Abstract
Background
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death around the world. The affected patients suffer not only from impaired lung function, but also from a wide variety of comorbidities. Their cardiac comorbidities, in particular, lead to increased mortality.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed, including guidelines from Germany and abroad.
Results
The usual diagnostic criteria for COPD are a post-bronchodilator FEV1/FVC quotient below the fixed threshold of 0.7, or, preferably, below the lower limit of normal (LLN) according to the GLI reference values for the avoidance of over- and underdiagnosis. The overall prognosis is markedly affected by comorbidities of the lung itself and those that involve other organs; in particular, many persons with COPD die of heart disease. The potential presence of heart disease must be borne in mind in the evaluation of patients with COPD, as lung disease can impair the detection of heart disease.
Conclusion
As patients with COPD are often multimorbid, the early diagnosis and adequate treatment not only of their lung disease, but also of their extrapulmonary comorbidities are very important. Well-established diagnostic instruments and well-tested treatments are available and are described in detail in the guidelines concerning the comorbidities. Preliminary observations suggest that more attention should be paid to the potential positive effects of treating comorbidities on the lung disease itself, and vice versa.
Chronic obstructive pulmonary disease (COPD) is associated with a high burden of disease; in 2030, the WHO predicts, it will become the third leading cause of death (e1). It is significantly more prevalent in current and ex-smokers and its prevalence increases with age. According to the Robert Koch Institute, the annual prevalence of COPD in Germany is 5.8%, and it affects men and in women in roughly equal numbers (1). If we compare these figures with the results of the international BOLD study, which showed a 10% prevalence of COPD (at least 20% reduction in spirometric forced expiratory lung volume) among its more than 9000 participants (2), it is clear that COPD is being underdiagnosed. A key feature of COPD is that the individual’s prognosis is significantly influenced by comorbidities. Among the most common of these are cardiovascular disease, osteoporosis and muscle weakness, endocrinological and metabolic disorders, anxiety disorders and depression, and cancer (3). The causes of COPD are multifactorial: in addition to age-related comorbidities, smoking and other inhaled noxa as a shared risk factor, and systemic inflammation of pulmonary origin (e2), other contributors to COPD include physical inactivity, poor nutrition, and hypoxemia. Patients need to be carefully examined for concomitant disease, as dyspnea, for example, may be viewed as a symptom of lung disease and cardiovascular disease be overlooked.
Prevalence.
The WHO predicts that in 2030 COPD will become the third leading cause of death. According to the Robert Koch Institute, the annual prevalence in Germany is 5.8% and the disease affects men and in women in roughly equal numbers.
Disease burden.
COPD is associated with a high disease burden, and comorbidities have a significant impact on the overall prognosis.
Germany’s largest observational study of COPD, COSYCONET, aimed to investigate the interaction between lung disease, systemic inflammation, and comorbidities (e3). In this study, it was found that 30% to 40% of patients with COPD and echocardiographic changes did not have a medical diagnosis of cardiovascular disease (4). This underlines the need for a wide-ranging differential diagnostic workup of dyspnea as a symptom. It is also essential for any comorbidities to be included in the diagnostic workup as well as in the treatment and management of the patient’s disease (5, e4).
This review provides information about the diagnosis and treatment of the most common comorbidities in patients with COPD. It takes a closer look at how these comorbidities interact with COPD and discusses practical approaches to treatment.
Learning objectives
Comorbidities.
It is important to note any comorbidities in the patient’s medical history, so that these can be included in the diagnostic workup and in the treatment and management of the patient.
After reading this article, the reader should be able to:
explain the diagnostic criteria, risk factors, and symptoms of COPD,
name the major underlying pathomechanisms and currently accepted treatment options for COPD, and
classify the various comorbidities of COPD and describe their impact on the disease and on the patient’s overall prognosis.
Method
A selective literature search was carried out, which included the German National Clinical Guideline on COPD (2nd edition, version 1) (6) as well as international recommendations (5, 7, e4). Expert opinions (as published in review articles) were also taken into account during the preparation of this manuscript (8).
Definition
Definition.
COPD is a chronic, progressive disease of the airways and lungs characterized by bronchial obstruction that is not fully reversible by bronchodilators.
COPD is a chronic, progressive disease of the airways and lungs characterized by bronchial obstruction that is not fully reversible by the administration of bronchodilators (6). Two main phenotypes exist:
The airway type, which is the predominant type and is characterized by chronic obstructive bronchitis with persistent cough and sputum expecto-ration for at least 12 months;
The emphysema type, with rarefaction of peripheral pulmonary vessels, decreasing gas exchange area and exercise capacity, and cough and/or sputum expectoration.
Additional types have also been defined recently based on etiology (table 1) (e4). Exacerbations are episodes of acute worsening of the respiratory situation lasting at least 48 h and accompanied by a need for treatment intensification (6).
Table 1. Classification of COPD based on etiology.
| COPD classification | Description |
| Genetic COPD (COPD-G) | α1-Antitrypsin deficiency (AATD) Other genetic variants with smaller effects acting in combination |
| COPD due to abnormal lung development (COPD-D) | Early life events, e.g., prematurity and low birth weight |
| Environmental COPD – Cigarette smoking COPD (COPD-C) – Biomass and pollution exposure COPD (COPD-P) |
– Exposure to cigarette smoke, including in utero and passive smoking – Vaping or using E-cigarettes – Use of cannabis – Exposure to household pollution, ambient air pollution, gas emissions from open fires, occupational exposure (in Germany, the proportion of occupationally caused COPD in non-smokers is estimated at up to about 30%, with causes such as, e.g., quartz dust and cadmium, or other dusts such as, in agriculture, wood dust or biomass) |
| COPD due to infections (COPD-I) | Childhood infections, tuberculosis, HIV |
| COPD and asthma (COPD-A) | Childhood asthma in particular |
| COPD of unknown cause (COPD-U) |
Adapted from the current GOLD guideline (e4).
2022, 2023, Global Initiative for Chronic Obstructive Lung Disease, available from www.goldcopd.org, published in Deer Park, IL, USA.
COPD, chronic obstructive lung disease
Diagnosis
Diagnosis.
In addition to exposure history, the patient should be asked about the symptoms listed and about smoking status, including number of pack-years, and should also undergo a clinical examination (indicative signs being, for example, prolonged expiration, barrel chest, or expiratory wheezing).
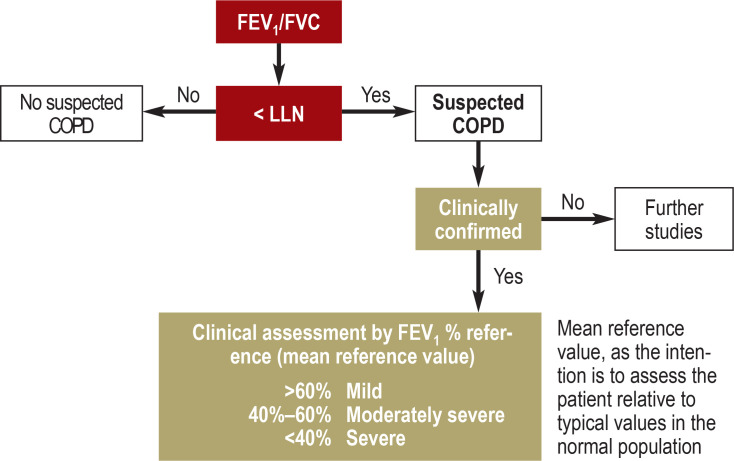
In addition to exposure history (in Germany, primarily smoking and workplace exposure), the patient should be asked about the symptoms listed above and about smoking status including number of pack-years, and should also undergo a clinical examination (indicative signs being, for example, prolonged expiration, barrel chest, or expiratory wheezing). In terms of lung function analysis, diagnosis is based on an irreversible airway obstruction showing that the ratio of the forced expiratory volume during the first second and the forced vital capacity of the lungs (FEV1/FVC) after administration of a bronchodilator is below the lower limit of normal (LLN, lower 5th percentile), preferably using Global Lung Function Initiative (GLI) reference values, see Figure (9). If these are not available, a fixed ratio of FEV1/FVC < 0.7 can be used. Blood gas analysis can be used to diagnose respiratory failure. Whole-body plethysmography can be used to assess static lung volumes and hence the degree of lung hyperinflation, while the diffusing capacity for inhaled carbon monoxide can provide valuable information about the severity of emphysema. A chest radiograph can be used for further differential diagnosis. The current GOLD guideline suggests the term “pre-COPD” for patients with respiratory symptoms and pre-existing structural changes, for example they may have emphysematous changes but preserved spirometry (e4). For this reason, patients in these groups with classic COPD-type symptoms and risk exposure should be referred for a single consultation with a pulmonary specialist, with the possibility of more extensive diagnostic testing of lung function.
Disease severity grading and treatment
In 2011, the international GOLD recommendations introduced, in addition to the assessment of COPD based on spirometric severity (GOLD grades 1–4), the GOLD groups A–D classification based on symptoms and exacerbation history (5). The latter proved superior in predicting disease progression (5), and therefore, since 2017, the ABCD classification has been the primary tool for treatment management according to the German National Clinical Guideline for COPD. Pharmacological and nonpharmacological treatments are shown in Table 3 and in the Box.
Pulmonary and extrapulmonary comorbidities
Comorbidities are associated with a poorer quality of life, increased risk of repeated hospital admissions, and higher mortality (13, 14, e6, e7). On average, more than 90% of patients with COPD have at least one comorbidity, and nearly 50% have more than three (e8). Comorbidities can be divided into the pulmonary and the extrapulmonary (15). The basic treatment of comorbidities should be guided by the clinical guidelines from the respective professional societies. The discussion below covers particularly important aspects and recommendations for patients with COPD.
Major pulmonary comorbidities
Disease severity grading and treatment.
Since 2017, the ABCD classification has been the primary tool to guide treatment.
Bronchial asthma
Bronchial asthma is the most common pulmonary comorbidity. The prevalence of asthma–COPD overlap syndrome (ACO) has been estimated at 27% in observational studies (e9), but the concept of ACO as an entity is not universally accepted. For example, the GOLD guideline (5) recommends that asthma and COPD should be considered as independent disease entities, whereas the UK NICE guideline refers to asthmatic features/features suggesting responsiveness to steroids (inhaled or systemic) in patients with COPD (7). These features include a confirmed medical diagnosis of bronchial asthma, an atopic tendency, variable airway obstruction as measured by 1-second capacity or circadian rhythm of peak expiratory flow, and peripheral eosinophilia (>300 cells/µL) (6, 7). This patient group should be treated early with inhaled corticosteroids (ICS), especially if exacerbations occur.
Bronchiectasis
The prevalence of bronchiectasis in COPD has been investigated in many studies, with sometimes contradictory findings as to rates (4% to 72%) (e10). Clinically significant bronchiectasis is associated with productive cough, bronchial infections, and morphologically abnormal dilatation of the bronchi as confirmed by computed tomography (CT). In patients with COPD, bronchiectasis is associated with a reduced body mass index and more advanced age, and with increased sputum production and exacerbations (e10). These patients also show increased colonization or limited infection with P. aeruginosa, increased signs of local and systemic inflammation, and raised mortality (hazard ratio 2.54) (16, 17, e11, e12). Identifying clinically significant bronchiectasis – as opposed to bronchiectatic changes that are only apparent on CT in the absence of corresponding clinical signs – is important (18). For bronchiectasis requiring treatment, further diagnostic tests include microbiological study of sputum for typical and atypical mycobacteria, ruling out of α1-antitrypsin and immunoglobulin deficiency, and Quantiferon testing to rule out tuberculosis infection. Inhaled steroids should not be used, especially in the case of bacterial exacerbations (17).
Extrapulmonary comorbidities
Bronchial asthma.
Bronchial asthma is the most common pulmonary comorbidity in COPD. The prevalence of bronchial asthma in patients with COPD has been estimated at 27% in observational studies.
Bronchiectasis.
Bronchiectasis is associated with productive cough, bronchial infections, and abnormal bronchial dilatation. In patients with COPD, bronchiectasis is associated with a reduced body mass index, more advanced age, and increased sputum production and exacerbations.
Cardiovascular disease
Cardiovascular disease is common in COPD; in COSYCONET, Germany’s largest COPD cohort, 18% of patients had known heart failure, coronary artery disease, or a history of myocardial infarction (4), while data from the German DACCORD registry put the prevalence of cardiovascular disease including hypertension at more than 50%, increasing further with age (e13, e14). Because of the lung disease, functional interrelationships exist between the heart and the lungs that manifest in interactions between hemodynamics and respiratory mechanics. Classical cor pulmonale describes the increase in resistance in the pulmonary arterial pathway that results in right heart strain and is designated as group 3 precapillary pulmonary hypertension (19). The pathophysiology underlying this form of pulmonary hypertension is rarefaction of the pulmonary capillary vessels together with chronic vasoconstriction in the presence of chronic hypoxemia; for further details see the literature (e15). The most frequent indicators of additional pulmonary arterial hypertension are a particularly low diffusing capacity of the lungs – given the severity of the COPD – and low arterial partial pressure of carbon dioxide (reference value Paco2 < 35 mm Hg) as an expression of hyperventilation (e16, e17). In this case, further diagnostic steps should be initiated, but there is currently no clear recommendation for initiating treatment specifically aimed at pulmonary arterial hypertension (19). Conversely, remodeling processes in the left heart triggered by COPD are less well known, but studies have shown a relationship between reduced left ventricular mass and left ventricular ejection fraction on the one hand and increased airway obstruction and extent of emphysema on the other (e18, e19). In addition to vascular remodeling, lung hyperinflation leads to an increase in intrathoracic pressure, which can exceed venous pressure and lead to a fall in blood volume in both ventricles (e18). In addition, obstruction and lung hyperinflation result in higher ventricular wall stress (20). Both prospective randomized controlled trials and longitudinal observation data have shown beneficial effects of effective inhaled bronchodilators in terms of improved left ventricular filling (21, 22, e20). These results make it clear that treating COPD in accordance with guidelines not only improves respiratory outcomes, but also has an effect on left ventricular cardiac function.
Cardiovascular disease.
Cardiovascular disease is common in COPD; for example, in COSYCONET, Germany’s largest COPD cohort, 18% of patients had known heart failure, coronary artery disease, or a history of myocardial infarction.
Heart failure
Improving left ventricular function is of prime clinical importance, because heart failure in particular is seen in 32.8% of patients hospitalized for a COPD exacerbation (e21, e22). As shown by examples in the studies cited, heart failure is associated with increased risk of repeat exacerbations (hazard ratio [HR] 1.45 [e23]) and hospitalization (HR 1.22 [e24]) and thus of increased mortality (HR 1.75 [e24]). Diagnostic tests for heart failure should include determination of pro-BNP levels and echocardiography. As described, inhaled COPD therapy can have protective effects on the left heart without increasing the risk of cardiac complications. At the same time, the use of cardioselective ß-blockers in patients with COPD who also have heart failure is associated with reduced mortality (HR 0.63 [24]). The conclusion to be drawn from all this is that optimally treating both diseases according to current guidelines is of benefit to both.
GOLD group grading.
GOLD groups A–E, which are based on symptom and exacerbation history, essentially determine pharmacological treatment. Patients should also be offered nonpharmacological treatment options.
Coronary heart disease
Patients with COPD have an increased risk of coronary heart disease (CHD): a large meta-analysis found a hazard ratio of 1.24 (e25). CHD is also the most common cause of death in these patients (e26). Identifying CHD is especially difficult in patients with COPD because dyspnea is common to both conditions (4), and there are also gender differences in the pattern of symptoms. For example, men are more likely to report fatigue and women to describe chest tightness (e27). Therefore, if dyspnea persists despite appropriate treatment, the possibility of coronary artery disease should be considered. A particularly important fact appears to be that after exacerbations the risk of an acute coronary event increases considerably (HR 2.63) (25). The two randomized controlled trials ETHOS (budesonide/glycopyrrolate/formoterol fumarate in two doses of budesonide versus glycopyrrolate/formoterol fumarate) and IMPACT (fluticasone furoate/umeclidinium/ vilanterol versus umeclidinium/vilanterol) studied the effects of inhaled triple therapy in patients at increased risk of exacerbations and showed a reduction in all-cause mortality in the LABA/LAMA/ICS treatment arm compared with LABA/LAMA combination therapy without ICS (26). In ETHOS, the number needed to treat to prevent a death was 80 (26), and a reduction in serious cardiovascular events was also found (1.4% for triple versus 2.1% for LABA/LAMA). Thus, the studies confirmed previously observed beneficial effects of ICS in the form of reduced systemic inflammation and lung hyperinflation together with improved cardiac function (e28, e29, 27). In contrast, LABA/ICS combination therapy did not alter mortality in patients without prior exacerbations (SUMMIT Trial) (27). These results underscore the efficacy of adequate COPD treatment in patients with coronary artery disease.
Heart failure.
Improving left ventricular function is of prime clinical importance, as heart failure in particular is seen in 32.8% of patients hospitalized for a COPD exacerbation.
Atrial fibrillation
The prevalence of atrial fibrillation (AF) in the general population is 1% to 2% and increases with age (e30). In COPD, the risk increases to 4.4-fold (e31), and patients with COPD who have AF also have an increased mortality risk (HR 2.2) (e30). AF often occurs in association with exacerbations, but also with greater severity of COPD. The reason for this, apart from underlying cardiovascular disease and cardiac mechanics that have been altered by airway obstruction, may be excessive, irregular use of short-acting anticholinergics (SAMA) or ß-agonists (SABA). It is important, therefore, always to ask about how often on-demand medication is being used, in order to identify both mild exacerbations and any potential adverse side effects.
Peripheral artery disease
Peripheral artery disease (PAD) is associated with reduced quality of life and exercise capacity; smoking is a risk factor (28). Data from the COSYCONET COPD cohort revealed a prevalence of PAD of approximately 9% in the clinical examination; this was higher than in a statistically adjusted control group without COPD (1.8%) (28). Patients with both COPD and PAD also have increased mortality (hazard ratio 1.4) (e32). Importantly, it appears that more than two-thirds of patients did not report having had this diagnosis before their clinical examination in the COSYCONET study. In view of this, a simple blood pressure measurement taken at two sites (ankle–brachial index < 0.9) would be a practicable screening method to detect PAD (28).
Metabolic disorders
The prevalence of diabetes mellitus in patients with stable COPD is around 15% to 17% (29, e14), which is higher than in the general population. Apart from any side effects of respiratory medication (especially oral glucocorticoids, but also inhaled ones [e33]), effects of systemic inflammation due to the underlying COPD (30) are under discussion as a possible cause. Recent observational studies showed that antidiabetic medication, especially metformin, was associated with a smaller loss of functional diffusing capacity in the lungs and less progression of pulmonary emphysema, presumably owing to its anti-inflammatory effect (29, e34, e35). Although hyperlipidemia is a risk factor for the development of cardiovascular comorbidities, interestingly, its presence does not appear to be associated with a worse outcome of COPD. To reduce the cardiovascular risk profile, treatment of concomitant hyperlipidemia should be based on the recommendations of the relevant professional associations (e36).
Renal insufficiency, hyperuricemia, and anemia
Renal insufficiency, hyperuricemia, and anemia are among the comorbidities easily detected by blood tests that have an impact on mortality (e37– e39). For renal insufficiency and hyperuricemia, the hazard ratio is 2.3 in each case (e40, e41); for anemia it is 1.31 (e42). Anemia appears to act synergistically with low oxygen saturation and hence low blood oxygen levels (34, e43). According to currently available data, none of these three conditions is influenced by COPD-specific therapy, and their treatment should follow guidelines based on their etiology.
Psychiatric and neurological disorders
Peripheral artery disease.
Data from the COSYCONET COPD cohort showed a prevalence of PAD of about 9%. Patients with both COPD and PAD have an increased mortality risk.
Anxiety disorders, depression, and dementia syndromes are among the common comorbidities seen in COPD. In advanced COPD, the overall prevalence of these three disorders can be as high as 20% to 25% and increases with severity of COPD (e44, 35, 36). These disorders are all associated with increased exacerbation rates and reduced functional status (e44, 35, 36). Mortality risk also increases: for example, for depression as a comorbidity the relative risk is 1.83, and for anxiety disorder it is 1.27 (e45). Because treatment of COPD on an outpatient basis requires a high degree of self-management, treatment planning should take account of patients’ individual cognitive and mental ability to understand and carry out their treatments. All three types of disorder can be relatively easily assessed using established questionnaires. Examples include the COPD Anxiety Questionnaire (CAF), Dementia Detection Test (DemTect), and the Patient Health Questionnaire-9 (PHQ-9) for depression. When using the last, it should be remembered that COPD symptoms can be misinterpreted as symptoms of depression (37).
For treating psychiatric disorders, in addition to behavioral therapy, COPD-specific rehabilitation programs (e46) and pharmacological treatment may be useful. In terms of psychiatric medication, potential drug interactions with theophylline (which still is prescribed, against recommendations) and roflumilast should be borne in mind. In addition, tricyclic antidepressants may potentiate the adverse cardiovascular effects of ß-agonists and anticholinergics, but their use in patients on COPD inhaled therapy is not regarded as contraindicated (e47).
Obstructive sleep apnea
Patients should always be asked about sleep disorders, snoring, and daytime sleepiness as part of their COPD assessment. Obstructive sleep apnea (OSA) occurs in 8% of patients (e14, e48). It is readily treatable, e.g., with continuous positive airway pressure (CPAP) therapy, and is regarded as relevant to the prognosis because treatment reduces the risk of cardiovascular events. Conversely, there is evidence that the use of benzodiazepines is associated with respiratory depression and also with increased rates of pneumonia and acute exacerbations (e49).
Osteoporosis
Psychiatric and neurological disorders.
Anxiety disorders, depression, and dementia syndromes are among the common comorbidities seen in COPD. In advanced COPD, the overall prevalence of these three disorders can be as high as 20% to 25% and increases with severity of COPD.
Diabetes mellitus.
The prevalence of diabetes mellitus in patients with stable COPD is around 15% to 17%. Apart from any side effects of respiratory medication, effects of systemic inflammation due to the underlying COPD are under discussion as a possible cause.
Patients with COPD have a 2.8-fold increased risk of osteoporosis (e50). The prevalence of osteoporosis in those whose COPD is stable is around 7% to 15% (e14, 38); COSYCONET reports it affecting 24% of patients in GOLD group D (38). The causes underlying its association with COPD exacerbations are systemic inflammation (30), increasing physical inactivity with increasing severity of COPD, and also COPD-specific treatment itself. For example, the risk of manifest osteoporosis rises with the use of oral glucocorticoids (e51), and rises further with inhaled steroids in a dose-dependent manner (e52). Vitamin D deficiency is particularly common in patients with severe exacerbations (36%) (e53). In addition, there is an association with spirometric grade: for example, mean vitamin D levels were 33% lower in patients with GOLD grade 4 disease than in healthy smokers (e54). Vitamin D also has an immunomodulatory effect on the innate and adaptive immune systems, so patients with severe COPD exacerbations should have their vitamin D levels measured (e4), in addition to bone density measurements in those with an increased risk of osteoporosis. COPD patients with osteoporosis also benefit from rehabilitation therapy to improve their physical activity (39). In addition, vitamin D and calcium deficiency should be primarily compensated for by nutrition if possible, and antiresorptive therapy, e.g., with bisphosphonates, should be given if necessary (e53, e55, e56).
Lung cancer
Cigarette smoking is the most important shared risk factor in the development of COPD and lung cancer. It is therefore not surprising that a significant proportion of patients with COPD develop lung cancer, with an age-dependent prevalence between 2% and 25% (e57 - e60, e14). This is of high prognostic significance both for the course of the COPD and for the treatment for the lung cancer. For instance, in a patient with impaired lung function, the risks attaching to invasive diagnostic examinations go up, and options for treatment in terms of surgery and radiotherapy are quite limited. Since the National Lung Screening Study showed that lung cancer screening with low-dose CT reduced cancer mortality by 20% (40), annual screening has been introduced in the USA. The screening recommendation there applies to current smokers and ex-smokers who have stopped within the past 15 years and have a smoking history of at least 30 pack-years; furthermore, screening is recommended only for persons between 50 and 80 years of age (8). Lung cancer screening is also likely to be introduced in Germany in the future. The increasing use of CT to enable phenotyping of COPD and, conversely, its use in screening studies of smokers and ex-smokers opens up the prospects of earlier detection of both these diseases, which hopefully should benefit the prognosis of both.
Lung cancer screening.
Increasing use of CT to enable phenotyping of COPD and, conversely, its possible future use in screening for lung cancer in smokers and ex-smokers opens up the prospect of earlier detection of both these diseases.
Supplementary Material
Case study
A 74-year-old ex-smoker (34 pack-years) with GOLD group B, GOLD grade 2 COPD diagnosed in 2018 presented for follow-up. History included a moderate exacerbation 2 years previously requiring a short course of oral prednisolone. CT showed diffuse emphysema and bronchiectasis in all lobes, but since starting dual bronchodilator therapy with LABA/LAMA the patient reported no sputum expectoration or repeat exacerbations. Other known diagnoses included obstructive sleep apnea with CPAP therapy, peripheral artery disease, and chronic renal insufficiency most likely due to underlying arterial hypertension. The patient reported a marked increase in dyspnea, chest pain, and new-onset leg edema was also evident. Pulmonary function testing gave stable findings showing no significant change over 2 years (FEV1 61% of reference, RV/TLC 126% of reference, Pao2 68 mm Hg, Paco2 39 mm Hg). Because of the reported chest pain and dyspnea symptoms, blood chemistry studies were performed and showed troponin T (hs) 0.320 ng/mL, creatinine 1.9 mg/dL, and d-dimer < 0.5 mg/dL. ECG gave no evidence of severe arrhythmias or repolarization abnormalities. However, echocardi-ography demonstrated severe aortic valve stenosis with values of dPmax 80 mm Hg, dPmean 51 mm Hg, valve orifice area 0.8 cm² with left ventricular hypertrophy and preserved pump function. With an initial diagnosis of symptomatic severe aortic valve stenosis, the patient was admitted to hospital immediately for cardiac catheterization and treatment planning for the stenotic aortic valve. Later, right coronary artery stenosis was found that did not require intervention, and transfemoral implantation of an Edwards Sapien prosthetic aortic valve was carried out. The patient had an uneventful postinterventional course; both the dyspnea and the chest pain rapidly settled, and he was then discharged for cardiac rehabilitation.
Figure.
Chronic obstructive pulmonary disease (German Respiratory League/German Respiratory Society): Interpretation of spirometry findings based on the recommendations of the German Respiratory League (e5). Suspected chronic obstructive pulmonary disease is defined by a decrease in the age-related Tiffeneau index (FEV1/FVC) to values below the 5th percentile (Z-score < –1.645). Clinical assessment is based on the mean FEV1% reference value. GLI values are preferred to the previously used reference values, some of which are now 40 years old, because they describe the normal population much better and ensure better comparability.
COPD, chronic obstructive pulmonary disease; DGP, German Respiratory Society; FEV1, 1-second capacity; GLI, Global Lung Function Initiative; LLN, lower limit of normal
Table 2. Five-year all-cause mortality in patients with COPD.
| GOLD grade | GOLD group | |||
| A | B | C | D | |
| Spirometry 1 | 3.4% | 9.8% | 16.2% | 5.5% |
| Spirometry 2 | 7% | 14.9% | 8.3% | 22.1% |
| Spirometry 3 | 13% | 23.7% | 17.7% | 32.6% |
| Spirometry 4 | 16.7% | 39.1% | 29.5% | 46% |
Five-year all-cause mortality in patients with COPD, based on spirometric GOLD grades 1–4 and the GOLD groups of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2019 ABCD classification.
Adapted from García Castillo E, Alonso Pérez T, Ancochea J, et al. Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2015 and GOLD 2019 staging: a pooled analysis of individual patient data. By kind permission, ERS 2023: ERJ Open Research 6 (4) 00253–2020; DOI: 10.1183/23120541.00253–2020 Published 2 November 2020
Table 3. Pharmacological treatment for chronic obstructive lung disease*.
| GOLD group | Symptoms more important | Exacerbations more important | Initial therapy | Follow-up therapy | |||
| Mild to moderate symptoms | Moderate to severe symptoms | Symptoms more important | Exacerbations more important | ||||
|
mMRC < 2
cat < 10 |
mMRC ≥
2 CAT ≥ 10 |
0–1 | ≥ 2 or 1 severe (hospital admission) | ||||
| Group A | x | x | 1 Bronchodilator | LABA + LAMA | LABA + LAMA | ||
| Group B | x | x | LABA or LAMA or LABA + LAMA |
LABA + LAMA | LABA + LAMA | ||
| Group E(formerly C and D) | x | x | x | LAMA or LABA + LAMA or LABA + ICS*1 LABA + LAMA + ICS*1 |
LABA + LAMALABA + LAMA + ICS Symptoms have another cause? Device appropriate for the patient? |
LABA + LAMA + ICS*2 roflumilast*3 azithromycin*4 |
|
*In the new GOLD recommendation, GOLD groups ABE are determined on the basis of symptom and exacerbation history (e4). This classification merges the former GOLD groups C and D based on exacerbations alone to form a group designated E. The main aim of the GOLD classifications has been to provide an efficient but also practical way to assess disease progression and the treatment goals of reduced symptom burden, improved quality of life, and avoidance of exacerbations. Initial therapy and follow-up therapy are carried out based on GOLD group or the leading problem in the individual case (in terms of symptoms or exacerbations). The fact that treatment is based primarily on symptom and exacerbation history underscores the central importance of history-taking in patient care. The table is based on the recommendations of the German National Clinical Guideline for COPD (6) and the international GOLD recommendations of the current GOLD Report 2023 (e4). The current GOLD recommendations for initial therapy are shown in bold; according to these, initial treatment in severely symptomatic patients should be with a LABA/LAMA combination, and initial therapy in patients with exacerbations and a peripheral blood eosinophil count > 300 cells/µL should be with a LABA/LAMA/ICS combination.
CAT, COPD Assessment Test; ICS, inhaled steroid; LABA, long-acting ß-agonist; LAMA, anticholinergic; mMRC, modified Medical Research Council Dyspnoea Scale *1 If peripheral blood eosinophilia ≥ 300 cells/µL, *2 If peripheral blood eosinophilia ≥ 100 cells/µL, *3 If peripheral blood eosinophilia < 100 cells/µL, FEV1 < 50% of reference value, and chronic bronchitis, *4 In ex-smokers, off-label use – not currently recommended in German National Clinical Guideline for COPD but included in the GOLD recommendations.
BOX. Nonpharmacological treatment of COPD.
-
Smoking cessation*1
Advice should be offered to all current smokers.
Behavioral therapy and pharmacological support are available.
Use of digital health apps may be helpful.
-
Immunizations*2
Ensure adequate immunization based on the recommendations of the Standing Committee on Vaccination (STIKO) (especially COVID-19, pneumococcus, influenza, pertussis).
Influenza: Annual immunization in the fall with an inactivated quadrivalent vaccine with the current antigen combination as recommended by the WHO.
Pneumococcus: Immunization with the 23-valent polysaccharide vaccine (PPSV23); if necessary, repeat vaccinations with PPSV23 at intervals of at least 6 years.
Pertussis: Adults should, at the time of their next due Td vaccination, receive the Tdap combination vaccine instead.
COVID-19: According to current STIKO recommendations, basic immunity should be provided by three antigen contacts (vaccination or infection, but at least 2 doses of vaccine), and in high-risk patients, such as those with COPD, further booster vaccinations should be given – usually after an interval of ≥12 months since the last antigen contact, and preferably in the fall.
-
Physical exercise*3
All patients, regardless of age and severity of illness, benefit from physical exercise.
Exercise should be adapted to the individual’s capacity.
-
Long-term oxygen therapy*4
If Spo2 ≤ 92%, assess whether long-term oxygen therapy (LTOT) is indicated
LTOT is indicated in patients with repeated resting hypoxemia Po2 ≤ 55 mm Hg, or Po2 56–60 mm Hg with concomitant polycythemia or cor pulmonale; hypercapnia should also be taken into consideration
LTOT is indicated in patients with stress hypoxemia (e.g., 6-minute walk test)
Current smoking is not an absolute contraindication, but patients should be encouraged to quit smoking, and potential hazards such as burns and explosion risk should be pointed out.
Noninvasive ventilation*5
-
Indicated for patients with:
Chronic daytime hypercapnia Paco2 ≥ 50 mm Hg, and/or
Nocturnal hypercapnia Paco2 ≥ 55 mm Hg, and/or
Mild daytime hypercapnia 46–50 mm Hg and rise in transcutaneous Pco2 values by ≥ 10 mm Hg during sleep.
Following acute respiratory acidosis requiring ventilation, if hypercapnia (Paco2 > 53 mm Hg) is still present at least 14 days after acute ventilation has ceased.
-
Lung volume reduction*6
Review options for lung volume reduction in patients with severe hyperinflation (residual volume > 175% of reference) after all conservative treatments have failed.
Evaluation of the next step should be by a multidisciplinary emphysema team including both surgeons and interventional bronchoscopists; this is essential because of the complexity involved.
Lung transplantation*7
-
A transplant may be offered to selected patients with end-stage COPD and limited life expectancy in cases where:
Conservative treatment options have been used up.
The patient has abstained from smoking for > 12 months.
The patient shows enough physical and psychological potential for rehabilitation.
Active malignant disease and other severe organ dysfunction have been ruled out.
High-dose long-term therapy with oral glucocorticoids is not given.
The patient is no older than about 65 years.
*1 Because smoking is by far the strongest risk factor for the development and progression of COPD, smokers should repeatedly be offered help to quit smoking (6).
*2 To avoid infection-related exacerbations, it is desirable to ensure that patients are adequately immunized according to the recommendations of the Standing Committee on Vaccination (10).
*3 The current clinical guideline also emphasizes the positive effects of physical exercise to increase activity and maintain ability to carry out activities of daily living.
*4 The criteria for initiating long-term oxygen therapy and noninvasive ventilation are based on the relevant guidelines (11, 12) and the National Clinical Guideline on COPD.
*5 For critically ill patients with respiratory insufficiency type 1 (hypoxemia) or type 2 (hypercapnia), long-term oxygen therapy and/or noninvasive ventilation should be initiated.
*6 In addition, after all conservative measures have failed, patients with massive pulmonary hyperinflation should be reviewed for consideration of possible lung volume reduction therapy (e4).
*7 Lung transplantation may also be considered in selected cases.
Further information on CME.
Access to the CME certification program is only over the internet: cme.aerzteblatt.de. The closing date for submissions is 22 June 2024. Submissions by letter, email or fax cannot be accepted.
Once a new CME module comes online, it remains available for 12 months. The results can be accessed 4 weeks after you start work on a module. Please note the closing date for each module, which can be found at cme.aerzteblatt.de.
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN), which is found on the CME card (8027XXXXXXXXXXX). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or else entered in “Meine Daten,” and the participant must agree to communication of the results.
CME Credit for this unit can be obtained via cme.aerzteblatt.de until 22 June 2024. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is the annual prevalence of COPD in Germany, according to figures from the Robert Koch Institute?
3.8%
4.8%
5.8%
6.8%
7.8%
Question 2
Which of the following is a common psychiatric comorbidity in patients with COPD?
Anxiety disorder
Obsessive-compulsive disorder
Bipolar disorder
Autism
Lack of impulse control
Question 3
Which clinical sign is an indication that a patient with COPD may also have bronchial asthma?
Peak expiratory flow does not show a circadian rhythm.
Change in 1-second capacity < 5%/predicted after inhalation of a bronchodilator.
Very good clinical and pulmonary functional response to inhaled steroids.
Eosinophils < 200 cells/µL in peripheral blood.
Hypertension > 160/90 mm Hg
Question 4
What is bronchiectasis commonly associated with in patients with COPD?
Restless leg syndrome
Reduced body mass index
Eating disorders
Hypertension
Atrial fibrillation
Question 5
Psychiatric disorders are common in patients with COPD and affect their overall prognosis. Several questionnaires are widely used to identify the presence of these disorders. Which of these is appropriate for identifying depression?
SF-36
Patient Health Questionnaire-9 (PHQ-9)
Mini Mental State Examination
COPD Assessment Test
Heidelberg Short Questionnaire
Question 6
Patients with COPD and severe exacerbations are often found to be deficient in which of the following vitamins?
Vitamin B6
Vitamin C
Vitamin A
Vitamin D
Vitamin B12
Question 7
The recommendation in the USA for annual lung cancer screening by chest CT applies to which of the following groups?
Current or ex-smokers (< 15 years) with a history of at least 30 pack-years
Current smokers over the age of 40
Ex-smokers between 50 and 80 years of age irrespective of their number of pack-years
Ex-smokers between 50 and 80 years of age who gave up smoking at least 30 years ago
Current smokers with a history of at least 10 pack-years
Question 8
Which genetic factor can lead to COPD?
Plasminogen activator inhibitor type 1 deficiency
α1-Antitrypsin deficiency
α2-Antiplasmin deficiency
α2-Macroglobulin deficiency
α1-Antichymotrypsin deficiency
Question 9
Which of the following is used for first-line therapy in patients with GOLD group A COPD?
Theophylline
Roflumilast
A bronchodilator
An inhaled steroid
Supportive oxygen therapy
Question 10
Many patients with COPD also have cardiovascular disease. The risk of a cardiovascular event increases particularly after an exacerbation. Which of the following statements about COPD therapy and its impact on cardiac function or overall survival is true?
In patients with numerous exacerbations, dual bronchodilator therapy with LABA/LAMA increases all-cause mortality.
The combination of LABA/LAMA with oral ß-blockers to treat heart failure is contraindicated.
The use of LABA/LAMA/ICS combination therapy reduces all-cause mortality in patients with a history of severe exacerbations.
Frequent use of short-acting ß-agonists reduces the incidence of arrhythmias.
Patients with frequent exacerbations should be started on treatment for pulmonary arterial hypertension.
►Access is possible only via: cme.aerzteblatt.de
Footnotes
Conflict of interest statement
KK has received consultancy fees and fees for presentations from Astra Zeneca, GSK, and Chiesi.
TW has received research grants from GSK and Astra Zeneca. He has received consultancy fees from Astra Zeneca, Berlin-Chemie, Chiesi, GSK, Novartis, and Böhringer-Ingelheim.
The other authors declare that no conflict of interest exists.
References
- 1.Steppuhn H, Kuhnert R, Scheidt-Nave C. 12-month prevalence of known chronic obstructive pulmonary disease (COPD) in Germany Robert Koch-Institut, Epidemiologie und Gesundheitsberichterstattung. https://edoc.rki.de/handle/176904/2821 (last accessed on 5 June 2023) [Google Scholar]
- 2.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 3.Rabe KF, Wedzicha JA. Controversies in treatment of chronic obstructive pulmonary disease. Lancet. 2011;378:1038–1047. doi: 10.1016/S0140-6736(11)61295-6. [DOI] [PubMed] [Google Scholar]
- 4.Alter P, Mayerhofer BA, Kahnert K, et al. Prevalence of cardiac comorbidities, and their underdetection and contribution to exertional symptoms in COPD: results from the COSYCONET cohort. Int J Chron Obstruct Pulmon Dis. 2019;14:2163–2172. doi: 10.2147/COPD.S209343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2022. https://goldcopd.org/2022-gold-reports/ (last accessed on 5 June 2023) [Google Scholar]
- 6.Nationale VersorgungsLeitlinie COPD (2021) / www.leitlinien.de/themen/copd (last accessed on 5 June 2023) 2019 [Google Scholar]
- 7.National Institut of Health. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. www.nice.org.uk/guidance/ng115 (last accessed on 6 June 2023) [Google Scholar]
- 8.Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet. 2022;400:921–972. doi: 10.1016/S0140-6736(22)01273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lungfunction 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joean O, Welte T. Vaccination and modern management of chronic obstructive pulmonary disease - a narrative review. Expert Rev Respir Med. 2022;16:605–614. doi: 10.1080/17476348.2022.2092099. [DOI] [PubMed] [Google Scholar]
- 11.AWMF. Leitlinie zur Langzeit-Sauerstofftherapie. https://register.awmf.org/assets/guidelines/020-002l_S2k_Langzeit_Sauerstofftherapie_2020-08.pdf (last accessed on 5 June 2023) 2020 [Google Scholar]
- 12.Windisch W, Dreher M, Geiseler J, et al. [Guidelines for non-invasive and invasive home mechanical ventilation for treatment of chronic respiratory failure - update 2017] Pneumologie. 2017;71:722–795. doi: 10.1055/s-0043-118040. [DOI] [PubMed] [Google Scholar]
- 13.Miravitlles M, Guerrero T, Mayordomo C, Sanchez-Agudo L, Nicolau F, Segu JL. Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000;67:495–501. doi: 10.1159/000067462. [DOI] [PubMed] [Google Scholar]
- 14.Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147:1219–1226. doi: 10.1378/chest.14-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trudzinski FC, Jorres RA, Alter P, et al. Sex-specific associations of comorbidome and pulmorbidome with mortality in chronic obstructive pulmonary disease: results from COSYCONET. Sci Rep. 2022;12 doi: 10.1038/s41598-022-12828-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-García MA, de la Rosa Carrillo D, Soler-Cataluna JJ, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:823–831. doi: 10.1164/rccm.201208-1518OC. [DOI] [PubMed] [Google Scholar]
- 17.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017 doi: 10.1183/13993003.00629-2017. [DOI] [PubMed] [Google Scholar]
- 18.Kahnert K, Jorres RA, Kauczor HU, et al. Relationship between clinical and radiological signs of bronchiectasis in COPD patients: results from COSYCONET. Respir Med. 2020;172 doi: 10.1016/j.rmed.2020.106117. 106117. [DOI] [PubMed] [Google Scholar]
- 19.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 20.Alter P, Jörres RA, Watz H, et al. Left ventricular volume and wall stress are linked to lung function impairment in COPD. Int J Cardiol. 2018;261:172–178. doi: 10.1016/j.ijcard.2018.02.074. [DOI] [PubMed] [Google Scholar]
- 21.Hohlfeld JM, Vogel-Claussen J, Biller H, et al. Effect of lung deflation with indacaterol plus glycopyrronium on ventricular filling in patients with hyperinflation and COPD (CLAIM): a double-blind, randomised, crossover, placebo-controlled, single-centre trial. Lancet Respir Med. 2018;6:368–378. doi: 10.1016/S2213-2600(18)30054-7. [DOI] [PubMed] [Google Scholar]
- 22.Kellerer C, Kahnert K, Trudzinski FC, et al. COPD maintenance medication is linked to left atrial size: results from the COSYCONET cohort. Respir Med. 2021;185 doi: 10.1016/j.rmed.2021.106461. 106461. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–922. doi: 10.2147/COPD.S130482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coiro S, Girerd N, Rossignol P, et al. Association of beta-blocker treatment with mortality following myocardial infarction in patients with chronic obstructive pulmonary disease and heart failure or left ventricular dysfunction: a propensity matched-cohort analysis from the High-Risk Myocardial Infarction Database Initiative. Eur J Heart Fail. 2017;19:271–279. doi: 10.1002/ejhf.647. [DOI] [PubMed] [Google Scholar]
- 25.Campo G, Pavasini R, Malagu M, et al. Chronic obstructive pulmonary disease and ischemic heart disease comorbidity: overview of mechanisms and clinical management. Cardiovasc Drugs Ther. 2015;29:147–157. doi: 10.1007/s10557-014-6569-y. [DOI] [PubMed] [Google Scholar]
- 26.Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of Budesonide/Glycopyrrolate/Formoterol for chronic obstructive pulmonary disease A randomized, double-blind, multicenter, parallel-group study. Am J Respir Crit Care Med. 2021;203:553–564. doi: 10.1164/rccm.202006-2618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817–1826. doi: 10.1016/S0140-6736(16)30069-1. [DOI] [PubMed] [Google Scholar]
- 28.Houben-Wilke S, Jorres RA, Bals R, et al. Peripheral artery disease and its clinical relevance in patients with Chronic Obstructive Pulmonary Disease in the COPD and Systemic Consequences-Comorbidities Network Study. Am J Respir Crit Care Med. 2017;195:189–197. doi: 10.1164/rccm.201602-0354OC. [DOI] [PubMed] [Google Scholar]
- 29.Kahnert K, Lucke T, Biertz F, et al. Transfer factor for carbon monoxide in patients with COPD and diabetes: results from the German COSYCONET cohort. Respir Res. 2017;18 doi: 10.1186/s12931-016-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 31.Kahnert K, Lucke T, Huber RM, et al. Relationship of hyperlipidemia to comorbidities and lung function in COPD: results of the COSYCONET cohort. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177501. e0177501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raymakers AJN, Sadatsafavi M, Sin DD, De Vera MA, Lynd LD. The impact of statin drug use on all-cause mortality in patients with COPD: a population-based cohort study. Chest. 2017;152:486–493. doi: 10.1016/j.chest.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Zhang Y, Li CW, Jones P, Wang C, Fan Y. Effect of statins on COPD: a meta-analysis of randomized controlled trials. Chest. 2017;152:1159–1168. doi: 10.1016/j.chest.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Trudzinski FC, Jorres RA, Alter P, et al. Associations of oxygenated hemoglobin with disease burden and prognosis in stable COPD: results from COSYCONET. Sci Rep. 2020;10 doi: 10.1038/s41598-020-67197-x. 10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144:766–777. doi: 10.1378/chest.12-1911. [DOI] [PubMed] [Google Scholar]
- 36.Almagro P, Cabrera FJ, Diez J, et al. Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD: the EPOC en Servicios de medicina interna (ESMI) study. Chest. 2012;142:1126–1133. doi: 10.1378/chest.11-2413. [DOI] [PubMed] [Google Scholar]
- 37.von Siemens SM, Jorres RA, Behr J, et al. Effect of COPD severity and comorbidities on the result of the PHQ-9 tool for the diagnosis of depression: results from the COSYCONET cohort study. Respir Res. 2019;20 doi: 10.1186/s12931-019-0997-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahnert K, Jörres RA, Lucke T, et al. Lower prevalence of osteoporosis in patients with COPD taking anti-inflammatory compounds for the treatment of diabetes: results from COSYCONET. Int J Chron Obstruct Pulmon Dis. 2021;16:3189–3199. doi: 10.2147/COPD.S335029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Gao H, Zhao L, Wang J. Osteoporosis in COPD patients: risk factors and pulmonary rehabilitation. Clin Respir J. 2022;16:487–496. doi: 10.1111/crj.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aberle DR, Adams AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (last accessed on 11 October 2022) [Google Scholar]
- E2.MacNee W. Systemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary disease. Ann Med. 2013;45:291–300. doi: 10.3109/07853890.2012.732703. [DOI] [PubMed] [Google Scholar]
- E3.Karch A, Vogelmeier C, Welte T, et al. The German COPD cohort COSYCONET: aims, methods and descriptive analysis of the study population at baseline. Respir Med. 2016;114:27–37. doi: 10.1016/j.rmed.2016.03.008. [DOI] [PubMed] [Google Scholar]
- E4.Global strategy for prevention, diagnosis and managment of COPD. Report. https://goldcopd.org/2023-gold-report-2/ (last accessed on 5 June 2023) 2023 [Google Scholar]
- E5.Criee CP, Baur X, Berdel D, et al. [Standardization of spirometry: 2015 update Published by German Atemwegsliga, German Respiratory Society and German Society of Occupational and Environmental Medicine] Pneumologie. 2015;69:147–164. doi: 10.1055/s-0034-1391345. [DOI] [PubMed] [Google Scholar]
- E6.Spece LJ, Epler EM, Donovan LM, et al. Role of comorbidities in treatment and outcomes after chronic obstructive pulmonary disease exacerbations. Ann Am Thorac Soc. 2018;15:1033–1038. doi: 10.1513/AnnalsATS.201804-255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147:1219–1226. doi: 10.1378/chest.14-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E8.Sharafkhaneh A, Petersen NJ, Yu HJ, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125–132. doi: 10.2147/copd.s8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Alshabanat A, Zafari Z, Albanyan O, Dairi M, FitzGerald JM. Asthma and COPD Overlap Syndrome (ACOS): A Systematic Review and Meta Analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136065. e0136065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Martinez-Garcia MA, Miravitlles M. Bronchiectasis in COPD patients: more than a comorbidity? Int J Chron Obstruct Pulmon Dis. 2017;12:1401–1411. doi: 10.2147/COPD.S132961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:400–407. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- E12.Tulek B, Kivrak AS, Ozbek S, Kanat F, Suerdem M. Phenotyping of chronic obstructive pulmonary disease using the modified Bhalla scoring system for high-resolution computed tomography. Can Respir J. 2013;20:91–96. doi: 10.1155/2013/727523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Kardos P, Vogelmeier C, Buhl R, Criee CP, Worth H. The Prospective Non-Interventional DACCORD Study in the National COPD Registry in Germany: design and methods. BMC Pulm Med. 2015;15 2. doi: 10.1186/1471-2466-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E14.Worth H, Buhl R, Criee CP, Kardos P, Mailander C, Vogelmeier C. The ‚real-life‘ COPD patient in Germany: the DACCORD study. Respir Med. 2016;111:64–71. doi: 10.1016/j.rmed.2015.12.010. [DOI] [PubMed] [Google Scholar]
- E15.McGettrick M, Peacock A. Group 3 pulmonary hypertension: challenges and opportunities. Glob Cardiol Sci Pract. 2020;2020 doi: 10.21542/gcsp.2020.6. e202006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E16.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127:1531–1536. doi: 10.1378/chest.127.5.1531. [DOI] [PubMed] [Google Scholar]
- E17.Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189–194. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- E18.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E19.Smith BM, Prince MR, Hoffman EA, et al. Impaired left ventricular filling in COPD and emphysema: is it the heart or the lungs? The Multi-Ethnic Study of Atherosclerosis COPD Study. Chest. 2013;144:1143–1151. doi: 10.1378/chest.13-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E20.Stone IS, Barnes NC, James WY, et al. Lung deflation and cardiovascular structure and function in chronic obstructive pulmonary disease A randomized controlled trial. Am J Respir Crit Care Med. 2016;193:717–726. doi: 10.1164/rccm.201508-1647OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E21.Almagro P, Cabrera FJ, Diez J, et al. Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD: the EPOC en Servicios de medicina interna (ESMI) study. Chest. 2012;142:1126–1133. doi: 10.1378/chest.11-2413. [DOI] [PubMed] [Google Scholar]
- E22.Fonarow GC, Heywood JT, Heidenreich PA, et al. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2007;153:1021–1028. doi: 10.1016/j.ahj.2007.03.012. [DOI] [PubMed] [Google Scholar]
- E23.Axson EL, Bottle A, Cowie MR, Quint JK. Relationship between heart failure and the risk of acute exacerbation of COPD. Thorax. 2021;76:807–814. doi: 10.1136/thoraxjnl-2020-216390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E24.Axson EL, Ragutheeswaran K, Sundaram V, et al. Hospitalisation and mortality in patients with comorbid COPD and heart failure: a systematic review and meta-analysis. Respir Res. 2020;21 doi: 10.1186/s12931-020-1312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E25.Wang JJ. Risk of coronary heart disease in people with chronic obstructive pulmonary disease: a meta-analysis. Int J Chron Obstruct Pulmon Dis. 2021;16:2939–2944. doi: 10.2147/COPD.S331505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E26.Putcha N, Drummond MB, Wise RA, Hansel NN. Comorbidities and chronic obstructive pulmonary disease: prevalence, influence on outcomes, and management. Semin Respir Crit Care Med. 2015;36:575–591. doi: 10.1055/s-0035-1556063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Trudzinski FC, Kellerer C, Jorres RA, et al. Gender-specific differences in COPD symptoms and their impact for the diagnosis of cardiac comorbidities. Clin Res Cardiol. 2021 doi: 10.1007/s00392-021-01915-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E28.Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons? Eur Respir Rev. 2018 doi: 10.1183/16000617.0057-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E29.Sin DD, Lacy P, York E, Man SF. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:760–765. doi: 10.1164/rccm.200404-543OC. [DOI] [PubMed] [Google Scholar]
- E30.Romiti GF, Corica B, Pipitone E, et al. Prevalence, management and impact of chronic obstructive pulmonary disease in atrial fibrillation: a systematic review and meta-analysis of 4,200,000 patients. Eur Heart J. 2021;42:3541–3554. doi: 10.1093/eurheartj/ehab453. [DOI] [PubMed] [Google Scholar]
- E31.Ye J, Yao P, Shi X, Yu X. A systematic literature review and meta-analysis on the impact of COPD on atrial fibrillation patient outcome. Heart Lung. 2022;51:67–74. doi: 10.1016/j.hrtlng.2021.09.001. [DOI] [PubMed] [Google Scholar]
- E32.Terzikhan N, Lahousse L, Verhamme KMC, et al. COPD is associated with an increased risk of peripheral artery disease and mortality. ERJ Open Res. 2018;4:00086–02018. doi: 10.1183/23120541.00086-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E33.Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010;123:1001–1006. doi: 10.1016/j.amjmed.2010.06.019. [DOI] [PubMed] [Google Scholar]
- E34.Kahnert K, Andreas S, Kellerer C, et al. Reduced decline of lung diffusing capacity in COPD patients with diabetes and metformin treatment. Sci Rep. 2022;12 doi: 10.1038/s41598-022-05276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E35.Polverino F, Wu TD, Rojas-Quintero J, et al. Metformin: experimental and clinical evidence for a potential role in emphysema treatment. Am J Respir Crit Care Med. 2021;204:651–666. doi: 10.1164/rccm.202012-4510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E36.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- E37.Kahnert K, Alter P, Welte T, et al. Uric acid, lung function, physical capacity and exacerbation frequency in patients with COPD: a multi-dimensional approach. Respir Res. 2018;19 doi: 10.1186/s12931-018-0815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E38.Cote C, Zilberberg MD, Mody SH, Dordelly LJ, Celli B. Haemoglobin level and its clinical impact in a cohort of patients with COPD. Eur Respir J. 2007;29:923–929. doi: 10.1183/09031936.00137106. [DOI] [PubMed] [Google Scholar]
- E39.Fabbian F, De Giorgi A, Manfredini F, et al. Impact of renal dysfunction on in-hospital mortality of patients with severe chronic obstructive pulmonary disease: a single-center Italian study. Int Urol Nephrol. 2016;48:1121–1127. doi: 10.1007/s11255-016-1272-5. [DOI] [PubMed] [Google Scholar]
- E40.Trudzinski FC, Alqudrah M, Omlor A, et al. Consequences of chronic kidney disease in chronic obstructive pulmonary disease. Respir Res. 2019;20 doi: 10.1186/s12931-019-1107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E41.Zhang X, Liu L, Liang R, Jin S. Hyperuricemia is a biomarker of early mortality in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:2519–2523. doi: 10.2147/COPD.S87202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E42.Park SC, Kim YS, Kang YA, et al. Hemoglobin and mortality in patients with COPD: a nationwide population-based cohort study. Int J Chron Obstruct Pulmon Dis. 2018;13:1599–1605. doi: 10.2147/COPD.S159249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E43.Hinke CF, Jörres RA, Alter P, et al. Prognostic value of oxygenated hemoglobin assessed during acute exacerbations of chronic pulmonary disease. Respiration. 2021;100:387–394. doi: 10.1159/000513440. [DOI] [PubMed] [Google Scholar]
- E44.Lecheler L, Richter M, Franzen DP, et al. The frequent and underrecognised co-occurrence of acute exacerbated COPD and depression warrants screening: a systematic review. Eur Respir Rev. 2017;26 doi: 10.1183/16000617.0026-2017. 170026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E45.Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144:766–777. doi: 10.1378/chest.12-1911. [DOI] [PubMed] [Google Scholar]
- E46.Tselebis A, Bratis D, Pachi A, et al. A pulmonary rehabilitation program reduces levels of anxiety and depression in COPD patients. Multidiscip Respir Med. 2013;8 doi: 10.1186/2049-6958-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E47.Yohannes AM, Alexopoulos GS. Pharmacological treatment of depression in older patients with chronic obstructive pulmonary disease: impact on the course of the disease and health outcomes. Drugs Aging. 2014;31:483–492. doi: 10.1007/s40266-014-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E48.Kahnert K, Alter P, Young D, et al. The revised GOLD 2017 COPD categorization in relation to comorbidities. Respir Med. 2018;134:79–85. doi: 10.1016/j.rmed.2017.12.003. [DOI] [PubMed] [Google Scholar]
- E49.Chung WS, Lai CY, Lin CL, Kao CH. Adverse respiratory events associated with hypnotics use in patients of chronic obstructive pulmonary disease: a population-based case-control study. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000001110. e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E50.Chen YW, Ramsook AH, Coxson HO, Bon J, Reid WD. Prevalence and risk factors for osteoporosis in individuals with COPD: a systematic review and meta-analysis. Chest. 2019;156:1092–1110. doi: 10.1016/j.chest.2019.06.036. [DOI] [PubMed] [Google Scholar]
- E51.Amiche MA, Albaum JM, Tadrous M, et al. Fracture risk in oral glucocorticoid users: a Bayesian meta-regression leveraging control arms of osteoporosis clinical trials. Osteoporos Int. 2016;27:1709–1718. doi: 10.1007/s00198-015-3455-9. [DOI] [PubMed] [Google Scholar]
- E52.Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66:699–708. doi: 10.1136/thx.2011.160028. [DOI] [PubMed] [Google Scholar]
- E53.Malinovschi A, Masoero M, Bellocchia M, et al. Severe vitamin D deficiency is associated with frequent exacerbations and hospitalization in COPD patients. Respir Res. 2014;15 doi: 10.1186/s12931-014-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E54.Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- E55.Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10 doi: 10.1002/14651858.CD001347.pub2. CD001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E56.Prophylaxe, Diagnostik und Therapie der OSTEOPOROSE. https://register.awmf.org/assets/guidelines/183-001l_S3_Osteoporose-Prophylaxe-Diagnostik-Therapie_2019-02.pdf (last accessed on 5 June 2023) [Google Scholar]
- E57.Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax. 2005;60:570–575. doi: 10.1136/thx.2004.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E58.Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: data From the First National Health and Nutrition Examination Survey follow-up. Arch Intern Med. 2003;163:1475–1480. doi: 10.1001/archinte.163.12.1475. [DOI] [PubMed] [Google Scholar]
- E59.Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E60.Fabbri LM, Luppi F, Beghe B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31:204–212. doi: 10.1183/09031936.00114307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Case study
A 74-year-old ex-smoker (34 pack-years) with GOLD group B, GOLD grade 2 COPD diagnosed in 2018 presented for follow-up. History included a moderate exacerbation 2 years previously requiring a short course of oral prednisolone. CT showed diffuse emphysema and bronchiectasis in all lobes, but since starting dual bronchodilator therapy with LABA/LAMA the patient reported no sputum expectoration or repeat exacerbations. Other known diagnoses included obstructive sleep apnea with CPAP therapy, peripheral artery disease, and chronic renal insufficiency most likely due to underlying arterial hypertension. The patient reported a marked increase in dyspnea, chest pain, and new-onset leg edema was also evident. Pulmonary function testing gave stable findings showing no significant change over 2 years (FEV1 61% of reference, RV/TLC 126% of reference, Pao2 68 mm Hg, Paco2 39 mm Hg). Because of the reported chest pain and dyspnea symptoms, blood chemistry studies were performed and showed troponin T (hs) 0.320 ng/mL, creatinine 1.9 mg/dL, and d-dimer < 0.5 mg/dL. ECG gave no evidence of severe arrhythmias or repolarization abnormalities. However, echocardi-ography demonstrated severe aortic valve stenosis with values of dPmax 80 mm Hg, dPmean 51 mm Hg, valve orifice area 0.8 cm² with left ventricular hypertrophy and preserved pump function. With an initial diagnosis of symptomatic severe aortic valve stenosis, the patient was admitted to hospital immediately for cardiac catheterization and treatment planning for the stenotic aortic valve. Later, right coronary artery stenosis was found that did not require intervention, and transfemoral implantation of an Edwards Sapien prosthetic aortic valve was carried out. The patient had an uneventful postinterventional course; both the dyspnea and the chest pain rapidly settled, and he was then discharged for cardiac rehabilitation.