Abstract
Objective
To investigate the effect of an academic detailing intervention on the utilisation of type 2 diabetes medication among general practitioners.
Design
We developed an academic detailing campaign based on the revised national treatment guideline for diabetes and the best available evidence. General practitioners were offered a 20-minute one-to-one visit by a trained academic detailer.
Setting and subjects
A total of 371 general practitioners received a visit and represented the intervention group. The control group consisted of 1282 general practitioners not receiving a visit.
Main outcome measures
Changes in prescribing from 12 months before to 12 months after the intervention. The primary endpoint was a change in metformin. Secondary endpoints were changes in other groups of Type 2 diabetes medication and of these drugs in total.
Results
Prescribing of metformin increased by 7.4% in the intervention group and 5.2% in the control group (p = .043). Sodium-glucose cotransporter-2 inhibitors increased by 27.6% in the intervention group and 33.8% in the control group (p = .019). For sulfonylureas there was a decrease of 3.6% in the intervention group vs. 8.9% in the control group (p = .026). The total amount of prescribed medications for type 2 diabetes increased by 9.1% in the intervention group and 7.3% in the control group (p = .08).
Conclusion
Academic detailing initiated a small but statistically significant increase in the prescription of metformin. For a complex subject like type 2 diabetes, we recommend reserving more time in the visit than the 20 min our campaign aimed for.
Keywords: Primary care, type 2 diabetes, antidiabetic drugs, physician prescribing pattern, continuing medical education, educational outreach, academic detailing
Key points
Academic detailing is a validated method for facilitating changes in prescribing, via interactive one-to-one meetings with a trained academic detailer.
General practitioners who received a 20-minute visit on the treatment of type 2 diabetes prescribed more metformin, compared to the control group.
For a complex interventions like the present, we recommend setting aside more than 20 minutes, to ensure sufficient time for discussion and reflection.
Academic detailing can impact prescribing, even for a complex subject like the treatment of Type 2 Diabetes.
Introduction
Diabetes is one of the most serious and frequent chronic diseases today, with an increasing prevalence over the last decades. In 2021, it was estimated that 537 million people were living with diabetes, with type 2 diabetes (T2D) accounting for more than 90% of all cases [1]. Based on data from 2017, an estimated 4.1% of the Norwegian population have T2D [2]. Due to its severity, even small improvements in the quality of treatment can have a substantial impact on both patients’ lives and costs for society.
Over the last decade, numerous novel treatment options for T2D have been marketed, including sodium-glucose cotransporter-2 inhibitors (SGLT2 inhibitors), glucagon-like peptide-1 receptor agonists (GLP-1 analogues) and dipeptidyl peptidase-4 inhibitors (DPP-4 inhibitors). Together with more traditional treatments like insulin, metformin, sulfonylureas and others, this adds up to a large number of possible treatments that physicians need to know and take into consideration when treating a patient with T2D. For hospital specialists working in diabetes care, this may be manageable, but for general practitioners (GPs) the number of treatment options can be perceived as overwhelming.
In Norway, an update of the national guideline for the treatment of T2D was published in 2018 [3]. This guideline offered advice on how to choose treatment for different subgroups of patients with T2D. The guideline was in line with international guidelines at the time [4,5], and promoted metformin as the first-line treatment for T2D [3]. It is, however, well known that new knowledge about treatment (e.g. from guidelines) is often not translated to change in clinical practice [6].
Academic detailing (AD) is a method to improve prescription behaviour by presenting a condensed summary of the best evidence on a subject in a one-to-one meeting with the prescriber [7,8]. In the meeting, conducted by trained professionals (typically pharmacists, nurses, or physicians), interaction is a key factor. Then the visitor (often called an academic detailer) can identify the individual needs of each prescriber and adjust the message according to the response. AD has been showed to facilitate changes in prescribing behaviour for a large number of treatments, including antibiotics, anticholinergic drugs and analgesics [7–10].
AD has also been used to improve the evidence-based use of newer glucose-lowering medications in primary care [11], but as far as we know there is only one published study that has evaluated the change in prescribing following an AD intervention on T2D [12]. In that study, no significant differences in prescribing trends between prescribers and matched controls one year after an AD intervention were found. The authors suggested the reason could be that both groups had been exposed to educational outreach programmes on diabetes also before the intervention. In addition, there had been a broad dissemination of current treatment guidelines for diabetes. The study [12] was conducted in 2013-14 and since then, several new antidiabetic drugs have been marketed and the treatment complexity of T2D has further increased.
The aim of this study was to evaluate the impact of an AD intervention on the prescription of medicines for the treatment of T2D in primary care in Norway.
Methods
GPs in selected municipalities in three Norwegian Health Regions (Northern Norway, Western Norway and South-Eastern Norway) were invited to participate in the study. The geographical areas where GPs were offered visits were mainly based on travel distance from the participating AD centres. When an area was chosen, we invited all GPs in that area. We did invite GPs in a wide range of locations, both in cities and in rural areas.
The AD visit consisted of a one-to-one meeting between the GP and the academic detailer. A total of 18 persons, 10 physicians (including four specialists in clinical pharmacology and two specialists in geriatric medicine) and 8 pharmacists, participated as academic detailers. Training of the academic detailers included a three-day general course in the method AD and additional training in the treatment of T2D and the specific content of the campaign. All academic detailers were employed at publicly funded university hospitals and performed visits as part of their regular hours.
Visits were planned for 20 min in the GP’s office during office hours, the duration was chosen to correspond with the length of consultations in general practice. A four-page brochure was used to support the oral message and highlight the key points conveyed. The primary message was to utilize metformin as first-line treatment, but the information was also given on the choices of second and third-line treatments, primary and secondary prevention for cardiovascular disease, non-pharmacological interventions and recommended monitoring. The content of the campaign was consistent with the recently revised national Norwegian treatment guideline at the time [3].
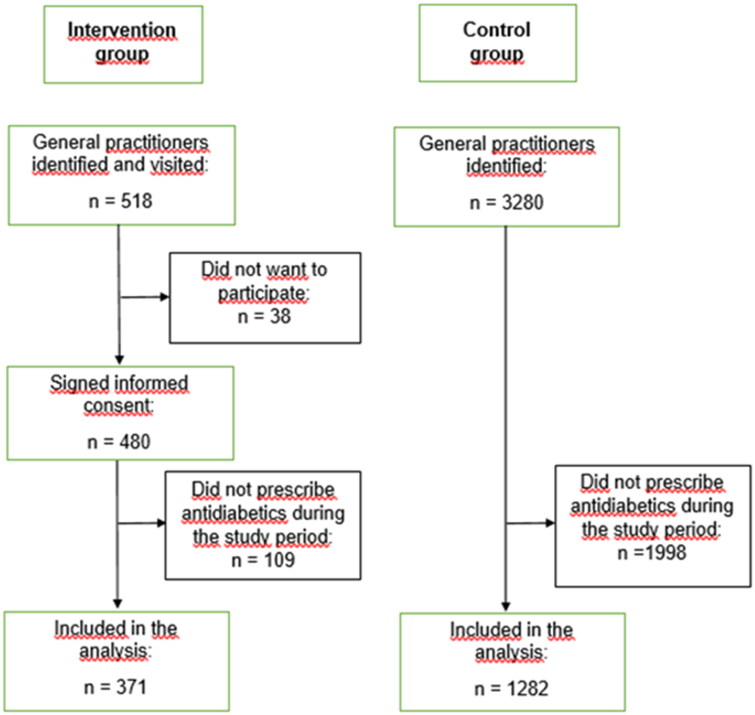
All GPs visited were asked to sign an informed consent, allowing us to obtain their encrypted prescription data from The Norwegian Prescription Database (NorPD) from 12 months before to 12 months after the date of the visit. Visits were conducted between August 28th and November 30th, 2018. The GPs received no financial incentive to participate. A total of 518 GPs were visited, of whom 480 signed the consent form to participate in the study (Figure 1). After the visit, all GPs received an anonymous electronic evaluation form with five questions related to the visit.
Figure 1.
Flow chart of the inclusion process for the intervention and the control groups.
Some of the visited GPs were not actively prescribing antidiabetic drugs during the whole study period. For the analyses, we therefore included only those GPs who met the following criteria: (i) first dispensed prescription of an antidiabetic drug minimum 364 d before the date of intervention, and (ii) last dispensed prescription of an antidiabetic drug to a new patient or of a new antidiabetic drug to an existing patient minimum 364 d after intervention. Based on these criteria, 371 GPs were included in the analyses (Figure 1).
The control group consisted of GPs from a selection of municipalities where none of the GPs had been offered a visit. We excluded municipalities adjacent to those having GPs in the intervention group. GPs in Norway use the International Classification of Primary Care (ICPC)-2 diagnostic codes to classify patient data and for reimbursement prescriptions, whereas physicians in hospitals mainly use the ICD-10 classification. To avoid including possible hospital physicians working part-time in primary care in the control group we included only prescribers mainly using ICPC-2 diagnostic codes in their prescriptions.
A total of 3280 prescribers were identified in the control municipalities. After the exclusion of those who did not prescribe antidiabetic drugs throughout the study period, 1282 GPs were included in the analyses of the control group (Figure 1).
The study was approved by the Regional Committee for Medical and Health Research Ethics (Ref. No. 12709 REK South-East C).
As a proxy for prescribing data, dispensing data from the Norwegian Prescription Database (NorPD), a validated population-based data source which contains all dispensing from Norwegian pharmacies were used [13–15]. In Norway, most prescriptions for chronic diseases like T2D are valid for repeated dispensing up to one year after the date of prescribing. We, therefore, evaluated changes in dispensing from each GP from 364 d before to 364 d after the date of intervention. Periods of 364 and not 365 d was chosen to include equal numbers of Saturdays and Sundays in all calculations irrespective of the weekday of intervention, as pharmacy sales on these days clearly differ from average. Since there was no intervention in the control group, intervention dates from the intervention group were randomly assigned as a fictitious intervention day to each GP in the control group and used in the analyses.
T2D drugs were defined as drugs belonging to Anatomical Therapeutic Chemical (ATC) Classification System group A10B Blood glucose lowering drugs, excl. insulins. We included patients being prescribed insulin (ATC group A10A) if they also were dispensed any drug from A10B, thereby excluding patients with type 1 diabetes [16]. For the analyses we used the total amount of Defined Daily Doses (DDDs) dispensed for each relevant antidiabetic drug for each prescriber. DDD is defined as the assumed average maintenance dose per day for a drug used for its main indication in adults [16]. DDDs for fixed combination drugs were recalculated giving a DDD count as if each of the active ingredients had been administered separately.
The following key information was retrieved from NorPD for all prescriptions including T2D drugs: Demographic information on the prescribers (gender, age and speciality) and the patients (gender, age and municipality), in addition to information related to the dispensed drugs (date of dispensing, ATC-code and amount dispensed in DDD).
The primary endpoint of the analysis was the change in the amount of prescribing of metformin. Secondary endpoints were changes in prescribing of the other groups of T2D drugs and of T2D drugs in total.
Statistical analyses
Year-over-year changes in daily dispensed drugs were computed on a daily basis from day 1 (the day after intervention) through day 364 for each of the two group. These changes were consistent with an identical independent normal distribution for the changes in each group. Year-over-year change in yearly dispensed medication for each group was assessed using the one-sample t-test. A comparison of year-over-year percentage change in yearly dispersed medication between the two groups was done using the two-sample t-test.
Data preparation and aggregation were done using MySQL version 5.7.34. Statistical analysis on aggregated data were done using R version 3.6.3. The level of significance was taken at 5%.
Results
A total of 371 GPs in the intervention group and 1282 GPs in the control group were included in the final analyses (Figure 1). Demographic data of the GPs and the patients included are presented in Table 1. The mean duration of the visits in the intervention group was 22 min (variation 7–84 min), and 84.5% had a duration of 20–25 min.
Table 1.
Demographic characteristics of the general practitioners and patients included in the intervention group and the control group.
| Intervention group | Control group | |
|---|---|---|
| General practitioners | ||
| Number included | 371 | 1282 |
| Mean age (years)a | 47 (SD 11) | 47 (SD 12) |
| Female gender, n (%) | 177 (47.7%) | 489 (38.1%) |
| Specialist in general practice, n (%) | 255 (68.7%) | 788 (61.5%) |
| Patients | ||
| Number included | 13 697 | 36 445 |
| Mean age (years)a | 65 (SD 14) | 63 (SD 14) |
| Female gender, n (%) | 5808 (42.4%) | 15 439 (42.4%) |
SD: standard deviation.
aAge per 31 December, 2018.
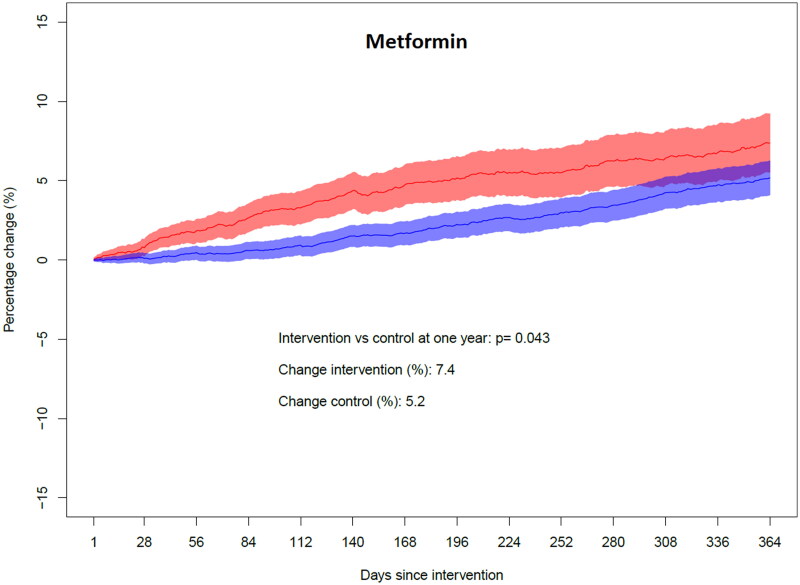
For metformin, including fixed combinations with other T2D drugs, the total amount prescribed measured in DDD increased by 7.4% in the intervention group the year after the intervention, compared to the year before the intervention. In the control group the increase was 5.2% (p = .043). The time pattern of the changes in the intervention and control groups is displayed in Figure 2.
Figure 2.
Year-over-year change in yearly dispensed amount for metformin. Fixed combinations with other drugs are included. The red colour indicates the intervention group and the blue colour indicates the control group. The shaded areas represent 95% confidence intervals of the day-to-day estimates. On the y-axis, a plus sign denotes an increase whereas a minus sign denotes a decrease.
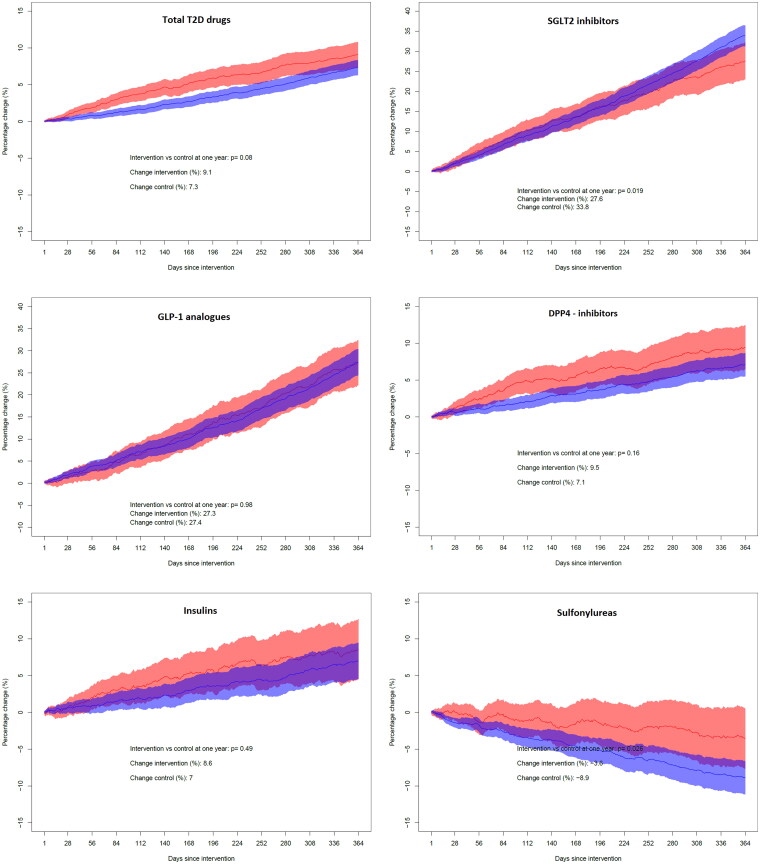
For sulfonylureas there was a decrease of 3.6% in the intervention group and 8.9% in the control group the year after the intervention (p = .026). The use of SGLT2 inhibitors increased by 27.6% in the intervention group and 33.8% in the control group (p = .019). For GLP-1 analogues, DPP-4 inhibitors and insulin, the changes in the two study groups were similar, and the differences did not reach statistical significance (Table 2). The time patterns of the changes in the intervention and control groups for these drug groups are displayed in Figure 3. The total amount of prescribed medications for the treatment of T2D increased by 9.1% in the intervention group the year after the intervention, compared to 7.3% in the control group (p = .08) (Table 2, Figure 3).
Table 2.
Changes in the amount prescribed for subgroups of antidiabetic drugs and antidiabetic drugs in total in the year after the intervention, compared to the year before the intervention.
| Drug/drug group | Intervention group | Control group | p-value |
|---|---|---|---|
| Metformina | +7.4% | +5.2% | 0.043 |
| Sulfonylureas | −3.6% | −8.9% | 0.026 |
| SGLT2 inhibitorsb | +27.6% | +33.8% | 0.019 |
| DPP-4 inhibitorsc | +9.5% | +7.1% | 0.16 |
| GLP-1 analoguesd | +27.3% | +27.4% | 0.98 |
| Insulina | +8.6% | +7.0% | 0.49 |
| Total | +9.1% | +7.3% | 0.08 |
A plus sign denotes an increase, a minus sign denotes a decrease.
aIncluding fixed combinations with other antidiabetic drugs.
bSGLT2 = sodium-glucose cotransporter-2.
cDPP-4 = dipeptidyl peptidase-4.
dGLP-1 = glucagon-like peptide-1.
Figure 3.
Year-over-year change in the yearly dispensed amount of various drug groups used for type 2 diabetes. The panels from top left represent total T2D drugs (ATC group A10B, plus insulins to patients with T2D), SGLT2 inhibitors (sodium-glucose cotransporter-2 inhibitors), GLP-1 analogues (glucagon-like peptide-1 receptor agonists), DPP-4 inhibitors (dipeptidyl peptidase-4 inhibitors), insulins (ATC group A10A, only to patients also prescribed any drug from ATC group A10B) and sulfonylureas. For all graphs, red colour indicates the intervention group and blue colour indicates the control group. The shaded areas represent 95% confidence intervals of the day-to-day estimates. On the y-axis, a plus sign denotes an increase whereas a minus sign denotes a decrease.
Of the 518 visited GPs, 301 (58.1%) responded to the electronic evaluation. In total, 74.4% of respondents considered that there was the appropriate time for the visit, 0.7% answered there was too much time, and 24.9% felt there was too little time. When asked whether the visit would initiate a change of prescribing practice, 13.7% answered to a large degree, 62.3% to some degree and 18.0% to a small degree, whereas only 6.0% expected no change. A total of 96.3% considered academic detailing as a suitable or very suitable method for information about pharmacotherapy, and 97.0% answered they were likely or very likely to accept another visit on a different therapeutic area.
Discussion
The principal finding in the present study was a significant increase in the prescribing of metformin among GPs that received an AD visit compared to the control group. Changes in the total prescribing of antidiabetic drugs were not significantly different between groups, although there was a trend towards a larger increase in the intervention group. The increased prescribing could be explained by either that more new patients were treated for T2D, that existing patients were given higher doses of T2D drugs and/or more drugs, or a combination of these factors.
The main message of the campaign was to utilize metformin as first-line treatment for T2D, including information about how to maintain metformin in patients with impaired or declining kidney function. The use of metformin increased in the control group as well, but less than the intervention group. As Figure 2 indicates, the difference seemed to be largest about six months after the intervention and thereafter declined. As there is an inherent delay from behaviour change among the GPs until all patients have received a new prescription and drugs are dispensed at the pharmacy, the gradual increase over six months could, in fact, indicate an immediate effect in the intervention group. However, our results also show that this effect was waning over time.
The campaign did not deliver clear advice on which drug should be preferred as second-line treatment when metformin could not be used or add-on when metformin alone was not sufficient. The reason was that the Norwegian treatment guidelines at the time did not differentiate between second-line treatments, and sulfonylureas, GLP-1 analogues, SGLT2 inhibitors, DPP-4 inhibitors and insulin were presented as equal options. During the study period, GLP-1 analogues, SLGT2 inhibitors and DPP-4 inhibitors were all heavily marketed by the manufactures. The use of those drugs increased in both study groups. In contrast, the use of sulfonylureas declined in both groups, but the intervention group did maintain sulfonylurea treatment for their patients to a larger degree than the control group. One can speculate that the increase in dispensing of SGLT2 inhibitors was connected to the decline in sulfonylureas.
The evaluation confirmed that the GPs were satisfied with the visits and found them helpful. Still, only 13.7% expected that the visit would initiate a large change in prescribing. This is thus in line with the modest changes in prescribing that was found.
This study has some weaknesses, but also some strengths that should be acknowledged. Strengths include the access to individual data on the prescriber level, and the high number of participants. With 371 prescribers in the intervention group, this is one of the largest published studies on AD with individual prescription data, allowing us to identify even modest differences between study groups.
Weaknesses include the use of dispensing data instead of actual prescribing data. Prescriptions for chronic diseases are valid for one year in Norway. Thus, after a prescriber has changed behaviour, it may take up to one year to show a full effect on dispensing. Unfortunately, the date of prescription is not included in the NorPD, only the date of dispensing. Another weakness is the selection of the control group. As we did not have individual inclusions of prescribers in the control group, we cannot verify that all prescribers actually worked as GPs, although the selection of reimbursement codes used in general practice and not in hospitals indicates that most prescribers were GPs.
Prescribers in the intervention group were more often female than in the control group, and there was a somewhat higher share of specialists in general practice in the intervention than in the control group. In order to evaluate whether gender influenced our results, we performed a sensitivity analysis by examining changes in prescribing separately for male and female prescribers. These results were principally the same as the combined analyses presented here, indicating that the gender imbalance did not affect the results.
The relative differences in change in prescribing behaviour between the study groups were generally small. In the only previously published study on AD intervention in T2D [12], there were no significant differences in the proportions of patients prescribed metformin or the proportions of patients prescribed long-acting sulfonylureas between the intervention group and the control group. The authors suggested the reason could be that both groups had been exposed to educational outreach programmes on diabetes before the intervention. In addition, there had been a broad dissemination of treatment guidelines for diabetes. The use of metformin has been the first-line treatment for T2D for several years prior to the intervention also in Norway, and a large proportion on patients with T2D were treated with metformin before our study was conducted [17]. Although we found a statistically significant intervention effect for metformin, the relatively small difference between the groups can support a tendency towards a ceiling effect, similar to that described in the previous study [12].
The total use of T2D drugs increased in both study groups. This could be caused by an increased prevalence of T2D, increased detection of T2D and/or an increased utilization of T2D drugs. The use of metformin had been increasing in Norway in the decade before our study was conducted [18], and the results of the present study indicate that this trend has continued. Both increased focus on diagnostics and treatment of T2D, increased general knowledge on the safety margin of continuing metformin in advanced age and reduced kidney function, as well as knowledge about the combination of two or three different T2D drugs may have contributed to the increased use of metformin. The marketing of newer T2D drugs is also likely to increase the total use of T2D drugs, as these drugs were recommended used in addition to, and not instead of metformin [3].
In a review on four AD programmes for diabetes [11], it was suggested that AD for diabetes is more complex and that visits were more didactic than AD for other clinical topics. This finding is similar to the general impression amongst the academic detailers in our campaign, that the complexity of T2D treatment, with many newer drug classes that the GPs could be unfamiliar with, was high. This was also reflected in the extensive content presented during the visit. In an internal, anonymous evaluation among the academic detailers conducted shortly after finishing our campaign, all reported that the amount of information in the brochure was too much to present in a 20-minute meeting (67% answered ‘some too much’ and 33% ‘way too much’), and only 61% agreed that the brochure had key messages that were easy to deliver during the visit.
In retrospect it is likely that the amount of information intended to be delivered in 20 min was too comprehensive, and that too little room was left for discussion and reflection, which is a crucial part AD as a method. It is likely that behaviour change after the intervention would have been more profound if more time had been reserved for discussion and reflection in the AD visits, and, correspondingly, if more effort had been put into presenting an even more focused message in the brochure.
Our group has previously shown a large reduction in the prescribing of diclofenac following a previous AD intervention [10]. In contrast to the complex subject discussed in the present campaign, that intervention had a simple and clear message, which was both easy to deliver to the GPs and easy for the GPs to act on (i.e. ‘use naproxen instead of diclofenac’). Thus, it is not surprising that the effect of that study was larger than the changes found in the present study.
Conclusion
Academic detailing for T2D among GPs initiated a small but statistically significant increase in the prescription of metformin. For a complex subject as T2D, we would recommend reserving more time for discussion and reflection in the AD visit than the 20 min our campaign aimed for.
Acknowledgements
We are grateful to all the academic detailers who performed the visits, and to the general practitioners who accepted a visit and signed consent to participate in our study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.International Diabetes Federation . IDF diabetes atlas. 10th ed. Brussels, Belgium: International Diabetes Federation; 2021. [Google Scholar]
- 2.NIPH . Diabetes in Norway [Internet]. Oslo, Norway: Norwegian Institute of Public Health; 2017. Available from: https://www.fhi.no/en/op/hin/health-disease/diabetes-in-norway–-public-health [Google Scholar]
- 3.Diabetes . National treatment guideline [Internet]. Oslo, Norway: The Norwegian Directorate of Health; 2018. Available from https://www.helsedirektoratet.no/retningslinjer/diabetes [Google Scholar]
- 4.American Diabetes Association . 9 Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2021. Diabetes Care. 2020;44(Suppl 1):S111–S124. [DOI] [PubMed] [Google Scholar]
- 5.Buse JB, Wexler DJ, Tsapas A, et al. . 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(2):221–228. doi: 10.1007/s00125-019-05039-w. [DOI] [PubMed] [Google Scholar]
- 6.Avorn J, Fischer M.. Bench to behavior’: translating comparative effectiveness research into improved clinical practice. Health Aff. 2010;29(10):1891–1900. doi: 10.1377/hlthaff.2010.0696. [DOI] [PubMed] [Google Scholar]
- 7.Avorn J. Academic detailing: “marketing” the best evidence to clinicians. JAMA. 2017;317(4):361–362. doi: 10.1001/jama.2016.16036. [DOI] [PubMed] [Google Scholar]
- 8.Dyrkorn R, Langaas HC, Giverhaug T, et al. . Academic detailing as a method of continuing medical education. Adv Med Educ Pract. 2019;10:717–725. doi: 10.2147/AMEP.S206073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien MA, Rogers S, Jamtvedt G, et al. . Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2007;2007(4):Cd000409. doi: 10.1002/14651858.CD000409.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langaas HC, Hurley E, Dyrkorn R, et al. . Effectiveness of an academic detailing intervention in primary care on the prescribing of non-steroidal anti-inflammatory drugs. Eur J Clin Pharmacol. 2019;75(4):577–586. doi: 10.1007/s00228-018-02611-y. [DOI] [PubMed] [Google Scholar]
- 11.Luo J, Dancel E, Bains S, et al. . Academic detailing in the new era of diabetes medication management. Curr Diab Rep. 2019;19(12):140. doi: 10.1007/s11892-019-1252-0. [DOI] [PubMed] [Google Scholar]
- 12.Trombetta DP, Heller DA.. Can academic detailing move the needle for patients with diabetes in a State-Based prescription drug benefit program? American Health & Drug Benefits. 2019;12(2):94–102. [PMC free article] [PubMed] [Google Scholar]
- 13.The Norwegian Prescription Database (NorPD) (Reseptregisteret) [Internet]. 2017. Available from: www.reseptregisteret.no.
- 14.Furu K. Establishment of the nationwide norwegian prescription database (NorPD) – new opportunities for research in pharmacoepidemiology in Norway. Norsk Epidemiologi. 2009;18(2):129–136. [Google Scholar]
- 15.Wettermark B, Zoëga H, Furu K, et al. . The nordic prescription databases as a resource for pharmacoepidemiological research–a literature review. Pharmacoepidemiol Drug Saf. 2013;22(7):691–699. doi: 10.1002/pds.3457. [DOI] [PubMed] [Google Scholar]
- 16.Methodology WCCfDS . Guidelines for ATC classification and DDD assignment 2022. Oslo, Norway: WHO; 2021. [Google Scholar]
- 17.Sommerschild HB, Blix HS, Dansie LS, et al. . Drug consumption in Norway 2016–2020. Data from norwegian drug wholesales statistics and the norwegian prescription database. Oslo: Norwegian Institute of Public Health; 2021. [Google Scholar]
- 18.Bakke Å, Cooper JG, Thue G, et al. . Type 2 diabetes in general practice in Norway 2005–2014: moderate improvements in risk factor control but still major gaps in complication screening. BMJ Open Diabetes Res Care. 2017;5(1):e000459. doi: 10.1136/bmjdrc-2017-000459. [DOI] [PMC free article] [PubMed] [Google Scholar]