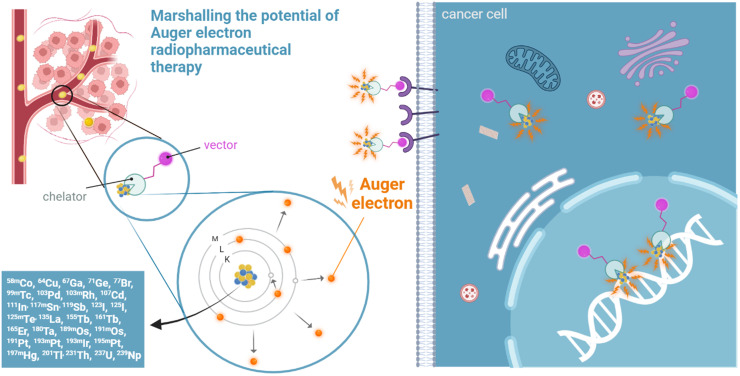
Visual Abstract
Keywords: radiopharmaceutical therapy, Auger electrons emitters, radiopharmaceuticals, recommendations
Abstract
Auger electron (AE) radiopharmaceutical therapy (RPT) may have the same therapeutic efficacy as α-particles for oncologic small disease, with lower risks of normal-tissue toxicity. The seeds of using AE emitters for RPT were planted several decades ago. Much knowledge has been gathered about the potency of the biologic effects caused by the intense shower of these low-energy AEs. Given their short range, AEs deposit much of their energy in the immediate vicinity of their site of decay. However, the promise of AE RPT has not yet been realized, with few agents evaluated in clinical trials and none becoming part of routine treatment so far. Instigated by the 2022 “Technical Meeting on Auger Electron Emitters for Radiopharmaceutical Developments” at the International Atomic Energy Agency, this review presents the current status of AE RPT based on the discussions by experts in the field. A scoring system was applied to illustrate hurdles in the development of AE RPT, and we present a selected list of well-studied and emerging AE-emitting radionuclides. Based on the number of AEs and other emissions, physical half-life, radionuclide production, radiochemical approaches, dosimetry, and vector availability, recommendations are put forward to enhance and impact future efforts in AE RPT research.
Auger electron (AE) radiopharmaceutical therapies (RPTs) are predicted to have efficacy similar to that of α-particles for oncologic small disease, with the added advantage of estimated lower risks of unwanted normal-tissue toxicity. Distinctly different from α-particles, AE emissions originate from the electron shells of an atom after it undergoes internal conversion or electron capture (Fig. 1). Such decay of radionuclides creates a vacancy in an inner atomic shell, most often in the K shell, that is filled by an electron from a higher shell, in turn creating a new vacancy. This leads to a cascade of atomic electron transitions. Each inner atomic shell electron transition results in the emission of either an x-ray or an Auger, Coster–Kronig, or super Coster–Kronig monoenergetic electron (collectively called AEs) (Fig. 1). From 2 and up to more than 30 AEs can be emitted per decay, with energies ranging from a few electron volts to tens of kiloelectron volts (1). AE radionuclides that decay by internal conversion also emit γ-rays and conversion electrons.
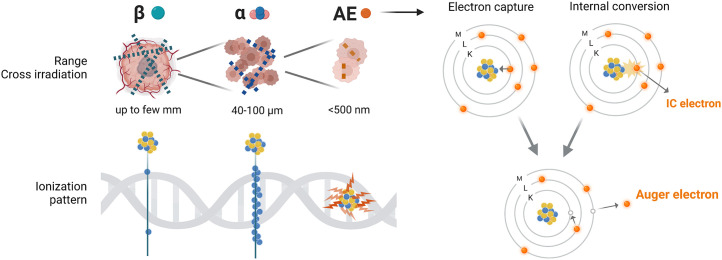
FIGURE 1.
Range, cross irradiation, and ionization patterns of β, α, and AE on scale of tumor/tumor cells (upper left) and DNA (lower left). Emission of Auger and conversion electrons after electron capture and internal conversion (right). IC = internal conversion.
Although some AEs can have maximal energies of tens of kiloelectron volts, for example, 78.2 keV with a maximal range of 87 μm for 195mPt, most AEs have very low energy (<1 keV). Those energies are deposited over less than 500 nm in tissues, a far shorter range than for α-particles (50–100 μm) (Fig. 1) (1,2). When copious low-energy AEs are emitted during rapid atomic relaxation processes (∼10−15 s), the shower of emitted AEs effectively leads to highly localized energy deposition within about 10 nm of the decay site. AEs’ linear energy transfer is high, between 4 and 26 keV/μm. The dense shower of AEs therefore leads to high-linear-energy-transfer–type radiotoxicity in the form of complex molecular modifications, including complex DNA lesions, lipid oxidation, and protein oxidation. This is especially impactful when energy depositions occur in certain subcellular targets, possibly driving the cellular outcome, high tumor cell killing efficiency, and correspondingly high radiobiologic effectiveness (3–5). The seeds of using AE emitters for RPT were planted by Ludwig Feinendegen in 1968 (6). Several decades have since passed, and much has been learned about the potency of the biologic effects caused by the intense shower of these low-energy AEs (7–9). However, the promise of AE RPT remains theoretic because only a few agents have been evaluated in clinical trials and no radiopharmaceuticals for AE RPT have received regulatory approval for clinical use.
There is renewed interest in radionuclides emitting AEs and other low-energy electrons based on recent work using a wide range of radionuclides: 161Tb, 197m/gHg, 119Sb, 103Pd, 195mPt, 193mPt, 191Pt, 165Er, 67Ga, 71Ge, 201Tl, and 155Tb. This is in addition to the historically well-studied 117mSn, 123/125I, 111In, and 99mTc (10). Many of the promising AE-emitting radionuclides can be produced with low-energy cyclotrons, providing worldwide accessibility; this is in contrast to α- and β-emitting radionuclides. Identification of the ideal radionuclides and optimal amalgamation of radionuclide, dosimetry, radiochemistry, and vector design need careful consideration, as well as the targeted epitope, disease type, and stage. We present an overview of hurdles in the development of a select group of AE-emitting radionuclides and specific recommendations for future AE RPT research. The selection process for preferred AE-emitting radionuclides favored those with a higher number of AEs emitted, preferred half-life, imageable emissions, current worldwide availability, target availability, ease of radiochemical separation, chelator availability, molar activity, vector availability, and overall dosimetry score. This work is a result of an international collaboration between experts in the field who gathered at the International Atomic Energy Agency “Technical Meeting on Auger Electron-Emitters for Radiopharmaceutical Developments” in Vienna in September 2022.
RATING OF RADIONUCLIDES FOR AE RPT
Supplemental Table 1 lists the criteria that define the advantages and disadvantages of AE-emitting radionuclides, with emphasis on those Auger emitters that not only are therapeutically promising but also have the capacity to clearly demonstrate the therapeutic efficacy of AE cascades (supplemental materials are available at http://jnm.snmjournals.org). Thus, the highest scores were awarded to radionuclides with high yields of AE and no β-particles, and lower scores were given to radionuclides that emit AEs and β-particles. The colors in the table indicate increasing favorability: red (unfavorable), yellow (somewhat favorable), light green (favorable), and dark green (highly favorable). With the criteria scored red, it is our goal to highlight the need for further development or research, if the variable is changeable.
Supplemental Table 2 shows the favorability for each criterion for candidate AE radionuclides.
Number of AEs per Decay
The numbers of AEs emitted per decay for the AE-emitting radionuclides (Supplemental Table 2) were extracted from International Commission on Radiological Protection publication 107 (11). High-Z radionuclides with multiple internal conversion and electron capture processes emit the most AEs per decay, for example, 125I, 201Tl, 193mPt, and 195mPt. 201Tl and 195mPt emit an average of 20.9 and 36.5 AEs per decay, whereas 161Tb and 64Cu emit only 10.9 and as low as 1.8 AEs per decay, respectively. Experimental evidence shows that fewer decays are required for high-yield AE radionuclides to achieve the same cell-killing efficacy as low-yield AE-emitting radionuclides (3,12,13). Given the difficulties in delivering enough decays to sterilize an entire population of tumor cells, it stands to reason that high yields of AEs are preferred over low yields. A requirement for fewer decays implies that lower activities can be administered without impacting therapeutic efficacy, should all other variables remain equal. This would be beneficial not only from a healthy-tissue toxicity point of view but also logistically. There is, however, insufficient evidence to define a yield below which the Auger effect vanishes. The closest answer may come from a series of studies performed with 77Br, 123I, and 125I incorporated similarly into the DNA (3,12,13). Results showed a radiobiologic effectiveness of approximately 7 for all 3 AE-emitting radionuclides compared with acute photon irradiation. These data suggest that every decay matters, independent of the number of electrons emitted and where along the DNA the radionuclide is located. Therefore, an average AE yield of 20 or more per decay is preferred for AE RPT. Although there is insufficient experimental evidence to confidently claim that similar arguments hold when the AE emitter is localized in the cytoplasm or on the cell surface, it stands to reason that one decay of a weak AE emitter will not be equivalent to 1 decay of a prolific AE emitter.
Coemission of Conversion Electrons, β-Particles, and Photons
Pure AE emitters are effective against not only single cells and micrometastases but also tumor nodules up to 1 mm in diameter (14). However, pure AE emitters are rare; most radionuclides emit concomitant, relatively more energetic conversion electrons or β-particles (Supplemental Table 2). Characteristic x-rays and γ-rays are emitted in competition with AE and conversion electron emissions, respectively. Even using AE emitters to treat micrometastases, energy may also be deposited outside the intended area. Accordingly, it cannot always be stated that AE RPT will have no consequences for off-target tissues.
The number of conversion electrons emitted per decay likely needs to be considered for most radionuclides studied for AE RPT. The percentage of β-particles emitted per decay is also described in Supplemental Table 2, when relevant. The main AE-emitting radionuclides that emit β-particles are 161Tb, 239Np, 180Ta, 231Th, 237U, and 64Cu.
In column 5 of Supplemental Table 2, radionuclides are annotated in red if they do not emit imageable photon emissions or light green if less than 5% of the γ-rays have an energy of 60–300 keV. A dark green score indicates radionuclides emitting more than 50% 60- to 300-keV γ-rays for SPECT or emitting positrons suitable for PET (Supplemental Table 1). Most traditional AE emitters, such as 111In, 99mTc, 67Ga, and 64Cu, are considered imageable. This clearly both has advantages, for example, dosimetry purposes, and has disadvantages, for example, healthy-tissue toxicity and protection from occupational exposure to staff involved. Here, we did not take into account the availability of theranostic pairs in which the imaging radionuclide differs from the therapeutic one, which could have its own advantages. Instead, we focused on the properties of the AE emitters themselves and our ability to image and follow distribution and accumulation within tissues directly.
Physical Half-Life
In Supplemental Table 2, the following categories based on physical half-life are indicated: red (<12 h and >20 d), yellow (<24 h), light green (1–2 d), and dark green (3–20 d). The physical half-life of the AE-emitting radionuclide should allow enough time for transport to radiolabeling facilities to maximize molar activity and to allow the radiolabeling (if required), quality control, and radiopharmaceutical administration. It should also be amenable for distribution to geographical regions with insufficient infrastructure for cancer therapy. Such regions are not likely to have external-beam therapy, and RPT offers a viable alternative with the added benefit of treating residual disease. Furthermore, the stricter control of administering prescribed activities for therapy makes scheduling patients and the arrival of their prescribed administered activity a more difficult task for radionuclides with short physical half-lives. The required physical half-life is also influenced by the pharmacokinetics of the radiopharmaceutical in terms of both effective uptake and clearance times in the tumor and normal tissues. Radiopharmaceutical vectors with slow uptake in the tumor require radionuclides with physical half-lives longer than the time required for peak uptake of the vector to avoid most of the decays occurring in normal tissues (e.g., bone marrow) before the agent has peaked in the tumor. In contrast, radiopharmaceuticals with fast tumor uptake can accommodate radionuclides with shorter physical half-lives. Longer physical half-lives are also acceptable; however, dose-rate effects should be considered for radionuclides with high yields of energetic β-particles, or conversion electron and radioactive waste handling becomes more complex in the case of very long-lived nuclides (15). Therefore, the physical half-life should preferably match the biologic half-times of the vector in the tumor. Generally, a physical half-life of several days is preferred to accommodate all the above aspects. It should also be noted that if a large population of cells is eradicated on successful targeting before most of the radionuclide decay, the radionuclide’s chemical form can be altered and redistributed to healthy tissues. However, we could argue that this is a possible advantage of AE emitters, whose toxicity is likely to be relatively smaller than α- or β-emitters unless they are brought into healthy cells (and perhaps all the way into healthy cells’ nuclei). Investment in radionuclides with short physical half-lives, such as 161Ho with a 2.5-h physical half-life, requires additional and compelling justification (e.g., achievable yields, molar activity, chelation, 2-step targeting, supply logistics, and dosimetry).
Worldwide Availability, Production Methods, Target Availability
In Supplemental Table 2, the selected AE-emitting radionuclides are scored for their worldwide availability and target availability. The worldwide availability values are among the lowest in the table, notably lower than that of the target availability column, suggesting research and development of viable production routes as a remedy. Historical uses and production routes are broadly indicative of availability; clinically used single-photon emitters made with proton-induced reactions score high, namely 67Ga, 111In, 123I, and 201Tl. Although reactor production offers unparalleled scalability, the presence of unreacted target material can limit the specific activity of the desired reaction product (e.g., 194Pt(n,γ)195mPt). Several other candidates made with proton-induced nuclear reactions have been the subject of recent research and can often be obtained through collaborative research networks in multiple countries, notably 64Cu (whose clinical promise as a PET-imageable radionuclide is a bellwether of promise for other radionuclides in the chart), 58mCo, 155Tb, and 135La, all of which are available sporadically in North America and Europe. These most available candidate AE-emitting radionuclides benefit from the distributed global infrastructure of small- to medium-sized cyclotrons (≤30 MeV H+). With clinical success of AE-based treatments, their availability is expected to scale in response as the hundreds of global hospital-based and research institution–based cyclotrons devote their considerable capacity to production.
Unfortunately, some of the most promising AE emitters with favorable decay characteristics have lower availability. The challenging separation and chelation chemistries of 71Ge, 119Sb, 165Er, and 197m/gHg presently throttle their exploration beyond fundamental physical and chemical research. Target material cost, handling, and availability limit work with refractory precious metal radioisotopes of platinum, iridium, and osmium. Work with actinides is limited by special nuclear materials restrictions, complex decay schemes, and the challenge of obtaining useful purities with chemical processing (e.g., 231Th, 237U, and 239Np).
Separation Chemistry, Chelation Chemistry, Molar Activity
Radiochemical separation of the desired radionuclide from the irradiated target material is an important step that applies to all production strategies. The main requirement for using the product radionuclide for the AE RPT is to minimize its contamination with target material and other stable or radioactive impurities associated with the irradiation or separation process. Also, high molar activity is very important as it enables a maximum number of radioactive atoms to be delivered to its target site and reach optimal therapeutic efficacy.
The selected AE-emitting radionuclides are categorized into 4 groups in terms of their ease of separation (Supplemental Table 2). Generally, charged particle–induced reactions and (n, γ) reactions followed by β decay form a product of a different Z than the target material, providing the possibility to separate no-carrier-added product radionuclide from the target material with sufficient purity for radiopharmaceutical application. Notably, most of the most promising AE-emitting radionuclides discussed here still lack efficient radiochemical purification; it is of the utmost importance to develop efficient purification procedures.
Precipitation, liquid–liquid extraction, distillation, ion exchange, and solid-phase extraction chromatography are among the major separation methods used. Among these methods, the ion exchange and solid-phase extraction separation methods are the most convenient and can be easily applied in the hot cell for mass production to increase the activity concentration of the final product. However, it is sometimes difficult to have a suitable resin material or selective eluting solvent for the desired radionuclide. Often a combination of several steps and methods is required to achieve the required separation (factors 108–109) of the desired product from the target element (16). Besides these chemical separation methods, mass separation provides another means for the separation of the no-carrier-added radionuclides; however, both the number of facilities available to perform mass separation and the scalability of this technique remain limited.
Most of the AE emitters in Supplemental Table 2 are radiometals. Efficient, stable chelation of these radiometals is a critical step in the synthesis of the respective AE-emitting radiopharmaceuticals. Stability and selectivity are 2 important criteria to guide the development of suitable chelators, together with their amenability to functionalization with targeting biomolecules. High thermodynamic stability of the complex is crucial to maintain the radiometal associated with the targeting vector, and the selectivity might facilitate the achievement of a high apparent molar activity by avoiding the chelation of possible metal contaminants. The molar activity was considered in different ranges, from less than 1 GBq/μmol (red in Supplemental Tables 1 and 2) to more than 100 GBq/μmol (dark green in Supplemental Tables 1 and 2). The radiopharmaceutical chemistry of several AE-emitting radiometals, namely radiolanthanides, is well established, and several acyclic or cyclic chelators are available to form kinetically inert and stable complexes, such as DOTA, TETA, and NOTA derivatives (17). In contrast, chelation chemistry for many promising AE emitters (e.g., 119Sb, 197mHg, 103Pd, and 195mPt) is less developed, and suitable chelators are still missing. Chelator availability was scored in Supplemental Table 2 from red (none) to yellow (complex radiolabeling), light green (most likely available), and dark green (routine).
Vector, Targeting, Cellular Dosimetry
Due to the very short range of AEs, the risk of normal-tissue toxicity from these electrons themselves is expected to be limited, provided that the vector carrying the radionuclide is not incorporated into normal tissues. Even in the worst-case scenario in which the radionuclide dissociates from its targeting vector, it is assumed that healthy-tissue toxicity—for example, bone marrow, kidneys, salivary glands, liver, and guts—will be minimal provided that the radionuclide does not concentrate within stem cells or other key subpopulations of cells. In addition, on incorporation into the bone, bone marrow toxicity should remain at acceptable levels because of their short range.
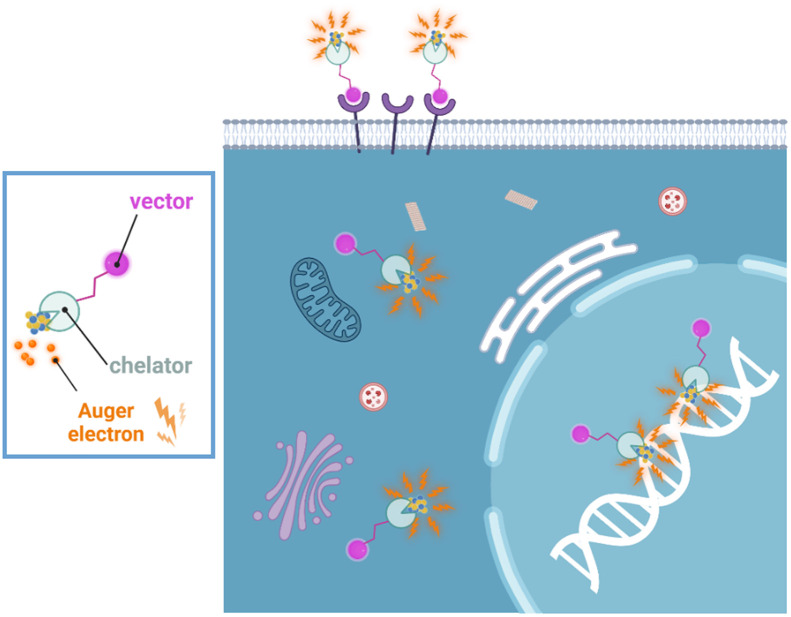
The short range of AE emissions may at first appear to be a negative trait. It necessitates targeting of the radiolabeled compound to a specific subcellular structure, such as nuclear DNA, the nucleolus, or, to a lesser extent, the nucleus, to obtain maximal beneficial effects. Approaches to targeting AE-emitting radionuclides to other subcellular compartments such as the cell membrane or mitochondria can also be considered (Fig. 2) (5,18). A higher number of decays per cell is needed for therapeutic efficacy from AEs when located outside the nucleus; however, bystander effects induced by cell membrane irradiation could compensate, at least partly, for the anticipated inferior efficacy in the absence of nuclear targeting, particularly when vectors do not gain access to every tumor cell. In Supplemental Tables 1 and 2, vector availability was scored red if no vectors for the selected radionuclide have been studied, whereas a high score (dark green) was applied if a considerable number of vectors have been studied.
FIGURE 2.
Although nucleus and DNA are typically the primary cellular targets of radiation damage, internalization into cancer cells and delivery to cell nucleus is not required for cell killing with AE-emitting radionuclides. Targeting of cell membrane can be an effective strategy for killing cancer cells with AEs (18). AEs can also initiate strong bystander response that significantly participates in cell killing (23,29,30).
Cellular dosimetry and macroscopic dosimetry (including photons) for the AE radionuclides are provided in Supplemental Table 3 (19). An overall dosimetry score for each radionuclide was calculated:
Scores of 1–5 were assigned for each dosimetry category. Category scores included the number of AEs emitted per decay , physical half-life, self-dose to the cell nucleus per decay in the cell nucleus , ratio of self-dose to the nucleus to total absorbed dose to the nucleus , and ratio of self-dose from particles to self-dose from all radiations including photons for a 6.2-mm radius sphere of water . The #AE and the 2 ratios were considered to be of more dosimetric importance than physical half-life and self-absorbed dose per decay in the context of using AE emitters for therapy. The argument for #AE has already been discussed above at length. The ratio speaks to maximizing the absorbed dose to the target cells and minimizing the absorbed dose to surrounding normal cells. The emphasizes the importance of minimizing the cross irradiation from photons. These were weighted more heavily by squaring their scores. The absorbed doses were calculated using MIRDcell V3.12 software and are presented in Supplemental Figures 1–6 and Supplemental Tables 3 and 4 (20).
DISCUSSION AND RECOMMENDATIONS
Number of AEs and Coemissions, Physical Half-Life
Ideally, a pure AE emitter such as 165Er would be pursued for AE RPT. Alternatively, the radionuclides that emit the highest number of AEs per decay should also be considered; these would include 201Tl, 119Sb, 125I, 193mPt, 195mPt, 231Th, 237U, and 125mTe. However, availability or simplicity of radiochemistry and targeting requirements currently limit the choice of AE-emitting radionuclides to those that coemit conversion electrons, photons, or β-particles.
The main coemissions that can impact clinical use are photons. For example, in the case of the AE-emitting radionuclides 111In and 125I, the photon-to-electron ratio is 11.8 and 2.16, respectively. But the photon-to-electron ratio ranges all the way from 0.05 for 103mRh to 54.8 for 94Tc (21). AE emitters with high yields of AEs and low photon yields are preferred. Administering high activities for AEs with high photon yields has radiation safety implications and can lead to undesirable irradiation of normal tissues, complications for radiation protection, and diminished acceptance by patients and the medical community. However, too low a photon yield may compromise concurrent imaging and make it difficult with current clinical instrumentation to measure the activity to be administered.
Available radionuclides with a promising photon-to-electron ratio for effective theranostics are 123I, 119Sb, 197mHg, 193mPt, 195mPt, and 125mTe. Among these, 119Sb, 193mPt, and 195mPt also have optimal physical half-lives.
Worldwide Availability, Production Methods, Target Availability
Despite the accessibility of many reaction routes to the broad palette of potentially therapeutic AE-emitting radionuclides, there are significant production challenges associated with several leading candidates. Of the selected AE-emitting radionuclides, 65.5% have limited or no worldwide availability (Supplemental Table 2). Some notable examples are 58mCo and 189mOs. For 58mCo (half-life, 9.4 h), the metastable isomer decays 100% to its long-lived ground state, 58gCo (half-life, 70 d), a radionuclide impurity that can only be accounted for with the pharmacokinetics of the targeting vector and the biologic elimination of the radiopharmaceutical. For 189mOs, the most desirable routes to formation of this radionuclide (half-life, 5.81 h) are by electron capture or β-decay of its 2 parents, 189Ir (half-life, 13.3 d) and 189Re (half-life, 24.3 h), which populate the metastable state with 7.5% and 8% of their decays, respectively, limiting the achievable molar activity of 189mOs. The situation with platinum radionuclides is similar but less fraught, since 193mPt and 195mPt, usually produced together, are both AE emitters of significant interest and have half-lives of about 4 d. High-purity production of the very interesting SPECT/AE-emitting radionuclide 155Tb requires a more highly enriched target 155Gd than is presently available (∼90% isotopic enrichment). Without higher 155Gd enrichments or mass separation, 156Tb content poses challenging dosimetry questions for patient studies.
Many AE-emitting radionuclides require target materials with high isotopic enrichments. Most of these materials are commonly sourced from a small number of commercial vendors worldwide who purchase or repurchase, mostly from Russian suppliers. The U.S. Department of Energy and the European Union have programs to reestablish enrichment capabilities, but global social and geopolitical instability contribute to rising material costs and diminishing availabilities exacerbated by an undiversified supply chain. Future large-scale production of AE radionuclides depends on multiple isotopic enrichment efforts for production of target materials on a scale of tens to hundreds of grams.
Separation Chemistry, Chelation Chemistry, Molar Activity
For several interesting AE-emitting candidates, no transmutation reaction is available; a method of mass-based separation will therefore have to be used to achieve high molar activities. These include, especially, 191Os, neutron- and γ-produced radionuclides, and several less investigated actinide radionuclides such as 231U, 229Pa, 231Th, and 237U (22). Improving the availability of AE-emitting radionuclides through development of production methods will advance the field. Focus should go to those radionuclides with a high AE yield, a favorable AE–to–γ-emission ratio, and a physical half-life that allows wide distribution and the development of their radiochemistry. Production using widely available technology (such as smaller cyclotrons, which are disseminated throughout the world) will also increase availability and adoption by the field. Advancement in production technologies for medical radionuclides; engaging and scaling production methods based on irradiation with protons, neutrons, and other particles (e.g. electron linear accelerators); and advancement of mass separation technology will enable expansion of the list of promising candidates and achieve the required molar activities for many promising AE emitters.
Vector, Targeting, Cellular Dosimetry
Positional effects (location of decay site in the cell) of AE emitters have been studied, but more remains to be learned. To date, targeting the DNA in the cell nucleus has proven to be the most effective for therapy, with the least number of decays required for cell inactivation. Evidence to date supports membrane targeting as the next best option, followed by cytoplasmic localization (Fig. 2). In all cases, radiation-induced bystander effects appear to play a role that merits further exploration (23). However, so far, there are no suitable AE-emitting radiopharmaceuticals that achieve both specific targeting of cancer cells and delivery of a cytocidal number of decays to sterilize the entire population of tumor cells (24). It remains to be seen whether other subcellular targets can be exploited through precision targeting with appropriate vectors to achieve similar or greater cytotoxicity (Table 1). The specificity and selectivity of the delivery vector or of the radiolabeled compounds should be evaluated in detail using cancer cells, including definition of subcellular localization and targeting specificity, in combination with the most optimal radionuclide for a disease-specific biologic target. Clinical evaluation should be informed through preclinical evaluation. A deep understanding of the biologic behavior and radiobiologic effects of AE-emitting radiopharmaceuticals is needed to select the optimal compounds for clinical investigation.
TABLE 1.
Available Vectors for Different Subcellular Targets
| Vector internalized | Vector targeting nucleus | Vector noninternalized |
|---|---|---|
| Trastuzumab anti-HER2 mAb (5) | CPP: for example, TAT (5) | Anti-CEA mAb 35A7: after transfecting cells with CEA (5,14,18) |
| mAb 425: binding EGFR and internalized | MAP (33) | (DOTA-LM3): SSTR antagonist that localizes at the cell membrane (34) |
| (anti-HER1) 125I-m225 (14,18) | PARPi: 125I-KX1 and 123I-MAPi (35,36) | |
| Membrane NAT receptor: cells transfected with NAT gene to enable active uptake of MIBG in cells (36) | IUdR: thymidine analogs that are incorporated into DNA in S phase (37) | |
| DOTATOC: SSTR agonists that localize in cytoplasm (34) | DOTATOC-NLS: SSTR agonists that localize to cellular nucleus (34); 125I-labeled Hoechst and acridine orange derivatives (38–40) | |
| F3 peptide: binds nucleolin, expressed in nuclei of normal cells but is also on membrane of some cancer cells (41) |
HER2 = human epidermal growth factor receptor 2; CPP = cell-penetrating peptides; CEA = carcinoembryonic antigen; EGFR = epidermal growth factor receptor; MAP = model amphipathic peptide; LM3 = p-Cl-Phe-cyclo(D-Cys-Tyr-D-4-amino-Phe(carbamoyl)-Lys-Thr-Cys)D-Tyr-NH2 and SSTR; HER1 = human epidermal growth factor receptor 1; PARPi = poly-ADP ribose polymerase inhibitor; KX1 = 1-(4-(iodophenyl)-8,9-dihydro-2,7,9a-triazabenzo[cd]azulen-6(7H)-one; MAPi = model amphipathic peptide inhibitor; NAT = noradrenaline transporter; MIBG = meta-iodobenzylguanidine; IUdR = 5-iodo-2′-deoxyuridine; SSTR = somatostatin receptor; NLS = nuclear localization sequence.
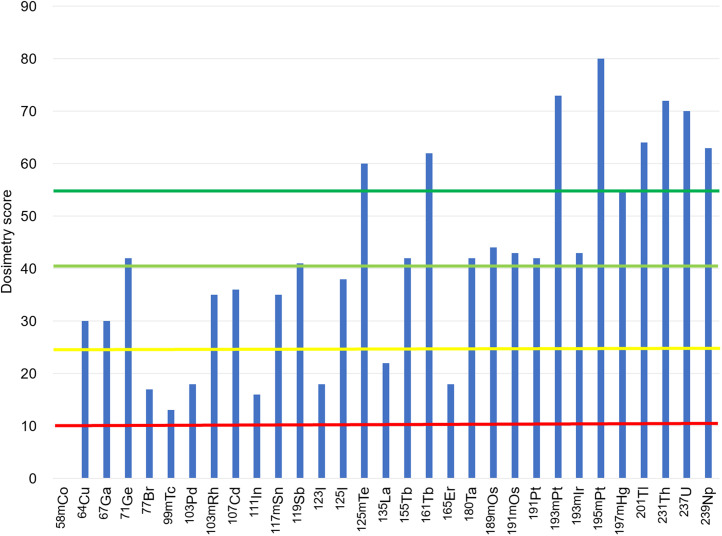
Another pressing problem to overcome clinical implementation of AE emitters is the inherent nonuniform distribution of radiopharmaceuticals in tumor tissues. A nonuniform distribution is present in each case and can be due to heterogeneous target expression or the natural variation (typically log-normal) that is present among even a clonal population of cells in suspension. Approaches to overcoming the nonuniform distribution of radiopharmaceuticals are being developed that involve the use of 2 or more radiopharmaceuticals (or other agents) to permit sterilization of circulating tumor cells (25,26), disseminated tumor cells, and micrometastases. More importantly, software tools (20,27) are continuously being developed to implement these approaches that are ultimately intended to provide personalized treatment. However, the development of standardized dosimetry practices is necessary for AE RPT (28). As pointed out by the International Commission on Radiation Units and Measurements, image-derived dosimetry may not suffice for AE emitters, given their short range or, when plentiful γ-particles are coemitted, because the ultimate application may be when treated lesions are so small that they fall beneath the detection and resolution limits of scanners. AE radionuclide–dedicated subcellular and multicellular biopsy-based dosimetry may be necessary (28). Figure 3 shows a histogram of the overall dosimetry score for the AE radionuclides. In general, the AE radionuclide candidates with a high atomic number are generally preferred dosimetrically because of the large number of AEs emitted, the small number of decays required to deliver sterilizing absorbed doses, and the low abundance of photons relative to particle radiations (Fig. 3).
FIGURE 3.
Overall dosimetry score for radionuclides for AE RPT: unfavorable (10–26), somewhat favorable (26–41), favorable (41–56), and highly favorable (≥55).
Preclinical Evaluation and Translation into the Clinic and Applications
Initial translational and clinical evaluations should focus on treatment of micrometastases and disseminated tumor cells (with the disease site and phenotype informing selection of the biologic target) and on design of a vector mechanism that selectively delivers the AE to cancer cells. We recommend that a higher likelihood of successful therapy can be gained from the treatment of early-stage, small disease; small residual disease (after other treatments, such as surgery or external-beam radiotherapy); or minimal recurrent disease, even at the occult stage. Detection methods other than anatomic or molecular imaging may be necessary, such as monitoring of circulating tumor cells, analysis of disseminated tumor cells in lymph nodes, and analysis of other biochemical markers. Contrarily, larger tumors may have a lower probability of success, with less possibility of indisputably establishing the clinical efficacy of AE RPT. The high-linear-energy-transfer property also makes radionuclides emitting α-particles, and likely AE emitters, theoretically less dependent on the oxygenation state of the tumor environment. This usual dependency is mitigated by considering their final subcellular localization and, to some extent, their ability to also induce an oxygen-dependent bystander response (18). AE emitters could therefore not only overcome hypoxia-related treatment resistance but also produce an enhanced therapeutic response in the form of radiation-induced bystander effects (23,29,30). Currently, different strategies including nanoparticle-based delivery constructs are adapted to achieve delivery of AE emitters to the nucleus and preferential subcellular targets. Also, the use of radiopharmaceutical cocktails is expected to maximize the cytocidal effect with AE RPT and minimize injected activity. This, in turn, can minimize normal-tissue toxicity. However, the translation of these strategies to the clinic requires extensive challenging preclinical evaluation (31,32). Although AE RPT will always be an adjuvant therapy in the clinic, preclinical research with pure AEs such as 71Ge, 119Sb, and 165Er will advance knowledge of the potential of AE RPT probes.
Well-designed clinical trials are necessary to demonstrate the merit of AE RPT for patients. These trials should consider preferential use in patients with small disease, comparison of standard-of-care treatment regimens versus the addition of AE RPT, and the use of relevant readouts, such as progression-free survival, overall survival, or recurrence of disease. Evaluation of late toxicity may become necessary at a later stage, to inform radiation protection of healthy tissues. This will be particularly important when comparing against radiopharmaceuticals that emit α-particles. Aside from the potential oncologic applications of AE RPT in treating small-volume diseases, there may be other viable applications, including treating infections, musculoskeletal disease, and cardiovascular and neurologic disorders.
KEY ASPECTS TO CONSIDER WHEN TRANSLATING AE RPT TO CLINICAL PRACTICE
High-yield AE emitters should be used to minimize the number of decays required. Many decays are needed to sterilize tumor cells with AE emitters. Because delivery of sufficient decays to all tumor cells is challenging it is desirable to develop radiopharmaceuticals that require as few decays as possible.
AE emitters can impart high-linear-energy-transfer–type radiotoxicity with no dose rate effect.
The physical characteristics of AE are well defined and promising, but more research is needed on the ideal delivery systems and their availability.
Radionuclides with low photon yields should be preferentially selected to avoid normal-tissue toxicity, minimize radiation protection issues, and gain acceptance of the therapy by patients and medical practitioners. A low (scoring criteria are in Supplemental Table 1) photon yield with energies of about 100 keV is desirable for SPECT imaging.
Combinations of AE RPT and other therapeutic modalities, such as chemotherapy and immunomodulatory therapy, will maximize cytocidal effect and minimize injected activity. This, in turn, can minimize normal-tissue toxicity.
Radiopharmaceuticals can degrade in the body, potentially resulting in distribution of radionuclides to normal tissues. Clinical experience with 223Ra-dichloride suggests that radionuclides that emit short-range radiation have a good safety profile when localized on bone surfaces. Therefore, AE-emitting radionuclides that are natural bone-surface seekers may be a good option for improving patient safety.
Unwanted cytotoxicity caused by AE emitters to healthy tissue can be countered with radical scavengers, unlike for α-particles. This implies that, like external-beam radiation therapy with photons, irradiated normal tissues may benefit from DNA repair to a greater degree than tumor tissue. Therefore, AE RPT has a potential added benefit not possible for α-RPT.
Most AE-emitting radionuclides can be produced with low-energy cyclotrons.
Stable accelerator target materials and nuclear reactions for AE emitters’ production are more available than those needed for α-emitters.
Pure AE emitters, such as 71Ge or 119Sb, could answer remaining radiobiologic questions pertaining to the therapeutic effectiveness of AE, but chelation chemistry is needed to incorporate these nuclides into radiopharmaceuticals.
DISCLOSURE
Roger Howell is supported in part by grant 1R01CA245139 from the U.S. National Cancer Institute (NCI). Samantha Terry is supported by the EPSRC Program for Next Generation Molecular Imaging and Therapy with Radionuclides (EP/S032789/1, “MITHRAS”) and core funding from the Wellcome/EPSRC Centre for Medical Engineering (WT203148/Z/16/Z). Valery Radchenko is supported by the Canadian Institute for Health Research (CIHR) via research project GR021373 and by the Natural Sciences and Engineering Research Council (NSERC) of Canada via Discovery grant RGPIN-2018-04997. TRIUMF receives funding via a contribution agreement with the National Research Council of Canada. Julie Bolcaen and Roger Howell hold a patent related to the area of work in this article. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENT
This review was informed by “Technical Meeting on Auger Electron Emitters for Radiopharmaceutical Developments,” held September 5–9, 2022, with support from the International Atomic Energy Agency. Figures were created with BioRender (biorender.com).
KEY POINTS
QUESTION: AE RPT may have the same therapeutic efficacy as α-particles for oncologic small disease, with lower risks of normal-tissue toxicity. However, what are the next steps for impactful AE RPT?
PERTINENT FINDINGS: The production of some AEs with highly desirable characteristics is not yet developed. Careful consideration of all parameters, including decay properties, nuclear chemistry, radiochemistry, dosimetry, and radiobiology, is essential to successful design of AE-emitting radiopharmaceuticals. An average AE yield of 20 or more per decay may be preferred for AE RPT.
IMPLICATIONS FOR PATIENT CARE: AE RPT might have efficacy similar to that of α-particles for oncologic small disease, with the advantage of lower risks of normal-tissue toxicity. The clinical success of AE RPT treatments can take advantage of the availability of hundreds of global hospital-based and research institution–based cyclotrons to produce AE-emitting radionuclides, facilitating their worldwide spread at a more economical cost.
REFERENCES
- 1. Howell RW. Radiation spectra for Auger-electron emitting radionuclides: report no. 2 of AAPM Nuclear Medicine Task Group No. 6. Med Phys. 1992;19:1371–1383. [DOI] [PubMed] [Google Scholar]
- 2. Kassis AI. Molecular and cellular radiobiological effects of Auger emitting radionuclides. Radiat Prot Dosimetry. 2011;143:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kassis AI, Sastry KS, Adelstein SJ. Kinetics of uptake, retention, and radiotoxicity of 125IUdR in mammalian cells: implications of localized energy deposition by Auger processes. Radiat Res. 1987;109:78–89. [PubMed] [Google Scholar]
- 4. Kassis AI, Fayad F, Kinsey BM, Sastry KS, Adelstein SJ. Radiotoxicity of an 125I-labeled DNA intercalator in mammalian cells. Radiat Res. 1989;118:283–294. [PubMed] [Google Scholar]
- 5. Pouget JP, Santoro L, Raymond L, et al. Cell membrane is a more sensitive target than cytoplasm to dense ionization produced by Auger electrons. Radiat Res. 2008;170:192–200. [DOI] [PubMed] [Google Scholar]
- 6. Biological Effects of Transmutation and Decay of Incorporated Radioisotopes, Panel Proceedings Series . International Atomic Energy Agency; 1968. [Google Scholar]
- 7. Hofer KG. Biophysical aspects of Auger processes: a review. Acta Oncol. 1996;35:789–796. [DOI] [PubMed] [Google Scholar]
- 8. Howell RW. Advancements in the use of Auger electrons in science and medicine during the period 2015-2019. Int J Radiat Biol. 2023;99:2–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Idrissou MB, Pichard A, Tee B, Kibedi T, Poty S, Pouget JP. Targeted radionuclide therapy using auger electron emitters: the quest for the right vector and the right radionuclide. Pharmaceutics. 2021;13:980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ku A, Facca VJ, Cai Z, Reilly RM. Auger electrons for cancer therapy: a review. EJNMMI Radiopharm Chem. 2019;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eckerman K, Endo A. ICRP publication 107. Nuclear decay data for dosimetric calculations. Ann ICRP. 2008;38:7–96. [DOI] [PubMed] [Google Scholar]
- 12. Kassis AI, Adelstein SJ, Haydock C, Sastry KSR, McElvany KD, Welch MJ. Lethality of Auger electrons from the decay of bromine-77 in the DNA of mammalian cells. Radiat Res. 1982;90:362–373. [PubMed] [Google Scholar]
- 13. Makrigiorgos GM, Kassis AI, Baranowska-Kortylewicz J, et al. Radiotoxicity of 5-[123I]iodo-2′-deoxyuridine in V79 cells: a comparison with 5-[125I]iodo-2′-deoxyuridine. Radiat Res. 1989;118:532–544. [PubMed] [Google Scholar]
- 14. Santoro L, Boutaleb S, Garambois V, et al. Noninternalizing monoclonal antibodies are suitable candidates for 125I radioimmunotherapy of small-volume peritoneal carcinomatosis. J Nucl Med. 2009;50:2033–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Solanki JH, Tritt T, Pasternack JB, et al. Cellular response to exponentially increasing and decreasing dose rates: implications for treatment planning in targeted radionuclide therapy. Radiat Res. 2017;188:221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radchenko V, Baimukhanova A, Dmitry F. Radiochemical aspects in modern radiopharmaceutical trends: a practical guide. Solvent Extr Ion Exch. 2021;39:714–744. [Google Scholar]
- 17. Herrero Álvarez N, Bauer D, Hernández-Gil J, Lewis JS. Recent advances in radiometals for combined imaging and therapy in cancer. ChemMedChem. 2021;16:2909–2941. [DOI] [PubMed] [Google Scholar]
- 18. Paillas S, Ladjohounlou R, Lozza C, et al. Localized irradiation of cell membrane by Auger electrons is cytotoxic through oxidative stress-mediated nontargeted effects. Antioxid Redox Signal. 2016;25:467–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vaziri B, Wu H, Dhawan AP, Du P, Howell RW. MIRD pamphlet no. 25: MIRDcell V2.0 software tool for dosimetric analysis of biologic response of multicellular populations. J Nucl Med. 2014;55:1557–1564. [DOI] [PubMed] [Google Scholar]
- 20. MIRDcell V3. Rutgers website. https://mirdcell.njms.rutgers.edu/. Accessed July 14, 2023.
- 21. Uusijärvi H, Bernhardt P, Ericsson T, Forssell-Aronsson E. Dosimetric characterization of radionuclides for systemic tumor therapy: influence of particle range, photon emission, and subcellular distribution. Med Phys. 2006;33:3260–3269. [DOI] [PubMed] [Google Scholar]
- 22. Filosofov D, Kurakina E, Radchenko V. Potent candidates for targeted Auger therapy: production and radiochemical considerations. Nucl Med Biol. 2021;94-95:1–19. [DOI] [PubMed] [Google Scholar]
- 23. Kishikawa H, Wang K, Adelstein SJ, Kassis AI. Inhibitory and stimulatory bystander effects are differentially induced by iodine-125 and iodine-123. Radiat Res. 2006;165:688–694. [DOI] [PubMed] [Google Scholar]
- 24. Rosenkranz AA, Slastnikova TA, Georgiev GP, Zalutsky MR, Sobolev AS. Delivery systems exploiting natural cell transport processes of macromolecules for intracellular targeting of Auger electron emitters. Nucl Med Biol. 2020;80–81:45–56. [DOI] [PubMed] [Google Scholar]
- 25. Akudugu JM, Howell RW. Flow cytometry-assisted Monte Carlo simulation predicts clonogenic survival of cell populations with lognormal distributions of radiopharmaceuticals and anticancer drugs. Int J Radiat Biol. 2012;88:286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pasternack JB, Domogauer JD, Khullar A, Akudugu JM, Howell RW. The advantage of antibody cocktails for targeted alpha therapy depends on specific activity. J Nucl Med. 2014;55:2012–2019. [DOI] [PubMed] [Google Scholar]
- 27. Katugampola S, Wang J, Rosen A, Howell RW. MIRD pamphlet no. 27: MIRDcell V3, a revised software tool for multicellular dosimetry and bioeffect modeling. J Nucl Med. 2022;63:1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sgouros G, Bolch WE, Chiti A, et al. ICRU report 96, dosimetry-guided radiopharmaceutical therapy. J ICRU. 2021;21:1–212. [Google Scholar]
- 29. Howell RW, Bishayee A. Bystander effects caused by nonuniform distributions of DNA-incorporated 125I. Micron. 2002;33:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xue LY, Butler NJ, Makrigiorgos GM, Adelstein SJ, Kassis AI. Bystander effect produced by radiolabeled tumor cells in vivo. Proc Natl Acad Sci USA. 2002;99:13765–13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sobolev AS. Modular nanotransporters for nuclear-targeted delivery of Auger electron emitters. Front Pharmacol. 2018;9:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bavelaar BM, Lee BQ, Gill MR, Falzone N, Vallis KA. Subcellular targeting of theranostic radionuclides. Front Pharmacol. 2018;9:996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zaro JL, Vekich JE, Tran T, Shen WC. Nuclear localization of cell-penetrating peptides is dependent on endocytosis rather than cytosolic delivery in CHO cells. Mol Pharm. 2009;6:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borgna F, Haller S, Rodriguez JMM, et al. Combination of terbium-161 with somatostatin receptor antagonists: a potential paradigm shift for the treatment of neuroendocrine neoplasms. Eur J Nucl Med Mol Imaging. 2022;49:1113–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pirovano G, Jannetti SA, Carter LM, et al. Targeted brain tumor radiotherapy using an Auger emitter. Clin Cancer Res. 2020;26:2871–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee H, Riad A, Martorano P, et al. PARP-1-targeted Auger emitters display high-LET cytotoxic properties in vitro but show limited therapeutic utility in solid tumor models of human neuroblastoma. J Nucl Med. 2020;61:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boyd M, Ross SC, Dorrens J, et al. Radiation-induced biologic bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with α-, β-, and Auger electron–emitting radionuclides. J Nucl Med. 2006;47:1007–1015. [PubMed] [Google Scholar]
- 38. Fourie H, Nair S, Miles X, et al. Estimating the relative biological effectiveness of Auger electron emitter 123I in human lymphocytes. Front Phys. 2020;8:1–14. [Google Scholar]
- 39. Pereira E, do Quental L, Palma E, et al. Evaluation of acridine orange derivatives as DNA-targeted radiopharmaceuticals for Auger therapy: influence of the radionuclide and distance to DNA. Sci Rep. 2017;7:42544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Balagurumoorthy P, Xu X, Wang K, Adelstein SJ, Kassis AI. Effect of distance between decaying 125I and DNA on Auger-electron induced double-strand break yield. Int J Radiat Biol. 2012;88:998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cornelissen B, Waller A, Target C, Kersemans V, Smart S, Vallis KA. 111In-BnDTPA-F3: an Auger electron-emitting radiotherapeutic agent that targets nucleolin. EJNMMI Res. 2012;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]