.31 | Group 1 > Group 2 | |||
| pb (w0-w2) | 0.007 | 0.001 | 0.048 | p=0.011e | |||
| pb (w0-w12) | <0.001 | <0.001 | 0.117 | Group 1 > Group 3 | |||
| pb (w2-w12) | <0.001 | 0.394 | 1 | p<0.001e | |||
| VAS: Visual analog scale; w0: Before treatment; w2: 2 weeks after treatment; w12: 12 weeks after treatment; a Friedman test; b Wilcoxon Signed Ranks test; c Kruskal Wallis test; d Mann-Whitney-U test with Bonferroni correction; e Between groups differences at follow-up, Kruskal Wallis test; Group 1 > Group 2 means treatment superiority. | |||||||
Table 3. Comparison of the physical examinations.
| Variables | Group 1 | Group 2 | Group 3 | Between-group analysis | |||
| n | % | n | % | n | % | p* | |
| Tinel test | |||||||
| Before treatment | 30 | 83 | 36 | 90 | 35 | 92 | 0.466 |
| 2 weeks after treatment | 20a | 56a | 27a | 68a | 23a | 61a | 0.582 |
| 12 weeks after treatment | 13b | 36b | 20b | 50b | 17b | 45b | 0.469 |
| Phalen test | |||||||
| Before treatment | 26 | 72 | 32 | 80 | 33 | 87 | 0.293 |
| 2 weeks after treatment | 14a | 39a | 24a | 60a | 22b | 58b | 0.134 |
| 12 weeks after treatment | 11b | 31b | 15b | 38b | 18b | 47b | 0.327 |
| Hand elevation test | |||||||
| Before treatment | 20 | 56 | 29 | 73 | 30 | 79 | 0.080 |
| 2 weeks after treatment | 11a | 31a | 17a | 43a | 18b | 47b | 0.318 |
| 12 weeks after treatment | 4b | 11b | 7b | 18b | 10b | 26b | 0.237 |
| * Chi-square test; a McNemar test, p<0.05; b McNemar test, p<0.001. | |||||||
In Group 1, a statistically significant improvement was observed in ENMG parameters, such as mSNV, mSNDL, and mMNDL, at two weeks after the treatment (p<0.001) and at 12 weeks (p<0.001). In Groups 2 and 3, no significant difference in ENMG parameters was observed during follow-up (Table 4).
Table 4. Comparison of electrophysiological parameters.
| Variables | Group 1 | Group 2 | Group 3 |
| Mean±SD | Mean±SD | Mean±SD | |
| Median sensory nerve velocity | |||
| Before treatment | 42.76±4.30 | 40.95±4.49 | 40.10±5.40 |
| 2 weeks after treatment | 47.02±3.73a | 41.02±4.48 | 41.32±5.54 |
| 12 weeks after treatment | 50.33±4.51b | 40.65±4.78 | 40.64±5.47 |
| Median sensory nerve distal latency | |||
| Before treatment | 3.06±0.36 | 3.02±0.29 | 3.18±0.43 |
| 2 weeks after treatment | 2.79±0.25a | 2.98±0.28 | 3.11±0.42 |
| 12 weeks after treatment | 2.64±0.27b | 3.00±0.29 | 3.16±0.45 |
| Median motor velocity | |||
| Before treatment | 56.93±4.18 | 55.86±4.07 | 55.57±3.72 |
| 2 weeks after treatment | 57.49±3.80 | 54.39±3.67 | 54.84±3.92 |
| 12 weeks after treatment | 57.64±4.04 | 55.77±4.58 | 55.25±3.85 |
| Median motor distal latency | |||
| Before treatment | 3.84±0.47 | 3.87±0.52 | 3.74±0.65 |
| 2 weeks after treatment | 3.61±0.51c | 3.86±0.55 | 3.73±0.49 |
| 12 weeks after treatment | 3.34±0.42b | 4.02±0.58 | 3.85±0.51 |
| Median nerve motor amplitude | |||
| Before treatment | 10.39±2.34 | 10.25±2.25 | 10.69±2.17 |
| 2 weeks after treatment | 10.58±2.75 | 11.08±2.70 | 10.10±1.72 |
| 12 weeks after treatment | 10.21±2.65 | 10.56±2.29 | 10.90±1.86 |
| Median nerve sensory amplitude | |||
| Before treatment | 37.86±15.02 | 34.64±23.17 | 31.16±17.27 |
| 2 weeks after treatment | 39.94±16.27 | 36.70±16.67 | 32.29±16.03 |
| 12 weeks after treatment | 34.67±15.64 | 34.29±13.40 | 33.97±14.59 |
| SD: Standard deviation; a p<0.001: Repeated measures ANOVA with post hoc Bonferroni correction (Comparison of outcomes before and two weeks after treatment); b p<0.001: Repeated measures ANOVA with post hoc Bonferroni correction (Comparison of outcomes before and 12 weeks after treatment); c p=0.008: Repeated measures ANOVA with post hoc Bonferroni correction (Comparison of outcomes before and two weeks after treatment). | |||
Discussion
In the present study, which aimed to determine the efficiency of therapeutic underwater pulsed US in the conservative management of mild-to-moderate CTS treatment, in each of the three groups, a statistically significant improvement was detected in VAS for pain, clinical assessment parameters, and PQAS at two weeks after treatment and at 12 weeks. The group with active underwater pulsed US treatment was found to be superior to the other groups in terms of changes in VAS pain and PQAS scores. There was no improvement in the ENMG results in Groups 2 and 3, while a significant improvement was observed in Group 1 at two weeks after treatment and at 12 weeks.
There are many studies on the efficacy of therapeutic US, which has been used in the treatment of musculoskeletal diseases for more than 75 years in the conservative treatment of CTS.[18,20-22] However, the type, intensity, and frequency of US treatment and the methodological differences of the studies demonstrating the effectiveness of US reveal that the effectiveness of this treatment should be supported by placebo-controlled studies.[9,18-22]
In a study by Ebenbichler et al.,[23] 45 patients with mild-to-moderate bilateral CTS were divided into two groups. The first group was given 1:4 pulsed US with an intensity of 1.0 W/cm2 , while the second group received placebo US. In the group receiving active US, a statistically significant improvement in symptoms, grip, and pinch strength, and electrophysiological parameters, such as motor distal latency and sensory conduction velocity, were observed. This effect was observed until the sixth month. According to previously mentioned studies, it was reported that pulsed US is efficient and also leads to electrophysiological changes. The heating effect and an increase in nerve conduction velocity due to this heating effect are not expected with pulsed or sham US.[6] The present study also showed that underwater pulsed US treatment resulted in improvement in both clinical and ENMG parameters. In these patients without neurological deficits, the primary symptom was pain with a predominant neuropathic component and a secondary loss of grasping power. The subnormal levels of motor amplitude (>5 mV) in the electromyography values of the patients indicate that there is no loss of axons.[24] Thenar atrophy and loss of muscle strength are not expected in the absence of axonal loss. Positive sensory symptoms, such as numbness and tingling, are observed with sensory involvement due to demyelination in mild-to-moderate CTS. Both the decrease in VAS pain and PQAS scores are consistent with the positive change in electromyography, particularly in mSNDL and mSNV. Although there is no true weakness in mild-to-moderate CTS patients, it is well known that there is a decrease in HGS secondary to pain. Therefore, positive sensory symptoms that improve first in treatment-induced recovery might be expected to increase grip strength. In our study, a statistically significant increase in HGS was observed, although its clinical significance was doubtful. It can be thought that this modest increase observed in a short time is due to the decrease in pain symptoms. Indeed, Krause et al.[25] found a minimal clinically important difference value of 1.64 for VAS pain. We believe the improvement in clinical and ENMG parameters can be attributed to the greater stimulation of tissue healing and acceleration of edema formation and resolution by underwater administration of pulsed US.[26]
The micro-massage effect of therapeutic US can increase the release of endogenous opioids and exert a pain-relieving effect.[27] This possible effect may influence study results when using sham US studies that have not been conducted underwater. In a sham US-controlled study, Armagan et al.[28] compared the efficacy of continuous and pulsed US in 46 patients with mild-to-moderate CTS. As a result of this study, in which a splint was given to all three groups, similar clinical improvements were detected at the end of the treatment (three weeks). Although electrophysiological improvement was observed in the active group with continuous and pulsed US groups, it was not found to be superior to the placebo group. In a randomized study very similar to this study, 44 CTS patients were treated with continuous (1 MHz, 1 W/cm2 ), pulsed (1 MHz, 1 W/cm2 in 1:4 pulsed), or sham US therapy for five sessions of 10 min for two weeks, in addition to splint treatment.[29] Sham US showed similar clinical, electrophysiological, and ultrasonographic improvement with pulsed and continuous US. In our study, the pulsed US treatment was administered underwater, different from other studies. Therefore, sham US and active pulsed US did not have a local massaging effect due to underwater administration (no probe contact). The similar treatment results of the splint plus sham US group and the splint-only group can be attributed to the efficacy of the wrist splint's effectiveness in symptomatic relief. In the active pulsed US treatment group, unlike the other groups, electrophysiological improvement was observed, and a greater reduction in pain was detected, indicating the effectiveness of underwater pulsed US treatment. In this study, US was administered underwater at an intensity of 1 W/cm2 and in 1:4 pulsed mode. The pulsed US facilitates the emission of sound waves without increasing the temperature in the tissues. This feature is the reason why many studies are performed with low intensity in pulsed mode. Low-intensity pulsed US can decrease inflammation and oxidative stress and facilitate peripheral nerve regeneration.[30] At this point, as it has been shown in many studies, low-intensity pulsed US application, which is accepted to have an intensity below 1 W/cm2 due to underwater application, may have caused a positive change in electrophysiologic studies by having anti-inflammatory and regenerative effects on the stimulation of the median nerve conduction.[6,31,32]
The pressure inside the carpal tunnel increases when the wrist is in flexion and extension. A static hand-wrist splint that keeps the wrist in a neutral position reduces the pressure on the median nerve. Owing to this biomechanical effect, splints that control the involuntary movements of the wrist at night, keep the wrist neutral position, and allow the movement of the fingers are the most frequently used first-line treatment in the conservative treatment of mild-to-moderate CTS. In a randomized controlled study, Walker et al.[33] reported that the use of a splint at night ensured improvement in symptom severity and nerve conduction, while continuous use of a splint led to more improvement in median nerve conduction compared to use at night, but no difference was detected in the improvement in symptom severity. Although many studies have shown the effectiveness of splinting in treating mild-to-moderate CTS, there is not enough evidence to say which splint design should be used in the treatment of CTS and for what period.[34] It is generally accepted that the night splint is effective in the short term, despite limited evidence, but its long-term efficacy (>6 months) is uncertain.[35,36] In many randomized controlled studies, a splint is used as a control group treatment.[37,38] In this study, which we conducted to evaluate the effectiveness of active underwater pulsed US therapy more clearly, we administered splints to all groups, and an improvement was observed in VAS, clinical assessment parameters, HGS, and PQAS in all groups. However, as other treatment types were also administered to two groups in addition to splinting, we could not attribute the success only to the splint alone. In the group treated only with the splint, improvement was observed in all parameters except for ENMG. These results were similar to the results of previous studies and demonstrated that splinting was efficient in CTS.[33,34]
This study has some limitations. The most important limitation is the lack of long-term follow-up results. The fact that the Boston Carpal Tunnel Questionnaire, which is the most frequently used questionnaire in the evaluation of symptoms and functional status in CTS patients, was not used may be a partial limitation. However, considering that the VAS for pain is used quite similarly to the Boston Carpal Tunnel Questionnaire in CTS (60% vs. 51%) and reflects the subjective symptoms shortly and clearly, we believe that this limitation will not pose a significant problem.[38] Other limitations include the lack of assessment of a group administered with continuous US treatment and a group that only received therapeutic pulsed underwater US treatment without splints to compare against the splint-only group. Despite these limitations, to our knowledge, the present study is the first randomized, sham-controlled study to investigate the efficacy of underwater pulsed US in the treatment of CTS. We believe that the strength of the present study lies in the fact that the active treatment group (pulsed US) was compared with both the sham US group and the splint group (control), carefully measuring subjective and objective outcomes.
In conclusion, improvements in pain symptoms and HGS were observed in all three groups in the conservative treatment of mild-to-moderate CTS. However, the change in electrophysiological parameters in the therapeutic underwater pulsed US group was significant and superior to that in the other groups. Therapeutic underwater pulsed US may be an effective, safe, and easy-to-apply treatment option in the management of CTS. Randomized controlled trails comparing only underwater US therapy with splint therapy alone will increase the level of evidence for our results.
Acknowledgments.
The authors thank all patients, physicians, and therapists who participated in the study.
Footnotes
Ethics Committee Approval: The study was approved by the Konya University Meram Faculty of Medicine Non-Interventional Clinical Research Ethics Committee (date: 16.03.2012, no: 2012/38). Its procedure was in compliance with the Declaration of Helsinki guidelines.
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Idea/concept, design, control/ supervision: S.B., N.Ş., R.Y.; Data collection and/or processing: S.B., N.Ş., R.Y.; Analysis and/or interpretation, writing the article, literature review: S.B., R.Y.; Critical review, references, and fundings, materials, other: : S.B., N.Ş., R.Y.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
Patient Consent for Publication: A written informed consent was obtained from each patient.<br><br><b>Data Sharing Statement:</b><br> The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Yunoki M, Kanda T, Suzuki K, Uneda A, Hirashita K, Yoshino K. Importance of recognizing carpal tunnel syndrome for neurosurgeons: A review. Neurol Med Chir (Tokyo) 2017;57:172–183. doi: 10.2176/nmc.ra.2016-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bongers FJ, Schellevis FG, van den Bosch WJ, van der Zee J. Carpal tunnel syndrome in general practice (1987 and 2001): Incidence and the role of occupational and nonoccupational factors. Br J Gen Pract. 2007;57:36–39. [PMC free article] [PubMed] [Google Scholar]
- 3.Jiménez Del Barrio S, Bueno Gracia E, Hidalgo García C, Estébanez de Miguel E, Tricás Moreno JM, Rodríguez Marco S, et al. Conservative treatment in patients with mild to moderate carpal tunnel syndrome: A systematic review. Neurologia (Engl Ed) 2018;33:590–601. doi: 10.1016/j.nrl.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Hernández-Secorún M, Montaña-Cortés R, Hidalgo-García C, Rodríguez-Sanz J, Corral-de-Toro J, Monti-Ballano S, et al. Effectiveness of conservative treatment according to severity and systemic disease in carpal tunnel syndrome: A systematic review. Int J Environ Res Public Health. 2021;18:2365–2365. doi: 10.3390/ijerph18052365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speed CA. Therapeutic ultrasound in soft tissue lesions. Rheumatology (Oxford) 2001;40:1331–1336. doi: 10.1093/rheumatology/40.12.1331. [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira Perrucini PD, Poli-Frederico RC, de Almeida Pires-Oliveira DA, Dragonetti Bertin L, Beltrão Pires F, Shimoya-Bittencourt W, et al. Anti-inflammatory and healing effects of pulsed ultrasound therapy on fibroblasts. Am J Phys Med Rehabil. 2020;99:19–25. doi: 10.1097/PHM.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 7.Jiang X, Savchenko O, Li Y, Qi S, Yang T, Zhang W, et al. A review of low-intensity pulsed ultrasound for therapeutic applications. IEEE Trans Biomed Eng. 2019;66:2704–2718. doi: 10.1109/TBME.2018.2889669. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Qian Y, Sun Z, Liu W, Sun G, Liu J, et al. Effectiveness of therapeutic ultrasound for the treatment of carpal tunnel syndrome (the USTINCTS trial): Study protocol for a three-arm, prospective, multicentre, randomised controlled trial. e057541BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-057541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page MJ, O'Connor D, Pitt V, Massy-Westropp N. Therapeutic ultrasound for carpal tunnel syndrome. CD009601Cochrane Database Syst Rev. 2013;2013 doi: 10.1002/14651858.CD009601.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huisstede BM, Hoogvliet P, Franke TP, Randsdorp MS, Koes BW. Carpal tunnel syndrome: Effectiveness of physical therapy and electrophysical modalities. An updated systematic review of randomized controlled trials. Arch Phys Med Rehabil. 2018;99:1623–1634. doi: 10.1016/j.apmr.2017.08.482. [DOI] [PubMed] [Google Scholar]
- 11.Casarotto RA, Adamowski JC, Fallopa F, Bacanelli F. Coupling agents in therapeutic ultrasound: Acoustic and thermal behavior. Arch Phys Med Rehabil. 2004;85:162–165. doi: 10.1016/s0003-9993(03)00293-4. [DOI] [PubMed] [Google Scholar]
- 12.Király M, Varga Z, Szanyó F, Kiss R, Hodosi K, Bender T. Effects of underwater ultrasound therapy on pain, inflammation, hand function and quality of life in patients with rheumatoid arthritis - a randomized controlled trial. Braz J Phys Ther. 2017;21:199–205. doi: 10.1016/j.bjpt.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDermid JC, Wessel J. Clinical diagnosis of carpal tunnel syndrome: A systematic review. J Hand Ther. 2004;17:309–319. doi: 10.1197/j.jht.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Ceceli E, Gumruk S, Okumus M, Kocaoglu S, Goksu H, Karagoz A. Comparison of 2 methods of neuropathic pain assessment in carpal tunnel syndrome and hand functions. Neurosciences (Riyadh) 2018;23:23–28. doi: 10.17712/nsj.2018.1.20170345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen MP, Gammaitoni AR, Olaleye DO, Oleka N, Nalamachu SR, Galer BS. The pain quality assessment scale: Assessment of pain quality in carpal tunnel syndrome. J Pain. 2006;7:823–832. doi: 10.1016/j.jpain.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Şahin N, Bodur S, Salli A, Uğurlu H. Reliability and validity of the Turkish version of the pain quality assessment scale in patients with carpal tunnel syndrome. Nobel Medicus. 2010;6:26–33. [Google Scholar]
- 17.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9:222–226. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 18.Yildiz N, Atalay NS, Gungen GO, Sanal E, Akkaya N, Topuz O. Comparison of ultrasound and ketoprofen phonophoresis in the treatment of carpal tunnel syndrome. J Back Musculoskelet Rehabil. 2011;24:39–47. doi: 10.3233/BMR-2011-0273. [DOI] [PubMed] [Google Scholar]
- 19.Gökoğlu F, Fındıkoğlu G, Yorgancoğlu ZR, Okumuş M, Ceceli E, Kocaoğlu S. Evaluation of iontophoresis and local corticosteroid injection in the treatment of carpal tunnel syndrome. Am J Phys Med Rehabil. 2005;84:92–96. doi: 10.1097/01.phm.0000151942.49031.dd. [DOI] [PubMed] [Google Scholar]
- 20.Bakhtiary AH, Rashidy-Pour A. Ultrasound and laser therapy in the treatment of carpal tunnel syndrome. Aust J Physiother. 2004;50:147–151. doi: 10.1016/s0004-9514(14)60152-5. [DOI] [PubMed] [Google Scholar]
- 21.Baysal O, Altay Z, Ozcan C, Ertem K, Yologlu S, Kayhan A. Comparison of three conservative treatment protocols in carpal tunnel syndrome. Int J Clin Pract. 2006;60:820–828. doi: 10.1111/j.1742-1241.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- 22.Dincer U, Cakar E, Kiralp MZ, Kilac H, Dursun H. The effectiveness of conservative treatments of carpal tunnel syndrome: Splinting, ultrasound, and low-level laser therapies. Photomed Laser Surg. 2009;27:119–125. doi: 10.1089/pho.2008.2211. [DOI] [PubMed] [Google Scholar]
- 23.Ebenbichler GR, Resch KL, Nicolakis P, Wiesinger GF, Uhl F, Ghanem AH, et al. Ultrasound treatment for treating the carpal tunnel syndrome: Randomised “sham” controlled trial. BMJ. 1998;316:731–735. doi: 10.1136/bmj.316.7133.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang CW, Wang YC, Chang KF. A practical electrophysiological guide for non-surgical and surgical treatment of carpal tunnel syndrome. J Hand Surg Eur Vol. 2008;33:32–37. doi: 10.1177/1753193408087119. [DOI] [PubMed] [Google Scholar]
- 25.Krause D, Roll SC, Javaherian-Dysinger H, Daher N. Comparative efficacy of the dorsal application of Kinesio tape and splinting for carpal tunnel syndrome: A randomized controlled trial. J Hand Ther. 2021;34:351–361. doi: 10.1016/j.jht.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Young S. Electrotherapy: Evidence based practice. 11. London: Churchill Livingstone; 2002. Ultrasound therapy; pp. 211–230. [Google Scholar]
- 27.Oztas O, Turan B, Bora I, Karakaya MK. Ultrasound therapy effect in carpal tunnel syndrome. Arch Phys Med Rehabil. 1998;79:1540–1544. doi: 10.1016/s0003-9993(98)90416-6. [DOI] [PubMed] [Google Scholar]
- 28.Armagan O, Bakilan F, Ozgen M, Mehmetoglu O, Oner S. Effects of placebo-controlled continuous and pulsed ultrasound treatments on carpal tunnel syndrome: A randomized trial. Clinics (Sao Paulo) 2014;69:524–528. doi: 10.6061/clinics/2014(08)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Çatalbaş N, Akkaya N, Atalay NS, Sahin F. Ultrasonographic imaging of the effects of continuous, pulsed or sham ultrasound treatments on carpal tunnel syndrome: A randomized controlled study. J Back Musculoskelet Rehabil. 2018;31:981–989. doi: 10.3233/BMR-160652. [DOI] [PubMed] [Google Scholar]
- 30.Bilir-Yildiz B, Sunay FB, Yilmaz HF, Bozkurt-Girit O. Low-intensity low-frequency pulsed ultrasound ameliorates sciatic nerve dysfunction in a rat model of cisplatin-induced peripheral neuropathy. Sci Rep. 2022;12:8125–8125. doi: 10.1038/s41598-022-11978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuhfried O, Vukanovic D, Kollmann C, Pieber K, Paternostro-Sluga T. Effects of pulsed ultrasound therapy on sensory nerve conduction parameters and the pain threshold perceptions in humans. PM R. 2017;9:781–786. doi: 10.1016/j.pmrj.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Ilham SJ, Chen L, Guo T, Emadi S, Hoshino K, Feng B. In vitro single-unit recordings reveal increased peripheral nerve conduction velocity by focused pulsed ultrasound. Biomed Phys Eng Express. 2018;4:045004–045004. doi: 10.1088/2057-1976/aabef1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker WC, Metzler M, Cifu DX, Swartz Z. Neutral wrist splinting in carpal tunnel syndrome: A comparison of night-only versus full-time wear instructions. Arch Phys Med Rehabil. 2000;81:424–429. doi: 10.1053/mr.2000.3856. [DOI] [PubMed] [Google Scholar]
- 34.Weng C, Dong H, Chu H, Lu Z. Clinical and electrophysiological evaluation of neutral wrist nocturnal splinting in patients with carpal tunnel syndrome. J Phys Ther Sci. 2016;28:2274–2278. doi: 10.1589/jpts.28.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page MJ, Massy-Westropp N, O'Connor D, Pitt V. Splinting for carpal tunnel syndrome. CD010003Cochrane Database Syst Rev. 2012;2012 doi: 10.1002/14651858.CD010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karjalanen T, Raatikainen S, Jaatinen K, Lusa V. Update on efficacy of conservative treatments for carpal tunnel syndrome. J Clin Med. 2022;11:950–950. doi: 10.3390/jcm11040950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khosrawi S, Emadi M, Mahmoodian AE. Effectiveness of splinting and splinting plus local steroid injection in severe carpal tunnel syndrome: A Randomized control clinical trial. Adv Biomed Res. 2016;5:16–16. doi: 10.4103/2277-9175.175902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mertz K, Lindsay SE, Morris A, Kamal RN. Outcome metrics in the treatment of carpal tunnel syndrome: A systematic review. Hand (N Y) 2022;17:659–667. doi: 10.1177/1558944720949951. [DOI] [PMC free article] [PubMed] [Google Scholar]