Abstract
Kartogenin, a small and heterocyclic molecule, has emerged as a promising therapeutic agent for incorporation into biomaterials, owing to its unique physicochemical and biological properties. It holds potential for the regeneration of cartilage-related tissues in various common conditions and injuries. Achieving sustained release of kartogenin through appropriate formulation and efficient delivery systems is crucial for modulating cell behavior and tissue function. This review provides an overview of cutting-edge kartogenin-functionalized biomaterials, with a primarily focus on their design, structure, functions, and applications in regenerative medicine. Initially, we discuss the physicochemical properties and biological functions of kartogenin, summarizing the underlying molecular mechanisms. Subsequently, we delve into recent advancements in nanoscale and macroscopic materials for the carriage and delivery of kartogenin. Lastly, we address the opportunities and challenges presented by current biomaterial developments and explore the prospects for their application in tissue regeneration. We aim to enhance the generation of insightful ideas for the development of kartogenin delivery materials in the field of biomedical therapeutics and regenerative medicine by providing a comprehensive understanding of common preparation methods.
Keywords: Kartogenin, biomaterials, delivery system, controlled release, tissue engineering
1. Introduction
The field of regenerative medicine holds great promise for restoring structure and function to damaged, diseased, or degenerated cells, tissues, and organs (Berthiaume et al., 2011; Sun & Kurtzberg, 2015). Osteoarthritis, a degenerative disease of the joints, is caused by mechanical stress, ligamentous injury, and genetic factors affecting cartilage and bone. It leads to pain, inflammation, and impaired of joint function (Hunter, 2011). In the context of regenerative medicine, an ideal approach to treating osteoarthritis would involve stimulating chondrogenic differentiation of stem cells and the formation of new cartilage tissue. Although various methods have been proposed to promote cartilage regeneration and prevent the progression of osteoarthritis, including the use of growth factors and biomaterials, there is still no highly effective pharmacological treatment available (Wu et al., 2014; Wang et al., 2019). Growth factors, particularly transforming growth factors are commonly employed for cartilage regeneration. However, due to their instability and short half-lives, achieving long-term induction has proven challenging (Lo et al., 2014; Li et al., 2016). To solve this, Johnson et al. utilized an image-based, high-throughput screening approach to evaluate over 22,000 structurally diverse, heterocyclic, drug-like molecules that mimic natural ligands involved in cell signaling and differentiation. Among these molecules, they identified kartogenin, one small molecule that demonstrated a dose-dependent promotion of chondrocyte differentiation from mesenchymal stem cells (Johnson et al., 2012; Marini & Forlino, 2012). Furthermore, kartogenin’s in vivo efficacy was assessed in two murine osteoarthritis models, both of which showed the formation of new articular cartilage, reduced serum levels of cartilage breakdown products, and improved load-bearing capacity. As a result, kartogenin has emerged as a small, functionally active molecule with regenerative and protective effect on cartilage tissue, and is now being investigated in the field of regenerative medicine.
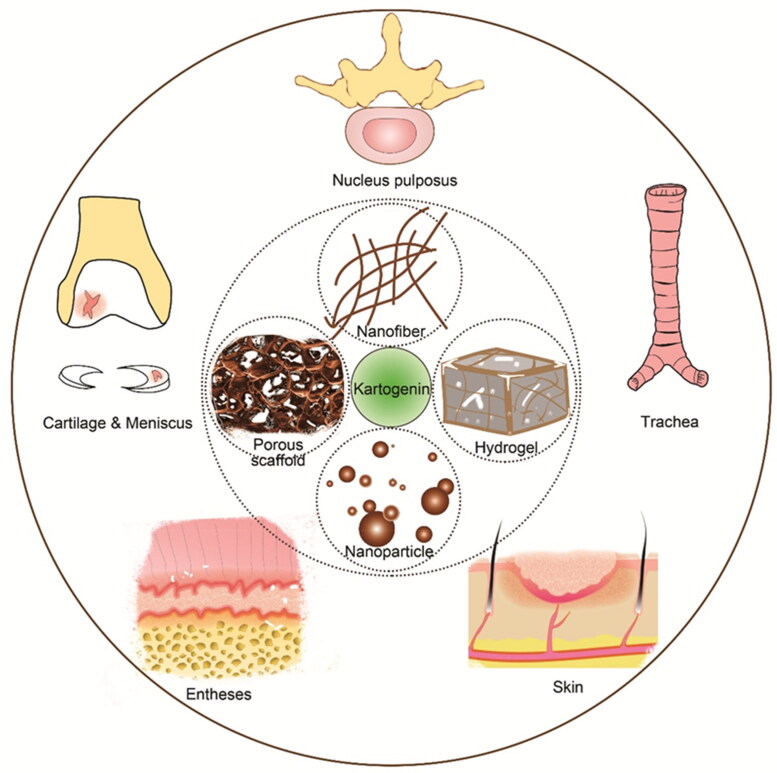
Since its initial discovery and subsequent characterization, kartogenin has undergone extensive investigation for various applications, particularly in tissue engineering and regenerative medicine. These applications encompass a range of anatomical sites, including cartilage, nucleus pulposus, meniscus, trachea, skin, and entheses (Figure 1) (Yin et al., 2017; Zhu et al., 2017; Im, 2018; Cai, Liu et al., 2019; Cai, Zhang et al., 2019). However, the physical and chemical properties of kartogenin, such as its hydrophobicity and low bioavailability, often hinder its standalone effectiveness. Therefore, a more prudent approach involves combining kartogenin with biomaterials, which holds promise for guiding tissue regeneration (Makris et al., 2015; Stejskalová & Almquist, 2017; Armiento et al., 2018). Notably, the structure and properties of kartogenin, particularly its carboxyl groups and hydrophobicity nature, make it amenable to incorporation into a diverse range of biomaterials. Introducing small, functional molecules into scaffolds represents an economical and effective strategy for developing bioactive materials for tissue engineering (Lu & Atala, 2014; Sun et al., 2019; Xuan et al., 2020). Moreover, the combination of kartogenin with other materials can yield synergistic effects. Overcoming limitations in the drug’s physical and chemical properties, thereby enhancing its bioavailability and reducing side effects. Simultaneously, it can augment the biological activity and functionality of scaffolds intended for tissue regeneration.
Figure 1.
Schematic diagram of kartogenin-Combinative biomaterials, including nanoparticles, nanofibers, porous scaffold, hydrogels etc. and their potential applications in regenerative medicine such as cartilage, meniscus, skin, entheses.
Since its initial report in 2012, there have been over 150 related studies related to kartogenin. However, existing reviews of kartogenin are limited in scope and fail to provide a comprehensive overview of the current state of the field. In this review, we extensively discuss the physicochemical properties and biological function of kartogenin. Additionally, we provide an in-depth examination of state-of-the-art functionalized biomaterials, with a particular focus on their design, structure, function, and application. Furthermore, we address the necessary properties for further translational applications in diverse biomaterials. Finally, we outline potential research directions for the utilization of kartogenin and similar bioactive molecules, elucidating their prospects in the realms of biomaterials and regenerative medicine.
2. Properties of kartogenin
Since its discovery, numerous studies have extensively investigated the properties and underlying biological mechanisms of kartogenin (Figure 2). In this section, we provide a comprehensive overview of its fundamental physicochemical properties and biological functions.
Figure 2.
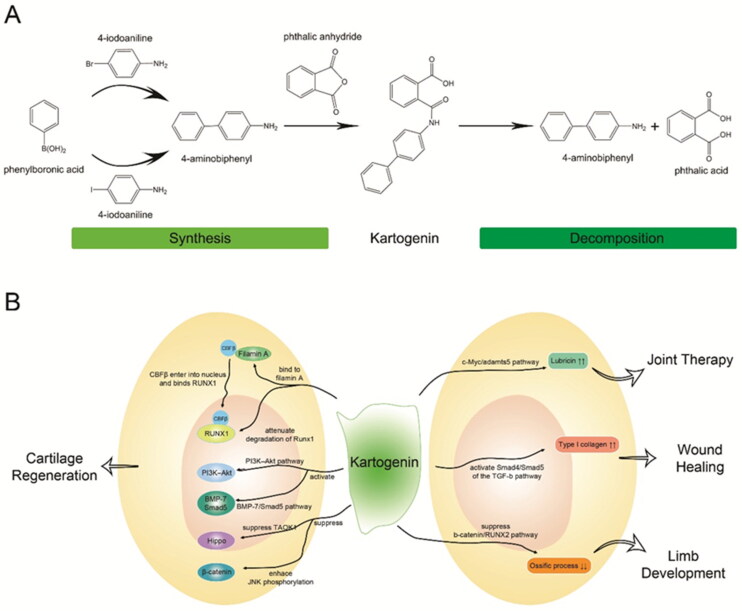
The structure and function of kartogenin. A) Schematic diagram of synthesis and decomposition of kartogenin. kartogenin can be synthesized through the chemical reaction of 4-aminobiphenyl and phthalic anhydride, and hydrolyzed into 4-aminobiphenyl and phthalic acid. B) Signaling pathways and cell receptors of kartogenin involved in regenerative medicine. Each arrow indicates a specific signaling pathway or tissue engineering.
2.1. Physicochemical properties of kartogenin
Kartogenin is a small, heterocyclic molecule (Figure 2A) with a molecular weight of 317.3 g/mol. Structurally, it consists of 4-aminobiphenyl (4-ABP) and phthalic acid (PA) linked by an amide bond. Additionally, kartogenin exhibits hydrophobic characteristics, making it readily soluble in dimethyl sulfoxide while remaining insoluble in water. Moreover, it demonstrates stable storage properties at room temperature. Synthesis of kartogenin involves the utilization of phthalic anhydride and 1,1′-biphenyl-4-amine (Johnson et al., 2012), which can be prepared through the combination of phenylboronic acid with 4-iodoaniline or 4-bromoaniline (Shi et al., 2016; Massaro et al., 2019). Various analytical methods that can be employed to confirm or detect kartogenin based on its structure (Hu et al., 2017), such as hydrogen/carbon nuclear magnetic resonance (1H/13C NMR), mass spectrometry (MS), Fourier transform infrared spectroscopy (FTIR), high performance liquid chromatography (HPLC), ultraviolet-visible spectroscopy (UV–Vis), differential scanning calorimetry (DSC), and X-ray diffraction (XRD) (see Table 1). Conversely, kartogenin can undergo hydrolysis by amide bond-breaking substances like amidases and peptidases, liberating its hydrolysates 4-ABP and PA, with 4-ABP playing a pivotal role in chondrogenic differentiation (Zhang et al., 2019).
Table 1.
The common detection methods and characteristics of kartogenin.
| Methods | Characteristics | Refs. | ||||
|---|---|---|---|---|---|---|
| 1H NMR | (DMSO-d6, δ, ppm) 7.36 (tt, 1H), 7.47 (tt, 2H), 7.59 (m, 2H), 7.66 (m, 5H), 7.81 (dt, 2H), 7.91 (dd, 1H), 10.45 (brs, 1H, NH); 13.05(brs, 1H, COOH). The chemical shifts of the 13 hydrogen atoms connected with benzene ring are at 6.0-7.0 ppm. So far, no attribution of the chemical shifts of these hydrogen atoms has been reported. | (Johnson et al., 2012) | ||||
| (CDCl3) δ: 8.02-7.99 (m, 2H, CH): 7.85-7.81 (m, 2H, CH), 7.77-7.73 (m, 2H, CH); 7.76-7.56 (m, 2H, CH); 7.54-7.47 (m, 4H, CH); 7.43-7.39 (m, 1H, CH); These 13 hydrogens are all of benzene rings | (Massaro et al., 2019) | |||||
| Prominent resonance peaks at δ = 7.3–7.9 ppm for protons of the benzene ring substituted with dicarboxylic acid. | (Hu et al., 2017; Kang et al., 2014) | |||||
| 13C NMR | (DMSO-d6, δ, ppm) 163.9, 139.0, 136.2, 133.1, 132.4, 129.1, 128.5, 120.5, 118.3, 113.8, 20.5. | (Johnson et al., 2012) | ||||
| MS | (ESI+) [M + H]+ m/z(318.1130) C20H16NO3+ (318.352) | (Johnson et al., 2012) | ||||
| (ESI-) [M - H]- m/z(316.1039) C20H14NO3- (316.336) | (Hu et al., 2017) | |||||
| FTIR | 3056 cm−1 H-C = C stretching; 1787 cm−1 HO-C = O stretching; 1702 cm−1 H2N-C = O stretching; 1494 cm−1 C = C stretching; 1531 cm−1 C = C bending; 1710 cm−1 C = O stretching; 1650 cm−1 C-N stretching. | (Massaro et al., 2019; Kang et al., 2016; Yang, Zheng et al., 2019) | ||||
| UV–Vis | λmax = 261 nm | (Massaro et al., 2019) | ||||
| HPLC | Column | Solvent | Wavelength | Flow rate | Retention time | |
| C18 | Acetonitrile/water (v:v = 35:65) + 0.1% formic acid | 274nm | 1mL/min | (Kang et al., 2014; Shi et al., 2016) | ||
| C18 | Gradient, A: acetonitrile + 0.1% H3PO4; B: water + 0.1% H3PO4 | 280nm | 0.4 mL/min | 21min | (Zhang et al., 2017) | |
| XRD | High specific peak is at 5.7° on the 2θ scale. | (Maudens et al., 2018) | ||||
| DSC | Endothermic event (Tm)-melting temperature = 198 °C. | (Maudens et al., 2018) | ||||
Furthermore, kartogenin possesses two hydrogen bond donor sites and three hydrogen bond acceptor groups. This property allows effective but reversible attachment to the matrix structure through hydrogen bonding when polysaccharides are employed in scaffold production. Consequently, costly or cumbersome binding strategies are not required (Kang et al., 2014, 2016; Westin et al., 2017). Additionally, its carboxyl group enables chemically crosslinking with other materials through covalent bonding, such as amidation or esterification with amine and hydroxyl groups, respectively. These physical and chemical properties facilitate the application of kartogenin in biomaterial engineering, utilizing either physical encapsulation or chemical conjugation methods.
2.2. Biological functions of kartogenin
Initially, Johnson and colleagues discovered that kartogenin induces chondrogenesis by binding to the carboxyl end of the actin-binding protein, filamin A, which disrupts its interaction with the transcription factor ‘core-binding factor β’ (CBFβ) subunit (Johnson et al., 2012). This interaction frees CBFβ to enter the nucleus and form a transcriptional complex with runt-related transcription factor 1 (RUNX1), facilitating cartilage differentiation (Figure 2B). Subsequent research has identified additional cell signaling pathways and receptors involved in kartogenin-induced chondrogenic differentiation. Zhou et al. demonstrated that kartogenin activates the BMP-7/Smad5 pathway, inducing differentiation of mesenchymal stem cells (MSCs) into chondrocytes (Zhou et al., 2019). Liu et al. suggested that KGN promotes cartilage regeneration by stimulating differentiation of cartilage stem/progenitor cells and enhancing proliferation via the IL-6/Stat3 signaling pathway (Liu et al., 2020). Fan et al. revealed that kartogenin attenuates the degradation of RUNX1, which physically interacts with p-Smad3 in the nucleus (Fan et al., 2020). Jing et al. discovered that kartogenin directs human umbilical cord MSCs (hUCMSCs) toward a precartilaginous stage by enhancing JNK phosphorylation and suppression of β-catenin (Jing et al., 2019). Furthermore, they found that small extracellular vesicles (sEvs) derived from kartogenin-preconditioned hUCMSCs are rich in miR-381-3p, which directly suppresses TAOK1 and inhibits the Hippo signaling pathway, thereby promoting chondrogenesis (Jing et al., 2020). A study by Zhang et al. suggested that 4-ABP targets the RPS6KA2 and PI3K–Akt pathways, with PI3K–Akt activation promoting osteoarthritic repair (Zhang et al., 2019).
Apart from its effects on cartilage, kartogenin also influences other tissues. It enhances lubricin accumulation through the c-Myc and adamts5 pathways (Liu et al., 2015), thereby slowing the degeneration of intervertebral disks (Huang et al., 2018) and reducing pain (Kwon et al., 2018). Wang and colleagues found that kartogenin could promotes type I collagen synthesis through activating Smad4/Smad5 of the TGF-β signaling pathway, suggesting its potential applications in wound healing (Wang et al., 2014). It has also been found to regulate hair follicles growth and hair growth cycle transition by inhibiting TGF-β2/Smad signaling (Chen et al., 2022). Additionally, kartogenin stimulates limb development through various key signaling pathways, particularly TGF-β (Decker et al., 2014) and inhibits the ossification process by suppressing the β-catenin/RUNX2 pathway (Jing et al., 2019). Furthermore, kartogenin has been utilized for fibrocartilage repair, particularly in the context of tendon-to-bone repair (Zhou et al., 2019; Chen et al., 2021).
Kartogenin exhibits good cytocompatibility and significantly enhances the proliferation of MSCs in a concentration-dependent manner (Zhang & Wang, 2014; Spakova et al., 2018; Wang et al., 2019). However, its effects on different cells vary, with 10 μM concentration yielding optimal effect on MSCs and 100 μM concentration promoting the proliferation tendon stem cells (TSCs) most effectively (Huang et al., 2017). Kartogenin can be administered directly through injection or oral. While most studies have focused on injection, one study has detected its distribution and decomposition products in vivo following oral administration (Zhang et al., 2019). The results showed the presence of kartogenin in the blood, while 4-ABP was detected in the cartilage, indicating bioabsorption through the oral route. However, despite the advantages of in situ injection in attenuating or avoiding liver metabolism and associated side effects, the limitations of low retention, short duration, the need for multiple injections and low bioavailability remain significant drawbacks. .
To date, extensive research has been conducted to investigate the receptors and signaling pathways affected by kartogenin at the cellular and molecular level. Its biological application, particularly in the manipulation and therapy of endogenous cells, have garnered widespread recognition. Efforts to minimize side effects and maximize its biological function, guided by an understanding of its physicochemical properties, have led to successful utilization of kartogenin in the field of material science, demonstrating its immense potential in regenerative medicine (Table 2). Thoughtful studies have also yielded good results in tissue engineering, which will be discussed in the following sections.
Table 2.
Summary of reported kartogenin delivery systems in regenerative medicine.
| Type | Component | Combination mode | Potential application | Refs. |
|---|---|---|---|---|
| Nanoparticles | Chitosan; | Crosslink | Cartilage regeneration | (Kang et al., 2014, 2016) |
| Chitosan/mesoporous silica | Microfluidic technology | Cartilage regeneration | (Yuan et al., 2022) | |
| Silk fibroin; | Encapsulation | Osteochondral regeneration | (Zhang et al., 2020) | |
| Upconversion; | Crosslink | Osteoarthritis | (Li et al., 2016) | |
| Polyamidoamine; | Crosslink | Osteoarthritis | (Hu et al., 2017) | |
| Poly(lactic-co-glycolic acid) (PLGA); | Encapsulation | Cartilage regeneration | (Asgari et al., 2020) | |
| PLGA and PLGA-PEG; | Mixture | Cartilage regeneration | (Almeida et al., 2020) | |
| PLGA-PEG-Hyaluronic acid (HA); | Crosslink | Cartilage regeneration | (Almeida et al., 2020) | |
| Ultrasmall superparamagnetic iron-oxide (USPIO); | Crosslink | Cartilage regeneration | (Yang, Zheng et al., 2019; Yang, Zhu et al., 2019) | |
| Poly(lactide-co-glycolide); | Double emulsion-solvent evaporation; | Osteoarthritis | (Sun et al., 2018) | |
| Polyurethane; | Crosslink; | Cartilage regeneration | (Fan et al., 2018, 2020) | |
| Isocyanatoethyl acrylate-modified β-cyclodextrin (β-CD-AOI2); | Host-guest interaction; | Osteochondral regeneration | (Liu et al., 2020) | |
| Nanovesicles | Small extracellular vesicles | Precondition; | Cartilage regeneration | (Jing et al., 2020) |
| Exosome | Electroporation. | Cartilage regeneration | (Xu et al., 2021) | |
| Microspheres | PLGA | Oil-in-water emulsion-solvent evaporation | Meniscus regeneration | (Li et al., 2021) |
| Polyamidoamine and hyaluronic acid methacrylate | Microfluidic technology | Osteoarthritis | (Lin et al., 2022) | |
| PEG-PCL-TSPBA | Microfluidic technology and crosslink | Osteoarthritis | (Yu et al., 2022) | |
| ALG, BSA and CH | Multiple emulsion | Cartilage regeneration | (Min et al., 2022) | |
| Silk fibroin and polyethylene glycol (PEG) | Mixture | Osteochondral regeneration | (Jiang et al., 2022) | |
| PLGA | Oil-in-water emulsion and premix membrane emulsification | Cartilage regeneration | (Teng et al., 2021; Yuan et al., 2021) | |
| PLGA | Emulsification–evaporation | cartilage regeneration | (Dai et al., 2023) | |
| Chitosan | Crosslink | Osteochondral regeneration | (Ji et al., 2022) | |
| Nanofibers | Poly(L-lactic acid-co-caprolactone) and collagen | Mixture | Tracheal cartilage regeneration | (Yin et al., 2017) |
| Silk fibroin and polydopamine | Crosslink | Interface tissue regeneration | (Chen et al., 2021) | |
| PCL and PLGA | Mixture | Cartilage regeneration | (Elder et al., 2022) | |
| Hydrogels | Gelatin methacryloyl (GelMA) | Mixture and crosslink | Interface tissue regeneration | (Huang et al., 2020) |
| Supramolecular gelatin | Mixture & Host-guest interaction | Osteochondral regeneration | (Xu et al., 2019) | |
| Gelatin and GelMA | Mixture | Nucleus pulposus regeneration | (Zhu et al., 2017) | |
| Chitosan and GelMA | Mixture | Cartilage regeneration | (Zhang et al., 2022) | |
| Chitosan-Hyaluronic acid | Physical absorb | Interface tissue regeneration | (Zhang et al., 2017) | |
| Platelet-rich plasma (PRP) | Mixture | Cartilage regeneration | (Yang, Zhu et al., 2019) | |
| Cellulose nanocrystal and dextran | Integration | Osteoarthritis | (Massaro et al., 2019) | |
| Halloysite nanotubes and laponite | Mixture | Cartilage regeneration | (Fan et al., 2020) | |
| Aldehyde methylene sodium alginate and amino gelatin | Encapsulation | Osteochondral regeneration | (Liu et al., 2020) | |
| GelMA and β-cyclodextrin | Host–guest interaction | Osteochondral regeneration | (Wei et al., 2023) | |
| Hydroxypropyl chitin and β-cyclodextrin | Crosslink & Host–guest interaction | Cartilage regeneration | (Yuan et al., 2023) | |
| Hyaluronic acidmethacryloyl (HAMA); Chitosan | Crosslink & Host-guest inclusion; Mixture | Cartilage regeneration | (Dehghan-Baniani et al., 2020; Min et al., 2022) | |
| Porous scaffolds | Chitosan and xanthan | Mixture | Cartilage lesions therapy | (Westin et al., 2017) |
| Collagen and Cellulose nanocrystals | Integration and absorption | Cartilage regeneration | (Yang, Zheng et al., 2019) | |
| GelMA and hydroxyapatite | 3D bioprinting | Osteochondral regeneration | (Zhang et al., 2023) | |
| methacrylated collagen and mineral oil | 3D bioprinting | Cartilage regeneration | (Kim & Kim, 2022) | |
| Collagen, chitosan, and hyaluronic acid sodium | Mixture and lyophilization | Osteoarthritis | (Sun et al., 2018) | |
| poly (glycerol sebacate) and poly (1,3- propylene sebacate) | Covalent incorporation | Cartilage regeneration | (Xuan et al., 2020) | |
| Others | Silk and chitosan | Integration and absorption | Cartilage regeneration | (Dehghan Baniani et al., 2021) |
| Nanographene oxide | π–π stacking and hydrophobic interactions | Osteoarthritis | (Zeng et al., 2021) | |
| Nanomicelles | Encapsulation | Osteoarthritis | (Su et al., 2022) |
3. Nanoscale materials for kartogenin
Nanodelivery, as a cutting-edge controlled drug delivery approach, offers the advantages of biodegradability, biocompatibility, and non-toxicity. It allows for the design of systems that achieve precise release kinetics, regulate biodistribution, and minimize toxic side effects, thereby improving the therapeutic index of the administered drug (Jiang et al., 2014; Wang et al., 2019). In this section, we provide a comprehensive review and discussion of nanomaterials in the context of kartogenin, with a specific emphasis on nanoparticles, nanofibers, and extracellular vesicles.
3.1. Nanoparticles
Nanoparticles, with their diverse forms and wide range of functions, have found extensive applications in various areas such as bioimaging, anti-microbial and antitumor targeting, and gene and drug delivery (Zhu et al., 2017; Jin et al., 2018; Song et al., 2021). These particles offer the ability to protect bioactive agents, control their release profiles, minimize side effects, and efficiently deliver them to target cells, maximizing their therapeutic effects. Notably, nanoparticles can be precisely synthesized in terms of shape, size and surface morphology to enhance solubility, immunocompatibility, and cellular uptake (Goldberg et al., 2007; Somiya & Kuroda, 2015). Furthermore, encapsulating active molecules within nanoparticles provides protection against degradation in biological fluids, enhaces solubility, and enables sustained and controlled release over time and space (Martinez et al., 2015; Parodi et al., 2017).
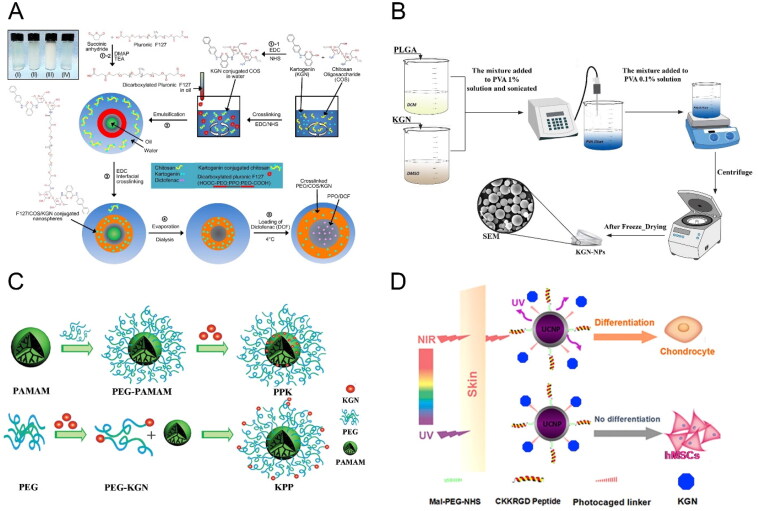
Chitosan, a chitin derivative with polycationic characteristics and the presence of an amino group, can be conjugated to kartogenin via the catalytic synthesis of amide bonds, using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide in combination with N-hydroxysuccinimide (EDC/NHS). It is then fabricated into nanoparticles using an ionic gelation method, using the tripolyphosphate anion which interacts with cationic chitosan via electrostatic forces (Zhang et al., 2010; Kang et al., 2014). Im’s group demonstrated that these kartogenin-conjugated chitosan nanoparticles improve the aqueous solubility and biocompatibility of hydrophobic kartogenin, exhibiting sustained in vitro release for up to 7 weeks. These polymer-drug conjugates hold promise as a drug delivery system for osteoarthritis treatment (Kang et al., 2014). They also developed thermo-responsive polymeric nanoparticles based on chitosan (Figure 3A) in which kartogenin was covalently cross-linked to the outer part of a dual drug-release nanoparticle, with independent control of release achieved through temperature change (Kang et al., 2016).
Figure 3.
(A) Illustration of the procedure and chemistry to synthesize the F127/COS/KGNDCF nanospheres. Reproduced with permission from (Kang et al., 2016) ©elsivier; (B) Schematic illustration for the synthesis of KGN-PLGA nanoparticles. Reproduced with permission from (Zare et al., 2021) ©elsivier; (C) Schematic illustrations of construction of KGN-PAMAM conjugates. Reproduced with permission from (Hu et al., 2017) ©elsivier; (D) Tissue penetration of NIR-triggered release of KGN from RGD-KGN-UCNP@SiO2 to induce the chondrogenic differentiation of hMSCs in vitro and in vivo. Reproduced with permission from (Li et al., 2016) ©elsivier.
Polymeric nanoparticles with hydrophobic regions have the advantage of encapsulating hydrophobic molecules, offering good dispersion and high drug loading capacity, making them suitable for nano-delivery systems. Poly(lactic-co-glycolic acid) (PLGA), a synthetic polypeptide known for its biocompatibility, biodegradability, and nonimmunogenicity, has been extensively employed in drug delivery applications (Zhang et al., 2022). As a hydrophobic molecule, kartogenin can be easily loaded into PLGA carriers to enhance its solubility and stability. Pariya and colleagues encapsulated kartogenin in PLGA nanoparticles using an emulsion-based formulation method (Figure 3B) and encapsulated the loaded nanoparticles into a composite scaffold, achieving linear and sustained release of kartogenin for up to 30 days with reduced initial burst release (Zare et al., 2021). Similarly, Asgari and colleagues developed KGN-loaded PLGA nanoparticles using an emulsion/solvent evaporation method, achieving an encapsulation efficiency of 70%.The release of kartogenin from PLGA nanoparticles lasted for 32 days with an initial burst release on the first day (Asgari et al., 2020).
In addition to polymeric nanoparticles, certain ions or metal nanoparticles can be employed for imaging purposes. Upconversion nanoparticles (UCNPs), composed of host lattices of ceramic materials, can absorb near-infrared light and convert it to weak UV or visible light, making them effective tools for light-mediated drug delivery in biomedical applications (Haase & Schäfer, 2011; Wang et al., 2011; Chen et al., 2014). Li and colleagues developed multifunctional nanoparticles based on UCNPs, where kartogenin is conjugated via a photocaged linker on the surface (Figure 3D) (Li et al., 2016). Under local exposure to NIR light, the drug could be released from the UCNPs internalized by cells, inducing chondrogenic differentiation at reduced dosage compared to other mehtods. Ultrasmall super-paramagnetic iron-oxide (USPIO) has also gained attention as a carrier due to its biocompatibility, non-toxicity, and non-immunogenicity in biological systems (Ramaswamy et al., 2009; Hachani et al., 2016). Yang and colleagues synthesized amino-functionalized USPIO nanoparticles through surface modification and then grafted kartogenin onto the surface, providing stable magnetic resonance signal during cartilage regeneration (Yang, Zheng et al., 2019; Yang, Zhu et al., 2019).
Polyamidoamine, with its well-defined nanoscale architecture, multivalency, and structural versatility, can also serve as a carrier for drug delivery (Cheng et al., 2015; Hu et al., 2016). Hu and colleagues fabricated and compared two different kartogenin-polyamidoamine conjugates (Figure 3C): one with the drug bound to the surface of nanoparticles (PPK) and another with the drugy conjugated to the end group of PEG (KPP). The KPP conjugate induced a higher expression of chondrogenic markers compare to PPK, attributed to the shielding effect of PEG for PPK (Hu et al., 2017).
The composition, structure, and biological activity of nanoparticles can be manipulated to regulate the loading and release of kartogenin, as well as their suspension stability and cellular uptake, ultimately controlling their functional efficacy.
3.2. Nanofibers
The extracellular matrix (ECM) is a collection of bio-macromolecules that have a filamentous architecture. It plays a crucial role in directing cell attachment, proliferation, and maintaining cell phenotype through structural support and biochemical cues (Theocharis et al., 2016; Prince & Kumacheva, 2019). Nanofibers, as a sub-class of biomaterials, exhibit structural similarity to the natural ECM. Their high surface area to volume ratio makes them suitable for surface modification, which enhances cell attachment, enables high drug loading, and improves mass transfer properties (Sill & von Recum, 2008; Ku & Park, 2010; Ding et al., 2019).
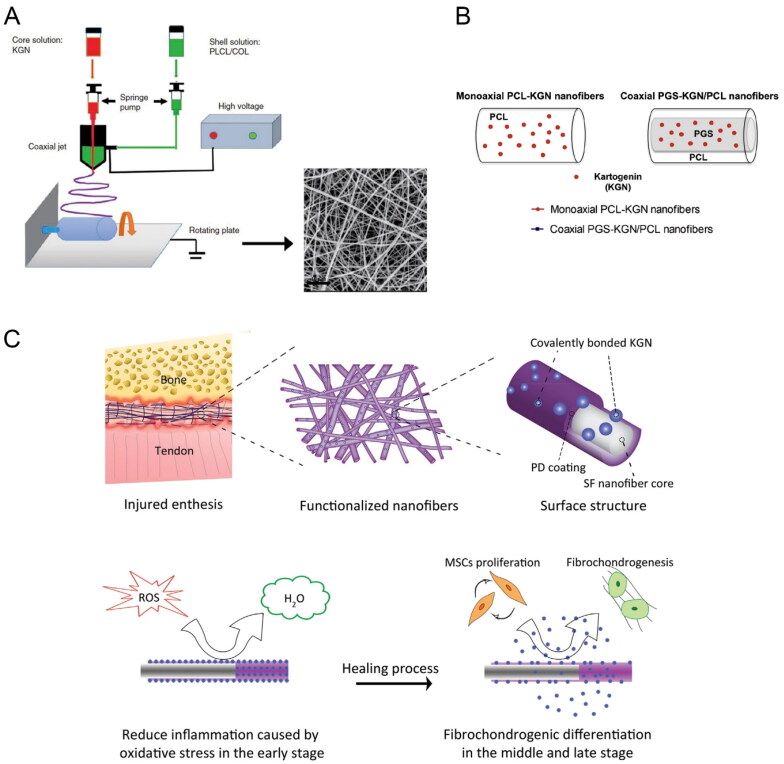
Nanofibers can be produced by electrospinning or extrusion methods (Li et al., 2012; Wang et al., 2014). Various modification strategies have been developed, including co-electrospinning (Li et al., 2011; Samavedi et al., 2011), physical absorption (Qian et al., 2018; Han et al., 2019), and chemical immobilization (Wu et al., 2019). Electrospinning, in particular, is a simple and efficient method for fabricating multifunctional nanofibers. It utilizes electric fields to pull out charged fibers with diameters in the range of several hundred nanometers. Several bioactive drugs, including kartogenin, have been successfully incorporated into electrospun nanofibers for controlled release (Jiang et al., 2014). Yin et al. designed coaxial electrospun nanofibers with kartogenin encapsulated in the core (Figure 4A), allowing for sustained and stable release over a period of approximately two months (Yin et al., 2017). Similarly, Silva et al. developed kartogenin-loaded coaxial poly (glycerol sebacate)/PCL aligned nanofibers (Figure 4B), which exhibited sustained release of kartogenin and induced chondrogenic differentiation of MSCs (Silva et al., 2020). Chemical cross-linking has also been employed for loading kartogenin onto nanofibers. In our previous research, a multi-functional nanofiber scaffold for interface tissue regeneration was developed, where kartogenin was cross-linked to the polydopamine coating on the surface of silk fibroin nanofibers (Figure 4C) (Chen et al., 2021). This core-shell nanofiber scaffold shows promise for the integration of tendon-bone. Furthermore, Zhu et al. fabricated an engineered scaffold for regenerating tendon–bone enthesis in rotator cuff tear (RCT) by utilizing nanofibers’ structural guidance and the biological effects of kartogenin (Zhu et al., 2019). Their results demonstrated that scaffold loaded with 100 μM KGN significantly promoted chondrogenic and tenogenic differentiation of rat bone marrow stromal cells. This engineered scaffold holds potential as a tissue engineering approach to enhance tendon–bone healing in RCTs.
Figure 4.
(A) Schematic illustration of the fabrication process of KGN@PC nanofibrous scaffold. Reproduced with permission from (Yin et al., 2017) ©SAGE; (B) Schematic representation and monoaxial PCL-KGN fibers. Reproduced with permission from (Silva et al., 2020) ©elsivier; (C) Schematic diagram of integration and regeneration of bone-tendon interface by using a kartogenin- and polydopamine-functionalized silk fibroin nanofibrous scaffold. Reproduced with permission from (Chen et al., 2021). ©elsivier.
3.3. Nanovesicles
Extracellular vesicles (EVs) are naturally released lipid bilayer-delimited particles, including exosomes and microvesicles, with diameters ranging from 50 to 200 nm. These vesicles have the capability to encapsulate various bioactive molecules, including proteins and nucleic acids, which can be transferred to recipient cells, modulating their cellular functions (Théry et al., 2018; Mathieu et al., 2019).
Exosomes, in particular, have gained significant attention as crucial mediators of intercellular communication and molecular trafficking (Edgar, 2016). Jing et al. investigated the potential of utilizing small extracellular vesicles (sEVs) as a biomimetic tool to induce chondrogenesis in MSCs (Jing et al., 2020). Their findings revealed that sEVs released from kartogenin-preconditioned hUCMSCs contained specific miRNAs that could be transferred to native MSCs, promoting their chondrogenic differentiation by targeting TAOK1, a negative regulator of the Hippo pathway. Xu et al. developed a method to load kartogenin into exosomes through electroporation. The encapsulated KGN could be released from exosomes, with approximately 50% release observed within 24 hours. Utilizing exosomes enhanced the effective concentration of KGN within cells and significantly promoted the chondrogenesis of MSCs both in vitro and in vivo (Xu et al., 2021). Despite the growing research on the effects of EVs as a delivery platform, certain limitations exist. For instance, native EVs are not inherently designed for targeted cargo delivery; instead, cargos are non-selectively delivered to various cell types in vivo. However, with a deeper understanding of EVs, it is expected that better strategies will be developed to optimize their design and functionality.
4. Macroscopic materials for kartogenin
In order to achieve specific cellular responses and facilitate new tissue formation, macroscopic materials used for cell or chemical agent delivery should possess biomimetic properties and incorporate components of the extracellular matrix (ECM) (Ravindran et al., 2012). In the field of regenerative medicine and tissue engineering, the implantation of biodegradable and biocompatible scaffolds containing growth factors or other active molecules could be implanted in target areas can gradually promote tissue healing and restore its original function (Zhang et al., 2009). This section focuses on the review and discussion of kartogenin-loaded macroscopic materials and their applications.
4.1. Hydrogels
Hydrogels are a class of large molecules composed of interconnected hydrophilic polymer chains that maintain a high water content while retaining their 3D structure and physical integrity through physiochemical crosslinks. These hydrogels exhibit a soft consistency, low interfacial tension, and high biocompatibility, closely resembling native tissue (Li & Mooney, 2016). Their ability to be tailored for site-specific and sustained drug delivery has made them widely utilized in regenerative medicine (Hoffman, 2012; Gaharwar et al., 2014; Liang et al., 2022). Particularly in tissue engineering, hydrogels are highly suitable for promoting tissue healing and regeneration (Drury & Mooney, 2003; Tan & Marra, 2010; Alge et al., 2013; Zhang & Khademhosseini, 2017).
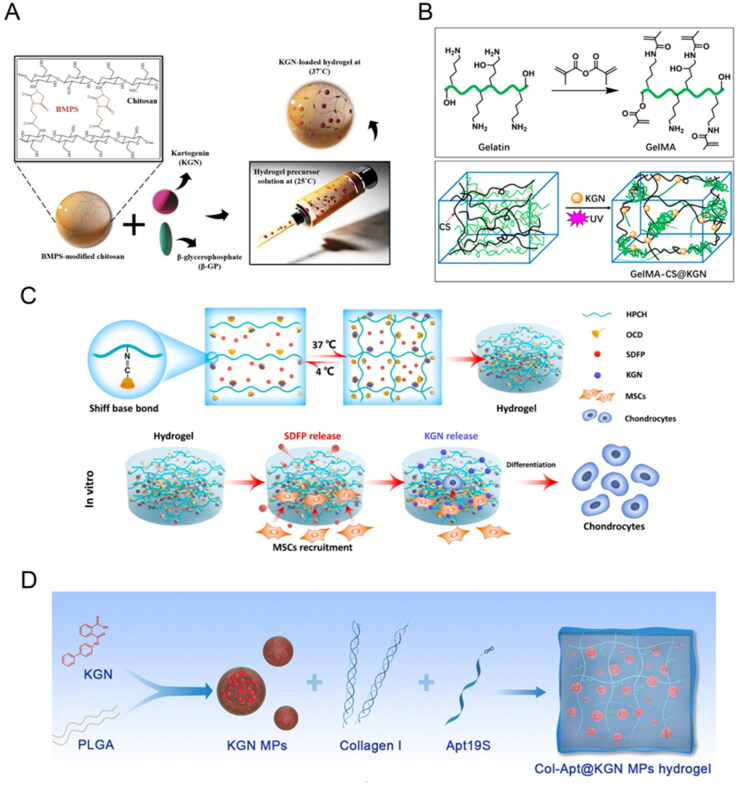
Hyaluronic acid (HA), a major component of ECM in the skin and various connective tissues, is a natural, biocompatible, biodegradable polysaccharide that exhibits low immunogenicity. It serves as an ideal hydrogel material for culturing and transplanting various cells (Tan et al., 2009; Ha et al., 2015). For instance, Zhu et al. developed an injectable kartogenin-conjugated chitosan/HA hydrogel with sustained kartogenin release, which effectively promoted differentiation in the nucleus pulposus of intervertebral disks (Zhu et al., 2017). Similarly, Yuan et al. loaded hydrophobic kartogenin into the hydrophobic cavity of β-CD modified with an aldehyde group β-CD-CHO (OCD) through host–guest interactions. The kartogenin-loaded OCD was then immobilized on the HPCH via a Schiff-base reaction, leading to sustained release and subsequent differentiation of MSCs into chondrocytes (Figure 5C) (Yuan et al., 2023). Additionally, Liu et al. achieved sustained release of kartogenin by integrating it into the hydrophobic internal cavity of β-CD-AOI2 (Liu et al., 2020). These drug-loaded nanoboxes were covalently photo-crosslinked with methacryloyl HA to incorporate them into the hydrogel’s covalent network, triggering chondrogenesis. Based on this, the research group developed a semi-embedded, biomimetic, biphasic osteochondral scaffold by combining 3D-printed hydroxyapatite scaffold with layer-specific release of stem cell differentiation inducers, achieving simultaneous reconstruction of osteochondral defects (Liu et al., 2020).
Figure 5.
(A) Schematic illustration of the current research strategy for cartilage tissue engineering. It consists of chitosan modification with BMPS following by addition of β-GP as a physical Crosslinker together with KGN as a small biomolecule for chondrogenesis promotion of hAMSCs. The resulting mixture is injectable at 25 °C which gels upon temperature enhancement to 37 °C. Reproduced with permission from (Dehghan-Baniani et al., 2020). ©elsivier; (B) Schematic illustrations of synthesis pathways of (top) GelMA macromolecule and (bottom) KGN-loaded GelMA-CS@KGN composite hydrogel. Reproduced with permission from (Zhang et al., 2022) ©frontiers; (C) Schematic diagram of the SDFP and KGN co-loaded HPCH for cartilage regeneration for stem-cell recruitment and chondrogenic differentiation. Reproduced with permission from (Yuan et al., 2023) ©elsivier; (D) Fabrication of Col-Apt@KGN MPs functional hydrogel. Reproduced with permission from (Dai et al., 2023). © the royal society of chemistry 2023.
Incorporating nanomaterials with hydrogels offers advantages in drug delivery. For example, in Dai’s study, kartogenin was loaded into PLGA microspheres through the emulsification–evaporation method, which were then embedded in collagen-based hydrogel (Figure 5D) (Dai et al., 2023). Similarly, Fan et al. grafted kartogenin onto polyurethane nanoparticles through the EDC/NHS condensation reaction, achieving a loading efficiency of 14%. These nanoparticles were encapsulated into aldehyde methacrylate sodium alginate and amino gelatin hydrogels for in situ cartilage repair (Fan et al., 2018, 2020). Another approach involves loading the drug onto the nanomaterial before integrating it with the hydrogel, combining the advantages of both. For instance, Xu et al. fabricated a unique gelatin supramolecular hydrogel via a ‘Host-Guest Macromer’ approach without chemical modification or direct crosslinking of the biopolymers (Feng et al., 2017). Kartogenin was mixed into the supramolecular hydrogel, leading to enhanced in situ osteochondral regeneration through the sustained delivery of chondrogenic molecules (Xu et al., 2019).
There are other types of hydrogel. Halloysite nanotubes and laponite were used as a novel carrier system for kartogenin delivery by Massaro et al. (2019). Halloysite, which offers selective functionalization of its inner and outer surfaces, is frequently employed for drug carrying and delivery (Massaro et al., 2018), while laponite has the ability to form injectable thixotropic hydrogel in aqueous solution (Boyer et al., 2018). Dextran, with its high water absorption and good biocompatibility, can be cross-linked to from ECM-like hydrogels similar to chondroitin sulfate in structure (Ito et al., 2007). Yang et al. used dextran hydrogels as a carrier for kartogenin-modified USPIO for cartilage repair. Additionally, Dehghan et al. incorporated kartogenin into the N-(β-maleimidopropyloxy) succinimide ester modified chitosan hydrogel, adding β-Glycerophosphate to achieve both thermosensitivity and sustained kartogenin release (Figure 5A) (Dehghan-Baniani et al., 2020). In Zhang’s work, kartogenin was incorporated into a composite hydrogel comprised of positively charged chitosan (CS) and methacrylated gelatin (GelMA) polymers (Figure 5B) (Zhang et al., 2022). This composite hydrogel achieved well-control release of kartogenin, satisfying the initial high drug concentration requirement while maintaining long-term sustained release.
4.2. Porous scaffolds
Porous scaffolds play a crucial role in creating three-dimensional (3D) environments that support cell survival and have shown great potential in tissue regeneration, as evidenced by various clinical trials (Seo et al., 2014; Offeddu et al., 2016). Several techniques, such as 3D printing (Li et al., 2017; Bittner et al., 2019), salt-leaching (Landau et al., 2017; Mahapatra et al., 2019), and freeze drying (Yang et al., 2015; Sun et al., 2018), have been employed to fabricate porous scaffolds. These scaffolds offer advantages such as increased drug loading capacity and the ability to achieve customized release profiles, creating an optimal environment for controlling cell fate (Oh et al., 2007).
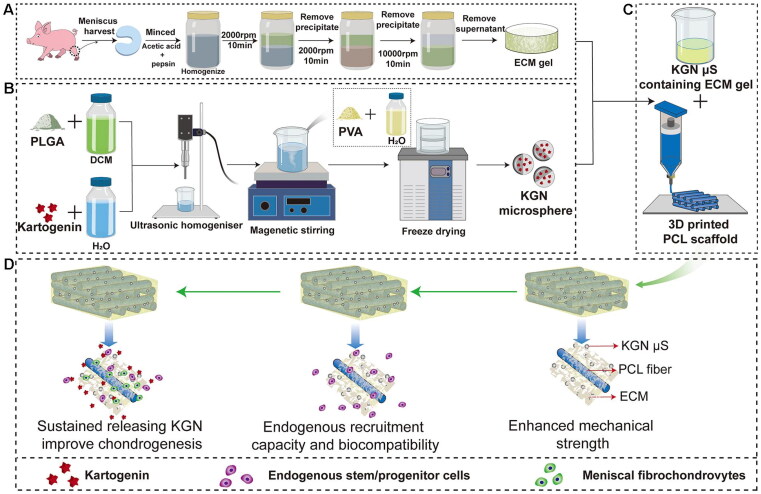
In a study by Westin, a porous chitosan-xanthan gum matrix was utilized, and kartogenin was reversibly attached to the scaffold to evaluate its effects on the chondrogenic differentiation of dental pulp stem cell (Westin et al., 2017). The results demonstrated the potential of kartogenin in the treatment of cartilage related lesions. Sun et al. incorporated kartogenin-loaded PLGA microspheres into collagen/chitosan/hyaluronic acid sodium salt porous scaffolds, creating a biomaterial with chondrogenic capacity for cartilage repair and the treatment of osteoarthritis (Sun et al., 2018). Additionally, Xuan et al. designed and fabricated shape-memory ternary scaffolds with chondrogenic capacity for cartilage repair, incorporating immobilized kartogenin and enabling minimally invasive implantation and restoration at body temperature (Xuan et al., 2020). These responsive scaffolds hold promise not only for cartilage repair but also for the delivery of other active molecules or protein drugs. Li et al. presented an alternative approach using 3D printing to create a poly(ɛ-caprolactone) (PCL) scaffold as a backbone, which was subsequently modified with KGN-loaded poly(lactic-co-glycolic acid) (PLGA) microspheres. This strategy enhanced meniscus regeneration through controlled release of kartogenin from the 3D porous scaffold (Figure 6) (Li et al., 2021).
Figure 6.
Schematic representation of the preparation process of the scaffolds. Flow chart of the preparation of the (A) MECM gel, (B) KGN-containing PLGA microspheres and (C) PCL/MECM-KGN mS scaffold; (D) Possible mechanism of meniscus regeneration. Enhanced mechanical strength, endogenous stem cells and sustained releasing KGN contributed to meniscus regeneration in these experiments. Abbreviations: MECM, meniscus extracellular matrix; KGN, kartogenin; PLGA, poly(lactic-co-glycolic) acid; mS, microspheres. Reproduced with permission from (Liu et al., 2020) © frontiers.
These studies demonstrate the potential of porous scaffolds as vehicles for kartogenin delivery in tissue regeneration applications. The incorporation of kartogenin within the scaffolds enhances their chondrogenic properties and promotes cartilage repair. The use of various techniques, such as reversible attachment, microsphere embedding, and 3D printing, provides versatility in scaffold design and drug delivery strategies. Further research and development in this field hold promise for advancing cartilage regeneration and the treatment of cartilage-related disorders.
5. Future perspectives
In this review, we have provided a comprehensive overview of kartogenin, highlighting its physical, chemical, and biological properties, as well as its regulatory abilities in cell differentiation and potential for application in tissue repair. We have emphasized recent advancements in the design and fabrication of kartogenin-functionalized biomaterials, both at the nanoscale and macroscopic levels, and discussed their applications in regenerative medicine. Kartogenin exhibits improved functionality when combined with biomaterials that modify its physicochemical and surface properties, leading to enhanced synergistic effects. Over the past decade, kartogenin has witnessed significant progress in its biofunctions and applications, expanding our understanding and promoting its therapeutic potential.
Despite the achievements of kartogenin, several scientific challenges and potential issues remain. Firstly, further exploration of its induction mechanism is necessary, although it is widely acknowledged that kartogenin possesses the ability to induce cell differentiation and offers advantages in cartilage-related tissue repair and regeneration compared to other biological factors. However, conflicting reports exist regarding the efficacy of kartogenin. For instance, Doran et al. found that the chondrogenic induction by kartogenin was far weaker than that of TGF, suggesting its limitations as a drug for cartilage regeneration (Music et al., 2020). Furthermore, Miyatake et al. discovered that kartogenin did not effectively promote the synthesis of superficial zone protein, an essential component for joint homeostasis on the cartilage surface, and they concluded that its chondrogenic effects depend on cellular phenotype and differentiation status (Miyatake et al., 2016).
Currently, most kartogenin research focuses on in situ injection, but oral administration offers a simpler and noninvasive route of application, which should be further investigated, particularly in combination with various biomaterials. Additionally, although various materials have been designed and functionalized with kartogenin, and in vitro release profiles have been studied, there is a scarcity of reports regarding the decomposition products or the actual drug dose in vivo. It is crucial to evaluate the effective utilization and safety of kartogenin and its degradation products before progressing to clinical use.
Furthermore, there is a need to explore and develop novel biomaterials for incorporating, carrying, and releasing kartogenin. This includes the exploration of composites combining nanoscale and 3D materials, as well as materials responsive to temperature, pH, or light, to enhance targeting accuracy and synergistic effects. We believe that advanced strategies for biomaterial fabrication will enable the development of new functionalized carriers, overcoming the limitations of conventional carriers and providing safe and effective platforms for kartogenin delivery in the future.
Funding Statement
This work was supported in part by grants from the National Natural Science Foundation of China [12202089], the Research Foundation of Chongqing University of Science and Technology [ckrc2021020], the Innovation-entrepreneurship Seed Foundation of Chongqing Engineering Laboratory of Nano/Micro Biological Medicine Detection Technology [1155108202108], and Chongqing Talents Project [CQYC20210302379].
Author contributions
P. Chen: Writing - Original Draft, Project administration. X. Liao: Writing - review & editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- Alge DL, Azagarsamy MA, Donohue DF, Anseth KS. (2013). Synthetically tractable click hydrogels for three-dimensional cell culture formed using tetrazine–norbornene chemistry. Biomacromolecules 14:1–15. doi: 10.1021/bm4000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida B, Wang Y, Shukla A. (2020). Effects of nanoparticle properties on kartogenin delivery and interactions with mesenchymal stem cells. Ann Biomed Eng 48:2090–2102. doi: 10.1007/s10439-019-02430-x. [DOI] [PubMed] [Google Scholar]
- Armiento AR, Stoddart MJ, Alini M, Eglin D. (2018). Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater 65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- Asgari N, Bagheri F, Eslaminejad MB, et al. (2020). Dual functional construct containing kartogenin releasing microtissues and curcumin for cartilage regeneration. Stem Cell Res Ther 11:289. doi: 10.1186/s13287-020-01797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume F, Maguire TJ, Yarmush ML. (2011). Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng 2:403–30. doi: 10.1146/annurev-chembioeng-061010-114257. [DOI] [PubMed] [Google Scholar]
- Bittner SM, Smith BT, Diaz-Gomez L, et al. (2019). Fabrication and mechanical characterization of 3D printed vertical uniform and gradient scaffolds for bone and osteochondral tissue engineering. Acta Biomater 90:37–48. doi: 10.1016/j.actbio.2019.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer C, Figueiredo L, Pace R, et al. (2018). Laponite nanoparticle-associated silated hydroxypropylmethyl cellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Acta Biomater 65:112–122. doi: 10.1016/j.actbio.2017.11.027. [DOI] [PubMed] [Google Scholar]
- Cai G, Liu W, He Y, et al. (2019). Recent advances in kartogenin for cartilage regeneration. J Drug Target 27:28–32. [DOI] [PubMed] [Google Scholar]
- Cai J, Zhang L, Chen J, Chen S. (2019). Kartogenin and its application in regenerative medicine. Curr Med Sci 39:16–20. doi: 10.1007/s11596-019-1994-6. [DOI] [PubMed] [Google Scholar]
- Chen G, Qiu H, Prasad PN, Chen X. (2014). Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem Rev 114:5161–214. doi: 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Wang S, Huang Z, et al. (2021). Multi-functionalized nanofibers with reactive oxygen species scavenging capability and fibrocartilage inductivity for tendon-bone integration. J Mater Sci Technol 70:91–104. doi: 10.1016/j.jmst.2020.09.006. [DOI] [Google Scholar]
- Chen Y, Zhou L, Ding Y, et al. (2022). Kartogenin regulates hair growth and hair cycling transition. Int J Med Sci 19:537–45. doi: 10.7150/ijms.68434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Hu Q, Cheng L, et al. (2015). Construction and evaluation of PAMAM–DOX conjugates with superior tumor recognition and intracellular acid-triggered drug release properties. Colloids Surf B Biointerfaces 136:37–45. doi: 10.1016/j.colsurfb.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Dai W, Liu Q, Li S, et al. (2023). Functional injectable hydrogel with spatiotemporal sequential release for recruitment of endogenous stem cells andin situ cartilage regeneration. J Mater Chem B 11:4050–4064. doi: 10.1039/d3tb00105a. [DOI] [PubMed] [Google Scholar]
- Decker RS, Koyama E, Enomoto-Iwamoto M, et al. (2014). Mouse limb skeletal growth and synovial joint development are coordinately enhanced by Kartogenin. Dev Biol 395:255–67. doi: 10.1016/j.ydbio.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan Baniani D, Mehrjou B, Chu PK, Wu H. (2021). A biomimetic nano‐engineered platform for functional tissue engineering of cartilage superficial zone. Adv Healthc Mater 10:e2001018. doi: 10.1002/adhm.202001018. [DOI] [PubMed] [Google Scholar]
- Dehghan-Baniani D, Chen Y, Wang D, et al. (2020). Injectable in situ forming kartogenin-loaded chitosan hydrogel with tunable rheological properties for cartilage tissue engineering. Colloids Surf B Biointerfaces 192:111059. doi: 10.1016/j.colsurfb.2020.111059. [DOI] [PubMed] [Google Scholar]
- Ding Y, Li W, Zhang F, et al. (2019). Electrospun fibrous architectures for drug delivery, tissue engineering and cancer therapy. Adv Funct Mater 29:1802852. doi: 10.1002/adfm.201802852. [DOI] [Google Scholar]
- Drury JL, Mooney DJ. (2003). Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24:4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- Edgar JR. (2016). Q&A: what are exosomes, exactly? BMC Biol 14:46. doi: 10.1186/s12915-016-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder S, Roberson JG, Warren J, et al. (2022). Evaluation of electrospun PCL-PLGA for sustained delivery of kartogenin. Molecules 27:3739. doi: 10.3390/molecules27123739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Li J, Yuan L, et al. (2018). Intra-articular injection of kartogenin-conjugated polyurethane nanoparticles attenuates the progression of osteoarthritis. Drug Deliv 25:1004–1012. doi: 10.1080/10717544.2018.1461279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Yuan L, Li J, et al. (2020). Injectable double-crosslinked hydrogels with kartogenin-conjugated polyurethane nano-particles and transforming growth factor β3 for in-situ cartilage regeneration. Mater Sci Eng C Mater Biol Appl 110:110705. doi: 10.1016/j.msec.2020.110705. [DOI] [PubMed] [Google Scholar]
- Feng Q, Wei K, Lin S, et al. (2017). Corrigendum to “Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration” [Biomaterials 101C (2016) 217–228]. Biomaterials 112:346–347. doi: 10.1016/j.biomaterials.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Gaharwar AK, Peppas NA, Khademhosseini A. (2014). Nanocomposite hydrogels for biomedical applications. Biotechnol Bioeng 111:441–53. doi: 10.1002/bit.25160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M, Langer R, Jia X. (2007). Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Ed 18:241–68. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha C, Park Y, Chung J, Park Y. (2015). Cartilage repair using composites of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel in a minipig model. Stem Cells Transl Med 4:1044–51. doi: 10.5966/sctm.2014-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase M, Schäfer H. (2011). Upconverting nanoparticles. Angew Chem Int Ed Engl 50:5808–29. doi: 10.1002/anie.201005159. [DOI] [PubMed] [Google Scholar]
- Hachani R, Lowdell M, Birchall M, et al. (2016). Polyol synthesis, functionalisation, and biocompatibility studies of superparamagnetic iron oxide nanoparticles as potential MRI contrast agents. Nanoscale 8:3278–87. doi: 10.1039/c5nr03867g. [DOI] [PubMed] [Google Scholar]
- Han F, Zhang P, Wen X, et al. (2019). Bioactive LbL-assembled multilayer nanofilms upregulate tenogenesis and angiogenesis enabling robust healing of degenerative rotator cuff tendons in vivo. Biomater Sci 7:4388–4398. doi: 10.1039/c9bm00413k. [DOI] [PubMed] [Google Scholar]
- Hoffman AS. (2012). Hydrogels for biomedical applications. Adv Drug Deliver Rev 64:18–23. doi: 10.1016/j.addr.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Hu Q, Ding B, Yan X, et al. (2017). Polyethylene glycol modified PAMAM dendrimer delivery of kartogenin to induce chondrogenic differentiation of mesenchymal stem cells. Nanomedicine 13:2189–98. doi: 10.1016/j.nano.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Hu W, Hou X, Xia Z, et al. (2016). Genome-wide survey and expression analysis of the calcium-dependent protein kinase gene family in cassava. Mol Genet Genomics 291:241–53. doi: 10.1007/s00438-015-1103-x. [DOI] [PubMed] [Google Scholar]
- Huang C, Zhang X, Luo H, et al. (2020). Effect of kartogenin-loaded gelatin methacryloyl hydrogel scaffold with bone marrow-stimulation for enthesis healing in rotator cuff repair. J Shoulder Elb Surg 30:544–553. doi: 10.1016/j.jse.2020.06.013. [DOI] [PubMed] [Google Scholar]
- Huang H, Xu H, Zhao J. (2017). A novel approach for meniscal regeneration using kartogenin-treated autologous tendon graft. Am J Sports Med 45:3289–3297. doi: 10.1177/0363546517721192. [DOI] [PubMed] [Google Scholar]
- Huang Y, Jiang T, Chen J, et al. (2018). Effects of kartogenin on the attenuated nucleus pulposus cell degeneration of intervertebral discs induced by interleukin-1beta and tumor necrosis factor-alpha. Int J Mol Med 41:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DJ. (2011). Pharmacologic therapy for osteoarthritis—the era of disease modification. Nat Rev Rheumatol 7:13–22. doi: 10.1038/nrrheum.2010.178. [DOI] [PubMed] [Google Scholar]
- Im G. (2018). Application of kartogenin for musculoskeletal regeneration. J Biomed Mater Res A 106:1141–8. doi: 10.1002/jbm.a.36300. [DOI] [PubMed] [Google Scholar]
- Ito T, Yeo Y, Highley C, et al. (2007). Dextran-based in situ cross-linked injectable hydrogels to prevent peritoneal adhesions. Biomaterials 28:3418–26. doi: 10.1016/j.biomaterials.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Ji X, Shao H, Li X, et al. (2022). Injectable immunomodulation-based porous chitosan microspheres/HPCH hydrogel composites as a controlled drug delivery system for osteochondral regeneration. Biomaterials 285:121530. doi: 10.1016/j.biomaterials.2022.121530. [DOI] [PubMed] [Google Scholar]
- Jiang J, Xie J, Ma B, et al. (2014). Mussel-inspired protein-mediated surface functionalization of electrospun nanofibers for pH-responsive drug delivery. Acta Biomater 10:1324–32. doi: 10.1016/j.actbio.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Xiang X, Song M, et al. (2022). An all-silk-derived bilayer hydrogel for osteochondral tissue engineering. Mater Today Bio 17:100485. doi: 10.1016/j.mtbio.2022.100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Luo Z, Zhang B, Pang Z. (2018). Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B 8:23–33. doi: 10.1016/j.apsb.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, Zhang X, Gao M, et al. (2019). Kartogenin preconditioning commits mesenchymal stem cells to a precartilaginous stage with enhanced chondrogenic potential by modulating JNK and β-catenin − related pathways. Faseb J 33:5641–53. doi: 10.1096/fj.201802137RRR. [DOI] [PubMed] [Google Scholar]
- Jing H, Zhang X, Luo K, et al. (2020). miR-381-abundant small extracellular vesicles derived from kartogenin-preconditioned mesenchymal stem cells promote chondrogenesis of MSCs by targeting TAOK1. Biomaterials 231:119682. doi: 10.1016/j.biomaterials.2019.119682. [DOI] [PubMed] [Google Scholar]
- Johnson K, Zhu S, Tremblay MS, et al. (2012). A stem cell-based approach to cartilage repair. Science 336:717–21. doi: 10.1126/science.1215157. [DOI] [PubMed] [Google Scholar]
- Kang M, Kim J, Im G. (2016). Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater 39:65–78. doi: 10.1016/j.actbio.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Kang ML, Ko J, Kim JE, Im G. (2014). Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials 35:9984–94. doi: 10.1016/j.biomaterials.2014.08.042. [DOI] [PubMed] [Google Scholar]
- Kim W, Kim GH. (2022). Highly bioactive cell-laden hydrogel constructs bioprinted using an emulsion bioink for tissue engineering applications. Biofabrication 14:045018. doi: 10.1088/1758-5090/ac8fb8. [DOI] [PubMed] [Google Scholar]
- Ku SH, Park CB. (2010). Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials 31:9431–7. doi: 10.1016/j.biomaterials.2010.08.071. [DOI] [PubMed] [Google Scholar]
- Kwon JY, Lee SH, Na HS, et al. (2018). Kartogenin inhibits pain behavior, chondrocyte inflammation, and attenuates osteoarthritis progression in mice through induction of IL-10. Sci Rep 8:13832. doi: 10.1038/s41598-018-32206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau S, Szklanny AA, Yeo GC, et al. (2017). Tropoelastin coated PLLA-PLGA scaffolds promote vascular network formation. Biomaterials 122:72–82. doi: 10.1016/j.biomaterials.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Li H, Liao Z, Yang Z, et al. (2021). 3D printed poly(ε-caprolactone)/meniscus extracellular matrix composite scaffold functionalized with kartogenin-releasing PLGA microspheres for meniscus tissue engineering. Front Bioeng Biotech 9:662381. doi: 10.3389/fbioe.2021.662381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lee WY, Wu T, et al. (2016). Near-infrared light-triggered release of small molecules for controlled differentiation and long-term tracking of stem cells in vivo using upconversion nanoparticles. Biomaterials 110:1–10. doi: 10.1016/j.biomaterials.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Li J, Mooney DJ. (2016). Designing hydrogels for controlled drug delivery. Nat Rev Mater 1:16071. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li H, Qian Y, et al. (2011). Electrospun poly (ɛ-caprolactone)/silk fibroin core-sheath nanofibers and their potential applications in tissue engineering and drug release. Int J Biol Macromol 49:223–32. doi: 10.1016/j.ijbiomac.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Li L, Qian Y, Jiang C, et al. (2012). The use of hyaluronan to regulate protein adsorption and cell infiltration in nanofibrous scaffolds. Biomaterials 33:3428–45. doi: 10.1016/j.biomaterials.2012.01.038. [DOI] [PubMed] [Google Scholar]
- Li S, Xu Y, Yu J, Becker ML. (2017). Enhanced osteogenic activity of poly(ester urea) scaffolds using facile post-3D printing peptide functionalization strategies. Biomaterials 141:176–187. doi: 10.1016/j.biomaterials.2017.06.038. [DOI] [PubMed] [Google Scholar]
- Li X, Ding J, Zhang Z, et al. (2016). Kartogenin-incorporated thermogel supports stem cells for significant cartilage regeneration. ACS Appl Mater Interfaces 8:5148–59. doi: 10.1021/acsami.5b12212. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang K, Li J, et al. (2022). Injectable protocatechuic acid based composite hydrogel with hemostatic and antioxidant properties for skin regeneration. Mater Design 222:111109. doi: 10.1016/j.matdes.2022.111109. [DOI] [Google Scholar]
- Lin J, Chen L, Yang J, et al. (2022). Injectable double positively charged hydrogel microspheres for targeting‐penetration‐phagocytosis. Small 18:e2202156. doi: 10.1002/smll.202202156. [DOI] [PubMed] [Google Scholar]
- Liu C, Ma X, Li T, Zhang Q. (2015). Kartogenin, transforming growth factor-?1 and bone morphogenetic protein-7 coordinately enhance lubricin accumulation in bone-derived mesenchymal stem cells. Cell Biol Int 39:1026–35. doi: 10.1002/cbin.10476. [DOI] [PubMed] [Google Scholar]
- Liu T, Li X, Wang T, et al. (2020). Kartogenin mediates cartilage regeneration by stimulating the IL-6/Stat3-dependent proliferation of cartilage stem/progenitor cells. Biochem Bioph Res Coummun 532:385–92. [DOI] [PubMed] [Google Scholar]
- Liu X, Chen Y, Mao AS, et al. (2020). Molecular recognition-directed site-specific release of stem cell differentiation inducers for enhanced joint repair. Biomaterials 232:119644. doi: 10.1016/j.biomaterials.2019.119644. [DOI] [PubMed] [Google Scholar]
- Liu X, Wei Y, Xuan C, et al. (2020). A biomimetic biphasic osteochondral scaffold with layer‐specific release of stem cell differentiation inducers for the reconstruction of osteochondral defects. Adv Healthc Mater 9:e2000076. doi: 10.1002/adhm.202000076. [DOI] [PubMed] [Google Scholar]
- Lo KWH, Jiang T, Gagnon KA, et al. (2014). Small-molecule based musculoskeletal regenerative engineering. Trends Biotechnol 32:74–81. doi: 10.1016/j.tibtech.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Atala A. (2014). Small molecules and small molecule drugs in regenerative medicine. Drug Discov Today 19:801–8. doi: 10.1016/j.drudis.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Mahapatra C, Kim J, Lee J, et al. (2019). Differential chondro- and osteo-stimulation in three-dimensional porous scaffolds with different topological surfaces provides a design strategy for biphasic osteochondral engineering. J Tissue Eng 10:2041731419826433. doi: 10.1177/2041731419826433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris EA, Gomoll AH, Malizos KN, et al. (2015). Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 11:21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini JC, Forlino A. (2012). Replenishing cartilage from endogenous stem cells. N Engl J Med 366:2522–4. doi: 10.1056/NEJMcibr1204283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JO, Evangelopoulos M, Bhavane R, et al. (2015). Multistage nanovectors enhance the delivery of free and encapsulated drugs. Curr Drug Targets 16:1582–90. doi: 10.2174/1389450115666141015113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro M, Buscemi G, Arista L, et al. (2019). Multifunctional carrier based on halloysite/laponite hybrid hydrogel for kartogenin delivery. ACS Med Chem Lett 10:419–24. doi: 10.1021/acsmedchemlett.8b00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro M, Cavallaro G, Colletti CG, et al. (2018). Chemical modification of halloysite nanotubes for controlled loading and release. J Mater Chem B 6:3415–3433. doi: 10.1039/c8tb00543e. [DOI] [PubMed] [Google Scholar]
- Mathieu M, Martin-Jaular L, Lavieu G, Théry C. (2019). Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- Maudens P, Seemayer CA, Thauvin C, et al. (2018). Nanocrystal-polymer particles: extended delivery carriers for osteoarthritis treatment. Small 14:1703108. doi: 10.1002/smll.201703108. [DOI] [PubMed] [Google Scholar]
- Min Q, Tian D, Zhang Y, et al. (2022). Strong and elastic chitosan/silk fibroin hydrogels incorporated with growth-factor-loaded microspheres for cartilage tissue engineering. Biomimetics-Basel 7:41. doi: 10.3390/biomimetics7020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake K, Iwasa K, McNary SM, et al. (2016). Modulation of superficial zone protein/lubricin/PRG4 by kartogenin and transforming growth factor-?1 in surface zone chondrocytes in bovine articular cartilage. Cartilage 7:388–97. doi: 10.1177/1947603516630789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Music E, Klein TJ, Lott WB, Doran MR. (2020). Transforming growth factor-beta stimulates human bone marrow-derived mesenchymal stem/stromal cell chondrogenesis more so than kartogenin. Sci Rep 10:8340. doi: 10.1038/s41598-020-65283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offeddu GS, Ashworth JC, Cameron RE, Oyen ML. (2016). Structural determinants of hydration, mechanics and fluid flow in freeze-dried collagen scaffolds. Acta Biomater 41:193–203. doi: 10.1016/j.actbio.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Oh SH, Park IK, Kim JM, Lee JH. (2007). In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 28:1664–71. doi: 10.1016/j.biomaterials.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Parodi A, Molinaro R, Sushnitha M, et al. (2017). Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials 147:155–168. doi: 10.1016/j.biomaterials.2017.09.020. [DOI] [PubMed] [Google Scholar]
- Prince E, Kumacheva E. (2019). Design and applications of man-made biomimetic fibrillar hydrogels. Nat Rev Mater 4:99–115. doi: 10.1038/s41578-018-0077-9. [DOI] [Google Scholar]
- Qian Y, Li L, Song Y, et al. (2018). Surface modification of nanofibrous matrices via layer-by-layer functionalized silk assembly for mitigating the foreign body reaction. Biomaterials 164:22–37. doi: 10.1016/j.biomaterials.2018.02.038. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Greco JB, Uluer MC, et al. (2009). Magnetic resonance imaging of chondrocytes labeled with superparamagnetic iron oxide nanoparticles in tissue-engineered cartilage. Tissue Eng Part A 15:3899–910. doi: 10.1089/ten.tea.2008.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran S, Gao Q, Kotecha M, et al. (2012). Biomimetic extracellular matrix-incorporated scaffold induces osteogenic gene expression in human marrow stromal cells. Tissue Eng Part A 18:295–309. doi: 10.1089/ten.TEA.2011.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavedi S, Olsen HC, Guelcher SA, et al. (2011). Fabrication of a model continuously graded co-electrospun mesh for regeneration of the ligament-bone interface. Acta Biomater 7:4131–8. doi: 10.1016/j.actbio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Seo S, Mahapatra C, Singh RK, et al. (2014). Strategies for osteochondral repair: focus on scaffolds. J Tissue Eng 5:2041731414541850. doi: 10.1177/2041731414541850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Xu X, Ye Y, et al. (2016). Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. Acs Nano 10:1292–9. doi: 10.1021/acsnano.5b06663. [DOI] [PubMed] [Google Scholar]
- Sill TJ, von Recum HA. (2008). Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Silva JC, Udangawa RN, Chen J, et al. (2020). Kartogenin-loaded coaxial PGS/PCL aligned nanofibers for cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl 107:110291. doi: 10.1016/j.msec.2019.110291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somiya M, Kuroda S. (2015). Development of a virus-mimicking nanocarrier for drug delivery systems: the bio-nanocapsule. Adv Drug Deliv Rev 95:77–89. doi: 10.1016/j.addr.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Song W, Zhang Y, Yu D, et al. (2021). Efficient synthesis of folate-conjugated hollow polymeric capsules for accurate drug delivery to cancer cells. Biomacromolecules 22:732–742. doi: 10.1021/acs.biomac.0c01520. [DOI] [PubMed] [Google Scholar]
- Spakova T, Plsikova J, Harvanova D, et al. (2018). Influence of kartogenin on chondrogenic differentiation of human bone marrow-derived MSCs in 2D culture and in co-cultivation with OA osteochondral explant. Molecules 23:181. doi: 10.3390/molecules23010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stejskalová A, Almquist BD. (2017). Using biomaterials to rewire the process of wound repair. Biomater Sci 5:1421–34. doi: 10.1039/c7bm00295e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Huang C, Liu H. (2022). Evaluation and preparation of a designed kartogenin drug delivery system (DDS) of hydrazone-linkage-based pH responsive mPEG-Hz-b-PCL nanomicelles for treatment of osteoarthritis. Front Bioeng Biotech 10:816664. doi: 10.3389/fbioe.2022.816664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Kurtzberg J. (2015). Chapter 13. Emerging uses of cord blood in regenerative medicine—neurological applications. In: Stavropoulos-Giokas C, Charron D, Navarrete C, ed. Cord blood stem cells and regenerative medicine, London: Academic Press, 167–77. [Google Scholar]
- Sun L, Wang M, Chen S, et al. (2019). Molecularly engineered metal-based bioactive soft materials – neuroactive magnesium ion/polymer hybrids. Acta Biomater 85:310–9. doi: 10.1016/j.actbio.2018.12.040. [DOI] [PubMed] [Google Scholar]
- Sun X, Wang J, Wang Y, Zhang Q. (2018). Collagen-based porous scaffolds containing PLGA microspheres for controlled kartogenin release in cartilage tissue engineering. Artif Cells Nanomed Biotechnol 46:1957–1966. doi: 10.1080/21691401.2017.1397000. [DOI] [PubMed] [Google Scholar]
- Tan H, Marra KG. (2010). Injectable, biodegradable hydrogels for tissue engineering applications. Materials 3:1746–1767. doi: 10.3390/ma3031746. [DOI] [Google Scholar]
- Tan H, Ramirez CM, Miljkovic N, et al. (2009). Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials 30:6844–53. doi: 10.1016/j.biomaterials.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng B, Zhang S, Pan J, et al. (2021). A chondrogenesis induction system based on a functionalized hyaluronic acid hydrogel sequentially promoting hMSC proliferation, condensation, differentiation, and matrix deposition. Acta Biomater 122:145–159. doi: 10.1016/j.actbio.2020.12.054. [DOI] [PubMed] [Google Scholar]
- Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. (2016). Extracellular matrix structure. Adv Drug Deliv Rev 97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Théry C, Witwer KW, Aikawa E, et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liu Q, Ma X, Dai G. (2019). Levels of matrix metalloproteinase-2 in committed differentiation of bone marrow mesenchymal stem cells induced by kartogenin. J Int Med Res 47:3261–70. doi: 10.1177/0300060519853399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Deng R, Wang J, et al. (2011). Tuning upconversion through energy migration in core–shell nanoparticles. Nat Mater 10:968–73. doi: 10.1038/nmat3149. [DOI] [PubMed] [Google Scholar]
- Wang J, Langhe D, Ponting M, et al. (2014). Manufacturing of polymer continuous nanofibers using a novel co-extrusion and multiplication technique. Polymer 55:673–685. doi: 10.1016/j.polymer.2013.12.025. [DOI] [Google Scholar]
- Wang J, Wang Y, Sun X, et al. (2019). Biomimetic cartilage scaffold with orientated porous structure of two factors for cartilage repair of knee osteoarthritis. Artif Cells Nanomed Biotechnol 47:1710–21. doi: 10.1080/21691401.2019.1607866. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhou J, Zhang N, et al. (2014). A heterocyclic molecule kartogenin induces collagen synthesis of human dermal fibroblasts by activating the smad4/smad5 pathway. Biochem Biophys Res Commun 450:568–74. doi: 10.1016/j.bbrc.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Wang Y, Feng L, Wang S. (2019). Conjugated polymer nanoparticles for imaging, cell activity regulation, and therapy. Adv Funct Mater 29:1806818. doi: 10.1002/adfm.201806818. [DOI] [Google Scholar]
- Wei W, Liu W, Kang H, et al. (2023). A one‐stone‐two‐birds strategy for osteochondral regeneration based on a 3d printable biomimetic scaffold with kartogenin biochemical stimuli gradient. Adv Healthc Mater 12:e2300108. [DOI] [PubMed] [Google Scholar]
- Westin CB, Trinca RB, Zuliani C, et al. (2017). Differentiation of dental pulp stem cells into chondrocytes upon culture on porous chitosan-xanthan scaffolds in the presence of kartogenin. Mater Sci Eng C Mater Biol Appl 80:594–602. doi: 10.1016/j.msec.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Wu J, Cao L, Liu Y, et al. (2019). Functionalization of silk fibroin electrospun scaffolds via BMSC affinity peptide grafting through oxidative self-polymerization of dopamine for bone regeneration. ACS Appl Mater Interfaces 11:8878–8895. doi: 10.1021/acsami.8b22123. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhu S, Wu C, et al. (2014). A Bi-lineage conducive scaffold for osteochondral defect regeneration. Adv Funct Mater 24:4473–83. doi: 10.1002/adfm.201304304. [DOI] [Google Scholar]
- Xu J, Feng Q, Lin S, et al. (2019). Injectable stem cell-laden supramolecular hydrogels enhance in situ osteochondral regeneration via the sustained co-delivery of hydrophilic and hydrophobic chondrogenic molecules. Biomaterials 210:51–61. doi: 10.1016/j.biomaterials.2019.04.031. [DOI] [PubMed] [Google Scholar]
- Xu X, Liang Y, Li X, et al. (2021). Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 269:120539. doi: 10.1016/j.biomaterials.2020.120539. [DOI] [PubMed] [Google Scholar]
- Xuan H, Hu H, Geng C, et al. (2020). Biofunctionalized chondrogenic shape-memory ternary scaffolds for efficient cell-free cartilage regeneration. Acta Biomater 105:97–110. doi: 10.1016/j.actbio.2020.01.015. [DOI] [PubMed] [Google Scholar]
- Yang SY, Hwang TH, Che L, et al. (2015). Membrane-reinforced three-dimensional electrospun silk fibroin scaffolds for bone tissue engineering. Biomed Mater 10:035011. doi: 10.1088/1748-6041/10/3/035011. [DOI] [PubMed] [Google Scholar]
- Yang W, Zheng Y, Chen J, et al. (2019). Preparation and characterization of the collagen/cellulose nanocrystals/USPIO scaffolds loaded kartogenin for cartilage regeneration. Mater Sci Eng C Mater Biol Appl 99:1362–73. doi: 10.1016/j.msec.2019.02.071. [DOI] [PubMed] [Google Scholar]
- Yang W, Zhu P, Huang H, et al. (2019). Functionalization of novel theranostic hydrogels with kartogenin-grafted USPIO nanoparticles to enhance cartilage regeneration. ACS Appl Mater Interfaces 11:34744–34754. doi: 10.1021/acsami.9b12288. [DOI] [PubMed] [Google Scholar]
- Yin H, Wang J, Gu Z, et al. (2017). Evaluation of the potential of kartogenin encapsulated poly(L-lactic acid-co-caprolactone)/collagen nanofibers for tracheal cartilage regeneration. J Biomater Appl 32:331–41. doi: 10.1177/0885328217717077. [DOI] [PubMed] [Google Scholar]
- Yu H, Huang C, Kong X, et al. (2022). Nanoarchitectonics of cartilage-targeting hydrogel microspheres with reactive oxygen species responsiveness for the repair of osteoarthritis. ACS Appl Mater Interfaces 14:40711–40723. doi: 10.1021/acsami.2c12703. [DOI] [PubMed] [Google Scholar]
- Yuan F, Wang H, Guan J, et al. (2021). Fabrication of injectable chitosan-chondroitin sulfate hydrogel embedding kartogenin-loaded microspheres as an ultrasound-triggered drug delivery system for cartilage tissue engineering. Pharmaceutics 13:1487. doi: 10.3390/pharmaceutics13091487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Wan J, Yang Y, et al. (2023). Thermosensitive hydrogel for cartilage regeneration via synergistic delivery of SDF-1α like polypeptides and kartogenin. Carbohydr Polym 304:120492. doi: 10.1016/j.carbpol.2022.120492. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Lyu Z, Zhang W, et al. (2022). Porous bioactive prosthesis with chitosan/mesoporous silica nanoparticles microspheres sequentially and sustainedly releasing platelet-derived growth factor-BB and kartogenin: a new treatment strategy for osteoarticular lesions. Front Bioeng Biotech 10:839120. doi: 10.3389/fbioe.2022.839120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare P, Pezeshki-Modaress M, Davachi SM, et al. (2021). Alginate sulfate-based hydrogel/nanofiber composite scaffold with controlled Kartogenin delivery for tissue engineering. Carbohydr Polym 266:118123. doi: 10.1016/j.carbpol.2021.118123. [DOI] [PubMed] [Google Scholar]
- Zeng W, Zhang Y, Wang D, et al. (2021). Intra-articular injection of kartogenin-enhanced bone marrow–derived mesenchymal stem cells in the treatment of knee osteoarthritis in a rat model. Am J Sports Med 49:2795–2809. doi: 10.1177/03635465211023183. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang JH. (2014). Kartogenin induces cartilage-like tissue formation in tendon–bone junction. Bone Res 2:14008. doi: 10.1038/boneres.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xia W, Liu P, et al. (2010). Chitosan modification and pharmaceutical/biomedical applications. Mar Drugs 8:1962–87. doi: 10.3390/md8071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yuan T, Zheng N, et al. (2017). The combined use of kartogenin and platelet-rich plasma promotes fibrocartilage formation in the wounded rat Achilles tendon entheses. Bone Joint Res 6:231–44. doi: 10.1302/2046-3758.64.BJR-2017-0268.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hu J, Athanasiou KA. (2009). The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng 37:1–57. doi: 10.1615/critrevbiomedeng.v37.i1-2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Chen J, Sun Y, et al. (2023). A 3D multifunctional bi-layer scaffold to regulate stem cell behaviors and promote osteochondral regeneration. J Mater Chem B 11:1240–1261. doi: 10.1039/d2tb02203f. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hu P, Liu T, et al. (2019). Kartogenin hydrolysis product 4-aminobiphenyl distributes to cartilage and mediates cartilage regeneration. Theranostics 9:7108–21. doi: 10.7150/thno.38182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Chen R, Xu X, et al. (2022). Construction of biocompatible hydrogel scaffolds with a long-term drug release for facilitating cartilage repair. Front Pharmacol 13:922032. doi: 10.3389/fphar.2022.922032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ling C, Zhang A, et al. (2020). An all-silk-derived functional nanosphere matrix for sequential biomolecule delivery and in situ osteochondral regeneration. Bioact Mater 5:832–843. doi: 10.1016/j.bioactmat.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Song W, Lu Y, et al. (2022). Recent advances in poly(α-L-glutamic acid)-based nanomaterials for drug delivery. Biomolecules 12:636. doi: 10.3390/biom12050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YS, Khademhosseini A. (2017). Advances in engineering hydrogels. Science 356:f3627. doi: 10.1126/science.aaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Zhang J, Yuan S, et al. (2019). A new insight of kartogenin induced the mesenchymal stem cells (MSCs) selectively differentiate into chondrocytes by activating the bone morphogenetic protein 7 (BMP-7)/Smad5 pathway. Med Sci Monit 25:4960–7. doi: 10.12659/MSM.916696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Huo D, Chen Q, et al. (2017). A eutectic mixture of natural fatty acids can serve as the gating material for near-infrared-triggered drug release. Adv Mater 29:1703702. doi: 10.1002/adma.201703702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Ma Z, Li H, et al. (2019). Enhancement of rotator cuff tendon–bone healing using combined aligned electrospun fibrous membranes and kartogenin. RSC Adv 9:15582–15592. doi: 10.1039/c8ra09849b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tan J, Zhu H, et al. (2017). Development of kartogenin-conjugated chitosan–hyaluronic acid hydrogel for nucleus pulposus regeneration. Biomater Sci 5:784–91. doi: 10.1039/c7bm00001d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.