Abstract
Background:
People who inject drugs (PWID) are at high risk for HCV infection and its complications in many developing countries including Iran. The aims of this study were to measure HCV prevalence and evaluate the impact of an on-site community-based HCV model of care among PWID in Kerman, Iran.
Methods:
Using respondent-driven sampling (RDS) method, we recruited PWID (≥18 years and reported drug injection in last 12 months) in Rostam study. Self-reported data were collected on socio-demographics, drug use behaviors and treatment services, and previous access to HCV care and treatment. HCV and HIV antibody were tested using rapid tests; reactive results were confirmed using RT-PCR and 4th generation ELISA, respectively. Individuals diagnosed with HCV were visited by an on-site general physician for eligibility assessment and counseling for 12 weeks of direct-acting antiviral therapy. Recruitment, interview, HCV screening, diagnosis and treatment were all conducted within the DIC.
Results:
A total of 171 PWID (median age; 39 years and 89.5% male) were recruited between July 2018, and May 2019. A considerable proportion had a history of homelessness (88.2%) and 24.5% reported incarceration in the last 12 months. Around 33.5% reported daily injection in the last 3 months. Only 11.8% reported ever being tested for HCV, and of those reporting being HCV RNA positive, only 30.0% reported ever receiving HCV treatment. A total of 62 individuals were anti-HCV positive, of whom 47 (75.8%) were HCV RNA positive. Of RNA positive individuals, 36 (76.6%) returned for treatment eligibility assessment. Of all the 36 participants eligible for treatment, 33 (91.7%) initiated treatment. Of those who initiated treatment and had an RNA test available 12 weeks after treatment, 30 (91.0%) achieved SVR12.
Conclusion:
Despite the high levels of marginalization and low levels of history of HCV testing and treatment, our integrated on-site community-based HCV care model within a DIC setting suggested that HCV care can be successfully delivered outside of hospital or specialized clinics; a model which is more likely to reach PWID and a step towards to HCV elimination.
Keywords: Hepatitis C, HCV prevalence, HCV treatment, people who inject drugs, community-based model, integrated model of care, DAA therapy, HCV elimination, Iran
Introduction
As a major transit country for drug trafficking [1], Iran has always had a high prevalence of opioid use disorders [2]. The recent decades, however, have witnessed significant changes in the patterns of drug use in Iran leading to an increased number of people who inject drugs (PWID) [3, 4]. Iran is currently hosting the highest population proportion of PWID in Middle East and North Africa [5]. A history of injecting drug use has been consistently reported as the main transmission risk for hepatitis C virus (HCV) infection in Iran [6, 7]. Notably, recent data suggests that one in two PWID are tested positive for HCV antibody in Iran [8].
Prevention and treatment of injection drug use have improved significantly in Iran since the scale up of harm reduction programs in 2005 [1]. A large nation-wide network of drug treatment and harm reduction [9, 10] was put into place by September 2014 including 580 centers delivering free needles and syringes and 5983 centers offering opioid agonist therapy (OAT) to PWID in Iran [11]. Besides ensuring access to sterile needle and syringe programs (NSP) and OAT among PWID, harm reduction programs have expanded to include risk reduction education, voluntary HIV counseling and testing, and free condom distribution among this population [12]. The provision of HCV care and treatment, however, occurs largely in tertiary healthcare settings and continues to fail in controlling the epidemic of HCV among Iranian PWID [13, 14].
The availability of highly effective interferon-free direct-acting antiviral (DAA) treatments has the potential to substantially increase HCV treatment uptake among PWID [15] and could provide a breakthrough in the efforts to address HCV epidemic among this population in Iran. Among PWID, effectiveness of DAA therapy can be impacted by low rates of linkage to care and poor adherence to HCV treatment [16]. Further, the lack of a publicly funded programs for HCV management and limited insurance coverage for HCV diagnosis and treatment would hinder any HCV control and prevention strategy, particularly among marginalized populations such as PWID [13]. Given all of this, HCV “test and treat” initiatives that are tailored to the specific needs of PWID are required to progress towards HCV elimination targets set by World Health Organization [17, 18].
The potential role of integrated models providing multidisciplinary care for PWID has been the subject of increased scientific interest [19] and shown promising results in HCV management in recent years. Integrating HCV care and treatment into existing harm reduction infrastructures where PWID are already receiving services, enhances HCV assessment and treatment among this population [20–22]. In Iran, Alavi et al., demonstrated that HCV care provision within the drug treatment and harm reduction settings facilitates HCV diagnosis and treatment uptake among people who use drugs [23]. However, the impact of these care models on HCV outcomes in Iran and particularly among PWID needs further assessments and research.
In Rostam study, we used a community-based drop-in center (DIC) that provides OAT and harm reduction supplies for PWID in Kerman, Iran as our site for HCV testing and treatment services. The aim of the current investigation was to assess the impact of an integrated on-site model for HCV services on HCV testing and treatment initiation among PWID. A secondary aim was to estimate response to HCV DAA therapy among PWID with HCV chronic infection.
Methods
Study design
Rostam was a three-part study that included A) a cross-sectional behavioral survey and HCV screening among PWID, B) a non-randomized treatment trial of PWID with HCV chronic infection and C) an observational cohort study of HCV negative (antibody negative or antibody positive/RNA negative) PWID with recent injecting (i.e. within the last 6 month) (Figure 1). The main aims of each study component were respectively as follows: to measure the prevalence of HCV infection (anti-HCV antibody and HCV RNA prevalence) among PWID, to assess the impact of an integrated on-site community-based intervention including on-site HCV rapid antibody testing, venipuncture for HCV RNA testing and treatment eligibility assessment on HCV testing and treatment initiation among PWID, and to evaluate the incidence of HCV infection (primary and reinfection) among a cohort of HCV RNA negative (antibody positive or negative) PWID with recent injecting drug use. The current paper will focus on results drawn from the A and B components of Rostam study.
Figure 1.
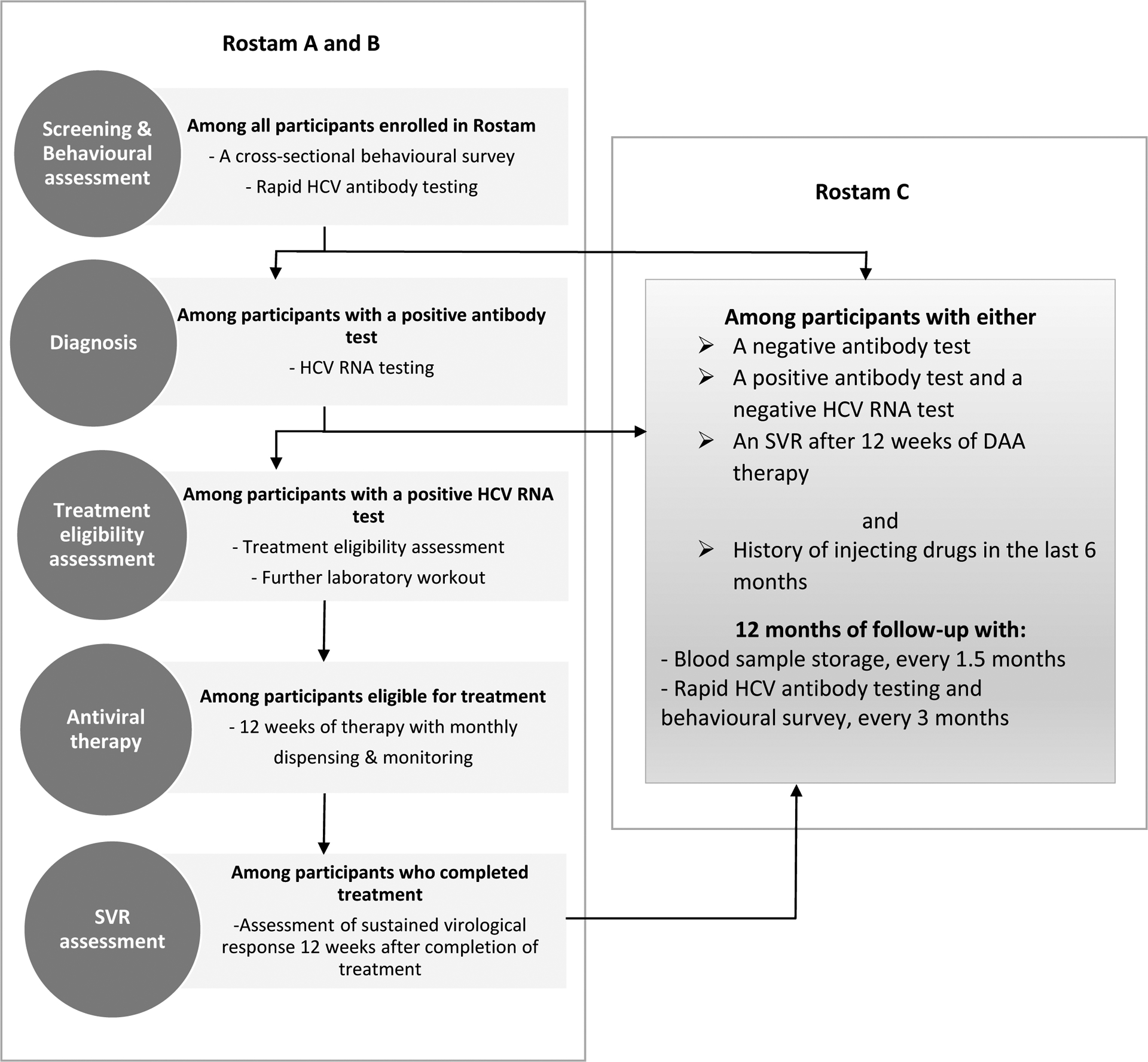
Rostam study design
Study site and participants
The study site was Monadian-Salamat community-based DIC which is one of the four DICs serving PWID under the supervision of Kerman University of Medical Sciences. Community-based DICs in Kerman provide free access to sterile needle and syringes for injection, condoms, warm meals, and personal hygiene facilities. Additional services including HIV screening and sexual health education are often provided for people visiting DICs as well. Recruitment, interview, HCV screening, diagnosis and treatment were all conducted at the Monadian-Salamat DIC, as a single-location setting. Kerman is a city with a population of 632,000 residents [24], located in the southeast of Iran.
Using Respondent-Driven Sampling (RDS) and 12 seeds, individuals were enrolled between July 10, 2018, and May 12, 2019, into the cross-sectional part of the study (Rostam-A). Inclusion criteria required ≥18 years of age, self-report history of injecting drug use (with verification of drug injecting knowledge and injection scars/markers by research staff) for non-medical purposes in the last 12 months and having been a resident of Kerman for the past 6 months with no intention to move out in the next 3 months. Recruited participants received 3$ (~150,000 Rials) as compensation for their time and were given three coupons to invite three peers to study with an additional 2$ (~100,000 Rials) incentive per each successful peer recruitment.
The study protocol was registered at Iranian Registry of Clinical Trials (IRCT) under the reference number of IRCT20170213032547N1. The ethics committees of Kerman University of Medical Sciences (# IR.KMU.REC.1396.2422) and University of California San Francisco (# 16–18626) approved the study protocol and procedures.
Study procedures
At enrolment, eligible participants who provided written informed consent were interviewed by same gender trained interviewers. All interviews were conducted face-to-face using a standardized risk assessment questionnaire, collecting information on participants’ social network size, socio-demographic characteristics, homelessness and incarceration, alcohol and drug use, injection risk behaviors, drug overdose, history of drug treatment and access to OAT programs, and HCV testing and treatment history.
Following interview, study participants who provided written informed consent were visited by a certified nurse counselor for a brief HCV/HIV counseling session and collection of a finger-stick capillary whole-blood sample for rapid diagnostic HCV (SD Bioline, Korea), and HIV (SD Bioline, Korea) antibody testing. Rapid test results were prepared in 20 minutes. Individuals with non-reactive HCV and HIV rapid test results were recruited into Rostam-C if they reported injecting drugs in the last six months, otherwise excluded from the study. For those with reactive HCV rapid test result (HIV reactive or non-reactive), the staff member managed post-test counselling and then collected a 10 ml whole blood sample for confirmatory laboratory testing using RT-PCR for HCV RNA diagnosis (AmpliSens® HCV-FRT, Russia) and if needed, two 4th generation ELISA kits for HIV antibody/antigen testing (Adaltis, Italy and Apdia, Belgium). Confirmatory laboratory test results were available in two weeks on average. Finally, if a participant was only HIV reactive, they were referred to HIV care and treatment clinics in Kerman.
All PWID with HCV chronic infection (i.e. HCV RNA detected) were visited by a trained general physician in the study site to receive their laboratory results and counseling for diagnosis. They were then invited to participate in an open-label single arm trial of 12-week HCV treatment (Rostam-B). If the person provided consent for the trial, treatment eligibility was evaluated and further laboratory workout (i.e. complete blood count, alanine aminotransferase, aspartate aminotransferase [AST], creatinine, hepatitis B surface antigen and beta‐human chorionic gonadotropin [β‐hCG; collected only for female participants]) was performed. All individuals with hepatitis B virus infection (HBV), tuberculosis, cirrhosis (AST-to-platelet ratio index >2 [25]), pregnancy (positive β‐hCG) and renal dysfunction (estimated Glomerular Filtration Rate [eGFR]<30 [26]) were planned to exclude from the trial and were referred to a specialized liver clinic for hepatitis management in Kerman. The results from further laboratory workout were also available in in two weeks on average.
Within a week of the day the treatment eligibility was confirmed, treatment initiation visits were scheduled or re-scheduled for participants who could not attend their initial appointment. At treatment initiation, participants received the first four weeks of a daily fixed dose combination of Sofosbuvir 400mg/ Daclatasvir 60mg (Datex®, Sobhan Medicine Trade Development Co, Iran or Daclasfovir Shari®, Shari/Bakhtar Bioshimi Co (BBpharmaco), Iran). Dose of Daclatasvir was adjusted based on the HIV anti-retroviral therapy in HIV/HCV co-infected patients. Following dispensing 28 HCV pills on the first visit of the trial, participants were asked to return in 4 and 8 weeks for their second- and third-treatment visits (to measure side effects and receive their subsequent pills), and then in 12 weeks at treatment completion (to measure side effects). At 12 weeks following treatment completion, blood sample was collected for HCV RNA testing using RT-PCR to evaluate the sustained virologic response (SVR12) as an indicator of response to DAA therapy. If any patient did not attend this visit, a later appointment was arranged for them. If less than 10 doses were missed, the treatment was prolonged by the number of missed doses. If more than 10 doses were missed, the condition was considered as treatment termination and HCV RNA was evaluated 12 weeks after treatment termination.
Participants in the trial received a small value incentive in cash ($2 equal to ~100,000 Rials) for every visit as a compensation for their time and travel costs. Physician visits, laboratory assessments, and HCV treatment were provided at no costs. Participants received either in-person (by outreach team) or phone call reminders for their next visits one week and two days beforehand.
Study outcomes
The primary outcomes in this analysis were HCV diagnosis and treatment initiation. HCV prevalence was measured by detection of HCV antibodies among all and HCV RNA among those with a positive antibody test result. HCV treatment initiation was evaluated among individuals with chronic HCV infection (HCV RNA detected) and was defined as receipt of four-week supply of HCV medication.
Secondary outcome was treatment response. Response to therapy was measured by sustained virological response (SVR) which was defined as an undetectable HCV RNA 12 weeks post-treatment (SVR12). If HCV RNA assessment was not possible at exactly 12 weeks post-treatment, the result of the next available HCV RNA assessment was used for SVR. SVR12 was calculated by intention to treat (ITT), including participants who initiated a 12-week course of DAA therapy.
Statistical analysis
Descriptive statistics consisting of medians and interquartile ranges (IQRs) for continuous variables and frequencies and percentages for categorical variables, were used to summarize participants’ characteristics and risk exposures at enrolment.
The proportions of individuals with positive HCV antibody and positive HCV RNA tests were measured among all participants. Since data analysis of studies using RDS method requires an adjustment for network size and homophily within networks, we used RDS package in R software [27] and Gile’s successive sampling estimator [28], to generate weighted prevalence estimates for HCV chronic infection overall and in subgroups of demographics and risk exposures among study participants.
Treatment initiation was evaluated among all participants with current HCV infection. Treatment response was evaluated among those who initiated DAA therapy within timeframe to have completed SVR12 assessment.
HCV care cascade was assessed by four interlinked steps including 1) numbers ever exposed to HCV infection (HCV antibody positive), 2) numbers and proportion with current HCV infection (HCV RNA positive) among who tested HCV antibody-positive, 3) numbers and proportion ever initiated treatment among those with current HCV infection, and 4) numbers and proportion who achieved SVR12 among those who initiated treatment.
Results
Participant’s characteristics
Between July 10, 2018, and May 12, 2019, 171 participants were recruited into Rostam study. The median (range) of network size of recruited PWID was 7 (3– 17), and the longest chain length was 12 people. The median (range) of age was 39 years (34– 47), and 89.5% were male (Table 1). Most participants had some or completed secondary or high school education (56.1%), were currently employed (77.8%), had a monthly income less than $100 (75.1%) and were not currently living with a spouse/partner (85.9%). A considerable proportion of participants had ever experienced homelessness (88.2%) and 24.5% reported incarceration in the last 12 months.
Table 1.
Selected characteristics of participants enrolled in Rostam and crude and weighted prevalence of chronic HCV infection, with row percentages as a proportion of the total enrolled.
| Characteristics | Total (%) | HCV chronic infection | |
|---|---|---|---|
| Crude n (%) |
Weighted n (%) |
||
| N= 171 | 47 (27.5%) | 47 (23.5%) | |
| Age (years) | |||
| Median (IQR) | 39 (34, 47) | 42 (38, 50) | 42 (38, 50) |
| 18–29 | 18 (10.5) | 3 (16.7) | 3 (18.0) |
| 30–39 | 69 (40.3) | 15 (21.7) | 15 (24.3) |
| ≥40 | 84 (49.2) | 29 (34.5) | 29 (30.2) |
| Sex | |||
| Male | 153 (89.5) | 44 (28.7) | 44 (32.3) |
| Female | 18 (10.5) | 3 (16.7) | 3 (13.1) |
| Education | |||
| Primary or less | 61 (35.7) | 16 (26.2) | 16 (20.3) |
| Some or completed secondary or high school | 96 (56.1) | 27 (28.1) | 27 (39.0) |
| University | 14 (8.2) | 4 (28.6) | 4 (23.6) |
| Unemployed, current | |||
| Yes | 38 (22.2) | 12 (31.6) | 12 (30.1) |
| No | 133 (77.8) | 35 (26.3) | 35 (28.0) |
| Monthly incomea | |||
| Under 1,000,000T (<$100) | 127 (75.1) | 39 (30.7) | 39 (36.4) |
| 1,000,000–4,999,999T ($100–499) | 39 (23.1) | 7 (17.9) | 7 (11.9) |
| 5,000,000T or more (>$500) | 3 (1.8) | - | - |
| Living with their spouse/partner, current | |||
| Yes (i.e., married, live with partners) | 24 (14.1) | 5 (20.8) | 5 (17.4) |
| No (i.e., single, married but live alone, widowed) | 147 (85.9) | 42 (28.6) | 42 (32.0) |
| Homelessness, evera | |||
| Yes | 150 (88.2) | 38 (25.3) | 38 (30.8) |
| No | 20 (11.8) | 9 (45.0) | 9 (39.5) |
| Incarceration, the last 12 months | |||
| Yes | 42 (24.5) | 9 (21.4) | 9 (19.4) |
| No | 129 (75.5) | 38 (29.5) | 38 (31.5) |
| Any alcohol use, the last 12 monthsa | |||
| Yes | 41 (24.4) | 15 (36.6) | 15 (31.4) |
| No | 127 (75.6) | 32 (25.2) | 32 (30.4) |
| HIV status | |||
| Positive | 6 (3.5) | 6 (100.0) | 6 (100.0) |
| Negative | 165 (96.5) | 41 (24.8) | 41 (17.4) |
| Years since first injection | |||
| Median (IQR) | 27 (22, 33) | 24 (22, 31) | 24 (22.31) |
| Injecting frequency, the last 3 monthsa | |||
| Daily | 57 (33.5) | 22 (38.6) | 22 (31.7) |
| Weekly (i.e. Not daily but at least weekly) | 36 (21.2) | 9 (25.0) | 9 (23.8) |
| Less than weekly | 53 (31.2) | 10 (18.9) | 10 (9.7) |
| None | 24 (14.1) | 6 (25.0) | 6 (42.2) |
| Injected with another person, the last 12 monthsa | |||
| Yes | 119 (70.0) | 37 (31.1) | 37 (38.2) |
| No | 51 (30.0) | 10 (19.6) | 10 (23.5) |
| Receptive syringe sharing, the last 12 months | |||
| Yes | 13 (7.6) | 3 (23.1) | 3 (20.7) |
| No | 158 (92.4) | 44 (27.8) | 44 (30.1) |
| Receptive injecting equipment sharing, the last 12 months | |||
| Yes | 26 (15.2) | 14 (53.8) | 14 (55.5) |
| No | 145 (84.8) | 33 (22.7) | 33 (21.1) |
| Drug overdose, evera | |||
| Yes | 73 (42.9) | 24 (32.9) | 24 (34.6) |
| No | 97 (57.1) | 23 (23.7) | 23 (25.4) |
| Received OAT, last 12 months | |||
| Yes | 160 (93.6) | 44 (27.5) | 44 (29.8) |
| No | 11 (6.4) | 3 (27.3) | 3 (25.0) |
| Easy access to free needle/syringe, currenta | |||
| Yes (very easy/somehow easy) | 112 (69.6) | 34 (30.3) | 34 (33.2) |
| No (very difficult/somehow difficult) | 49 (30.4) | 13 (26.5) | 13 (23.6) |
| HCV testing, evera,b | |||
| Yes | 20 (11.8) | 11 (55.0) | 11 (57.6) |
| No | 133 (78.7) | 32 (24.1) | 32 (22.5) |
| Do not know | 16 (9.5) | 4 (25.0) | 4 (24.0) |
| HCV treatment uptake, evera,b,c | |||
| Yes | 5 (45.5) | 3 (60.0) | 3 (61.0) |
| No | 6 (54.5) | 5 (83.3) | 5 (80.0) |
Among participants with available information
Self-reported
Among 11 individuals who reported ever being HCV RNA positive
Abbreviations- IQR; interquartile range, OAT; opioid agonist therapy
Around 24.4% reported any alcohol use in the last 12 months. Median years (range) since first injection was 27 (22–33). With regard to injection frequency, 33.5% and 21.2% reported respectively daily and weekly injection in the last 3 months. Seventy percent reported injection with another person in the last 12 months. The prevalence of receptive sharing of syringe and injection equipment was respectively 7.6% and 15.2% in last 12 months and 42.9% reported ever having a drug overdose. Receiving OAT in last 12 months was reported by a high proportion (93.6%) and 69.6% of participants reported that access to free needle/syringe is very to somehow easy for them.
HCV testing and treatment history
A total of 20 individuals (11.8%) reported ever being tested for HCV, of which 55.0% (11/20) reported being HCV RNA positive at the time of testing and of those reporting ever being HCV RNA positive, 45.5% (5/11) reported ever initiating HCV therapy.
Hepatitis C diagnosis
Sixty-two of 171 (36.2%) participants enrolled in the study were anti-HCV positive, of whom 47 (75.8%) were HCV RNA positive leading to an overall crude prevalence of HCV chronic infection of 27.5%. Overall, HIV prevalence was 3.5% leading to an HIV/HCV co-infection prevalence of 3.5%.
The crude and weighted prevalence of HCV chronic infection are presented overall and in subgroups of participants in Table 1. The weighted prevalence of HCV chronic infection was 23.5% overall and was considerably higher among PWID who were male (32.3% vs. 13.1% among females), who had a monthly income less than $100 (36.4% vs. 11.9% among those who had a monthly income between $100 and $499), with a positive status of HIV (100.0% vs. 17.4% in HIV negative individuals), who reported receptive sharing of injecting equipment in the last 12 months (55.5% vs. 21.1% among those who did not), with a history of HCV testing (57.6% vs. 22.5% among those never tested) and with no history of HCV treatment uptake (80.0% vs. 61.0% among those who were never treated for HCV).
Hepatitis C treatment initiation
Among 47 individuals diagnosed with chronic HCV infection in Rostam study, 36 (76.6%) returned for receiving their HCV RNA test results and 11 (23.4%) were lost to follow up. Treatment eligibility assessment and further laboratory workout were performed for these 36 individuals, and all were eligible for treatment. Of the 36 eligible individuals, 2 (5.5%) were lost to follow up again and 34 (94.4%) returned and initiated HCV antiviral therapy at the Rostam site.
Compared to those who initiated treatment, those who did not (Table 2) were less frequently ever homeless (69.2% vs. 85.3%), had more frequently an income less than $100 (92.3% vs. 76.5%) and more frequently reported injecting in the last 3 months (69.2% vs. 58.8%). However, no differences were significant.
Table 2.
Characteristics of participants diagnosed with chronic HCV in subgroups who started HCV treatment and who did not, N= 47
| Characteristics | Not started treatment N = 13 (27.7%) |
Initiated treatment N = 34 (72.3%) |
P-value |
|---|---|---|---|
| Male sex | 13 (100) | 31 (91.2) | 0.2 |
| Mean age [SD], Years | 45.3 [13.2] | 46.3 [15.1] | - |
| Ever being homeless | 9 (69.2) | 29 (85.3) | 0.2 |
| Low education (primary or less) | 8 (61.5) | 22 (64.7) | 0.8 |
| living with their spouse (no) | 11 (84.6) | 31 (91.2) | 0.5 |
| Having an employment (no) | 9 (69.2) | 23 (67.6) | 0.8 |
| Monthly income (Under 1,000,000T (<$100)) | 12 (92.3) | 26 (76.5) | 0.2 |
| Injecting frequency reported in the last 3 months (at least weekly) | 9 (69.2) | 20 (58.8) | 0.5 |
| Injected with another person in the last 12 months | 11 (84.6) | 27 (79.4) | 0.6 |
| Reported OAT in last 12 months | 10 (76.9) | 25 (73.5) | 0.7 |
Abbreviations- SD; standard deviation, OAT; opioid agonist therapy
HCV treatment response
Treatment response was evaluated among participants who initiated treatment and returned to have assessments of SVR12. From 34 individuals who initiated treatment, six were lost to follow-up during the treatment and two never returned for SVR assessment. A total 26 people completed treatment and returned for their SVR assessments.
The assessment was performed for 26 individuals and 24 achieved SVR12 leading to a treatment response of 92.3% (24/26).
HCV care cascade
Figure 2 shows all the steps in the HCV care cascade along with numbers of lost to follow up in each step in our study. A total of 62 individuals were anti-HCV positive, of whom 47 (75.8%) were HCV RNA positive. Of RNA positive individuals, 36 (76.6%) returned for treatment eligibility assessment and 11 were lost. Of all the 36 participants eligible for treatment, 34 (94.4%) initiated treatment and 2 were lost. Of those who initiated treatment, 26 (76.5%) completed treatment and returned for SVR assessments and 8 were lost. Finally, from 26 individuals who had an RNA test available 12 weeks after treatment, 24 (92.3%) achieved SVR12 and 2 did not.
Figure 2.
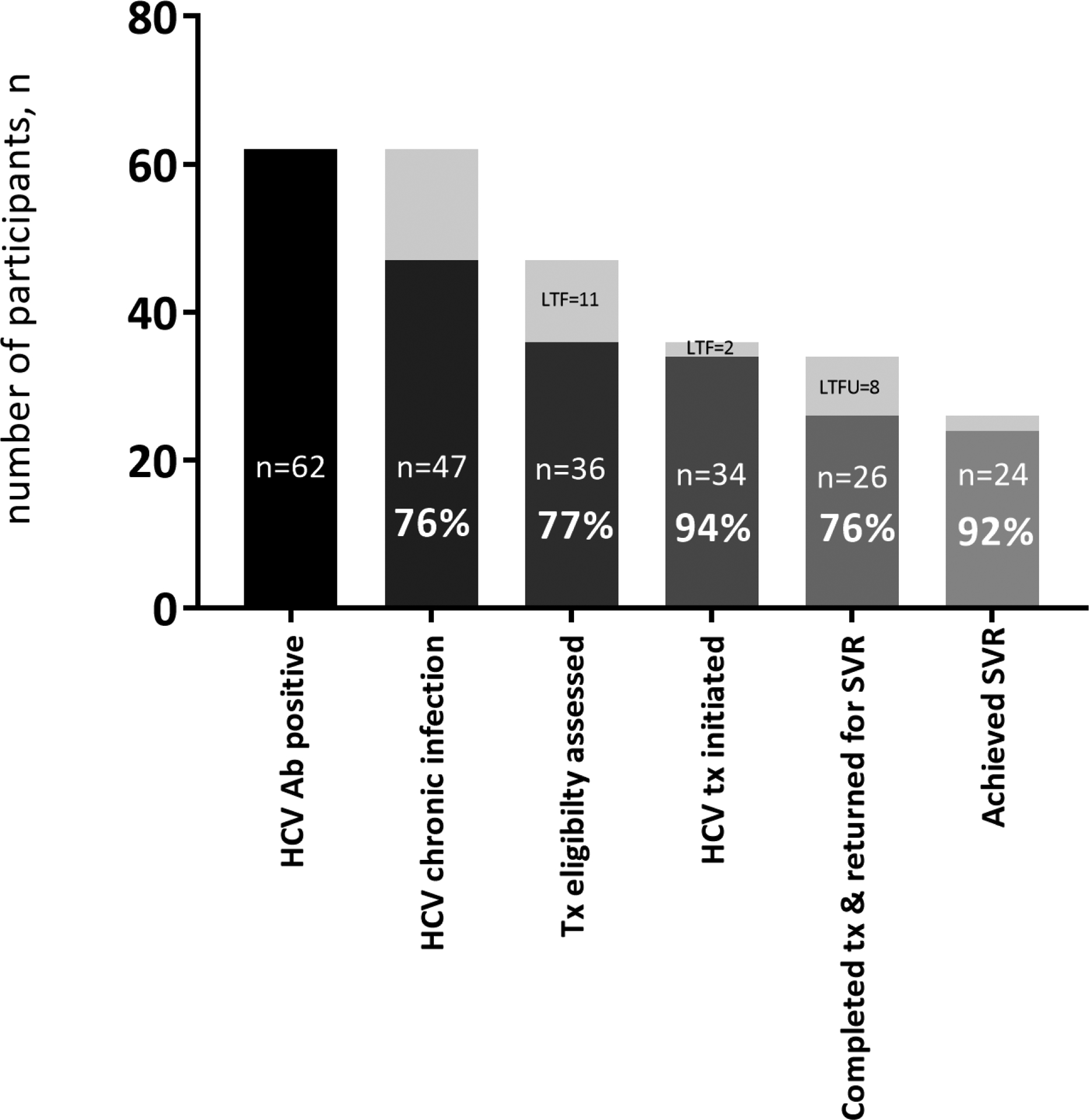
Hepatitis C virus screening, diagnosis, treatment eligibility assessment, treatment initiation, treatment completion and SVR achievement in people who inject drugs in Rostam study, n= 171
Abbreviations- Ab; antibody, Tx; treatment, SVR; sustained virological response
Discussion:
The present study was part of a larger project and focused on the prevalence of HCV infection and impact of an on-site intervention designed to increase HCV testing and treatment uptake among PWID in Iran. The study showed that more than one third of PWID had been exposed to HCV infection at least once in their life and almost a quarter were living with chronic HCV infection. Despite the very low proportions of PWID reporting ever being tested or treated for HCV, our model revealed encouraging levels of HCV testing and treatment uptake among this population. These outcomes suggest that HCV care can be effectively delivered to PWID outside hospital-based settings and through community-based efforts. The results can also be reflecting on the trusting relationship between providers of care in such settings and PWID, lack of which has been addressed as an important barrier to HCV care among PWID [29].
HCV prevalence among our PWID population (23.5%) was higher than what found (13.5%) from a similar setting study carried out among clients of seven DICs between 2006 and 2011 in Shiraz, Iran [30]. HCV antibody prevalence in our study, on the other hand, was lower than estimates previously reported (~52%) for Iranian PWID in two meta-analyses [31, 32]. The studies included in these two meta-analyses, however, had recruited participants mostly from clinical settings and through convenience sampling. In current study, we applied RDS, a method that has been demonstrated to improve statistical reliability and validity of outcomes and reflects better on the prevalence estimates in hard-to-reach populations such as PWID [27, 33]. The other likely reason for the observed lower antibody prevalence might be the difference in recency of injecting drug use between our study (any injection in last 12 months) and studies included in the meta-analyses (active or ongoing injection).
High levels of marginalization (i.e., homelessness) and low levels of previous HCV testing and treatment uptake were reported among our study population. However, we observed that our intervention of integrating on-site rapid HCV antibody testing, RNA testing and on-site dispensing of DAA therapy within a DIC setting was highly acceptable among PWID, with more than 90% of those eligible, initiating treatment. Unrestricted access to DAA therapy might have encouraged those more eager for treatment to participate in our study as results from earlier papers indicated high levels of unwillingness to receive interferon-based HCV treatments within the same settings among this population [34]. DIC settings in Iran mainly provide low-threshold harm reduction services, such as distribution of sterile needles and syringes, OAT and recently HIV rapid testing and counselling. Integration of other services such as psychoeducation interventions for people who use amphetamine-type substances [35] has recently been piloted in 10 DICs in Iran and shown promising results in engaging people in such programs. With the high prevalence of HCV infection among clients of DICs and OAT clinics and with the extensive network of drug treatment and harm reduction in Iran [9, 10], integration of HCV care and treatment within these settings has the potential to identify and treat a large cohort of HCV-infected PWID.
There are a number of limitations in this study. We assessed the integration of HCV care only within one DIC and in one city in Iran. Therefore, the generalizability of our findings to other settings used by PWID and other populations of PWID remains undetermined. Next, similar to previous research in similar settings [23, 36], women were underrepresented in our study population, which has been suggested to be an indicator of higher levels of stigma around drug use and therefore more restricted access to harm reduction services among women [37]. The sample size was not powered enough to study factors associated with HCV testing or treatment uptake among our study population. Further, we tried but failed to locate and conduct interviews with those who did not return for their HCV test results or initiation of their treatment, which could have provided more insights for HCV care barriers in PWID population.
In conclusion, the integration of on-site HCV care within a community-based drop-in-center among PWID in Kerman, Iran was successful and led to high levels of HCV testing and treatment uptake among this marginalized population. Our model can provide important information for the development and implementation of future interventions within other settings to enhance HCV assessment and treatment outcomes among PWID. Given that only a few individuals in this study had ever been tested or treated for HCV infection, provision of HCV care within the existing infrastructure for harm reduction and drug treatment has the potential to reach a large population at risk of HCV infection. However, further research is needed to understand the next steps in expanding these models of care to PWID populations in Iran, particularly now that highly effective interferon-free DAA therapies have made the elimination of hepatitis C a possibility.
Acknowledgments
This research was supported by a grant from the National Institutes of Health, University of California, San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research (P30-AI027763), AIDS Research Institute Strategic Support, and the Iranian Ministry of Health (Mental Health and HIV national program). The Sobhan Drug Company donated the HCV medications. We would like to express our sincere gratitude to Monadian-Salamat DIC manger (Farahnaz Farahbakhsh) and all the DIC staff for their outstanding collaboration and supports. We acknowledge in particular the help and support of our research staff (Soheil Mehmandoost, Mehrdad Khezri, Mohammadreza Shojaie and Majid Mirzaie) and our scientific consultants (Alireza Noroozi, Mehdi Shafiei and Willi McFarland).
Footnotes
Conflicts of interest
None.
References
- 1.Zafarghandi MBS, Jadidi M, and Khalili N, Iran’s activities on prevention, treatment and harm reduction of drug abuse. International journal of high risk behaviors & addiction, 2015. 4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin‐Esmaeili M, et al. , Epidemiology of illicit drug use disorders in Iran: prevalence, correlates, comorbidity and service utilization results from the Iranian Mental Health Survey. Addiction, 2016. 111(10): p. 1836–1847. [DOI] [PubMed] [Google Scholar]
- 3.Amin-Esmaeili M, et al. , Profile of people who inject drugs in Tehran, Iran. Acta Medica Iranica, 2016: p. 793–805. [PubMed] [Google Scholar]
- 4.Amin-Esmaeili M, et al. , Factors correlated with hepatitis C and B virus infections among injecting drug users in Tehran, IR Iran. Hepatitis monthly, 2012. 12(1): p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mumtaz GR, et al. , HIV among people who inject drugs in the Middle East and North Africa: systematic review and data synthesis. PLoS Med, 2014. 11(6): p. e1001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moradi G, et al. , Drug use and risk behaviour profile, and the prevalence of HIV, hepatitis C and hepatitis B among people with methamphetamine use in Iran. International Journal of Drug Policy, 2019. 73: p. 129–134. [DOI] [PubMed] [Google Scholar]
- 7.Hariri S, et al. , Continuum of hepatitis C care cascade in prison and following release in the direct-acting antivirals era. Harm Reduction Journal, 2020. 17(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajabi A, Sharafi H, and Alavian SM, Harm reduction program and hepatitis C prevalence in people who inject drugs (PWID) in Iran: an updated systematic review and cumulative meta-analysis. Harm Reduction Journal, 2021. 18(1): p. 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alam-Mehrjerdi Z, et al. , Drug use treatment and harm reduction programs in Iran: A unique model of health in the most populated Persian Gulf country. Asian journal of psychiatry, 2015. 16: p. 78–83. [DOI] [PubMed] [Google Scholar]
- 10.Nissaramanesh B, Trace M, and Roberts M, The rise of harm reduction in the Islamic Republic of Iran. Beckley Foundation Drug Policy Programme, Briefing Paper, 2005. 8. [Google Scholar]
- 11.National AIDS Committee Secretariat, M.o.H.a.M.E., Islamic Republic of Iran AIDS Progress Report On Monitoring of the United Nations General Assembly Special Session on HIV and AIDS. March 2015.
- 12.Karamouzian M, et al. , Improving the quality and quantity of HIV data in the Middle East and North Africa: key challenges and ways forward. International journal of health policy and management, 2017. 6(2): p. 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malekinejad M, et al. , High hepatitis C virus prevalence among drug users in Iran: systematic review and meta-analysis of epidemiological evidence (2001–2012). International Journal of Infectious Diseases, 2015. 40: p. 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massah O, et al. , Barriers to hepatitis C treatment among women in methadone treatment: A study from Iran, the most populous Persian Gulf country. Addiction & health, 2017. 9(4): p. 229. [PMC free article] [PubMed] [Google Scholar]
- 15.Hajarizadeh B, Generic direct acting antiviral treatment: the first step towards elimination of hepatitis C in Iran. Hepatitis Monthly, 2017. 17(1): p. 1. [Google Scholar]
- 16.Hajarizadeh B, et al. , Hepatitis C treatment as prevention: evidence, feasibility, and challenges. The lancet Gastroenterology & hepatology, 2016. 1(4): p. 317–327. [DOI] [PubMed] [Google Scholar]
- 17.Hesamizadeh K, et al. , Next steps toward eradication of hepatitis C in the era of direct acting antivirals. Hepatitis monthly, 2016. 16(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharafi H, et al. , Performance of a rapid diagnostic test for screening of hepatitis C in a real-life prison setting. Journal of Clinical Virology, 2019. 113: p. 20–23. [DOI] [PubMed] [Google Scholar]
- 19.Socías ME, et al. , Integrated models of care for people who inject drugs and live with hepatitis C virus: A systematic review. International Journal of Drug Policy, 2019. 72: p. 146–159. [DOI] [PubMed] [Google Scholar]
- 20.Grebely J, et al. , Treatment for hepatitis C virus infection among people who inject drugs attending opioid substitution treatment and community health clinics: the ETHOS Study. Addiction, 2016. 111(2): p. 311–319. [DOI] [PubMed] [Google Scholar]
- 21.Lindenburg CE, et al. , Hepatitis C testing and treatment among active drug users in Amsterdam: results from the DUTCH-C project. European journal of gastroenterology & hepatology, 2011. 23(1): p. 23–31. [DOI] [PubMed] [Google Scholar]
- 22.Harris KA Jr, Arnsten JH, and Litwin AH, Successful integration of hepatitis C evaluation and treatment services with methadone maintenance. Journal of addiction medicine, 2010. 4(1): p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alavi M, et al. , An intervention to improve HCV testing, linkage to care, and treatment among people who use drugs in Tehran, Iran: The ENHANCE study. International Journal of Drug Policy, 2019. 72: p. 99–105. [DOI] [PubMed] [Google Scholar]
- 24.Iran., S.c.o., national population and housing census. Retrieved from: https://www.amar.org.ir/english/Population-and-Housing-Censuses. 2016.
- 25.Organization WH, Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. 2018. [PubMed]
- 26.Alavian SM, et al. , Recommendations for the clinical management of hepatitis C in Iran: a consensus-based national guideline. Hepatitis monthly, 2016. 16(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Handcock M, Fellows I, and Gile K, RDS: Respondent-Driven Sampling, Version 0.8–1. Project home page at http://hpmrg.org, URL https://CRAN.R-project.org/package=RDS., 2012. 10: p. 2017. [Google Scholar]
- 28.Gile KJ, Improved inference for respondent-driven sampling data with application to HIV prevalence estimation. Journal of the American Statistical Association, 2011. 106(493): p. 135–146. [Google Scholar]
- 29.Mirzazadeh A, et al. , Barriers and motivators to participation and retention in HIV/HCV cohort studies among people who inject drugs: a community consultation in Iran. Substance abuse treatment, prevention, and policy, 2020. 15(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salehi A, et al. , Prevalence of HIV, HCV, and high-risk behaviors for substance users in drop in centers in southern Iran. Journal of addiction medicine, 2015. 9(3): p. 181–187. [DOI] [PubMed] [Google Scholar]
- 31.Bagheri Amiri F, Mostafavi E, and Mirzazadeh A, HIV, HBV and HCV coinfection prevalence in Iran-a systematic review and meta-analysis. PloS one, 2016. 11(3): p. e0151946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmud S, Akbarzadeh V, and Abu-Raddad LJ, The epidemiology of hepatitis C virus in Iran: systematic review and meta-analyses. Scientific reports, 2018. 8(1): p. 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaghaghi A, Bhopal RS, and Sheikh A, Approaches to recruiting ‘hard-to-reach’populations into research: a review of the literature. Health promotion perspectives, 2011. 1(2): p. 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alam-Mehrjerdi Z, et al. , Willingness to receive treatment for hepatitis C among injecting drug users on methadone program: implications for education and treatment. Addiction & health, 2016. 8(2): p. 90. [PMC free article] [PubMed] [Google Scholar]
- 35.Radfar SR, Mohsenifar S, and Noroozi A, Integration of methamphetamine harm reduction into opioid harm reduction services in Iran: preliminary results of a pilot study. Iranian Journal of Psychiatry and Behavioral Sciences, 2017. 11(2). [Google Scholar]
- 36.Rahnama R, et al. , Access to harm reduction programs among persons who inject drugs: findings from a respondent-driven sampling survey in Tehran, Iran. International Journal of Drug Policy, 2014. 25(4): p. 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolan K, et al. , Characteristics of Iranian women seeking drug treatment. Journal of women’s health, 2011. 20(11): p. 1687–1691. [DOI] [PubMed] [Google Scholar]


