Abstract
A standard bismuth quadruple therapy, a fluoroquinolone-containing triple (or quadruple) therapy or a proton pump inhibitor (PPI)-amoxicillin high-dose dual therapy has been recommended as a second-line treatment for Helicobacter pylori infection by the Maastricht VI/Florence Consensus Report. The major shortcoming of levofloxacin-amoxicillin triple therapy is low cure rate for eradicating levofloxacin-resistant strains. With the rising prevalence of levofloxacin-resistant strains, levofloxacin-amoxicillin triple therapy cannot reliably achieve a high eradication rate for second-line treatment of H. pylori infection in most countries now. The present article aims to review current second-line eradication regimens with a per-protocol eradication rate exceeding 85% in most geographic areas. Recently, a novel tetracycline-levofloxacin quadruple therapy consisting of a PPI, bismuth, tetracycline, and levofloxacin for rescue treatment of H. pylori infection has been developed. The new therapy achieved a higher per-protocol eradication rate than levofloxacin-amoxicillin triple treatment in a randomized controlled trial (98% versus 69%). Additionally, the tetracycline-levofloxacin quadruple therapy also exhibits a higher eradication rate than amoxicillin-levofloxacin quadruple therapy. High-dose dual PPI-amoxicillin therapy is another novel second-line treatment for H. pylori infection. The new therapy can achieve an eradication rate of 89% by per-protocol analysis for the second-line treatment in Taiwan. Recently, levofloxacin-based sequential quadruple therapy and potassium-competitive acid blocker have also been applied in the second-line treatment of H. pylori infection. A meta-analysis revealed that a vonoprazan-based regimen has significant superiority over a PPI-based regimen for second-line H. pylori eradication therapy. In conclusion, the eradication rate of levofloxacin-amoxicillin triple therapy is suboptimal in the second-line treatment of H. pylori infection now. Currently, a standard bismuth quadruple therapy (tetracycline-metronidazole quadruple therapy), a tetracycline-levofloxacin quadruple therapy, an amoxicillin-levofloxacin quadruple therapy, a levofloxacin-based sequential quadruple therapy or a high-dose PPI-amoxicillin dual therapy is recommended for the second-line treatment of H. pylori infection.
Keywords: amoxicillin-levofloxacin quadruple therapy, bismuth quadruple therapy, Helicobacter pylori, high-dose dual therapy, second-line treatment, tetracycline-levofloxacin quadruple therapy
Introduction
Helicobacter pylori is an important pathogen that can induce chronic gastritis, peptic ulcer, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma.1–3 Currently, the eradication rate using standard triple therapy for H. pylori has decreased to less than 80% worldwide.4,5 The main causes of eradication failure by standard triple therapy include antibiotic resistance, poor adherence, low gastric pH, a high bacterial load and the rapid metabolism of proton pump inhibitor (PPI).4–6 Recently, several strategies including bismuth quadruple therapy, concomitant therapy, sequential therapy, hybrid therapy, reverse hybrid therapy, high-dose dual therapy, and vonoprazan-based therapy have been proposed to improve the eradication rate of H. pylori infection,7–9 but H. pylori eradication still fails in 3–24% of infected patients.8–11 This present article aims to review current second-line eradication regimens with a per-protocol eradication rate exceeding 85% in most geographic areas.
Current antibiotic resistance in the second-line treatment of H. pylori infection
Antibiotic resistance is a critical factor predicting the outcome of eradication therapy.12,13 Therefore, empiric second-line treatment for H. pylori infection should be guided by local resistance profiles of the pathogen and cure rates of eradication therapies. Previous reports reveal the rates of antibiotic resistances to clarithromycin, amoxicillin, metronidazole, tetracycline and levofloxacin were 56–71%, 0–8%, 35–74%, 0–4%, and 21–43%, respectively.14–19 The data suggest that amoxicillin and tetracycline are effective antibiotics for use in the second-line anti-H. pylori therapy. A recent study investigated the trend of annual antibiotic resistance rates in the treatment of H. pylori infection from 2013 to 2019 in Taiwan. 16 The report showed that progressively higher resistance rates were observed for levofloxacin (from 37% to 52%) and metronidazole (from 41% to 77%) among patients who received second-line eradication therapy. The trend of changes in antibiotic resistances has great impact on the eradication efficacy of salvage regimens containing levofloxacin and metronidazole for the treatment of H. pylori infection.
Second-line therapies for H. pylori infection
A standard bismuth quadruple therapy, a fluoroquinolone-containing quadruple (or triple) therapy or a PPI-amoxicillin high-dose dual therapy have been recommended as second-line treatment for H. pylori infection by the Maastricht VI/Florence Consensus Report. 18 Recently, tetracycline-levofloxacin quadruple therapy and vonoprazan-based therapy have proposed to increase the eradication rate.14,20 Table 1 lists the regimens and durations of these recommended second-line therapies.
Table 1.
Regimens for second-line anti-H. pylori therapy.
| Regimens | Drug | Duration of therapy (days) | |||||
|---|---|---|---|---|---|---|---|
| PPI | Bismuth | Lev | Amo | Tet | Met | ||
| Standard bismuth quadruple therapy | SD, bid |
300 mg qid |
500 mg qid |
500 mg tid |
10–14 | ||
| Fluoroquinolone-amoxicillin triple therapy |
SD, bid |
500 mg qd |
1 g, bid |
14 | |||
| Fluoroquinolone-amoxicillin quadruple therapy |
SD, bid |
300 mg qid |
500 mg qd |
500 mg, qid |
14 | ||
| Levofloxacin-tetracycline quadruple therapy |
SD, bid |
300 mg qid |
500 mg qd |
500 mg qid |
10–14 | ||
| High-dose dual therapy | SD, qid |
750 mg, qid | 14 | ||||
Amo, amoxicillin; Bismuth, tripotassium dicitrato bismuthate; Lev, levofloxacin; Met, metronidazole; PPI, proton pump inhibitor; SD, standard dose; Tet, tetracycline.
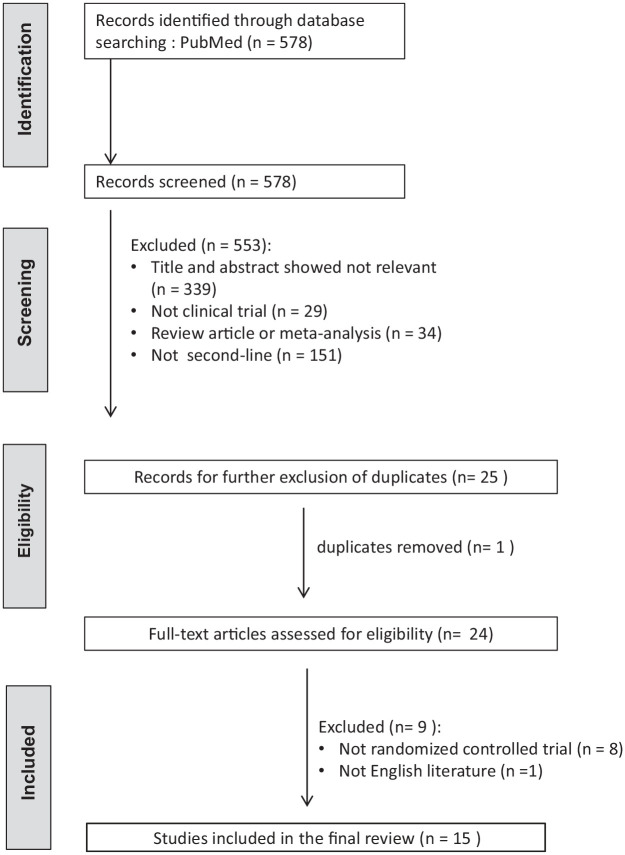
To investigate the efficacies of current second-line eradication regimens, randomized controlled trials published in English language articles reporting the eradication rates of second-line anti-H. pylori therapy were eligible for literature search. The status of H. pylori infection before and after treatment should be verified by at least one of the following tests: urea breath test, rapid urease test, histology or culture. The post-treatment H. pylori status should be assessed at least 4 weeks after eradication therapies. Medical literatures were searched from PubMed (1 March 2013–1 March 2023). We identified the eligible literatures with the keywords of ‘Helicobacter pylori’ or ‘H. pylori’ or ‘H pylori’, and ‘therapy’ or ‘treatment’. ‘Clinical trial’ was set as filter. The titles and abstracts were screened by CAS and CBS to exclude irrelevant studies. The full articles of potentially eligible studies were further reviewed by CAS and CBS. Literatures published in the abstract form only were excluded. Studies that did not report the results according to the intention-to-treat and per-protocol analyses were also excluded. Two investigators, CAS and CBS, reviewed the literatures under the above criteria independently using pre-designed data extraction form. Disagreements were resolved through discussion with PIH to reach consensus. Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the literature search. Table 2 summarizes the intention-to-treat and per-protocol eradication rates of various H. pylori eradication regimens including standard bismuth quadruple therapy (tetracycline-metronidazole quadruple therapy), fluoroquinolone-amoxicillin triple therapy, fluoroquinolone-amoxicillin quadruple therapy, tetracycline-fluoroquinolone quadruple therapy, high-dose dual therapy, levofloxacin-based sequential therapy, tetracycline-metronidazole-amoxicillin concomitant therapy, furazolidone-tetracycline quadruple therapy, furazolidone-amoxicillin quadruple therapy, amoxicillin-clarithromycin-metronidazole tetracycline quintuple therapy, and tetracycline-metronidazole-ofloxacin quintuple therapy.14,19,21–33
Figure 1.
PRISMA diagram of the literature search.
Table 2.
Eradication rates of second-line anti-H. pylori therapies in the randomized controlled trials from 2013 to 2023.
| Type of therapy | Country | Population | Previous treatment | Regimen | Eradication rate | |
|---|---|---|---|---|---|---|
| ITT | PP | |||||
| Standard bismuth quadruple therapy (Tetracycline-metronidazole quadruple therapy) | Taiwan 19 | Adult patients with dyspepsia (n = 74) | Standard triple therapy | Esomeprazole 40 mg bid, bismuth 300 mg qid, tetracycline 500 mg qid, metronidazole 500 mg qid |
80% | 91% |
| Taiwan 21 | Adult patients (n = 261) |
Clarithromycin-based therapy | Esomeprazole 40 mg bid bismuth 300 mg qid tetracycline 500 mg qid metronidazole 500 mg tid for 10 days |
88% | 93% | |
| Israel 22 | Adult patients (n = 51) |
Clarithromycin- triple therapy | Lansoprazole 30 mg bid bismuth 525 mg qid metronidazole 500 mg tid tetracycline 500 mg qid /doxycycline 100 mg bid for 14 days |
43% | 69% | |
| Taiwan 23 | Adult patients (n = 63) |
Standard triple therapy | Rabeprazole 20 mg bid, bismuth 120 mg qid, tetracycline 500 mg qid, metronidazole 250 mg qid for 10 days |
92% | 93% | |
| China 24 | Adult patients with dyspepsia (n = 143) |
Clarithromycin-based triple or quadruple therapy | Lansoprazole 30 mg bid, bismuth 240 mg bid, tetracycline 500 mg qid, metronidazole 400 mg qid for 14 days |
85% | 91% | |
| Fluoroquinolone- amoxicillin Triple therapy |
Taiwan 14 | Adult patients (n = 52) |
Clarithromycin triple, non-bismuth quadruple or bismuth quadruple therapy | Esomeprazole 40 mg bid, amoxicillin 500 mg qid, levofloxacin 500 mg qd for 10 days |
69% | 69% |
| China 26 | Adult patients (n = 89) |
Clarithromycin-based or metronidazole-based triple or bismuth quadruple therapy | Esomeprazole 20 mg bid, amoxicillin 1000 mg bid, antofloxacin 200 mg qd for 14 days |
88% | 91% | |
| China 26 | Adult patients (n = 89) |
Clarithromycin-based or metronidazole-based triple or bismuth quadruple therapy | Esomeprazole 20 mg bid, amoxicillin 1000 mg bid, levofloxacin 500 mg qd for 14 days |
69% | 70% | |
| Taiwan 27 | Adult patients with dyspepsia (n = 34) |
Standard triple therapy | Rabeprazole 20 mg bid, amoxicillin 1000 mg bid, levofloxacin 500 mg qd for 10 days |
68% | 67% | |
| Taiwan 28 | Adult patients (n = 82) |
Standard triple, therapy | Esomeprazole 40 mg bid, amoxicillin 1000 mg bid levofloxacin 500 mg qd, for 10 days |
81% | 82% | |
| Taiwan 30 | Adult patients with chronic gastritis or peptic ulcers (n = 56) |
(not described) | Rabeprazole 20 mg bid, amoxicillin 1000 mg bid levofloxacin 500 mg qd, for 7 days |
79% | 79% | |
| Fluoroquinolone- amoxicillin quadruple therapy |
Taiwan 25 | Adult patients (n = 56) |
Standard triple, non-bismuth quadruple and bismuth quadruple therapies) | Esomeprazole 40 mg bid, bismuth 300 mg qid amoxicillin 1000 mg qid levofloxacin 500 mg qd for 10 days |
69% | 69% |
| Taiwan 27 | Adult patients with dyspepsia (n = 33) |
Standard triple therapy | Rabeprazole 20 mg bid, bismuth 120 mg qid amoxicillin 1000 mg bid levofloxacin 500 mg qd for 10 days |
85% | 84% | |
| Taiwan 30 | Adult patients (n = 300) |
Clarithromcyin-based therapies | Lansoprazole 30 mg bid, amoxicillin 1000 mg bid levofloxacin 500 mg qd, for 10 days |
75% | 79% | |
| China 24 | Adult patients with dyspepsia (n = 141) | Clarithromycin-based triple or quadruple therapy | Lansoprazole 30 mg bid, bismuth 240 mg bid, amoxicillin, 1000 mg bid, levofloxacin 500 mg qd |
83% | 88% | |
| Tetracycline-fluoroquinolone quadruple therapy | Taiwan 19 | Adult patients with dyspepsi a (n = 76) | Standard triple therapy | Esomeprazole 40 mg bid, bismuth 300 mg qid, tetracycline 500 mg qid, levofloxacin 500 mg qd for 10 days |
79% | 87% |
| Taiwan 14 | Adult patients (n = 50) |
Standard triple, non-bismuth quadruple and bismuth quadruple therapies | Esomeprazole 40 mg bid, bismuth 300 mg qid tetracycline 500 mg qid levofloxacin 500 mg qd for 10 days |
98% | 98% | |
| Taiwan 25 | Adult patients (n = 56) |
Standard triple, non-bismuth quadruple and bismuth quadruple therapies | Esomeprazole 40 mg bid, bismuth 300 mg qid tetracycline 500 mg qid levofloxacin 500 mg qd for 10 days |
89% | 89% | |
| Turkey 31 | Adult patients with peptic ulcer or non-ulcer dyspepsia (n = 75) |
Standard triple therapy | Pantoprazole 40 mg bid, bismuth 300 mg qid tetracycline 500 mg qid levofloxacin 500 mg qd for 10 days |
91% | 93% | |
| High-dose dual therapy | China 32 | Adult patients (n = 329) |
Standard triple, bismuth-containing quadruple therapy, or non-bismuth quadruple therapy | Esomeprazole 40 mg tid, amoxicillin 1000 mg tid for 14 days |
75% | 81% |
| Taiwan 29 | Adult patients (n = 56) |
(not described) | Rabeprazole 20 mg qid, amoxicillin 750 mg qid for 14 days |
89% | 89% | |
| Levofloxacin- based sequential quadruple therapy |
Taiwan 21 | Adult patients (n = 280) | Clarithromycin-based therapy | Esomeprazole 40 mg bid and amoxicillin 1000 mg bid for 7 days, then esomeprazole 40 mg bid, metronidazole 500 mg bid, and levofloxacin 250 mg for 7 days, |
88% | 90% |
| Taiwan 28 | adult patients (n = 82) |
standard triple, therapy | esomeprazole 40 mg bid, amoxicillin 1000 mg bid for 5 days, then esomeprazole 40 mg bid, levofloxacin 500 mg qd, metronidazole 500 mg tid for 5 days |
90% | 91% | |
| Taiwan 30 | Adult patients (n = 300) |
Clarithromcyin-based therapies | Lansoprazole 30 mg bid, amoxicillin 1000 mg bid for 5 days, then lansoprazole 30 mg bid, levofloxacin 500 mg qd, metronidazole 500 mg tid for 5 days |
84% | 86% | |
| Turkey 31 | Adult patients with peptic ulcer or non-ulcer dyspepsia (n = 70) | Standard triple therapy | Pantoprazole 40 mg bid, amoxicillin 1000 mg bid for 5 days, then pantoprazole 30 mg bid, levofloxacin 500 mg qd, metronidazole 500 mg tid for 5 days |
82% | 86% | |
| Tetracycline- metronidazole- amoxicillin concomitant therapy |
Taiwan 23 | Adult patients (n = 61) |
Standard triple therapy | Rabeprazole 20 mg bid, amoxicillin 1000 mg bid, tetracycline 500 mg qid, metronidazole 250 mg qid for 10 days |
90% | 89% |
| Furazolidone-tetracycline quadruple therapy | China 32 | Adult patients (n = 329) |
Standard triple, bismuth-containing quadruple therapy, or non-bismuth quadruple therapy | Esomeprazole 40 mg tid, bismuth 220 mg bid, furazolidone 100 mg bid, tetracycline 500 mg tid for 14 days |
78% | 85% |
| Amoxicillin-clarithromycin-metronidazole quintuple therapy |
Iran 33 | Adult patients with dyspepsia (n = 104) |
Non-bismuth quadruple therapy | Omeprazole 20 mg bid, bismuth 240 mg bid, amoxicillin 1000 mg bid, clarithromycin 500 mg bid, tinidazole 500 mg bid for 7 days |
76% | 76% |
| Tetracycline- metronidazole- ofloxacin quintuple therapy |
Iran 33 | Adult patients with dyspepsia (n = 104) |
Non-bismuth quadruple therapy | Omeprazole 20 mg bid, bismuth 240 mg bid, tetracycline 500 mg qid, metronidazole 500 mg bid, ofloxacin 200 mg bid for 7 days |
87% | 87% |
ITT, intention-to-treat; PP, per-protocol.
Standard bismuth quadruple therapy
The regimen of standard bismuth quadruple therapy comprises a PPI twice daily, a bismuth four times daily, tetracycline 500 mg four times daily and metronidazole 500 mg three times daily for 10–14 days. 21 A meta-analysis including 12 comparative trials demonstrated that bismuth quadruple therapy achieved an 89% cure rate [95% confidence interval (CI): 86%−93%, I 2 = 78%] in the second-line treatment for H. pylori infection. 34 Hp-EuReg found that bismuth quadruple therapy could achieve an optimal eradication (≧90%) after failure of first-line clarithromycin-containing therapies, but cure rate of levofloxacin-based triple therapy was suboptimal. 35 A recent 15-year prospective study from Korea showed that the overall per-protocol eradication rate of 14-day bismuth-containing quadruple therapy in second-line treatment was 89.5% (95% CI: 86.3–92.7%). 36 Despite high rates of metronidazole resistance, no significant decline in annual eradication rates was observed during the study period. Therefore, bismuth-containing quadruple therapy is a pivotal second-line treatment for H. pylori infection, especially in areas with high quinolone resistance. Several recent studies revealed that treatment duration is an important factor related to the eradication efficacy of bismuth quadruple therapy. 36 A recent randomized controlled trial study also showed a per-protocol eradication rate of 77.2% versus 93.6% between the 7-day and 14-day groups that received a bismuth-containing quadruple therapy as second-line treatment. 37 Therefore, it is reasonable to encourage bismuth-containing quadruple therapy for 14 days in the second-line treatment of H. pylori infection.
Fluoroquinolone-based triple and quadruple therapies
The most commonly used fluoroquinolone-based triple therapy is composed of a PPI (standard dose) twice daily, amoxicillin 1 gm twice daily, and levofloxacin 500 mg daily for 7–14 days.27,37–39 Several previous meta-analyses have demonstrated that fluoroquinolone-based triple therapy and bismuth quadruple therapy have comparable eradication rates, and the former induces fewer adverse events than the latter in patients receiving first-line eradication treatment with PPI-clarithromycin-amoxicillin triple regimens. 39 However, an increased prevalence of levofloxacin resistance in the second-line treatment of H. pylori infection has been reported,16,38 reducing the efficacies of levofloxacin-containing regimens. A systemic review and meta-analysis showed that levofloxacin-amoxicillin triple therapy achieved an overall eradication rate of 77% (95% CI: 75−80%, I 2 = 68%) in the salvage treatment of H. pylori infection. 40 Several studies revealed that the cure rate of levofloxacin triple therapy was markedly reduced in facing levofloxacin-resistant strains.40,41 In a systemic review and meta-analysis, 40 levofloxacin-amoxicillin triple therapy achieved an eradication rate of 81% (95% CI: 68–90%) for levofloxacin-sensitive strains, but the cure rate dropped to 36% (95% CI: 25−49%) for levofloxacin-resistant strains.
Bismuth salts have synergistic effects on antibiotics and have been used to increase eradication efficacy. 42 A randomized controlled trial demonstrated that a 14-day PPI-bismuth-levofloxacin-amoxicillin quadruple therapy had a superior eradication rate for levofloxacin-resistant strains than a 14-day PPI-levofloxacin-amoxicillin triple therapy (71% versus 37%) in the treatment of H. pylori infection. 43 Another randomized controlled trial showed that a 14-day PPI-bismuth-amoxicillin-levofloxacin quadruple therapy and classical bismuth-containing quadruple therapy had comparable eradication rate (intention-to-treat: 83% versus 88%; per-protocol: 85% versus 91% in the second-line treatment of H. pylori infection. 24
PPI-amoxicillin high-dose dual therapy
High-dose dual therapy is another novel second-line treatment for H. pylori infection. 29 The new therapy includes high-dose PPI and amoxicillin (Table 1), which maintain gastric pH above 6.5 and stabilize plasma concentrations of amoxicillin above the minimum inhibitory concentration of H. pylori regardless of CYP2C19 genotype. 44 A randomized controlled trial showed that a 14-day high-dose dual therapy and a 7-day levofloxacin-amoxicillin triple therapy had comparable eradication rate (89% versus 79%) in the second-line treatment. 29 Another randomized controlled trial from Germany also demonstrated that a 14-day high-dose dual therapy and a 14-day bismuth-containing quadruple therapy had comparable efficacy in the rescue treatment of H. pylori infection (76% versus 81%). 45
Tetracycline–levofloxacin quadruple therapy
Recently, a novel tetracycline-levofloxacin quadruple therapy has developed for rescue treatment of H. pylori infection. 46 It consists of esomeprazole 40 mg b.i.d, tripotassium dicitrato bismuthate 300 mg q.i.d., tetracycline 500 mg q.i.d., and levofloxacin 500 mg q.d. for 10–14 days. The pilot study of tetracycline-levofloxacin quadruple therapy demonstrated that the novel therapy could achieve a very high eradication rate (95.8%) in the second-line treatment of H. pylori infection. 14 Table 3 lists the comparative randomized controlled trials of tetracycline-levofloxacin quadruple therapy from 2013 to 2023. A randomized controlled trial demonstrated that the cure rate of a 10-day tetracycline-levofloxacin quadruple therapy was markedly higher than that of a 10-day PPI-amoxicillin-levofloxacin triple therapy in the second-line treatment for H. pylori infection (98.0% versus 69.2%). 14 Another randomized controlled study showed that a 10-day tetracycline-levofloxacin quadruple therapy was more effective than a 10-day amoxicillin-levofloxacin quadruple therapy (per-protocol analysis: 89% versus 70%) in the second-line treatment of H. pylori infection in a population with high levofloxacin resistance (51%). 25 The novel therapy can maintain a high eradication rate against levofloxacin-resistant strains (93%) for the salvage treatment of H. pylori infection.14,25 Recently, a randomized controlled trial also demonstrated that a 10-day tetracycline-levofloxacin quadruple therapy and a 10-day standard bismuth quadruple therapy have comparable efficacy (per-protocol analysis: 87% versus 91%) in second-line treatment of H. pylori infection. 19 These findings suggest that tetracycline-levofloxacin quadruple therapy can reliably provide a per-protocol eradication >85%, and is a good option in the rescue treatment of H. pylori infection.
Table 3.
Comparison of tetracycline-levofloxacin quadruple therapy and other recommended therapies in randomized control trials from 2013 to 2023.
| Author (year) | No. of cases | Duration, day | Regimens | Eradication rate | |
|---|---|---|---|---|---|
| Intention-to-treat | Per-protocol | ||||
| Kuo et al.
20
(2013) |
76 | 10 | esomeprazole 40 mg bid, bismuth subcitrate 300 mg qid, tetracycline 500 mg qid, levofloxacin 500 mg qd |
78.9% (60/76) | 87.0% (60/69) |
| 74 | 10 | esomeprazole 40 mg bid, bismuth subcitrate 300 mg qid, tetracycline 500 mg qid, metronidazole 500 mg qid |
79.7% (59/74) | 90.8% (59/65) | |
| Calhan et al.
32
(2013) |
75 | 10 | pantoprazole 40 mg bid, bismuth subcitrate 300 mg qid, tetracycline 500 mg qid, levofloxacin 500 mg qd |
90.6% (68/75) | 93.1% (68/73) |
| 73 | 10 | pantoprazole 40 mg bid, amoxicillin 1000 mg bid for 5 days, then pantoprazole 30 mg bid, levofloxacin 500 mg qd, metronidazole 500 mg tid for 5 days |
82.2% (60/73) | 85.7% (60/70) | |
| Hsu et al.
15
(2017) |
50 | 10 | esomeprazole 40 mg bid, bismuth subcitrate 300 mg qid, tetracycline 500 mg qid, metronidazole 500 mg qid |
98.0% (49/50) a | 97.8% (44/45) b |
| 52 | 10 | esomeprazole 40 mg bid, amoxicillin 500 mg qid, levofloxacin 500 mg qd |
69.2% (36/52) a | 68.6% (35/51) b | |
| Hsu et al.
26
(2021) |
56 | 10 | esomeprazole 40 mg bid, tripotassium dicitrato bismuthate 300 mg qid, tetracycline 500 mg qid, levofloxacin 500 mg qd |
89.3% (50/56) c | 89.1% (49/55) d |
| 56 | 10 | esomeprazole 40 mg bid, tripotassium dicitrato bismuthate 300 mg qid, tetracycline 500 mg qid, metronidazole 250 mg qid |
69.6% (39/56) c | 69.8% (37/53) d | |
p < 0.001. bp < 0.001. cp = 0.010. dp = 0.013.
Vonoprazan-based second-line therapy
Vonoprazan is a potassium-competitive acid blocker (P-CAB). The new gastric acid suppression agent inhibits gastric acid secretion via reversible suppressing H+/K+-ATPase. Its efficacy of acid inhibition is superior to that of PPI. 40 Vonoprazan is majorly metabolized through CYP 3A4 in the liver and partially by SULT2A1, CYP2C19, CYP2B6, and CYP2D6.47,48 Several randomized controlled studies and non-randomized controlled studies demonstrated that a 7-day vonoprazan-based triple therapy was superior to a 7-day PPI-based triple therapy in the first-line H. pylori treatment.20,49 However, a randomized controlled trial from Japan was conducted to compare the efficacies of vonoprazan-amoxicillin-metronidazole and rabeprazole-amoxicillin-metronidazole triple therapies in second-line treatment. 50 The results showed no significant differences in eradication rates (90% versus 85% by per-protocol analysis) between the two second-line therapies. Nonetheless, a meta-analysis of non-RCT studies by Shinozaki et al. showed that a vonoprazan-based regimen has significant superiority over a PPI-based regimen for second-line H. pylori eradication therapy. 51 The current Japanese guidelines on the management of H. pylori infections recommend replacing PPI with vonoprazan for first-line and second-line eradication therapies. 52
Levofloxacin-based sequential quadruple therapy
Recently, a 14-day treatment regimen has been developed to improve eradication rate in the second-line anti-H. pylori therapy. A multicentre, randomized controlled trial from Taiwan investigated the efficacies of a 14-day levofloxacin-based sequential quadruple therapy (esomeprazole 40 mg and amoxicillin 1 g for 7 days, followed by esomeprazole 40 mg, metronidazole 500 mg, and levofloxacin 250 mg for 7 days, all twice-daily) and a 10-day bismuth-based quadruple therapy (esomeprazole 40 mg twice daily, bismuth tripotassium dicitrate 300 mg four times a day, tetracycline 500 mg four times a day, and metronidazole 500 mg three times a day) in adult patients with persistent H. pylori infection after first-line clarithromycin-based therapy. 21 In per-protocol analysis, 246 (90%) of 273 participants in the levofloxacin-based sequential quadruple group and 245 (93%) of 264 participants in the bismuth-based quadruple achieved H. pylori eradication. There were no differences in eradication rates between groups. Another randomized controlled trial also confirmed that levofloxacin-based sequential quadruple therapy could achieve a high per-protocol eradication rate (93%) in Turkey. 31
Miscellaneous second-line therapies
Several other strategies have been developed to increase the eradication rates of H. pylori infection, including rifaburin-containing triple therapy, 53 tetracycline-metronidazole-amoxicillin concomitant therapy, 23 furazolidone-tetracycline quadruple therapy, 32 amoxicillin-clarithromycin-metronidazole quintuple therapy, 33 and tetracycline-metronidazole-ofloxacin quintuple therapy 33 (Table 2). Fiorini et al. applied a 12-day PPI-amoxicillin-rifabutin triple therapy (esomeprazole 40 mg bid, amoxicillin 1 g bid, and rifabutin 150 mg qd) to treat 55 dyspeptic patients with eradication failure by first-line anti-H. pylori therapy who harbored triple-resistant (clarithromycin, metronidazole, levofloxacin) strains. 53 The novel therapy could achieve a very high per-protocol eradication rate (92%) in second-line treatment of H. pylori infection for patients infected with multidrug-resistant strains. A 7-day once-daily triple therapy with esomeprazole, moxifloxacin, and rifabutin had also been used for second-line therapy of H. pylori infection. 54 The per-protocol eradication rate in 24 patients with eradication failure by first-line treatment was 78% (61%−95%). Recently, rifabutin-containing triple therapies were also applied in the third-line rescue treatment for multidrug-resistant strains of H. pylori.55,56 In countries where bismuth is unavailable, tetracycline-metronidazole-amoxicillin concomitant therapy could be a valid treatment for H. pylori infection after failure of first-line therapy. A randomized controlled trial demonstrated that a 10-day tetracycline-metronidazole-amoxicillin concomitant therapy and a 10-day standard bismuth quadruple therapy had comparable efficacy (per-protocol analysis: 89% versus 93%) in second-line treatment of H. pylori infection. 23 The data suggested that the novel concomitant therapy could be an alternative second-line treatment in countries where bismuth is unavailable.
Treatment algorithms in the second-line therapy for H. pylori infection
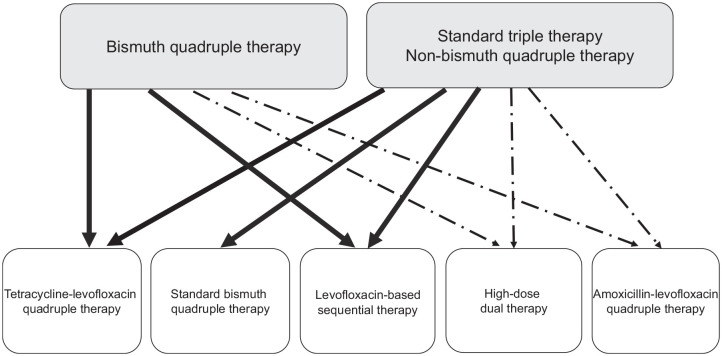
Figure 2 shows the treatment algorithm in current second-line therapies for H. pylori infection. With the rising prevalence of levofloxacin resistance, the eradication rate of levofloxacin-amoxicillin triple therapy cannot reliably achieve an eradication rate >85% for second-line treatment of H. pylori infection in most countries.40,41 Standard bismuth quadruple therapy, tetracycline-levofloxacin quadruple therapy, levofloxacin-based sequential quadruple therapy, amoxicillin-levofloxacin quadruple therapy, and high-dose dual therapy can maintain a high eradication rate in the era of high levofloxacin resistance. Therefore, it is reasonable to recommend the five rescue treatments after failure of clarithromycin-containing triple therapy or non-bismuth quadruple therapy. In H. pylori-infected patients with treatment failure by bismuth quadruple therapy, tetracycline-levofloxacin quadruple therapy, levofloxacin-based sequential quadruple therapy, amoxicillin-levofloxacin quadruple therapy and high-dose dual therapy can be recommended for rescue treatment of H. pylori infection.
Figure 2.
Algorithm of second-line therapy for Helicobacter pylori infection. The solid lines denote second-line therapy with a per-protocol eradication rate exceeding 85% in most geographic areas. The dashed lines denote second-line therapy with a per-protocol eradication rate exceeding 85% in some geographic areas.
Conclusion
With the rising prevalence of levofloxacin resistance, the eradication rate of levofloxacin-amoxicillin triple therapy is suboptimal in the second-line treatment of H. pylori infection now. Currently, a standard bismuth-containing quadruple therapy, a tetracycline-levofloxacin quadruple therapy, a levofloxacin-based sequential quadruple therapy, an amoxicillin-levofloxacin quadruple therapy, or a high-dose dual therapy is recommended for the second-line treatment of H. pylori infection.
Acknowledgments
None.
Footnotes
ORCID iD: Seng-Kee Chuah  https://orcid.org/0000-0002-8934-3223
https://orcid.org/0000-0002-8934-3223
Contributor Information
Chih-An Shih, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Antai Medical Care Corporation, Antai Tian-Sheng Memorial Hospital, Pingtung County; Department of Nursing, Meiho University, Pingtung County.
Chang-Bih Shie, Division of Gastroenterology, Department of Internal Medicine, An Nan Hospital, China Medical University, Tainan.
Wei-Chen Tai, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, and Chang Gung University College of Medicine, Taoyuan.
Seng-Kee Chuah, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, and Chang Gung University College of Medicine, Taoyuan.
Hsi-Chang Lee, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Renai Branch, Taipei City Hospital, 10, Section 4, Ren’ai Road, Da’an District 106, Taipei.
Ping-I Hsu, Division of Gastroenterology and Hepatology, Department of Internal Medicine, An Nan Hospital, China Medical University, No. 66, Sec. 2, Changhe Road., Annan Dist., Tainan City 70965.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Chih-An Shih: Writing – original draft.
Chang-Bih Shie: Writing – original draft.
Wei-Chen Tai: Writing – review & editing.
Seng-Kee Chuah: Writing – review & editing.
Hsi-Chang Lee: Writing – review & editing.
Ping-I Hsu: Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the Ministry of Science and Technology, Executive Yuan, Taiwan, ROC (Grant numbers: MOST 109-2314-B075B-007, MOST 110-2314-B-039-045 and MOST 111-2314-B-039-066), and the An Nan Hospital, China Medical University (Grant Numbers: ANHRF110-18, ANHRF ANHRF111-34 and ANHRF 111-44).
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology 2007; 133: 985–1001. [DOI] [PubMed] [Google Scholar]
- 2. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002; 347: 1175–1186. [DOI] [PubMed] [Google Scholar]
- 3. Zucca E, Dreyling M; ESMO Guidelines Working Group. Gastric marginal zone lymphoma of MALT type: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009; 20(Suppl. 4): 113–114. [DOI] [PubMed] [Google Scholar]
- 4. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010; 59: 1143–1153. [DOI] [PubMed] [Google Scholar]
- 5. Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology 2019; 157: 44–53. [DOI] [PubMed] [Google Scholar]
- 6. Flores-Treviño S, Mendoza-Olazarán S, Bocanegra-Ibarias P, et al. Helicobacter pylori drug resistance: therapy changes and challenges. Expert Rev Gastroenterol Hepatol 2018; 12: 819–827. [DOI] [PubMed] [Google Scholar]
- 7. Megraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 2004; 53: 1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hsu PI, Chen KY, Tai WC, et al. Hybrid, high-dose dual and bismuth quadruple therapies for first-line treatment of Helicobacter pylori infection in Taiwan: a multicenter, open-label, randomized trial. Am J Gastroenterol 2023; 118: 1184–1195. [DOI] [PubMed] [Google Scholar]
- 9. Gatta L, Vakil N, Leandro G, et al. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol 2009; 104: 3069–3079; quiz 1080. [DOI] [PubMed] [Google Scholar]
- 10. Sardarian H, Fakheri H, Hosseini V, et al. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter 2013; 18: 129–134. [DOI] [PubMed] [Google Scholar]
- 11. Molina–Infante J, Romano M, Fernandez–Bermejo M, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology 2013; 145: 121–128.e1. [DOI] [PubMed] [Google Scholar]
- 12. Camargo MC, García A, Riquelme A, et al. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Rom J Gastroenterol 2014; 109: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saleem N, Howden CW. Update on the management of Helicobacter pylori infection. Curr Treat Options Gastroenterol 2020; 18: 476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsu PI, Tsai FW, Kao SS, et al. Ten-Day quadruple therapy comprising proton pump inhibitor, bismuth, tetracycline, and levofloxacin is more effective than standard levofloxacin triple therapy in the second-line treatment of Helicobacter pylori infection: a randomized controlled trial. Am J Gastroenterol 2017; 112: 1374–1381. [DOI] [PubMed] [Google Scholar]
- 15. Wu IT, Chuah SK, Lee CH, et al. Five-year sequential changes in secondary antibiotic resistance of Helicobacter pylori in Taiwan. World J Gastroenterol 2015; 21: 10669–10674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang CM, Tai WC, Hsu PI, et al. Trend of changes in antibiotic resistance in Helicobacter pylori from 2013 to 2019: a multicentre report from Taiwan. Therap Adv Gastroenterol 2020; 13: 1756284820976990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gisbert JP, Molina-Infante J, Marin AC, et al. Second-line rescue triple therapy with levofloxacin after failure of non-bismuth quadruple “sequential” or “concomitant” treatment to eradicate H. pylori infection. Scand J Gastroenterol 2013; 48: 652–656. [DOI] [PubMed] [Google Scholar]
- 18. Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut 2022; 71: 1724–1762. [Google Scholar]
- 19. Kuo CH, Hsu PI, Kuo FC, et al. Comparison of 10 day bismuth quadruple therapy with high-dose metronidazole or levofloxacin for second-line Helicobacter pylori therapy: a randomized controlled trial. J Antimicrob Chemother 2013; 68: 222–228. [DOI] [PubMed] [Google Scholar]
- 20. Murakami K, Sakurai Y, Shiino M, et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy forHelicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 2016; 65: 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liou JM, Jiang XT, Chen CC, et al. Second-line levofloxacin-based quadruple therapy versus bismuth-based quadruple therapy for Helicobacter pylori eradication and long-term changes to the gut microbiota and antibiotic resistome: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol 2023; 8: 228–241. [DOI] [PubMed] [Google Scholar]
- 22. Munteanu D, Etzion O, Ben-Yakov G, et al. Efficacy and safety of sequential versus quadruple therapy as second-line treatment for helicobacter pylori infection—a randomized controlled trial. PLoS One 2017; 12: e0183302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jheng GH, Wu IC, Shih HY, et al. Comparison of second-line quadruple therapies with or without bismuth for Helicobacter pylori infection. Biomed Res Int 2015; 2015: 163960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cao Z, Chen Q, Zhang W, et al. Fourteen-day optimized levofloxacin-based therapy versus classical quadruple therapy for Helicobacter pylori treatment failures: a randomized clinical trial. Scand J Gastroenterol 2015; 50: 1185–1190. [DOI] [PubMed] [Google Scholar]
- 25. Hsu P, Tsay F, Kao JY, et al. Tetracycline-levofloxacin versus amoxicillin-levofloxacin quadruple therapies in the second-line treatment of Helicobacter pylori infection. Helicobacter 2021; 26: e12840. [DOI] [PubMed] [Google Scholar]
- 26. He XJ, Zeng XP, Jiang CS, et al. Efficacy and safety of antofloxacin-based triple therapy for Helicobacter pylori eradication failure in China. Dig Dis Sci 2022; 67: 208–215. [DOI] [PubMed] [Google Scholar]
- 27. Wu TS, Hsu PI, Kuo CH, et al. Comparison of 10-day levofloxacin bismuth-based quadruple therapy and levofloxacin-based triple therapy for Helicobacter pylori. J Dig Dis 2017; 18: 537–542. [DOI] [PubMed] [Google Scholar]
- 28. Chuah SK, Liang CM, Lee CH, et al. A randomized control trial comparing 2 levofloxacin-containing second-line therapies for Helicobacter pylori eradication. Medicine 2016; 95: e3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang JC, Lin CJ, Wang HL, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol 2015; 13: 895–905.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liou JM, Bair MJ, Chen CC, et al. Levofloxacin sequential therapy vs levofloxacin triple therapy in the second-line treatment of Helicobacter pylori: a randomized trial. Rom J Gastroenterol 2016; 111: 381–387. [DOI] [PubMed] [Google Scholar]
- 31. Calhan T, Kahraman R, Sahin A, et al. Efficacy of two levofloxacin-containing second-line therapies for Helicobacter pylori: a pilot study. Helicobacter 2013; 18: 378–383. [DOI] [PubMed] [Google Scholar]
- 32. Bi H, Chen X, Chen Y, et al. Efficacy and safety of high-dose esomeprazole-amoxicillin dual therapy for Helicobacter pylori rescue treatment: a multicenter, prospective, randomized, controlled trial. Chin Med J 2022; 135: 1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mansour-Ghanaei F, Joukar F, Naghipour MR, et al. Seven-day quintuple regimen as a rescue therapy for Helicobacter pylori eradication. World J Gastroenterol 2015; 21: 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nyssen OP, McNicholl AG, Gisbert JP. Meta-analysis of three-in-one single capsule bismuth-containing quadruple therapy for the eradication of Helicobacter pylori. Helicobacter 2019; 24: e12570. [DOI] [PubMed] [Google Scholar]
- 35. Nyssen OP, Vaira D, Aisa AP. Empirical second-line therapy in 5000 patients of the European Registry on Helicobacter pylori management (Hp-EuReg). Clin Gastroenterol Hepatol 2022; 20: 2243–2257. [DOI] [PubMed] [Google Scholar]
- 36. Yoon K, Kim N, Lee JW, et al. Annual eradication rate of bismuth-containing quadruple therapy as second-line treatment for Helicobacter pylori infection: A 15-year prospective study at a tertiary hospital in Korea. Helicobacter 2020; 25: e12685. [DOI] [PubMed] [Google Scholar]
- 37. Yang HJ, Jung HK, Kang SJ, et al. Salvage regimens after failure of previous Helicobacter pylori eradication therapy: a systematic review and meta-analysis. Korean J Helicobacter Up Gastrointest Res 2021; 21: 59–71. [Google Scholar]
- 38. Lee BH, Kim N, Hwang TJ, et al. Bismuth-containing quadruple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate in Korea. Helicobacter 2010; 15: 38–45. [DOI] [PubMed] [Google Scholar]
- 39. Gisbert JP. Optimization strategies aimed to increase the efficacy of Helicobacter pylori eradication therapies with quinolones. Molecules 2020; 25: 5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen PY, Wu MS, Chen CY, et al. Systematic review with meta-analysis: the efficacy of levofloxacin triple therapy as the first- or second-line treatments of Helicobacter pylori infection. Aliment Pharmacol Ther 2016; 44: 427–437. [DOI] [PubMed] [Google Scholar]
- 41. Di Caro S, Fini L, Daoud Y, et al. Levofloxacin/amoxicillin-based schemes vs quadruple therapy for Helicobacter pylori eradication in second-line. World J Gastroenterol 2012; 18: 5669–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goodwin CS, Marshall BJ, Blincow ED, et al. Prevention of nitroimidazole resistance in Campylobacter pylori by coadministration of colloidal bismuth subcitrate: clinical and in vitro studies. J Clin Pathol 1988; 41: 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liao J, Zheng Q, Liang X, et al. Effect of fluoroquinolone resistance on 14-day levofloxacin triple and triple plus bismuth quadruple therapy. Helicobacter 2013; 18: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sugimoto M, Furuta T, Shirai N, et al. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther 2004; 76: 290–301. [DOI] [PubMed] [Google Scholar]
- 45. Miehlke S, Kirsch C, Schneider-Brachert W, et al. A prospective, randomized study of quadruple therapy and high-dose dual therapy for treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter 2003; 8: 310–319. [DOI] [PubMed] [Google Scholar]
- 46. Hsu PI, Chen WC, Tsay FW, et al. Ten-day quadruple therapy comprising proton-pump inhibitor, bismuth, tetracycline, and levofloxacin achieves a high eradication rate for Helicobacter pylori infection after failure of sequential therapy. Helicobacter 2014; 19: 74–79. [DOI] [PubMed] [Google Scholar]
- 47. Kagami T, Sahara S, Ichikawa H, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther 2016; 43: 1048–1059. [DOI] [PubMed] [Google Scholar]
- 48. Wang Y, Wang C, Wang S, et al. Cytochrome p450-based drug-drug interactions of vonoprazan in vitro and in vivo. Front Pharmacol 2020; 11: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miftahussurur M, Pratama Putra B, Yamaoka Y. The potential benefits of vonoprazan as Helicobacter pylori infection therapy. Pharmaceuticals 2020; 13: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hojo M, Asaoka D, Takeda T, et al. Randomized controlled study on the effects of triple therapy including vonoprazan or rabeprazole for the second-line treatment of Helicobacter pylori infection. Therap Adv Gastroenterol 2020; 13: 1756284820966247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shinozaki S, Kobayashi Y, Osawa H, et al. Effectiveness and safety of Vonoprazan versus proton pump inhibitors for second-line Helicobacter pylori eradication therapy: systematic review and meta-analysis. Digestion 2021; 102: 319–325. [DOI] [PubMed] [Google Scholar]
- 52. Kato M, Ota H, Okuda M, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 Revised Edition. Helicobacter 2019; 24: e12597. [DOI] [PubMed] [Google Scholar]
- 53. Fiorini G, Zullo A, Vakil N, et al. Rifabutin triple therapy is effective in patients with multidrug-resistant strains of Helicobacter pylori. J Clin Gastroenterol 2018; 52: 137–140. [DOI] [PubMed] [Google Scholar]
- 54. Miehlke S, Schneider-Brachert W, Kirsch C, et al. One-week once-daily triple therapy with esomeprazole, moxifloxacin, and rifabutin for eradication of persistent Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter 2008; 13: 69–74. [DOI] [PubMed] [Google Scholar]
- 55. Zullo A, De Francesco V, Manes G, et al. Second-line and rescue therapies for Helicobacter pylori eradication in clinical practice. J Gastrointest Liver Dis 2010; 19: 131–134. [PubMed] [Google Scholar]
- 56. Lim HC, Lee YJ, An B, et al. Rifabutin-based high-dose proton-pump inhibitor and amoxicillin triple regimen as the rescue treatment for Helicobacter pylori. Helicobacter 2014; 19: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]