Abstract
The therapeutic landscape for inflammatory bowel disease (IBD) has changed considerably over the past two decades, owing to the development and widespread penetration of targeted therapies, including biologics and small molecules. While some conventional treatments continue to have an important role in the management of IBD, treatment of IBD is increasingly moving towards targeted therapies given their greater efficacy and safety in comparison to conventional agents. Early introduction of these therapies—particularly in persons with Crohn’s disease—combining targeted therapies with traditional anti-metabolite immunomodulators and targeting objective markers of disease activity (in addition to symptoms), have been shown to improve health outcomes and will be increasingly adopted over time. The substantially increased costs associated with targeted therapies has led to a ballooning of healthcare expenditure to treat IBD over the past 15 years. The introduction of less expensive biosimilar anti-tumour necrosis factor therapies may bend this cost curve downwards, potentially allowing for more widespread access to these medications. Newer therapies targeting different inflammatory pathways and complementary and alternative therapies (including novel diets) will continue to shape the IBD treatment landscape. More precise use of a growing number of targeted therapies in the right individuals at the right time will help minimize the development of expensive and disabling complications, which has the potential to further reduce costs and improve outcomes.
Keywords: Crohn’s disease, Biologic, Biosimilar, Management, Therapy, Ulcerative colitis
Key Points.
Over the past two decades, targeted therapies, including biologics and small molecules, have dramatically changed the treatment landscape and improved quality of life for persons with IBD.
Strategies to optimize the effectiveness of available therapies, such as introducing biologic therapy early in the course of Crohn’s disease, targeting normalization of objective markers of disease remission, and using therapeutic drug monitoring to guide treatment decisions have the potential to improve long-term prognosis and longevity of current medical treatment options.
Randomized controlled trials and real-world studies have demonstrated that biologic therapies are effective and safe for treating IBD. However, proactive use of these therapies early in the disease course and optimizing these therapies based on treatment response along with therapeutic drug monitoring may further improve their effectiveness in clinical practice. Increasing knowledge on how to identify the right therapy for the right person at the right time should further improve long-term outcomes and cost-effectiveness of treatment strategies for persons with IBD.
The latest targeted therapies introduced into clinical practice, including selective anti-interleukin-23 inhibitors, sphingosine-1-phosphate agonists, and Janus kinase-1 inhibitors, have shown promising results in randomized controlled trials and may further improve treatment outcomes for persons with IBD.
Biosimilar anti-tumour necrosis factor agents have shown similar treatment response rates and safety as their bio-originator counterparts. Biosimilars typically have a substantially lower cost, which may favourably bend the cost curve and promote more widespread access to targeted therapies in clinical practice.
Surgery continues to play an important role in IBD management, particularly for stricturing and penetrating complications of Crohn’s disease, perianal fistulizing Crohn’s disease, medically refractory IBD, and intestinal cancers.
Evolving therapies directed at modifying gut bacterial flora (e.g., modified diets, probiotics, and faecal microbiota transplant) have shown promise as potential therapies for IBD, although further research is required in these areas before they can be widely recommended.
INTRODUCTION
The goals of inflammatory bowel disease (IBD) treatment are to induce and maintain disease remission, reduce disease-related complications, prevent permanent bowel damage, and improve quality of life (1). Medical IBD treatment is largely focused on moderating the body’s aberrant immune response to commensal gut bacteria using immunosuppressive therapies. Dietary and alternative medicine treatment strategies, which help modulate inflammation by modifying the gut microbiome, hold promise as therapeutic adjuncts. Additionally, updated goals for IBD treatment and monitoring, including treating to a combined target of sustained bowel healing and elimination of clinical symptoms, may help to improve the long-term IBD course. Despite advances in medical management, surgery continues to play an important role in managing bowel strictures, penetrating complications, perianal disease, and medically refractory intestinal inflammation.
Compared to older non-targeted immunosuppressive therapies, newer classes of IBD treatments, including biologics and small molecules, have shown greater potential to alter the disease trajectory. Newer agents on the horizon will hopefully continue to improve efficacy, safety and tolerability of IBD treatments. The emergence of biosimilar therapies, which have similar effectiveness and safety to bio-originator medications but are typically marketed at a substantially lower cost, may help bend the overall cost curve of biologic therapies downward and improve access to targeted treatments in the future.
In this article, we review current IBD treatments, strategies to optimize available treatments, emerging therapies, and key data supporting current treatment decisions, with a focus on Canadian data where available.
What Are the Available Medical Treatment Options for People with IBD?
The IBD treatment landscape has evolved rapidly over the last several decades, and the pace of this evolution is increasing (2). Prior to the 1960s, corticosteroids were the only medical therapy available to treat IBD. Five-aminosalicylic acid (5-ASA/mesalamine) and immunomodulators were introduced in the 1960s and 1970s. The first anti-tumour necrosis factor α (anti-TNF) biologic therapy (infliximab) was approved for use in Canada in 2001, followed by a second (adalimumab) in 2004. Since then, several additional biologic therapies and small molecules that target specific inflammatory pathways have been introduced (3). A summary of medications to treat IBD that are approved for use in Canada, their routes of administration, primary indications, and major adverse events is provided in Table 1. In general, biologic and other targeted therapies have demonstrated greater efficacy and improved safety as compared to non-targeted immunosuppressive agents. In particular, approved anti-integrin and anti-interleukin (IL) 12/23/anti-IL 23 agents have achieved a very high standard for safety.
Table 1.
Currently available treatments for IBD in Canada
| Drug | Route of administration | Standard maintenance dose schedule | Treatment phase | Type of IBD | Common side effects and serious adverse reactions (selected) |
|---|---|---|---|---|---|
| Topical anti-inflammatory therapies | |||||
| 5-Aminosalicylates (5-ASA, mesalamine):Older generation (oral) • Sulfasalazine (SSZ) Newer generation (oral) • Pentasa • Salofalk • Mezavant • Octasa • Teva 5-ASA Enemas • Salofalk • Pentasa • Mezera Suppositories • Salofalk • Pentasa |
Oral, rectal (suppositories, foams, and enemas) | Oral: Daily Rectal: Daily, though twice weekly has been shown to be beneficial for maintenance of remission |
Induction, maintenance | Mild-to-moderate ulcerative colitis (oral); rectal therapy may be used in distal ulcerative colitis. May be beneficial in mild colonic Crohn’s disease, but this is controversial. |
Common side effects: • Nausea/vomiting • Paradoxical diarrhoea • Allergic hypersensitivity reactions (causing skin rash and mild fever) • Male infertility (SSZ) Serious adverse reactions (rare): • Serositis (pericarditis, serositis, mesenteritis) • Allergic interstitial nephritis • Cytopenias • Pancreatitis • Hepatitis |
| Topical corticosteroids: Budesonide (Entocort, Cortiment) |
Oral, rectal | Oral: Daily Rectal: Daily |
Induction | Mild-to-moderate Crohn’s disease (Entocort) or ulcerative colitis (Cortiment) | See below for corticosteroids N.B. Risk of steroid-related side effects markedly lower than prednisone or solumedrol |
| Non-targeted immunosuppressive therapies | |||||
| Corticosteroids (prednisone, prednisolone, methylprednisolone) | Oral (prednisone) or intravenous (prednisolone, methylprednisolone) | Daily | Induction | Crohn’s disease, ulcerative colitis | Common side effects: • Sleep disturbance • Irritability, mood swings • Mild anxiety or depression • Fluid retention • Increased appetite • Weight gain • Acne • Linear growth delay (children) • Myalgias, arthralgias Serious adverse reactions: • Opportunistic infections • Osteonecrosis • Osteoporosis • Diabetes mellitus • Hypertension • Cataracts • Muscle atrophy • Body fat redistribution (Cushingoid) |
| Cyclosporine | Oral or intravenous | Daily | Induction | Ulcerative colitis | Common side effects: • Hirsutism • Tremor • GI upset • Headache Serious adverse reactions: • Opportunistic infections • Seizures • Renal toxicity • Hypertension |
| Thiopurines (azathioprine, 6-mercaptopurine) | Oral | Daily | Maintenance | Crohn’s disease, ulcerative colitis | Common side effects: • GI upset • Hypersensitivity skin and joint reactions Serious adverse reactions: • Opportunistic infections • Pancreatitis • Bone marrow toxicity • Hepatotoxicity • Lymphoma • Non-melanoma skin cancer |
| Methotrexate | Oral, subcutaneous | Weekly | Maintenance | Crohn’s disease | Common side effects: • Flu-like symptoms • GI upset • Nausea, vomiting • Mucositis Serious adverse reactions: • Bone marrow toxicity • Hepatotoxicity, hepatic fibrosis • Pneumonitis, lung fibrosis |
| Targeted immuno-active therapies | |||||
| Biologics | |||||
| Anti-TNFs | |||||
| Adalimumab (Humira, Abrilada, Adalimumab injection, Amgevita, Hadlima, Hadlima Pushtouch, Hyrimoz, Hulio, Idacio, Simlandi, Hadlima, Yuflyma) | Subcutaneous | Every two weeks | Induction, maintenance | Crohn’s disease, ulcerative colitis | Common side effects: • Injection site reactions • GI upset • Hypersensitivity reactions (skin, joints) • Upper respiratory tract infections • Headache • Nausea Serious adverse reactions: • Opportunistic infections • Drug-induced lupus • Cardiomyopathy • Demyelinating neuropathy • Lymphoma • Melanoma |
| Golimumab (Simponi) | Subcutaneous | Every four weeks | Induction, maintenance | Ulcerative colitis | As per Adalimumab |
| Infliximab (Remicade Inflectra, Ixifi, Renflexis, Remsima, Remsima SC, Avsola) |
Intravenous | Every eight weeks | Induction, maintenance | Crohn’s disease, ulcerative colitis | As per Adalimumab Acute infusion reactions (including anaphylaxis) |
| Anti-integrins | |||||
| Vedolizumab (Entyvio) | Intravenous | Every eight weeks | Induction, maintenance | Crohn’s disease, ulcerative colitis | Common side effects: • Acute infusion reactions (IV) • Injection site reactions (SC) • GI upset • Hypersensitivity reactions (skin, joints) • Upper respiratory tract infections • Headache |
| Anti-IL-12/23s; Anti-IL-23s | |||||
| Ustekinumab (Stelara) (Anti-IL-12/23) |
Intravenous induction followed by subcutaneous maintenance | Every eight weeks | Induction, maintenance | Crohn’s disease, ulcerative colitis | Common side effects: • Injection site reactions • Upper respiratory tract infections • Headache |
| Risankizumab (Skyrizi) (Anti-IL-23) |
Intravenous induction followed by subcutaneous maintenance | Every eight weeks | Induction, maintenance | Crohn’s disease | Common side effects: • Injection site reactions • Upper respiratory tract infections • Headache |
| Small molecules | |||||
| Janus kinase (JAK) inhibitors | |||||
| Tofacitinib (JAK-1/2/3, TYK-2) |
Oral | Twice daily | Induction, maintenance | Ulcerative colitis | Common side effects: • GI upset • Hypersensitivity reactions • Upper respiratory tract infections • Headache • Elevated liver enzymes • Hypercholesterolemia Serious adverse reactions: • Opportunistic infections • Herpes Zoster (shingles) • Venous thromboembolism* • Major cardiovascular events* • Cancers* |
| S1P receptor modulators | |||||
| Ozanimod | Oral | Daily | Induction, maintenance | Ulcerative colitis | Common side effects: • GI upset • Upper respiratory tract infections • Pyrexia • Headache • Elevated liver enzymes Serious adverse reactions:† • Opportunistic infections • Hypertension • Bradycardia (rare) • Progressive multifocal leukoencephalopathy (rare) |
*Only demonstrated in studies in rheumatoid arthritis patients.
†Until more data available, extrapolated from studies in tofacitinib.
What Do Large Canadian Studies Tell Us About the Impact of Newer Anti-TNF Therapies on IBD Outcomes?
Most (4–9), but not all (10), Canadian real-world studies have demonstrated declining trends in IBD-related hospitalizations and/or intestinal surgeries in parallel with the introduction of biologic therapies into the marketplace. However, a recent population-based study in Ontario that corrected for secular trends was not able to demonstrate a significant change in the rates of IBD-specific hospitalizations or intestinal surgeries corresponding to the period following marketplace introduction of infliximab among persons with Crohn’s disease over a 10-year period or among persons with ulcerative colitis over five-year period; this suggests that factors other than anti-TNF therapy may have also contributed to the observed trends in earlier studies (7). Data from Ontario and Manitoba have also shown increasing penetration of anti-TNF therapy in persons with Crohn’s disease over the first decade following market introduction, but very little uptake in individuals with ulcerative colitis; this suggests that underuse of biologic therapies may limit the population-level impact on ulcerative colitis disease course (11, 12). An ongoing multi-provincial study by the Canadian Gasto-Intestinal Epidemiology Consortium with longer follow-up aims to further evaluate the impact of more widespread uptake of biologic and other targeted therapies on IBD outcomes across Canada.
A population-based study from Manitoba further showed that initiation of anti-TNF treatment in the first two years following a diagnosis of Crohn’s disease was associated with 4.5 fewer IBD-specific hospitalizations (95% CI: 2.10), and 10.4 fewer all-cause hospitalizations (95% CI: 3.7, 17.0) per 100 person-years, over the five years following the start of therapy (13). The decrease in IBD-specific and all-cause hospitalizations was most prominent in the latter half of the five-year follow-up period. The adjusted cumulative surgery rate over the five years after beginning anti-TNF therapy was not significantly different between those who began the therapy early or late in the follow-up period (5.7 vs 7.3 operations per 100 person-years; risk difference, −1.6 [95% CI, −4.5, 1.3]). However, when the first year of follow-up after starting anti-TNF therapy is excluded, early anti-TNF therapy was associated with 3.6 fewer surgeries per 100 person-years (95% CI, 1.9, 5.3). Similarly, data from a multicentre study in 552 persons younger than age 17 diagnosed with inflammatory (non-penetrating, nonstricturing) Crohn’s disease between 2008 and 2012 at 28 paediatric gastroenterology centres in North America found that treatment with anti-TNFα therapy within three months of diagnosis was superior to early treatment with an immunomodulator alone (85.3% vs. 60.3% in remission; relative risk: 1.41; 95% CI: 1.14 to 1.75; p = 0.002) in achieving clinical remission at one year (14). A landmark pragmatic randomized controlled trial (RCT) conducted in Belgian and Canadian non-academic centres showed that early introduction of combined immunosuppression with an anti-TNF agent and an anti-metabolite immunomodulator in persons with Crohn’s disease reduced the risk of surgery, hospital admission, and/or serious disease-related complications at 24 months as compared to conventional step-up therapy (27.7% and 35.1%, absolute difference: 7.3%, hazard ratio: 0.73; 95% CI: 0.62 to 0.86; p < 0.001) (15).
A population-based study from Manitoba further showed an annual reduction in corticosteroid use of 3.8% over the past two decades in persons with Crohn’s disease (most marked after 2007) and of 2.5% in persons with ulcerative colitis, which could relate to increasing penetration of biologic therapies and greater recognition of the potential adverse events associated with of long-term corticosteroid use (16). Similar findings were observed in a population-based study from Alberta, with an average annual decline in corticosteroid use of more than 18% among persons with IBD between 2010 and 2015, coinciding with increasing penetration of anti-TNF therapy (17).
Given the relatively recent introduction of other classes of biologic and targeted therapies, limited real-world Canadian data exist for these therapies. Such studies will hopefully inform a future review of the IBD treatment landscape.
HOW DO WE OPTIMALLY USE IBD TREATMENTS TO IMPROVE LONG-TERM DISEASE OUTCOMES?
Several strategies have emerged over the past 10 to 15 years that have improved our ability to optimize the effectiveness of targeted therapies used to treat IBD.
Treat-to-Target
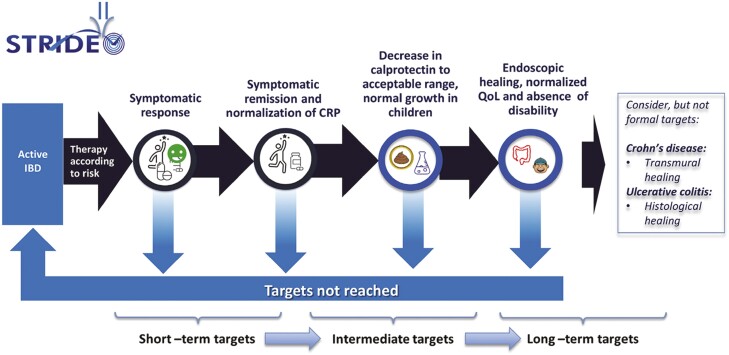
Selecting Therapeutic Targets in Inflammatory Bowel Disease-II (STRIDE-II) was a landmark paper guiding treatment targets in adults and children with IBD (1). In those with IBD experiencing a flare of disease activity, the short-to-intermediate-term goal is to achieve rapid symptomatic response followed by clinical remission and normalization of C-reactive protein (CRP) and faecal calprotectin. The longer-term targets include endoscopic healing, normalization of growth (in children), normal quality of life, and absence of disability (Figure 1). Specific timelines for meeting these targets were not provided in the STRIDE-II document as they may vary across disease phenotypes and treatment agents. In general, short term may be considered as four to six weeks, intermediate term as three to six months and longer term as six to 12 months and beyond. When these targets are not met, a new strategy of managing IBD is required, such as optimizing the dose of therapy, using adjuvant therapies, changing medications, or surgery.
Figure 1.
STRIDE-II.
Achieving treat-to-target (T2T) goals requires regular disease activity surveillance that evaluates a combination of symptoms, blood work, faecal calprotectin, bowel imaging, and endoscopy. The most notable innovation has been targeting objective markers of disease remission, particularly faecal calprotectin and endoscopic and radiographic healing, in combination with reducing symptoms (18–21). Importantly, gastrointestinal symptoms that persist despite achieving objective disease remission are common and may relate to chronic bowel damage or superimposed irritable bowel syndrome that does not require treatments that target IBD (22).
Multiple studies have demonstrated the value of endoscopic healing on long-term IBD prognosis (23–26). A T2T strategy has further been shown to increase the probability of endoscopic healing (18,21). An RCT, the effect of tight control management on Crohn’s disease (CALM), demonstrated that adjusting medical management based on regular disease activity surveillance using symptoms and biomarkers, such as CRP and faecal calprotectin, improves clinical and endoscopic outcomes out to three-years (21). A follow-up Canadian study further demonstrated that this treatment approach is cost-effective relative to symptom-based management (27).
Therapeutic Drug Monitoring
Therapeutic drug monitoring (TDM) of biologic agents has permitted a more scientific method to guide treatment decisions. Such monitoring is most relevant for the anti-TNF class of therapy, as the chimeric nature of the molecules leads to greater potential for the development of anti-drug antibodies and permanent loss of efficacy (28). In the setting of on-going or recurrent disease activity during treatment with a biological agent, the presence of high plasma levels of drug or anti-drug antibodies indicates either a mechanistic switch of disease activity (to another immune phenotype) or the development of irreversible immunogenicity, associated with markedly reduced drug effectiveness and the need to switch to another agent (29,30). Conversely, the presence of low circulating levels of drug or of anti-drug antibodies in the setting of a disease flare may be managed, at least in the short term, through optimizing drug dosage (29,30). An expert international panel unanimously voted a reactive TDM strategy should be used for all biologics to help manage both primary non-response and secondary loss of response (30); this approach has been demonstrated to be cost-effective relative to empiric drug optimization (31). Furthermore, it was recommended that treatment discontinuation should not be considered for infliximab or adalimumab until a drug concentration of at least 10–15 μg/mL is achieved (30). Importantly, the target drug concentration to effect disease activity may vary depending on the disease phenotype, the assay used, and the desired therapeutic outcome; thus, treatment decisions based on TDM should be individualized (30). Some individuals may require higher-than-established drug concentrations to establish optimal long-term disease control, such as individuals with perianal fistulizing Crohn’s disease or small bowel Crohn’s disease (32).
Proactive TDM, a strategy in which biologic treatment escalation or de-escalation is guided purely by plasma drug and/or anti-drug antibody levels, in the absence of objective disease activity, has not demonstrated convincing evidence of benefit with respect to health outcomes and is associated with a substantially higher cost than conventional management (33). However, a large RCT demonstrated that proactive dose adjustment of adalimumab when treating paediatric Crohn’s disease was associated with a higher rate of corticosteroid-free clinical remission, a higher rate of composite sustained corticosteroid-free clinical remission, normal CRP, and normal faecal calprotectin at all visits from weeks eight to 72 when compared to reactive TDM (34). The personalized anti-TNF therapy in Crohn’s disease study, a large prospective UK study in anti-TNF-naïve people aged ≥6 years with active luminal Crohn’s disease at first exposure to infliximab (n = 955) or adalimumab (n = 655), found that week 14 drug concentration was independently associated with the probability of week 14 primary non-response (infliximab, odds ratio [OR]: 0.35; 95% CI: 0.20, 0.62; adalimumab, OR: 0.13; 95% CI: 0.06, 0.28); week 54 non-remission (infliximab, OR: 0.29; 95% CI: 0.16, 0.52; adalimumab, OR: 0.03; 95% CI: 0.01, 0.12) and the development of anti-drug antibodies (infliximab: 62.8%; 95% CI: 59.0, 66.3; adalimumab: 28.5%; 95% CI: 24.0, 32.7) (35). This study further reported that the optimal week 14 drug concentrations associated with remission at both week 14 and week 54 were 7 mg/L for infliximab and 12 mg/L for adalimumab. In light of these data, an international panel voted strongly in favour of proactive TDM once post-induction, at least once during maintenance therapy, after any reactive TDM-based treatment change, and before and after any dose escalation or de-escalation to guide drug dosing of anti-TNF therapy; the panel noted the need for more data to support proactive TDM for other biologic agents (30).
Step-up Versus Top-down Approaches to Therapy With Anti-TNFs
Multiple RCTs have demonstrated that early combined immunosuppression of an anti-TNF and a thiopurine early in the disease course can improve disease outcomes compared with a conventional step-up treatment strategy or anti-TNF monotherapy (15, 36–38). Population-level data from Manitoba and other Canadian provinces have similarly demonstrated a positive impact of early combined immunosuppression on the risk of future IBD-related complications (39,40), as well as earlier introduction of anti-TNF therapy on healthcare utilization in persons with Crohn’s disease (13). The long-term impact on colectomy of early medical intervention for individuals with ulcerative colitis is less clear. At present, the considerably higher costs of targeted therapies as compared to conventional agents limits access to early combined immunosuppression for individuals dependent on public drug coverage. Fortunately, some provinces have recently approved access to biosimilar anti-TNF agents and vedolizumab without the requirement for loss of effectiveness of an immunomodulator.
Choosing the Right Therapy for the Right Person at the Right Time
Given the increasing variety of drugs available to treat IBD, there is great interest in understanding how to choose the right drug for the right person at the right time. To date, only two RCTs have directly compared approved targeted therapies for IBD. In the VARSITY trial, vedolizumab was superior to adalimumab at standard dosing for achieving clinical remission (although not steroid-free remission) in persons with moderate-to-severe ulcerative colitis (41). In the SEAVUE trial, ustekinumab was equivalent to adalimumab in attaining symptomatic and endoscopic remission in persons with Crohn’s disease. While there are other head-to-head trials ongoing, these will only assess a fraction of the possible comparisons that can potentially be made between drugs. Network meta-analyses (NMA) have tried to fill in some of these data gaps through indirect comparisons. Recent NMAs have suggested that anti-TNFs and anti-IL-23 therapies may be superior to other therapies for Crohn’s disease, and anti-TNF (infliximab), anti-IL-23, and Janus kinase (JAK) inhibitor therapies (tofacitinib) may be most effective for ulcerative colitis, while anti-integrin therapy (vedolizumab) ranks highest for safety (42–44). Importantly, differences in the design and populations of studies included in NMAs may account for some of the apparent differences. Further research will be required to identify which specific sequence or combination of available agents, possibly in conjunction with other strategies of medical treatment (such as modulation of gut bacteria), optimally impacts long-term disease prognosis in individuals.
HOW WILL BIOSIMILAR AGENTS IMPACT TREATMENT OPTIONS FOR CANADIANS WITH IBD?
A biosimilar medication must show a high degree of similarity to the original product and have no clinically meaningful differences in safety, purity, or potency (45). As several anti-TNF bio-originator compounds have now exhausted their patency, multiple biosimilar anti-TNF agents have been developed and are now commercially available, often at lower prices than their originator molecules. Given that spending on biologic medications is currently responsible for the majority of direct healthcare spending in IBD (see Kuenzig et al., this volume), the availability of less expensive alternatives could yield substantial cost savings. As a result, many provinces and insurers have been instituting policies that favour the use of biosimilar formulations of infliximab and adalimumab in place of their bio-originator forms (Remicade and Humira, respectively). In addition, six Canadian provinces have either instituted or have imminent plans to institute a mandatory switch policy, whereby the majority of current users of Remicade and Humira will be required to switch to a corresponding biosimilar to maintain insurance coverage.
Most studies comparing bio-originator to biosimilar anti-TNF therapies for anti-TNF-naïve individuals have not shown any meaningful differences in objective IBD-related or safety outcomes (46–48). A systematic review of clinical trials that investigated biosimilar infliximab in anti-TNF-naïve people with IBD and in people with IBD who switched from originator infliximab also did not show any significant differences in efficacy or safety between the originator infliximab and its biosimilar. However, a recent systematic review and position statement released by the Canadian Association of Gastroenterology and Crohn’s and Colitis Canada reported that very low-quality evidence, according to GRADE criteria, does not support nonmedical switching to biosimilar infliximab in persons who have stable IBD and are doing well on the originator product due to an increased risk of worsening disease, dose escalation, and/or switching to an alternative. (45). This position is shared by some, but not all, national societies. Nonetheless, given the potential cost savings and minimal risk of disease recurrence associated with non-medical switching, most North American and European societies, concur that this is an acceptable approach if agreed upon by both the physician and the individual (45,49–53). Conversely, the overwhelming majority of societies do not support the mandatory substitution of a biosimilar agent for the originator agent in all individuals due to a paucity of evidence for the efficacy and safety of this approach (45,49). Crohn’s and Colitis Canada has suggested the use of a risk matrix (https://crohnsandcolitis.ca/Get-Involved/Advocating-for-change/Non-Medical-Switch-Biosimilars) to guide biosimilar switching in persons with IBD. The position statement by the Canadian Association of Gastroenterology and Crohn’s and Colitis Canada did provide a weak recommendation that an infliximab biosimilar could be started in people with active Crohn’s disease who are naïve to anti-TNF therapy, for cost reasons, but noted that there were insufficient data to recommend the use of biosimilars in people with active ulcerative colitis naïve to infliximab.
Importantly, it has been reported that 10%–20% of individuals may experience a nocebo effect with biosimilar switching (an increase in symptoms that follow a perception of a change in therapy) (54,55), particularly those who have high levels of anxiety and a tendency toward catastrophization (56). The impact of nocebo effects can be mitigated through involvement of the individual in the decision-making process, setting expectations of a positive outcome, and identification of persons in who may be at higher risks for developing nocebo effects (54).
While more data are needed, in the absence of a significant observable deterioration in individual health outcomes in most studies to date evaluating biosimilar start or switch, biosimilar agents offer an attractive solution to the ballooning costs associated with IBD therapies. What is less clear is to what extent these cost savings will be re-invested to the betterment of IBD care and research. At a minimum, the lower costs associated with biosimilar agents, alongside the parallel lowering of costs of their bio-originator counterparts, should allow for improved access to these agents. Several provincial drug formularies have now agreed to fund biosimilar anti-TNF therapies for individuals with IBD irrespective of prior failure of conventional antimetabolite immunomodulating agents, which is not the case for most bio-originator drugs.
WHAT’S ON THE HORIZON FOR IBD TREATMENT?
In the last year, two new drugs were approved by Health Canada for the treatment of IBD. Risankizumab (Skyrizi) was approved for the treatment of moderate-to-severe Crohn’s disease, and ozanimod (Zeposia) was approved for the treatment of moderate-to-severe ulcerative colitis. Upadacitinib (Rinvoq) is also being considered for approval in Canada.
Risankizumab is the first drug that specifically targets IL-23 approved for Crohn’s disease (57,58). It has been approved in Canada since 2019 for psoriatic arthritis and plaque psoriasis, and, thus far, has not demonstrated any increased risk of serious complications in comparison to other medications (59). A head-to-head trial comparing risankizumab to ustekinumab in Crohn’s disease is underway (60). A head-to-head trial of these agents in persons with plaque psoriasis has demonstrated superiority of risankizumab over ustekinumab (61).
Ozanimod is an oral sphingosine-1-phosphate (S1P) inhibitor, and is the first drug in its class approved in Canada for moderate-to-severe ulcerative colitis (62). It has been approved in Canada since 2017 for multiple sclerosis and has been shown to have an excellent safety profile over the long-term in this condition (63,64).
Upadacitinib is a JAK-1-specific inhibitor that, in its initial trials, has yielded remission and response rates higher than those seen with other drugs (65). Though there have not been head-to-head comparisons, indirect comparisons with other therapies suggest that upadacitinib may be one of the more effective therapies currently available for ulcerative colitis (66). Concerns have been raised about JAK inhibitor users being at higher risk of cardiovascular, cancerous, and thromboembolic complications based on studies evaluating another JAK-inhibitor (tofacitinib) in elderly persons with rheumatoid arthritis (67). The only serious adverse event that has been consistently associated with this class of medications in persons with ulcerative colitis is herpes zoster (i.e., shingles), typically with higher dose, long-term usage (68). However, this risk is also elevated in people using anti-TNFs and may be reduced through vaccination against herpes zoster virus, as suggested for all adults with IBD on immunosuppression by clinical practice guidelines from the Canadian Association of Gastroenterology (69).
Agents that currently have active research programs in IBD and may become available in coming years include other selective JAK inhibitors (filgotinib for ulcerative colitis and Crohn’s disease (70,71), upadacitinib for Crohn’s disease (72)), other selective IL-23 inhibitors (mirikizumab and guselkumab for Crohn’s disease and ulcerative colitis (73,74)), other S1P receptor modulators (ozanimod for Crohn’s disease, etrasimod for ulcerative colitis (75)) and mesenchymal stem cell treatment for perianal fistulizing Crohn’s disease (76). Health Canada approval of these agents will depend on their efficacy and safety in ongoing clinical trials, and public drug coverage will depend on the ability to demonstrate that these agents will achieve meaningful improvements in the quality of life for Canadians with IBD at a reasonable cost. Most likely, these agents will initially be funded as second or third-line agents in public drug benefits programs across Canada. Crohn’s and Colitis Canada will continue to advocate for Canadians living with IBD to receive the best available treatments when they are required.
WHAT IS THE ROLE OF ALTERNATIVE AND MICROBIOME-ALTERING THERAPIES IN IBD CARE?
The application of treatments that are neither Health Canada-approved immune-modulating medications nor surgery in the management of IBD, known as complementary and alternative medicine (CAM), are common among persons with IBD. The prevalence of CAM use has been estimated to be as high as 50% in some studies (77–80). There are many potential contributors to the use of CAM in these individuals, including lack of efficacy of conventional therapies, safety concerns with conventional therapies, and improved sense of control over the disease (79). Despite the high prevalence of CAM use, there is limited data demonstrating its efficacy in the treatment of IBD. In recent years, however, there has been emerging data evaluating microbiome-altering therapies such as faecal microbiota transplantation (FMT) and probiotics.
The composition of the gut microbiome has been shown to have a significant influence on the body’s immune response, and changes in the microbiome have been implicated in the development of IBD and flares (81). As a result, there is great interest in emerging therapies that seek to restore a healthy microbiome, such as FMT and probiotics, in the hopes that this will decrease intestinal inflammation and reduce symptom burden. In FMT, faecal material from a healthy individual is introduced via enema, colonoscopy, or nasogastric tube into the intestine of someone with IBD; the aim is to supplant the microbiome of the recipient with that of the healthy donor. A recent meta-analysis of six RCTs found that FMT was associated with higher odds of clinical and endoscopic remission as compared to placebo in persons with ulcerative colitis (OR: 4.11; 95% CI: 2.19, 7.72), with no difference in the risk of side effects (82). A more recent study of 66 people with ulcerative colitis in clinical remission who were randomized to either the combination of FMT and an anti-inflammatory diet or standard medical therapy noted higher rates of clinical and endoscopic remission at 48 weeks (25% vs. 0%, p = 0.007) in the FMT arm (83). There is less evidence supporting the role of FMT in maintenance of remission in ulcerative colitis or in the treatment of Crohn’s disease. Two RCTs evaluating the role of FMT in Crohn’s disease have shown improvements in short-term clinical remission rates (84,85). Most other studies evaluating the role of FMT in Crohn’s disease are limited by small study size, heterogeneity, and publication bias. Though the FMT data is promising in ulcerative colitis, it is still not available as a therapeutic strategy for IBD outside of clinical trials.
Probiotics, defined as products containing specific strains of live microorganisms that can be taken orally, are also commonly used by individuals with IBD, despite a lack of convincing evidence on effectiveness or safety. The American Gastroenterology Association recently issued Clinical Practice Guidelines stating that there is no evidence of benefit to any probiotic for either induction or maintenance of remission and suggested that probiotics should only be used in the context of clinical trials. This document did make a conditional recommendation for a specific 8-strain probiotic combination in the treatment of individuals with pouchitis based on a review of seven studies (86), four of which supported a role of this probiotic in the prevention of pouchitis flares (87–90). The quality of evidence was rated as very low.
WHAT IS THE ROLE OF DIET IN IBD?
Many people look to dietary therapy as either an alternative or an adjuvant treatment to conventional IBD management. To date, the strongest evidence to support dietary therapy is in paediatric IBD. Exclusive enteral nutrition (EEN), whereby individuals receive all nutritional intake through a formula for up to 12 weeks delivered orally, via nasogastric tube, or a gastrostomy tube, has been shown to be effective in induction of remission in Crohn’s disease (91,92). While this treatment requires a significant commitment from both the individual and their caregivers and adherence can be a challenge, some guidelines favour it over corticosteroids, particularly in children with a history of delayed growth (93,94). Partial enteral nutrition is another nutritional therapy that may be better tolerated than EEN. A 2019 study evaluated a combination of partial enteral nutrition with a specific whole foods-based exclusion diet (the Crohn’s disease exclusion diet [CDED]) (95). Participants were randomized to receive partial enteral nutrition and the CDED for 12 weeks or EEN for six weeks, then transition to partial enteral nutrition and a free diet. The CDED consists of a diet that avoided or reduced exposure to foods containing animal/dairy fat, high fat from other sources, wheat, red or processed meat and protein sources rich in taurine, emulsifiers, artificial sweeteners, carrageenans, and sulphites. The second phase step-down diet involves higher exposure to fruits, vegetables, and legumes along with some foods that are reintroduced with restrictions to increase food flexibility and relieve monotony. Among 74 participants at week six, 75% of children given CDED plus partial enteral nutrition were in corticosteroid-free remission versus 59% given EEN. At week 12, 75.6% of children given CDED plus partial enteral nutrition were in corticosteroid-free remission versus 45.1% given EEN. Partial nutrition and CDED were also better tolerated than EEN.
The data to support of the use of dietary therapy in adults with IBD is not as strong. A Cochrane systematic review on diet for induction of remission in Crohn’s disease concluded that all studies provided low or very low-quality evidence (96). One of the most common therapeutic diets used by adults is the Specific Carbohydrate Diet (SCD) (97). The Mediterranean diet has also become increasingly popular and several studies have identified a lower risk of Crohn’s disease among populations consuming this diet, which consists of fruits, vegetables, nuts, fish, whole grains, and use of olive oil as the predominant fat source (98). A recent study compared these two diets (99). Adults with Crohn’s disease were randomly assigned 1:1 to consume the Mediterranean or SCD for 12 weeks. The primary outcome was symptomatic remission at week six. Among the 194 participants, SCD was not superior to Mediterranean diet to achieve symptomatic remission, faecal calprotectin response, or CRP response. The authors concluded that the greater ease of following the Mediterranean diet and its other health benefits make it preferred to the SCD for most individuals with Crohn’s disease.
WHAT IS THE ROLE OF SURGERY IN IBD MANAGEMENT?
Surgery, once the mainstay of IBD treatment, continues to play an important role in the management of IBD due to failure or inadequate usage of medical therapy or the development of disease-related complications. Often, surgery complements medical therapy, particularly for perianal fistulizing disease and fibrostenotic small bowel disease. Common reasons for IBD surgery include perianal abscesses or persistently draining fistula tracts, fibrotic intestinal strictures resulting in bowel obstruction, penetrating intestinal complications (such as intra-abdominal abscess or enteric fistulas), treatment-refractory disease resulting in persistent and/or rapidly escalating disease activity (sometimes associated with complications such as toxic megacolon or bowel perforation), and intestinal cancer. A multicenter RCT from the Netherlands and the United Kingdom in persons with non-stricturing ileocecal Crohn’s disease affecting <40 cm of small bowel, in whom conventional therapy had failed, demonstrated that surgical resection was associated with similar health outcomes and quality of life as infliximab treatment and was more cost-effective in persons with limited small bowel Crohn’s disease (100,101). Close collaboration between medical and surgical IBD specialists is important for the management of complex IBD phenotypes.
CONCLUSION
The IBD therapeutic landscape and treatment goals have changed dramatically over the past two decades, and we can anticipate further changes in the years to come as more drugs with different mechanisms of action and a greater number of biosimilar agents gain Health Canada approval. An improved understanding of matching drugs and treatment strategies to individuals may lead to better health outcomes, prevent complications, and improve quality of life.
KNOWLEDGE GAPS AND FUTURE RESEARCH DIRECTIONS
Understanding the factors that may predict individual-level response to drugs with specific mechanisms of action will allow physicians to choose the right therapies for the right individual at the right time.
Understanding the changes in the immune system that lead to loss of response to a previously effective medication may help mitigate loss of effectiveness or change the choice of individual therapy.
RCTs and real-world evidence to understand the comparative effectiveness of different types and combinations of medical therapies in persons with specific IBD subtypes, as well as pragmatic trials and real-world evidence to better understand how to optimize the usage and sequencing of medical therapies in clinical practice will better enable T2T and personalized treatment strategies.
Real-world evidence is required to inform the effectiveness and safety of newer biosimilar agents that are entering the marketplace.
Future clinical trials and observational studies of IBD therapies should aim to include under-represented populations, such as Indigenous peoples, pregnant people, paediatrics, seniors, and immigrants.
PATIENT AND CAREGIVER PARTNER PERSPECTIVE
This article provided patient partners with hope and peace of mind particularly related to the ongoing research towards developing new medication options to treat IBD. Partners were also reassured from the research related to the safety, and efficacy of biosimilars as more provinces enforce a non-medical switch from biologics. The availability of biosimilars can enhance access to life-changing medications for persons with IBD. Patient partners encouraged greater education for patients and caregivers related to the safety and efficacy of switching from biologic to biosimilar medications to decrease anxiety related to non-medical switching. Partners encouraged individuals with IBD who are non-medically switching to biosimilar medications to maintain positive thoughts about the switch to prevent nocebo effects. Selecting the right therapy for the right person at the right time was seen as an important strategy to adopt. Also, combination therapies for certain individuals could result in better outcomes. Partners recognized that complementary therapies have potential roles as treatment adjuncts, particularly in individuals living with ulcerative colitis.
POLICY IMPLICATIONS AND KEY ADVOCACY OUTCOMES
All Health Canada-approved therapies to treat IBD should be accessible to individuals when deemed necessary to control their disease by prescribing physicians. Advocacy should target barriers to accessing the safest and most effective medications as part of shared decision-making between the individual and the physician.
Increasing acceptance of biosimilar agents by individuals with IBD and their practitioners should be encouraged to help control the rate of rising drug costs to treat IBD, enhance competitive pricing of biotherapies and improve access to biotherapies.
Non-medical switch policies should consider the patient experience and integrate both the risk of increased disease activity and the impact of disease on the individual and family. Use of Crohn's and Colitis Canada' Risk Matrix to help guide non-medical switch policies implemented by provincial health ministries is encouraged. Vigilance should be maintained to ensure that new biosimilar agents that enter the marketplace are held to the same standards for efficacy and safety as their bio-originator counterparts.
Healthcare practitioners and afflicted individuals should be informed of the nocebo effect and tools (including mental health support) should be introduced to help mitigate it in those forced to switch.
The cost savings realized from increasing use of biosimilar agents should be redirected towards improving access to targeted therapies and diagnostic testing for persons with IBD and increasing research funding for IBD.
Tools required to monitor individuals according to a T2T approach, including, but not limited to endoscopy, cross-sectional imaging, and faecal calprotectin should be readily available to practitioners to assist with IBD management.
Scientific literature on the evidence for IBD treatment options should be free and accessible to all Canadians to better inform individuals and health care providers as to the options and modifiable behaviours for disease control.
SUPPLEMENT SPONSORSHIP
This article appears as part of the supplement “The Impact of Inflammatory Bowel Disease in Canada in 2023”, sponsored by Crohn’s and Colitis Canada, and supported by Canadian Institutes of Health Research Project Scheme Operating Grant (Reference number PJT-162393).
Contributor Information
Sanjay K Murthy, Department of Medicine, University of Ottawa, Ottawa, Ontario, Canada; The Ottawa Hospital IBD Centre, Ottawa, Ontario, Canada.
Adam V Weizman, Division of Gastroenterology and Hepatology, Mount Sinai Hospital, University of Toronto, Toronto, Ontario, Canada; Department of Medicine, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada.
M Ellen Kuenzig, SickKids Inflammatory Bowel Disease Centre, Division of Gastroenterology, Hepatology, and Nutrition, The Hospital for Sick Children, Toronto, Ontario, Canada; Child Health Evaluative Sciences, SickKids Research Institute, The Hospital for Sick Children, Toronto, Ontario, Canada.
Joseph W Windsor, Department of Medicine, University of Calgary, Calgary, Alberta, Canada; Department of Community Health Sciences, University of Calgary, Calgary, Alberta, Canada.
Gilaad G Kaplan, Department of Medicine, University of Calgary, Calgary, Alberta, Canada; Department of Community Health Sciences, University of Calgary, Calgary, Alberta, Canada.
Eric I Benchimol, SickKids Inflammatory Bowel Disease Centre, Division of Gastroenterology, Hepatology, and Nutrition, The Hospital for Sick Children, Toronto, Ontario, Canada; Child Health Evaluative Sciences, SickKids Research Institute, The Hospital for Sick Children, Toronto, Ontario, Canada; ICES, Toronto, Ontario, Canada; Department of Paediatrics, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Institute of Health Policy, Management, and Evaluation, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada.
Charles N Bernstein, Department of Internal Medicine, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Manitoba, Canada; University of Manitoba IBD Clinical and Research Centre, Winnipeg, Manitoba, Canada.
Alain Bitton, Division of Gastroenterology and Hepatology, McGill University Health Centre IBD Centre, McGill University, Montréal, Quebec, Canada.
Stephanie Coward, Department of Medicine, University of Calgary, Calgary, Alberta, Canada; Department of Community Health Sciences, University of Calgary, Calgary, Alberta, Canada.
Jennifer L Jones, Departments of Medicine, Clinical Health, and Epidemiology, Dalhousie University, Halifax, Nova Scotia, Canada.
Kate Lee, Crohn’s and Colitis Canada, Toronto, Ontario, Canada.
Juan-Nicolás Peña-Sánchez, Department of Community Health and Epidemiology, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
Noelle Rohatinsky, College of Nursing, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
Sara Ghandeharian, Crohn’s and Colitis Canada, Toronto, Ontario, Canada.
Nasruddin Sabrie, Department of Medicine, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada.
Sarang Gupta, Department of Medicine, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada.
Gurmun Brar, Department of Medicine, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada.
Rabia Khan, SickKids Inflammatory Bowel Disease Centre, Division of Gastroenterology, Hepatology, and Nutrition, The Hospital for Sick Children, Toronto, Ontario, Canada; Child Health Evaluative Sciences, SickKids Research Institute, The Hospital for Sick Children, Toronto, Ontario, Canada; ICES, Toronto, Ontario, Canada.
James H B Im, SickKids Inflammatory Bowel Disease Centre, Division of Gastroenterology, Hepatology, and Nutrition, The Hospital for Sick Children, Toronto, Ontario, Canada; Child Health Evaluative Sciences, SickKids Research Institute, The Hospital for Sick Children, Toronto, Ontario, Canada.
Tal Davis, SickKids Inflammatory Bowel Disease Centre, Division of Gastroenterology, Hepatology, and Nutrition, The Hospital for Sick Children, Toronto, Ontario, Canada; Child Health Evaluative Sciences, SickKids Research Institute, The Hospital for Sick Children, Toronto, Ontario, Canada.
Jake Weinstein, SickKids Inflammatory Bowel Disease Centre, Division of Gastroenterology, Hepatology, and Nutrition, The Hospital for Sick Children, Toronto, Ontario, Canada; Child Health Evaluative Sciences, SickKids Research Institute, The Hospital for Sick Children, Toronto, Ontario, Canada.
Joëlle St-Pierre, Department of Medicine, University of Calgary, Calgary, Alberta, Canada.
Roxana Chis, Department of Gastroenterology and Hepatology, Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada.
Saketh Meka, Department of Neuroscience, McGill University, Montreal, Quebec, Canada.
Eric Cheah, Department of Gastroenterology and Clinical Nutrition, The Royal Children’s Hospital Melbourne, Parkville, Australia.
Quinn Goddard, Department of Medicine, University of Calgary, Calgary, Alberta, Canada; Department of Community Health Sciences, University of Calgary, Calgary, Alberta, Canada.
Julia Gorospe, Department of Medicine, University of Calgary, Calgary, Alberta, Canada; Department of Community Health Sciences, University of Calgary, Calgary, Alberta, Canada.
Jack Kerr, Department of Medicine, Memorial University of Newfoundland, St John’s Newfoundland, Canada.
Kayla D Beaudion, Department of Interdisciplinary Science, McMaster University, Hamilton, Ontario, Canada.
Ashley Patel, Crohn’s and Colitis Canada, Toronto, Ontario, Canada.
Sophia Russo, Department of Anesthesiology, Pharmacology, and Therapeutics, University of British Columbia, Vancouver, British Colombia, Canada.
Jonathan Blyth, Crohn’s and Colitis Canada, Toronto, Ontario, Canada.
Stephanie Blyth, Crohn’s and Colitis Canada, Toronto, Ontario, Canada.
Diane Charron-Bishop, Crohn’s and Colitis Canada, Toronto, Ontario, Canada.
Laura E Targownik, Division of Gastroenterology and Hepatology, Mount Sinai Hospital, University of Toronto, Toronto, Ontario, Canada.
FUNDING
Funding for this report was supported by AbbVie Corporation, Janssen Canada, Pfizer Canada, Bristol Myers Squibb Canada, Amgen Canada and Takeda. None of the funders influenced the content of the report.
CONFLICT OF INTEREST
Sanjay K. Murthy has previously participated in advisory board meetings for AbbVie, Janssen, Takeda, Pfizer, Shire and Ferring and as a speaker at educational events sponsored by Janssen, AbbVie and Pfizer. Adam V. Weizman has been a speaker for AbbVie, Janssen, Viatris, Amgen and Pendopharm and is on the advisory boards for AbbVie, Takeda and Ferring. Ellen Kuenzig is a member of the Scientific and Medical Advisory Council of Crohn’s and Colitis Canada. Gilaad G. Kaplan has received honoraria for speaking or consultancy from AbbVie, Janssen, Pfizer and Takeda. He has received research support from Ferring, Janssen, AbbVie, GlaxoSmith Kline, Merck and Shire. He has been a consultant for Gilead. He shares ownership of a patent: TREATMENT OF INFLAMMATORY DISORDERS, AUTOIMMUNE DISEASE, AND PBC. UTI Limited Partnership, assignee. Patent WO2019046959A1. PCT/CA2018/051098. September 7, 2018. He is a member of the Scientific and Medical Advisory Council of Crohn’s and Colitis Canada. Eric I. Benchimol holds the Northbridge Financial Corporation Chair in Inflammatory Bowel Disease, a joint Hospital-University Chair between the University of Toronto, The Hospital for Sick Children and the SickKids Foundation. He has acted as a consultant for the Dairy Farmers of Ontario and McKesson Canada for matters unrelated to medications used to treat inflammatory bowel disease. He is Past Chair of the Scientific and Medical Advisory Council of Crohn’s and Colitis Canada and Editor-in-Chief of the Journal of the Canadian Association of Gastroenterology (JCAG). Charles N. Bernstein is supported in part by the Bingham Chair in Gastroenterology. He is on Advisory Boards for AbbVie Canada, Amgen Canada, Bristol Myers Squibb, JAMP Pharmaceuticals, Lilly Canada, Janssen Canada, Pfizer Canada, Roche Canada, Sandoz Canada, Takeda Canada. He is a Consultant for Mylan Pharmaceuticals and Takeda. He has received educational grants from AbbVie Canada, Pfizer Canada, Takeda Canada, Janssen Canada and Bristol Myers Squibb Canada. He is on the speaker’s panel for AbbVie Canada, Janssen Canada, Pfizer Canada and Takeda Canada. Received research funding from AbbVie Canada, Amgen Canada, Pfizer Canada, Sandoz Canada. Alain Bitton has participated in advisory boards with AbbVie, Janssen, Takeda, McKesson, BioJamp, Bristol Myers Squibb Hoffman-LaRoche, Amgen. He has received research support from AbbVie. He is on the speaker’s panel for Janssen, Takeda, AbbVie and has participated in educational activities supported by Viatris, has received educational support from Fresenius Kabi, Amgen and Takeda. Jennifer L. Jones has received honoraria for speaking and consulting for AbbVie, Janssen, Pfizer, Shire and Takeda. She is the co-chair of the Scientific and Medical Advisory Council of Crohn’s and Colitis Canada. Kate Lee has received honoraria from AbbVie Corporation and Bristol Myers Squibb Canada. Ashley Patel has received an AbbVie IBD scholarship. Sophia Russo has received an AbbVie IBD scholarship. Laura E. Targownik has received research funding from AbbVie Canada, Takeda Canada, Sandoz Canada, Amgen Canada, Gilead Canada, Roche Canada and Pfizer Canada, and has been on Advisory Boards for Janssen Canada, AbbVie Canada, Takeda Canada, Pfizer Canada, Merck Canada, Roche Canada, Sandoz Canada, Organon Canada, Fresesnius Kabi Canada, Eli Lilly Canada and Amgen Canada. She is a member of the Scientific and Medical Advisory Council of Crohn’s and Colitis Canada. None: Joseph W. Windsor, Stephanie Coward, Juan-Nicolás Peña-Sánchez, Noelle Rohatinsky, Sara Ghandeharian, Nasruddin Sabrie, Sarang Gupta, Gurmun Brar, Rabia Khan, James H. B. Im, Tal Davis, Jake Weinstein, Joëlle St-Pierre, Roxana Chis, Saketh Meka, Eric Cheah, Quinn Goddard, Julia Gorospe, Jack Kerr, Kayla D. Beaudoin, Jonathan Blyth, Stephanie Blyth, and Diane Charron-Bishop.
DATA AVAILABILITY
No new data were generated or analyzed in support of this review.
REFERENCES
- 1. Turner D, Ricciuto A, Lewis A, et al. ; International Organization for the Study of IBD. STRIDE-II: An update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): Determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–83. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 2. Mulder DJ, Noble AJ, Justinich CJ, Duffin JM.. A tale of two diseases: The history of inflammatory bowel disease. J Crohns Colitis. 2014;8:341–8. doi: 10.1016/j.crohns.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 3. Baumgart DC, Le Berre C.. Newer biologic and small-molecule therapies for inflammatory bowel disease. N Engl J Med. 2021;385:1302–15. doi: 10.1056/NEJMra1907607. [DOI] [PubMed] [Google Scholar]
- 4. Ma C, Moran GW, Benchimol EI, et al. Surgical rates for Crohn’s disease are decreasing: A population-based time trend analysis and validation study. Am J Gastroenterol. 2017;112:1840–8. doi: 10.1038/ajg.2017.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moore SE, McGrail KM, Peterson S, et al. Infliximab in ulcerative colitis: The impact of preoperative treatment on rates of colectomy and prescribing practices in the province of British Columbia, Canada. Dis Colon Rectum. 2014;57:83–90. [DOI] [PubMed] [Google Scholar]
- 6. Reich KM, Chang HJ, Rezaie A, et al. The incidence rate of colectomy for medically refractory ulcerative colitis has declined in parallel with increasing anti-TNF use: A time-trend study. Aliment Pharmacol Ther. 2014;40:629–38. [DOI] [PubMed] [Google Scholar]
- 7. Murthy SK, Begum J, Benchimol EI, et al. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: A population-based interrupted time series study. Gut. 2020;69:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dittrich AE, Sutton RT, Haynes K, Wang H, Fedorak RN, Kroeker KI.. Incidence rates for surgery in Crohn’s disease have decreased: A population-based time-trend analysis. Inflamm Bowel Dis. 2020;26:1909–16. doi: 10.1093/ibd/izz315. [DOI] [PubMed] [Google Scholar]
- 9. Dheri AK, Kuenzig ME, Mack DR, et al. Shifting health care use from hospitalisations and surgeries to outpatient visits in children with inflammatory bowel disease: A population-based cohort study from Ontario, Canada. J Crohns Colitis. 2021;15:1991–2000. doi: 10.1093/ecco-jcc/jjab095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verdon C, Reinglas J, Coulombe J, et al. No change in surgical and hospitalization trends despite higher exposure to anti-tumor necrosis factor in inflammatory bowel disease in the Québec provincial database from 1996 to 2015. Inflamm Bowel Dis. 2021;27:655–61. doi: 10.1093/ibd/izaa166. [DOI] [PubMed] [Google Scholar]
- 11. Targownik LE, Tennakoon A, Leung S, Lix LM, Singh H, Bernstein CN.. Temporal trends in initiation of therapy with tumor necrosis factor antagonists for patients with inflammatory bowel disease: A population-based analysis. Clin Gastroenterol Hepatol. 2017;15:1061–70.e1. doi: 10.1016/j.cgh.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 12. Murthy SK, Begum J, Benchimol EI, et al. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: A population-based interrupted time series study. Gut. 2020;69:274–82. doi: 10.1136/gutjnl-2019-318440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Targownik LE, Bernstein CN, Benchimol EI, et al. Earlier anti-TNF initiation leads to long-term lower health care utilization in Crohn’s disease but not in ulcerative colitis. Clin Gastroenterol Hepatol. 2022;20:2607–18.e14. doi: 10.1016/j.cgh.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 14. Walters TD, Kim MO, Denson LA, et al. ; PRO-KIIDS Research Group. Increased effectiveness of early therapy with anti-tumor necrosis factor-α vs an immunomodulator in children with Crohn’s disease. Gastroenterology. 2014;146:383–91. doi: 10.1053/j.gastro.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 15. Khanna R, Bressler B, Levesque BG, et al. ; REACT Study Investigators. Early combined immunosuppression for the management of Crohn’s disease (REACT): A cluster randomised controlled trial. Lancet. 2015;386:1825–34. doi: 10.1016/S0140-6736(15)00068-9. [DOI] [PubMed] [Google Scholar]
- 16. Targownik LE, Bernstein CN, Benchimol EI, et al. Trends in corticosteroid use during the era of biologic therapy: A population-based analysis. Am J Gastroenterol. 2021;116:1284–93. doi: 10.14309/ajg.0000000000001220. [DOI] [PubMed] [Google Scholar]
- 17. Seow CH, Coward S, Kroeker KI, et al. Declining corticosteroid use for inflammatory bowel disease across Alberta: A population-based cohort study. J Can Assoc Gastroenterol. 2022;5:276–86. doi: 10.1093/jcag/gwac021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: A proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13:1042–50.e2. [DOI] [PubMed] [Google Scholar]
- 19. Bouguen G, Levesque BG, Pola S, et al. Endoscopic assessment and treating to target increase the likelihood of mucosal healing in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:978–85. [DOI] [PubMed] [Google Scholar]
- 20. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 21. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–89. [DOI] [PubMed] [Google Scholar]
- 22. Fairbrass KM, Costantino SJ, Gracie DJ, Ford AC.. Prevalence of irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease in remission: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:1053–62. doi: 10.1016/S2468-1253(20)30300-9. [DOI] [PubMed] [Google Scholar]
- 23. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 24. Froslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group. Mucosal healing in inflammatory bowel disease: Results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–22. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 25. Arias MT, Vande CN, Vermeire S, et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:531–8. [DOI] [PubMed] [Google Scholar]
- 26. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15:1295–301. [DOI] [PubMed] [Google Scholar]
- 27. Lakatos PL, Kaplan GG, Bressler B, et al. Cost-effectiveness of tight control for Crohn’s disease with adalimumab-based treatment: Economic evaluation of the CALM trial from a Canadian perspective. J Can Assoc Gastroenterol. 2022;5:169–76. doi: 10.1093/jcag/gwac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strand V, Balsa A, Al-Saleh J, et al. Immunogenicity of biologics in chronic inflammatory diseases: A systematic review. BioDrugs. 2017;31:299–316. doi: 10.1007/s40259-017-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khanna R, Sattin BD, Afif W, et al. Review article: A clinician’s guide for therapeutic drug monitoring of infliximab in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:447–59. [DOI] [PubMed] [Google Scholar]
- 30. Cheifetz AS, Abreu MT, Afif W, et al. A comprehensive literature review and expert consensus statement on therapeutic drug monitoring of biologics in inflammatory bowel disease. Am J Gastroenterol. 2021;116:2014–25. doi: 10.14309/ajg.0000000000001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marquez-Megias S, Nalda-Molina R, Sanz-Valero J, et al. Cost-effectiveness of therapeutic drug monitoring of anti-TNF therapy in inflammatory bowel disease: A systematic review. Pharmaceutics. 2022;14:1009. doi: 10.3390/pharmaceutics14051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grossberg LB, Cheifetz AS, Papamichael K.. Therapeutic drug monitoring of biologics in Crohn’s disease. Gastroenterol Clin North Am. 2022;51:299–317. doi: 10.1016/j.gtc.2021.12.007. [DOI] [PubMed] [Google Scholar]
- 33. Nguyen NH, Solitano V, Vuyyuru SK, et al. Proactive therapeutic drug monitoring versus conventional management for inflammatory bowel diseases: A systematic review and meta-analysis. Gastroenterology. 2022;163:937–49.e2. doi: 10.1053/j.gastro.2022.06.052. [DOI] [PubMed] [Google Scholar]
- 34. Assa A, Matar M, Turner D, et al. Proactive monitoring of adalimumab trough concentration associated with increased clinical remission in children with Crohn’s disease compared with reactive monitoring. Gastroenterology. 2019;157:985–96.e2. doi: 10.1053/j.gastro.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 35. Kennedy NA, Heap GA, Green HD, et al. ; UK Inflammatory Bowel Disease Pharmacogenetics Study Group. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: A prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341–53. doi: 10.1016/S2468-1253(19)30012-3. [DOI] [PubMed] [Google Scholar]
- 36. D’Haens G, Baert F, Van AG, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: An open randomised trial. Lancet. 2008;371:660–7. [DOI] [PubMed] [Google Scholar]
- 37. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 38. Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400.e3. doi: 10.1053/j.gastro.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 39. Targownik LE, Benchimol EI, Bernstein CN, et al. Upfront combination therapy, compared with monotherapy, for patients not previously treated with a biologic agent associates with reduced risk of inflammatory bowel disease-related complications in a population-based cohort study. Clin Gastroenterol Hepatol. 2019;17:1788–98.e2. doi: 10.1016/j.cgh.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 40. Targownik LE, Benchimol EI, Bernstein CN, et al. Combined biologic and immunomodulatory therapy is superior to monotherapy for decreasing the risk of inflammatory bowel disease-related complications. J Crohns Colitis. 2020;14:1354–63. doi: 10.1093/ecco-jcc/jjaa050. [DOI] [PubMed] [Google Scholar]
- 41. Sands BE, Peyrin-Biroulet L, Loftus EV, et al. ; VARSITY Study Group. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019;381:1215–26. doi: 10.1056/NEJMoa1905725. [DOI] [PubMed] [Google Scholar]
- 42. Barberio B, Gracie DJ, Black CJ, et al. Efficacy of biological therapies and small molecules in induction and maintenance of remission in luminal Crohn’s disease: Systematic review and network meta-analysis. Gut. 2023;72:264–74. [DOI] [PubMed] [Google Scholar]
- 43. Singh S, Murad MH, Fumery M, et al. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn’s disease: A systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:1002–14. doi: 10.1016/S2468-1253(21)00312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ.. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: An updated network meta-analysis. Clin Gastroenterol Hepatol. 2020;18:2179–91.e6. doi: 10.1016/j.cgh.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moayyedi P, Benchimol EI, Armstrong D, Yuan C, Fernandes A, Leontiadis GI.. Joint Canadian Association of Gastroenterology and Crohn’s Colitis Canada Position statement on biosimilars for the treatment of inflammatory bowel disease. J Can Assoc Gastroenterol. 2020;3:e1–9. doi: 10.1093/jcag/gwz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hanauer S, Liedert B, Balser S, Brockstedt E, Moschetti V, Schreiber S.. Safety and efficacy of BI 695501 versus adalimumab reference product in patients with advanced Crohn’s disease (VOLTAIRE-CD): A multicentre, randomised, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6:816–25. doi: 10.1016/S2468-1253(21)00252-1. [DOI] [PubMed] [Google Scholar]
- 47. Ye BD, Pesegova M, Alexeeva O, et al. Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn’s disease: An international, randomised, double-blind, phase 3 non-inferiority study. Lancet. 2019;393:1699–707. doi: 10.1016/S0140-6736(18)32196-2. [DOI] [PubMed] [Google Scholar]
- 48. Goll GL, Jørgensen KK, Sexton J, et al. Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: Open-label extension of the NOR-SWITCH trial. J Intern Med. 2019;285:653–69. doi: 10.1111/joim.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Danese S, Fiorino G, Raine T, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease-an update. J Crohns Colitis. 2017;11:26–34. doi: 10.1093/ecco-jcc/jjw198. [DOI] [PubMed] [Google Scholar]
- 50. Crohn’s and Colitis Canada. Crohn’s and Colitis Canada’s Biosimilar Position Statement: Updated September 2019, 2019.
- 51. British Society of Gastroenterology. BSG Guidance on the Use of Biosimilar Infliximab CT-P13 in Inflammatory Bowel Disease, 2016.
- 52. Crohn's & Colitis Foundation of America. Biosimilars: Position Statement, 2019.
- 53. European Society for Paediatric Gastroenterolgy, Hepatology and Nutrition. 2019 Use of Biolsimilars in Pediatric Inflammatory Bowel Disease, 2019.
- 54. D’Amico F, Solitano V, Peyrin-Biroulet L, Danese S.. Nocebo effect and biosimilars in inflammatory bowel diseases: What’s new and what’s next? Expert Opin Biol Ther. 2021;21:47–55. doi: 10.1080/14712598.2020.1817374. [DOI] [PubMed] [Google Scholar]
- 55. Boone NW, Liu L, Romberg-Camps MJ, et al. The nocebo effect challenges the non-medical infliximab switch in practice. Eur J Clin Pharmacol. 2018;74:655–61. doi: 10.1007/s00228-018-2418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Corsi N, Colloca L.. Placebo and nocebo effects: The advantage of measuring expectations and psychological factors. Front Psychol. 2017;8:308. doi: 10.3389/fpsyg.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: Results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015–30. doi: 10.1016/S0140-6736(22)00467-6. [DOI] [PubMed] [Google Scholar]
- 58. Ferrante M, Panaccione R, Baert F, et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: Results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet. 2022;399:2031–46. doi: 10.1016/S0140-6736(22)00466-4. [DOI] [PubMed] [Google Scholar]
- 59. Gordon KB, Lebwohl M, Papp KA, et al. Long-term safety of risankizumab from 17 clinical trials in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2022;186:466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Study Comparing Intravenous (IV)/ Subcutaneous (SC) Risankizumab to IV/ SC Ustekinumab to Assess Change in Crohn’s Disease Activity Index (CDAI) in Adult Participants With Moderate to Severe Crohn’s Disease (CD) (SEQUENCE), Vol. 2023. [Google Scholar]
- 61. Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551–60. [DOI] [PubMed] [Google Scholar]
- 62. Sandborn WJ, Feagan BG, D’Haens G, et al. ; True North Study Group. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021;385:1280–91. doi: 10.1056/NEJMoa2033617. [DOI] [PubMed] [Google Scholar]
- 63. Cohen JA, Comi G, Selmaj KW, et al. ; RADIANCE Trial Investigators. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): A multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019;18:1021–33. doi: 10.1016/S1474-4422(19)30238-8. [DOI] [PubMed] [Google Scholar]
- 64. Comi G, Kappos L, Selmaj KW, et al. ; SUNBEAM Study Investigators. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): A multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019;18:1009–20. doi: 10.1016/S1474-4422(19)30239-X. [DOI] [PubMed] [Google Scholar]
- 65. Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399:2113–28. doi: 10.1016/S0140-6736(22)00581-5. [DOI] [PubMed] [Google Scholar]
- 66. Barberio B, Gracie DJ, Black CJ, Ford AC.. Efficacy of biological therapies and small molecules in induction and maintenance of remission in luminal Crohn’s disease: Systematic review and network meta-analysis. Gut. 2023;72:264–74. doi: 10.1136/gutjnl-2022-328052. [DOI] [PubMed] [Google Scholar]
- 67. Ytterberg SR, Bhatt DL, Mikuls TR, et al. ; ORAL Surveillance Investigators. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–26. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 68. Din S, Selinger CP, Black CJ, et al. Systematic review with network meta-analysis: Risk of Herpes zoster with biological therapies and small molecules in inflammatory bowel disease. Aliment Pharmacol Ther 2023;57:666–75. [DOI] [PubMed] [Google Scholar]
- 69. Jones JL, Tse F, Carroll MW, et al. Canadian Association of Gastroenterology Clinical Practice Guideline for immunizations in patients with inflammatory bowel disease (IBD)-part 2: Inactivated vaccines. J Can Assoc Gastroenterol. 2021;4:e72–91. doi: 10.1093/jcag/gwab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Feagan BG, Danese S, Loftus EV Jr, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): A phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021;397:2372–84. doi: 10.1016/S0140-6736(21)00666-8. [DOI] [PubMed] [Google Scholar]
- 71. Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): Results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266–75. doi: 10.1016/S0140-6736(16)32537-5. [DOI] [PubMed] [Google Scholar]
- 72. D’Haens G, Panés J, Louis E, et al. Upadacitinib was efficacious and well-tolerated over 30 months in patients with Crohn’s disease in the CELEST extension study. Clin Gastroenterol Hepatol. 2022;20:2337–46.e3. doi: 10.1016/j.cgh.2021.12.030. [DOI] [PubMed] [Google Scholar]
- 73. Sands BE, Peyrin-Biroulet L, Kierkus J, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Crohn’s disease. Gastroenterology. 2022;162:495–508. doi: 10.1053/j.gastro.2021.10.050. [DOI] [PubMed] [Google Scholar]
- 74. Sandborn WJ, D’Haens GR, Reinisch W, et al. ; GALAXI-1 Investigators. Guselkumab for the treatment of Crohn’s disease: Induction results from the phase 2 GALAXI-1 study. Gastroenterology. 2022;162:1650–64.e8. doi: 10.1053/j.gastro.2022.01.047. [DOI] [PubMed] [Google Scholar]
- 75. Sandborn WJ, Peyrin-Biroulet L, Zhang J, et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158:550–61. doi: 10.1053/j.gastro.2019.10.035. [DOI] [PubMed] [Google Scholar]
- 76. Panés J, Bouma G, Ferrante M, et al. INSPECT: A retrospective study to evaluate long-term effectiveness and safety of darvadstrocel in patients with perianal fistulizing Crohn’s disease treated in the ADMIRE-CD trial. Inflamm Bowel Dis. 2022;28:1737–45. doi: 10.1093/ibd/izab361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hung A, Kang N, Bollom A, Wolf JL, Lembo A.. Complementary and alternative medicine use is prevalent among patients with gastrointestinal diseases. Dig Dis Sci. 2015;60:1883–8. doi: 10.1007/s10620-014-3498-3. [DOI] [PubMed] [Google Scholar]
- 78. Yoon SL, Grundmann O, Smith KF, Mason SR.. Dietary supplement and complementary and alternative medicine use are highly prevalent in patients with gastrointestinal disorders: Results from an online survey. J Diet Suppl. 2019;16:635–48. doi: 10.1080/19390211.2018.1472712. [DOI] [PubMed] [Google Scholar]
- 79. Hilsden RJ, Verhoef MJ, Rasmussen H, et al. Use of complementary and alternative medicine by patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:655–62. [DOI] [PubMed] [Google Scholar]
- 80. Hilsden RJ, Verhoef MJ, Best A, Pocobelli G.. Complementary and alternative medicine use by Canadian patients with inflammatory bowel disease: Results from a national survey. Am J Gastroenterol. 2003;98:1563–8. doi: 10.1111/j.1572-0241.2003.07519.x. [DOI] [PubMed] [Google Scholar]
- 81. Kamada N, Seo SU, Chen GY, Núñez G.. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–35. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 82. El Hage Chehade N, Ghoneim S, Shah S, et al. Efficacy of fecal microbiota transplantation in the treatment of active ulcerative colitis: A systematic review and meta-analysis of double-blind randomized controlled trials (online ahead of print on June 29, 2022). Inflamm Bowel Dis. doi: 10.1093/ibd/izac135. [DOI] [PubMed] [Google Scholar]
- 83. Kedia S, Virmani S, Vuyyuru S K, et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: A randomised controlled trial. Gut. 2022;71:2401–13. [DOI] [PubMed] [Google Scholar]
- 84. Sokol H, Landman C, Seksik P, et al. ; Saint-Antoine IBD Network. Fecal microbiota transplantation to maintain remission in Crohn’s disease: A pilot randomized controlled study. Microbiome. 2020;8:12. doi: 10.1186/s40168-020-0792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sood A, Mahajan R, Singh A, et al. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: A pilot study. J Crohns Colitis. 2019;13:1311–7. doi: 10.1093/ecco-jcc/jjz060. [DOI] [PubMed] [Google Scholar]
- 86. Preidis GA, Weizman AV, Kashyap PC, Morgan RL.. AGA technical review on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020;159:708–38.e4. doi: 10.1053/j.gastro.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: A double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–9. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 88. Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: A double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–9. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 89. Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–14. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pronio A, Montesani C, Butteroni C, et al. Probiotic administration in patients with ileal pouch-anal anastomosis for ulcerative colitis is associated with expansion of mucosal regulatory cells. Inflamm Bowel Dis. 2008;14:662–8. doi: 10.1002/ibd.20369. [DOI] [PubMed] [Google Scholar]
- 91. Borrelli O, Cordischi L, Cirulli M, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: A randomized controlled open-label trial. Clin Gastroenterol Hepatol. 2006;4:744–53. doi: 10.1016/j.cgh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 92. Critch J, Day AS, Otley A, King-Moore C, Teitelbaum JE, Shashidhar H; NASPGHAN IBD Committee. Use of enteral nutrition for the control of intestinal inflammation in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2012;54:298–305. doi: 10.1097/MPG.0b013e318235b397. [DOI] [PubMed] [Google Scholar]
- 93. van Rheenen PF, Aloi M, Assa A, et al. The medical management of paediatric Crohn’s disease: An ECCO-ESPGHAN guideline update (Online ahead of print on October 7, 2020). J Crohns Colitis. doi: 10.1093/ecco-jcc/jjaa161. [DOI] [PubMed] [Google Scholar]
- 94. Sandhu BK, Fell JM, Beattie RM, Mitton SG, Wilson David C, Jenkins H; IBD Working Group of the British Society of Paediatric Gastroenterology, Hepatology, and Nutrition. Guidelines for the management of inflammatory bowel disease in children in the United Kingdom. J Pediatr Gastroenterol Nutr. 2010;50(Suppl 1):S1–13. doi: 10.1097/MPG.0b013e3181c92c53. [DOI] [PubMed] [Google Scholar]
- 95. Levine A, Wine E, Assa A, et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. 2019;157:440–50.e8. doi: 10.1053/j.gastro.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 96. Limketkai BN, Iheozor-Ejiofor Z, Gjuladin-Hellon T, et al. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst Rev. 2019;2:CD012839. doi: 10.1002/14651858.CD012839.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Goens D, Micic D.. Role of diet in the development and management of Crohn’s disease. Curr Gastroenterol Rep. 2020;22:19. doi: 10.1007/s11894-020-0755-9. [DOI] [PubMed] [Google Scholar]
- 98. Khalili H, Håkansson N, Chan SS, et al. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: Results from two large prospective cohort studies. Gut. 2020;69:1637–44. doi: 10.1136/gutjnl-2019-319505. [DOI] [PubMed] [Google Scholar]
- 99. Lewis JD, Sandler RS, Brotherton C, et al. ; DINE-CD Study Group. A randomized trial comparing the specific carbohydrate diet to a Mediterranean diet in adults with Crohn’s disease. Gastroenterology. 2021;161:837–52.e9. doi: 10.1053/j.gastro.2021.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ponsioen CY, de Groof EJ, Eshuis EJ, et al. ; LIR!C study group. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: A randomised controlled, open-label, multicentre trial. Lancet Gastroenterol Hepatol. 2017;2:785–92. doi: 10.1016/S2468-1253(17)30248-0. [DOI] [PubMed] [Google Scholar]
- 101. de Groof EJ, Stevens TW, Eshuis EJ, et al. ; LIR!C study group. Cost-effectiveness of laparoscopic ileocaecal resection versus infliximab treatment of terminal ileitis in Crohn’s disease: The LIR!C trial. Gut. 2019;68:1774–80. doi: 10.1136/gutjnl-2018-317539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this review.