Abstract
Saffron, botanically known as Crocus sativus L., is renowned as the world’s most expensive spice and has been utilized in various fields since ancient times. Extensive scientific research has been conducted on Crocus sativus (C. sativus), focusing on its phytochemical composition, diverse applications, and biological activities. C. sativus phytochemicals consist mainly of three compounds, namely crocin, picrocrocin, and safranal, which are responsible for most of its properties. Saffron is rich in bioactive compounds, more than 150 of which have been isolated. Owing to its unique composition and properties, saffron is used in various fields, such as the food industry, perfumery, cosmetics, pharmaceutics, and medicine. However, the high economic value of saffron makes it susceptible to adulteration and various fraudulent practices. To deal with this issue, a number of methods and techniques have been developed to authenticate and determine adulterants in saffron. This paper presents a bibliometric study of saffron based on the Web of Science database, analyzing 3,735 studies published between 2000 and 2021. The study also examined author participation and collaboration networks among countries. Production, transformation, chemical composition, methods of adulteration detection, uses, and health properties of saffron are also discussed.
Keywords: adulteration, chemical composition, heart disease, saffron
INTRODUCTION
Saffron (Crocus sativus L.) is a monocotyledonous herbaceous triploid plant with a chromosome count of 3n=24 (the basic chromosome number is 8). It belongs to the Iridaceae family and constitutes one of the 85 species of the Crocus genus [1]. Crocus sativus L. (C. Sativus) is known to be the world's most expensive spice. It has been cultivated for a long time in several countries in Asia, the Mediterranean basin, Europe, and South Africa [2]. Iran holds the leading position in saffron production and exportation, with 404 tons in 2018 [3]. The highly valued part of the plant is the stigma of its flowers [4], which is grown in greenhouses and fields of different soils. Saffron grows from a corm, which is considered the nutrient reserve of the plant [4].
From a chemical standpoint, saffron contains more than 150 chemical compounds, including flavonoids, carotenoids, flavonoid glycosides, monoterpenes and monoterpenoid derivatives, monocyclic aromatic hydrocarbons, amino acids, and alkaloids [5]. This remarkable composition contributed to its broad spectrum of medicinal effects [5–7]. Its use can be traced back to ancient civilizations such as the Egyptians and traditional Chinese medicine [8,9]. The precise chemical constitution of saffron and its health-promoting characteristics, such as antioxidant, hypolipidemic, antihypertensive, immunomodulatory, antimicrobial, antitumor, and antidepressant properties, have been determined and recently reviewed [10,11].
In addition to its medicinal, soporific, and culinary benefits, it is an important source of food dye as well as a perfume ingredient. Nowadays, saffron is used in many fields, including in the food industry (as a flavoring and coloring agent), in perfume compositions, medicine, healthcare, and cosmetics [7]. Due to its exceptional composition, extensive use, and the difficulties linked to its production, saffron is the most expensive spice on the world market. Approximately 60,000 flowers are required to produce 1 kg of dried stigma, estimated at 10,000$ USD [8]. This high price makes this product a target for various types of adulterations. To address this issue, different fraud detection methods have been developed [12]. While several reviews have been published on various aspects of saffron, none have comprehensively compiled its production, chemical composition, transformation, biological activities, health-healing properties, and quality assessment. This review aimed to fill that gap by providing a bibliometric analysis of the existing literature, highlighting saffron production, chemical composition, and biological activities, and focusing on strategies and emerging methods for ensuring its authenticity and quality control. In addition, we discussed the different uses of saffron.
MATERIAL AND METHODS
The present study utilized the Web of Science (WoS) database as the primary data source, given its extensive collection of scholarly papers (n=3735). The investigation aimed to analyze scientific research studies published between 2000 and 2021, employing a bibliometric approach. Various aspects of the research were examined, including the global publication trend, identification of journals with the highest number of publications, assessment of prolific authors, evaluation of the most productive countries, and analysis of commonly used keywords. To conduct this study, the search string used was: (TS = ("saffron") OR TS = ("saffron") OR TS = ("Crocus sativus*")) AND PY = (2000-2021). VOS viewer and Origin Pro (version 2021) software were used to process bibliographic data.
RESULTS
Global publication trend (2000–2021)
The analysis of the WoS database yielded a total of 3,735 records on C. sativus published between 2000 and 2021. The global literature output on saffron showed a consistent increase, with the number of publications rising from 39 in 2000 to 413 in 2021, representing an average annual growth rate of approximately 11.05%. The highest number of publications (451 papers) was recorded in 2020. A total of 849 conference papers and original articles were published from 2000 to 2010. This represents only 22.74% of the total number of publications. It is noteworthy that the number of papers increased every year from 2011 to 2021, except for 2012, 2019, and 2021 when the number of publications decreased (Figure 1). Starting from the same period, the overall number of publications has grown intermittently, with over 2000 publications, highlighting a developing focus on the saffron plant. The quantity of research output in this period suggests an intensified concern for C. sativus. This may be ascribed to its significance and potential applications in other fields. Many studies have reported that every part of the saffron plant has important benefits [13–16]. This growing consciousness is resulting in expanded uses of saffron in pharmacology, pharmacy, food science and technology, agronomy, microbiology, and horticulture, among other uses.
Figure 1.
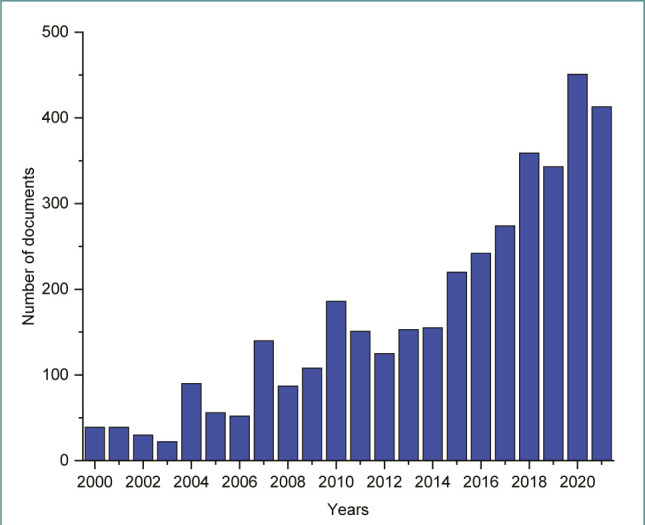
Global publication trend on C. sativus (2000-2021)
Authors' participation (2000-2021)
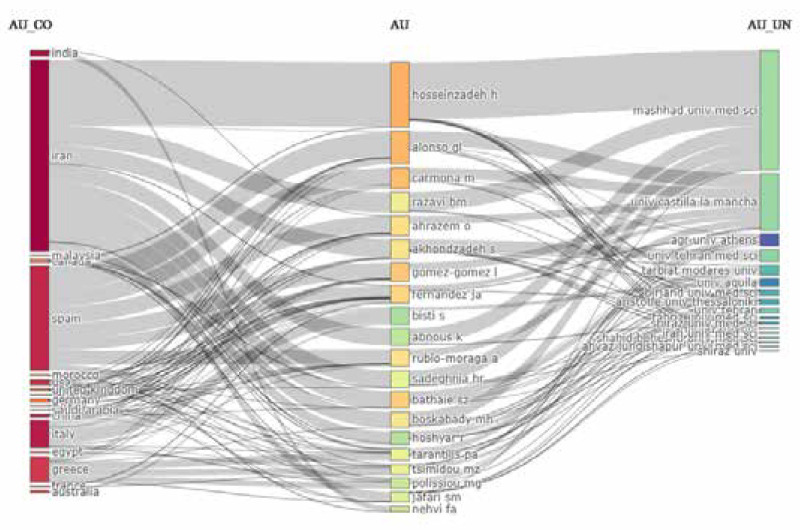
Based on the WoS data, 13 authors had over 30 published papers on the saffron plant between 2000–2021. These authors collectively published 619 articles, with an average of about 47 articles per author. Three authors published more papers than the above-mentioned average. These authors surpassed the average publication count: H. Hosseinzadeh with 126 articles, G.L. Alonso with 78, and M. Carmona with 55 (Table 1). Considering article quality/impact, the top 13 authors were cited 19,709 times in 619 articles, with an overall average of 30.3 citations per article between 2000 and 2021.
Table 1.
Most productive authors publishing papers on saffron (2000-2021)
| Authors | Number of published papers | Total citations | Average citation per paper | h-index |
|---|---|---|---|---|
| Hosseinzadeh H | 126 | 5452 | 43.3 | 43 |
| Alonso GL | 78 | 2110 | 27.1 | 29 |
| Carmona M | 55 | 1739 | 31.6 | 25 |
| Gomez-Gomez L | 43 | 1393 | 32.4 | 20 |
| Bathaie SZ | 41 | 1402 | 34.2 | 20 |
| Fernandez JA | 41 | 597 | 14.6 | 15 |
| Ahrazem O | 40 | 919 | 23.0 | 19 |
| Rubio-moraga A | 34 | 874 | 25.7 | 18 |
| Akhondzadeh S | 33 | 1571 | 47.6 | 18 |
| Boskabady MH | 33 | 933 | 28.3 | 18 |
| Razavi BM | 33 | 818 | 24.8 | 16 |
| Tsimidou MZ | 32 | 605 | 18.9 | 15 |
| Tarantilis PA | 30 | 1296 | 43.2 | 19 |
| Total | 619 | 19709 | 30.3 | 21 |
Figure 2 shows a three-field graph for the 20 most productive authors, countries, and universities. Iran, Spain, and Italy were the top-performing countries, while Meshhad Med Science University (Iran) and Castilla La Mancha University (Spain) were the top organizational contributors. The authors H. Hosseinzadeh (mainly with Mashhad Med Science University), G.L. Alonso, and M. Carmona (mainly with Castilla La Mancha University) did not demonstrate a significant collaborative trend for either institutions or countries.
Figure 2.
Most 20 productive authors, countries, and universities on C. sativus research (2000–2021)
Collaboration network of countries
The collaborative networks of the selected countries, with at least 5 published papers, were examined with the VOS viewer. Only fifty-nine countries met the minimum threshold and were aggregated into 7 clusters (Figure 3). The combination of the individual circles results in a cluster, and these are connected with lines to represent the collaborative relationships or networks and their strengths [17,18]. The top-ranked countries are illustrated with larger circles than the low-performing countries. For the 59 outputs, the analysis resulted in a total link strength of 1,176 and 436 links. Iran (264), the United States (210), and Italy (174) had the greatest total link strengths.
Figure 3.
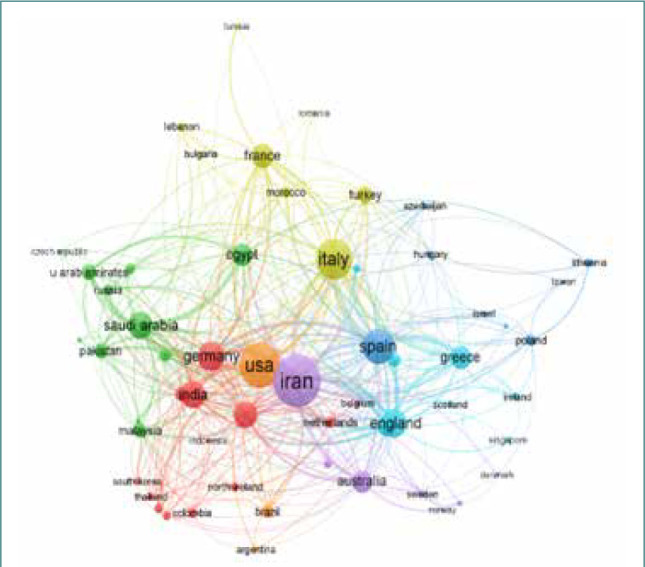
International collaborations network with countries with at least 5 publications (based on the WoS database)
Cluster 1 (red) contains 12 countries. Germany and India had the highest collaborative network with other countries in this cluster. Their total link strengths are 103 and 94, respectively. In cluster 2, 11 countries are represented in green. Saudi Arabia and Egypt have the greatest total link strengths of 94 and 67, respectively, while in cluster 3 (9 countries pictured in blue), Spain leads with a link strength of 167. In cluster 4 (8 countries depicted in yellow), Italy has the greatest collaborative network with a total link strength of 174. In cluster 5 (represented in purple), composed of 8 countries, Iran has the strongest collaborative networks. This is expected since the country has the maximum number of publications. In clusters 6 (turquoise) and 7 (orange), England (102) and the USA (210) have the highest total link strength. In summary, the majority of the 59 countries working on saffron research are collaborating. However, there is a continuing need to develop additional collaborative networks among countries with lower rankings and those with higher rankings to better exploit the potential of saffron.
Journal participation in C. sativus research (2000-2021)
Table 2 represents the most important journals in which saffron research articles were published. According to the WoS database, 1,508 journals and proceedings published research studies on C. sativus between 2000 and 2021. This section focuses only on journals with a minimum of 30 documents. There were 10 journals that met this threshold, with the Journal of Food Chemistry, at 76 outputs, reporting the highest number of papers on saffron, followed by the Planta Medica Journal, with 55 documents. Others include Molecules (49), Iranian Journal of Basic Medical Sciences (48), Phytotherapy Research (48), Avicenna Journal of Phytomedicine (44), and Journal of Agricultural and Food Chemistry (44).
Table 2.
Most important journals publishing world papers on saffron (2000-2021)
| Journals | Total publications | Total citations | Average citations per journal |
|---|---|---|---|
| Food Chemistry | 76 | 2504 | 32.9 |
| Planta Medica | 55 | 656 | 11.9 |
| Molecules | 49 | 735 | 15.0 |
| Iranian Journal of Basic Medical Sciences | 48 | 1501 | 31.3 |
| Phytotherapy Research | 48 | 2398 | 50.0 |
| Avicenna Journal of Phytomedicine | 44 | 761 | 17.3 |
| Journal of Agricultural and Food Chemistry | 44 | 2139 | 48.6 |
| Food Science Technology and Nutrition | 37 | 79 | 2.1 |
| Saffron Science Technology and Health | 36 | 66 | 1.8 |
| Industrial Crops and Products | 32 | 524 | 16.4 |
The number of citations per journal serves as a strong index of influence and research [19]. The average citation per journal of the Journal of Phytotherapy Research was on top (50). This was followed by the Journal of Agricultural and Food Chemistry (48.6) and Food Chemistry (32.9) (Table 2).
Co-occurrence of keywords related to C. sativus
To make the bubble map more efficient, words/terms appearing at least 5 times in the published papers were examined and visualized. Of 13,258 keywords used, 1,067 reached the selected threshold, and 2 were removed manually. The results obtained are displayed in the form of network visualization and density diagrams. According to the terms and density maps (Figures 4 and 5), seven clusters were generated. However, the most significant keywords were saffron, with 1,333 occurrences, followed by crocin, oxidative stress, crocetin, and Crocus sativus L., with 563, 410, 408, and 430 occurrences, respectively.
Figure 4.
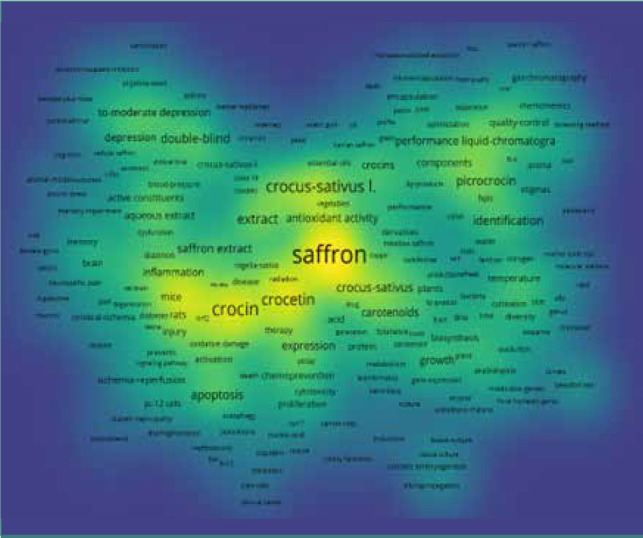
Density map of clusters based on WoS database
Figure 5.
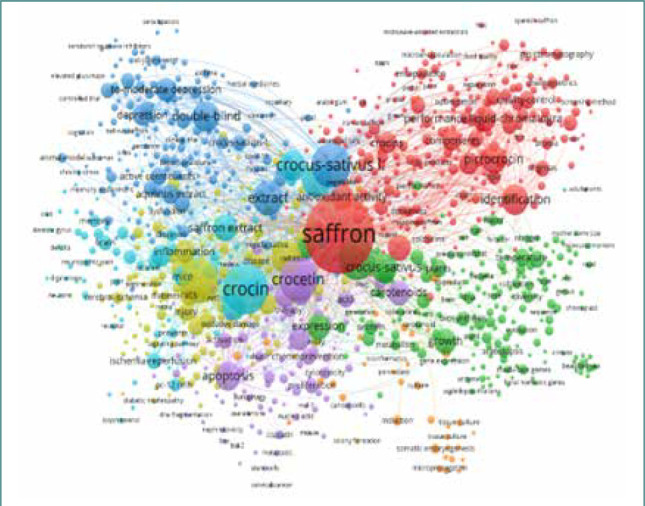
Terms map based on WoS database, made in VOS viewer software
Saffron production and transformation
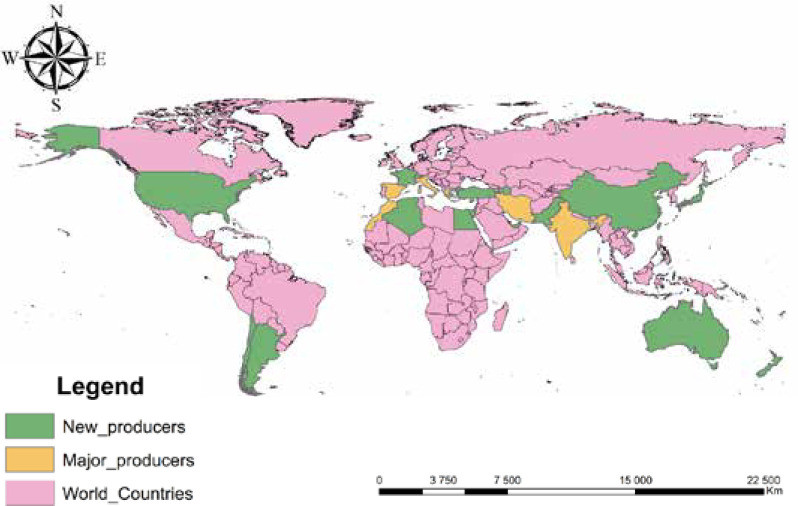
Saffron is cultivated in different areas and territories across the globe (Figure 6). The main producing countries are the following: Iran (mainly Khorasan, Fars, Kerman, and Yazd), India (Kashmir, Jammu, Uttar, Pradesh, and Himachal Pradesh), Spain (Castile-La Mancha), Italy (Aquila, Cerdeña, San Gimignano, and Emilia-Romagna), Morocco (mainly in the Taznakhte and Taliouine areas), and Greece (Kozani). Recently, there have been new areas of saffron cultivation which include Australia, Azerbaijan, Egypt, France, Israel, Japan, Turkey, Pakistan, United States, New Zealand, China, Argentina, Chili, United Arab Emirates, and Switzerland [1,2,20]. Cultivating saffron in all these areas with different climatic conditions demonstrates its adaptability and undemanding nature.
Figure 6.
World map with saffron production countries
Saffron is a perennial plant that takes birth from a corm and develops in a wide variety of ecological edaphic and climatic conditions, including altitudes ranging from sea level to 2000m [21]. Saffron corms have a 5-month dormancy period, during which they do not require much water. In fact, saffron cultivation is suitable in arid or semi-arid areas where the water deficit is extreme in summer and temperatures range from –20/–15ºC in winter to 35–45 ºC in summer [2,21].
According to Menia et al., saffron grows on a range of soil types but prefers well-drained, loose clay calcareous soils that are friable, allowing for easy root penetration [22]. A recent study published by Pirasteh-Anosheh et al. shows that it is possible to cultivate saffron in semi-saline water (2.9 dS m−1) and in saline soil (5.8 dS m−1). Conventional cultivation of saffron is initiated by ploughing the soil. Deep ditches of about 20 cm or more are created, and saffron corms are placed at the bottom and then covered with soil. Every country has a different planting season, but usually, it takes place in late May to early October. Harvesting is generally carried out from October to November and consists of plucking the flowers and separating the stigmas. In most countries, it is usually performed manually by women [2]. The next step is drying. The quality of the produced stigmas is influenced by this extremely sensitive process [23]. Depending on the country, different drying techniques are used. In Morocco, Iran, and India, the traditional methods of dehydrating stigmas in the sun or the shade for 3–10 days until the humidity content is reduced to about 8–10% are still used. In other countries, drying is carried out employing oak embers, a gas cooker, live vine shoot charcoal, or, to a lesser extent, an electric coil [21]. A recent study by Chen et al. showed that freeze drying, infrared drying, and microwave drying were better for preserving the bioactive and aroma compounds of saffron [24].
Currently, global saffron production worldwide is projected at 408 tons. Iran, India, Greece, Afghanistan, Morocco, and Spain are the major producers and exporters. Iran is ranked first with about 90% of world production and 108000 ha of cultivated areas [25].
In addition to the stigma, the most important part of saffron, a large quantity of saffron by-products is produced during stigma processing. In fact, for the production of 1 kg of dried saffron stigma, around 80 kg of C. sativus flowers are needed [26]. Unfortunately, such by-products are less valorized and less important economically. They are mainly used as fertilizer or animal feed [27]. Few studies have focused on the other parts of the saffron plant, although the presence of kaempferol and antioxidant activities has been reported by many researchers. Many studies have shown that saffron petals present immense potential for phytopharmaceutical and nutraceutical purposes [28, 29].
Chemical composition
Major compounds
The chemical composition of saffron is well documented. Many previous studies carried out in different countries have reported the proximate composition of C. sativus as follows: water (10%), moisture (10%), various sugars (63%), proteins (12–14%), amino acids (12%), minerals (5%), fats (5-8%), starch (6–7%), pectins (6%), gums and dextrins (9–10%), vitamins (B1 (riboflavin), B2 (thiamine) (0.3–1.38%), glucose (7-8%), fructose (1-2%), pentosanes (6-7%), pigments, alkaloids, and essential oils (0.3%) [21,26,30–33]. Minerals, heavy metals, and amino acids reported in saffron stigmas are summarized in Table 3 [34–36].
Table 3.
Minerals, heavy metals, and amino acids in saffron (C. sativus)
| Compounds | Content (mg/100 g) | Compounds | Content |
|---|---|---|---|
| Aspartic acid | 0.04 – 0.048 | Iron | 53 – 160 µg/g |
| Glutamic acid | 0.017 – 0.036 | Nickel | 1.19 – 2.8 µg/g |
| Threonine | 0.019 – 0.026 | Potassium | 8200 – 12200 µg/g |
| Serine | 0.016 – 0.023 | Sodium | 25 – 87 µg/g |
| Alanine | 0.13 – 0.17 | Zinc | 16 – 22.7 µg/g |
| Proline | 0.057 – 0.08 | Copper | 6 – 10 µg/g |
| Leucine | 0.017 – 0.020 | Manganese | 13 – 23 µg/g |
| Valine | 0.022 – 0.037 | Lithium | 27 – 188 ng/g |
| Isoleucine | 0.012 – 0.019 | Calcium | 218 – 568 µg/g |
| Glycine | 0.007 – 0.016 | Magnesium | 1130 – 1760 µg/g |
| Lysine | 0.026 – 0.027 | Boron | 13.1 – 17.1 µg/g |
| Histidine | 0.012 – 0.019 | Chromium | 0.574 – 1.8 µg/g |
| Arginine | 0.007 – 0.012 | Cobalt | 54 – 171 ng/g |
| Tyrosine | 0.008 – 0.011 | Cadmium | 15 – 181 ng/g |
| Phenylalanine | 0.012 – 0.03 | Arsenic | 11 – 63 ng/g |
| Phosphorus | 3223 – 4367 ng/g |
The three main chemicals found in saffron stigmas are crocin, safranal, and picrocrocin [26]. These three compounds have undergone substantial research, and multiple studies have indicated that they are responsible for the unique properties and several biological activities of saffron [33]. Crocin is produced when crocetin is esterified with various glucosides and is responsible for the golden yellow-orange color of saffron. Safranal (C10H14O, 2,6,6-trimethyl-1,3-cyclohexadiene-1-carboxaldehyde) and picrocrocin are produced from the degradation of zeaxanthin and belong to the monoterpenoid class [26, 37, 38]. The characteristics of crocin, picrocrocin, and safranal are summarized in Table 4.
Table 4.
Frequency of liver hydatid cysts according to Gharbi WHO Classification [10]
| Compounds | Main characteristics | References |
|---|---|---|
| Crocin (C44H64O24) MW: 976.96 g/mol |
- Hydrophilic carotenoid is the most abundant in saffron, with about 80% of the total chemical constituents of saffron. - Responsible for the dazzling golden–yellow–red shade of the spice. - Crocin exhibits an antioxidant effect and protects cells and tissues from oxidation (against oxidative stress). - Crocin is stable in hard conditions. - Crocin presents a maximum absorbance at 440nm. - Crocin is water soluble and is used in many industries as a coloring agent. - Total crocin amount might vary from 6 to 16% of the dry saffron mass. |
[2] [26, 39] |
| Safranal (C10H14O) MW: 150. 21 g/mol |
- Constitutes the main constituent of the essential oil of saffron, about 30 to 70%. - Responsible for the specific aroma of saffron. - It is a monoterpene aldehyde aglycon of picrocrocin formed during dehydration and storage after harvest because of the action of β-glucosidase.\ - The λmax for safranal is at 330 nm. - It is made from 0.001 to 0.006% of saffron dry matter. - Safranal amount may range from 0.04 to 0.48%. |
[40–44] |
| Picrocrocin (C16H26O7) MW: 330.37 g/mol |
- Responsible for the specific flavor and bitter taste of saffron. - Makes approximately 1 to 13% of the dry matter of saffron. - Picrocrocin-a precursor of safranal is a monoterpene glycoside found in the essential oil of saffron. It is the second most abundant compound in the essential oil of saffron. - Presents a maximum absorbance at λ=254 nm. |
[41, 44, 45] |
Secondary metabolites
Recent studies have reported that the saffron stigma is an excellent reserve of bioactive compounds, with more than 150 compounds identified [2, 9, 32]. Most of these compounds belong to different classes, namely, carotenoids, flavonoids, monoterpenes, and monocyclic aromatic hydrocarbons.
Carotenoids identified in saffron are as follows: crocin, crocetin (8,8' -Diapo-ψ,ψ-carotenedioic acid), dimethyl-crocetin, methyl-crocetin, crocetin-di-(β-D-glucosyl)-ester, 13Z-crocin, Zeaxanthin, crocetin-di- (β-neapolitanosyl)-ester, crocetin-1-all-O-β-gentiobiosyl-ester, lycopene (ψ,ψ-carotene), cis-crocetin- (β-gentiobiosyl)-(β-neapolitanosyl)-ester, cis-crocetin (β-D-triglucoside)-(β-D-gentibiosyl) ester, crocetin- (β-gentiobiosyl)- (β-neapolitanosyl)-ester, α-carotene, crocetin-mono-(β-D-glucosyl)-ester, crocetin (β-D-neapolitanose)-(β-D-glucosyl) ester, β-carotene (β, β-carotene), cis-crocetin (β-D-neapolitanose)-(β-D-glucosyl) ester, crocetin-mono-(β-D-gentiobiosyl)-ester, crocetin (β-D-triglucoside)-(β-D-gentibiosyl) ester, γ-carotene (β,ψ-carotene), crocetin-(β-D-gentiobiosyl)-(β-D-glucosyl)-ester, Phytoene, Lycopersene, ζ-carotene (Tetrahydrolycopene), and Phytofluene [6, 9, 40, 46–50].
Many compounds belonging to the flavonoids family were identified in saffron as follows: Kaempferol, Kaempferide, Astragalin, kaempferol-3-O-sophoroside-7-O-glucoside, kaempferol-3,7,4' -triglucoside, kaempferol-7-O-sophoroside, sophoraflavonoloside, kaempferol-3-O-β-D-(2-O-β-D-6-O-acetylglucosyl)-glucopyranoside, kaempferol 3-O-β-D-(6-O-acetyl)-glucopyranoside, kaempferol 7-O-β-D-glucopyranoside, kaempferol 3,7-di-O-β-D-glucopyranoside, kaempferol-3-O-β-D-(6-O-acetyl)glucopyranoside-7-O-β-Dglucopyranoside, kaempferol-3-O-β-D-(2-O-β-D-6-acetylglucosyl)glucopyranoside-7-O-β-D-glucopyranoside, isorhamnetin-4' -O-α-L-rhamnopyranosyl(1 → 2)-β-Dglucopyranoside (crosatoside A), Helichrysoside, isorhamnetin-3,4' -diglucoside, isorhamnetin-3-O-robinobioside, isorhamnetin-3-O-glucoside, Rutin, Apigenin, vitexin, isoorientin, naringenin, orientin, populin, myricetin, quercetin, and rhamnetin [6, 9, 26, 50–55].
Numerous studies have reported many monoterpenes isolated from saffron including: (4R)-4-hydroxy-2,6,6-trimethylcyclohex-1- enecarbaldehyde 4-O -[βDglucopyranosyl (1 → 3)-β-Dglucopyranoside], (4S)-4-(Hydroxymethyl)-3,5,5-trimethylcyclohex-2-enone-β-Dglucopyranoside, (4R)-4-hydroxy-2,6,6-trimethylcyclohex-1-enecarbaldehyde O-β-D- gentiobioside, (4R)-4-hydroxy-2,6,6-trimethylcyclohex-1-enecarboxylic acid O-β-D-glucopyranoside, 6-hydroxy-3-(hydroxymethyl)-2,4,4-trimethylcyclohexa-2,5-dienone 6-O-β-D-glucopyranoside, (5S)-5-hydroxy-7,7-dimethyl-4,5,6,7-tetrahydro-3Hisobenzofuran-1-one O-β-Dglucopyranoside, (1R,5S,6R)-5-(hydroxymethyl)-4,4,6-trimethyl-7-oxabicyclo[4.1.0] heptan-2-one O-β-Dglucopyranoside, 5-hydroxy-7,7-dimethyl-4,5,6,7-tetrahydro-3Hisobenzo-Furanone 5-O-β-D-gentibioside, 4-hydroxymethyl-3,5,5-trimethylcyclohex-2-en-1-one 4-O-β-D-gentibioside, (4R)-4-hydroxy-3,5,5-trimethylcyclohex-2-enone 4-O-β-Dglucopyranoside, (4S)-4-hydroxy-3,5,5-trimethylcyclohex-2-enone 4-O-β-Dglucopyranoside, (2Z)-3-methylpent-2-enedioic acid 1-[1-(2,4,4-trimethyl-3,6-dioxocyclohexenyloxy)-O-β-Dglucopyranosid-6-yl] ester, (1R)- 3,5,5-trimethylcyclohex-3-enol O-β-D-glucopyranoside, (4S,3' R)-4-Hydroxy-4-(3' -hydroxy-1' -butenyl)-3,5,5-trimethyl-2- cyclohexen-1-one 3' -O-β-Dglucopyranoside, safranal (2,6,6-trimethyl-1,3-cyclohexadiene-1-carboxaldehyde), 2,6,6-trimethyl-1,4-cyclohexadiene-1-carboxaldehyde, 2,6,6-trimethyl-4-hydroxy-1- cyclohexen-1-carboxaldehyde, 2,4,4-trimethyl-3-formyl-6-hydroxy-2,5-cylohexadien-1-one, 4-hydroxymethyl-3,5,5-trimethylcyclohex-3-enol, crocusatin-B, crocusatin-C, crocusatin-D, crocusatin-F, crocusatin-G, crocusatin-H, crocusatin-I, crocusatin-J, crocusatin-K, crocusatin-L, 3-hydroxy-β-ionone, isophorone, 3,5,5-trimethyl-4-hydroxy-1-cyclohexanon-2-ene, 3,5,5-trimethyl-1,4-cyclohexadione, 3,5,5-trimethyl-1,4-cyclohexadion-2-ene, 3,5,5-trimethyl-2-hydroxy-1,4-cyclohexadion-2-ene, crocusatin-A, crocusatin-E [6, 9, 40, 50, 53, 56–58].
Monocyclic aromatic hydrocarbons found in saffron are sodium (2S)-(O-hydroxyphenyl) lactate, methylparaben, protocatechuic acid methyl ester, protocatechuic acid, 4-hydroxybenzoic acid, 4-hydroxyphenethyl alcohol, benzoic acid, 1-O-(4-hydroxybenzoyl)-β -D-glucose, p-coumaric acid, vanillic acid, methylvanillate, 3-hydroxy-4-methoxybenzoic acid, β-(p-hydroxyphenyl) ethanol-α-O-α-Lrhamnopyranosyl(1 → 2)-β Dglucopyranoside(crosatoside B), 2,4-dihydroxy-6-methoxyacetophenone-2-β-D-glucopyranoside, 2,3,4-trihydroxy-6-methoxyacetopenone-3-β-D-glucopyranoside, benzyl O-β-D-glucopyranoside, 2-phenylethyl O-β-D-glucopyranoside [9, 52, 53, 57, 59]. Several nitrogen-containing compounds were also identified in saffron namely 5-methyluracil, pyridin-3-ylmethanol, uracil, nicotinamide, adenosine, harman, tribulusterine, and 1-(9H-β-carbolin-1-yl)-3,4,5- trihydroxypentan-1-one [9, 52, 53].
Saffron quality control and authenticity
The quality of saffron is related to its low water content and high content of particular scents (safranal) and specific coloring (crocin and picrocrocin). The International standards ISO 3632-1 and ISO 3632-2 2011 have been developed to monitor the quality of marketed saffron.
Also known as red gold, the high price of saffron is due to many factors, including its large number of applications, increased demand in the market, limited areas of production, and for the most part, manual means of production and low yield. Due to its high market value, saffron is one of the most targeted products for fraudulent practices. Disparate methods are employed by cheaters to maximize profit and delude consumers by adding foreign materials (biological, artificial, or synthetic adulterants) that have a similar appearance to C. sativus. Adding similar plants such as Calendula officinalis L., Buddleja officinalis Maxim., Gardenia jasminoides Ellis., Curcuma longa L., and Carthamus tinctorius L, and mixing with low-quality saffron or adding various parts of the saffron plant such as petals and stamens or even other species of crocus like Crocus vernus and Crocus speciosus, are common fraudulent practices [91-94,100]. Incorporating chemicals such as potassium nitrate, starch, potassium hydroxide, sodium, lactose, monopotassium tartrate, glucose, barium sulphate, sodium borate, and calcium carbonate is another common illegal behavior. Powdered saffron is usually adulterated by adding various synthetic dye and chemicals like allura red, magenta III (new fuchsin), azorubine, rocelline, carminic acid, cochineal A red, tartrazine, naphthol yellow, sudan II, sunset yellow, picric acid, quinoline, rhodamine B, emaranth, ponceau 4R and red 2G, martins yellow, tropeolina, erythrosine, and fucsina [62-64,73,100].
In addition to the aforementioned methods of adulteration, there are several unusual practices employed to increase the weight of saffron. These include soaking saffron stigmas in syrups, honey, glycerin, or olive oil and adding animal tissue such as dried meat fibers [100].
Researchers have made significant efforts to combat these fraudulent practices. Numerous techniques have been developed to detect adulterants, as outlined in Table 5. These techniques encompass various approaches, including physical, chromatographic, molecular, DNA-based, sensor-based, and spectroscopic strategies [101]. Physical procedures are the oldest, most fundamental, and most straightforward ways to obtain physicochemical information regarding the purity of saffron, including its ash content, morphological properties (size, shape, appearance, etc.), color, and flavor. One of the physical ways for saffron adulteration detection is using microscopic and macroscopic equipment to identify impurities in saffron. The main advantages of physical procedures are their simplicity, ease of use, and lack of sample preparation requirements. However, these methods have certain limitations, including the inability to quantify adulterants and a lack of sensitivity, precision, specificity, and reproducibility. Chromatographic techniques are widely employed for saffron authentication and are considered among the most commonly used methods. Several chromatographic tools are used, including HPLC (High-Performance Liquid Chromatography), GC (Gas Chromatography), and TLC (Thin Layer Chromatography). In addition to the standalone chromatographic techniques mentioned earlier, various combinations of these techniques with other techniques and detectors are utilized in saffron authentication. These include HPLC-DAD, LC-MS/MS, HPLC/PDA/MS, UHPLC-MS/MS, GC/MS/MS, and HS-GC-FID [62–67]. These combinations offer several advantages in detecting a wide range of adulterants, including biological, synthetic, and chemical adulterants, both volatile and non-volatile compounds.
Table 5.
Techniques used to detect adulteration in saffron
| Methods category | Technique used | Description | Targeted compounds or adulterants | References |
|---|---|---|---|---|
| Physical methods | Coloration Ashing Microscopic analysis |
The best-known method is to immerse the saffron in a water solution and see the color released; the pure saffron releases yellow-orange color slowly, giving the aroma of saffron. Diphenylamine (DPA) is used to check the purity of saffron; after adding saffron, the solution becomes blue and then dark reddish brown. Ash content is used to check inorganic contaminations; ash should be inferior to eight percent. Cell types and tissue structure could also be used to check the purity of saffron using microscopic analysis. |
Biological adulterants Inorganic salts Chemical adulterants |
[60,61] |
| Chromatographic and electrophoresis techniques | Thin Layer Chromatography (TLC) High-performance liquid chromatography (HPLC) Gas chromatography Electrophoresis |
TLC technique detects different adulterants in saffron, especially artificial colorants. Layer chromatography combined with other techniques such as image analysis (TLC-IA), Raman spectroscopy, and chemometrics, high-performance thin-layer chromatography (HPTLC) coupled with multivariate image analysis (MIA) were proposed as reliable and fast techniques to check the authenticity of saffron. HPLC-DAD UHPLC-DAD-MS method for detecting adulteration of saffron using gardenia. HPLC in conjunction with PDA-ESI-MS (Photodiode-Array-Electrospray Ionization- Mass Spectrometry) Ultra-High-Performance Liquid Chromatography- High-Resolution Tandem Mass Spectrometry) LC-MS (liquid chromatography-mass spectrometry) Liquid Chromatography-Quadrupole-Time of flight-Mass Spectrometry LC-QTOF-MS Gas chromatography-mass spectrometry GC-MS. Gas chromatography-combustion- isotopic ratio mass spectrometry GC-C-IRMS combined with chemometrics. Headspace Gas-Chromatography with Flame Ionization Detector in conjunction with multivariate data analysis techniques HS-GC-FID. Solid-phase microextraction gas chromatography-mass spectrometric, SPME-GC-MS. 1D-Gel Electrophoresis for proteins determination |
Artificial colorants. Amino-acids. Natural adulterants (safflower, marigold, and turmeric) Metabolic fingerprints Gardenia kaempferol derivatives Safranal and 2-caren-10-al No targeted compounds Volatile profile. Plant adulterants |
|
| Spectroscopic techniques | UV–Vis Spectroscopy Fourier Transform Infrared Spectroscopy (FTIR) Nuclear Magnetic Resonance (NMR) Mass spectrometry (MS) Raman Spectroscopy Near-infrared (NIR) spectroscopy Fluorescence |
UV–Vis spectrometry UV–Vis spectrophotometry combined with linear discriminant analysis (LDA) Transmittance Fourier Transform Infrared (FT-IR) Spectra Attenuated Total Reflectance Fourier Transform Infrared (ATR)-FTIR Diffuse Reflectance Infrared Fourier-transform spectroscopy (DRIFTS) combined with PLSDA and PLS. FT-IR spectroscopy, in combination with other techniques, colorimetry, UV–Vis spectrometry, Reverse Phase-High Performance Liquid Chromatography-Photodiode Array Detector (RP-HPLC-DAD) 1H NMR technique H NMR combined with modelling techniques (OPLS-DA, PLS-DA, and PCA). Matrix-assisted laser desorption/ ionization - mass spectrometry MALDI-MS/MS UHPLC-ESI/QTOF mass spectrometry Inductively coupled plasma mass spectrometry (ICPMS) Isotope-ratio mass spectrometry (IRMS) Direct analysis in real time-high resolution mass spectrometry (DART-HRMS) Proton transfer-mass spectrometry (PTR-MS) Raman spectroscopy combined with chemo-metrics Thin-layer chromatography (TLC) combined with Raman spectroscopy Near-infrared spectroscopy combined with multivariate data analysis Spectrofluorometer |
Synthetic red colorants According to ISO Normative 3632 Various artificial food colorants Crocin content Plant-based adulterants Carminic acid Crocetin and picrocrocin Plant-based adulterants Gardenia, turmeric (Other parts of the flower) Mineral adulterants, Volatile compounds Plant-based adulterants Plant-based adulterants Synthetic dyes |
[73, 74] [75 - 79] [80] [81, 82] [83 - 85] [86, 87] [77] [63] [88] |
| Molecular techniques | Loop-mediated isothermal amplification (LAMP) PCR technique Bar-HRM DNA-based plastid genes matK High-resolution melt analysis Conjoined with universal DNA barcoding regions (Bar-HRM), DNA barcodes Recombinase polymerase amplification in conjunction with lateral flow immunoassay strip (RPA-LFD) |
Plant-based adulterants | [89- 94] | |
| Sensor-based technique | Electronic nose (E-nose), voltammetric electronic tongue (VE-tongue), E-nose based on metal oxide sensors (MOX), E-tongue, | Volatile compounds, safflower, pale yellow style of Saffron, corn fibres dyed with extract of beetroot, dyed corn silk, Tartrazine, and Methyl orange | [95 - 99] | |
| Elemental analysis | Elemental analyzer | C–H–N elemental analyzer | Bio-element stable isotope | [100] |
Nevertheless, they are time-consuming, require sample preparation, have higher costs, and require highly trained personnel. The most popular spectroscopic techniques used in saffron authentication are UV–VIS spectroscopy, Raman spectroscopy, fluorescence spectroscopy, near-infrared spectroscopy (NIR), laser-induced breakdown spectroscopy (LIBS), Fourier-transform infrared spectroscopy (FTIR), mid-infrared spectroscopy (MIR), ion mobility spectrometry (IMS), nuclear magnetic resonance spectroscopy (NMR). Molecular and DNA-based approaches have recently gained popularity in saffron adulteration detection due to their relatively low cost. These approaches include techniques such as polymerase chain reaction (PCR), arrays (DNA array and protein array), hybridization, and sequencing techniques [91-98]. Sensor-based techniques and elemental analysis are also employed in saffron adulteration detection [95–100].
In addition to the techniques already in use, several emerging procedures have significant potential in detecting food adulteration and could be used in the future to authenticate and detect saffron adulteration. Among others, hyperspectral imaging (HSI), electron spin resonance (ESR) spectroscopy, and terahertz spectroscopy are worth mentioning [100-102]. Alighaleh et al. proposed a new approach based on deep neural networks and RGB photos taken under uncontrolled/unstructured conditions to detect genuine saffron [103]. In another work, a simple, non-chemical, non-destructive, fast, and accessible method using visible-near infrared hyperspectral imaging (Vis-NIR-HSI) combined with a new chemometric strategy based on mean-filed independent component analysis (MF-ICA) and multivariate classification techniques is proposed to detect fake saffron [104].
Saffron uses
Therapeutic uses
Due to its specific composition, several studies of saffron have reported a broad spectrum of biological activities. These include: antioxidant, anti-tumor, antianxiety, antiviral, anti-inflammatory, antigenotoxic, anticancer, anti-atherosclerotic, anti-diabetic, hypotensive, hypoglycemic, antihyperlipidemic, antidegenerative, anti-tumor, insulin resistant reducers, anti-convulsant activity, anti-nociceptive, anti-Alzheimer, aphrodisiac, stimulant, anti-poison, livotonic, lactogogue, anticancer, nervine tonic, cardiac tonic, carminative, immune stimulator, diaphoretic, diuretic, sedative, emmenagogue, relaxant, febrifuge, anti-stress, antiulcer, antimutagenic, antigenotoxic, memory, and learning enhancer, anticonvulsant, antidepressant, blood pressure regulator, oxygen boosting of tissues, and bronchodilator [105–116]. These properties are basically due to apocarotenoids, which are bioactive chemicals and include crocin, crocetin, and safranal. Some studies have indicated that saffron can prevent Alzheimer's disease [39], heart disease [117], mild-to-moderate depression [118, 119], cardiovascular diseases [120], gastrointestinal disorders, severe headaches [121], neurodegenerative and psychiatric disorders [122,123], premenstrual syndrome, and anxiety [116]. It is also considered effective in improving memory and learning skills [25, 124].
Saffron is used in folk medicine and the Ayurvedic health system as a sedative, expectorant, anti-asthma, emmenagogue, and adaptogenic agent [125]. Additional virtues ascribed to saffron are around the gastro-intestinal and genital system, in particular, the ability to stimulate the stomach, decrease appetite, cure hemorrhoids, prolapse of the anus, restrain intestinal fermentations, and help in the treatment of amenorrhea [126]. Saffron has been recommended for use in the reduction of pain and inflammation. It has also been proposed as an analgesic agent [127]. Moreover, saffron extracts have been used to treat fever, wounds, back pain, abscesses, gingivitis, and pain related to the eruption of first teeth in children [121]. It is often used to reduce blood cholesterol levels and thus the severity of atherosclerosis, resulting in a reduced risk of myocardial infarction [128]. A study by Mashmoul et al. proved the effectiveness of a cream using an extract of saffron in treating burns induced by heat [129]. Finally, Khazdair et al. [130] showed that saffron effectively improves sleep quality.
Cosmetology and perfumery uses
In addition to therapeutic uses, saffron is used for cosmetics and perfumery [2]. It is reported to have an anti-sun effect, so it can be used as a natural UV absorber to protect the skin from harmful rays [7, 131]. As mentioned in therapeutic uses, saffron has anti-carcinogenic properties and can prevent skin cancers and depigmentation. Moreover, saffron has been suggested to exhibit an anti-rhythmic effect on human skin by reducing melanin. Saffron has long been used in cosmetics as a natural pigment, very effective in lightening the skin [132–134]. Clinical trials on the anti-pruritic effects of saffron and its considerable overall positive effect on the skin were observed [135]. Mzabri et al. [7] discussed saffron’s anti-aging effects, its use in treating blemishes such as acne, and its aptitude in exfoliating and improving blood circulation for facial skin.
Culinary uses
Culinary purposes are one of the main uses of saffron. Worldwide, most of the saffron is used in cooking for flavoring and as a dye. Its unique flavor is described by chefs and saffron specialists [2]. Its three main compounds, crocin, picrocrocin, and safranal, determine the intensity of the color, the power of the flavor, and the strength of the aroma, respectively. Saffron is used to color a large variety of dishes and drinks in many countries. In India, it is used mainly in some dishes such as “Kheer,” “Biryani,” “Kashmiri pulao,” and "Kehwa." Italy uses it in “Risotto alla Milanese,” France in “Bouillabaisse,” and Spain in “Paella Valenciana” [22]. It is also a key ingredient in a variety of pastries and bread, many of which celebrate a secular or religious holiday, such as the Gugelhupt cake in Europe, St. Lucia buns in Sweden, buns and bread in Cornwall (England), Christmas bread in Estonia, sweets on the Greek islands, and rice pudding during Iranian Shi'ite as well as Jewish holidays [21]. In Morocco, saffron is used to aromatize tea instead of mint and also as a spice in the preparation of various traditional dishes such as “koftas” (meatballs and tomatoes) and “mrouzia” (a sweet and salty dish made with mutton and dill). Saffron is also a central ingredient in the herbal mixture known as chermoula, which flavors many Moroccan dishes [136].
Other uses
In addition to the above-mentioned applications, several studies have reported many other potential uses of saffron. Khoulati et al. reported that saffron extract could be a bio-stimulant source for some plants, such as tomatoes [137]. Saffron is also used as a dye in the textile industry, though this use has practically disappeared with the development of artificial coloring [138].
DISCUSSION
In recent years, there has been a significant increase in scientific interest surrounding the saffron plant. This is evident from the growing number of research papers published on saffron-related topics over the past two decades. Analysis of the data from the WoS database reveals a substantial rise in the total number of saffron publications, exceeding 2000 papers. This may be attributed to its significance and potential applications in many fields. Regarding authors' contribution and collaboration network of countries, thirteen authors were found to be the most productive, and six authors exceeded the average impact (30.3), with S. Akhondzadeh (47.6 citations), H. Hosseinzadeh (43.3 citations), and P.A. Tarantilis (43.2 citations) S.Z Bathaie. (34.2 citations), L. Gomez-Gomez (32.4 citations), and M. Carmona (31.6 citations). When comparing these authors using the h-index, three reached a higher level than the average (21) for the same period. As illustrated in Table 1, these authors are H. Hosseinzadeh (43), G.L. Alonso (29), and M. Carmona (25). Iran, Spain, and Italy were the high-performing nations, while Meshhad Med Science and Castilla La Mancha were the top contributor universities. Despite the good production achieved so far, it would be advantageous if these researchers collaborate and connect with international researchers in the future. This would enhance their production and citation.
The saffron industry has witnessed an expansion in the number of producing countries in recent years. This can be attributed to the relatively low input requirements of the saffron plant, making it feasible for cultivation in various regions. Approximately 408 tons of saffron stigma are produced annually worldwide, with Iran holding the leading position as the largest producer. During the stigma processing, a significant amount of saffron by-products are generated. In addition to the stigma, these by-products have also attracted the attention of researchers, and many studies have been carried out on them, showing an immense potential to be used for phytopharmaceutical and nutraceutical purposes [28, 29].
The composition of C. sativus stigma is well documented [9, 32]. Crocin, safranal, and picrocrocin are the three principal compounds present in saffron stigmas. The secondary metabolites composition of C. Sativus has also been explored, and more than 150 compounds with important biological activities have been isolated. It should be mentioned that the chemical composition of saffron is influenced by many factors, such as soil composition, climatic conditions, location, agronomic practices, harvest time, method processing, and conservation [37,38].
Certainly, the quality of saffron is influenced by its composition, especially water content and particular compounds like safranal, crocin, and picrocrocin. Because of its high price, saffron is known to be one of the most targeted products for different types of adulterations. Tricksters add foreign substances (biological, artificial, or synthetic adulterants) that have a similar aspect to C. sativus in order to maximize profit and mislead consumers. To suppress this phenomenon, an international standard, ISO 3632 (ISO 3632-1; ISO 3636-2), has been developed to evaluate the quality of marketed saffron using spectrophotometric and chromatographic measurements. In addition to the international standard ISO, and because of the emergence of pollutants that are difficult to detect and the development of falsification techniques, researchers have proposed several other methods involving chromatographic, molecular, DNA-based, sensor-based, and spectroscopic techniques. More recently, some emerging approaches involving new techniques include hyperspectral imaging (HSI), electron spin resonance (ESR) spectroscopy, terahertz spectroscopy, deep neural network, and RGB photos [91-98, 95-102]. More deep research is needed to explore the effectiveness of such techniques.
The potential uses of saffron have attracted significant scientific research interest. Studies have explored a wide range of applications for saffron, highlighting its diverse biological potential. With a rich history spanning over 3000 years in traditional medicine, saffron has been revered as a panacea in numerous cultures, offering treatment for an extensive range of approximately 90 illnesses [32,108,109]. This demonstrates the excellent pharmaceutical nature of C. sativus. Numerous studies have highlighted its potential efficacy in addressing different types of cancers [108, 110], including breast and lung cancer [111], cervical cancer [112], and colorectal cancer [113].
Additionally, saffron has been traditionally used to alleviate symptoms of asthma, cough, flatulence, stomachic disorders, colic, insomnia, chronic uterine hemorrhage, amenorrhea, smallpox, bronchospasm, sexual dysfunction, and infertility [7, 32, 114–116]. Beyond its medicinal use, saffron is used in several cosmetic and perfumery products to care for the skin [7, 131–134]. Culinary uses are one of the most important applications of saffron stigma, used in cooking for flavoring and as a dye in many countries. Based on the available information, the therapeutic uses of saffron are still being explored, and further research is needed to determine its specific applications and effectiveness in treating various conditions. There is a growing trend in the cosmetology and perfumery industries to incorporate natural and healthy elements into their products.
CONCLUSION
Saffron is the most expensive spice in the world, rich in bioactive compounds, and useful in many fields. Although many studies have been conducted on this plant, some gaps exist. There is an ongoing need to develop collaborative networks in research between countries with lower rankings and those with higher rankings to explore better and utilize the potential of saffron. Expanding the cultivation of saffron to more nations is a viable option since the plant is adaptable to a wide range of pedoclimatic conditions. This expansion can help meet the growing market demand for C. sativus.
Furthermore, research on the mechanization of saffron production and processing is essential. Implementing mechanized techniques would improve efficiency, increase availability, and reduce costs associated with saffron production. Introducing other types of drying, such as freeze-drying, would also help obtain a consistently high-quality product. Studies exploring additional saffron by-products could valorize them while increasing the amount farmers earn. Developing more practical, inexpensive, and responsive approaches to detect adulterants that can be routinely used on an industrial scale would greatly enhance consumer confidence, augment interest, and increase the marketability of premium saffron.
Acknowledgments
Conflict of interest
The authors declare no conflict of interest.
Funding
This work was performed in the frame of two projects: Project 1: Projets de Recherche Citoyenne, financially supported by Cowater, ANDZOA, and REFARM. Project 2: Valorization of Medicinal and Aromatic Plants (3rd edition, VPMA3 2021/09), funded by the National Agency of Medicinal and Aromatic Plants (Taounate, Morocco) and National Center for Scientific and Technical Research (CNRST, Morocco).
Authorship
MI, HAB, LB, SO, EHS, SJ, and SG contributed to the manuscript concept, acquisition, data interpretation, draft of the article, and final approval. MI, RA, AH, KWG, LCM, LHE, AB, and SG contributed to data interpretation, critical revision, and final approval of the manuscript and equally contributed to the manuscript.
References
- 1.Kumar R, Singh V, Devi K, Sharma M, et al. State of Art of Saffron (Crocus sativus L.) Agronomy: A Comprehensive Review. Food Rev Int. 2008;25(1-2):44–85. doi: 10.1080/87559120802458503. [DOI] [Google Scholar]
- 2.Kothari D, Thakur R, Kumar R. Saffron (Crocus Sativus L.): Gold of the Spices—a Comprehensive Review. Hortic Environ Biotechnol. 2021;62(5):661–677. doi: 10.1007/s13580-021-00349-8. [DOI] [Google Scholar]
- 3.Shahnoushi N, Abolhassani L, Kavakebi V, Reed M, Saghaian S. Economic Analysis of Saffron Production. In: Shrivastava S, Chatterjee S, editors. Saffron. Elsevier; 2020. pp. 337–356. [DOI] [Google Scholar]
- 4.Shahandeh H. Soil Conditions for Sustainable Saffron Production. In: Shrivastava S, Chatterjee S, editors. Saffron. Elsevier; 2020. pp. 59–66. [DOI] [Google Scholar]
- 5.Broadhead GK, Chang A, Grigg J, McCluskey P. Efficacy and Safety of Saffron Supplementation: Current Clinical Findings. Crit Rev Food Sci Nutr. 2016;56(16):2767–2776. doi: 10.1080/10408398.2013.879467. [DOI] [PubMed] [Google Scholar]
- 6.Christodoulou E, Kadoglou NP, Kostomitsopoulos N, Valsami G. Saffron: A Natural Product with Potential Pharmaceutical Applications. J Pharm Pharmacol. 2015;67(12):1634–1649. doi: 10.1111/jphp.12456. [DOI] [PubMed] [Google Scholar]
- 7.Mzabri I, Addi M, Berrichi A. Traditional and Modern Uses of Saffron (Crocus Sativus) Cosmetics. 2019;6(4):63. doi: 10.3390/cosmetics6040063. [DOI] [Google Scholar]
- 8.Kafi M, Kamili AN, Husaini AM, Ozturk M, Altay V. An Expensive Spice Saffron (Crocus Sativus L.): A Case Study from Kashmir, Iran, and Turkey. In: Reddy DS, editor. Global Perspectives on Underutilized Crops. Springer International Publishing; 2018. pp. 109–149. [DOI] [Google Scholar]
- 9.Xing B, Li S, Yang J, Lin D, et al. Phytochemistry, Pharmacology, and Potential Clinical Applications of Saffron: A Review. J Ethnopharmacol. 2021;281:114555. doi: 10.1016/j.jep.2021.114555. [DOI] [PubMed] [Google Scholar]
- 10.Mykhailenko O, Kovalyov V, Goryacha O, Ivanauskas L, Georgiyants V. Biologically Active Compounds and Pharmacological Activities of Species of the Genus Crocus: A Review. Phytochemistry. 2019;16:56–89. doi: 10.1016/j.phytol.2019.100080. [DOI] [PubMed] [Google Scholar]
- 11.Bellachioma L, Marini E, Magi G, Pugnaloni A, et al. Phytochemical Profiling, Antibacterial and Antioxidant Properties of Crocus Sativus Flower: A Comparison between Tepals and Stigmas. Open Chemistry. 2022;20:431–443. doi: 10.1515/chem-2022-0155. [DOI] [Google Scholar]
- 12.Ordoudi SA, Tsimidou MZ. Consideration of Fluorescence Properties for the Direct Determination of Erythrosine in Saffron in the Presence of Other Synthetic Dyes. Food Additives & Contaminants: Part A. 2011;28:417–422. doi: 10.1080/19440049.2010.551423. [DOI] [PubMed] [Google Scholar]
- 13.Chen N, Wang W, Xiang J, Li T, et al. The Anti-hyperuricemic Effect of Flavonoid Extract of Saffron By-product and Its Pharmacokinetics in Rats after Oral Administration. Journal of Separation Science. 2022;45(4):856–873. doi: 10.1002/jssc.202100776. [DOI] [PubMed] [Google Scholar]
- 14.Soureshjani HE, Heidari M. In Vitro Variation in Antibacterial Activity Plant Extracts on Glau-cium Elegans and Saffron (Crocus Sativus) Bangladesh Journal of Pharmacology. 2014;9:275–278. doi: 10.3329/bjp.v9i3.18875. [DOI] [Google Scholar]
- 15.Kuchta K, Jin H, Wang R, He H, et al. Quality Control of Saffron (Crocus Sativus L.) via a Novel HPLC Method. Planta Med. 2016;81(S1):S1–S381. doi: 10.1055/s-0036-1597038. [DOI] [Google Scholar]
- 16.Zhou T, Qiu X, Zhao L, Yang W, et al. Optimal Light Intensity and Quality Increased the Saffron Daughter Corm Yield by Inhibiting the Degradation of Reserves in Mother Corms during the Reproductive Stage. Industrial Crops and Products. 2022;176:114396. doi: 10.1016/j.indcrop.2021.114396. [DOI] [Google Scholar]
- 17.Obileke K, Onyeaka H, Omoregbe O, Makaka G, et al. Bioenergy from Bio-Waste: A Bibliometric Analysis of the Trend in Scientific Research from 1998–2018. Biomass Conv. Bioref. 2022;12:1077–1092. doi: 10.1007/s13399-020-00832-9. [DOI] [Google Scholar]
- 18.Salmerón-Manzano E, Garrido-Cardenas JA, Manzano-Agugliaro F. Worldwide Research Trends on Medicinal Plants. International Journal of Environmental Research and Public Health. 2020;17:3376. doi: 10.3390/ijerph17103376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George TT, Obilana AO, Oyenihi AB, Rautenbach FG. Moringa Oleifera through the Years: A Bibliometric Analysis of Scientific Research (2000-2020) South African Journal of Botany. 2021;141:12–24. doi: 10.1016/j.sajb.2021.04.025. [DOI] [Google Scholar]
- 20.Ramezani M, Dourandish A, Jamali Jaghdani T, Aminizadeh M. The Influence of Dense Planting System on the Technical Efficiency of Saffron Production and Land Use Sustainability: Empirical Evidence from Gonabad County, Iran. Agriculture. 2022;12:92. doi: 10.3390/agriculture12010092. [DOI] [Google Scholar]
- 21.Alonso GL, Zalacain A, Carmona M. Saffron. In: Peter KV, editor. Handbook of Herbs and Spices. Elsevier; 2012. pp. 469–498. [DOI] [Google Scholar]
- 22.Monika M, Sadaf I, Aashq H. Production Technology of Saffron for Enhancing Productivity. Pharmacogn Phytochem. (7th ed) 2018;7:p1033–1039. [Google Scholar]
- 23.El Midaoui A, Ghzaiel I, Vervandier-Fasseur D, Ksila M, et al. Saffron (Crocus Sativus L.): A Source of Nutrients for Health and for the Treatment of Neuropsychiatric and Age-Related Diseases. Nutrients. 2022;14:597. doi: 10.3390/nu14030597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen D, Xing B, Yi H, Li Y, et al. Effects of Different Drying Methods on Appearance, Microstructure, Bioactive Compounds and Aroma Compounds of Saffron (Crocus Sativus L.) LWT. 2020;120:108913. doi: 10.1016/j.lwt.2019.108913. [DOI] [Google Scholar]
- 25.Cardone L, Castronuovo D, Perniola M, Cicco N, et al. Saffron (Crocus Sativus L.) the King of Spices: An Overview. Scientia Horticulturae. 2020;272:109560. doi: 10.1016/j.scienta.2020.109560. [DOI] [Google Scholar]
- 26.Abu-Izneid T, Rauf A, Khalil AA, Olatunde A. Nutritional and Health Beneficial Properties of Saffron (Crocus Sativus L): A Comprehensive Review. Critical Reviews in Food Science and Nutrition. 2022;62:2683–2706. doi: 10.1080/10408398.2020.1857682. [DOI] [PubMed] [Google Scholar]
- 27.Jadouali SM, Atifi H, Mamouni R, Majourhat K. Composition of Saffron By-Products (Crocus Sativus) in Relation to Utilization as Animal Feed. Agricultural Science Digest. 2022;42(4):475–481. doi: 10.18805/ag.D-360. [DOI] [Google Scholar]
- 28.Menghini L, Leporini L, Vecchiotti G, Locatelli M, et al. Crocus Sativus L. Stigmas and By-products: Qualitative Fingerprint. Antioxidant Potentials and Enzyme Inhibitory Activities. Food Research International. 2018;109:91–98. doi: 10.1016/j.foodres.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 29.Righi V, Parenti F, Tugnoli V, Schenetti L, Mucci A. Crocus Sativus Petals: Waste or Valuable Resource? The Answer of High-Resolution and High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance. Journal of Agricultural and Food Chemistry. 2015;63:8439–8444. doi: 10.1021/acs.jafc.5b03284. [DOI] [PubMed] [Google Scholar]
- 30.Belyagoubi L, Loukidi B, Belyagoubi BN, Gismondi A, et al. Valorization of Algerian Saffron: Stigmas and Flowers as Source of Bioactive Compounds. Waste and Biomass Valorization. 2021 doi: 10.1007/s12649-021-01454-6. [DOI] [Google Scholar]
- 31.D’Archivio AA, Di Vacri ML, Ferrante M, Maggi MA. Geographical Discrimination of Saffron (Crocus Sativus L.) Using ICP-MS Elemental Data and Class Modeling of PDO Zafferano Dell’Aquila Produced in Abruzzo (Italy) Food Analytical Methods. 2019;12:2572–2581. doi: 10.1007/s12161-019-01610-8. [DOI] [Google Scholar]
- 32.Pandita D. Medicinal and Aromatic Plants. Elsevier; 2021. Saffron (Crocus Sativus L.): Phytochemistry, Therapeutic Significance and Omics-Based Biology; pp. 325–396. [DOI] [Google Scholar]
- 33.Zhang A, Shen Y, Cen M, Hong X, et al. Polysaccharide and Crocin Contents, and Antioxidant Activity of Saffron from Different Origins. Industrial Crops and Products. 2019;133:111–117. doi: 10.1016/j.indcrop.2019.03.009. [DOI] [Google Scholar]
- 34.Perini M, Pianezze S, Ziller L, Ferrante M, et al. Stable Isotope Ratio Analysis Combined with Inductively Coupled Plasma-Mass Spectrometry for Geographical Discrimination between Italian and Foreign Saffron. Journal of Mass Spectrometry. 2020:e4595. doi: 10.1002/jms.4595. [DOI] [PubMed] [Google Scholar]
- 35.Priscila del Campo C, Garde-Cerdán T, Sánchez AM, Maggi L, et al. Determination of Free Amino Acids and Ammonium Ion in Saffron (Crocus Sativus L.) from Different Geographical Origins. Food Chemistry. 2009;114(4):1542–1548. doi: 10.1016/j.foodchem.2008.11.034. [DOI] [Google Scholar]
- 36.Ibourki M, Gharby S, Sakar EH, Hani OE, et al. Elemental Profiling and Geographical Differentiation of Saffron (Crocus Sativus L.) Using Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) and Principal Component Analysis. Chemical Data Collections. 2022;41:100937. doi: 10.1016/j.cdc.2022.100937. [DOI] [Google Scholar]
- 37.Caballero-Ortega H, Pereda-Miranda R, Abdullaev FI. HPLC Quantification of Major Active Components from 11 Different Saffron (Crocus Sativus L.) Sources. Food Chemistry. 2007;100:1126–1131. doi: 10.1016/j.foodchem.2005.11.020. [DOI] [Google Scholar]
- 38.Mohtashami L, Amiri MS, Ramezani M, Emami SA, Simal-Gandara J. The Genus Crocus L.: A Review of Ethnobotanical Uses. Phytochemistry and Pharmacology. Industrial Crops and Products. 2021;171:113923. doi: 10.1016/j.indcrop.2021.113923. [DOI] [Google Scholar]
- 39.Christodoulou E, Kadoglou NP, Kostomitsopoulos N, Valsami G. Saffron: A Natural Product with Potential Pharmaceutical Applications. Journal of Pharmacy and Pharmacology. 2015;67:1634–1649. doi: 10.1111/jphp.12456. [DOI] [PubMed] [Google Scholar]
- 40.Carmona M, Zalacain A, Sánchez AM, Novella JL, Alonso GL. Crocetin esters, picrocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS. J Agric Food Chem. 2006;54(3):973–9. doi: 10.1021/jf052297w. [DOI] [PubMed] [Google Scholar]
- 41.Kabiri M, Rezadoost H, Ghassempour AA. Comparative Quality Study of Saffron Constituents through HPLC and HPTLC Methods Followed by Isolation of Crocins and Picrocrocin. LWT. 2017;84:1–9. doi: 10.1016/j.lwt.2017.05.033. [DOI] [Google Scholar]
- 42.Maggi L, Carmona M, Zalacain A, Kanakis CD, et al. Changes in Saffron Volatile Profile According to Its Storage Time. Food Res Int. 2010;43:1329–1334. doi: 10.1016/j.foodres.2010.03.025. [DOI] [Google Scholar]
- 43.Shahi T, Assadpour E, Jafari SM. Main Chemical Compounds and Pharmacological Activities of Stigmas and Tepals of 'Red Gold'; Saffron. Trends Food Sci Technol. 2016;58:69–78. doi: 10.1016/j.tifs.2016.10.010. [DOI] [Google Scholar]
- 44.Lage M, Cantrell CL. Quantification of Saffron (Crocus Sativus L.) Metabolites Crocins. Picrocrocin and Safranal for Quality Determination of the Spice Grown under Different Environmental Moroccan Conditions. Scientia Horticulturae. 2009;121:366–373. doi: 10.1016/j.scienta.2009.02.017. [DOI] [Google Scholar]
- 45.Alonso GL, Salinas MR, Garijo J, Sánchez-Fernández MA. Composition of Crocins and Picrocrocin from Spanish Saffron (Crocus Sativus L.) J Food Qual. 2001;24:219–233. doi: 10.1111/j.1745-4557.2001.tb00604.x. [DOI] [Google Scholar]
- 46.Pfister S, Meyer P, Steck A, Pfander H. Isolation and Structure Elucidation of Carotenoid-Glycosyl Esters in Gardenia Fruits (Gardenia Jasminoides Ellis) and Saffron (Crocus Sativus Linne) J Agric Food Chem. 1996;44:2612–2615. doi: 10.1021/jf950713e. [DOI] [Google Scholar]
- 47.Pitsikas N, Boultadakis A, Georgiadou G, Tarantilis PA, et al. Effects of the Active Constituents of Crocus Sativus L. Crocins, in an Animal Model of Anxiety. Phytomedicine. 2008;15:1135–1139. doi: 10.1016/j.phymed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Shahi T, Assadpour E, Jafari SM. Main chemical compounds and pharmacological activities of stigmas and tepals of ‘red gold’; saffron. Trends Food Sci Technol. 2016;58:69–78. doi: 10.1016/j.tifs.2016.10.010. [DOI] [Google Scholar]
- 49.Tarantilis PA, Beljebbar A, Manfait M, Polissiou M. FT-IR, FT-Raman Spectroscopic Study of Carotenoids from Saffron (Crocus Sativus L.) and Some Derivatives. Spectrochim Acta A. 1998;54:651–657. doi: 10.1016/s1386-1425(98)00024-9. [DOI] [Google Scholar]
- 50.Tung NH, Shoyama Y. New Minor Glycoside Components from Saffron. J Nat Med. 2013;67:672–676. doi: 10.1007/s11418-012-0721-4. [DOI] [PubMed] [Google Scholar]
- 51.Ahrazem O, Rubio-Moraga A, Nebauer SG, Molina RV, Gómez-Gómez L. Saffron: Its Phytochemistry, Developmental Processes, and Biotechnological Prospects. J Agric Food Chem. 2015;63:8751–8764. doi: 10.1021/acs.jafc.5b03194. [DOI] [PubMed] [Google Scholar]
- 52.Li CY, Lee EJ, Wu TS. Antityrosinase Principles and Constituents of the Petals of Crocus Sativus. J Nat Prod. 2004;67:437–440. doi: 10.1021/np0302854. [DOI] [PubMed] [Google Scholar]
- 53.Li CY, Wu TS. Constituents of the Stigmas of Crocus Sativus and Their Tyrosinase Inhibitory Activity. J Nat Prod. 2002;65:1452–1456. doi: 10.1021/np020188v. [DOI] [PubMed] [Google Scholar]
- 54.Slimestad R, Andersen ØM, Francis GW, Marston A, Hostettmann K. Syringetin 3-O-(6″-Acetyl)-β-Glucopyranoside and Other Flavonols from Needles of Norway Spruce, Picea Abies. Phytochemistry. 1995;40:1537–1542. doi: 10.1016/0031-9422(95)00383-i. [DOI] [Google Scholar]
- 55.Veit M, Beckert C, Höhne C, Bauer K, Geiger H. Interspecific and Intraspecific Variation of Phenolics in the Genus Equisetum Subgenus Equisetum. Phytochemistry. 1995;38:881–891. doi: 10.1016/0031-9422(94)00658-g. [DOI] [Google Scholar]
- 56.Condurso C, Cincotta F, Tripodi G, Verzera A. Bioactive Volatiles in Sicilian (South Italy) Saffron: Safranal and Its Related Compounds. J Essent Oil Res. 2017;29:221–227. doi: 10.1080/10412905.2016.1244115. [DOI] [Google Scholar]
- 57.Straubinger M, Jezussek M, Waibel R, Winterhalter P. Novel Glycosidic Constituents from Saffron. J Agric Food Chem. 1997;45:1678–1681. doi: 10.1021/jf960861k. [DOI] [Google Scholar]
- 58.Zarghami NS, Heinz DE. Monoterpene Aldehydes and Isophorone-Related Compounds of Saffron. Phytochemistry. 1971;10:2755–2761. doi: 10.1016/S0031-9422(00)97275-3. [DOI] [Google Scholar]
- 59.Gao W, Li Y, Zhu D. Phenolic Glucosides and a γ-Lactone Glucoside from the Sprouts of Crocus Sativus. Planta Med. 1999;65:425–427. doi: 10.1055/s-1999-14020. [DOI] [PubMed] [Google Scholar]
- 60.Ordoudi S, Tsimidou M. Detection of Artificial Red Colorants in Saffron Using UV-Vis Spectrometry and Tristimulus Colorimetry. Acta Horticulturae. 2004;650:331–338. doi: 10.17660/ActaHortic.2004.650.41. [DOI] [Google Scholar]
- 61.Sudha Revathy S, Rathinamala R, Murugesan M. Authentication methods for drugs used in Ayurveda, Siddha, and Unani systems of medicine: an overview. IJPSR. 2012;3(8):2352–2361. doi: 10.13040/IJPSR.0975-8232.3(8).2352-2361. [DOI] [Google Scholar]
- 62.Sereshti H, Poursorkh Z, Aliakbarzadeh G, Zarre S. Quality Control of Saffron and Evaluation of Potential Adulteration by Means of Thin Layer Chromatography-Image Analysis and Chemometrics Methods. Food Control. 2018;90:48–57. doi: 10.1016/j.foodcont.2018.02.026. [DOI] [Google Scholar]
- 63.Dai H, Gao Q, He L. Rapid Determination of Saffron Grade and Adulteration by Thin-Layer Chromatography Coupled with Raman Spectroscopy. Food Anal Methods. 2020;13:2128–2137. doi: 10.1007/s12161-020-01828-x. [DOI] [Google Scholar]
- 64.Amirvaresi A, Rashidi M, Kamyar M, Amirahmadi M, et al. Combining Multivariate Image Analysis with High-Performance Thin-Layer Chromatography for Development of a Reliable Tool for Saffron Authentication and Adulteration Detection. J Chromatogr A. 2020;1628:461461. doi: 10.1016/j.chroma.2020.461461. [DOI] [PubMed] [Google Scholar]
- 65.Moras B, Loffredo L, Rey S. Quality Assessment of Saffron (Crocus Sativus L.) Extracts via UHPLC-DAD-MS Analysis and Detection of Adulteration Using Gardenia Fruit Extract (Gardenia Jasminoides Ellis) Food Chem. 2018;257:325–332. doi: 10.1016/j.foodchem.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 66.Rubert J, Lacina O, Zachariasova M, Hajslova J. Saffron Authentication Based on Liquid Chromatography High Resolution Tandem Mass Spectrometry and Multivariate Data Analysis. Food Chem. 2016;204:201–209. doi: 10.1016/j.foodchem.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Guijarro-Díez M, Castro-Puyana M, Crego AL, Marina ML. Detection of Saffron Adulteration with Gardenia Extracts through the Determination of Geniposide by Liquid Chromatography–Mass Spectrometry. J Food Compos Anal. 2017;55:30–37. doi: 10.1016/j.jfca.2016.11.004. [DOI] [Google Scholar]
- 68.Farag MA, Hegazi N, Dokhalahy E, Khattab AR. Chemometrics Based GC-MS Aroma Profiling for Revealing Freshness, Origin and Roasting Indices in Saffron Spice and Its Adulteration. Food Chem. 2020;331:127358. doi: 10.1016/j.foodchem.2020.127358. [DOI] [PubMed] [Google Scholar]
- 69.Ghiasi S, Parastar H. Chemometrics-Assisted Isotope Ratio Fingerprinting Based on Gas Chromatography/Combustion/Isotope Ratio Mass Spectrometry for Saffron Authentication. J Chromatogr A. 2021;1657:462587. doi: 10.1016/j.chroma.2021.462587. [DOI] [PubMed] [Google Scholar]
- 70.Morozzi P, Zappi A, Gottardi F, Locatelli M, Melucci DA. Quick and Efficient Non-Targeted Screening Test for Saffron Authentication: Application of Chemometrics to Gas-Chromatographic Data. Molecules. 2019;24(14):2602. doi: 10.3390/molecules24142602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rocchi R, Mascini M, Faberi A, Sergi M, et al. Comparison of IRMS, GC-MS and E-Nose Data for the Discrimination of Saffron Samples with Different Origin, Process, and Age. Food Control. 2019;106:106736. doi: 10.1016/j.foodcont.2019.106736. [DOI] [Google Scholar]
- 72.Paredi G, Raboni S, Marchesani F, Ordoudi SA, et al. Insight of Saffron Proteome by Gel-Electrophoresis. Molecules. 2016;21(2):167. doi: 10.3390/molecules21020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ordoudi SA, Staikidou C, Kyriakoudi A, Tsimidou MZ. A Stepwise Approach for the Detection of Carminic Acid in Saffron with Regard to Religious Food Certification. Food Chem. 2018;267:410–419. doi: 10.1016/j.foodchem.2017.04.096. [DOI] [PubMed] [Google Scholar]
- 74.D'Archivio AA, Maggi MA. Geographical identification of saffron (Crocus sativus L.) by linear discriminant analysis applied to the UV-visible spectra of aqueous extracts. Food Chem. 2017;219:408–413. doi: 10.1016/j.foodchem.2016.09.169. [DOI] [PubMed] [Google Scholar]
- 75.Karimi S, Feizy J, Mehrjo F, Farrokhnia M. Detection and Quantification of Food Colorant Adulteration in Saffron Sample Using Chemometric Analysis of FT-IR Spectra. RSC Adv. 2016;6:23085–23093. doi: 10.1039/C5RA25983E. [DOI] [Google Scholar]
- 76.Lee FY, Htar TT, Akowuah GA. ATR-FTIR and Spectrometric Methods for the Assay of Crocin in Commercial Saffron Spices (Crocus Sativus L.) Int J Food Prop. 2015;18(8):1773–1783. doi: 10.1080/10942912.2014.923911. [DOI] [Google Scholar]
- 77.Varliklioz S, Eksi-Kocak H, Yetim H, Boyaci IH. Novel Spectroscopic Method for Determination and Quantification of Saffron Adulteration. Food Anal Methods. 2017;10(5):1547–1555. doi: 10.1007/s12161-016-0710-4. [DOI] [Google Scholar]
- 78.Petrakis EA, Polissiou MG. Assessing Saffron (Crocus Sativus L.) Adulteration with Plant-Derived Adulterants by Diffuse Reflectance Infrared Fourier Transform Spectroscopy Coupled with Chemometrics. Talanta. 2017;162:558–566. doi: 10.1016/j.talanta.2016.10.072. [DOI] [PubMed] [Google Scholar]
- 79.Ordoudi SA, Tsimidou MZ. Detection of Artificial Red Colorants in Saffron Using UV-Vis Spectrometry and Tristimulus Colorimetry. Acta Hortic. 2004;650:331–338. doi: 10.17660/ActaHortic.2004.650.41. [DOI] [Google Scholar]
- 80.Yilmaz A, Nyberg NT, Mølgaard P, Asili J, et al. 1H NMR Metabolic Fingerprinting of Saffron Extracts. Metabolomics. 2010;6(4):511–517. doi: 10.1007/s11306-010-0221-z. [DOI] [Google Scholar]
- 81.Dowlatabadi R, Farshidfar F, Zare Z, Pirali M. Detection of Adulteration in Iranian Saffron Samples by 1H NMR Spectroscopy and Multivariate Data Analysis Techniques. Metabolomics. 2017;13(1):19. doi: 10.1007/s11306-016-1155-x. [DOI] [Google Scholar]
- 82.Petrakis EA, Cagliani LR, Polissiou MG, Consonni R. Evaluation of Saffron (Crocus sativus L.) Adulteration with Plant Adulterants by 1H NMR Metabolite Fingerprinting. Food Chemistry. 2015;173:890–896. doi: 10.1016/j.foodchem.2014.10.107. [DOI] [PubMed] [Google Scholar]
- 83.Aiello D, Siciliano C, Mazzotti F, Di Donna L. A Rapid MALDI MS/MS Based Method for Assessing Saffron (Crocus sativus L.) Adulteration. Food Chemistry. 2020;307:125527. doi: 10.1016/j.foodchem.2019.125527. [DOI] [PubMed] [Google Scholar]
- 84.Senizza B, Rocchetti G, Ghisoni S, Busconi M. Identification of Phenolic Markers for Saffron Authenticity and Origin: An Untargeted Metabolomics Approach. Food Research International. 2019;126:108584. doi: 10.1016/j.foodres.2019.108584. [DOI] [PubMed] [Google Scholar]
- 85.Wakefield J, McComb K, Ehtesham E, Van Hale R. Chemical Profiling of Saffron for Authentication of Origin. Food Control. 2019;106:106699. doi: 10.1016/j.foodcont.2019.06.025. [DOI] [Google Scholar]
- 86.Nenadis N, Heenan S, Tsimidou MZ, Van Ruth S. Applicability of PTR-MS in the Quality Control of Saffron. Food Chemistry. 2016;196:961–967. doi: 10.1016/j.foodchem.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 87.Silvis ICJ, Luning PA, Klose N, Jansen M, van Ruth SM. Similarities and differences of the volatile profiles of six spices explored by Proton Transfer Reaction Mass Spectrometry. Food Chem. 2019 Jan 15;271:318–327. doi: 10.1016/j.foodchem.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 88.Shawky E, Abu El-Khair RM, Selim DA. NIR Spectroscopy-Multivariate Analysis for Rapid Authentication, Detection and Quantification of Common Plant Adulterants in Saffron (Crocus sativus L.) Stigmas. LWT. 2020;122:109032. doi: 10.1016/j.lwt.2020.109032. [DOI] [Google Scholar]
- 89.Zhao M, Shi Y, Wu L, Guo L, et al. Rapid Authentication of the Precious Herb Saffron by Loop-Mediated Isothermal Amplification (LAMP) Based on Internal Transcribed Spacer 2 (ITS2) Sequence. Scientific Reports. 2016;6:25370. doi: 10.1038/srep25370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Villa C, Costa J, Oliveira MBPP, Mafra I. Novel Quantitative Real-Time PCR Approach to Determine Safflower (Carthamus tinctorius) Adulteration in Saffron (Crocus sativus) Food Chemistry. 2017;229:680–687. doi: 10.1016/j.foodchem.2017.02.136. [DOI] [PubMed] [Google Scholar]
- 91.Bosmali I, Ordoudi SA, Tsimidou MZ, Madesis P. Greek PDO Saffron Authentication Studies Using Species-Specific Molecular Markers. Food Research International. 2017;100:899–907. doi: 10.1016/j.foodres.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 92.Soffritti G, Busconi M, Sánchez R, Thiercelin JM, et al. Genetic and Epigenetic Approaches for the Possible Detection of Adulteration and Auto-Adulteration in Saffron (Crocus sativus L.) Spice. Molecules. 2016;21(3):343. doi: 10.3390/molecules21030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bansal S, Thakur S, Mangal M, Mangal AK, Gupta RK. Identification of suitable locus for specific detection of biological adulterants of saffron. Food Anal Methods. 2019;12:2509–17. doi: 10.1007/s12161-019-01604-6. [DOI] [Google Scholar]
- 94.Zhao M, Wang B, Xiang L, Xiong C, et al. A Novel Onsite and Visual Molecular Technique to Authenticate Saffron (Crocus sativus) and Its Adulterants Based on Recombinase Polymerase Amplification. Food Control. 2019;100:117–121. doi: 10.1016/j.foodcont.2019.01.011. [DOI] [Google Scholar]
- 95.Tahri K, Tiebe C, Bougrini M, Saidi T, et al. Characterization and Discrimination of Saffron by Multisensory Systems, SPME-GC-MS and UV-Vis Spectrophotometry. Analytical Methods. 2015;7(8):10328–10338. doi: 10.1039/C5AY01986A. [DOI] [Google Scholar]
- 96.Heidarbeigi K, Mohtasebi SS, Foroughirad A, Ghasemi VM, et al. Detection of Adulteration in Saffron Samples Using Electronic Nose. International Journal of Food Properties. 2015;18(7):1391–1401. doi: 10.1080/10942912.2014.915850. [DOI] [Google Scholar]
- 97.Heidarbeigi K, Mohtasebi SS, Serrano-Diaz J, Medina-Plaza C, et al. Flavour Characteristics of Spanish and Iranian Saffron Analysed by Electronic Tongue. Quality Assurance and Safety of Crops & Foods. 2016;8(3):359–368. doi: 10.1080/10942912.2014.915850. [DOI] [Google Scholar]
- 98.Kiani S, Minaei S, Ghasemi-Varnamkhasti M. A Portable Electronic Nose as an Expert System for Aroma-Based Classification of Saffron. Chemometrics and Intelligent Laboratory Systems. 2016;156:148–156. doi: 10.1016/j.chemolab.2016.05.013. [DOI] [Google Scholar]
- 99.Koocheki A, Milan E. Food Science. Technology and Nutrition: Woodhead Publishing; 2020. Saffron Adulteration; pp. 321–334. [DOI] [Google Scholar]
- 100.Maggi L, Carmona M, Kelly SD, Marigheto N, Alonso GL. Geographical Origin Differentiation of Saffron Spice (Crocus sativus L. Stigmas)–Preliminary Investigation Using Chemical and Multi-Element (H. C, N) Stable Isotope Analysis. Food Chemistry. 2011;128(2):543–548. doi: 10.1016/j.foodchem.2011.03.063. [DOI] [PubMed] [Google Scholar]
- 101.Kumari L, Jaiswal P, Tripathy SS. Various Techniques Useful for Determination of Adulterants in Valuable Saffron: A Review. Trends in Food Science & Technology. 2021;111:301–321. doi: 10.1016/j.tifs.2021.02.061. [DOI] [Google Scholar]
- 102.Lu X, Xia Z, Qu F, Zhu Z, Li S. Identification of Authenticity, Quality, and Origin of Saffron Using Hyperspectral Imaging and Multivariate Spectral Analysis. Spectroscopy Letters. 2020;53(1):76–85. doi: 10.1080/00387010.2019.1693403. [DOI] [Google Scholar]
- 103.Alighaleh P, Khosravi H, Rohani A, Saeidirad MH, Einafshar S. The detection of saffron adulterants using a deep neural network approach based on RGB images taken under uncontrolled conditions. Expert Systems with Applications. 2022;198:116890. doi: 10.1016/j.eswa.2022.116890. [DOI] [Google Scholar]
- 104.Hashemi-Nasab FS, Parastar H. Vis-NIR hyperspectral imaging coupled with independent component analysis for saffron authentication. Food Chemistry. 2022;393:133450. doi: 10.1016/j.foodchem.2022.133450. [DOI] [PubMed] [Google Scholar]
- 105.Hosseini A, Razavi BM, Hosseinzadeh H. Saffron (Crocus sativus) petal as a new pharmacological target: A review. Iranian Journal of Basic Medical Sciences. 2018;21(11):1091–1099. doi: 10.22038/IJBMS.2018.31243.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.José Bagur M, Alonso Salinas G, Jiménez-Monreal A, Chaouqi S. Saffron: An old medicinal plant and a potential novel functional food. Molecules. 2019;23(1):30. doi: 10.3390/molecules23010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nanda S, Madan K. The role of safranal and saffron stigma extracts in oxidative stress, diseases, and photoaging: A systematic review. Heliyon. 2021;7(10):e06117. doi: 10.1016/j.heliyon.2021.e06117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.) Exp Biol Med (Maywood) 2002;227(1):20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 109.Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76(7-8):722–724. doi: 10.1016/j.fitote.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 110.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detection and Prevention. 2004;28(6):426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 111.Samarghandian S, Borji A, Farahmand SK, Afshari R, Davoodi S. Crocus sativus L. (Saffron) stigma aqueous extract induces apoptosis in alveolar human lung cancer cells through caspase-dependent pathways activation. BioMed Research International. 2013;2013:417928. doi: 10.1155/2013/417928. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Abdullaev FI, Frenkel GD. Effect of saffron on cell colony formation and cellular nucleic acid and protein synthesis. Biofactors. 1992;3(3):201–204. doi: 10.1002/biof.5520030305. [DOI] [PubMed] [Google Scholar]
- 113.Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT, Shoyama CY, Yuan CS. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Experimental Oncology. 2007;29(3):175–180. [PMC free article] [PubMed] [Google Scholar]
- 114.Giaccio M. Crocetin from saffron: An active component of an ancient spice. Critical Reviews in Food Science and Nutrition. 2004;44(3):155–172. doi: 10.1080/10408690490441433. [DOI] [PubMed] [Google Scholar]
- 115.Makri OE, Ferlemi AV, Lamari FN, Georgakopoulos CD. Saffron administration prevents selenite-induced cataractogenesis. Molecular Vision. 2013;19:1188–1197. [PMC free article] [PubMed] [Google Scholar]
- 116.Melnyk JP, Wang S, Marcone MF. Chemical and biological properties of the world's most expensive spice: Saffron. Food Research International. 2010;43(8):1981–1989. doi: 10.1016/j.foodres.2010.07.033. [DOI] [Google Scholar]
- 117.Del-Angel DS, Martínez NLH, Cruz MEG, Urrutia EC, et al. Saffron extract ameliorates oxidative damage and mitochondrial dysfunction in the rat brain. Acta Horticulturae. 2007;(739):359–366. doi: 10.17660/ActaHortic.2007.739.47. [DOI] [Google Scholar]
- 118.Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, Amini H, et al. Crocus sativus L. in the treatment of mild to moderate depression: A double-blind, randomized and placebo-controlled trial. Phytotherapy Research. 2005;19(2):148–151. doi: 10.1002/ptr.1647. [DOI] [PubMed] [Google Scholar]
- 119.Siddiqui M, Saleh MM, Basharuddin SBB, Zamri SB. Saffron (Crocus sativus L.): As an antidepressant. Journal of Pharmacy and Bioallied Sciences. 2018;10(3):173. doi: 10.4103/0975-7406.243427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hosseinzadeh H, Modaghegh MH, Saffari Z. Crocus sativus L. (saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evidence-Based Complementary and Alternative Medicine. 2009;6(3):343–350. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mousavi SZ, Bathaie SZ. Historical uses of saffron: Identifying potential new avenues for modern research. Avicenna Journal of Phytomedicine. 2011;1(2):57–66. [Google Scholar]
- 122.Farkhondeh T, Samarghandian S, Shaterzadeh Yazdi H, Samini F. The protective effects of crocin in the management of neurodegenerative diseases: A review. American Journal of Neurodegenerative Disease. 2018;7(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 123.Pitsikas N. The effect of Crocus sativus L. and its constituents on memory: Basic studies and clinical applications. Evidence-Based Complementary and Alternative Medicine. 2015;2015:926284. doi: 10.1155/2015/926284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Akhondzadeh S, Shafiee Sabet M, Harirchian MH, Togha M. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer's disease. Psychopharmacology. 2010;207(4):637–643. doi: 10.1007/s00213-009-1706-1. [DOI] [PubMed] [Google Scholar]
- 125.Gohari A, Saeidnia S, Mahmoodabadi M. An overview on saffron, phytochemicals, and medicinal properties. Pharmacognosy Reviews. 2013;7(13):61–66. doi: 10.4103/0973-7847.112850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hosseinzadeh H, Nassiri-Asl M. Avicenna's (Ibn Sina) the Canon of Medicine and saffron (Crocus sativus): A review. Phytotherapy Research. 2013;27(4):475–483. doi: 10.1002/ptr.4784. [DOI] [PubMed] [Google Scholar]
- 127.Tamaddonfard E, Erfanparast A, Farshid AA, Imani M, et al. Safranal, a constituent of saffron, exerts gastro-protective effects against indomethacin-induced gastric ulcer. Life Sciences. 2019;224:88–94. doi: 10.1016/j.lfs.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 128.Zheng S, Qian Z, Tang F, Sheng L. Suppression of vascular cell adhesion molecule-1 expression by crocetin contributes to attenuation of atherosclerosis in hypercholesterolemic rabbits. Biochemical Pharmacology. 2005;70(8):1192–1199. doi: 10.1016/j.bcp.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 129.Mashmoul M, Azlan A, Yusof BNM, Khaza'ai H, et al. Effects of saffron extract and crocin on anthropometrical, nutritional, and lipid profile parameters of rats fed a high-fat diet. Journal of Functional Foods. 2014;8:180–187. doi: 10.1016/j.jff.2014.03.017. [DOI] [Google Scholar]
- 130.Khazdair MR, Anaeigoudari A, Hashemzehi M, Mohebbati R. Neuroprotective potency of some spice herbs: A literature review. Journal of Traditional and Complementary Medicine. 2019;9(2):98–105. doi: 10.1016/j.jtcme.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Golmohammadzadeh S, Jaafari MR, Hosseinzadeh H. Does saffron have antisolar and moisturizing effects? Iranian Journal of Pharmaceutical Research. 2010;9(2):133–140. [PMC free article] [PubMed] [Google Scholar]
- 132.Carmona M, Sánchez AM, Ferreres F, Zalacain A. Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: Comparative study of samples from different geographical origins. Food Chemistry. 2007;100(2):445–450. doi: 10.1016/j.foodchem.2005.09.065. [DOI] [Google Scholar]
- 133.Das I, Chakrabarty RN, Das S. Saffron can prevent chemically induced skin carcinogenesis in Swiss albino mice. Asian Pacific Journal of Cancer Prevention. 2004;5(1):70–76. [PubMed] [Google Scholar]
- 134.Das I, Das S, Saha T. Saffron suppresses oxidative stress in DMBA-induced skin carcinoma: A histopathological study. Acta Histochemica. 2010;112(4):317–327. doi: 10.1016/j.acthis.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 135.Gao XH, Zhang L, Wei H, Chen HD. Efficacy and safety of innovative cosmeceuticals. Clinics in Dermatology. 2008;26(4):367–374. doi: 10.1016/j.clindermatol.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 136.Modaghegh MH, Shahabian M, Esmaeili HA, Rajbai O, Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine. 2008;15(12):1032–1037. doi: 10.1016/j.phymed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 137.Khoulati A, Ouahhoud S, Mamri S, Alaoui K, et al. Saffron extract stimulates growth, improves the antioxidant components of Solanum lycopersicum L. and has an antifungal effect. Annals of Agricultural Sciences. 2019;64(2):138–150. doi: 10.1016/j.aoas.2019.10.002. [DOI] [Google Scholar]
- 138.Tsatsaroni E, Liakopoulou-Kyriakides M, Eleftheriadis I. Comparative study of dyeing properties of two yellow natural pigments: Effect of enzymes and proteins. Dyes and Pigments. 1998;37(4):307–315. doi: 10.1016/S0143-7208(97)00069-7. [DOI] [Google Scholar]