Abstract
Introduction:
Well-differentiated thyroid cancer (WDTC) is the most common thyroid malignancy, and the worldwide incidence is increasing. Early stage disease is curable with surgery. We hypothesized that patients who live at greater distances from health care institutions or have complicating socioeconomic barriers may present with more advanced diseases and have worse outcomes.
Methods:
The National Cancer Database (NCDB) was used to identify patients who were diagnosed with WDTC between 2004 and 2018. Race, ethnicity, insurance status, income status, and distance from residence to health care clinic of diagnosis (great circle distance [GCD]) were analyzed with respect to the severity of disease at presentation (stage) and outcomes. Binary logistic regression and Cox regression were used to determine associations between socioeconomic variables and tumor stage or survival.
Results:
The Hispanic (OR: 1.49, CI: 1.45–1.54, P < 0.001) and Asian (OR: 1.49, CI: 1.43–1.55, P < 0.001) populations had higher odds of developing an advanced disease when compared to the White population separately. Patients without insurance displayed higher odds of developing an advanced disease at diagnosis compared to those with insurance (OR: 1.39, CI: 1.31–1.47, P < 0.001). Adjusted-Cox regression analysis of survival revealed that Black patients had detrimental survival outcomes when compared to White patients (HR: 1.24, P < 0.001), and patients with private insurance had improved survival outcomes when compared to those without insurance (HR: 0.58, P < 0.001).
Conclusions:
Hispanic and Asian patients were found to be more likely to present with an advanced disease but also displayed greater overall survival when compared to the White population. The Black population, patients without insurance, and patients with lower income status exhibited worse survival outcomes.
Keywords: Ethnic, Follicular, Papillary, Racial, Socioeconomic, Well-differentiated
Introduction
Survival and disease stage at presentation for patients with thyroid cancer are influenced by a host of factors. Thyroid malignancies frequently have an indolent clinical course with minimal symptoms and may go undetected for extended periods of time.1–3 As these patients frequently have mild symptoms, those with less access to health care facilities may be less likely to seek medical attention, causing delays in diagnosis. These delays can certainly result in progression to advanced stages. Recent studies have drawn particular attention to racial and socioeconomic disparities that may negatively influence clinical outcomes in a variety of diseases.2,4,5 These studies suggest that delayed presentation at healthcare facilities among patients of minority racial groups is one of the primary factors leading to worse outcomes in these groups.4,6,7
Well-differentiated thyroid cancer (WDTC) encompasses papillary thyroid carcinoma and follicular thyroid carcinoma, which account for approximately 95% of thyroid cancer diagnoses in the United States.8,9 As the worldwide incidence of these cancers has risen sharply over the past decade, research efforts have been dedicated to determining predisposing factors for WDTC and providing greater insight into treatment options.8,10–12 While WDTCs tend to have favorable outcomes, there is little data characterizing the populations that are at risk for poor outcomes.13,14
Therefore, we sought to explore the association between socioeconomic factors and outcomes of WDTCs using data from the National Cancer Database (NCDB). We hypothesized that members of minority populations have less access to health care and less socioeconomic support, thereby making them more likely to present with advanced stages of disease and have worse outcomes when compared to their White peers.
Materials and Methods
In order to test these hypotheses, we examined these socioeconomic factors in the context of disease stage at presentation for WDTC’s. Subsequently, we examined survival results realizing that comorbidities are a complicating factor. We controlled for this factor using the Charlson-Deyo index score.15 The NCDB was used to identify patients who were diagnosed with thyroid cancer between 2004 and 2018. At our institution, publicly available datasets do not require institutional review board approval. We utilized the following ICD-0–3 codes classified as WDTC for our analysis: 8050 (papillary carcinoma), 8260 (papillary adenocarcinoma), 8330 (follicular adenoma), 8331 (follicular adenocarcinoma), 8332 (follicular adenocarcinoma, trabecular), 8335 (follicular carcinoma, minimally invasive), 8340 (papillary carcinoma, follicular variant), 8337 (insular carcinoma), 8341 (papillary micro-adenoma), 8342 (papillary carcinoma, oxyphilic), 8343 (papillary carcinoma, encapsulated) and, 8344 (papillary carcinoma, columnar cell).16 All other tumor types were excluded from further analysis. We excluded all patients with missing or blank values for tumor staging at diagnosis or missing vital status at the date of last contact or death. We also excluded patients with missing characteristics of interest such as race, ethnicity, insurance status, or grater circle distance (GCD). We excluded those with greater than 2802 miles GCD (the longest possible distance between two locations in the mainland United States) in order to focus on a patient population exclusive to the continental United States (Fig. 1). It is noteworthy that all patients who were staged by the AJCC 8th edition staging system were excluded as a result of the exclusion factors that were used in the study (Fig. 1). A table of the characteristics of the excluded patients is shown in the supplemental materials. In general, the excluded patients had similar race/ethnicity compared to included patients, but it is not possible to make firm comparisons since the excluded patients were missing relevant information. (See Supplemental Material).
Fig. 1 –
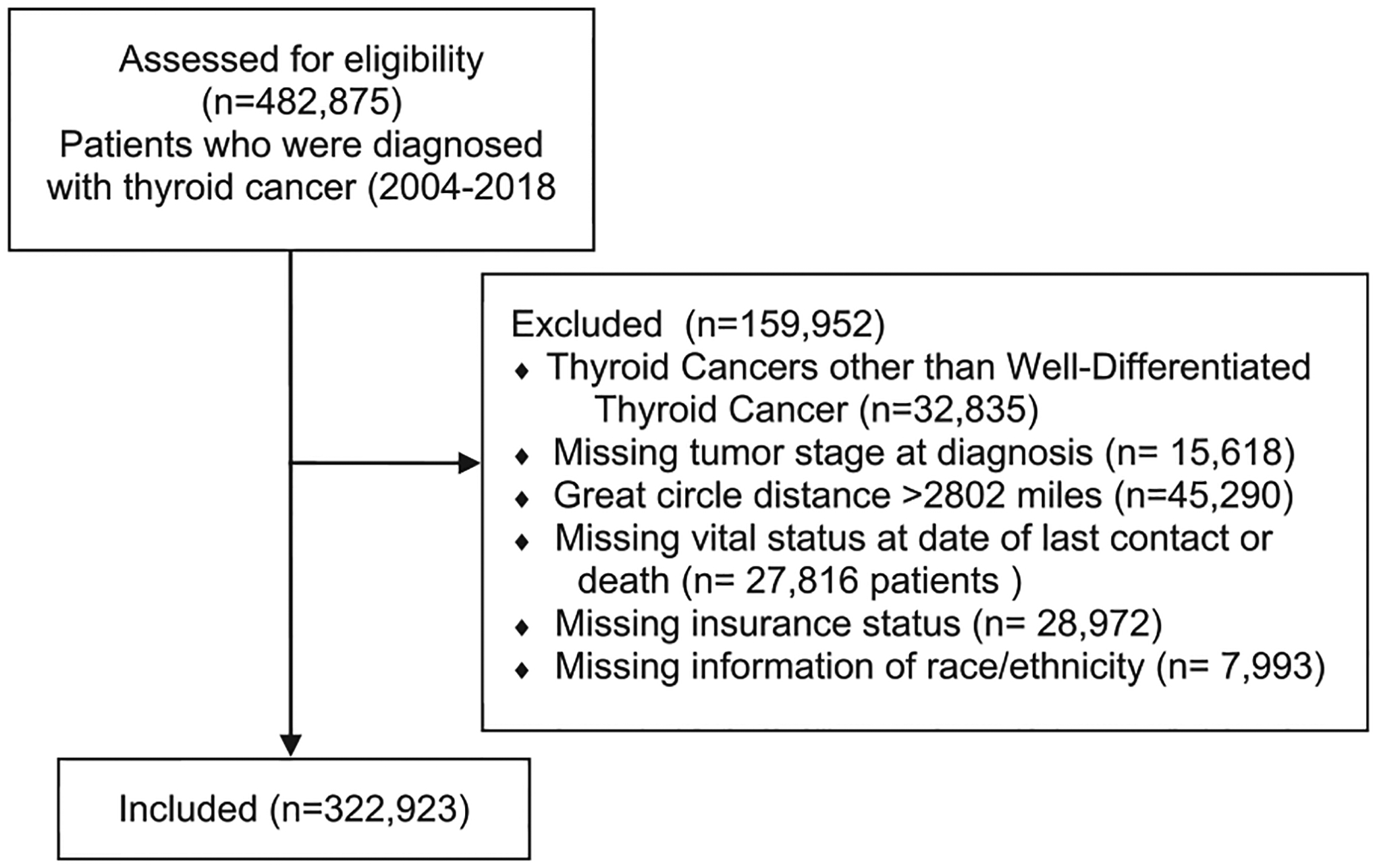
Consort flow diagram for patient inclusion: the consort flow diagram displays the criteria that were considered for excluding certain patient populations from this study as well as the number of patients that were excluded.
Next, the GCD variable was divided into quintiles. The aim of this methodology was to create 5 groups with similar numbers of patients. The divisions between categories of this variable were based on differences in distance between patient residence and their hospital of diagnosis. Patients who lived between 0 miles and 4.2 miles of their hospital of diagnosis were grouped into the first group. The mile markers for the other groups were 4.2–8.3 miles (group 2), 8.3–14.4 miles (group 3), 14.4–28.9 miles (group 4), and ≥28.9 miles (group 5).
Race and ethnicity were categorized based on definitions for racial and ethnic categories proposed by the National Institutes of Health in 2015 including White, Black, and Asian, with ethnicity defined as Hispanic or non-Hispanic.17 To determine whether patients should be classified as Hispanic or non-Hispanic, a new variable was generated to account for Hispanic ethnicity in addition to the racial categories above. Therefore, the final racial and ethnic categorical variable included the following categories: White, Black, Asian, and Hispanic.
Several variables were recoded into dichotomous values for binary logistic regression. The data obtained from the NCDB contained a categorical variable that included four overall stages to describe the tumors at diagnosis of the patients in the dataset. The staging system utilized by the NCDB is the standard Tumor (T), Nodes (N), and Metastasis (M) AJCC staging system.18 In order to create a dichotomous variable, a new variable was recoded to include two values: “early stage disease” (AJCC stage I or II) or an “advanced disease” (AJCC stage III or IV). Age was similarly recoded, with values representing patients diagnosed with WDTC at age ≤50 and those diagnosed at age >50. These cutoffs were selected because the mean and median age of diagnosis for the dataset was 50. The Charlson-Deyo comorbidity score was also recoded into a dichotomous variable. The original variable contained 4 values, with 0 indicating a lack of comorbidities, and values of 1–3 indicating comorbidities of increasing severity. This variable was dichotomized such that all values indicating the presence of comorbidities were grouped, and all values indicating a lack of comorbidities were grouped together (in a secondary analysis, stage was dichotomized as T1N0M0 or “higher stage” and used as a substitution for the above noted dichotomy of “early stage”/”advanced disease”.)
Statistical analysis
Analysis of associations between “demographic data” categories in relation to racial or ethnic status were conducted using chi-squared and one-way analysis of variance (one-way analysis of variance was only used for age as it was analyzed as a continuous variable) (Table 1). Subsequently, a binary endpoint of 50 years old was chosen for the age variable. These analyses were conducted between all four racial and ethnic groups of interest. Binary logistic regression was performed to determine the relationship between demographic variables and tumor staging at diagnosis. The demographic variables used for binary logistic regression of stage included age at diagnosis, insurance status, sex, income status, comorbidity score (Charlson-Deyo score), GCD, and race/ethnicity. Initially, univariate binary logistic regression analyses were conducted for each demographic variable. Multivariate binary logistic regression was then conducted using the same variables. Subsequently, all of the above variables were included for the race/ethnicity analysis. The White category was used as reference.
Table 1 –
Demographic data*.
| Demographic data | White | Black | Asian | Hispanic |
|---|---|---|---|---|
| Total patients: | 253,762 | 22, 978 | 16,761 | 29,422 |
| Sex (female): | 189,136 (74.5%) | 19,132 (83.3%) | 13,233 (79.0%) | 23,949 (81.4%) |
| Mean age at diagnosis (±SD): | 50.58 ± 15.28 | 50.40 ± 14.35 | 47.35 ± 14.67 | 46.30 ± 14.60 |
| Insurance status: | ||||
| Private | 181,867 (71.7%) | 13,544 (58.9%) | 11,902 (71.0%) | 17,372 (59.0%) |
| Government | 67,794 (26.7%) | 8423 (36.7%) | 4368 (26.1%) | 9109 (31.0%) |
| No insurance | 4101 (1.6%) | 1011 (4.4%) | 491 (2.9%) | 2941 (10.0%) |
| Charlson-Deyo score: | ||||
| 0 | 213,125 (84.0%) | 17,402 (75.7%) | 14,727 (87.9%) | 24,807 (84.3%) |
| 1 | 32,748 (12.9%) | 4237 (18.4%) | 1713 (10.2%) | 3796 (12.9%) |
| 2 | 5952 (2.3%) | 898 (3.9%) | 247 (1.5%) | 594 (2.0%) |
| ≥3 | 1937 (0.8%) | 441 (1.9%) | 74 (0.4%) | 225 (0.8%) |
| Tumor stage at diagnosis: | ||||
| Early stage disease (AJCC staging: 1–2) | 202,378 (79.8%) | 18,643 (81.1%) | 12,918 (77.1%) | 22,907 (77.9%) |
| Advanced stage (AJCC staging: 3–4) | 51,384 (20.2%) | 4335 (18.9%) | 3843 (22.9%) | 6515 (22.1%) |
| Great circle distance: | ||||
| 0–4.2 miles | 46,648 (18.4%) | 6610 (28.8%) | 4616 (27.5%) | 7667 (26.1%) |
| >4.2–8.3 miles | 47,839 (18.9%) | 5874 (25.6%) | 4409 (26.3%) | 7364 (25.0%) |
| >8.3–14.4 miles | 51,130 (20.1%) | 4446 (19.3%) | 3733 (22.3%) | 6439 (21.9%) |
| >14.4–28.9 miles | 53,532 (21.1%) | 3276 (14.3%) | 2483 (14.8%) | 4690 (15.9%) |
| >28.9 miles | 54,613 (21.5%) | 2772 (12.1%) | 1520 (9.1%) | 3262 (11.1%) |
| Median income for area of residence: | ||||
| ≤$50,353 | 75,541 (29.8%) | 13,093 (57.0%) | 3368 (20.1%) | 12,848 (43.7%) |
| >$50,353 | 175,606 (69.2%) | 9526 (41.5%) | 13,299 (79.3%) | 16,273 (55.3%) |
Using chi-squared analysis or one-way analysis of variance (only used for the continuous variable of age), it was determined that demographic factors showed significant differences (P < 0.001) between the four racial/ethnic groups. In subsequent analyses, the age variable was formatted as a binary variable rather than a continuous variable.
Following the above-noted binary analysis of tumor stage at diagnosis, the continuous variable of survival was analyzed. Cox regression was performed to conduct survival analyses. The covariates that were included in this analysis included race/ethnicity, age at diagnosis, insurance status, GCD, Charlson-Deyo score, median household income, and tumor stage at diagnosis. These factors were selected because they showed the greatest influence in univariate analysis. The time variable that was used for Cox regression contained the months from diagnosis until the last contact with the patient or death. These analyses produced several different survival analyses based on the above-mentioned covariates. Cox regression also produced hazard ratios that compared the survival of patients with various demographic characteristics. SPSS (version 27) statistical software was used to conduct all analyses for this study.
Results
There were 322,923 patients with WDTC included in this study. The patients’ demographic data are listed in Table 1. There were 253,762 (78.2%) White patients, 22,978 (7.1%) Black patients, 16,761 (5.2%) Asian patients, and 29,422 (9.1%) Hispanic patients. The Asian and Hispanic populations presented with the youngest mean ages of diagnosis at 47.35 and 46.30, respectively. Lack of health insurance was highest in the Hispanic population (10.0%), while government insurance use was highest in the Black population (36.7%). The Black population was found to have the highest rate of comorbid conditions when compared to the other populations in the study (24.3%). The Black population had the lowest rate of advanced disease at diagnosis (18.9%), and the Asian population had the highest rate of advanced disease at diagnosis (22.9%). The White population had the highest rate of living ≥28.9 miles (furthest distance measured) from the hospital of diagnosis (21.5%), and the Black population had the highest rates of living 0–4.2 miles (closest distance measured) from their hospital of diagnosis (28.8%). The Black population had the highest rate of living in areas where the median income was ≤$50,353 (57.0%), and the Asian population had the highest rate of living in areas where the median income was >$50,353 (79.3%).
Binary logistic regression analyses displayed differences between different categories of socioeconomic variables that were assessed in this analysis in relation to association with an advanced disease (AJCC tumor staging of III-IV) at the time of diagnosis. Univariate binary logistic regression showed that age at diagnosis younger than 50 (odds ratio [OR]: 0.21, 95%, confidence interval [CI]: 0.20–0.21, P < 0.001), absence of comorbidities (OR: 0.66, 95% CI: 0.65–0.68, P < 0.001), and GCD less than 28.9 miles (OR: 0.87, 95% CI: 0.85–0.89, P < 0.001) were associated with a lower likelihood of diagnosis with an advanced disease, while male sex (OR: 2.23, 95% CI: 2.19–2.27, P < 0.001) and lower income (OR: 1.03, 95% CI: 1.01–1.05, P < 0.001) were associated with increased likelihood of diagnosis with an advanced disease (Table 2). Multivariate binary logistic regression showed that age at diagnosis younger than 50 (OR: 0.22, 95%, CI: 0.21–0.22, P < 0.001), lower income status (OR: 0.98, 95%, CI: 0.96–0.99, P < 0.001), absence of comorbidities (OR: 0.90, 95%, CI: 0.88–0.92, P < 0.001), and GCD less than 28.9 miles (OR: 0.89, 95%, CI: 0.87–0.91, P < 0.001) were associated with a lower likelihood of diagnosis with an advanced disease, while male sex (OR: 1.96, 95%, CI: 1.92–1.99, P < 0.001) and lack of insurance (OR: 1.39, 95%, CI: 1.31–1.47, P < 0.001) were associated with increased likelihood of diagnosis with an advanced disease (Table 2). Lower income status was associated with more advanced diseases on univariate analysis and after controlling for other factors on multivariate analysis, lower income status was associated with less advanced disease. However, the OD for univariate and multivariate analyses of this factor were close to unity. It is noteworthy that a separate multivariate analysis (only changing the binary factor of stage to T1N0M0 versus “higher stage”) showed that patients younger than 50 were more likely to be diagnosed with disease greater than T1N0M0 staging when compared to patients older than 50 (P < 0.001), but as noted above, they were less likely to have stage III or IV disease (Tables 2 and 3).
Table 2 –
Binary logistic regression results for socioeconomic variables and association with stage of disease (Stage I/II vs. III/IV).
| Parameter | Univariate binary logistic regression (OR) | Multivariate binary logistic regression (OR) |
|---|---|---|
| Age at diagnosis ≤ 50 | 0.21 (CI: 0.202–0.211) (P < 0.001)* | 0.22 (CI: 0.213–0.221) (P < 0.001) |
| Insurance status (no insurance) | 1.03 (CI: 0.98–1.08) (P= 0.302) | 1.39 (CI: 1.31–1.47) (P < 0.001) |
| Sex (male) | 2.23 (CI: 2.19–2.27) (P < 0.001)* | 1.96 (CI: 1.92–1.99) (P < 0.001) |
| Income (lower income) | 1.03 (CI: 1.01–1.05) (P < 0.001)* | 0.98 (CI: 0.96–0.99) (P < 0.001) |
| Comorbidities/Charlson-Deyo score (absent) | 0.66 (CI: 0.65–0.68) (P < 0.001)* | 0.90 (CI: 0.88–0.92) (P < 0.001) |
| Great circle distance <28.9 miles | 0.87 (CI: 0.85–0.89) (P < 0.001)* | 0.89 (CI: 0.87–0.91) (P < 0.001) |
| Great circle distance <4.2 miles | 0.99 (CI: 0.97–1.02) (P= 0.56) | 1.01 (CI: 0.99–1.03) (P= 0.37) |
| Black versus White | 0.92 (CI: 0.89–0.95) (P < 0.001)* | 1.01 (CI: 0.97–1.05) (P= 0.72) |
| Hispanic versus White | 1.12 (CI: 1.09–1.16) (P < 0.001)* | 1.49 (CI: 1.45–1.54) (P < 0.001) |
| Asian versus White | 1.17 (CI: 1.13–1.22) (P < 0.001)* | 1.49 (CI: 1.43–1.55) (P < 0.001) |
OR >1 indicates greater likelihood of advanced disease.
Table 3 –
Binary logistic regression for socioeconomic variables and association with stage (T1N0M0 versus higher stage)*.
| Parameter | Univariate binary logistic regression (OR) | Multivariate binary logistic regression (OR) |
|---|---|---|
| Age at diagnosis ≤ 50 | 1.28 (CI: 1.26–1.30) (P < 0.001) | 1.36 (CI: 1.34–1.38) (P < 0.001) |
| Insurance status (no insurance) | 1.52 (CI: 1.45–1.59) (P < 0.001) | 1.49 (CI: 1.42–1.56) (P < 0.001) |
| Sex (male) | 1.90 (CI: 1.87–1.94) (P < 0.001) | 1.99 (CI: 1.96–2.03) (P < 0.001) |
| Income (lower income) | 0.99 (CI: 0.98–1.02) (P = 0.936) | 0.99 (CI: 0.98–1.01) (P = 0.72) |
| Comorbidities/Charlson-Deyo score (absent) | 0.92 (CI: 0.90–0.93) (P < 0.001) | 0.95 (CI: 0.93–0.97) (P < 0.001) |
| Great circle distance <28.9 miles | 0.91 (CI: 0.90–0.93) (P < 0.001) | 0.92 (CI: 0.90–0.93) (P < 0.001) |
| Great circle distance <4.2 miles | 0.99 (CI: 0.98–1.01) (P = 0.661) | 1.03 (CI: 1.01–1.05) (P = 0.004) |
| Black versus White | 0.97 (CI: 0.94–0.99) (P = 0.037)* | 1.05 (CI: 1.02–1.08) (P = 0.002) |
| Hispanic versus White | 1.52 (CI: 1.49–1.56) (P < 0.001)* | 1.54 (CI: 1.50–1.59) (P < 0.001) |
| Asian versus White | 1.45 (CI: 1.41–1.50) (P < 0.001)* | 1.48 (CI: 1.43–1.53) (P < 0.001) |
Patients with T1N0M0 disease included 151,795 patients, and higher stage disease included 141,672 patients. OR of >1 were associated with more advanced diseases.
Racial and ethnic status variables were also used in the above noted binary logistic regression analyses regarding tumor stage at presentation. Univariate binary logistic regression indicated that Black patients were less likely to be diagnosed with an advanced disease when compared to the White population (OR: 0.92, CI: 0.89–0.95, P < 0.001) (Table 2). Multivariate analysis (controlling for all factors listed in Table 2) did not produce significant results when comparing the likelihood of diagnosis with an advanced disease between the Black population and White population. Univariate and multivariate analyses showed that Hispanic patients were more likely to be diagnosed with advanced diseases when compared to the White population (univariate OR: 1.12, CI: 1.09–1.16, P < 0.001; multivariate OR: 1.49, CI: 1.45–1.54, P < 0.001) (Table 2). Univariate and multivariate analysis indicated that the Asian population was more likely to be diagnosed with advanced diseases when compared to the White population (univariate OR: 1.17, CI: 1.13–1.22, P < 0.001; multivariate OR: 1.49, CI: 1.43–1.55, P < 0.001) (Table 2). A separate multivariate analysis (only changing the binary factor of stage to T1N0M0 or “higher stage”) showed that the Black population had more “higher stage” (>T1N0M0) than the White population (P = 0.002) (Table 3).
Survival analysis using Cox regression indicated a survival advantage for Asian patients (HR: 0.77, P < 0.001) and Hispanic patients (HR: 0.82, P < 0.001) and a survival disadvantage for Black patients (HR: 1.27, P < 0.001) (Fig. 2, Table 4). The median income status of patients’ area of residences was also found to have an association with long-term survival. When compared to patients from areas with a median household income of less than $40,227 (lowest quartile of income among patients), patients living in areas with a median household income of greater than $40,227 were shown to have a survival advantage (Fig. 3, Table 4). Analysis of the GCD of patients’ residences to hospitals of diagnosis indicated that patients living less than 28.9 miles from the hospital of diagnosis were associated with improved survival when compared to patients living greater than 28.9 miles (HR: 0.90, P < 0.001) (Fig. 4, Table 4). Patients with private insurance and government insurance were shown to have a hazard ratios of 0.58 (P < 0.001) and 0.95 (P < 0.001), respectively (improved survival), when compared to patients without any form of insurance (Fig. 5, Table 4).
Fig. 2 –
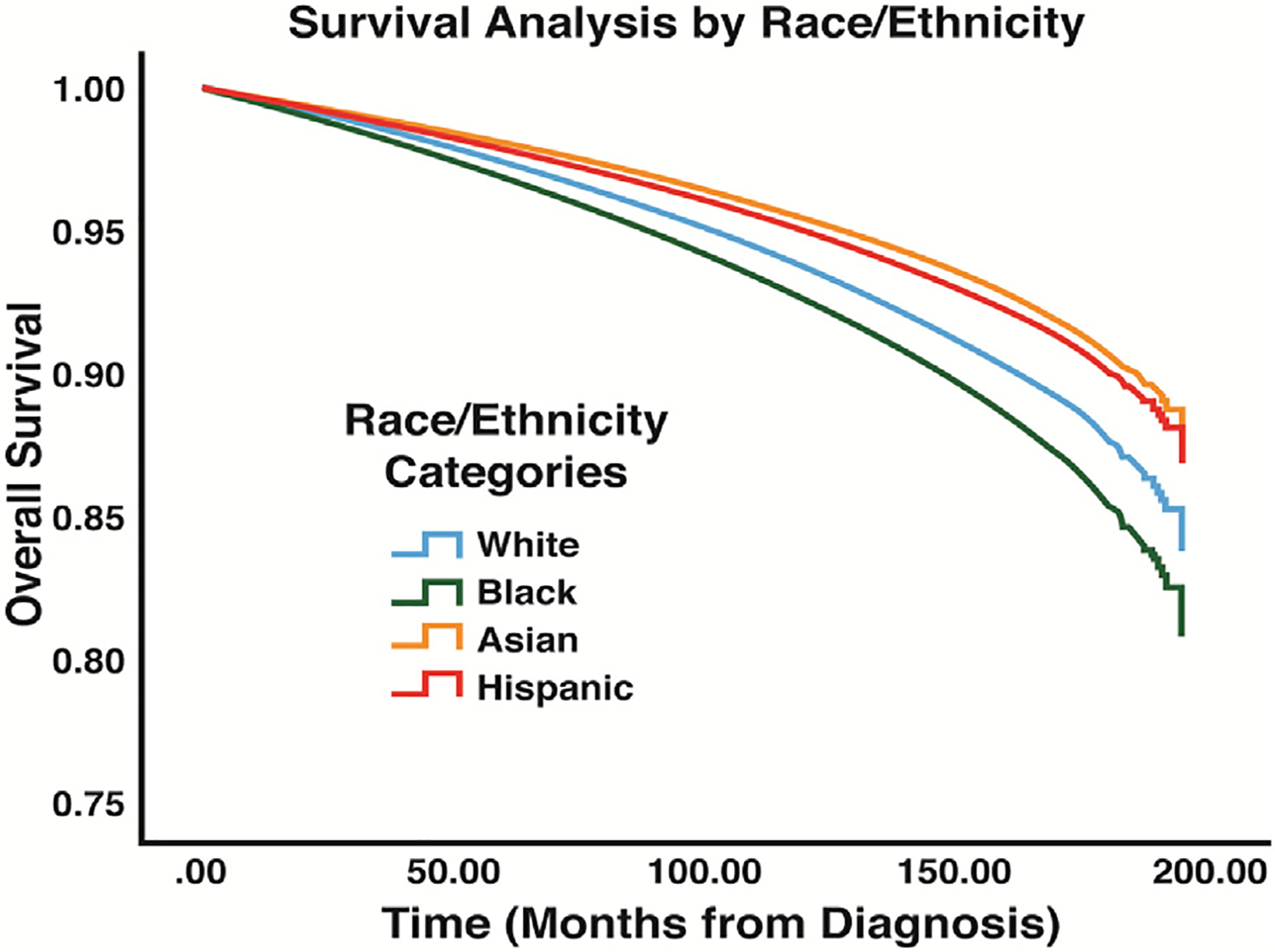
Survival results for race/ethnicity variables: analysis displays the differences in overall survival between the race/ethnicity categories that were compared in this study.
Table 4 –
Cox regression survival analysis results.
| Demographic categories: | Hazard ratios: | Significance (P-value) |
|---|---|---|
| Age | 1.08 | <0.001 |
| Race/Ethnicity with non-Hispanic White reference category | ||
| Non-Hispanic Black | 1.24 | <0.001 |
| Non-Hispanic Asian | 0.76 | <0.001 |
| Hispanic | 0.80 | <0.001 |
| Great circle distance with distance of ≥28.9 miles from hospital of diagnosis as reference category | ||
| <28.9 miles | 0.90 | <0.001 |
| Charlson-Deyo index score for comorbidities with a comorbidity score of 0 serving as the reference category | ||
| Charlson-Deyo score: 1 | 1.44 | <0.001 |
| Charlson-Deyo score: 2 | 2.14 | <0.001 |
| Charlson-Deyo score: 3 | 3.08 | <0.001 |
| Sex with male sex serving as the reference category | ||
| Sex: female | 0.66 | <0.001 |
| Insurance status with “no insurance” category serving as the reference category | ||
| Government insurance | 0.95 | 0.231 |
| Private insurance | 0.58 | 0.001 |
| Median income status with the lowest income status group (<$40,227) as reference category | ||
| $40,227–$50,353 | 0.95 | 0.010 |
| $50,354–$63,332 | 0.87 | <0.001 |
| ≥63,333 | 0.71 | <0.001 |
Fig. 3 –
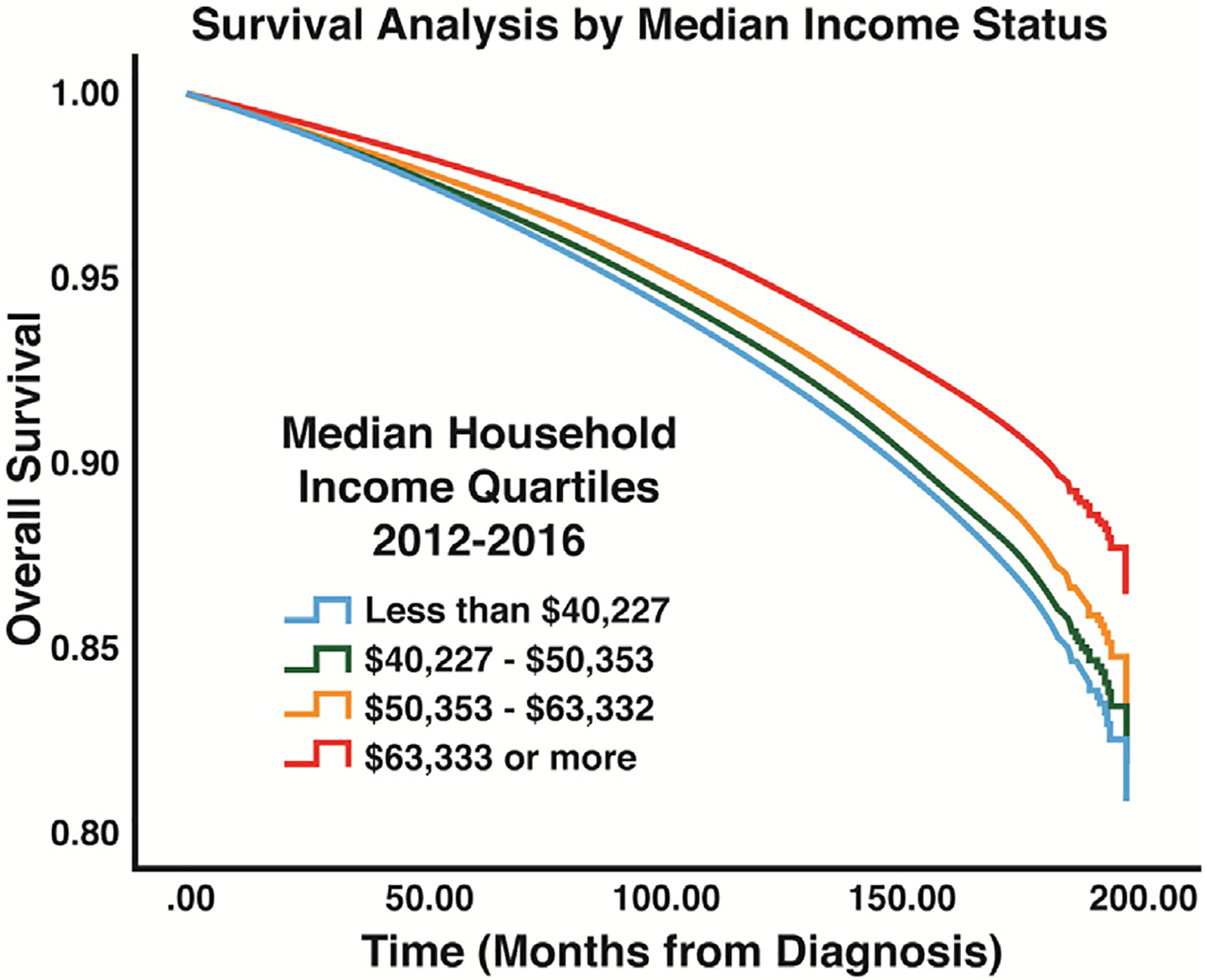
Survival results for income quartiles: analysis displays the differences in overall survival between the different income categories that were compared in this study.
Fig. 4 –
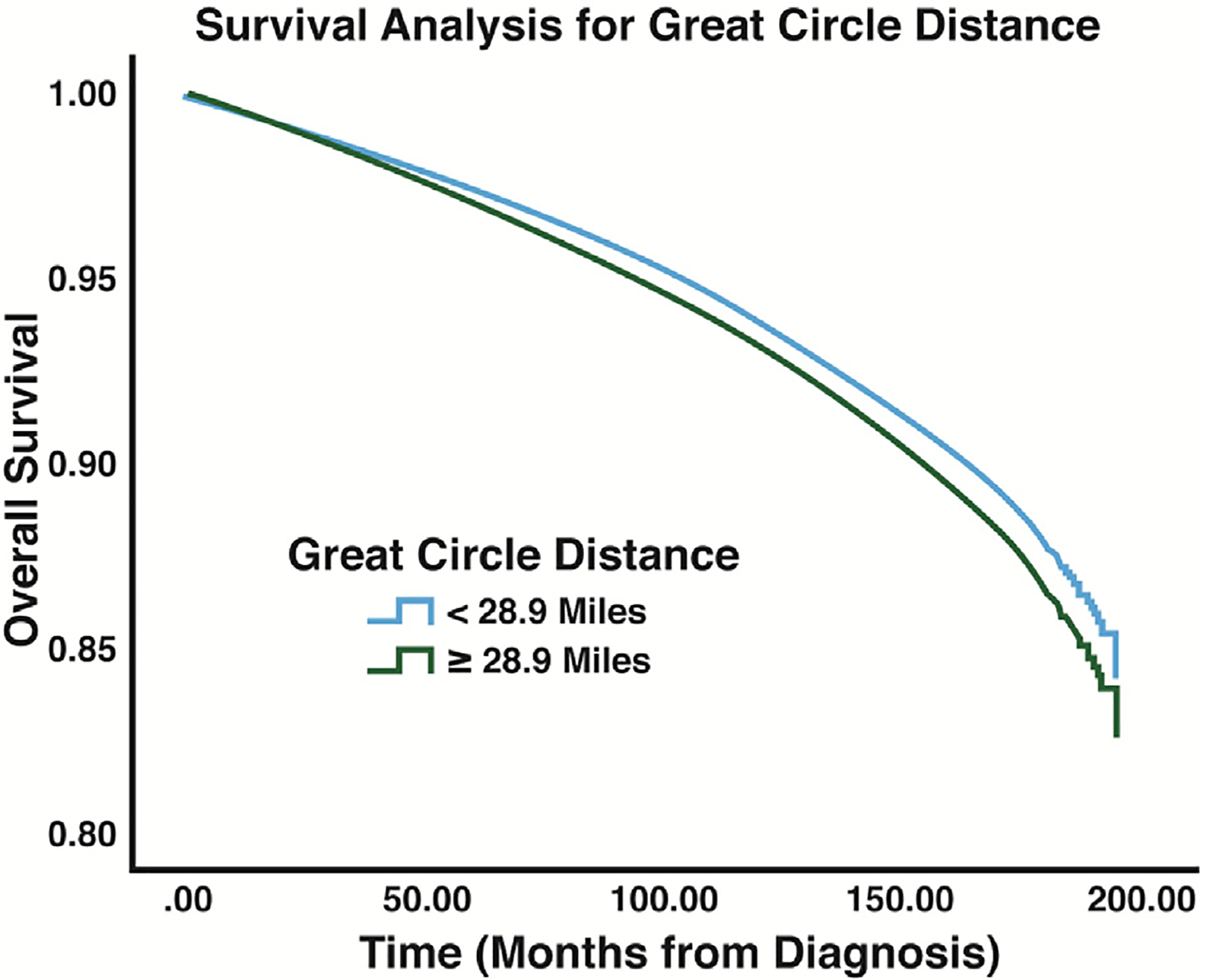
Survival results for GCD: analysis displays the differences in overall survival between patients who lived within 28.9 miles of the hospital of diagnosis and patients who lived beyond 28.9 miles of their hospital of diagnosis.
Fig. 5 –
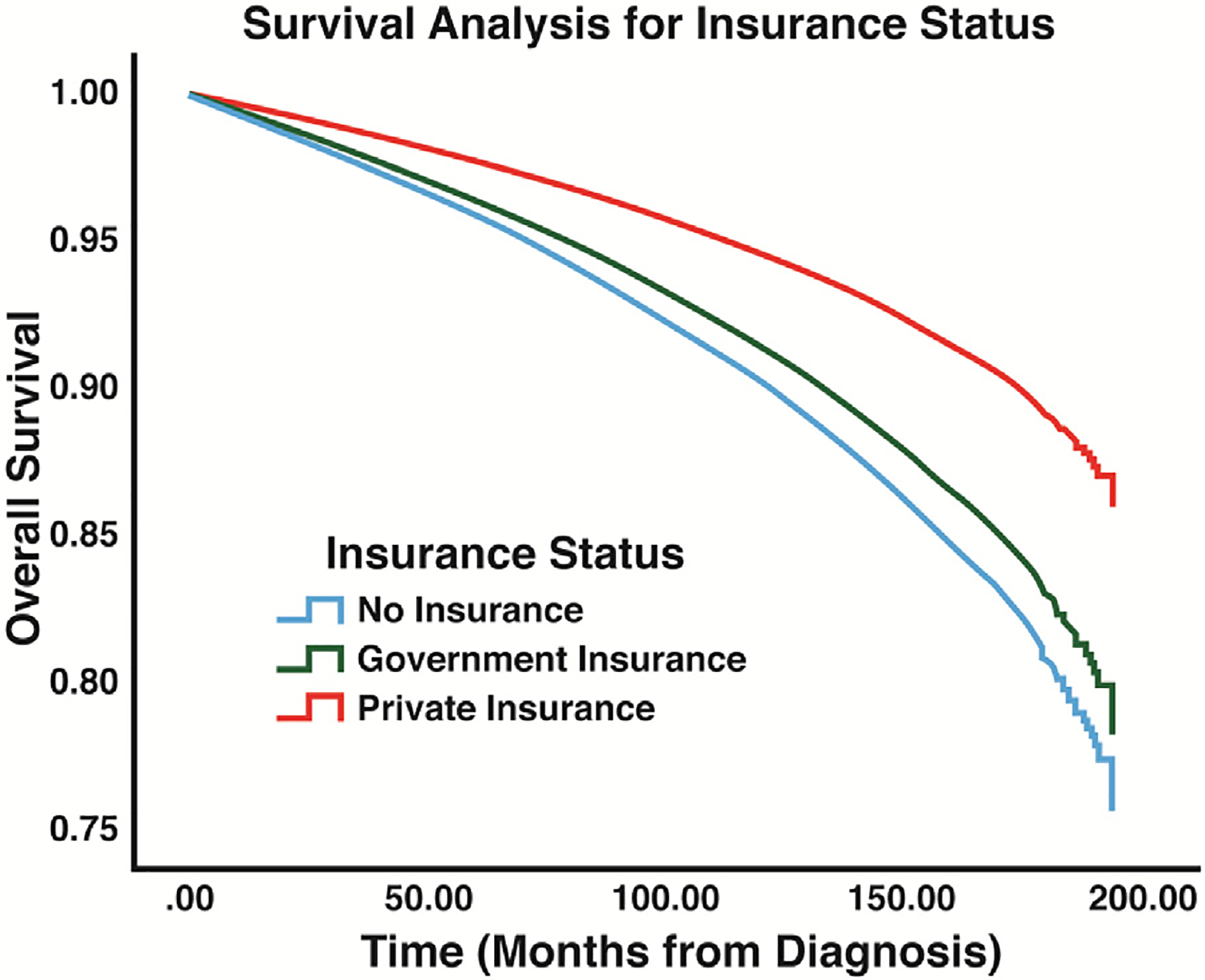
Survival results for insurance status: analysis displays the differences in overall survival between the different insurance categories that were compared in this study.
Discussion
This study examined associations between health care disparities and outcomes for patients with WDTCs. Our results demonstrated that certain race or ethnic statuses, income statuses, insurance statuses, and distances of residences from hospitals of diagnosis were associated with worse outcomes. This analysis highlights important realities of our health care system that have an effect on highly curable WDTCs. Our results reveal that key socioeconomic factors need to be addressed regarding the management of these WDTCs.
Differences in outcomes for patients with WDTCs relative to race and ethnic status were present in our analysis. The Black population was found to have a significantly decreased survival rate compared to the White population (Fig. 2). This finding is consistent with similar studies assessing thyroid cancer outcomes in the Black population, which have shown higher rates of advanced disease, more aggressive tumor growth, and poor survival outcomes.19 Our analysis did not show that the Black population was more likely to present with an advanced disease compared to the White population, but other studies have indicated that the Black population has an increased likelihood of being diagnosed with tumors >4 cm compared to the White population.19 Similarly, our study did show that the Black population had more disease greater than T1N0M0 (Table 3). Our findings that demonstrated decreased survival, but similar rates of advanced disease for Black patients compared to White patients were intriguing. These findings may suggest that the crude assessment of cancer stage from large national databases may have underestimated the rate of higher stage disease in Black patients. Alternatively, these findings may suggest that a cancer diagnosis has greater ramifications on overall physical and mental health in patients already burdened with socioeconomic stressors. Therefore, a WDTC diagnosis in the Black population may influence survival to a greater degree than these patients’ peers. Some demographic characteristics of the Black population may predispose this population to poorer survival outcomes such as higher rates of comorbidities, decreased rates of private insurance usage, and lower income among the Black population compared to other populations. Additionally, it is possible that racism plays a role in how patients navigate the health care system based on previous analyses that have focused on the effects of racism on outcomes in various health care settings.20,21
The Asian and Hispanic populations displayed the highest rates of overall survival, even though both populations had a higher likelihood of developing an advanced disease at diagnosis when compared to the White population. It is difficult to assess the factors that may be contributing to these contrasting results regarding survival and disease presentation. Based on our results regarding income and insurance status, it may be more important to consider socioeconomic factors when attempting to explain differences in disease progression between various groups of patients with WDTCs. On the other hand, it may also be important to determine whether there is a biologic basis for differences between racial and ethnic groups regarding disease progression. Many studies have already undertaken this effort by conducting molecular and genetic analyses to compare genetic factors that may predispose different populations to WDTC. For instance, it has been suggested that the Korean population may be more likely to develop papillary thyroid cancer due to an increased rate of v-Raf murine sarcoma viral oncogene homolog B1 mutations when compared to other populations around the world.22 Further molecular analysis will provide greater insight into factors that may be affecting disease progression in different racial and ethnic populations.
A prominent finding in our study was related to the income status of patients with WDTC. When controlling for several other variables, the median income status of patients’ residential areas (zip code) was associated with survival. It has long been known that counties with lower median incomes have worse cancer survival outcomes compared to higher income counties.23 Several studies have indicated that a patient’s income status may have an impact on his or her ability to receive quality health care. Additionally, in the field of endocrine surgery, patients with lower income have been shown to be more likely to receive care from low-volume endocrine surgeons who tend to have worse long term postoperative outcomes.24–27 This may help to explain the difference in outcomes despite less number of advanced diseases at diagnosis in the Black population. Patients with lower income were slightly less likely to present with an advanced aisease compared to wealthier patients. This finding contrasted with our results showing poorer survival outcomes for low-income patients compared to the wealthier population. Some researchers have indicated that low-income patients may delay medical care despite access to care for reasons such as misconceptions about the health care system cancer diagnosis.28 Based on these findings, it will be important to further elucidate factors that may contribute to the adverse survival outcomes among the low-income status patients other than tumor staging at diagnosis. Certainly, age and distance from cancer treatment sites are intertwined with income levels. Younger patients were more likely to present with disease greater than T1N0M0, but they had a lesser number of advanced diseases and better survival, as has been shown by others.29,30 Likewise, patients who lived in the furthest quintile of distance from treatment did poorly as previously reported.31,32 Further analysis will be necessary to decipher the interplay of income, age, and distance from treatment center as we seek to help patients gain better access to care.
Finally, patients with private health insurance were found to have improved survival. It is noteworthy that minority populations, such as Blacks and Hispanics, were more likely than the White population to utilize government insurance or live without insurance, which suggests that some intersectional factors may be contributing to poor outcomes related to insurance status. Future efforts should be focused on determining methods of directing (or navigating) patients to prompt, high-volume endocrine surgeons and other providers regardless of their insurance status.33–35
An additional consideration of this study is the underlying uncertainty of large databases. The NCDB is currently one of the best resources for information on large populations with various diagnoses of cancer. However, the study is limited by the potential for inaccurate staging of patients at diagnosis by individual providers as well as the inability of large databases to capture the entire milieu of comorbidities. We made efforts to mitigate these uncertainties by using two different staging cut-offs for patients at presentation and using standard comorbidity scores. However, these mitigation efforts potentially suffer from improper coding or inherent inaccuracies in staging and/or assessments of comorbidities. Also, we acknowledge that our study does not display causality between the socioeconomic factors that were analyzed and the outcomes of interest given the study design, but clarification of associations between different factors and outcomes should provide direction for further studies to assess causality.
Conclusions
The above-noted disparities in outcomes for minority patients, rural patients, and patients who lack private insurance are vital obstacles to the optimization of our health care system. This study used WDTC to illustrate this point, but unfortunately, these disparities are very prevalent among many tumor types. A recent study using the Surveillance, Epidemiology, and End Results database examined race and ethnicity with respect to outcomes in nine major cancers: prostate, ovarian, breast, stomach, pancreatic, lung, liver, esophageal, and colorectal cancers. This large study of more than 950,000 patients examined race as divided into White, Asian, Black, and Hispanic. Using a multivariate logistic regression to account for confounding factors, only Black patients demonstrated a statistically significant increased rate of metastases at diagnosis. The Asian population was found to have the lowest rate of metastases at diagnosis (OR: 1.14, P < 0.001). Both Black and Hispanic patients were less likely to receive definitive treatment (OR: 0.63, P < 0.001 and OR: 0.75, P < 0.001; respectively).36
The breadth and depth of disparities in cancer outcomes for disadvantaged socio-economic groups have been well-documented in the literature.36–38 Currently, researchers are exploring methods to mitigate disparities in cancer care outcomes. It is believed that prospective studies involving assessments of biological, social, and individual factors are needed. The multiethnic cohort study, which was started in the early 1990’s, has been funded by the National Cancer Institute to decipher racial and ethnic differences for patients diagnosed with cancer based on biological, social, and individual assessments.37 These study patients undergo baseline data collection (biological specimens as well as questionnaires involving multiple socioeconomic and lifestyle factors), and they are subsequently followed with multiple repeated assessments. Studies of this nature are beginning to unravel biological and socioeconomic root causes for disparate cancer outcomes, but more studies such as this are needed. For instance, researchers are examining other methodologies such as community-based participatory research.38 These efforts recruit community representatives to partner with health care researchers. This partnership is formed to develop a program that involves co-learning, shared decision-making, and mutual ownership. Community involvement can potentially enhance patient engagement, improve communication, and reduce misconceptions about health care.38
In order to build on the research efforts noted above, public policy programs will be needed to effect changes that are deemed necessary based on research findings. Esnaola et al. have identified five areas of policy that will need to be addressed in the future: improve access to care, increase diversity in the physician workforce, expand the use of patient navigators, expand the use of active comanagement, and increase adherence to best practices.38 Improving disparities in cancer outcomes will require much more outreach to under-served populations and these efforts will be the subject of intense work in the future.
Supplementary Material
Funding
The authors have indicated that they have no funding regarding the content of this article.
Footnotes
Supplementary Materials
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jss.2022.11.033.
Disclosure
None declared.
REFERENCES
- 1.Maceri DR, Babyak J, Ossakow SJ. Lateral neck mass. Sole presenting sign of metastatic thyroid cancer. Arch Otolaryngol Head Neck Surg. 1986;112:47–49. [DOI] [PubMed] [Google Scholar]
- 2.Al-Qurayshi Z, Randolph GW, Srivastav S, Kandil E. Outcomes in endocrine cancer surgery are affected by racial, economic, and healthcare system demographics. Laryngoscope. 2016;126:775–781. [DOI] [PubMed] [Google Scholar]
- 3.Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93:809–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catapano JS, Rumalla K, Srinivasan VM, et al. Delays in presentation and mortality among Black patients with mechanical thrombectomy after large-vessel stroke at a US hospital. Neurosurg Focus. 2021;51:E9. [DOI] [PubMed] [Google Scholar]
- 5.Paul A, Englert P, Varga M. Socio-economic disparities and COVID-19 in the USA. J Physiol. 2020;2:035017. [Google Scholar]
- 6.Harari A, Li N, Yeh MW. Racial and socioeconomic disparities in presentation and outcomes of well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2014;99:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evangelista LS, Dracup K, Doering LV. Racial differences in treatment-seeking delays among heart failure patients. J Card Fail. 2002;8:381–386. [DOI] [PubMed] [Google Scholar]
- 8.Park H, Park J, Park SY, et al. Clinical course from diagnosis to death in patients with well-differentiated thyroid cancer. Cancers (Basel). 2020;12:2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid. 2011;21:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Repplinger D, Bargren A, Zhang YW, Adler JT, Haymart M, Chen H. Is Hashimoto’s thyroiditis a risk factor for papillary thyroid cancer? J Surg Res. 2008;150:49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu XM, Wan Y, Sippel RS, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254:653–660. [DOI] [PubMed] [Google Scholar]
- 12.Dream S, Wang R, Lovell K, Iyer P, Chen H, Lindeman B. Outpatient thyroidectomy in the pediatric population. Am J Surg. 2020;219:890–893. [DOI] [PubMed] [Google Scholar]
- 13.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. [DOI] [PubMed] [Google Scholar]
- 14.Lin HW, Bhattacharyya N. Survival impact of treatment options for papillary microcarcinoma of the thyroid. Laryngoscope. 2009;119:1983–1987. [DOI] [PubMed] [Google Scholar]
- 15.D’Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996;49:1429–1433. [DOI] [PubMed] [Google Scholar]
- 16.Fritz AP, Percy CL, Jack A, Whelan S. International classification of Diseases for Oncology. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 17.Health NIO. Racial and ethnic categories and definitions for NIH diversity programs and for other reporting purposes. 2015. Available at: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-089.html. Accessed June 7, 2022.
- 18.Amin MBE, Greene FL, Edge SB. AJCC Cancer Staging Manual. New York City, NY: Springer; 2017. [Google Scholar]
- 19.Hollenbeak CS, Wang L, Schneider P, Goldenberg D. Outcomes of thyroid cancer in African Americans. Ethn Dis. 2011;21:210–215. [PubMed] [Google Scholar]
- 20.Abraham P, Williams E, Bishay AE, Farah I, Tamayo-Murillo D, Newton IG. The roots of structural racism in the United States and their manifestations during the COVID-19 pandemic. Acad Radiol. 2021;28:893–902. [DOI] [PubMed] [Google Scholar]
- 21.Stringer Smith C History of racism in healthcare: from medical mistrust to Black african-American dentists as moral exemplar and organizational ethicsd—a bioethical synergy awaits. Am J Bioeth. 2022;22:7–9. [DOI] [PubMed] [Google Scholar]
- 22.Song YS, Lim JA, Park YJ. Mutation profile of well-differentiated thyroid cancer in Asians. Endocrinol Metab. 2015;30:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connor JM, Sedghi T, Dhodapkar M, Kane MJ, Gross CP. Factors associated with cancer disparities among low-, medium-, and high-income US counties. JAMA Netw Open. 2018;1:e183146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDow AD, Roman BR, Saucke MC, et al. Factors associated with physicians’ recommendations for managing low-risk papillary thyroid cancer. Am J Surg. 2021;222:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melfa G, Porello C, Cocorullo G, et al. Surgeon volume and hospital volume in endocrine neck surgery: how many procedures are needed for reaching a safety level and acceptable costs? A systematic narrative review. Geka Chiryo. 2018;39:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aryanpour Z, Asban A, Boyd C, et al. A single institution experience with papillary thyroid cancer: are outcomes better at comprehensive cancer centers? Am J Surg. 2021;222:802–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wrenn SM, Wang TS, Toumi A, Kiernan CM, Solórzano CC, Stephen AE. Practice patterns for surgical management of low-risk papillary thyroid cancer from 2014 to 2019: a CESQIP analysis. Am J Surg. 2021;221:448–454. [DOI] [PubMed] [Google Scholar]
- 28.Nonzee NJ, Ragas DM, Ha Luu T, et al. Delays in cancer care among low-income minorities despite access. J Womens Health (Larchmt). 2015;24:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semrad TJ, Semrad AM, Farwell DG, Chen Y, Cress R. Initial treatment patterns in younger adult patients with differentiated thyroid cancer in California. Thyroid. 2015;25:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orosco RK, Hussain T, Brumund KT, Oh DK, Chang DC, Bouvet M. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the Surveillance, Epidemiology, and End Results database. Thyroid. 2015;25:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20:1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scoggins JF, Fedorenko CR, Donahue SM, Buchwald D, Blough DK, Ramsey SD. Is distance to provider a barrier to care for medicaid patients with breast, colorectal, or lung cancer? J Rural Health. 2012;28:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freund KM, Battaglia TA, Calhoun E, et al. National cancer Institute patient navigation research program: methods, protocol, and measures. Cancer. 2008;113:3391–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung JM, Martin JW, Jefferson FA, et al. Racial and socioeconomic disparities in bladder cancer survival: analysis of the California cancer registry. Clin Genitourin Cancer. 2019;17:e995–e1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opneja A, Cioffi G, Alahmadi A, et al. Adoption of single agent anticancer therapy for advanced hepatocellular carcinoma and impact of facility type, insurance status, and income on survival: analysis of the national cancer database 2004–2014. Cancer Med. 2021;10:4397–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang C, Zhang C, Wang Q, Li Z, Lin J, Wang H. Differences in stage of cancer at diagnosis, treatment, and survival by race and ethnicity among leading cancer types. JAMA Netw Open. 2020;3:e202950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zavala VA, Bracci PM, Carethers JM, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021;124:315–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esnaola NF, Ford ME. Racial differences and disparities in cancer care and outcomes: where’s the rub? Surg Oncol Clin N Am. 2012;21:417–437. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


