Abstract
Targeting the HER2 oncogene represents one of the greatest advances in the treatment of breast cancer. HER2 is one member of the ERBB-receptor family, which includes EGFR (HER1), HER3 and HER4. In the presence or absence of underling genomic aberrations such as mutations or amplification events, intricate interactions between these proteins on the cell membrane lead to downstream signaling that encourages cancer growth and proliferation. In this Review, we contextualize efforts to pharmacologically target the ErbB receptor family beyond HER2, with a focus on EGFR and HER3. Preclinical and clinical efforts are synthesized. We discuss successes and failures of this approach to date, summarize lessons learned, and propose a way forward that invokes new therapeutic modalities such as antibody drug conjugates (ADCs), combination strategies, and patient selection through rational biomarkers.
I. Introduction
The ErbB family is comprised of four transmembrane growth factor receptors which are closely related: EGFR (or HER1), HER2, HER3 and HER4 [1]. These proteins are critical for the development of normal cells, but when dysregulated, can promote disordered proliferation, invasion, and unchecked cell survival leading to the development of cancer [2]. Intricate and complex interactions between these receptors, in the presence or absence of extracellular ligands, results in activation of downstream signaling primarily via the PI3K/AKT, MAP kinase, and JAK/STAT pathways [3, 4] (figure 1). Such aberrant signaling is typically caused by mutations or amplification of ErbB family genes, leading to increased homo- or hetero-dimerization and constitutively active kinase activity with resultant downstream signaling [4]. A high-level structural homology exists between members of the ErbB receptor family, with an extracellular ligand binding domain, a transmembrane helix and a cytoplasmic domain which possesses enzymatic activity. However, notable exceptions include the fact that HER2 does not have a direct ligand and HER3 has impaired kinase activity [5]. Interactions between these proteins on the cell surface are highly interrelated, forming a complex signaling network where no individual member functions in isolation, and cooperation is the rule rather than the exception [6].
Figure 1.
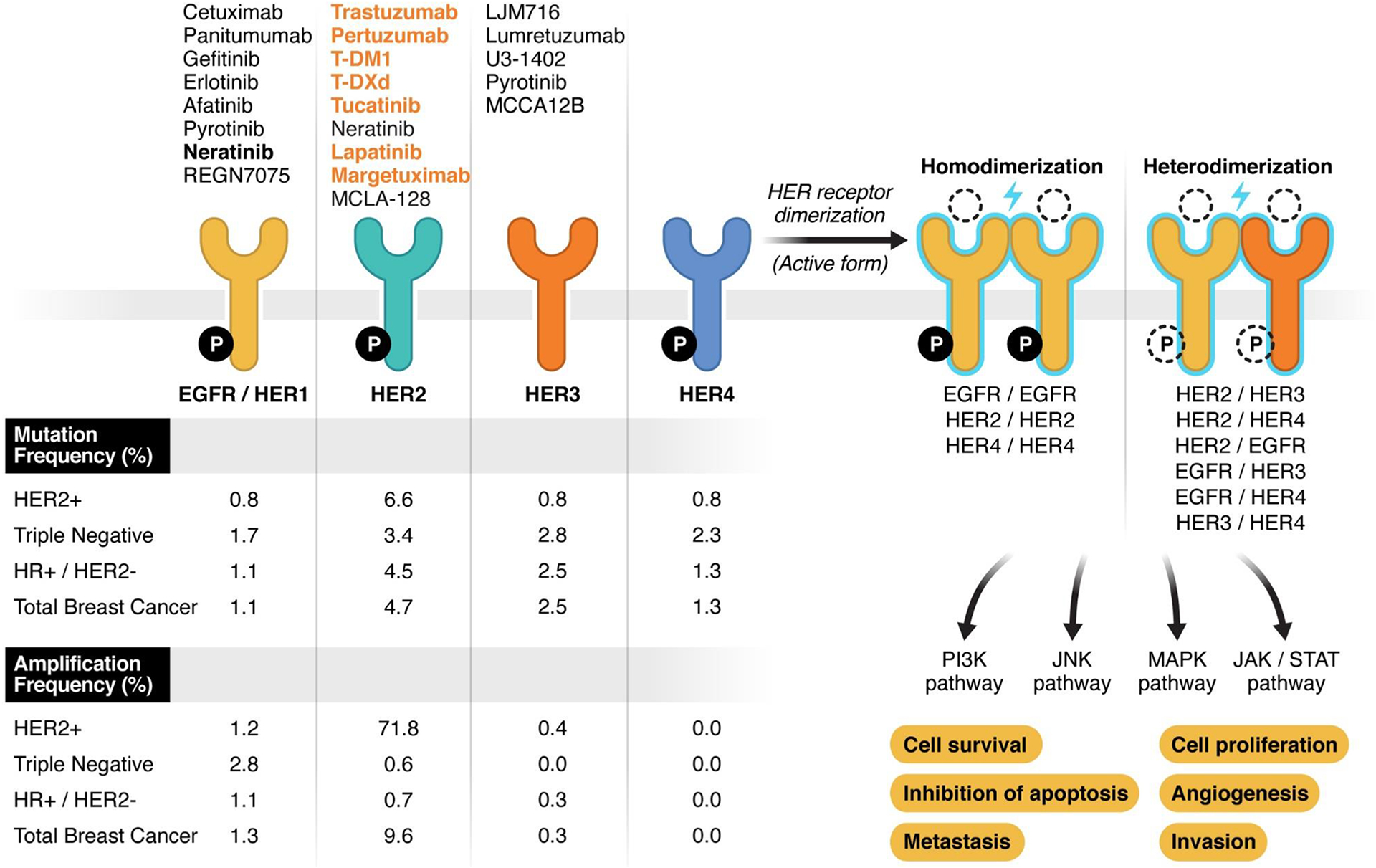
Schematic representation of ERBB-family receptors. On the right part of the figure, the 4 receptors have been represented: on upper side, FDA-approved (orange) and experimental (black) targeted agents for each receptor; on the lower side amplification and mutation frequency is reported. The genomic alterations frequency has been extracted from MSKCC sequencing data by MSK-IMPACT including 1918 breast cancer samples (published data, Razavi et al. Cancer Cell 2018 https://doi.org/10.1016/j.ccell.2018.08.008 ). ER/PR and HER2 status have been defined as the last status determined per SOC that guided the therapeutic choice at the time of the data collection. The right part of the figure represents all possible combinations of dimerization (active form) of ERBB-receptors along with the downstream molecular pathway and the effect of their activation on breast cancer cells. Ligand and intracellular phosphorylation (P) are represented with dotted lined because their status vary across the receptors/dimers. Specifically: all the receptors are active with the phosphorylation of the intracellular domain except HER3. In normal conditions, all receptors have a ligand-dependent activity except HER2.
In breast cancer, 15–20% of tumors specifically overexpress HER2 due to ERBB2 gene amplification, resulting in a cancer phenotype that is highly aggressive if left untreated [7]. The capability to pharmacologically target the HER2 oncoprotein represents one of the great triumphs of oncologic science, and has transformed the prognosis of this this disease, resulting in meaningful improvements in overall survival for patients with early or advanced HER2-positive breast cancers, as reviewed elsewhere [8]. Given the intricate relationships between the four ErbB family members in their individual/collective contribution to oncogenesis, renewed interest has arisen in how these interactions can be harnessed to improve upon the tissue specificity and cytotoxicity of existing therapies. In this review, we focus on how targeting ErbB proteins beyond HER2, including epidermal growth factor receptor (EGFR), HER3, and HER4 might improve outcomes for patients with breast cancer. We briefly review historical attempts to target these proteins as well as novel strategies, including monoclonal antibodies (mABs), tyrosine kinase inhibitors (TKIs) and antibody drug conjugates (ADCs).
II. EGFR (HER1)
EGFR, one of the most canonical oncogenes in cancer medicine, is encoded by the EGFR gene on chromosome 7p11, and was identified first in the 1970s [9]. Known ligands of EGFR are numerous, and include EGF, TGF-alpha, and amphiregulin, among several others [10]. In the presence or absence of a ligand, the major heterodimerization partner for EGFR is HER2. When paired, EGFR and HER2 form stable activated complexes, which are endocytosed at a slow rate and readily recycled to the cell surface (rather than degraded), resulting in strong and long-lived signaling activation [10]. Targeting EGFR with oral tyrosine kinase inhibitors or mABs has been a successful strategy in many cancer subtypes, including non-small lung cancer [11], colorectal cancer [12], and head and neck cancer [13].
EGFR is overexpressed in up to 66% of basal-like and triple negative breast cancers (TNBC); in the absence of hormone receptor expression, EGFR expression has been used historically as a lineage marker for these subtypes of breast cancer as well as a poor prognostic indicator [14, 15]. For these reasons, efforts to target EGFR in breast cancer have focused largely on TNBC [16]. It has been hypothesized that overexpression of EGFR in TNBC is largely attributable to post-transcriptional changes or gene duplication, since true gene amplifications and activating mutations are relatively rare, occurring in around 2% and 3% of TNBCs respectively [16, 17].
Preclinical studies have shown activity of EGFR antagonists against breast cancer cell lines, as well as potential synergies between EGFR targeted therapies and cytotoxic therapies in vitro [18]. However, clinical efforts to target EGFR in TNBC have largely been unsuccessful to date, characterized by increased toxicity, outweighing any additional clinical benefit observed [16]. Despite over a dozen phase I and II clinical trials investigating agents including the EGFR mABs cetuximab, panitumumab, and nimotuzumab, and TKIs such as gefitinib, erlotinib, afatinib, icotinib, no therapies that specifically target EGFR have been approved for the treatment of breast cancer, as monotherapy or part of a combinatorial strategy [16, 18]. For example, cetuximab has been investigated in metastatic triple negative breast cancer in combination with taxanes [19], platinum agents [20, 21], and irinotecan [22], without any signal for increased activity over single agent chemotherapy. Erlotinib monotherapy had very limited activity in an unselected population of advanced breast cancers [23]. Gefitinib has been investigated as a single agent in advanced breast cancers with very few if any responses [24, 25], with similar results observed with afatinib [26].
The reason for this outcome may be that despite observed overexpression in EGFR, few TNBCs in vivo are oncologically addicted to EGFR signaling in such a way that renders them sensitive to EGFR inhibition. Instead, TNBC is a genomically complex disease, characterized by loss of tumor suppressor genes and high mutational burden without single identifiable driver mutations or programs [27]. This stands in contrast to disease entities such as EGFR-mutant lung cancer, which is highly dependent on EGFR signaling, and highly sensitive to EGFR inhibitors [28]. While mABs offer the theoretical additional benefit of immunologic activity over TKIs via engagement of the fragment crystallizable (Fc) component by immune effector cells, any additional activity conferred by these agents has not yet proven to be clinically significant enough to warrant standard use in breast cancer.
Is it possible that the strategy of targeting EGFR in breast cancer is still viable, but the correct tumor subtype, drug combination, or treatment context has not yet been investigated? Perhaps looking past TNBC and/or investigating new drug partners could hold the answer. For example, EGFR alterations including gene amplification are enriched in ER+/HER2− breast cancers that are previously exposed to hormonal therapy; EGFR overexpression conferred resistance to hormonal blockade in vitro, which was rescued by EGFR inhibition with TKIs [29]. In the neoadjuvant setting with treatment naïve ER+/HER2− breast cancers, gefitinib did not add any clinical or biochemical benefit (by KI-67 reduction) to anastrozole alone [30], however in the metastatic setting, the addition of gefitinib to hormone therapies in ER+/HER2− breast cancer has exhibited mixed results in small phase 2 studies [31, 32]. It remains to be seen whether refractory ER+/HER2− breast cancer represents a new treatment context in which pharmacologic targeting of EGFR may prove useful in breast cancer. Building on this, EGFR is also frequently overexpressed in inflammatory breast cancer and metaplastic breast cancer, both highly aggressive disease subtypes with poor prognoses and urgent needs for better therapeutic options [15]. Lastly, it is worth noting that few of the targeted studies of EGFR inhibitors in breast cancer utilized a strategic biomarker for patient selection purposes, raising the possibility that a subset of responsive patients exists and have been diluted by existing trial data [15].
Of note, ADCs represent a new and promising strategy to target the ErbB receptors in breast cancer and beyond. ADCs are canonically comprised of a monoclonal antibody targeting a tumor-specific antigen, a highly potent cytotoxic payload, and a linker that connects the two [33]. ADCs accumulate in tumors, where they engage with the target antigen and are internalized, at which point the linker is broken and the payload is released, enacting it’s end-activity on cancer cells. ADCs targeting HER2 have made a profound impact in breast cancer treatment, including ado-trastuzumab emtansine (T-DM1) which is approved in the adjuvant [34] as well as metastatic [35] setting for HER2-positive breast cancers, and fam-trastuzumab deruxtecan, which is approved in metastatic HER2-positive breast cancer [36] and also recently exhibited substantial activity in HER2-low breast cancers [37].
ADCs targeting EGFR have been tested clinically in various settings [38–40], and early phase studies are underway in solid tumors with initial results expected in the coming months and years. While no EGFR-targeted ADC is currently approved for treatment, three main experimental candidates have emerged: MRG1003 (delivering MMAE with a cleavable linker), M1231 (a bispecific mAB targeting MUC1 and EGFR, delivering a hemiasterlin payload), and depatuxizumab mafodotin (delivering MMAF via a non-cleavable linker) [41]. Although many of the early trial efforts in these drugs are focusing on other EGFR-expressing cancers such as glioblastoma, gastrointestinal, and head/neck malignancies, preclinical efforts are underway to develop such ADCs alone or in combination therapy regimens specifically for use in TNBC [42, 43]. Of special note, this ADC approach may take advantage of EGFR as a ‘docking station’ for ADC internalization and ultimately payload delivery, and may be effective regardless of whether tumors are driven by downstream activity of the ADC target [33]. As discussed further below, the potential for on-target off-tumor toxicity of EGFR-targeted agents is another hurdle to be overcome by these agents, given the role that EGFR plays in normal tissue.
III. HER3
HER3 is encoded by the ERBB3 gene, located on chromosome 12q13; known ligands for this receptor include NRG-1 and NRG-2 [44]. Of special note, HER3 does not possess kinase activity on its own, however it is capable of forming heterodimers with HER2 (and/or EGFR), which dramatically increases transphosphorylation and activation of downstream signaling cascades, in perhaps the most mitogenic stimulus in human breast cancer [44, 45]. Moreover HER3 stands out among ErbB family members as a potent inducer of PI3K activity, due to direct binding with the PI3K p85 subunit [46]. Preclinically, HER3 expression is necessary for HER2-mediated signaling in HER2-overexpressing breast cancer cell lines, underlining the importance of heterodimerization for the ErbB receptor family in oncogenesis [47]. Overexpression of HER3 occurs in approximately 10–30% of primary breast carcinomas and is associated with worse outcomes in some studies [48]; however unified and consistent measurement of HER3 expression, which is often highly variable over time, has not been agreed upon in the research community, which qualifies the results of such studies.
Mechanistically, overexpression of HER3 is hypothesized to lead to de-novo and acquired resistance to HER2-targeted therapies in HER2-positive breast cancer [49, 50]. Indeed, pertuzumab, a HER2-targeted mAB which substantially prolongs overall survival in HER2-positive advanced breast cancer and increases rates of pathologic complete response in early HER2-positive breast cancer, is thought to add to the antiproliferative effects of the HER2-targeted mAB trastuzumab specifically by interfering with the heterodimerization of HER2 and HER3 [51]. HER3 overexpression has also been observed as a mechanism of feedback upregulation and therapy escape when PI3K is inhibited in HER2-positive breast cancers, showing how targeting downstream effectors of ErbB family genes can have unexpected consequences [52, 53].
Efforts to target HER3 in cancer have historically fallen into three categories: blocking the kinase activity of its relevant dimerization partners, blocking the dimerization process, or directly targeting the HER3 protein itself [46]. Because of its lack of intrinsic kinase activity, TKIs which target the ATP binding site should not effectively inhibit HER3, which likely undercuts the benefits of this approach [54]. Of note, mutations in ERBB3 occur in breast cancer, albeit relatively rarely with a prevalence of 1–2% [55]. Such mutations can be oncogenic in preclinical models, and can occur in the extracellular or kinase domains [56]. HER2 kinase inhibition with neratinib has been attempted in patients with HER3 mutant cancers in the SUMMIT basket trial, however no clinical responses were observed in this small subpopulation [57].
For these reasons, many efforts to target this protein have relied on mABs as a therapeutic strategy [58]. Example agents include seribantumab, lumretuzumab, elgemtumab, and patritumab, which have highly limited activity as single agents, and have thus far not demonstrated meaningful benefit when used in combination strategies [59]. Additionally, toxicities may emerge when these agents are employed in combinations. For example, the triplet of elgemtumab along with trastuzumab and alpelisib led to significant gastrointestinal toxicity in patients with PI3K-mutated HER2-positive advanced breast cancer, suggesting that care must be taken when combining multiple agents targeting this pathway, as on-target off-tumor side effects can occur [60].
This has paved the way for new platforms, which include bispecific antibodies as well as ADCs. For example, the HER3-targeted ADC patritumab deruxtecan (U3–1402) has shown signs of activity in advanced, heavily-pretreated breast cancers [61]. Most recent results at the time of this publication showed an ORR of 30.1% in hormone receptor positive (HR+)/HER2− breast cancer (n=113), 22.6% in TNBC (n=53), and 42.9% in HER2+ disease (n=14) in phase I/II studies [62]. Duration of response ranged from 5.9 to 8.3 months. In this study, HER3 expression on pre-treatment specimens did not seem to correlate well with response and temporal variation in HER3 expression may compromise its use as a biomarker. Of note, the SOLTI-TOT HER3 window of opportunity trial investigated the use of a single pre-operative dose of patritumab deruxtecan in HR+/HER2− breast cancer, finding an ORR of 45%, with increased tumor cellularity and tumor-infiltrating lymphocyte (CelTIL) score, and no apparent correlation between response and pre-treatment ERBB3 mRNA [63]. Several other HER3-targeted ADCs are in preclinical development [59]. Of note, while activation in parallel pathways and lack of oncogenic dependence are standard means of resistance to classical targeted therapies such as mABs and TKIs, ADCs may be able to overcome these mechanisms by using the target protein as a means of delivering a cytotoxic payload [64]. In this way, ADCs represent a promising strategy to overcome the compensatory signaling changes and oncologic complexity that have allowed cancers to evade therapies targeting the ErbB receptor family.
A few bispecific antibodies have been investigated which target HER3 in addition to other targets, though experience in breast cancer is limited. For example, duligotuzumab bispecifically targets EGFR and HER3, with the hope of overcoming acquired resistance to EGFR inhibition, and showed preclinical activity in TNBC [65]. This agent was investigated in metastatic colon cancer but unfortunately had increased toxicity without any improvement in efficacy compared to cetuximab, and no studies in breast cancer have been reported [66]. M-111 is a bispecific antibody-fusion protein targeting HER2 and HER3, which was investigated clinically in breast cancer over a decade ago in a phase 1 study [67]; MM-141 (istiratumab) targets HER3 and IGF-1R and advanced to phase 2 studies in pancreatic cancer [68], but has not been developed clinically in breast cancer. Of special note, HER2/3 bispecific antibodies have found a promising niche in a mechanistically distinct clinical setting: NRG1 gene rearrangements encoding for NRG1, the predominant ligand of HER3 and HER4, which can potently activate ERBB signaling [69]. Preclinical and preliminary clinical data strongly support further investigation of this strategy in the subset of patients with breast cancer harboring NRG1 rearrangements, demonstrating the importance of biomarker selection in therapy deployment.
IV. HER4
The ERBB4 gene is located on chromosome 22q33; known ligands of HER4 include neuregulins and epiregulin, among others [44, 70]. The role of HER4 in cancer development and signaling is a complex one, with many unanswered questions. HER4 is capable of homodimerization or heterodimerization, which may have contrasting effects on cell proliferation and survival. Some studies have shown a positive correlation between ERBB4 expression and breast cancer related outcomes [18, 71, 72], in that HER4 promotes differentiation and growth suppression [73]. However other studies have suggested that this relationship is highly context-dependent, and differs with membranous vs. nuclear staining, as well as homo- vs. heterodimerization [18, 74, 75]. These mixed results can be attributed to the complexity of HER4 biology, which involves at least four different receptor isoforms, some of which can be cleaved, forming soluble intracellular domain that can localize to the nucleus or cytoplasm and has numerous and diverse activities [44].
Accordingly, some have hypothesized that inhibiting HER4 could be deleterious and lift the brakes on the pro-apoptotic signals mediated by the protein [44, 76]. However, the cleaved intracellular domain, when localized in the nucleus, specifically appears to co-activate estrogen receptor activity, suggesting a potential role for HER4 biology in ER+ breast cancers [77]. Beyond this, structural differences between HER4 isoforms poses another challenge in drug design—target engagement must be carefully controlled to avoid unwanted downstream effects [78]. While HER4-targeted mABs have been investigated preclinically, none have entered clinical trials as of yet [78, 79].
V. A note on toxicity
EGFR, HER2, HER3 and HER4 are expressed widely in normal tissues, and play a major role in physiologic cell processes. Specifically, EGFR is expressed in the skin, gastrointestinal system, and kidney [80]; HER2 is expressed in the gastrointestinal, respiratory, reproductive tract, skin, breast, placenta, and heart [81]; HER3 is found at high levels in the gastrointestinal tract and central nervous system [82], and HER4 is found in skeletal muscle, heart, and central nervous system [70]. Inhibition of these proteins and their downstream activities can have significant consequences in normal tissue, as reflected in the toxicity patterns seen across ErbB-family targeted therapies. This has direct effects on the therapeutic window of drugs that that inhibit these proteins and is a critical consideration in the pharmacologic development of such agents.
Across tumor types, EGFR targeted therapies are known to cause skin toxicity, manifesting as an acneiform rash most commonly seen on the face; such a rash occurs in >80% of patients treated with cetuximab [83]. Gastrointestinal toxicity such as diarrhea and nausea, as well as hepatotoxicity are also relatively common, all due to normal tissue expression and function of the wild type EGFR protein [83]. Drugs that have a higher affinity for mutant EGFR, such as osimertinib, have a better therapeutic index in patients with EGFR-mutant cancers [84], however activating EGFR mutations are rare in breast cancer. The toxicity patterns of HER3-targeted agents may depend on the therapeutic modality and also combination therapy partners [85].
Turning to newer therapy modalities, toxicity patterns seen with ADCs are complex, and are influenced by payload and linker-specific factors, in addition to function of the target protein [33]. Patritumab deruxtecan, for example, appears to be associated primarily with payload-related hematologic toxicity, however drug-induced pneumonitis occurs as well [61]. Similar pneumonitis has also been observed with the HER2-targeted ADC trastuzumab deruxtecan in breast cancer [86], as well as the trop-2 targeted ADC datopotamab DXd in lung cancer (though curiously less so in breast cancer) [87, 88]. It remains to be seen whether this is due to target expression in or around lung tissue, or off-target payload effects.
VI. Future directions
To date, targeting EGFR, HER3 or HER4 in breast cancer with monoclonal antibodies or small molecule inhibitors has unfortunately born little fruit. This is most likely due to the complexity of interactions between the ErbB receptor family members as well as their downstream signaling cascades, which, besides HER2, function largely in a network fashion rather than as singular oncogenes in breast cancer. With some examples discussed above, it is quite possible that biomarkers can select the patients who would benefit from molecular inhibition of EGFR, HER3 or HER4, and preclinical studies should strive to elucidate the role of these players in resistance to existing therapies.
Moreover, the ability to target these proteins with ADCs has opened up a new pathway with real potential to improve patient outcomes. Because ADCs capitalize on the presence of a cell surface marker which may or may not be a functional oncogenic driver, they can overcome the mechanisms of intrinsic resistance to small molecular inhibitors, including parallel pathway signaling and feedback upregulation. The ultimate cytotoxicity of ADCs is largely due to the delivery of a chemotherapeutic payload, which can kill cells regardless of these complexities and the resilience of cell signaling interactions. Further, the bystander effect can overcome spatial heterogeneity of target expression, which may be of particular utility in EGFR and HER3, which may not be homogeneously or stably expressed in breast cancer. Perhaps the most promising example of this strategy, as discussed above, is patritumab deruxtecan, which is currently being investigated clinically in phase 2 studies in breast cancer.
VII. Conclusion
Despite many setbacks, strategies targeting ErbB proteins beyond HER2 still hold promise, particularly those which take advantage of new drug delivery platforms and biomarker selection. Herein, we highlight the progress and pitfalls of these efforts to date. Promising strategies include the use of ADCs and monoclonal or bispecific antibodies, and future studies would likely benefit from using biomarker selection in order to better balance the benefits of these drugs against their known toxicity. Further, translational work is required to identify synergistic partners to overcome primary and acquired resistance seen in breast cancers driven by ErbB family signaling. Ultimately, a comprehensive understanding of the intrinsic and extrinsic features of the ErbB ecosystem is critical to personalizing treatments and securing clinical benefit for patients with breast cancer.
Table 1:
Efficacy and safety of anti- ErbB family drugs (excluding HER2− selective therapies) in breast cancer
| Drug | Class | Target | Phase | Subtype/Setting | N | Combination Partners | Activity | Tox. of special Interest |
|---|---|---|---|---|---|---|---|---|
| Cetuximab | mAb | Amplified EGFR | II II II |
mTNBC [89] MBC [22] Early TNBC Neoadj setting[90] |
102 19 25 |
Alone Cet/Tax/carbo Cet/Docetaxel |
ORR: 6–16% pCR rate: 24% |
Rash ≥ G3: 3% |
| Panitumumab | mAb | Amplified EGFR | II | mTNBC[91] Early TNBC (neoadj) setting[92] |
71 60 |
Panitumumab/Carbo/Gem Panitumumab/FEC100→ docetaxel |
ORR:42% PFS: 4.4 mo pCR rate: 46.8% |
Skin tox ≥ G3: 43.3–70% |
| Gefinitib | TKI | Mutant EGFR | II | HR+/Her2+/− MBC[93] HR+/HER2− MBC (TAM-resistant)[94] HR+ HER2+/−MBC (TAM-naïve)[95] Early BC (neoadj) [96] Early HR+ BC (neoadj)[97] |
40 71 290 206 |
Monotherapy ANA + Gef or plac TAM + Gef/plac EP→ Gef/Plac ANA+Gef vs ANA |
ORR: 0% ORR: 22% vs 28% mPFS: 10.9 vs 8.8 pCR rate: 5% ki67 change 0–16w: mean 1.37 (p=.26) |
Diarrhea G≥3: 10–13% Rash: G≥3: 16.1–30% |
| Erlotinib | TKI | Mutant EGFR | II | MBC[23] | 69 | Monotherapy | ORR: 3% | Rash: G≥2: 6–13% |
| Afatinib | TKI | Mutant EGFR | II III |
HER2−MBC[26] HER2+ MBC[98] |
50 208 |
Monotherapy Afatinib+ Vino vs Trastuzumab+Vino |
ORR: 0% mPFS: 5.6 vs 5.5 mo |
Diarrhea G≥3:40% Diarrhea G≥3: 24% Rash: G≥3:11% |
| Pyrotinib | TKI | panErbB | III | HER2+ MBC[99] | 266 | Pyrotinib + Cape vs Lapatinib + Cape | mPFS: 12.5 vs 6.8 mo HR: 0.39 CI: 0.25–0.56 | Diarrhea G3: 31% |
| LJM716 | mAb | HER3 | I | HER2+ /PIK3CA mutant MBC[85] | 10 | LJM716 Trastuzumab and alpelisib | ORR: 1/21 (4.8%); CBR > 30 weeks: 4/21 | G ≥3 diarrhea (n = 6), hypokalemia (n = 3), abnormal liver enzymes (n = 3) |
| Lumretuzumab | mAb | HER3 | I I |
Highly pretreated HER3 amplified solid tumors [100] HER3 +, HER2-Low Breast Cancer [101] |
25 35 |
Pertuzumab + paclitaxel | CBR: 21.3% ORR: 38% - 55% (untreated pts) |
No DLT, diarrhea 46.8% G3 Diarrhea 50% |
| Patritumab deruxtecan | ADC | HER3 | I/II | HER3-high or low TNBC or HR+/HER2−[102] | 172 | HER3-high: ORR:10–30% HER3-low: 29–33% |
ILD: 5% |
Notes: TKI: tyrosine kinase inhibitor; N: number; Tax: ; MBC: metastatic breast cancer; BC: breast cancer; mTNBC: metastatic triple negative breast cancer; HR+: hormone receptor positive; mPFS: median progression free survival; ORR: overall response rate; ANA: anastrozole; TAM: tamoxifen; pCR: pathological complete response; CBR: clinical benefit rate; DLT: dose-limiting toxicity; Plac: placebo; G: grade; mo: months; EP: epirubicin and paclitaxel; vino: vinorelbine; neoadj: neoadjuvant: tox: toxicities; cape: capecitabine; ILD: interstitial lung disease; pts: patients; Lapa: lapatinib; Pyro: pyrotinib
Table 2:
Ongoing trials of anti- ErbB family drugs (excluding HER2− selective therapies) in breast cancer
| NCT Identifier | Phase | Drug | Target | Combination therapy | Drug class | Population | Primary endpoint |
|---|---|---|---|---|---|---|---|
| NCT02980341 | I/II | U3–1402 | HER3 | - | ADC | HER3+ | Safety |
| NCT04699630 | II | U3–1402 | HER3 | - | ADC | MBC | ORR and PFS6 |
| NCT04965766 | II | U3–1402 | HER3 | - | ADC | MBC | ORR |
| NCT04610528 | I Pre-surgery (WoO) |
U3–1402 | HER3 | - | ADC | mRNA-ERBB3 expressed eBC | CelTIL score |
| NCT04460430 | II | Neratinib | EGFR/HER3/HER4 | Fulv/TAM/EXE | TKI | HR+/HER2− /HER2 mutant MBC | PFS |
| NCT05154396 | II | Neratinib escalating dose | EGFR/HER3/HER4 | - | TKI | HR+/HER2−/HER4+ eBC (neadj) | Safety |
| NCT04965064 | II | Neratinib | EGFR/HER3/HER4 | Capecitabine | TKI | BM HER2− with HER2 CELsignia + | OS |
| NCT03065387 | II | Neratinib | HER2/EGFR | Palbociclib or Trametinib or everolimus | TKI | HER2 mutant | Safety of the combination |
| NCT04872985 | II | Pyrotinib | panErbB | - | TKI | HR+/HER2−/HER4+ eBC (neadj) | tpCR |
| NCT04582968 | II | Pyrotinib | panErbB | + RT | TKI | HER2+ MBC with BM | Safety CNS |
| NCT04001621 | II | Pyrotinib | panErbB | - | TKI | HER2+ MBC | PFS |
| NCT04681911 | II | Pyrotinib | panErbB | Inetetamab (ADC) + Chemo | TKI | HER2+ MBC | ORR |
| NCT04481932 | II | Pyrotinib | panErbB | TCbH | TKI | HER2+ early BC (neadj) | tpCR |
| NCT04255056 | II | Pyrotinib | AC | ||||
| NCT04646759 | III | Pyrotinib | panErbB | Fulvestrant | TKI | HR+/HER2+ MBC | PFS |
| NCT05255523 | II | Pyrotinib | panErbB | Trastuzumab | TKI | 2L HER2+ MBC | Incidence of first BM event |
| NCT04973319 | III | Pyrotinib | panErbB | Sequentially after HP | TKI | Non-pCR patient (post-neoad) | IDFS |
| NCT03321981 | II | MCLA-128 | HER3 | ET or H/Chemotherapy | TKI | HR+ or HER2+ | CBR 24w |
Note: ORR: overall response rate; ET: endocrine therapy; CBR 24w: clinical benefit rate at 24 weeks; CNS: central nervous system; TKI: tyrosine kinase inhibitor; ADC: antibody drug conjugate; tpCR: total pathological complete response; CNS: central nervous system; FULV: fulvestrant; TAM: tamoxifen; eBC: early breast; WoO: window of opportunity; CelTIL: tumor cellularity and TILS; BM: brain metastasis; HR: hormone receptor; EXE: exemestane; TCbH: Taxotere, Carboplatin, Herceptin; AC: adriamycin and cyclophosphamide; mTNBC: metastatic triple negative breast cancer; IDFS: invasive disease-free survival; HP: trastuzumab and pertuzumab
https://clinicaltrials.gov/ (last update on 4/05/2022)
Prior efforts to target the ErbB receptor family beyond HER2 have been inadequate
Novel therapeutic approaches such as antibody-drug conjugates hold promise
Better patient selection can improve the potential of these agents in breast cancer
COI Statement
Joshua Z Drago declares research funding from AstraZeneca, speaking fees from Virology Education, and consulting fees from Intellisphere LLC, Biotheranostics, Genagon, and AmMax Bio.
Emanuela Ferraro has no interests to declare.
Nour Abuhadra has no interests to declare.
Shanu Modi declares consulting/advisory fees from Genentech, Seagen, Daiichi, AstraZeneca, Macrogenics, Novartis, Glaxosmith kline, Zymneworks, honoraria/speaking engagements from Seagen, Daiichi, AstraZeneca, and research funding from Genentech, Seagen, Daiichi, and AztraZeneca.
REFERENCES
- 1.Ross JS and Fletcher JA, The HER-2/neu Oncogene in Breast Cancer: Prognostic Factor, Predictive Factor, and Target for Therapy. STEM CELLS, 1998. 16(6): p. 413–428. [DOI] [PubMed] [Google Scholar]
- 2.de Bono JS and Rowinsky EK, The ErbB receptor family: a therapeutic target for cancer. Trends in Molecular Medicine, 2002. 8(4): p. S19–S26. [DOI] [PubMed] [Google Scholar]
- 3.Riese II DJ and Stern DF, Specificity within the EGF family/ErbB receptor family signaling network. BioEssays, 1998. 20(1): p. 41–48. [DOI] [PubMed] [Google Scholar]
- 4.Hynes NE and MacDonald G, ErbB receptors and signaling pathways in cancer. Current Opinion in Cell Biology, 2009. 21(2): p. 177–184. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, ErbB Receptors and Cancer. Methods Mol Biol, 2017. 1652: p. 3–35. [DOI] [PubMed] [Google Scholar]
- 6.Yarden Y and Pines G, The ERBB network: at last, cancer therapy meets systems biology. Nature Reviews Cancer, 2012. 12(8): p. 553–563. [DOI] [PubMed] [Google Scholar]
- 7.Yarden Y, Biology of HER2 and its importance in breast cancer. Oncology, 2001. 61: p. 1–13. [DOI] [PubMed] [Google Scholar]
- 8.Loibl S and Gianni L, HER2-positive breast cancer. Lancet, 2017. 389(10087): p. 2415–2429. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter G, et al. , Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J Biol Chem, 1975. 250(11): p. 4297–304. [PubMed] [Google Scholar]
- 10.Yarden Y, The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer, 2001. 37 Suppl 4: p. S3–8. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Serrano A, et al. , Targeting EGFR in Lung Cancer: Current Standards and Developments. Drugs, 2018. 78(9): p. 893–911. [DOI] [PubMed] [Google Scholar]
- 12.Khan K, et al. , Targeting EGFR pathway in metastatic colorectal cancer- tumour heterogeniety and convergent evolution. Crit Rev Oncol Hematol, 2019. 143: p. 153–163. [DOI] [PubMed] [Google Scholar]
- 13.Xu MJ, Johnson DE, and Grandis JR, EGFR-targeted therapies in the post-genomic era. Cancer Metastasis Rev, 2017. 36(3): p. 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reis-Filho JS and Tutt AN, Triple negative tumours: a critical review. Histopathology, 2008. 52(1): p. 108–18. [DOI] [PubMed] [Google Scholar]
- 15.Masuda H, et al. , Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat, 2012. 136(2): p. 331–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakai K, Hung MC, and Yamaguchi H, A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am J Cancer Res, 2016. 6(8): p. 1609–23. [PMC free article] [PubMed] [Google Scholar]
- 17.Park HS, et al. , High EGFR gene copy number predicts poor outcome in triple-negative breast cancer. Mod Pathol, 2014. 27(9): p. 1212–22. [DOI] [PubMed] [Google Scholar]
- 18.Maennling AE, et al. , Molecular Targeting Therapy against EGFR Family in Breast Cancer: Progress and Future Potentials. Cancers (Basel), 2019. 11(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modi S, et al. , A phase I study of cetuximab/paclitaxel in patients with advanced-stage breast cancer. Clinical Breast Cancer, 2006. 7(3): p. 270–277. [DOI] [PubMed] [Google Scholar]
- 20.Baselga J, et al. , Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol, 2013. 31(20): p. 2586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey LA, et al. , TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol, 2012. 30(21): p. 2615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crozier JA, et al. , N0436 (Alliance): A Phase II Trial of Irinotecan With Cetuximab in Patients With Metastatic Breast Cancer Previously Exposed to Anthracycline and/or Taxane-Containing Therapy. Clin Breast Cancer, 2016. 16(1): p. 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickler MN, et al. , Efficacy and safety of erlotinib in patients with locally advanced or metastatic breast cancer. Breast Cancer Research and Treatment, 2009. 115(1): p. 115–121. [DOI] [PubMed] [Google Scholar]
- 24.Baselga J, et al. , Phase II and Tumor Pharmacodynamic Study of Gefitinib in Patients with Advanced Breast Cancer. Journal of Clinical Oncology, 2005. 23(23): p. 5323–5333. [DOI] [PubMed] [Google Scholar]
- 25.von Minckwitz G, et al. , A multicentre phase II study on gefitinib in taxane- and anthracycline-pretreated metastatic breast cancer. Breast Cancer Research and Treatment, 2005. 89(2): p. 165–172. [DOI] [PubMed] [Google Scholar]
- 26.Schuler M, et al. , A phase II trial to assess efficacy and safety of afatinib in extensively pretreated patients with HER2-negative metastatic breast cancer. Breast Cancer Research and Treatment, 2012. 134(3): p. 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foulkes WD, Smith IE, and Reis-Filho JS, Triple-negative breast cancer. N Engl J Med, 2010. 363(20): p. 1938–48. [DOI] [PubMed] [Google Scholar]
- 28.Ramalingam SS, et al. , Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. New England Journal of Medicine, 2019. 382(1): p. 41–50. [DOI] [PubMed] [Google Scholar]
- 29.Razavi P, et al. , The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell, 2018. 34(3): p. 427–438 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith IE, et al. , A Phase II Placebo-Controlled Trial of Neoadjuvant Anastrozole Alone or With Gefitinib in Early Breast Cancer. Journal of Clinical Oncology, 2007. 25(25): p. 3816–3822. [DOI] [PubMed] [Google Scholar]
- 31.Cristofanilli M, et al. , Phase II, Randomized Trial to Compare Anastrozole Combined with Gefitinib or Placebo in Postmenopausal Women with Hormone Receptor–Positive Metastatic Breast Cancer. Clinical Cancer Research, 2010. 16(6): p. 1904. [DOI] [PubMed] [Google Scholar]
- 32.Osborne CK, et al. , Gefitinib or Placebo in Combination with Tamoxifen in Patients with Hormone Receptor–Positive Metastatic Breast Cancer: A Randomized Phase II Study. Clinical Cancer Research, 2011. 17(5): p. 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drago JZ, Modi S, and Chandarlapaty S, Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Von Minckwitz G, et al. , Trastuzumab emtansine for residual invasive HER2-positive breast cancer. New England Journal of Medicine, 2019. 380(7): p. 617–628. [DOI] [PubMed] [Google Scholar]
- 35.Verma S, et al. , Trastuzumab emtansine for HER2-positive advanced breast cancer. New England journal of medicine, 2012. 367(19): p. 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortés J, et al. , Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. New England Journal of Medicine, 2022. 386(12): p. 1143–1154. [DOI] [PubMed] [Google Scholar]
- 37.Modi S, et al. , Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gan HK, et al. , Antibody–drug conjugates in glioblastoma therapy: the right drugs to the right cells. Nature Reviews Clinical Oncology, 2017. 14(11): p. 695–707. [DOI] [PubMed] [Google Scholar]
- 39.Lakhani N, et al. , Abstract CT056: A Phase Ia/IIa trial of AVID100, an anti-EGFR antibody-drug conjugate. 2019, AACR. [Google Scholar]
- 40.Xu R. h., et al. , First-in-human dose-escalation study of anti-EGFR ADC MRG003 in patients with relapsed/refractory solid tumors. 2020, American Society of Clinical Oncology. [Google Scholar]
- 41.Cai X, et al. , Antibody-Drug Conjugates Targeting the Human Epidermal Growth Factor Receptor (HER) Family in Cancers. Frontiers in Molecular Biosciences, 2022: p. 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zoeller JJ, et al. , Navitoclax enhances the effectiveness of EGFR-targeted antibody-drug conjugates in PDX models of EGFR-expressing triple-negative breast cancer. Breast Cancer Research, 2020. 22(1): p. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Si Y, et al. , Anti-EGFR antibody-drug conjugate for triple-negative breast cancer therapy. Engineering in Life Sciences, 2021. 21(1–2): p. 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koutras AK, et al. , The upgraded role of HER3 and HER4 receptors in breast cancer. Crit Rev Oncol Hematol, 2010. 74(2): p. 73–8. [DOI] [PubMed] [Google Scholar]
- 45.Alimandi M, et al. , Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene, 1995. 10(9): p. 1813–21. [PubMed] [Google Scholar]
- 46.Gala K and Chandarlapaty S, Molecular pathways: HER3 targeted therapy. Clin Cancer Res, 2014. 20(6): p. 1410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee-Hoeflich ST, et al. , A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res, 2008. 68(14): p. 5878–87. [DOI] [PubMed] [Google Scholar]
- 48.Chiu CG, et al. , HER-3 Overexpression Is Prognostic of Reduced Breast Cancer Survival A Study of 4046 Patients. Annals of Surgery, 2010. 251(6): p. 1107–1116. [DOI] [PubMed] [Google Scholar]
- 49.Gajria D and Chandarlapaty S, HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther, 2011. 11(2): p. 263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park YH, et al. , Role of HER3 expression and PTEN loss in patients with HER2-overexpressing metastatic breast cancer (MBC) who received taxane plus trastuzumab treatment. Br J Cancer, 2014. 110(2): p. 384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capelan M, et al. , Pertuzumab: new hope for patients with HER2-positive breast cancer. Ann Oncol, 2013. 24(2): p. 273–282. [DOI] [PubMed] [Google Scholar]
- 52.Serra V, et al. , PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene, 2011. 30(22): p. 2547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandarlapaty S, et al. , AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell, 2011. 19(1): p. 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang N, Saba NF, and Chen ZG, Advances in Targeting HER3 as an Anticancer Therapy. Chemother Res Pract, 2012. 2012: p. 817304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaiswal BS, et al. , Oncogenic ERBB3 mutations in human cancers. Cancer Cell, 2013. 23(5): p. 603–17. [DOI] [PubMed] [Google Scholar]
- 56.Kiavue N, et al. , ERBB3 mutations in cancer: biological aspects, prevalence and therapeutics. Oncogene, 2020. 39(3): p. 487–502. [DOI] [PubMed] [Google Scholar]
- 57.Hyman DM, et al. , HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature, 2018. 554(7691): p. 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu XL, et al. , Development of Effective Therapeutics Targeting HER3 for Cancer Treatment. Biological Procedures Online, 2019. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haikala HM and Jänne PA, Thirty Years of HER3: From Basic Biology to Therapeutic Interventions. Clinical Cancer Research, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jhaveri K, et al. , A Phase I study of alpelisib in combination with trastuzumab and LJM716 in patients with PIK3CA-mutated HER2-positive metastatic breast cancer. Clinical Cancer Research, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krop I, et al. , Abstract PD1–09: Safety and efficacy results from the phase 1/2 study of U3–1402, a human epidermal growth factor receptor 3 (HER3)-directed antibody drug conjugate (ADC), in patients with HER3-expressing metastatic breast cancer (MBC). Cancer Research, 2021. 81(4 Supplement): p. PD1–09. [Google Scholar]
- 62.Krop IE, et al. , Results from the phase 1/2 study of patritumab deruxtecan, a HER3-directed antibody-drug conjugate (ADC), in patients with HER3-expressing metastatic breast cancer (MBC). 2022, American Society of Clinical Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prat A, et al. , LBA3 Patritumab deruxtecan (HER3-DXd) in early-stage HR+/HER2-breast cancer: Final results of the SOLTI TOT-HER3 window of opportunity trial. Annals of Oncology, 2022. 33: p. S164. [Google Scholar]
- 64.Drago JZ, Modi S, and Chandarlapaty S, Unlocking the potential of antibody–drug conjugates for cancer therapy. Nature Reviews Clinical Oncology, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tao JJ, et al. , Antagonism of EGFR and HER3 enhances the response to inhibitors of the PI3K-Akt pathway in triple-negative breast cancer. Science signaling, 2014. 7(318): p. ra29–ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hill AG, et al. , Phase II Study of the Dual EGFR/HER3 Inhibitor Duligotuzumab (MEHD7945A) versus Cetuximab in Combination with FOLFIRI in Second-Line RAS Wild-Type Metastatic Colorectal Cancer. Clinical Cancer Research, 2018. 24(10): p. 2276–2284. [DOI] [PubMed] [Google Scholar]
- 67.Higgins MJ, et al. , A phase I/II study of MM-111, a novel bispecific antibody that targets the ErB2/ErB3 heterodimer, in combination with trastuzumab in advanced refractory HER2-positive breast cancer. Journal of Clinical Oncology, 2011. 29(15_suppl): p. TPS119–TPS119. [Google Scholar]
- 68.Kundranda M, et al. , Randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer (CARRIE). Annals of Oncology, 2020. 31(1): p. 79–87. [DOI] [PubMed] [Google Scholar]
- 69.Schram AM, et al. , Zenocutuzumab, a HER2xHER3 bispecific antibody, is effective therapy for tumors driven by NRG1 gene rearrangements. Cancer Discovery, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plowman GD, et al. , Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci U S A, 1993. 90(5): p. 1746–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sassen A, et al. , Cytogenetic analysis of HER1/EGFR, HER2, HER3 and HER4 in 278 breast cancer patients. Breast Cancer Research, 2008. 10(1): p. R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sassen A, et al. , Presence of HER4 associates with increased sensitivity to Herceptin™ in patients with metastatic breast cancer. Breast Cancer Research, 2009. 11(4): p. R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sartor CI, et al. , Her4 mediates ligand-dependent antiproliferative and differentiation responses in human breast cancer cells. Molecular and cellular biology, 2001. 21(13): p. 4265–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mohd Nafi SN, et al. , Nuclear HER4 mediates acquired resistance to trastuzumab and is associated with poor outcome in HER2 positive breast cancer. Oncotarget, 2014. 5(15): p. 5934–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujiwara S, et al. , The localization of HER4 intracellular domain and expression of its alternately-spliced isoforms have prognostic significance in ER+ HER2-breast cancer. Oncotarget, 2014. 5(11): p. 3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brockhoff G, Target HER four in breast cancer? Oncotarget, 2019. 10(34): p. 3147–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu Y, et al. , Coregulation of estrogen receptor by ERBB4/HER4 establishes a growth-promoting autocrine signal in breast tumor cells. Cancer research, 2006. 66(16): p. 7991–7998. [DOI] [PubMed] [Google Scholar]
- 78.Hollmén M, et al. , Suppression of breast cancer cell growth by a monoclonal antibody targeting cleavable ErbB4 isoforms. Oncogene, 2009. 28(10): p. 1309–19. [DOI] [PubMed] [Google Scholar]
- 79.Okazaki S, et al. , Development of an ErbB4 monoclonal antibody that blocks neuregulin-1-induced ErbB4 activation in cancer cells. Biochem Biophys Res Commun, 2016. 470(1): p. 239–244. [DOI] [PubMed] [Google Scholar]
- 80.Yano S, et al. , Distribution and function of EGFR in human tissue and the effect of EGFR tyrosine kinase inhibition. Anticancer Res, 2003. 23(5A): p. 3639–50. [PubMed] [Google Scholar]
- 81.Press MF, Cordon-Cardo C, and Slamon DJ, Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene, 1990. 5(7): p. 953–62. [PubMed] [Google Scholar]
- 82.Rajkumar T and Gullick W, A monoclonal antibody to the human c-erbB3 protein stimulates the anchorage-independent growth of breast cancer cell lines. British journal of cancer, 1994. 70(3): p. 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harandi A, et al. , Clinical efficacy and toxicity of anti-EGFR therapy in common cancers. Journal of Oncology, 2009. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Remon J, et al. , Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Annals of Oncology, 2018. 29: p. i20–i27. [DOI] [PubMed] [Google Scholar]
- 85.Jhaveri K, et al. , A Phase I Study of Alpelisib in Combination with Trastuzumab and LJM716 in Patients with PIK3CA-Mutated HER2-Positive Metastatic Breast Cancer. Clin Cancer Res, 2021. 27(14): p. 3867–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Modi S, et al. , Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. New England Journal of Medicine, 2019. 382(7): p. 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmid P, et al. , 166MO Datopotamab deruxtecan (Dato-DXd)+ durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): Initial results from BEGONIA, a phase Ib/II study. Annals of Oncology, 2022. 33: p. S199. [Google Scholar]
- 88.Meric-Bernstam F, et al. , TROPION-PanTumor01: Dose analysis of the TROP2-directed antibody-drug conjugate (ADC) datopotamab deruxtecan (Dato-DXd, DS-1062) for the treatment (Tx) of advanced or metastatic non-small cell lung cancer (NSCLC). 2021, Wolters Kluwer Health. [Google Scholar]
- 89.Carey LA, et al. , TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 2012. 30(21): p. 2615–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nabholtz JM, et al. , Multicentric neoadjuvant pilot Phase II study of cetuximab combined with docetaxel in operable triple negative breast cancer. International Journal of Cancer, 2016. 138(9): p. 2274–2280. [DOI] [PubMed] [Google Scholar]
- 91.Yardley DA, et al. , Panitumumab, Gemcitabine, and Carboplatin as Treatment for Women With Metastatic Triple-Negative Breast Cancer: A Sarah Cannon Research Institute Phase II Trial. Clin Breast Cancer, 2016. 16(5): p. 349–355. [DOI] [PubMed] [Google Scholar]
- 92.Nabholtz JM, et al. , Multicentric neoadjuvant phase II study of panitumumab combined with an anthracycline/taxane-based chemotherapy in operable triple-negative breast cancer: identification of biologically defined signatures predicting treatment impact. Ann Oncol, 2014. 25(8): p. 1570–7. [DOI] [PubMed] [Google Scholar]
- 93.Green MD, et al. , Gefitinib treatment in hormone-resistant and hormone receptor-negative advanced breast cancer. Annals of Oncology, 2009. 20(11): p. 1813–1817. [DOI] [PubMed] [Google Scholar]
- 94.Tryfonidis K, et al. , A European Organisation for Research and Treatment of Cancer randomized, double-blind, placebo-controlled, multicentre phase II trial of anastrozole in combination with gefitinib or placebo in hormone receptor-positive advanced breast cancer (NCT00066378). Eur J Cancer, 2016. 53: p. 144–54. [DOI] [PubMed] [Google Scholar]
- 95.Osborne CK, et al. , Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clinical cancer research : an official journal of the American Association for Cancer Research, 2011. 17(5): p. 1147–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guarneri V, et al. , Phase II, randomized trial of preoperative epirubicin-paclitaxel +/− gefitinib with biomarker evaluation in operable breast cancer. Breast Cancer Res Treat, 2008. 110(1): p. 127–34. [DOI] [PubMed] [Google Scholar]
- 97.Smith IE, et al. , A phase II placebo-controlled trial of neoadjuvant anastrozole alone or with gefitinib in early breast cancer. J Clin Oncol, 2007. 25(25): p. 3816–22. [DOI] [PubMed] [Google Scholar]
- 98.Harbeck N, et al. , Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1): an open-label, randomised, phase 3 trial. The Lancet Oncology, 2016. 17(3): p. 357–366. [DOI] [PubMed] [Google Scholar]
- 99.Xu B, et al. , Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. The Lancet Oncology, 2021. 22(3): p. 351–360. [DOI] [PubMed] [Google Scholar]
- 100.Meulendijks D, et al. , First-in-Human Phase I Study of Lumretuzumab, a Glycoengineered Humanized Anti-HER3 Monoclonal Antibody, in Patients with Metastatic or Advanced HER3-Positive Solid Tumors. Clinical Cancer Research, 2016. 22(4): p. 877–885. [DOI] [PubMed] [Google Scholar]
- 101.Schneeweiss A, et al. , Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Investigational New Drugs, 2018. 36(5): p. 848–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krop I, et al. , Abstract PD1–09: Safety and efficacy results from the phase 1/2 study of U3–1402, a human epidermal growth factor receptor 3 (HER3)-directed antibody drug conjugate (ADC), in patients with HER3-expressing metastatic breast cancer (MBC). Cancer Research, 2021. 81(4 Supplement): p. PD1–09–PD1–09. [Google Scholar]


