Abstract
Objectives
This study aims to examine the effect of high-frequency repetitive transcranial magnetic stimulation (rTMS) on gait parameters and lower extremity motor recovery in a more specific sample of individuals with chronic and traumatic incomplete spinal cord injury (iSCI).
Patients and methods
This double-blind, sham-controlled, randomized study included a total of 28 individuals (20 males, 8 females; mean age: 35.7±12.1 years; range, 18 to 45 years) with chronic (>1 year) traumatic iSCI. The participants were randomly allocated to either sham rTMS group (n=14) or real rTMS group (n=14). We compared the groups based on the lower extremity motor scores (LEMS), the temporal-spatial gait measurements using three-dimensional gait analysis, the Walking Index for SCI–II (WISCI-II), and 10-m walking test at baseline, three weeks (post-treatment) and five weeks (follow-up) after the treatment.
Results
The real rTMS group revealed a significant improvement in walking speed, LEMS score, and 10-m walking test after the treatment compared to baseline (p=0.001, p=0.002, and p=0.023, respectively). Changes in the LEMS score were significantly increased in the real rTMS group compared to the sham group at both three and five weeks (p=0.001 and p=0.001, respectively). No significant difference was observed in the other variables between the groups (p>0.05).
Conclusion
Our study findings support the therapeutic effectiveness of rTMS on motor recovery in chronic iSCI. The rTMS can be used as an adjuvant therapy to conventional physiotherapy in the rehabilitation of patients with iSCI.
Keywords: Gait, motor recovery, repetitive transcranial magnetic stimulation, spinal cord injury.
Introduction
Lower extremity motor recovery and regaining walking function are the most critical goals at the rehabilitation period for patients with spinal cord injury (SCI). Walking promotes musculoskeletal health by providing mechanical loading exercise, reduces systemic inflammation, enhances mental health, enables community involvement, and improves the efficency of daily living activities.[1] Patients with incomplete SCI (iSCI) have the potential to show prominent spontaneous functional recovery within several years after injury.[2] This recovery process basically depends on synaptic plasticity and reorganization of spared fiber tracts in the spinal cord, primary motor cortex, and corticospinal tract.[2,3] Motor recovery after iSCI can be potentiated by various rehabilitation modalities such as activitybased therapies.[4]
It is a non-invasive way to induce excitability changes in the motor cortex and the descending corticospinal pathway through repetitive transcranial magnetic stimulation (rTMS).[5] It can be used for therapeutic purposes, as it provides long-term changes in the excitability-like mechanisms involved in long-lasting potentiation and depression of synaptic transmission.[6] Stimulation of motor neurons from the cortex downwards can promote neuronal reorganization by increasing the corticospinal interaction activity to support the sprouting of novel synaptic connections in injured sites of the spinal cord.[7] On this basis, rTMS can potentially be used to enhance motor function in individuals with iSCI by induction of cortical and/or spinal cord neuroplasticity.[8,9]
Belci et al.[10] conducted the first study of rTMS that showed amelioration in motor function of upper extremity for patients with cervical iSCI. Subsequent studies reported promising results of the efficiency of rTMS in the treatment of hand motor functions after iSCI.[11-20] However, most of those studies were case series and did not provide high-quality evidence. The role of rTMS in the treatment of lower extremity and gait function has been studied relatively less. Previously, two studies showed an improvement in the lower extremity muscle strength and clinical walking tests after rTMS combined with gait training.[21,22] However, both studies had some methodological concerns such as having been conducted in the acute/subacute phase of iSCI and not excluding participants with iSCI of non-traumatic origin. Thus, confounding effects of spontaneous functional recovery during the early period of iSCI and the possibility of exacerbation in non-traumatic iSCI depending on the course of the disease that might have masked the benefits of rTMS could not be ruled out.
In the present study, we hypothesized that real rTMS would lead to significant motor recovery in the lower extremities and improve gait parameters compared to sham rTMS. We, therefore, aimed to examine the effect of high-frequency rTMS on lower extremity motor recovery in a more specific sample of individuals with chronic and traumatic iSCI. Temporospatial gait parameters were assessed using three-dimensional (3D) gait analysis system to accurately determine whether a potential increase in muscle strength could contribute to functional walking.
Patients and Methods
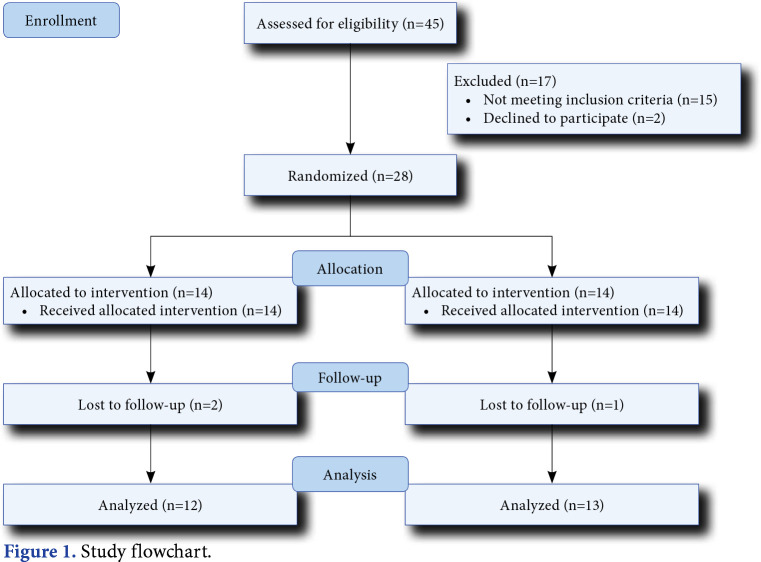
This double-blind, sham-controlled, randomized study was conducted at University of Health Sciences Türkiye, Gülhane Medical School, Gaziler Physical Therapy and Rehabilitation Training and Research Hospital, Department of Physical Medicine and Rehabilitation between October 2015 and April 2017. Initially, a total of 45 patients admitted to the SCI clinic were screened. Of them, 28 (20 males, 8 females; mean age: 35.7±12.1 years; range, 18 to 45 years) were included and randomly assigned to either real rTMS group (n=14) or sham rTMS group (n=14) using a computer-based randomization program. The study flowchart is shown in Figure 1. All participants received 15 sessions (five days a week for a total of three weeks) real/sham rTMS plus gait training and 10 sessions (five days a week) gait training without rTMS. Inclusion criteria were as follows: motor incomplete cervical/thoracic SCI Grade C-D according to the American Spinal Injury Association Impairment Scale (AIS); SCI of traumatic origin; minimum duration of one year after injury; age of 15 to 45 years (i.e., age range was limited given that all participants had similar neuroplasticity characteristics); and ability to walk at least 10 m independently or with an assistive device such as a cane. Exclusion criteria were as follows: the presence of any other musculoskeletal or neurological disease leading to walking disability; lower motor neuron lesions (e.g., cauda equina and conus medullaris); a history of epilepsy; presence of cranium defect, metallic implants in the cranium, or pacemaker; and pregnancy. The participants were allowed to withdraw from the study at any time for any reason. All the participants had a stable medication status and no additional medication was initiated during the study that would affect the parameters examined and the treatment process. All sessions applied to the patient were performed by a single investigator. It was known only by the researcher who applied the TMS, whether it was a real or sham application. Another investigator who made the evaluation was not in the laboratory during the three weeks of rTMS treatment and was blind to the group allocation.
Figure 1. Study flowchart.
rTMS procedure
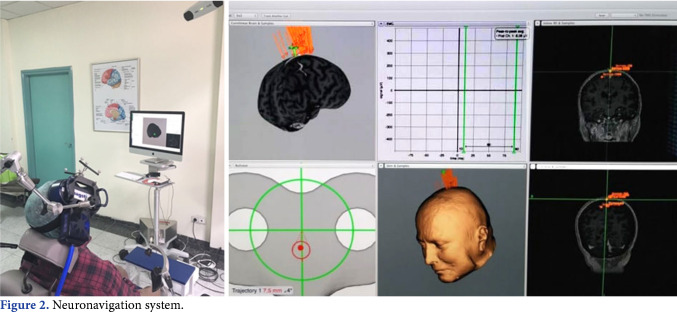
The motor cortex was stimulated using an 8-shaped coil with an outer loop diameter of 70 mm (Air Film Coil, Magstim, Whitland, Dyfed, UK) connected to Magstim Rapid2 Magnetic Stimulator (Magstim, Whitland, Dyfed, UK). 40 trains of 20-Hz pulses for 2 sec at an inter-train interval of 28 sec were applied, providing a total of 1,600 pulses in the real rTMS sessions. The stimulation frequency of 20 Hz was selected due to the effect on motor recovery demonstrated in previous studies.[21,22] Twenty min were allotted for each rTMS session. Resting motor threshold (RMT) was obtained from the contralateral first dorsal interosseous (FDI) muscle by stimulating the dominant hemisphere. The RMT was determined with 1% TMS machine output increment as the minimal stimulus intensity required to produce motor-evoked potentials (MEP) of >50 µV in 5 out of 10 consecutive trials.[23] The stimulation intensity of the rTMS to be applied was adjusted as 110% of RMT obtained in contralateral muscle of FDI by stimulating the dominant hemisphere. Given the damage in the descending pathways of spinal cord, it was mostly impossible to establish a motor threshold by stimulating lower extremity motor area. Even if we took a response in some case, this was probably not a true threshold due to the fact that we could elicit a response with a higher stimulation intensity to overcome the damage in nerve fibers. Therefore, we used upper extremity to decide RMT, consistent with previous studies. On the other hand, using 90% of the RMT was the preference of the researchers, which was not consistent with the current literature, even if there are some studies conducted above 100% of motor threshold. The coil intersected the scalp tangentially, pointing backwards over the vertex. The Brainsight TMS Navigation System (Rogue Research, Montreal, Canada) was used for coil orientation and motor cortex localization area related to the lower extremities on the participant’s cranial magnetic resonance imaging (MRI) scans. The participants received rTMS while sitting in a comfortable chair with a TMS coil holder, which was designed specifically for the neuronavigation system (Figure 2). The same protocol was applied with a sham coil (Sham Air Film Coil, Magstim Company Ltd., UK) which was seemingly identical to the active coil and produced similar noises during the sessions, but gave no active magnetic stimulation in the sham group. The participants and the researcher assessing the participants were blinded to the type of rTMS given.
Figure 2. Neuronavigation system.
Gait training
A daily exercise program was applied, consisting of 30 min of lower extremity strengthening and 30 min of overground walking and balance exercises under the supervision of a physiotherapist who was blinded to the group assignment. The rTMS sessions were immediately followed by the exercise program with the aim of potentially priming functional networks for the following exercise intervention through the mechanism of metaplasticity.[24,25]
Outcome measures
The primary outcome measures were (i) lower extremity motor score (LEMS) measured according to the standardized AIS clinical examination,[26] and (ii) the temporal-spatial gait measurements assessed with 3D gait analysis. Secondary outcome measures were (i) the Walking Index for SCI–II (WISCI-II)[27] scale to measure the gait functions of participants with iSCI, (ii) the 10-m walking test (time in seconds to walk 10 m at comfortable walking speed), and (iii) the Modified Ashworth Scale (MAS)[28] assessed on knee extensors, hip adductors, and ankle plantar flexors for spasticity evaluation. The MAS was measured bilaterally, and mean values were recorded separately for each muscle group. All outcome measures except for gait analysis were assessed at baseline, three weeks (post-treatment), and five weeks (follow-up).
Gait analysis
Gait analysis was performed in the gait and motion analysis laboratory using a 3D, seven-camera, Vicon 512 motion measurement system (Oxford Metrics Ltd., Oxford, UK). The Vicon Clinical Manager software was used to calculate and plot parameters. The pelvis, thigh, shin, and foot of the subject were marked with 15 reflective markers bilaterally. To determine appropriate anthropometric scales, we took measurements of height, weight, knee width, ankle width, and leg length. The participants were instructed to walk at a self-selected speed along a walkway with two pressure plates embedded in it after completing three or four practice sessions in the laboratory. To achieve a clean trial on at least three occasions, participants walked as many times as necessary. The walking speed (m per sec), cadence (steps per min), single support time (sec), double support time (sec), step length (m), and step time (sec) were included in temporal spatial measurements for analysis. Baseline and post-treatment gait analyses were performed.
Statistical analysis
In each group, 12 participants were calculated based on the estimation that the sample size would detect a difference of 3.5 in LEMS with type 1 error of 0.05 and 80% power.[21] Assuming 15% dropout, the final sample size required was calculated as a total of 28 participants with iSCI (14 participants in each group).
Statistical analysis was performed using the SPSS for Mac version 20.0 software (IBM Corp., Armonk, NY, USA). Descriptive data were expressed in mean ± standard deviation (SD) or median (min-max) for continuous variables and in number and frequency for categorical variables. The Kolmogorov-Smirnov test was used to determine whether the obtained parameters were appropriate for normal distribution. As the parameters were not normal, non-parametric analysis was used for comparisons. The chi-square test was used to compare categorical variables, while the Mann-Whitney U test to compare the changes in outcome measures between two groups. The comparisons of repeated measures within the groups were evaluated by using the Friedman test. The Wilcoxon signed-rank test was used for post-hoc analysis. A p value of <0.05 was considered statistically significant. To avoid type 1 error, the Bonferroni correction was performed in all multiple comparisons. After Bonferroni correction, a p value of <0.0083 for intra-group comparisons and a p value of <0.016 for inter-group comparisons were considered statistically significant.
Results
There were 14 participants in the sham rTMS group and 14 participants in the real rTMS group. Two participants in the real rTMS group and one participant in the sham rTMS group did not complete the rTMS sessions and excluded from the study. Therefore, 13 participants in the real rTMS group and 12 participants in the sham rTMS group were included. Both groups had comparable baseline characteristics (Table 1). All the participants tolerated the treatment well and did not report any side effects. The participants in both groups did not describe any sensorial symptoms (e.g., pain, tingling sensation) during real versus sham rTMS procedures.
Table 1. Baseline characteristics of patients.
| Real rTMS group (n=13) | Sham rTMS group (n=12) | p | |||
| n | Mean±SD | n | Mean±SD | ||
| Age (year) | 35.9±12.4 | 35.6±11.7 | 0.958 | ||
| Sex | |||||
| Female | 4 | 2 | |||
| Male | 9 | 10 | |||
| Body mass index (kg/m2) | 24.2±4.4 | 24.1±4.4 | 0.933 | ||
| Time since injury (month) | 28.8±15.3 | 34.5±27.5 | 0.533 | ||
| Type of injury | 0.763 | ||||
| Motor vehicle accident | 5 | 6 | |||
| Fall | 3 | 3 | |||
| Iatrogenic | 2 | 2 | |||
| Diving in shallow water | 2 | 1 | |||
| Gunshot wound | 1 | 0 | |||
| Level of injury | 0.363 | ||||
| Tetraplegic | 9 | 8 | |||
| Paraplegic | 4 | 4 | |||
| AIS | 0.588 | ||||
| C | 2 | 1 | |||
| D | 11 | 11 | |||
| rTMS: Repetitive transcranial magnetic stimulation; SD: Standard deviation; AIS: American Spinal Injury Association Impairment Scale. | |||||
The outcome measures are summarized in Table 2. The real rTMS group showed a significant increase in the LEMS post-treatment (p=0.002) and at follow-up (p=0.001), compared to baseline. However, no significant improvement in the LEMS was observed at any time points (p=0.034 for both post-treatment and follow-up) in sham rTMS group. The 10-m walking test result significantly improved compared to baseline in the real rTMS group (p=0.001 for both post-treatment and follow-up), but not in the sham rTMS group (p=0.248 for post-treatment and p=0.169 for follow-up). No significant improvement was observed in the WISCI-II score over time in either group (p>0.05). In the assessment of spasticity with MAS on knee extensor, hip adductor and ankle flexor muscle groups, no significant difference over time was observed in either group (p>0.05). Gait analysis revealed a significant improvement in walking speed post-treatment in the real rTMS group (p=0.002), but not in the sham rTMS group (p=0.113). No other temporal spatial parameters showed a significant difference in either group (p>0.05).
Table 2. Outcome measurements for real and sham rTMS groups.
| Baseline | After last session | Follow-up | |||||||
| Mean±SD | Median | Q1-Q3 | Mean±SD | Median | Q1-Q3 | Mean±SD | Median | Q1-Q3 | |
| LEMS | |||||||||
| Real rTMS group | 32.6±8.9 | 35.0 | 24.5-39.5 | 35.4±8.7 | 38.0 | 27.0-42.5 | 36.2±8.8 | 40.0 | 27.0-43.0 |
| Sham rTMS group 10 meters walking test | 36.0±11.0 | 39.0 | 30.0-44.0 | 36.5±11.2 | 39.0 | 30.0-45.0 | 36.7±11.3 | 39.0 | 30.0-45.7 |
| Real rTMS group | 37.5±28.5 | 28.1 | 15.2-58.4 | 32.9±18.9 | 18.9 | 14.7-45.8 | 33.0±26.2 | 19.9 | 14.4-44.8 |
| Sham rTMS group WISCI-II |
37.5±38.8 | 26.1 | 18.0-61.8 | 36.0±35.9 | 25.7 | 17.8-61.5 | 36.2±35.7 | 25.7 | 17.7-61.5 |
| Real rTMS group | 14.8±3.2 | 13.0 | 12.0-18.0 | 15.6±3.4 | 15.0 | 12.5-20.0 | 15.2±3.7 | 15.0 | 12.5-20.0 |
| Sham rTMS group MAS (knee extensor) | 15.6±3.9 | 14.5 | 13.0-20.0 | 15.6±3.9 | 14.5 | 13.0-20.0 | 15.6±3.9 | 14.5 | 13.0-20.0 |
| Real rTMS group | 0.73±1.16 | 0.0 | 0.0-2.0 | 0.42±0.90 | 0.0 | 0.0-0.5 | 0.42±0.90 | 0.0 | 0.0-0.5 |
| Sham rTMS group MAS (hip adductor) | 1.04±1.21 | 0.5 | 0.0-2.0 | 1.08±1.31 | 0.5 | 0.0-2.7 | 1.00±1.20 | 0.5 | 0.0-2.0 |
| Real rTMS group | 1.30±1.18 | 1.0 | 0.0-2.5 | 0.92±0.93 | 1.0 | 0.0-2.0 | 0.92±0.3 | 1.0 | 0.0-2.0 |
| Sham rTMS group MAS (ankle flexor) |
1.33±1.55 | 0.5 | 0.0-3.0 | 1.33±1.55 | 0.5 | 0.0-3.0 | 1.33±1.55 | 0.5 | 0.0-3.0 |
| Real rTMS group | 1.80±1.36 | 2.0 | 0.5-2.7 | 1.46±1.12 | 1.5 | 0.2-2.5 | 1.30±1.12 | 1.0 | 0.0-2.2 |
| Sham rTMS group Temporal spatial parameters Walking speed (m/sec) |
1.04±1.13 | 1.0 | 0.0-2.0 | 1.20±1.26 | 1.0 | 0.0-2.0 | 1.20±1.26 | 1.0 | 0.0-2.0 |
| Real rTMS group | 0.41±0.28 | 0.33 | 0.17-0.63 | 0.49±0.28 | 0.43 | 0.19-0.74 | - | - | - |
| Sham rTMS group Cadence (steps/min) |
0.37±0.30 | 0.27 | 0.13-0.58 | 0.42±0.35 | 0.22 | 0.15-0.69 | - | - | - |
| Real rTMS group | 60.9±26.2 | 62.2 | 35.1-72.3 | 65.0±26.7 | 74.5 | 34.3-89.6 | - | - | - |
| Sham rTMS group Double support time (sec) | 52.3±27.3 | 44.1 | 28.0-71.0 | 53.9±28.4 | 44.6 | 30.5-75.0 | - | - | - |
| Real rTMS group | 1.29±1.10 | 0.81 | 0.51-2.21 | 1.10±0.92 | 0.65 | 0.35-2.13 | - | - | - |
| Sham rTMS group Single support time (sec) | 1.66±1.08 | 1.67 | 0.56-2.83 | 1.43±0.94 | 1.60 | 0.52-2.23 | - | - | - |
| Real rTMS group | 0.55±0.13 | 0.55 | 0.48-0.64 | 0.56±0.12 | 0.59 | 0.47-0.68 | - | - | - |
| Sham rTMS group Step length (m) |
0.60±0.14 | 0.58 | 0.49-0.78 | 0.70±0.26 | 0.69 | 0.43-0.98 | - | - | - |
| Real rTMS group | 0.44±0.13 | 0.43 | 0.27-0.65 | 0.41±0.13 | 0.40 | 0.29-0.54 | - | - | - |
| Sham rTMS group Step time (sec) |
0.41±0.12 | 0.39 | 0.29-0.55 | 0.41±0.12 | 0.40 | 0.30-0.53 | - | - | - |
| Real rTMS group | 1.24±0.57 | 1.07 | 0.79-1.85 | 1.12±0.55 | 0.80 | 0.67-1.79 | - | - | - |
| Sham rTMS group | 1.72±1.16 | 1.34 | 1.01-2.12 | 1.51±0.95 | 1.38 | 0.79-1.93 | - | - | - |
| rTMS: Repetitive transcranial magnetic stimulation; SD: Standard deviation; Q: Quartile; LEMS: Lower extremity motor score; WISCI-II: Walking Index for SCI-II; MAS: Modified Ashworth scale. | |||||||||
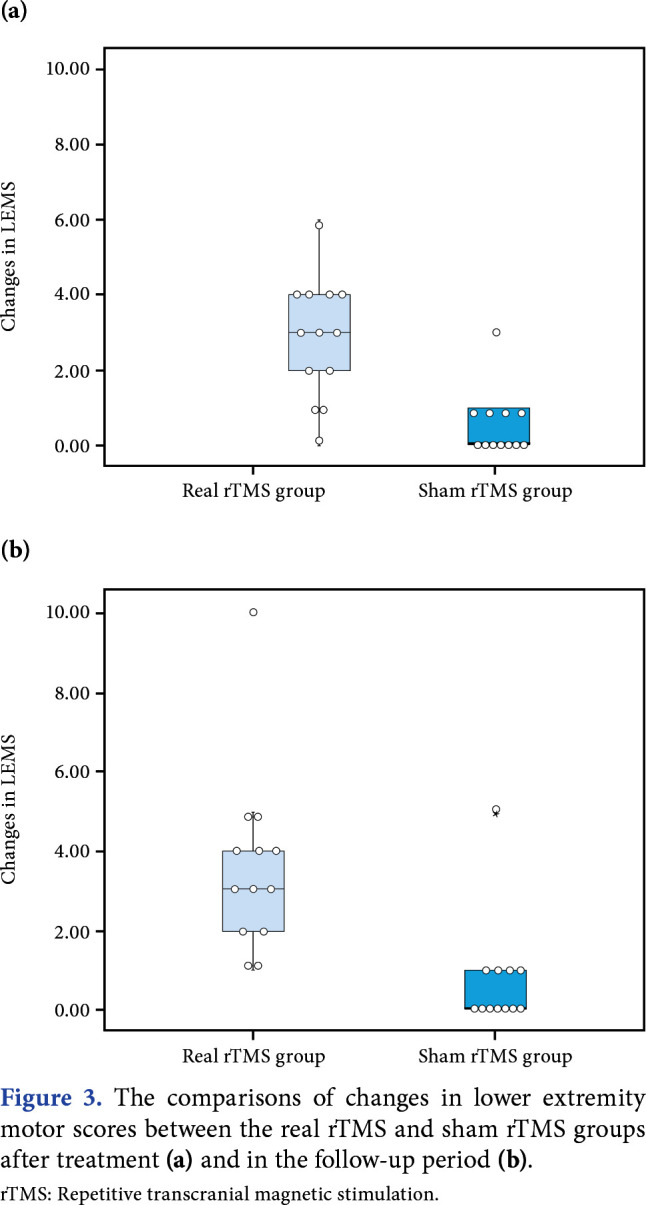
The changes in outcome measures compared to baseline were analyzed between the groups at each time point (Table 3). Accordingly, the changes in the LEMS were significantly higher at post-treatment (p=0.001) and follow-up (p=0.001) in the real rTMS group, compared to the sham rTMS group (Figure 3). Other outcome measures were not significantly different between the groups (p>0.05).
Table 3. Outcome measurements for real and sham rTMS groups.
| Real rTMS group | Sham rTMS group | ||||||
| Mean±SD | Median | Q1-Q3 | Mean±SD | Median | Q1-Q3 | p | |
| LEMS | |||||||
| Post-treatment | 2.84±1.62 | 3.00 | 1.50-4.00 | 0.58±0.90 | 0.00 | 0.00-1.00 | 0.001 |
| Follow-up | |||||||
| 10 meters walking test | 3.61±2.32 | 3.00 | 2.00-4.50 | 0.75±1.42 | 0.00 | 0.00-1.00 | <0.001 |
| Post-treatment | -4.57±7.50 | -1.35 | 6.36-0.43 | -1.52±5.05 | -0.47 | 3.52-0.41 | 0.270 |
| Follow-up | -4.50±7.84 | -1.35 | 6.32-0.91 | -1.51±4.98 | -0.47 | 2.91-0.00 | 0.295 |
| WISCI-II | |||||||
| Post-treatment | 0.76±1.48 | 0.00 | 0.00-1.50 | 0.00±0.00 | 0.00 | 0.00-0.00 | 0.347 |
| Follow-up | |||||||
| MAS (knee extensor) | 0.38±1.50 | 0.00 | 0.00-0.00 | 0.00±0.00 | 0.00 | 0.00-0.00 | 0.769 |
| Post-treatment | -0.30±0.63 | 0.00 | -0.50-0.00 | 0.04±0.33 | 0.00 | 0.00-0.00 | 0.347 |
| Follow-up | |||||||
| MAS (hip adductor) | -0.30±0.63 | 0.00 | -0.50-0.00 | -0.04±0.45 | 0.00 | 0.00-0.00 | 0.538 |
| Post-treatment | -0.38±0.84 | 0.00 | -0.51-0.00 | 0.00±0.00 | 0.00 | 0.00-0.00 | 0.205 |
| Follow-up | |||||||
| MAS (ankle flexor) | -0.38±0.84 | 0.00 | -0.51-0.00 | 0.00±0.00 | 0.00 | 0.00-0.00 | 0.205 |
| Post-treatment | -0.34±0.55 | 0.00 | -1.00-0.00 | 0.16±0.38 | 0.00 | 0.00-0.00 | 0.077 |
| Follow-up | |||||||
| Temporal spatial parameters Walking speed (m/sec) | -0.50±0.91 | 0.00 | -1.00-0.00 | 0.16±0.38 | 0.00 | 0.00-0.00 | 0.077 |
| Post-treatment | 0.07±0.10 | 0.04 | 0.01-0.18 | 0.05±0.13 | 0.02 | 0.01-0.04 | 0.459 |
| Cadence (steps/min) | |||||||
| Post-treatment | 4.05±8.51 | 3.10 | -2.60-10.10 | 1.62±3.91 | 3.20 | -1.75-4.40 | 0.820 |
| Double support time (sec) | |||||||
| Post-treatment | -0.18±0.35 | -0.08 | 0.29-0.03 | -0.22±0.47 | -0.07 | -0.45-0.01 | 0.865 |
| Single support time (sec s) | |||||||
| Post-treatment | 0.01±0.08 | 0.02 | -0.07-0.09 | 0.09±0.15 | 0.03 | -0.02-0.14 | 0.228 |
| Step length (m) | |||||||
| Post-treatment | -0.23±0.71 | 0.01 | -0.18-0.06 | 0.00±0.03 | 0.02 | -0.01-0.03 | 0.691 |
| Step time (sec) | |||||||
| Post-treatment | -0.12±0.26 | -0.04 | -0.16-0.01 | -0.21±1.04 | -0.11 | -0.21-0.04 | 0.820 |
| rTMS: Repetitive transcranial magnetic stimulation; SD: Standard deviation; Q: Quartile; LEMS: Lower extremity motor score; WISCI-II: Walking Index for SCI-II; MAS: Modified Ashworth scale. | |||||||
Figure 3. The comparisons of changes in lower extremity motor scores between the real rTMS and sham rTMS groups after treatment (a) and in the follow-up period (b). rTMS: Repetitive transcranial magnetic stimulation.
Discussion
In the present study, we report a sham-controlled trial of three weeks daily rTMS plus gait training exercises on the functions of lower extremity and gait in patients with chronic iSCI. All participants were assessed with 3D gait analysis. The rTMS led to a significant improvement in the LEMS, compared to the sham stimulation. However, there was no significant difference in the temporal spatial parameters, WISCI-II scale, and spasticity level.
A significant improvement in the LEMS is the main finding of this study. The LEMS improved more in the rTMS group than in the sham group, and this difference was maintained after two weeks of follow-up. In contrast to sham stimulation, rTMS enhanced walking speed. However, no significant difference in the walking speed improvement was observed between the groups. Similarly, previous studies have reported an improvement in the walking speed measured with the 10-meter walking test.[21,22] The unclear results in the current study may be due to the relatively small sample size. Nonetheless, the study provides evidence to support rTMS as an adjunct to physical training in chronic iSCI. We believe that further studies would better illustrate the impact of motor improvement after rTMS on walking in larger cohorts. In addition, we found no significant difference in the WISCI-II scale between the groups in our study. The WISCI-II scale usually reflects the gait capacity of participants with SCI. Those who walk with less support take higher scores according to the WISCI-II scale. Our study findings showed that the improvement in lower extremity muscle strength did not significantly reduce the need for support while walking. Although spasticity had a negative effect on gait function, we found a significant improvement in 10-m walking test and walking speed after real rTMS application; however, there was no significant improvement in the spasticity level. This can be attributed to the fact that our participants did not have high levels of spasticity before the treatment.
Although the mechanism underlying motor recovery with rTMS has not been well established yet, the following suggestions can be made. Neurophysiological studies have revealed that intracortical inhibition decreases naturally in those with iSCI whose motor function is improved.[29] The decrease in intracortical inhibition may lead to improved corticospinal connections of intact neurons. As rTMS has the capability of modulating the motor cortex excitability, it has been suggested that it could decrease intracortical inhibition after iSCI.[16,30] Due to the effect on the descending corticospinal tracts, rTMS can improve discordant and decrease segmental plasticity resulting from SCI.[31] It has also been proposed that some biochemical changes in the mechanism may account for motor recovery with rTMS. Hou et al.[32] found that the combination of rTMS and treadmill training improved spasticity and gait parameters after cervical SCI in a rat model. Upregulation of dopamine-beta-hydroxylase, glutamic acid decarboxylase 67, GABAB receptor, and brain neurotrophic factor were detected at the lumbar spinal cord level of the rats. It was suggested that these upregulation mechanisms were helpful to achieve improvements in adaptive plasticity after SCI.
In a recent meta-analysis, there was a significant amelioration in LEMS with high frequency rTMS after iSCI.[33] The current study demonstrated the efficacy of rTMS in a more specific sample of participants with iSCI, unlike previous randomized-clinical trials;[21,22] only participants with chronic iSCI of traumatic origin were included. Since spontaneous functional recovery significantly decreases in the chronic phase[34] and is unlikely to create a bias in methodological design, the present study suggests that corticospinal plasticity may be altered by rTMS, even in the chronic period after SCI. Nevertheless, it is necessary to conduct future studies to confirm the criteria for selecting appropriate participants and to determine the optimal time for rTMS after SCI.
The rTMS has been used to reduce spasticity after iSCI, but there are conflicting results in the literature. As in the present study, Kumru et al.[22] primarily investigated motor recovery with rTMS and found no reduction in spasticity assessed as a secondary outcome measure. However, the aforementioned authors showed a decrease in spasticity in their previous study[35] in which spasticity was the primary outcome measure and the baseline MAS scores of the participants were relatively higher. The effect of the baseline spasticity level on the benefits from rTMS could be a research topic for further studies.
The short follow-up time, unmeasured lower extremity MEP values, and relatively small sample size are the main limitations to this study. Future studies with longer follow-up periods and larger sample size should be designed to demonstrate the long-term effects of rTMS after iSCI. In addition, a figure-of-8-shaped coil was used in this study, as the researchers had a figure-of-8-shaped sham coil identical to the real coil for a strong placebo effect. However, as a double cone coil has a relatively deeper effect, it could be used to stimulate the lower extremity motor cortex, which is localized in the interhemispheric area. In a study by Schecklmann et al.,[36] a double cone coil generated a higher magnetic field with a greater depth of penetration than a figure-of-eight coil. On the other hand, the results of another study by Desbeaumes Jodoin et al.[37] revealed that more reliable and tolerable rTMS treatments could be expected with the double cone coil, and offered an alternative to figure-of-eight coil in certain cases.
In conclusion, our study findings support the therapeutic effectiveness of rTMS on motor recovery in chronic and traumatic iSCI. The rTMS can be used as an adjuvant therapy to conventional physiotherapy in the rehabilitation of participants with iSCI. However, there are still many unclear points such as the optimal stimulation protocol, the impact of the nature of the spinal injury, the optimal time for treatment, and long-term effects of rTMS. Further studies are needed to elucidate the controversies in rTMS, and further placebo-controlled trials may facilitate benefit level of rTMS after iSCI.
Acknowledgments.
We thank Berke Aras, MD for his valuable help in gait analysis.
Footnotes
Ethics Committee Approval: The study protocol was approved by the Ankara Numune Training and Research Hospital Ethics Committee (date: 08.01.2015, no: E-15-390). The study was retrospectively registered on the Clinical Trials Registry (ClinicalTrials.gov ID: NCT04372134). The study was reported according to the CONSORT guidelines.The study was conducted in accordance with the principles of the Declaration of Helsinki.
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Idea/concept, design: S.K.; Control/supervision, critical review: E.Y., B.Y.; Data collection and/or processing, writing the article, references and fundings, materials: S.K., A.U.; Analysis and/or interpretation: S.K., E.Y.; Literature review: A.U., B.Y.
Financial Disclosure: The study was supported by the Scientific and Technological Research Council of Turkey (115S917).
Patient Consent for Publication: A written informed consent was obtained from each patient.
References
- 1.Hardin EC, Kobetic R, Triolo RJ. Ambulation and spinal cord injury. Phys Med Rehabil Clin N Am. 2013;24:355–370. doi: 10.1016/j.pmr.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- 3.Oudega M, Perez MA. Corticospinal reorganization after spinal cord injury. J Physiol. 2012;590:3647–3663. doi: 10.1113/jphysiol.2012.233189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behrman AL, Harkema SJ. Physical rehabilitation as an agent for recovery after spinal cord injury. Phys Med Rehabil Clin N Am. 2007;18:183–202. doi: 10.1016/j.pmr.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Klomjai W, Katz R, Lackmy-Vallée A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS) Ann Phys Rehabil Med. 2015;58:208–213. doi: 10.1016/j.rehab.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation. Nat Rev Neurosci. 2007;8:559–567. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- 7.Gunduz A, Rothwell J, Vidal J, Kumru H. Non-invasive brain stimulation to promote motor and functional recovery following spinal cord injury. Neural Regen Res. 2017;12:1933–1938. doi: 10.4103/1673-5374.221143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tazoe T, Perez MA. Effects of repetitive transcranial magnetic stimulation on recovery of function after spinal cord injury. S145-55Arch Phys Med Rehabil. 2015;96(4 Suppl) doi: 10.1016/j.apmr.2014.07.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellaway PH, Vásquez N, Craggs M. Induction of central nervous system plasticity by repetitive transcranial magnetic stimulation to promote sensorimotor recovery in incomplete spinal cord injury. Front Integr Neurosci. 2014;8:42–42. doi: 10.3389/fnint.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belci M, Catley M, Husain M, Frankel HL, Davey NJ. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord. 2004;42:417–419. doi: 10.1038/sj.sc.3101613. [DOI] [PubMed] [Google Scholar]
- 11.Tolmacheva A, Savolainen S, Kirveskari E, Brandstack N, Mäkelä JP, Shulga A. Paired associative stimulation improves hand function after non-traumatic spinal cord injury: A case series. Clin Neurophysiol Pract. 2019;4:178–183. doi: 10.1016/j.cnp.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi H, Seo KC, Kim TU, Lee SJ, Hyun JK. Repetitive transcranial magnetic stimulation enhances recovery in central cord syndrome patients. Ann Rehabil Med. 2019;43:62–73. doi: 10.5535/arm.2019.43.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dongés SC, Boswell-Ruys CL, Butler JE, Taylor JL. The effect of paired corticospinal-motoneuronal stimulation on maximal voluntary elbow flexion in cervical spinal cord injury: An experimental study. Spinal Cord. 2019;57:796–804. doi: 10.1038/s41393-019-0291-3. [DOI] [PubMed] [Google Scholar]
- 14.Gharooni AA, Nair KPS, Hawkins D, Scivill I, Hind D, Hariharan R. Intermittent theta-burst stimulation for upper-limb dysfunction and spasticity in spinal cord injury: A single-blind randomized feasibility study. Spinal Cord. 2018;56:762–768. doi: 10.1038/s41393-018-0152-5. [DOI] [PubMed] [Google Scholar]
- 15.Gomes-Osman J, Field-Fote EC. Improvements in hand function in adults with chronic tetraplegia following a multiday 10-Hz repetitive transcranial magnetic stimulation intervention combined with repetitive task practice. J Neurol Phys Ther. 2015;39:23–30. doi: 10.1097/NPT.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuppuswamy A, Balasubramaniam AV, Maksimovic R, Mathias CJ, Gall A, Craggs MD, et al. Action of 5 Hz repetitive transcranial magnetic stimulation on sensory, motor and autonomic function in human spinal cord injury. Clin Neurophysiol. 2011;122:2452–2461. doi: 10.1016/j.clinph.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Alexeeva N, Calancie B. Efficacy of QuadroPulse rTMS for improving motor function after spinal cord injury: Three case studies. J Spinal Cord Med. 2016;39:50–57. doi: 10.1179/2045772314Y.0000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shulga A, Lioumis P, Zubareva A, Brandstack N, Kuusela L, Kirveskari E, et al. Long-term paired associative stimulation can restore voluntary control over paralyzed muscles in incomplete chronic spinal cord injury patients. Spinal Cord Ser Cases. 2016;2:16016–16016. doi: 10.1038/scsandc.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calabrò RS, Naro A, Leo A, Bramanti P. Usefulness of robotic gait training plus neuromodulation in chronic spinal cord injury: A case report. J Spinal Cord Med. 2017;40:118–121. doi: 10.1080/10790268.2016.1153275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tolmacheva A, Savolainen S, Kirveskari E, Lioumis P, Kuusela L, Brandstack N, et al. Long-term paired associative stimulation enhances motor output of the tetraplegic hand. J Neurotrauma. 2017;34:2668–2674. doi: 10.1089/neu.2017.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benito J, Kumru H, Murillo N, Costa U, Medina J, Tormos JM, et al. Motor and gait improvement in patients with incomplete spinal cord injury induced by high-frequency repetitive transcranial magnetic stimulation. Top Spinal Cord Inj Rehabil. 2012;18:106–112. doi: 10.1310/sci1802-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumru H, Benito-Penalva J, Valls-Sole J, Murillo N, Tormos JM, Flores C, et al. Placebo-controlled study of rTMS combined with Lokomat® gait training for treatment in subjects with motor incomplete spinal cord injury. Exp Brain Res. 2016;234:3447–3455. doi: 10.1007/s00221-016-4739-9. [DOI] [PubMed] [Google Scholar]
- 23.Säisänen L, Julkunen P, Niskanen E, Danner N, Hukkanen T, Lohioja T, et al. Motor potentials evoked by navigated transcranial magnetic stimulation in healthy subjects. J Clin Neurophysiol. 2008;25:367–372. doi: 10.1097/WNP.0b013e31818e7944. [DOI] [PubMed] [Google Scholar]
- 24.Jung P, Ziemann U. Homeostatic and nonhomeostatic modulation of learning in human motor cortex. J Neurosci. 2009;29:5597–5604. doi: 10.1523/JNEUROSCI.0222-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham WC. Metaplasticity: Tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387–387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 26.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011) J Spinal Cord Med. 2011;34:535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ditunno JF Jr, Barbeau H, Dobkin BH, Elashoff R, Harkema S, Marino RJ, et al. Validity of the walking scale for spinal cord injury and other domains of function in a multicenter clinical trial. Neurorehabil Neural Repair. 2007;21:539–550. doi: 10.1177/1545968307301880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craven BC, Morris AR. Modified Ashworth scale reliability for measurement of lower extremity spasticity among patients with SCI. Spinal Cord. 2010;48:207–213. doi: 10.1038/sc.2009.107. [DOI] [PubMed] [Google Scholar]
- 29.Davey NJ, Smith HC, Wells E, Maskill DW, Savic G, Ellaway PH, et al. Responses of thenar muscles to transcranial magnetic stimulation of the motor cortex in patients with incomplete spinal cord injury. J Neurol Neurosurg Psychiatry. 1998;65:80–87. doi: 10.1136/jnnp.65.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiscock A, Miller S, Rothwell J, Tallis RC, Pomeroy VM. Informing dose-finding studies of repetitive transcranial magnetic stimulation to enhance motor function: A qualitative systematic review. Neurorehabil Neural Repair. 2008;22:228–249. doi: 10.1177/1545968307307115. [DOI] [PubMed] [Google Scholar]
- 31.Nardone R, Höller Y, Brigo F, Orioli A, Tezzon F, Schwenker K, et al. Descending motor pathways and cortical physiology after spinal cord injury assessed by transcranial magnetic stimulation: A systematic review. Brain Res. 2015;1619:139–154. doi: 10.1016/j.brainres.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 32.Hou J, Nelson R, Nissim N, Parmer R, Thompson FJ, Bose P. Effect of combined treadmill training and magnetic stimulation on spasticity and gait impairments after cervical spinal cord injury. J Neurotrauma. 2014;31:1088–1106. doi: 10.1089/neu.2013.3096. [DOI] [PubMed] [Google Scholar]
- 33.Duan R, Qu M, Yuan Y, Lin M, Liu T, Huang W, et al. Clinical benefit of rehabilitation training in spinal cord injury: A systematic review and meta-analysis. E398-410Spine (Phila Pa 1976) 2021;46 doi: 10.1097/BRS.0000000000003789. [DOI] [PubMed] [Google Scholar]
- 34.Kirshblum S, Millis S, McKinley W, Tulsky D. Late neurologic recovery after traumatic spinal cord injury. Arch Phys Med Rehabil. 2004;85:1811–1817. doi: 10.1016/j.apmr.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Kumru H, Murillo N, Samso JV, Valls-Sole J, Edwards D, Pelayo R, et al. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair. 2010;24:435–441. doi: 10.1177/1545968309356095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schecklmann M, Schmaußer M, Klinger F, Kreuzer PM, Krenkel L, et al. Resting motor threshold and magnetic field output of the figure-of-8 and the double-cone coil. Sci Rep. 2020;10:1644–1644. doi: 10.1038/s41598-020-58034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desbeaumes Jodoin V, Miron JP, Lesperance P, Montplaisir L. Significant differences in motor threshold between figure-8 and double-cone coils for repetitive transcranial magnetic stimulation in patients with refractory depression. Eur J Psychiatry. 2018;32:195–196. [Google Scholar]