Abstract
Extracellular vesicle (EV) therapy recently had shown significant efficacy in various diseases. Serum starvation culture (SC) is one of the most widely used methods for collecting EVs. However, SC may cause inadvertent effects and eventually dampen the therapeutic potential of EVs. Therefore, we developed a novel method for EV collection, continuous nutrient supply culture (CC), which can provide an optimal condition for mesenchymal stem cells (MSCs) by continuously supplying essential nutrients to MSCs. By comparing with SC strategy, we revealed that CC could maintain CC-MSCs in a normal autophagy and apoptotic state, which reduced the shunting of EV precursors in cells and useless information material carried by EVs. In CC-MSCs, the expression levels of endosomal sorting complexes required for transport (ESCRT) and targeting GTPase27 (Rab27) were upregulated compared to those in SC-MSCs. Besides, we analyzed the membrane transport efficiency of EV formation, which demonstrated the CC strategy could promote the formation of EV precursors and the release of EVs. In addition, miRNA analysis revealed that CC-EVs were enriched with anti-chronic inflammatory factors, which could inhibit the nuclear factor kappa-B (NF-κB) pathway, mitigate chronic inflammation, and effectively repair skin photo-aging damage.
Keywords: Extracellular vesicles, continuous nutrient supply culture, autophagy, apoptosis, skin photo-aging
Graphical abstract

Introduction
Mesenchymal stem cells (MSCs) are widely used for treating many aging and degenerative diseases, 1 as well as traumas such as wound and bone defects, 2 because of their low immunogenicity and differentiation ability. 3 Extracellular vesicles derived from mesenchymal stem cells (MSCs-EVs) are important signaling molecules for regulating the surrounding tissue microenvironment. 4 In recent years, MSCs-EVs have been extensively studied, due to their similar functions to stem cells in the context of therapy. 5 Moreover, MSCs-EVs can avoid the risks of adverse inflammatory reactions, vascular occlusion, and disease transmission that stem cell therapy may cause, 6 because EVs contain various small molecule signaling factors, such as signaling proteins, microRNAs (miRNAs), ribonucleic acid (RNA), and deoxyribonucleic acid (DNA). These small molecules are important mediators of intercellular communication, 7 and the signals they transmit are closely related to the cells types and status. 8 Despite all types of somatic cells release EVs for information transmission, 9 EVs secreted by different types of cells carry different messages and perform different functions in the body. Meanwhile, the information carried by EVs secreted by the same cell in different states of apoptosis and autophagy are also different,10,11 and these differences may have different effects on their repair function.
The traditional method for EV culture is to use serum starvation culture (SC) for a period, and extracellular vesicles (SC-EVs) are collected from the cell supernatant. 12 The purpose of SC is to synchronize cell division, 13 reduce the batch difference between fetal bovine serum (FBS), and exclude the interference of EVs present in FBS. 14 However, SC slows the proliferation of stem cells, causing cellular injury, which make the supernatant collection is time-consuming until a desired number of cells are expanded. Moreover, apoptosis resulting from SC 15 hinders the stem cells from being reused, leading to higher production costs of EVs and limiting their industrialization process. In addition, EVs secreted from MSCs under SC may be different from those secreted under sufficient nutrition supply in vivo, which may lead to uncontrollable risks. 16 Besides, SC leads to apoptosis, and apoptotic cells produce apoptotic vesicles that distort the true EV production and therapeutic effect. 17 Also, SC can enhance intracellular autophagy and cause late endosomes to convert to the autophagy pathway (cell) to form an amphisome. 18 The autophagy pathway of endosomes and the multivesicular endosomes (MVEs) pathway of EVs have shared components and one may be inhibited while the other is activated. Enhanced autophagy may shift later endosomes to the autophagy pathway, resulting in reduced formation of EV precursors. These represent limitations of the stem cell SC method.
In recent years, many scholars have explored SC replacement methods.19,20 From serum-free medium containing animal derived proteins to xenobiotic-free medium with human blood derived components, 21 chemically defined medium without animal serum supplements has gradually conformed to clinical application requirements of MSCs. 22 Because serum-free medium and xenobiotic-free medium contain many animal derived proteins, 23 which lead to overnight centrifugation is still necessary to remove animal derived EVs interference when participating in EVs related research. For example, Luo et al. used knockout serum replacement (KSR) to culture stem cells to reduce the effect of FBS on stem cells. The particles obtained using this culture method had a similar shape, size, and biological activity to EVs, 19 which confirmed that a non-serum starvation culture method is feasible. However, it has been reported that SC may deplete energy sources and source substances required for EV synthesis and will have a greater impact on the cell state of MSCs. 24 In this state, the caspase in stem cells is activated, and poly ADP-ribose polymerase (PARP) is cleaved to lose its enzymatic activity, which accelerates the instability of cells and gradually induces apoptosis. 25 Besides, MSCs in a starvation state require endosomes to engulf proteins and other substances, and then fuse with lysosomes to degrade macromolecules to provide energy and raw materials for self-renewal. 24 Thus, the starved state will enhance the autophagy of MSCs. EVs act as signal carriers and are closely related to stem cell autophagy and apoptotic states; indeed, the EV formation process may carry distorted informative material of cell-programed death. Nevertheless, a normal nutrient supply is necessary for MSCs, which can carry various signaling molecules to maintain the stability of their surrounding microenvironment 26 and promote the regeneration and repair of damaged tissues. 27 Thus, new culture methods should be developed to replace SC, because FBS contains sugars, vitamins, lipids, growth factors, and other substances, which are crucial for cell growth.
Herin, we designed a novel SC replacement culture strategy of continuous nutrient supply culture (CC) that can continuously supply various nutrients and growth factors by using a chemically defined medium. The composition and concentration of this medium are definite, which can avoid the introduction of animal derived EVs. Then, we systematically compared the effects of SC and CC on the autophagy and apoptotic status of MSCs, MSCs-EV production, their therapeutic functions, and the potential mechanisms governing EV production.
Methods
MSCs separation and culture
MSCs were obtained from the umbilical cord of a human placenta delivered by cesarean section. 28 Clean the umbilical cord with PBS, cut off the arteries and veins, and then rinse again. After cutting the umbilical cord into 2 mm pieces, the cracked umbilical cord was divided into two parts. In the CC strategy, one part of the cracked umbilical cord tissue was cultured in UltraMedia® serum-free medium (Regenα-Geek, China) supplemented with 1% penicillin/streptomycin (P/S), and continuously supplied with growth factors, albumin, and other nutrients, while other part of the cracked umbilical cord was cultured in DMEM/F12 (Gibco, USA), supplemented with 10% FBS and 1% P/S. The cracked umbilical cord tissue was cultured in an incubator containing 5% CO2 at 37°C. When primary cells outgrowth from the cracked umbilical cord tissue and growth reaches 80% cell confluency, tissue pieces were removed. Subsequently, MSCs were passaged and cultured by the above two kinds of culture medium respectively.
Cell supernatant collection and EV extraction
When the MSCs reached 80% fusion in a 5% CO2 incubator at 37°C, the cell supernatant was collected directly and cells were passaged in the CC group. In the SC group, cells were washed with phosphate buffered saline (PBS, pH = 7.4) three times, and the cell supernatants were collected after starvation in serum-free DMEM/F12 medium for 24 h. EVs were isolated and extracted by differential centrifugation methord. 29 Briefly, the cell supernatant was centrifuged at 300g for 10 min to collect the supernatant to remove residual cells. Then, the supernatant was then collected by centrifugation of 2000g for 10 min to remove cell fragments. And cell fragments and large vesicles were separated by centrifugation at10,000g at 4°C for 30 min. After that the medium was added to the ultracentrifuge tube, and the cell supernatant was removed by ultracentrifugation (Beckman, America) at100,000g for 70 min at 4°C. 30 Finally, the EV pellet was resuspended of PBS, and the cell supernatant was removed by ultracentrifugation at100,000g at 4°C for 70 min to eliminate the interference of impure proteins. EV suspension is obtained by resuspending EV pellet in 50 μL PBS for subsequent experiments.
EV identification
The morphology of the EVs was captured by a transmission electron microscope (TEM, JEOL, Japan). Simply, the EVs (20 μL) were dropped onto a coated copper mesh and stained with phosphotungstic acid. After 1–2 min, a filter paper was used to cut into the sharp corners to absorb the excess staining solution for three times, and air-dried. The sample was then captured with a transmission electron microscope.
About nanoparticle tracking analysis, 200 μL of the EVs sample was injected into the sample chamber of nanoparticle tracking analyzer (Zeta view, Particle Metrix, Germany). Using the detection settings provided by the software developer, three groups of samples were repeatedly measured for 30 s each time. For data collection and analysis, the displacement of each detected particle is calculated from the recorded video. Single-particle tracking technology use a combination of classic electrophoresis technology and Brownian motion to analyze the size of EVs.
According to the instructions of the BCA protein detection kit (Beyotime, China), 20 μL EV suspension was added to a 96-well plate. BCA reagents A and B were mixed at a ratio of 1:50, and then 200 μL mixed reagents were added. After incubation at 37°C for 30 min, the absorbance was detected at 562 nm. Finally, the protein content was calculated according to the absorbance.
The EVs suspension (40 μg) was mixed with protein loading buffer at a ratio of 5:1, and the mixture was boiled for 5 min to denature the protein. The mixture was separated by 10% SDS-PAGE and transferred to nitrocellulose membrane. After blocking with 5% skim milk for 1 h, the membrane was incubated with the primary antibody of CD9, CD63, CD81, tumor susceptibility gene 101 protein (TSG101) and calnexin at 4°C overnight. Then washing three times with TBST, the membrane was incubated with the secondary antibody (Goat Anti-Rabbit IgG) (Abcam, UK) for 1 h at room temperature. Subsequently, washing the membrane, the signal of the western blot substrate was detected by chemiluminescence analysis. The obtained western blot signals were quantified with ImageJ software.
Examination of EV formation and secretion
To detect apoptosis, MSCs were cultured by the CC or SC strategy. First, the cell supernatant was collected and the adherent cells were washed with PBS buffer and trypsinized. Then, the adherent and suspension cells were obtained by centrifugation at 400g for 5 min at 4°C. Subsequently, 5 μL Annexin V and 10 μL propidium iodide (Beyotime, China) were added to the cell suspension and incubated in the dark for 20 min. The percentage of apoptosis cells rate was detected by flow cytometry.
To detect the number of plasma membrane (PM) in MSCs, the cells were obtained by the above method. Subsequently, the cells were incubated with direct fluorescent antibodies (CD9-PE, CD63-FITC and CD81-PE) (Solarbio, China) for 30 min. The cells were washed with PBS three times. Finally, the percentage of positive cells rate was detected by flow cytometry.
To detect apoptosis, autophagy and the whole cell tetraspanins content, MSCs were washed with PBS for three times. Then, MSCs were lysed with RIPA lysis buffer (Beyotime, China) containing 0.5 mM phenylmethanesulfonyl fluoride. The total protein extract was obtained by centrifugation at 12,000 rpm for 30 min. Finally, the total protein extract (40 μg) was separated and the protein expression of Protein 1A/1B-light chain I/II (LC3 I/II), PARP (ZenBioScience, China), CD9, CD63 and CD81 (Abcam, UK) were examined according to the previous method.
To detect the number of EV precursors synthesized by MSCs with different cultivation methods, CC-MSCs and SC-MSCs were digested and centrifuged, and the cell pellets were fixed with 2.5% glutaraldehyde and osmic acid for 2 h at 4°C. Following fixation, the pellets were dehydrated by 50%, 70%, 80%, and 90% ethanol for 20 min, soaked with acetone for 15 min three times. Subsequently, the cell pellets were embedded, sectioned, stained with uranyl acetate and lead citrate, and washed three times. Finally, images are captured by TEM and the number of EVs precursors was counted in a random cellular field of images.
Uptake of EVs by HEFs
According to the protein concentration, 200 μg EVs were incubated with 50 μL PKH26 red fluorescent staining solution (Umibio, China) for 10 min. Subsequently, 10 mL PBS was added to the complex, and EVs was isolated by the above isolated method. Then, HEFs were co-cultured with EVs labeled with PKH26 for 24 h, the HEFs were fixed with 4% paraformaldehyde, stained with 4′, 6-diamidino-2-phenylindole (DAPI) and washed with PBS. Finally, whether EVs labeled with PKH26 is aggregated in cytoplasm was observed under microscope.
Examination of EVs-treated cell proliferation and photo-aging expression
Photo-aging model of Human epithelial fibroblasts (HEFs) were seeded at a density of 105/cm2 cells per well. At 37°C and 5% CO2, HEFs were cultured with growth medium (DMEM/H containing 10% FBS and 1% P/S) until the cells reached 80% cell confluency. Then, the medium was removed, the cells were washed with PBS. The cells were exposes to ultraviolet radiation B (UVB) light at a dose of 0.2 J. Then the cells were co-cultured with growth medium for 24 h. The above process is repeated three times. At the same time, the cells were not irradiated by UVB light in the normal group. Subsequently, the cells were washed three times with PBS, and medium containing 1010 particles/mL EVs was added as the experimental group.
After 24 h of co-cultivation, 10 μL Cell counting kit-8 (CCK-8) reagent was added to each 100 μL medium, incubated for 2 h, and the absorbance was measured at 450 nm.
About cell scratch closure test, the cells were evenly scraped out with the tip of a 200 μL pipette tip, the exfoliated cells were washed with PBS, and medium containing 1010 particles/mL EVs was added to the experimental group. The widths of the scratches were recorded and imaged at 0 and 24 h, and the edge of the scar was measured using ImageJ software. The average width was counted, and the healing rate was calculated.
After co-culture with EVs for 24 h, the cells were fixed with a fixative for 30 min, adding β-galactosidase (β-gal) staining solution (Beyotime, China) and incubating overnight at 37°C. Following incubation, the cells were washed three times with PBS to remove the excess stain. The total number of cells and the number of stained cells were counted by using ImageJ software, and the rate of senescence staining was calculated.
About Enzyme-linked immunosorbent assays (ELISA), cells were disrupted by cryogenic sonication to extract cellular proteins. Human bromodeoxynucleoside uracil (BrdU), matrix metalloproteinase 1 (MMP-1), and collagen type I content determination was carried out according to the manufacturer’s method for ELISA. According to the instructions, add 50 μL of the proteins sample to be tested into the well. Then, 100 μL of horseradish peroxidase labeled detection antibody was added to each well. The reaction well was sealed with a plate sealing film, and incubated at 37℃ for 30 min. And then, the liquid was removed and washing liquid was added into each well (repeat the washing for five times). Subsequently, 50 μL of reaction substrate was added to and incubated at 37℃ in the dark for 15 min. Finally, 50 μL of stop solution was added to each well, and the OD value of each well was determined. The results of BCA protein quantification were used for standardized quantification.
Photo-aging model and treatment of Kun Ming (KM) mice
After 2 weeks of adaptive feeding, the back hair of the KM mice was removed with a razor, applying a depilatory cream to obliterate the hair. After the KM mice were anesthetized and their eyes were shielded, the mice were irradiated with UVB light at a dose of 450 J. The KM mice were irradiated three times per week for 8 weeks.
KM mice successfully modeled by photo-aging were randomly divided into CC-EV, SC-EV, and control groups. After the mice were depilated and anesthetized, six points in the same radiation range were obtained from the back skin. The mice in the experimental group were injected subcutaneously with 100 μL of 1010 particles/mL EVs suspension, and the mice in the control group were injected with the same amount of PBS. The mice continued to be irradiated with UVB and treated three times per week.
After 3 weeks of treatment, the mice were euthanized with an overdose of anesthetic. Subsequently, the skin of the damaged area of the KM mice was cut off and divided in half. For histological analysis, one part of the skin tissue on the back of the mice was fixed with formalin tissue fixative, embedded in paraffin, sectioned, and then stained with hematoxylin-eosin stain and Masson stain. The average thickness of the epidermis was measured using ImageJ software.
Another part of the skin tissue was divided into small pieces with ophthalmological scissors. The tissue was mixed with RIPA lysis buffer containing a mixture of protease inhibitors. Subsequently, the mixture was ground on ice, and centrifuged to remove the precipitate and obtain the supernatant. The total protein extract (70 μg) was separated by 10% SDS-PAGE. The photo-aging-related protein expression was examined according to the previous method.
Protein extraction and western blotting
Regarding the protein extraction method, RIPA lysis buffer (Solarbio, China), containing a mixture of protease inhibitors, was used to lyse the cells to obtain the protein. After divided the skin tissue into small pieces with ophthalmological scissors, the tissue was mixed with RIPA lysis buffer containing a mixture of protease inhibitors. The mixture was ground on ice, and centrifuged to remove the precipitate and obtain the supernatant. The supernatant was quantified with a BCA protein detection kit, and the total protein extract (cell protein extract: 40 μg, tissue protein extract: 70 μg) was separated by 10% SDS-PAGE and transferred to nitrocellulose membrane. After blocking with 5% skim milk for 1 h, the membrane was incubated with the primary antibody of interest at 4°C overnight. Then washing three times with TBST, the membrane was incubated with the secondary antibody for 1 h at room temperature. Subsequently, washing the membrane, the signal of the western blot substrate was detected by chemiluminescence analysis. The obtained western blot signals were quantified with ImageJ software.
RNA extraction and miRNA library construction
Total RNA was extracted from tissue with Trizol. Specifically, the sample was cryodesiccated with nitrogen, transferred to a tube with 1.5 mL triazole reagent, and centrifuged for 5 min at12,000g. The supernatant was collected and 0.3 mL chloroform and isoamyl alcohol (24:1) was added. Following centrifugation at12,000g for 10 min at 4°C, the supernatant was transferred to a 1.5 mL centrifuge tube, and equal volumes of isopropanol supernatant were added. The mixture was centrifuged at12,000g for 20 min at 4°C, and the supernatant was removed. After washing with 1 mL of 75% ethanol, the RNA pellet was air-dried in a biological safety cabinet, and 100 µL of DEPC-treated water was added to dissolve. Subsequently, the total RNA concentration was analyzed using a bioanalyzer (Thermo Fisher Scientific, USA). Libraries were prepared with 1 μg of total RNA per sample. Total RNA was purified and 18–30 nt small RNA bands were separated by gel electrophoresis using cDNA transcriptase, followed by several rounds of RNA amplification. PCR products were separated by agarose gel, and 130 bp PCR products were selected. The fragment size of the RNA library was measured by quantitative real-time PCR using a bioanalyzer. Sequencing was performed using the BGISEQ-500 platform (BGI-Shenzhen, China).
Statistical analysis
The data were statistically processed by Graphpad Prism 8.0 software. The average values of the two groups of data were compared by independent sample T test. The average values of multiple groups of data were compared by one-way ANOVA. All statistical results are statistically considered significant at p < 0.05.
Results
Characterization and production comparison of EVs
The cell supernatant was collected and the EVs was isolated by using the ultracentrifugation method to reduce the influence of the residual protein (Figure 1(a)). It could be found that the separated particles were round with double-layer membrane structures (Figure 1(b)). Most of these particles were 30–150 nm in size (Figure 1(c)), which is consistent with the EV particle size. 31 Western blotting illustrated that the separated particles had classic positive EV markers, including TSG101, CD9, CD63, and CD81, and no contaminant protein calnexin (Figure 1(d)). Based on the above research results, the particles isolated by differential centrifugation are EVs. Under the same volume of cell supernatant and separation method, the protein comparison showed that CC-EVs had a significantly higher protein content (Figure 1(e)). The number of EVs derived from each cell was calculated which based on the total number of cells and the amount of cell supernatant obtained. The number of EVs derived from each CC-MSCs was approximately 2.4 × 105 particles, which was about 1.4-fold of SC-MSCs (Figure 1(f)). Finally, the concentration of CC-EVs was approximately 1.4 × 1012 particles/mL, while that of SC-EVs was approximately 6.4 × 1011 particles/mL, showing a significant difference in the number of particles. The different numbers of EV particles in the 30–150 nm size range were the most obvious (Figure 1(g) and (h)).
Figure 1.
Characterization and production: comparison of EVs: (a) isolation method of EVs, (b) TEM images of EVs, (c) particles size distribution, (d) western blot analysis of the particles to detect the expression of EV protein markers (CD81, CD63, CD9 and TSG101), (e) protein concentration of EV suspensions, (f) the number of EV pellets derived from each cell, (g) EV particle concentration, and (h) the size distribution of CCEVs and SC-EVs. Data are expressed as mean plus and minus standard deviations (n = 3), ***p < 0.001.
The mechanism of EVs formation
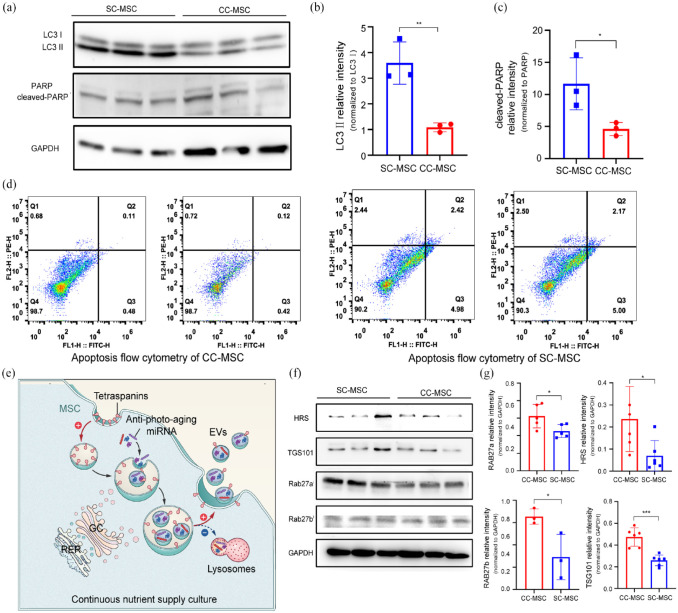
To study the mechanism by which the CC strategy promotes the formation of EVs, we firstly examined the effect of different MSCs culture methods on the cell state. LC3 II, a marker of the autophagy process,32,33 formed by enzymatic hydrolysis from LC3 I. It can be seen that the expression of LC3 II in SC-MSCs is significantly increased, showing that SC-MSCs has enhanced autophagy, while MSCs cultured with the CC strategy showed significantly attenuated autophagy (Figure 2(a) and (b)). PARP, a DNA repair enzyme, is an important marker of apoptosis. 34 SC treatment upregulates the expression of cleaved-PARP in MSCs (Figure 2(a) and (c)), suggesting the promotion of apoptosis. Moreover, the results of flow cytometry showed that 0.85% of CC-MSCs were early apoptotic and 0.6% were late apoptotic, with a total apoptosis rate of 0.115%. Compared to SC-MSCs, the total apoptosis rate decreased by 2.85% (Figure 2(d)). After that, the proteins involved in EVs formation and external release were analyzed. A membrane transporter, ESCRT, promotes endocytosis of the cell membrane and encapsulates DNA, miRNA, and signaling proteins (Figure 2(e)), while accelerating the formation of intraluminal vesicles (ILVs). 35 The CC strategy can upregulate the major proteins of ESCRT, such as HRS and TSG101, and accelerate the membrane transport of MSCs and the formation of EVs. CC-MSCs also expressed higher levels of Rab27a and Rab27b proteins (Figure 2(f) and (g)), indicating that the CC strategy stimulated the synthesis of Rab27 protein to accelerate the transport of EV precursors to the PM.
Figure 2.
Cell nutritional status affects autophagy, apoptosis status and transmembrane protein expression: (a) the expression of autophagy-related LC3 and apoptosis-related PARP in MSCs with different culture methods, (b) quantitative analysis of gray values, (c) flow cytometry analysis of MSCs stained with Annexin V/PI, (d) formation and release mechanisms of EVs, (e) the expression difference of membrane transporter protein (ESCRTs and Rab27) in different culture methods, and (f) quantitative analysis of gray values. Data are expressed as mean plus and minus standard deviations, *p < 0.05, ***p < 0.001.
Finally, we attempted to study the process of cell membrane fusion. To this end, the difference in the ratio of tetraspanins (CD81, CD9, and CD63) were analyzed in the PM and whole cells to quantify the number of successfully endocytosed membranes converted to MVEs. It was demonstrated CC-MSCs have a lower percentage of cells strongly positive for cell surface tetraspanins (Figure 3(a) and (b)). This indicated that CC reduced the content of tetraspanins on the surface of MSCs. It was hypothesized that CC-MSCs endocytosed more tetraspanins-containing PM to form the precursors of EVs, resulting in decreased cell surface tetraspanins. Interestingly, the content of tetraspanins in the whole cell proteins of CC-MSCs was significantly higher than that of SC-MSCs (Figure 3(c) and (d)), while the tetraspanins in the plasma membrane of CC-MSCs were found to be reduced in cytometry results, demonstrating that in CC-MSCs tetraspanins-containing PM were endocytosed in MSCs.
Figure 3.
Differential transport of tetraspanins in PM affects the formation of precursors of EVs (Intracellular vesicles content is equal to the whole membrane content minus cells surface membrane content): (a) immunofluorescence flow cytometry of tetraspanins (CD9, CD63, CD81) in PM of SC-MSCs and (b) CCMSCs, (c) expression of tetraspanins in whole cell lysates, (d) quantitative analysis of gray values, (e) images of cell sections captured in different culture methods by TEM, the upper and lower scale bar separately represents 2 μm and 500 nm, and (f) the number of MVEs of MSCs cultured in different ways, the budding ILVs within each MVE, and the membrane surface area of each MVE. Data are expressed as mean plus and minus standard deviations, *p < 0.05, **p < 0.01, ***p < 0.001.
To further demonstrate that tetraspanins-containing PM form EV precursors, we counted EV precursors in MSCs using electron microscopy. The captured images showed that the number of MVEs in CC-MSCs was highly abundant and the morphology was more mature, also with more vesicles (Figure 3(e) and (f)). These results indicated that the CC strategy can stimulate MSCs to accelerate membrane endocytosis, encapsulate intracellular small molecular information substances, and carry out transport to an external environment.
Thus, the CC strategy can inhibit the autophagy and apoptosis state of MSCs, upregulate the expression of EV-related membrane transporters, accelerate the transport and external release of small molecule signal substances in MSCs, and promote MSCs to release more EVs.
HEFs migration and proliferation
In order to observe that EVs can be absorbed by HEFs, HEFs were incubated with red fluorescent tracer PKH26-labeled EVs for 24 h. HEFs contained red fluorescent particles around the nucleus (in cytoplasm) under the microscope, which indicated that EVs could be successfully absorbed by HEFs (Figure 4(a)). Restoring the damaged skin caused by UVB mainly involves regenerating and migrating of HEFs. CC-EVs and SC-EVs both improve the cells proliferation damaged by UVB light, but CC-EVs have a better therapeutic effect (Figure 4(b)). In addition, the BrdU ELISA test was used for secondary verification. With CC-EVs, aging fibroblasts enter the S phase, synthesize DNA, and displayed improved proliferation (Figure 4(c)). Moreover, to determine how MSCs-EVs affected HEF migration, a scratch healing test was conducted. The results showed that photo-aging HEFs showed slower wound healing, and the aging cell group treated with CC-EVs had faster wound healing (Figure 4(d) and (e)).
Figure 4.
Verification of the promoting proliferation function of EVs in vitro: (a) HEFs co-incubated with PKH26-labeled EVs for 24 h, (b) CCK-8 analysis after co-incubating photo-aging HEFs with EVs for 24 h, (c) BrdUELISA on photo-aging HEFs co-cultured with EVs, (d) the images of the cell scratch area, the scale bar represents 500 μm, and (e) scratch test healing rate statistics, **p < 0.01, ***p < 0.001.
Anti-photo-aging effects of EVs on photo-aging HEFs
It has been reported that the activity of β-galactosidase (β-gal) increases significantly with cells aging. 36 β-gal staining showed that UVB irradiated induced senescence in the HEFs (Figure 5(a)). After treatment of aging cells with EVs for 48 h, the β-gal activity in the cells significantly reduced, indicating CC-EVs had a better anti-aging effect (Figure 5(b)). UVB increased the synthesis of matrix metalloproteinase (MMPs) in the tissue, leading to the decomposition of collagen and elastin. In the aging cells treated with CC-EVs, the expression of MMP1 was down-regulated (Figure 5(c)), and collagen type I syntheses were increased (Figure 5(d)), which indicated that CC-EVs also had a better anti-aging function. It is known that ultraviolet light damage cellular DNA, and high levels of the tumor protein p53 result in apoptosis and cell cycle arrest. Our study showed that EVs treatment reduced the p53 expression level in photo-aging cells (Figure 5(e) and (f)) and repaired the DNA damage in the cells.
Figure 5.
Verification of the anti-photo-aging repair function of EVs in vitro: (a) β-gal staining on photo-aging HEFs co-cultured with EVs, the scale bar represents 500 μm, (b) positive rate and statistics of β-gal staining cells, (c) MMP1-ELISA and (d) collagen typeⅠ-ELISA on photo-aging HEFs co-cultured with EVs, (e) expression of p53 associated with cellular DNA damage, and (f) quantitative analysis of gray values. Data are expressed as mean plus and minus standard deviations, *p < 0.05, ***p < 0.001.
CC-EVs inhibit chronic inflammation caused by photo-aging
After 8 weeks of UVB irradiation, the skin on the backs of the KM mice became rough, the epithelium thickened, and some scars were formed. Compared with normal mice of the same age, the skin of the model mice was obviously rough and thickened. The epithelium of the model mice was obviously thickened, the distribution of dermal fibers was disordered, and the skin appendages were decreased. The skin of the KM mice in CC-EVs and SC-EVs treatment groups became significantly smoother, and the skin rupture and scars from sunburns disappeared (Figure 6(a) and (b)). The CC-EV treatment group showed a significant reduction in epithelial thickness, which was only half of that observed in the control group (Figure 6(c) and (d)). In contrast, the epithelial thickness was decreases to a lesser extent in SC-EV mice. According to Masson’s staining, UVB irradiation caused much fragmented, disorganized collagen fibers in the subcutaneous tissue and the skin appendages were less disordered. The CC-EV treatment caused collagen fibers to regenerate in the subcutaneous tissue, and the skin appendages to regenerate and arrange evenly (Figure 6(c)). UVB irradiation causes oxidative stress on the skin, up-regulates the expression of tumor necrosis factor-α (TNF-α), activates the nuclear factor kappa-B (NF-κB) signaling pathway, and promotes inflammation, leading to chronic inflammatory exudation and collagen metabolism, and finally, skin aging. 37 To study the molecular mechanisms of CC-EVs and SC-EVs in the context of skin aging and collagen regeneration, the skin protein samples were analyzed. The results illustrated CC-EVs significantly inhibited the expression of TNF-α and phosphorylated protein kinase B (p-AKT) in tissues, activated the NF-κB signal transduction pathway, which inhibited the synthesis of MMP1, reducing the chronic inflammatory response. In addition, CC-EVs significantly up-regulated the expression of tumor transforming factor (TGF-β) promoted collagen I regeneration in skin tissue (Figure 6(e) and (f)).
Figure 6.
Verification of the anti-photo-aging repair function of EVs in vivo: (a) experimental protocol for photoaging mice modeling and EV treatment, (b) images captured from mice skin after 3 weeks of treatment with different EVs, (c) the degree of skin epithelium thickening and the degree of collagen disorder, the scale bar represents 500 μm, (d) statistics of mice epithelial thickness (n = 5), (e) expression of epithelial tissue aging and regeneration-related proteins, (f) and Quantitative analysis of gray values. Data are expressed as mean plus and minus standard deviations, *p < 0.05, **p < 0.01, ***p < 0.001.
NF-κB signaling pathway inhibition
In the miRNA analysis of the two EVs groups, the circles represented the 95% confidence interval. Principal component analysis (PCA) factor analysis of the CC-EVs and SC-EVs produced 10 components with eigenvalues greater than 1.0. PC1 and PC2 accounted for 78.04% and 16.47% of the total variance, respectively. The PCA factor showed that PC1 and PC2 could clearly separate the miRNA expression profiles of the two groups of EVs (Figure 7(a)). The Venn diagram showed that CC-EVs had 663 miRNAs and SC-EVs had 552 miRNAs, with 440 miRNAs of the same type (Figure 7(b)). In the comparison of the miRNA analysis of the five samples, the twofold up-regulated or down-regulated genes were regarded as differentially expressed, and the differentially expressed miRNA was plotted as a heat map (Figure 7(c)). The comparison between CC-EVs and SC-EVs showed 125 significantly differentially expressed miRNAs. Further analysis showed differentially expressed miRNAs in CC-EVs and SC-EVs. It had 55 miRNAs with a twofold change: 46 were up-regulated (83.6%) in CC-EVs, and 9 were down-regulated (17.4%) (Figure 7(e)). In addition, a miRNA interaction network related to the NF-κB signaling pathway was constructed. In the network, the miRNA with the highest degree was located in the middle of the network, and the number of links indicated the number of interactions between the miRNA and the target protein. As shown in Figure 7(d), multiple miRNAs in CC-EVs targeted the NF-κB signal pathway to down-regulate the aging-related genes MMPs and β-gal. At the same time, miR-493 and others acted on the target gene TNF-α 38 to inhibit the NF-κB signaling pathway, which could down-regulate the transcription of inflammatory factors in the nucleus and reduce the level of oxidative stress in the body. The results indicated that the CC strategy could change the cargo carried by cell vesicles so that CC-EVs contained more anti-aging factors and resists chronic inflammation caused by activation of the NF-κB signaling pathway (Figure 7(f)).
Figure 7.
Cell nutritional status affects the information substances carried by EVs, resulting in differences in the repair functions of EVs: (a) according to RNA analysis results, PCA of miRNAs carried by EVs, (b) Venn diagram of miRNA species carried by EVs, (c) MiRNA heat map of co-carried miRNAs in EVs, (d) network interaction map of photo-aging chronic inflammation-related miRNA/targeted miRNA interactions and target proteins, (e) Bar graph of miRNAs content with significant differences in EVs, and (f) the anti-photo-aging repair mechanism of EVs and the inhibition of NF-κB signaling pathway by miRNAs carried by EVs.
Discussion
SC and extraction of cell supernatants are the traditional method for obtaining EVs for isolation. 39 As EVs carry intercellular information to be communicated and transmit information about the characteristics and status of secretory cells, 40 nutritional deficiency will enhance the apoptosis and autophagy of MSCs in the state of SC for stem cells, and the function of MSCs-EVs will decrease accordingly. To investigate the effect of cellular nutritional status on EVs, we used the CC strategy, non-starved stem cells with continuous nutrient supply, to improve the deficiency of SC. The CC strategy could reduce both autophagy and apoptosis in stem cells, increase intracellular membrane transporters, enhance anti-photo-aging components of EVs, and boost EV formation.
The formation of the precursors of most EVs depends on the endosomal sorting complex ESCRT. 41 Cells form endosomes through cell membrane endocytosis, which selectively bind to ESCRT to integrate miRNAs, small DNA fragments, and signaling proteins into MVEs, which bud from the lumen of MVEs to form ILVs. 42 Late endosomes that are not bound to ESCRT are shunted to the autophagy pathway and degraded by lysosomes to maintain intracellular material stability. 43 Our study showed that cell starvation could down-regulates the expression of sorting complexes, which might be related to the enhanced autophagy induced by SC. Non-serum starvation culture could increase the expression of ESCRT subunit proteins HRS and TSG101 in MSCs. ESCRT determines the type and quantity of miRNAs, and other signaling molecules sorted into vesicles. ESCRT subunit proteins can promote the binding of endosomes to miRNAs and other molecules, 44 meaning that CC-EVs can carry a wider variety of miRNAs. SC promoted lysosomal degradation, reusing unwanted intracellular proteins. In contrast, the CC strategy inhibited autophagy and increased the intracellular levels of TSG101, which promoted the binding of late endosomes to ESCRT subunits and prevented the shunting of late endosomes from collateral degradation of lysosomes. 45 More late endosomes entered the EVs synthesis pathway, wrapping signaling molecules and converting them to MVEs. The number of MVEs and budding ILVs in CC-MSCs was significantly increased under an electron microscope, indicating that our CC strategy successfully inhibited autophagy-induced endosomal degradation, enhanced EV precursor formation, and EV production.
The process of cells transporting ILVs to the outside of the cell is inseparable from the important protein of membrane transport, Rab27, which regulates the fusion of ILVs and cell membranes. 46 Through autophagy, which depends on intracellular membrane transport, cells eliminate damaged organelles and proteins. Among them, Rab27 can prevent the fusion of MVEs and lysosomes to inhibit the degradation of ILVs. Rab27 that is not degraded by MVEs mediates the secretion of transport to the plasma membrane, fuses with the cell membrane, and transmits and releases biologically active substances to the extracellular environment. 47 Thus, Rab27 protein regulates MVEs toward two distinct ends, lysosomal degradation by autophagy and extracellular secretion of EVs. It was found that the non-serum-starved CC strategy can promote the expression of Rab27a and Rab27b, which could drives MVEs outward movement, inhibiting the degradation pathway and promoting membrane transport. 48 In this way, MSCs can secrete more EVs instead of degrading them intracellularly. The formation of endosomes depends on cell membrane endocytosis, and the cell membranes containing tetraspanins are closely related to EVs formed through the MVEs-PM pathway. 48 We determined that SC hindered the endocytosis of MSCs containing tetraspanins by analyzing the difference in the ratio of tetraspanins in the PM and whole cells to quantify the amount of successful endocytic conversion to MVEs at the membrane. Considering the nutritional deficiency of MSCs caused by SC, the energy source for cell membrane transport was reduced, resulting in inhibition of the membrane transport process of the cell, 49 while the lysosome degradation activity was increased to obtain nutrients for survival, the two factors both inhibited the synthesis of EVs.
SC slows the formation and release of EVs and affects material entry into the vesicles. We hypothesized that this was because of the abnormal autophagy and apoptosis states of MSCs. EVs secreted by different types of cells in the normal body carry different messages. As an information-storing material, EVs can transmit the information on the cell’s own characteristics. Indeed, glioma cell-derived EVs can enhance the M2 polarization of microglia, 50 and mast cell-derived EVs can inhibit allergy. 51 The characteristics of MSCs are that they can self-replicate and differentiate into various cells for tissue renewal and repair, and their derived EVs can accelerate tissue damage repair.52,53 Additionally, changes in the states of autophagy and apoptosis affect the information carried by EVs. Therefore, stem cell starvation, apoptosis, and autophagy resulting from nutrient deficiency may alter the nature and composition of EVs. Interestingly, MSCs of the same cell line that were starved or had sufficient nutrition showed different autophagy and apoptosis rates. Compared to CC-MSCs, starved MSCs had significantly enhanced autophagy, and the apoptosis rate was also much higher than that of CC-MSCs. Starved MSCs undergo autophagy and apoptosis, and the composition of the released EVs varies. CC-EVs contain 112 more miRNA species than SC-EVs, and the miRNA expression heat map shows that there are 125 miRNA species with considerable differences. This suggests that autophagy and apoptosis induced by SC not only reduce the secretion of EVs but also changes in the physiological state of cells can affect the composition of EVs. The complex miRNA sorting mechanism is worthy of further exploration. To avoid the influence of exogenous EVs, the use of SC strategy makes the autophagy and apoptosis status of SC-MSCs and normal MSCs very different, resulting in MSCs-EVs carrying unpredictable molecular compositions, 11 and a large number of abnormal information substances are released in target cells. Thus, SC-induced apoptosis alters the miRNA and protein composition of MSCs-EVs, and the characteristic therapeutic effect of MSCs-EVs is reduced. Therefore, using a medium that simulates MSCs in vivo to maintain the cells in a normal physiological state not only avoids interfering with exogenous EVs, but also reduces the changes in EVs components caused by starvation. Combining three-dimensional culture with CC may represent an excellent way to explore the functional map of MSCs-EVs in vivo.
It has been shown that various MSCs-EVs have anti-photo-aging activities, 54 most of which use SC treatment, before collecting the cell supernatant to isolate EVs. The EVs obtained using this method inhibit the activation of NF-κB signaling pathway and protect the skin from UVB-induced aging. 55 Our results reaffirmed these previous results, and further showed that non-serum starved CC-EVs had better anti-photo-aging therapeutic functions. Compared to SC-EVs, CC-EVs significantly down-regulated the expression level of p53 in cells, decreased the activity of β-gal, 56 a key enzyme of cellular senescence, and enhanced cell proliferation and migration. In addition, experiments in vivo showed that CC-EVs treatment had an anti-photo-aging effect in just 3 weeks, as demonstrated by the improvement in the roughness and thickness of the skin, and the disappearance of scarring and ulceration caused by sunburn. Compared to MSCs-EVs obtained by SC, 57 CC-EVs could restore photo-aging skin to normal skin level after only 3 weeks of treatment, which had high value in clinical application. CC-EVs promoted the growth of fibroblasts by up-regulating the expression of TGF-β, activating the Smad Interacting Protein pathway and promoting the synthesis of extracellular matrix, such as collagen and fibronectin. 58 CC-EVs also inhibited the chronic inflammation caused by the NF-κB signaling pathway, 59 and reduced the accumulation of chronic inflammation, and achieved the recovery of photo-aging skin. Through the miRNA analysis of EVs, we found that there are 46 miRNAs that exponentially increased the content of CC-EVs compared to SC-EVs. Among them, miR-125b and miR-196a are key molecules acting on the NF-κB signaling pathway to fight chronic inflammation.60,61 Subcutaneous pro-inflammatory cytokines, such as TNF-α, are elevated during photo-aging induction. Long-term activation of NF-κB signaling pathway leads to the accumulation of chronic inflammation, resulting in the deposition of subcutaneous inflammatory cells and the thickening and ulceration of the skin surface. We analyzed and produced the network interaction map of miRNA and NF-κB, and found 16 anti-photo-aging-related miRNA molecules that are highly expressed in CC-EVs. These miRNAs can act on the NF-κB-mediated inflammatory pathway and inhibit the inflammatory response of skin tissue, while only four miRNAs were detected in SC-EVs. The NF-κB signaling pathway mediates inflammation in different ways and is closely related to many human diseases, such as inflammatory bowel disease, rheumatoid arthritis, atherosclerosis, and prostate cancer, colon cancer, and other tumor-related diseases. 62 Although our study had limitations, and we did not continue to explore in-depth research-worthy issues such as age-related skin aging, CC-EVs contain more potent miRNAs than SC-EVs, which are able to inhibit NF-κB signaling activation and may have superior therapeutic potential for the above diseases.
Conclusion
In conclusion, we aim to construct a SC replacement culture strategy to reduce the time and cost of obtaining MSCs supernatant. Our designed CC strategy can maintain the normal autophagy and apoptosis status of MSCs, increase the production of EVs, reduce the cost of EV application, and improve the damage repair effect of EVs in chronic inflammation. MSCs culture method combining 3D culture strategy and CC strategy can shorten the culture time and improve the MSCs utilization rate, which may be an important way to promote the industrialization of EVs. The research provides a direction for the large-scale production of EVs, which promotes the application of EVs in large animal testing and clinical treatment.
Acknowledgments
Not applicable.
Footnotes
Authorship contribution statement: Lihao Chen: Conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing-original draft, writing-review & editing. Weihan Xie: Conceptualization, funding acquisition, validation, visualization, writing-review & editing. Keke Wu: Conceptualization, funding acquisition, validation, writing-review & editing. Yuan Meng: Conceptualization, funding acquisition, writing-review & editing. Yijun He: Investigation, methodology, writing-original draft, visualization. Jiawei Cai: Validation, visualization. Yuan Jiang: Investigation, methodology. Qi Zhao: Visualization, methodology. Yixi Yang: Investigation, methodology. Minru Zhang: Investigation, validation. Manping Lu: Methodology, software, validation. Shaozhang Lin: Investigation, validation. Lin Liang: Investigation, validation. Zhiyong Zhang: Data curation, project administration, supervision, funding acquisition, conceptualization. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (NSFC) (Nos. 82072415, 81772354, 81902189 and 32201077), the Clinical Innovation Research Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (No. 2018GZR0201001), the Science and Technology Innovation Project of Foshan City (No. 1920001000025), the Science Technology Project of Guangzhou City (No. 2019ZD15), Guangdong Basic and Applied Basic Research Foundation (Nos. 2022A1515011982, 2020A1515111046, 2020A1515110343, and 2020A1515110062) and the Collegiate Innovation and National Young Thousand-Talent Scheme to Zhiyong Zhang.
Ethical approval statement: The experimental animals were male SPF Kunming mice from the Guangdong Provincial Medical Laboratory Animal Center, animal production license number: SCXK (Guangdong) 2013-0002. All animal experiments were conducted in compliance with the “Regulations on the Administration of Laboratory Animals” of the People’s Republic of China. Human umbilical cord mesenchymal stem cells (hUCMSCs) were approved by the Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University. Normal full-term cesarean section umbilical cords were collected for primary culture, with the informed consent of the mothers and their families.
ORCID iD: Zhiyong Zhang  https://orcid.org/0000-0002-7222-2652
https://orcid.org/0000-0002-7222-2652
Data sharing: The manuscript and Supplemental Files include all the data.
References
- 1. XingHua P, QingKeng L, Xiang Y, et al. Umbilical cord mesenchymal stem cells protect thymus structure and function in aged C57 mice by downregulating aging-related genes and upregulating autophagy- and anti-oxidative stress-related genes. Aging 2020; 12(17): 16899–16920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Michal K, Anna FD, Wioletta L, et al. Bone defect repair using a bone substitute supported by mesenchymal stem cells derived from the umbilical cord. Stem Cells Int 2020; 9(4): 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Md Fadilah NI, Mohd Abdul, Kader Jailani MS, Badrul Hisham MAI, et al. Cell secretomes for wound healing and tissue regeneration: next generation acellular based tissue engineered products. J Tissue Eng 2022; 13: 20417314221114273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9(6): 654–659. [DOI] [PubMed] [Google Scholar]
- 5. Kong J, Wang F, Zhang J, et al. Exosomes of endothelial progenitor cells inhibit neointima formation after carotid artery injury. J Surg Res 2018; 232: 398–407. [DOI] [PubMed] [Google Scholar]
- 6. Wahlund CJE, Güclüler G, Hiltbrunner S, et al. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci Rep 2017; 7(1): 17095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics 2010; 73(10): 1907–1920. [DOI] [PubMed] [Google Scholar]
- 8. Guangpeng H, Xueqiang P, Shibo W, et al. Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications. Mol Cancer 2022; 21(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Niel G, Porto-Carreiro I, Simoes S, et al. Exosomes: a common pathway for a specialized function. J Biochem 2006; 140(1): 13–21. [DOI] [PubMed] [Google Scholar]
- 10. Gudbergsson JM, Johnsen KB. Exosomes and autophagy: rekindling the vesicular waste hypothesis. J Cell Commun Signal 2019; 13(4): 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kakarla R, Hur J, Kim YJ, et al. Apoptotic cell-derived exosomes: messages from dying cells. Exp Mol Med 2020; 52(1): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yan Z, Jia X, Siying L, et al. Embryonic stem cell-derived extracellular vesicles enhance the therapeutic effect of mesenchymal stem cells. Theranostics 2019; 9(23): 6976–6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kues WA, Anger M, Carnwath JW, et al. Cell cycle synchronization of porcine fetal fibroblasts: effects of serum deprivation and reversible cell cycle inhibitors. Biol Reprod 2000; 62(2): 412–419. [DOI] [PubMed] [Google Scholar]
- 14. Lehrich BM, Liang Y, Fiandaca MS. Foetal bovine serum influence on in vitro extracellular vesicle analyses. J Extracell Vesicles 2021; 10(3): e12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandag Z, Jung S, Quynh NTN, et al. Inhibitory role of TRIP-Br1/XIAP in necroptosis under nutrient/serum starvation. Mol Cells 2020; 43(3): 236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Bonacquisti EE, Brown AD, et al. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells 2020; 9(3): 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caruso S, Poon IKH. Apoptotic cell-derived extracellular vesicles: more than just debris. Front Immunol 2018; 9: 1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of exosome composition. Cell 2019; 177(2): 428–445.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ying L, Dunqin G, Peng W, et al. Optimized culture methods for isolating small extracellular vesicles derived from human induced pluripotent stem cells. J Extracell Vesicles 2021; 10(6): e12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van de Vlekkert D, Qiu X, Annunziata I, et al. Isolation and characterization of exosomes from skeletal muscle fibroblasts. J Vis Exp 2020; 16(159): e61127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu X, Kang H, Liu X, et al. Serum and xeno-free, chemically defined, no-plate-coating-based culture system for mesenchymal stromal cells from the umbilical cord. Cell Prolif 2016; 49(5): 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bui HTH, Nguyen LT, Than UTT. Influences of Xeno-free media on mesenchymal stem cell expansion for clinical application. Tissue Eng Regen Med 2021; 18(1): 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gomes FG, Andrade AC, Wolf M, et al. Synergy of human platelet-derived extracellular vesicles with secretome proteins promotes regenerative functions. Biomedicines 2022; 10(2): 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Back MJ, Ha HC, Fu Z, et al. Activation of neutral sphingomyelinase 2 by starvation induces cell-protective autophagy via an increase in Golgi-localized ceramide. Cell Death Dis 2018; 9(6): 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexandre D, Jean-Bernard D. Caspase-7 uses RNA to enhance proteolysis of poly(ADP-ribose) polymerase 1 and other RNA-binding proteins. Proc Natl Acad Sci USA 2019; 116(43): 21521–21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Di L, Yan X, Qiuli L, et al. Mesenchymal stem cell-macrophage crosstalk and maintenance of inflammatory microenvironment homeostasis. Front Cell Dev Biol 2021; 9: 681171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin H, Sohn J, Shen H, et al. Bone marrow mesenchymal stem cells: aging and tissue engineering applications to enhance bone healing. Biomaterials 2019; 203: 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Priscilla NA, David JF. An emerging frontier in intercellular communication: extracellular vesicles in regeneration. Front Cell Dev Biol 2022; 10: 849905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017; 546(7659): 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nguyen DC, Lewis HC, Joyner C, et al. Extracellular vesicles from bone marrow-derived mesenchymal stromal cells support ex vivo survival of human antibody secreting cells. J Extracell Vesicles 2018; 7(1): 1463778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Avalos PN, Forsthoefel DJ. An emerging frontier in intercellular communication: extracellular vesicles in regeneration. Front Cell Dev Biol 2022; 10: 849905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yingxue W, Maria R, Matthew J, et al. Control of infection by LC3-associated phagocytosis, CASM, and detection of raised vacuolar pH by the V-ATPase-ATG16L1 axis. Sci Adv 2022; 8(43): eabn3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li W, Luo LX, Zhou QQ, et al. Phospholipid peroxidation inhibits autophagy via stimulating the delipidation of oxidized LC3-PE. Redox Biol 2022; 55: 102421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xiaohui L, Wenxia J, Johannes R, et al. PARP inhibitors trap PARP2 and alter the mode of recruitment of PARP2 at DNA damage sites. Nucleic Acids Res 2022; 50(7): 3958–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yong J, Lele M, Wanying Z, et al. Extracellular signals regulate the biogenesis of extracellular vesicles. Biol Res 2022; 55(1): 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Valieva Y, Ivanova E, Fayzullin A, et al. Senescence-associated β-galactosidase detection in pathology. Diagnostics 2022; 12(10): 2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hee HS, Elaine B, SeYoung C, et al. Anti-photoaging effect of hydrolysates from pacific whiting skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 signaling pathway in UVB-induced human dermal fibroblasts. Mar Drugs 2022; 20(5): 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Du J, Li W, Wang B. Long non-coding RNA TUG1 aggravates cerebral ischemia and reperfusion injury by sponging miR-493-3p/miR-410-3p. Open Med 2021; 16(1): 919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clotilde T, Sebastian A, Graça R, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006; Chapter 3: Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 40. Wei Y, Shi M, Zhang J, et al. Autologous versatile vesicles-incorporated biomimetic extracellular matrix induces biomineralization. Adv Funct Mater 2020; 30(21): 2000015. [Google Scholar]
- 41. Denghui W, Weixiang Z, Ying G, et al. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res 2020; 31(2): 157–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 2018; 75(2): 193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marcelo FC, Osmar AM, Isabel CM. ATP is released from autophagic vesicles to the extracellular space in a VAMP7-dependent manner. Autophagy 2012; 8(12): 1741–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nkosi D, Sun L, Duke LC, et al. Epstein-Barr virus LMP1 promotes syntenin-1 and Hrs-induced extracellular vesicle formation for its own secretion to increase cell proliferation and migration. mBio 2020; 11(3): e00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lene M, Marie PN, Elisabeth SWC, et al. Cargo-dependent degradation of ESCRT-I as a feedback mechanism to modulate endosomal sorting. Traffic 2011; 12(9): 1211–1226. [DOI] [PubMed] [Google Scholar]
- 46. Hendrix A, De Wever O. Rab27 GTPases distribute extracellular nanomaps for invasive growth and metastasis: implications for prognosis and treatment. Int J Mol Sci 2013; 14(5): 9883–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zou W, Lai M, Zhang Y, et al. Exosome release is regulated by mTORC1. Adv Sci 2019; 6(3): 1801313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Daniel CS. Regulation of vesicular trafficking and leukocyte function by Rab27 GTPases and their effectors. J Leukoc Biol 2013; 94(4): 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thomas R, Sandra B, Benjamin G, et al. ER-to-Golgi transport in HeLa cells displays high resilience to Ca2+ and energy stresses. Cells 2020; 9(10): 2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ziwen P, Rongrong Z, Boyan L, et al. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Mol Cancer 2022; 21(1): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dimitris S, Hany GB, Michèle R, et al. Immunoregulatory properties of mast cell-derived exosomes. Mol Immunol 2002; 38(16–18): 1359–1362. [DOI] [PubMed] [Google Scholar]
- 52. Yang J, Chen Z, Pan D, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined Pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomedicine 2020; 15: 5911–5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kou X, Xu X, Chen C, et al. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci Transl Med 2018; 10(432): eaai8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Keda Z, Li Y, Fu-Rong L, et al. Topical application of exosomes derived from human umbilical cord mesenchymal stem cells in combination with sponge spicules for treatment of photoaging. Int J Nanomedicine 2020; 15: 2859–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peng X, Yu X, Zheng Z, et al. Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res Ther 2020; 11(1): 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ricardo IM, Hannah KD, Themistoklis V, et al. Senescence-associated β-galactosidase reveals the abundance of senescent CD8+ T cells in aging humans. Aging Cell 2021; 20(5): e13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shiqi H, Zhenhua L, Jhon C, et al. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano 2019; 13(10): 11273–11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang X, Zhou Y, Yu X, et al. Differential roles of cysteinyl cathepsins in TGF-β signaling and tissue fibrosis. iScience 2019; 19: 607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tang B, Tang F, Wang Z, et al. Upregulation of Akt/NF-κB-regulated inflammation and Akt/Bad-related apoptosis signaling pathway involved in hepatic carcinoma process: suppression by carnosic acid nanoparticle. Int J Nanomedicine 2016; 11: 6401–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fengting H, Jian T, Xiaohong Z, et al. MiR-196a promotes pancreatic cancer progression by targeting nuclear factor kappa-B-inhibitor alpha. PLoS One 2014; 9(2): e87897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Valmiki S, Ahuja V, Puri N, et al. MiR-125b and miR-223 contribute to inflammation by targeting the key molecules of NF-κB pathway. Front Med 2020; 6: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 2013; 12: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]