Abstract
Epithelial ovarian cancer (EOC) is often asymptomatic and presents clinically in an advanced stage as widespread peritoneal microscopic disease that is generally considered to be surgically incurable. Targeted α-therapy with the α-particle–emitting radionuclide 225Ac (half-life, 9.92 d) is a high-linear-energy-transfer treatment approach effective for small-volume disease and even single cells. Here, we report the use of human epidermal growth factor receptor 2 (HER2) 225Ac-pretargeted radioimmunotherapy (PRIT) to treat a mouse model of human EOC SKOV3 xenografts growing as peritoneal carcinomatosis (PC). Methods: On day 0, 105 SKOV3 cells transduced with a luciferase reporter gene were implanted intraperitoneally in nude mice, and tumor engraftment was verified by bioluminescent imaging (BLI). On day 15, treatment was started using 1 or 2 cycles of 3-step anti-HER2 225Ac-PRIT (37 kBq/cycle as 225Ac-Proteus DOTA), separated by a 1-wk interval. Efficacy and toxicity were monitored for up to 154 d. Results: Untreated PC-tumor–bearing nude mice showed a median survival of 112 d. We used 2 independent measures of response to evaluate the efficacy of 225Ac-PRIT. First, a greater proportion of the treated mice (9/10 1-cycle and 8/10 2-cycle; total, 17/20; 85%) survived long-term compared with controls (9/27, 33%), and significantly prolonged survival was documented (log-rank [Mantel–Cox] P = 0.0042). Second, using BLI, a significant difference in the integrated BLI signal area to 98 d was noted between controls and treated groups (P = 0.0354). Of a total of 8 mice from the 2-cycle treatment group (74 kBq total) that were evaluated by necropsy, kidney radiotoxicity was mild and did not manifest itself clinically (normal serum blood urea nitrogen and creatinine). Dosimetry estimates (relative biological effectiveness–weighted dose, where relative biological effectiveness = 5) per 37 kBq administered for tumors and kidneys were 56.9 and 16.1 Gy, respectively. One-cycle and 2-cycle treatments were equally effective. With immunohistology, mild tubular changes attributable to α-toxicity were observed in both therapeutic groups. Conclusion: Treatment of EOC PC-tumor–bearing mice with anti-HER2 225Ac-PRIT resulted in histologic cures and prolonged survival with minimal toxicity. Targeted α-therapy using the anti-HER2 225Ac-PRIT system is a potential treatment for otherwise incurable EOC.
Keywords: pretargeted radioimmunotherapy, 225Ac, peritoneal carcinomatosis
Epithelial ovarian cancer (EOC) is the most lethal ovarian cancer (1) and frequently presents as advanced-stage disease, such as peritoneal carcinomatosis (PC), where disease has spread throughout the peritoneal cavity (2). Advanced-stage disease (stage 3+) is associated with a poor prognosis and a 5-y overall survival ranging from 18% to 46% (2). Most patients die because of extensive peritoneal disease burden and malignant bowel obstruction (3).
Treatment options beyond the traditional surgeries or systemic chemotherapies, such as radiotherapy and immunotherapy in patients with advanced EOC, have had some success. Palliative radiation to metastatic EOC has been demonstrated to be well tolerated, with a 68% partial or complete response as defined as symptom control for over 3 mo in 1 study (4), indicative of the clinical radiosensitivity of EOC. Still, more studies are warranted to determine the role of radiation therapy in EOC. Similarly, there has been an explosion of immune and targeted therapies in the past few decades, such as those against human epidermal growth factor receptor 2, or HER2, which has been found to be overexpressed in breast, gastroesophageal, bladder, lung, colon, endometrial, ovarian, and head and neck cancers (5). HER2 is overexpressed in 11%–68% of EOC (6,7), though the role for HER2-directed therapy for EOC is still limited to clinical trials at this time (8).
Thus, there is increasing interest in the potential role of alternative, more innovative therapies to cure EOC, such as radioimmunotherapy, particularly in the PC setting. Radioimmunotherapy typically combines a tumor-targeting monoclonal antibody with a β- or α-particle–emitting radionuclide for molecularly targeted radiotherapy (9). To improve the therapeutic index (or tumor–to–normal-tissue absorbed dose ratios) of radioimmunotherapy, a pretargeted radioimmunotherapy (PRIT) approach can be used (9–11). We have developed a PRIT platform for treatment of human tumors that combines a nonradioactive antitumor antigen/anti-DOTA radiohapten bispecific antibody (BsAb) with a renally clearing radiohapten for efficient tumor targeting (12). We previously reported cures without radiotoxicity in nude mice bearing established flank human HER2-expressing human breast cancer xenografts (13). Furthermore, for treatment of PC, we established safe and curative intraperitoneal PRIT in nude mice bearing human colorectal PC xenografts (14).
For advanced, disseminated EOC, there is a strong rationale for radioimmunotherapy using α-emitting radionuclides such as 225Ac. The decay cascade of 225Ac (half-life, 9.92 d) to stable 209Bi yields 4 α-particles (5.8–8.4 MeV) with short ranges in tissue (47–85 μm) and thus is well suited for targeted α-therapy of PC and microscopic disease (15). However, a major limitation of targeted α-therapy with 225Ac-immunoconjugates has been off-target daughter toxicity, most notably to the kidneys in mice (16). Thus, we have adapted our PRIT system for targeted α-therapy with a novel 225Ac-radiohapten (225Ac-Proteus DOTA, or 225Ac-Pr) that clears rapidly from the body via excretion by the kidneys into the urine, demonstrating no acute or chronic toxicity, including nephrotoxicity, at curative doses (17).
The primary objective of our study was to demonstrate that anti-HER2 PRIT can be administered intraperitoneally with a sufficient therapeutic index to safely achieve complete responses without significant toxicity in nude mice bearing human EOC xenografts. Second, we assayed the internalization kinetics of the BsAb-pretargeted radiohapten and performed multicellular dosimetry calculations for 225Ac-Pr and 111In-Pr, its theranostic pair (17). We used an aggressive SKOV3-luciferase (SKOV3-luc) reporter PC nude mouse model to test the hypothesis that intraperitoneal anti-HER2 PRIT using 225Ac-Pr is effective against ovarian PC.
MATERIALS AND METHODS
Reagents and General Procedures
All experiments involving mice were performed in accordance with Memorial Sloan Kettering Cancer Center Institutional Animal Care and Use Committee protocol 00-03-053 for compliance with the requirements of the National Institutes of Health on use of laboratory animals. Details regarding all reagents and general procedures may be found in the supplemental materials (available at http://jnm.snmjournals.org). A schematic of the anti-HER2/anti-DOTA IgG-scFv BsAb (13) and structures of the DOTA(Y)-conjugated poly-N-acetylgalactosamine glycodendron clearing agent (18) and 225Ac-Pr (17) are provided in Supplemental Figure 1.
Radiolabeling of BsAb and Proteus
131I was obtained from Nordion, Inc., as 131I-NaI solution. BsAb was radioiodinated using the Iodogen (Thermo-Fisher Scientific) method (19) to a specific activity of 132 MBq/mg. Radiochemical purity was verified by size-exclusion chromatography to be greater than 98%.
The 225Ac used in this research was supplied by the U.S. Department of Energy Office of Science by the Isotope Program in the Office of Nuclear Physics. 225Ac-Pr was synthesized to a molar activity of approximately 50 kBq/nmol, as previously described (17). Radiochemistry measurements were made at secular equilibrium using a CRC-15R radioisotope calibrator (Capintec, Inc.) set at 775; the displayed activity values were multiplied by 5.
No-carrier-added 111In-InCl3 sterile solution was obtained from Nuclear Diagnostic Products, Inc. 111In-radioactivity measurements were made using the CRC-15R calibrator with the manufacturer’s recommended settings for the radionuclide. 111In-Pr was synthesized to a molar activity of approximately 12 MBq/nmol, as previously described (17).
Cell Culture and Flow Cytometry Assay of HER2 Antigen Expression
SKOV3 is a HER2-positive (+) human serous EOC cell line that expresses the estrogen receptor and is p53-null but is known to be resistant to estrogen and antiestrogen therapy (20). The SKOV3-luc cell line (21) was obtained from Dmitry Pankov. The human GD2+/HER2-negative neuroblastoma IMR32-luc cell line (22) was used as a negative control during in vitro studies of 111In-Pr internalization. Surface HER2 antigen expression was confirmed by flow cytometry analysis, as described previously (21). Data were analyzed using FlowJo, version 10, software and reported as geometric mean fluorescence intensity.
In Vitro Binding and Internalization of Tracer 131I-BsAb and BsAb-Pretargeted 111In-Pr
The internalization kinetics of tracer 131I-BsAb after binding to HER2+ SKOV3-luc cells were determined at 37°C for up to 24 h, as described previously (13). For kinetics analysis, data were curve-fitted using a nonlinear model with GraphPad Prism 8.1.0. To assay the internalization kinetics of BsAb-pretargeted 111In-Pr, a method based on Heskamp et al. (23) was used. Control BsAb included anti-GPA33 BsAb (24) and anti-GD2 BsAb (22).
Multicellular Dosimetry and Biologic-Response Modeling
Internalization assay data for BsAb-pretargeted 111In-Pr were input to MIRDcell, version 3 (25,26). For the purposes of MIRDcell calculations, the radii of the SKOV3 cells and nuclei were approximated visually with microscopy, measuring 9 and 3 μm, respectively, and the cell radius was confirmed on a Vi-CELL XR Cell Analyzer (Beckman Coulter). For the cell culture studies, dosimetry was conducted for 111In-Pr and then extrapolated to 225Ac-Pr with the assumption that the concentration of Proteus in the buffer, cellular distribution, and molar internalization of 111In-Pr and 225Ac-Pr is equivalent during BsAb pretargeting. As noted previously, the molar activities were 12 MBq/nmol and 50 kBq/nmol, respectively.
Radiopharmaceutical Therapy of PC
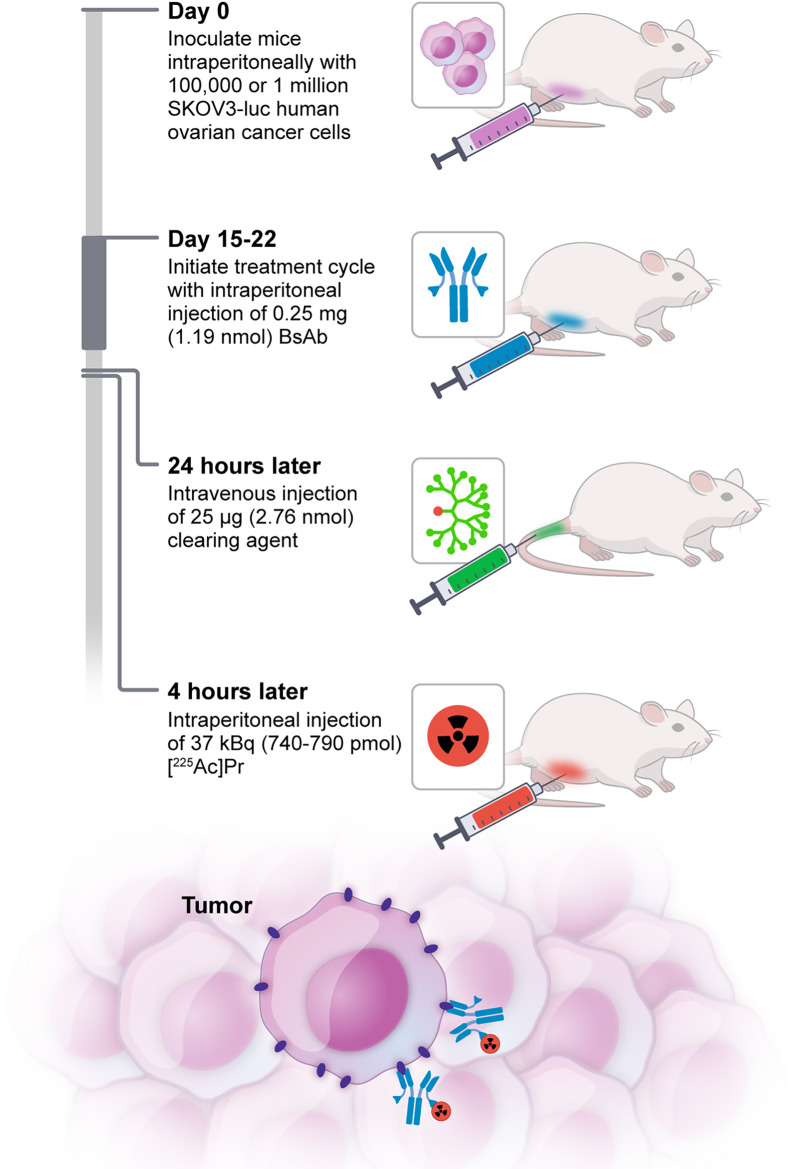
Preliminary studies to establish an aggressive SKOV3-luc PC nude mouse model are shown in Supplemental Figure 2. For in vivo 225Ac-PRIT experiments, the intraperitoneal route of administration was selected for intracavity delivery of BsAb and radiohapten to target PC, whereas the clearing agent was injected via intravenous administration. Injection timing of reagents was relative to the injection of radiohapten: 250 μg (1.19 nmol) of BsAb were injected intraperitoneally in the right lower quadrant at −28 h, followed by 25 μg (2.76 nmol) of clearing agent via the lateral tail vein at −4 h and 225Ac-Pr intraperitoneally in the right lower quadrant at 0 h. All reagents were formulated for injection in sterile normal saline up to a 250-μL volume. Mice bearing PC tumors were randomly divided into 5 groups of 8–10 on day 14 after bioluminescent imaging (BLI) before treatment. Treatment mice received 1 or 2 cycles of anti-HER2 225Ac-PRIT plus 37 kBq of 225Ac-Pr (cycle 1, 740 pmol; cycle 2, 790 pmol) at 15 and 22 d after tumor inoculation, respectively (both n = 10). Control groups included administration of BsAb only (n = 9), 1 cycle of off-targeted PRIT (with anti-GPA33 BsAb (24) in place of anti-HER2 BsAb; n = 10), or no treatment (n = 8). Weekly weights and PC tumor progression by BLI were monitored. Mice in the survival arms reached therapeutic endpoints if they had more than a 20% weight decrease from the pretreatment baseline, developed severe abdominal distension from palpable tumor or ascites, or appeared moribund to investigators or to the Research Animal Resource Center staff conducting daily monitoring. At 154 d after tumor inoculation (treatment initiated on day 15), a total of 15 surviving anti-HER2 225Ac-PRIT treatment mice (n = 7 from 1 cycle and n = 8 from 2 cycles) and 1 untreated control mouse were submitted for hematology, serum chemistry, and necropsy by the Laboratory of Comparative Pathology of Memorial Sloan Kettering Cancer Center to evaluate treatment effects and toxicity.
Data Analysis
Quantitative data were expressed as mean ± SD unless otherwise noted. Statistical analyses were performed using GraphPad Prism 8.1.0. Kaplan–Meier survival curves were analyzed with the Mantel–Cox test. Two-sided Student t tests were calculated, and a P value of less than 0.05 was considered to be statistically significant.
RESULTS
In Vitro Binding and Internalization of BsAb and BsAb-Pretargeted 111In-Pr
BsAb binding HER2+ SKOV-luc was assayed using flow cytometry (Supplemental Fig. 3). The mean fluorescence intensity for anti-HER2 BsAb binding and anti-GPA33 BsAb binding to SKOV3-luc cells was 4,736 and 133, respectively (for antibody isotope control 100). These data confirm HER2-specific binding of anti-HER2 BsAb and the HER2+/GPA33-negative antigenic phenotype of SKOV-luc cells.
In vitro radiotracer binding studies were performed with HER2+ SKOV3-luc cells to determine the internalization and cellular processing at 37 °C of 131I-BsAb (Supplemental Fig. 4). The mean surface-bound 131I-BsAb at 24 h, corresponding to the time interval between injections of BsAb and CA, was 9.45% of the added activity.
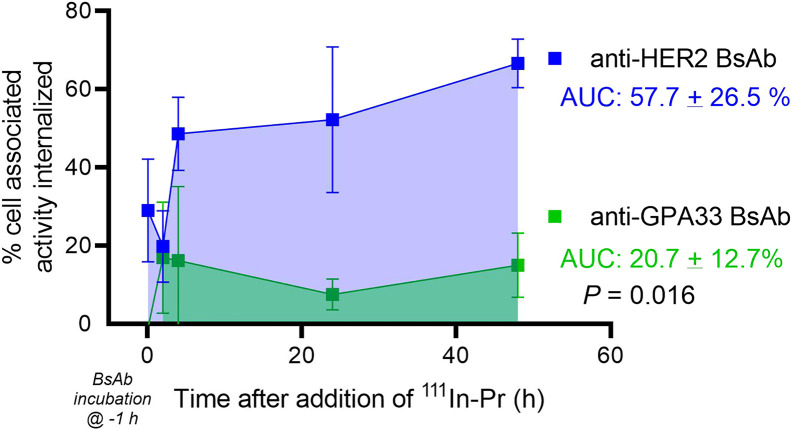
During the in vitro radiohapten internalization assay with HER2+ SKOV3-luc cells (data shown are n = 9 except for 48 h, which is n = 3; Fig. 1), anti-HER2 BsAb-pretargeted 111In-Pr rapidly localized to the cell surface and was internalized at 37 °C. Control experiments performed with HER2-negative IMR32-luc neuroblastoma verified that internalization of the anti-HER2 BsAb/111In-Pr complex is HER2-mediated (Supplemental Fig. 5). In summary, the HER2 system showed that an increased proportion of cell-associated activity was internalized in comparison to that with the GPA33 system (e.g., at 48 h, mean 67% vs. mean 15% for HER2 vs. GPA33, respectively). In the absence of BsAb, the average percentage added 111In-Pr activity that was internalized was less than 0.1% at all time points, confirming that pretargeted BsAb was required for 111In-Pr internalization.
FIGURE 1.
In vitro 111In-Pr internalization when pretargeted using anti-HER2 or anti-GPA33 BsAb. Area under curve was determined from 5 min to 48 h after addition of 111In-Pr. Data shown are n = 9 except for 48 h, which is n = 3. AUC = area under curve.
Multicellular Dosimetry
Using MIRDcell, the mean absorbed dose to the nucleus of the cells cultured in the 9.6 cm2 wells was calculated. Based on the internalization kinetics of BsAb-pretargeted 111In-Pr (Fig. 1; Supplemental Fig. 6), the average percent internalized over the 48-h period was 58% ± 27% for anti-HER2 BsAb. The in vitro dosimetry conducted for 111In was extrapolated to 225Ac and its daughters, assuming equivalent internalization of 225Ac-Pr, and a molar activity of 0.05 MBq/nmol. Calculations were performed similarly with MIRDcell and tabulated in Table 1 for various scenarios of subcellular distribution and absence or presence of daughter contributions to the absorbed dose. The calculated upper limit (i.e., assuming 100% internalization of 225Ac-Pr and its daughter radionuclides) of the absorbed dose to the nucleus was 3.28 Gy. With 58% internalization through our PRIT system, we were able to deliver an estimated 2.08 Gy, which is a dose to the nucleus 5 times greater than that delivered to a noninternalizing system (0.42 Gy). With improvement to a 100% internalizing system, we could potentially deliver a 7.8-times increase in absorbed dose to the nucleus.
TABLE 1.
Estimated Absorbed Doses to Cultured Cells Using MIRDcell Version 3.11
| Subcellular distribution | Absorbed dose (Gy per 1.1 kBq/mL) | |||
|---|---|---|---|---|
| Activity in cytoplasm | Activity on cell surface | 225Ac | 225Ac plus daughters | 0.58 × D (225Ac plus daughters in cyto) plus 0.42 × D (225Ac on cell surface) |
| 0% | 100% | 0.42 | 1.58 | |
| 58% | 42% | 0.70 | 2.57 | 2.08 |
| 100% | 0% | 0.90 | 3.28 | |
D = absorbed dose.
Doses are assuming 0–100% internalization for 111In-Pr in assay and then extrapolated to 225Ac without daughters (column 3) and with daughters (column 4). Column 5 gives absorbed dose when daughters are included for 225Ac decays in cytoplasm (58%) and daughters are excluded for decays on cell surface (42%).
Serial Biodistribution of Intraperitoneal Anti-HER2 225Ac-PRIT and Dosimetry Calculations
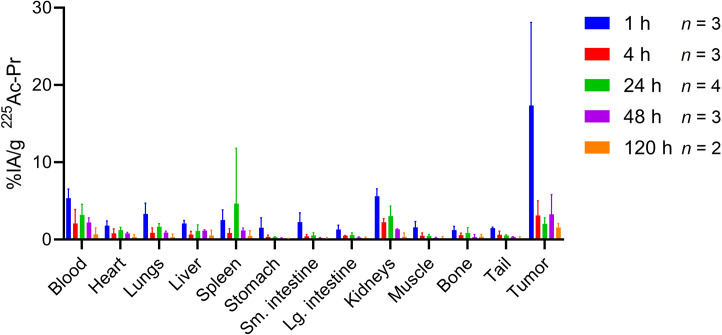
After we administered anti-HER2 225Ac-PRIT per the protocol in Figure 2, the radioactivity in the tumor peaked at 17.33 ± 10.77 percentage injected activity (%IA)/g at 1 h after injection with rapid washout, decreasing to 2.03 ± 0.80 %IA/g at 24 h after injection (Fig. 3; Supplemental Table 1). The estimated absorbed dose to the tumor and the relative biological effectiveness (RBE)–weighted dose to the tumor (where RBE is 5) were 11.4 and 56.9 Gy, respectively, per 37 kBq administered, assuming 58% internalization from the above studies (Supplemental Table 2). The kidneys had the highest radioactivity uptake of the normal organs, 5.62 ± 0.95 %IA/g at 1 h after injection, which steadily decreased to 0.37 ± 0.50 %IA/g by 120 h after injection and corresponded to an estimated RBE-weighted dose of 16.1 Gy/37 kBq.
FIGURE 2.
Schematic representation of 3-step HER2-targeted intraperitoneal 225Ac-PRIT concept.
FIGURE 3.
HER2-targeted intraperitoneal 225Ac-PRIT serial biodistribution data in mouse model of human EOC PC. Shown is ex vivo biodistribution assay after administration of pretargeted 225Ac-Pr (37 kBq, 773 pmol) into groups of nude mice bearing intraperitoneal SKOV3-luc xenografts (n = 2–4/group).
Therapy Studies
At pretreatment baseline, the average BLI was highest in the HER2 225Ac-PRIT 2-cycle group (4.51 × 105) and significantly larger than the BLI in the no-treatment group (1.74 × 105, P = 0.0188) (Supplemental Figs. 7 and 8). No significant differences were observed between the no-treatment group and the other groups (range of P = 0.095–0.752).
After the start of treatment, BLI values between treatment groups diverged significantly on area-under-the-curve analyses (Table 2). Although BLI values continued to increase in groups treated with either 1 cycle of off-targeted (irrelevant GPA33 target) 225Ac-PRIT or no treatment, they remained low or decreased in both 1- and 2-cycle anti-HER2 225Ac-PRIT treatment groups. Among anti-HER2 PRIT treatment groups, the BLI decrease was rapid, 47% within 7 d (P = 0.04). By 98 d after injection, the average BLI values of the treatment mice were significantly different from that of the off-targeted and no-treatment control mice (P = 0.001) (Supplemental Fig. 8).
TABLE 2.
Summary of 225Ac-PRIT Therapy Results
| Parameter | Long-term survivors* | nBLI AUC† | Median survival (d) | Log-rank P (vs. no treatment) | Log-rank P (vs. 1-cycle GPA33) | Log-rank P (vs. HER2 BsAb only) | Log-rank P (vs. 1-cycle HER2 only) |
|---|---|---|---|---|---|---|---|
| No treatment | 2/8 | 18,737 | 112 | ||||
| One-cycle GPA33 | 2/10 | 3,558 | 105 | 0.9397 | |||
| HER2 BsAb only | 6/9 | 1,036 | >154 | 0.1536 | 0.0923 | ||
| One-cycle HER2 | 8/10 | 0.97 | >154 | 0.0093 | 0.0047 | 0.2055 | |
| Two-cycle HER2 | 9/10 | 4.75 | >154 | 0.0170 | 0.0076 | 0.2763 | 0.6717 |
At 133 d after tumor inoculation.
Supplemental Figure 8 provides normalized BLI data.
nBLI = normalized BLI; AUC = area under curve.
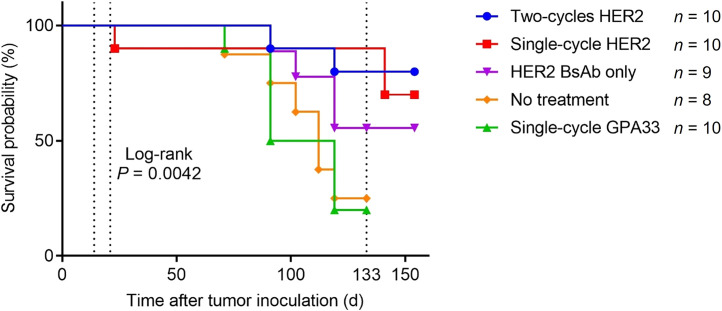
Prolonged survival was demonstrated in anti-HER2 225Ac-PRIT groups (17/20 at 133 d) compared with the other 3 groups of control mice (10/27 at 133 d, log-rank P < 0.05) (Fig. 4). Interestingly, the group treated with anti-HER2-BsAb alone did not reach median survival, suggesting there might be some treatment effect of the trastuzumab domains of the anti-HER2-BsAb on SKOV3-luc xenografts in mice, as described previously (27). Furthermore, this raises the possibility of synergism between anti-HER2 225Ac-PRIT and the anti-HER2-BsAb.
FIGURE 4.
Kaplan–Meier survival curves.
Although the untreated mouse had a high PC tumor burden, there was no histologic evidence of viable neoplasia in 15 of 15 mice in the treatment group on day 154. Histopathologic examination of PC lesions in a surviving no-treatment control mouse at 154 d after injection showed a carcinoma in which most cells displayed strong complete membrane immunoreactivity for HER2 by immunohistochemistry (Supplemental Fig. 9). With 225Ac-PRIT, residual fibrotic scars with mild chronic inflammation, suggestive of previously treated tumors, were identified on peritoneal surfaces, but no evidence of viable carcinoma cells was observed within any organs (Supplemental Fig. 9). The liver of the untreated mouse displayed marked inflammatory lesions of the bile ducts, which were likely caused by biliary obstruction by the peritoneal tumors observed on the liver and mesentery. The livers of treated mice had a similar but significantly milder pattern of inflammation, suggesting that this process was partially reversed with tumor treatment. The gross and histopathologic findings are summarized in Supplemental Table 3.
Toxicity
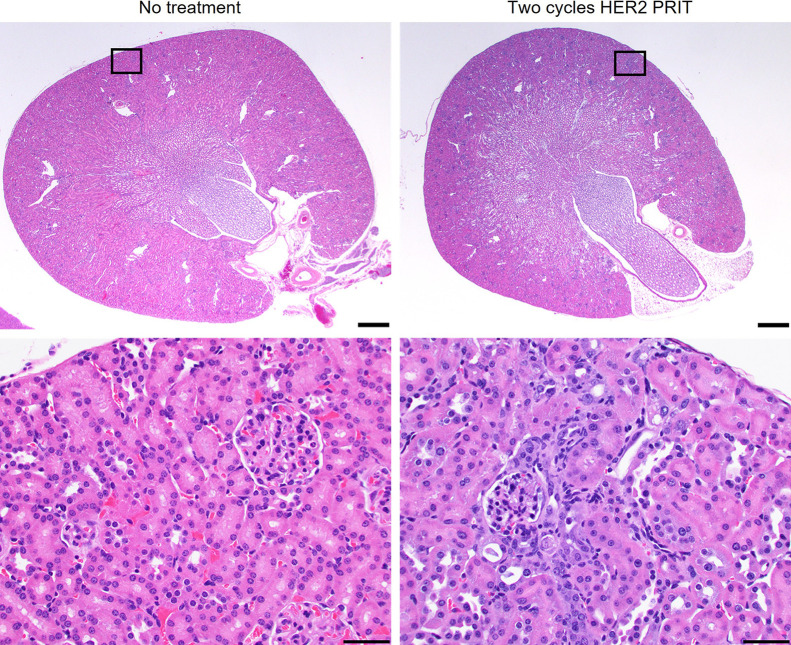
All treatments were well tolerated. Only a single mouse in the 1-cycle anti-HER2 225Ac-PRIT treatment group required removal from the study at 22 d after tumor inoculation (7 d after treatment initiation because of sudden weight loss). No significant weight loss was observed after treatment (Supplemental Fig. 10). On histopathologic examination, the only finding interpreted as organ injury caused by the treatment was minimal to mild renal tubular degeneration (Fig. 5; Supplemental Table 4), but this lesion did not affect renal function based on serum blood urea nitrogen or creatinine (Supplemental Table 5). All hematologic parameters were within reference limits for treated mice (Supplemental Table 6). Observations of both acute and chronic toxicity suggest that we did not reach the maximum tolerated dose and can consider further dose escalation.
FIGURE 5.
Minimal to mild radiation nephropathy was observed in mice treated with 1 (37 kBq) or 2 (74 kBq) cycles of HER2 225Ac-PRIT. Shown is representative hematoxylin and eosin staining of kidneys from nontreated control and mouse treated with 2 cycles of HER2 225Ac-PRIT at 154 d after tumor inoculation (treatment initiated on day 15). Scale bar for top images: 500 μm. Scale bar for bottom images: 50 μm.
DISCUSSION
In this paper, we demonstrate clinical and pathologic cures with minimal toxicities using 225Ac in the form of anti-HER2–pretargeted 225Ac-Pr. 225Ac-Pr is both efficiently targeted to the tumor and internalized via an anti-HER2/anti-DOTA BsAb as part of our 225Ac-PRIT regimen in an aggressive in vivo murine model of HER2+ SKOV3 EOC PC. Untreated mice developed rapid diffuse intraperitoneal spread with ascites that recapitulates aggressive, clinical peritoneal disease, leading to a median survival of about 4 mo after tumor inoculation in mice. When treated with 1 or 2 cycles of HER2 225Ac-PRIT (37 kBq/cycle), median survival was not reached by 154 d after tumor inoculation. Also, at the end of the study, a large subset of clinical cures (15/20 mice in the treatment groups) was confirmed with extensive histopathology analysis. Although there was evidence of previous tumor growth, as demonstrated by the fibrotic scars on peritoneal surfaces and mild multifocal hepatoportal lymphoplasmacytic infiltration (suggestive of recovery from prior biliary obstruction most likely secondary to prior tumor), no PC was found, demonstrating complete tumor eradication (histopathologic cure) in the subset.
Despite the pathologic cures in most animals, others in the 225Ac-PRIT groups showed persistent BLI. When these animals were necropsied, viable tumors were found. These residual tumors escaped 225Ac-PRIT partly because of the nonuniform distribution of the 225Ac in the nodule. MIRDcell, version 3.11, 3-dimensional calculations for spheric SKOV3 nodules with radii of 100, 200, 400, and 500 μm, with an exponential radial activity distribution (exponential factor, 0.035) and a drug penetration depth of 99 μm, yield mean absorbed doses of 41.4, 48.1, 50.7, and 51.2 Gy, respectively, for the average activity concentration measured in nodules of animals given an administered activity of 37 kBq. The corresponding surviving fraction of cells in the nodules was 0.0, 0.015, 0.22, and 0.31 when 225Ac plus daughters were used as the source radiation. In contrast, the surviving fraction of cells for a uniform activity distribution was 0 in all cases. These hypothetical cases suggest that limited penetration of anti-HER2 into the nodules could affect the outcome for some nodules. However, it is also possible that the nodules arose from individual cells suspended in the peritoneal cavity that expressed extremely low levels of HER2 on their cell surface. Such cells may have insufficient 225Ac to ensure cell killing. This can be overcome by combining multiple 225Ac-labeled agents (28).
Alternative PRIT approaches have been evaluated in preclinical EOC models. Frost et al. demonstrated PRIT against intraperitoneal ovarian NIH:OVCAR-3 microtumors using avidin-conjugated monoclonal antibody MX35 and 211At-labeled, biotinylated, and succinylated poly-l-lysine (29). Recently, Affibody (Affibody AB)-based peptide nucleic acid-mediated PRIT with complementary probe 177Lu-HP2 was shown to significantly prolong survival of mice bearing HER2+ SKOV3 cells (30,31). Although these PRIT approaches show promise, we now show that anti-HER2 DOTA-based PRIT is well suited for tumor targeting of 225Ac because of the extraordinarily high affinity of the anti-DOTA antibody and rapid whole-body clearance of 225Ac-Pr (17).
One advantageous characteristic of our anti-HER2 225Ac-PRIT regimen is that the surface BsAb/radiohapten complex is internalized. Our cellular dosimetry calculations demonstrated that our 225Ac-PRIT system was able to deliver an estimated dose to the nucleus 6.1 times greater than that with a noninternalizing system. It is conceivable that optimizing delivery of the radiohapten to the target (i.e., 100% internalization) could further increase the absorbed dose delivered to the target tumor tissues by a factor of 1.6, nearly doubling the therapeutic index. By sequestering the 225Ac decay daughters, such as nephrotoxic 213Bi, within the tumor cells, one might also reduce the unwanted trapping of the daughters by the kidneys or nontargeted tissues, providing a further safety margin.
The issue of α-dosimetry is fascinating but complex. In this paper, we have elected to focus on tumor dosimetry because it is apparent from the work of many investigators, including Sgouros et al. (32,33) and Liatsou et al. (34), that RBE is particularly important in this context. Complexity comes from the need to include the RBE for high-linear-energy-transfer α-particles, which are generally considered to be approximately 5 times as effective per absorbed dose as β-particles or photons. For tumors and kidneys, we estimated an RBE-weighted dose of 56.9 and 16.1 Gy, respectively, as described in Supplemental Table 2. Liatsou et al. reported a slightly higher RBE of 6.4 for bone marrow toxicity (cellularity) of anti-HER2 212Pb-IgG based on the equivalent dose for a 2 Gy/fraction treatment (34). As the biodistribution is so different in the context of pretargeting, we recognize that additional experiments are needed to clarify the RBE and its dependence on different organ systems. We refer to International Commission on Radiation Units and Measurements Report 96, which addresses the complex problems of α-dosimetry (32). Dosimetry of this kind requires special handling, especially at the level of multicellular dosimetry (35). Notably, for kidneys, we found subclinical toxicity, with no detectable abnormalities in the usual parameters of renal function (such as blood urea nitrogen or creatinine). However, mild renal histopathologic changes attributable to α-toxicity, such as those we previously reported, were seen during the current studies (17). In addition to β-dosimetry, α-dosimetry will be critical as we move toward the clinic, and this will require more detailed studies.
CONCLUSION
The anti-HER2 DOTA 225Ac-PRIT system may have potential in patients with HER2-expressing EOC PC. Temporal decoupling via PRIT and internalization of the radiohapten are 2 effective strategies to deliver an effective radioactive dose to tumors while minimizing toxicity to nontargeted tissues. Further studies to optimize targeting and therapeutic index in anticipation of clinical translation are under way.
DISCLOSURE
This research was funded in part by the Hedvig Hricak Chair in Radiology (to Steven Larson); the Enid A. Haupt Chair (to Nai-Kong Cheung); the Center for Targeted Radioimmunotherapy and Theranostics, Ludwig Center for Cancer Immunotherapy of MSKCC (to Steven Larson); and Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center of MSKCC (to Steven Larson). Steven Larson was also supported in part by P50-CA86438. This study also received support from R01-CA233896 (to Sarah Cheal). We also acknowledge P30-CA008748 for use of the Tri-Institutional Laboratory of Comparative Pathology, MSKCC, WCM, and Rockefeller University, New York, NY; technical services provided by the MSKCC Small-Animal Imaging Core Facility and Laboratory of Comparative Pathology; as well as the Molecular Cytology Core Facility. Both MSKCC and Nai-Kong Cheung have financial interest in Y-mAbs Therapeutics, Inc., Abpro-Labs, and Lallemand-Biotec Pharmacon. Nai-Kong Cheung reports receiving commercial research grants from Y-mAbs Therapeutics, Inc., and Abpro-Labs. Nai-Kong Cheung was named as inventor on multiple patents filed by MSKCC, including those licensed to Y-mAbs Therapeutics, Inc., Lallemand-Biotec Pharmacon, and Abpro-Labs. Nai-Kong Cheung is a scientific advisory board member for Eureka Therapeutics. Nai-Kong Cheung, Steven Larson, and Sarah Cheal were named as inventors in the following patent applications relating to GPA33: SK2014-074, SK2015-091, SK2017-079, SK2018-045, SK2014-116, SK2016-052, and SK2018-068 filed by MSK. Steven Larson reports receiving commercial research grants from Genentech, Inc., WILEX AG, Telix Pharmaceuticals Limited, and Regeneron Pharmaceuticals, Inc.; holding ownership interest/equity in Elucida Oncology, Inc., and Y-mAbs Therapeutics, Inc.; and holding stock in ImaginAb, Inc. Steven Larson is the inventor and owner of issued patents both currently unlicensed and licensed by MSKCC to Samus Therapeutics, Inc., Elucida Oncology, Inc., and Y-mAbs Therapeutics, Inc. Steven Larson serves or has served as a consultant to Cynvec LLC, Eli Lilly & Co., Prescient Therapeutics Limited, Advanced Innovative Partners, LLC, Gerson Lehrman Group, Progenics Pharmaceuticals, Inc., Y-mAbs Therapeutics, Inc., and Janssen Pharmaceuticals, Inc. Guangbin Yang and Ouathek Ouerfelli are listed as inventors and receive royalties from patents that were filed by MSKCC. Ouathek Ouerfelli is an unpaid member of the scientific advisory board of Angiogenex and owns shares in Angiogenex. Andrea Cercek reports receiving research funding from GSK, Seagen, and Inspirna and is a member of the advisory board of Bayer, GSK, Merck, Janssen, G1 Therapeutics, and Seagen. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can intraperitoneal HER2-targeted actinium radionuclide therapy safely and effectively treat human EOC growing as PC in a mouse model?
PERTINENT FINDINGS: 225Ac was efficiently and specifically delivered to intraperitoneal HER2-expressing SKOV3-luc xenografts using a HER2-DOTA bsAb-based approach in PRIT. Objective tumor response plus efficacy in tumor eradication was observed in small-volume tumor models with minimal or no myelo-, hepato-, or gastrointestinal toxicities. Nephrotoxicities attributed to α-emitters at approximately 20 wk were mild and far from dose-limiting.
IMPLICATIONS FOR PATIENT CARE: This study identified a new approach to treating HER2-expressing human EOC growing as PC. With its precision and potency, it may fulfill unmet needs in this devastating disease.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Prat J.; FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. [DOI] [PubMed] [Google Scholar]
- 3. Dvoretsky PM, Richards KA, Angel C, Rabinowitz L, Beecham JB, Bonfiglio TA. Survival time, causes of death, and tumor/treatment-related morbidity in 100 women with ovarian cancer. Hum Pathol. 1988;19:1273–1279. [DOI] [PubMed] [Google Scholar]
- 4. Butala AA, Patel RR, Manjunath S, et al. Palliative radiation therapy for metastatic, persistent, or recurrent epithelial ovarian cancer: efficacy in the era of modern technology and targeted agents. Adv Radiat Oncol. 2020;6:100624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21:283–290. [DOI] [PubMed] [Google Scholar]
- 7. Medl M, Sevelda P, Czerwenka K, et al. DNA amplification of HER-2/neu and INT-2 oncogenes in epithelial ovarian cancer. Gynecol Oncol. 1995;59:321–326. [DOI] [PubMed] [Google Scholar]
- 8. Thouvenin L, Charrier M, Clement S, et al. Ovarian cancer with high-level focal ERBB2 amplification responds to trastuzumab and pertuzumab. Gynecol Oncol Rep. 2021;37:100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of human tumours. Nat Rev Cancer. 2015;15:347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verhoeven M, Seimbille Y, Dalm SU. Therapeutic applications of pretargeting. Pharmaceutics. 2019;11:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bailly C, Bodet-Milin C, Rousseau C, Faivre-Chauvet A, Kraeber-Bodere F, Barbet J. Pretargeting for imaging and therapy in oncological nuclear medicine. EJNMMI Radiopharm Chem. 2017;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheal SM, Chung SK, Vaughn BA, Cheung N-KV, Larson SM. Pretargeting: a path forward for radioimmunotherapy. J Nucl Med. 2022;63:1302–1315. [DOI] [PubMed] [Google Scholar]
- 13. Cheal SM, Xu H, Guo H-F, et al. Theranostic pretargeted radioimmunotherapy of internalizing solid tumor antigens in human tumor xenografts in mice: curative treatment of HER2-positive breast carcinoma. Theranostics. 2018;8:5106–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chandler CS, Bell MM, Chung SK, et al. Intraperitoneal pretargeted radioimmunotherapy for colorectal peritoneal carcinomatosis. Mol Cancer Ther. 2022;21:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McDevitt MR, Sgouros G, Sofou S. Targeted and nontargeted alpha-particle therapies. Annu Rev Biomed Eng. 2018;20:73–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jaggi JS, Seshan SV, McDevitt MR, LaPerle K, Sgouros G, Scheinberg DA. Renal tubulointerstitial changes after internal irradiation with alpha-particle-emitting actinium daughters. J Am Soc Nephrol. 2005;16:2677–2689. [DOI] [PubMed] [Google Scholar]
- 17. Cheal SM, McDevitt MR, Santich BH, et al. Alpha radioimmunotherapy using 225Ac-proteus-DOTA for solid tumors: safety at curative doses. Theranostics. 2020;10:11359–11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheal SM, Patel M, Yang G, et al. An N-acetylgalactosamino dendron-clearing agent for high-therapeutic-index DOTA-hapten pretargeted radioimmunotherapy. Bioconjug Chem. 2020;31:501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salacinski PR, McLean C, Sykes JE, Clement-Jones VV, Lowry PJ. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3α,6α-diphenyl glycoluril (Iodogen). Anal Biochem. 1981;117:136–146. [DOI] [PubMed] [Google Scholar]
- 20. Hua W, Christianson T, Rougeot C, Rochefort H, Clinton GM. SKOV3 ovarian carcinoma cells have functional estrogen receptor but are growth-resistant to estrogen and antiestrogens. J Steroid Biochem Mol Biol. 1995;55:279–289. [DOI] [PubMed] [Google Scholar]
- 21. Lopez-Albaitero A, Xu H, Guo H, et al. Overcoming resistance to HER2-targeted therapy with a novel HER2/CD3 bispecific antibody. OncoImmunology. 2017;6:e1267891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheal SM, Xu H, Guo HF, Zanzonico PB, Larson SM, Cheung NK. Preclinical evaluation of multistep targeting of diasialoganglioside GD2 using an IgG-scFv bispecific antibody with high affinity for GD2 and DOTA metal complex. Mol Cancer Ther. 2014;13:1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heskamp S, Hernandez R, Molkenboer-Kuenen JDM, et al. α- versus β-emitting radionuclides for pretargeted radioimmunotherapy of carcinoembryonic antigen-expressing human colon cancer xenografts. J Nucl Med. 2017;58:926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheal SM, Xu H, Guo H-F, et al. Theranostic pretargeted radioimmunotherapy of colorectal cancer xenografts in mice using picomolar affinity 86Y- or 177Lu-DOTA-Bn binding scFv C825/GPA33 IgG bispecific immunoconjugates. Eur J Nucl Med Mol Imaging. 2016;43:925–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaziri B, Wu H, Dhawan AP, Du P, Howell RW, Committee SM. MIRD pamphlet no. 25: MIRDcell V2.0 software tool for dosimetric analysis of biologic response of multicellular populations. J Nucl Med. 2014;55:1557–1564. [DOI] [PubMed] [Google Scholar]
- 26. Katugampola S, Wang J, Rosen A, Howell RW. MIRD pamphlet no. 27: MIRDcell V3, a revised software tool for multicellular dosimetry and bioeffect modeling. J Nucl Med. 2022;63:1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luistro LL, Rosinski JA, Bian H, et al. Development and characterization of a preclinical ovarian carcinoma model to investigate the mechanism of acquired resistance to trastuzumab. Int J Oncol. 2012;41:639–651. [DOI] [PubMed] [Google Scholar]
- 28. Pasternack JB, Domogauer JD, Khullar A, Akudugu JM, Howell RW. The advantage of antibody cocktails for targeted alpha therapy depends on specific activity. J Nucl Med. 2014;55:2012–2019. [DOI] [PubMed] [Google Scholar]
- 29. Frost SH, Bäck T, Chouin N, et al. Comparison of 211At-PRIT and 211At-RIT of ovarian microtumors in a nude mouse model. Cancer Biother Radiopharm. 2013;28:108–114. [DOI] [PubMed] [Google Scholar]
- 30. Oroujeni M, Tano H, Vorobyeva A, et al. Affibody-mediated PNA-based pretargeted cotreatment improves survival of trastuzumab-treated mice bearing HER2-expressing xenografts. J Nucl Med. 2022;63:1046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Westerlund K, Altai M, Mitran B, et al. Radionuclide therapy of HER2-expressing human xenografts using Affibody-based peptide nucleic acid-mediated pretargeting: in vivo proof of principle. J Nucl Med. 2018;59:1092–1098. [DOI] [PubMed] [Google Scholar]
- 32. Sgouros G, Bolch WE, Chiti A, et al. ICRU report 96, dosimetry-guided radiopharmaceutical therapy. J ICRU. 2021;21:1–212. [Google Scholar]
- 33. Sgouros G, Frey E, Du Y, Hobbs R, Bolch W. Imaging and dosimetry for alpha-particle emitter radiopharmaceutical therapy: improving radiopharmaceutical therapy by looking into the black box. Eur J Nucl Med Mol Imaging. 2021;49:18–29. [DOI] [PubMed] [Google Scholar]
- 34. Liatsou I, Yu J, Bastiaannet R, et al. 212Pb-conjugated anti-rat HER2/neu antibody against a neu-N derived murine mammary carcinoma cell line: cell kill and RBE in vitro. Int J Radiat Biol. 2022;98:1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sgouros G, Roeske JC, McDevitt MR, et al. MIRD pamphlet no. 22 (abridged): radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J Nucl Med. 2010;51:311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]