Abstract
Purpose
To evaluate the long-term safety and efficacy of sequential canaloplasty and trabeculotomy combined with cataract surgery in patients with mild, moderate, and advanced open-angle glaucoma.
Patients and Methods
Case records of 171 consecutive patients (171 eyes) who had undergone cataract surgery followed by canaloplasty (≥180°) and trabeculotomy (≥90°) for mild, moderate, or advanced open-angle glaucoma (Shaffer grade ≥3) using the OMNI Surgical System (Sight Sciences, Inc., Menlo Park, CA) were analyzed retrospectively. Efficacy endpoints included change in mean IOP and number of medications from baseline to postoperative 12- and 24-months for the overall dataset and stratified by each stage of glaucoma. Kaplan–Meier survival analysis of success (eyes that did not require secondary surgical interventions (SSI)) by postoperative 24 months was also performed.
Results
Postoperatively, there was a statistically significant reduction in IOP (baseline of 17.2 mmHg on 1.3 medicines reduced to 14.3 on 0.8 medicines (12 months) and 14.0 on 0.9 medicines (24 months), p<0.001 for both time points). Eyes with advanced glaucoma (N=63) maintained significant IOP reduction (17.8 mmHg on 1.6 medicines at baseline reduced to 13.6 mmHg on 1.3 medicines (12 months) and 13.0 on 1.5 medicines (24 months), p<0.001). Kaplan–Meier analysis showed a 93.0% survival probability for the avoidance of SSI at 2 years after surgery.
Conclusion
Canaloplasty and trabeculotomy combined with cataract surgery provided effective IOP reduction for eyes with all stages of glaucoma at postoperative 12 and 24 months, and the procedure yielded a 93% survival rate for SSI avoidance at 2 years.
Keywords: open-angle glaucoma, OMNI surgical system, combined canaloplasty and trabeculotomy, implant-free MIGS, mild, moderate, advanced glaucoma
Introduction
Open-angle glaucoma (OAG) is a chronic, progressive, and irreversible optic neuropathy that is characterized by increased resistance to aqueous outflow in the trabecular meshwork (TM), despite an anatomically open iridocorneal angle. Elevated intraocular pressure (IOP) is considered an important risk factor leading to the development and progression of the disease. Surgical intervention for glaucoma aims to facilitate the egress of aqueous humor and restore IOP to levels consistent with optic nerve health, ideally restoring fluid dynamics to a normal equilibrium.1
About 50–70% of the trabecular outflow resistance is thought to be at proximal tissues such as the juxtacanalicular TM and inner wall of Schlemm’s canal (SC), and the remaining 30–50% at distal sites such as the back wall of SC and the collector channels.2–4 Nowadays, minimally invasive glaucoma surgeries (MIGS) have evolved to achieve IOP reduction by restoring flow through the trabecular outflow pathway.5–9 Addressing all sources of outflow resistance circumferentially would theoretically offer more consistent efficacy by treating all components of the conventional outflow pathway.
Glaucoma treatment with the OMNI surgical system (Sight Sciences, Inc., Menlo Park, CA) is an implant-free MIGS procedure that addresses all three sources of outflow resistance at the level of the TM, SC, and collector channels with a single device. It is the first MIGS device that facilitates the microcatheterization of all 360° of the SC from a single clear corneal incision to allow the surgeon to micro invasively perform ab interno canaloplasty, followed by trabeculotomy (if the surgeon deems it appropriate) using the same purpose-built device.10 By first performing canaloplasty, the tissues in the distal outflow pathway, such as collector channels and ostia, are dilated and potentially re-opened while subsequent trabeculotomy removes the resistance residing in the TM.
This ab interno procedure can be performed either as a standalone procedure or combined with cataract surgery. Combination procedures with cataract surgery allow early surgical intervention as cataract surgery creates the opportunity to also intervene with MIGS without adding significant risk to the patient.11
Recent studies of sequential canaloplasty and trabeculotomy, either as a standalone procedure or at the time of cataract surgery, have reported significant IOP reductions of 28% to 35% and medication reductions of 25–75%. However, these studies included only eyes with mild-moderate OAG.6,10–13 The purpose of the present study was to evaluate the real-world safety and effectiveness outcomes of the OMNI-assisted sequential canaloplasty and trabeculotomy performed in combination with phacoemulsification for the treatment of all stages of OAG (mild, moderate, and advanced), including pigmentary glaucoma and pseudoexfoliative glaucoma.
Materials and Methods
In this retrospective study, case records of consecutive patients who underwent phacoemulsification and intraocular lens (IOL) implantation followed by canaloplasty and trabeculotomy for OAG of any stage at Omni Eye Services of Atlanta from June 2018 to July 2020 were included. The stage of glaucoma was defined using the criteria from the Center for Medicare and Medicaid Services (CMS).14 Per the CMS criteria, glaucoma has been categorized into three stages.
Early or mild-stage glaucoma (code H40.1131): This is defined as optic nerve abnormalities consistent with glaucoma, and retinal nerve fiber layer changes, but with no visual field abnormalities. (The exception would be abnormalities only present on short-wavelength automated perimetry (SWAP) or frequency doubling technology (FDT) perimetry visual field testing).
Moderate-stage glaucoma (Code H40.1132): Medicare defines this as optic nerve abnormalities consistent with glaucoma and retinal nerve fiber layer changes, plus visual field abnormalities in one hemifield – but not within 5° of fixation.
Advanced/severe-stage glaucoma (Code H40.1133): This is defined as optic nerve abnormalities consistent with glaucoma, retinal nerve fiber layer changes, glaucomatous visual field abnormalities in both hemifields and/or vision loss within 5° of fixation in at least one hemifield.
Eyes with primary OAG (Shaffer grade ≥3), secondary glaucoma (pigmentary glaucoma and pseudoexfoliative glaucoma), and eyes with previously appositional chronic angle closure glaucoma (for which laser peripheral iridotomy had successfully opened the angle) were included in the analysis. All eyes underwent a minimum of 180° of canaloplasty and a minimum of 90° of trabeculotomy using the OMNI Surgical System by a single surgeon (AY). The study was performed in accordance with the tenets of the Declaration of Helsinki and its amendments and was approved by Salus Independent Review Board (Austin, TX, USA) with a waiver of informed consent as the data were recorded in patient charts as a part of routine clinical practice and only de-identified patient data were analyzed.
Primary efficacy endpoints included an assessment of the change in mean IOP and the mean number of medications from baseline to postoperative 12 months. Secondary endpoints included change in IOP, and medication use from baseline to postoperative 12 months stratified for each stage of glaucoma (mild, moderate, and advanced), change in IOP, and medication use from baseline to postoperative 24 months – overall as well as based on each stage of glaucoma. Kaplan–Meier survival analysis for secondary surgical interventions (SSI) during the 24-month follow-up period was also performed in the overall dataset as well as stratified by each stage of glaucoma. SSI would involve any secondary surgical intervention secondary to glaucoma surgery for the treatment of uncontrolled glaucoma. The need for SSI was determined based on the inability to achieve target IOP despite maximum tolerated medications, with the subsequent performance of an interventional glaucoma procedure such as a glaucoma tube shunt implantation or bleb forming procedure. Target IOP was determined at the discretion of the surgeon based on the eye’s glaucoma stage and prognosis. Postoperative incidence of adverse events (AE), such as IOP spike and hyphema, were also recorded. IOP spike was defined as IOP >10 mmHg from the previous visit and numerical hypotony as IOP <6 mmHg at any postoperative time point. Hyphema was considered an AE only when it was ≥1mm. Cyclodialysis cleft, an iatrogenically induced pathway between the anterior chamber and the suprachoroidal space, was also documented.
Surgical Procedure
The OMNI procedure was performed immediately following IOL implantation during cataract surgery. The TM was visualized under direct gonioscopy, and the OMNI tip was introduced through a clear corneal incision. The tip was inserted through the TM to create a micro-goniotomy to facilitate microcatheter advancement around 180° of SC. Upon retraction of the catheter, a fixed volume of OVD was automatically dispensed to dilate SC and the collector channels. Steps were repeated in the remaining 180° of SC to complete up to 360° canaloplasty. The same microcatheter was reinserted and advanced into the now-dilated SC, and the microcatheter was withdrawn in a manner to unroof the SC to achieve a minimum 90° of trabeculotomy. The degrees of canaloplasty and trabeculotomy performed were titrated based on the surgeon’s discretion and the patient’s medical needs. Varying extents of canaloplasty and trabeculotomy with OMNI was based on the goal of maximizing efficacy while minimizing the potential for intraoperative complications. Extent of treatment was sometimes limited due to patient non-cooperation during surgery, difficulty with access to all parts of Schlemm’s canal, or decreasing operative view of tissues from intraoperative hemorrhage.
Statistical Analysis
Data analysis was performed using SPSS software version 27.0 (IBM Corp, New York, USA). Descriptive statistics on continuous variables included mean, standard deviation, and range, where applicable. Categorical variables were reported in terms of percentages. The normality of scale data was checked using the Kolmogorov–Smirnov test and/or quantile–quantile plots. For normally distributed scale data, paired t-test was used to compare the mean baseline IOP and the number of medications with mean postoperative values at different time points through 24 months after surgery. In the case of not-normally distributed scale data, a non-parametric counterpart, Wilcoxon signed-rank test, was used. A p-value of <0.05 was considered statistically significant. The incidence of AE was summarized as percentages. Kaplan–Meier survival analysis SSI (24-months’ follow-up period) was also performed.
Results
This retrospective study analyzed 171 eyes of 171 patients. The mean age of 171 patients was 70.5 ± 8.2 years (55% females and 45% males). Preoperative glaucoma characteristics are shown in Table 1. Mild-stage OAG was present in 32.7% of the patients (56/171), moderate-stage in 30.4% (52/171), and advanced-stage in 36.8% (63/171) of the patients. Of the total 171 patients, 63% (108/171) underwent 360° of viscodilation and 180–360° of trabeculotomy and 25% (43/171) underwent 180° of viscodilation and 180° of trabeculotomy.
Table 1.
Preoperative Glaucoma Characteristics of Patients Who Underwent Combined Canaloplasty and Trabeculotomy with Cataract Surgery
| Characteristics | N | % |
|---|---|---|
| Glaucoma Type | ||
| Primary open-angle | 156 | 91.2 |
| Chronic angle-closure* | 9 | 5.3 |
| Pseudoexfoliation | 2 | 1.2 |
| Pigmentary | 1 | 0.6 |
| Other | 3 | 1.8 |
| Glaucoma severity | ||
| Mild | 56 | 32.7 |
| Moderate | 52 | 30.4 |
| Advanced | 63 | 36.8 |
Note: *After laser peripheral iridotomy has successfully opened the angle.
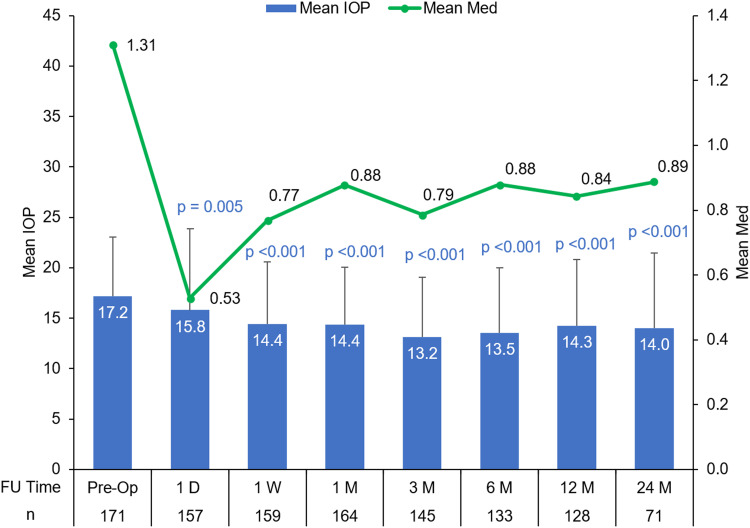
Of the total 171 patients included, 128 patients completed the first 12 months’ follow-up, and 71 patients completed the 24 months’ follow-up. The mean IOP and the mean number of medications at baseline and postoperative time points are shown in Figure 1. The reduction in mean IOP and the mean number of medications from baseline was statistically significant at each postoperative time point. At postoperative Month 12, mean IOP improved to 14.3 ± 5.5 mmHg with 82% of eyes achieving an IOP of <18 mmHg and the mean number of medications of 0.84 ± 0.8. At Month 24, mean IOP improved to 14.0 ± 7.4 mmHg with 86% of eyes achieving an IOP of <18 mmHg and the mean number of medications of 0.9 ± 0.8 (Figure 1). The results were similar when assessed in patients with primary open-angle glaucoma (POAG) that accounted for 91.2% of patients available in the full analysis dataset (at postoperative Month 12, mean IOP was 14.3 ± 6.7 mmHg with 80.5% of eyes having an IOP of <18 mmHg and the mean number of medications of 0.8 ± 0.8).
Figure 1.
Mean IOP and the mean number of medications at preoperative and postoperative time points through 24 months in the overall cohort. (p values represent the change in IOP from baseline to each postoperative time point).
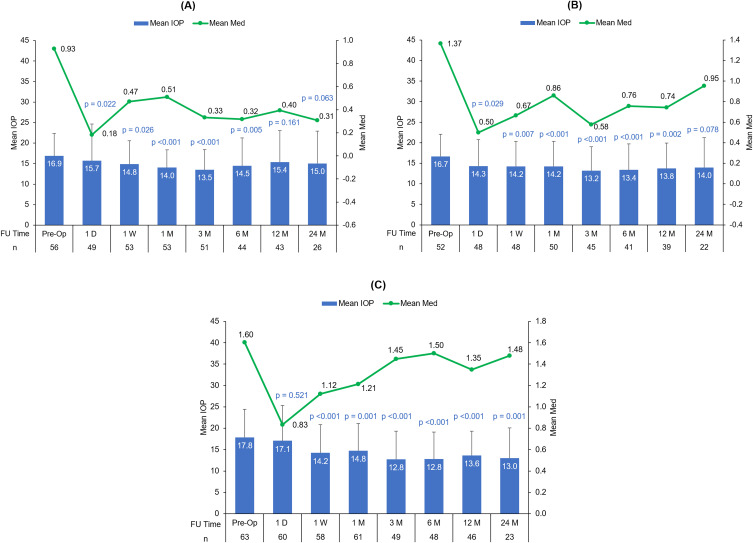
Figure 2A–C present the mean IOP and the mean number of medications at all postoperative time points for mild, moderate, and advanced glaucoma stages respectively. The percentage reduction in IOP and the number of medications were commensurate with the glaucoma stage. At postoperative Month 12, the percentage reduction in IOP for mild, moderate, and advanced glaucoma subgroups was 2.1%, 10.3%, and 14.7% respectively. At postoperative Month 24, the percentage reduction in IOP was 5.9%, 6.1%, and 21.2% for mild, moderate, and advanced glaucoma subgroups respectively (Table 2). Although the postoperative mean IOP was found to be in the range of low to mid-teens from 1 month onwards (13.5–15.0 in mild, 13.2–14.2 in moderate, and 12.8–14.8 mmHg in advanced glaucoma subgroups), the mean number of medications required to lower IOP was higher in eyes with moderate/advanced glaucoma (0.3–0.5 in mild, 0.6–1.0 in moderate, and 1.2–1.5 in advanced glaucoma subgroups).
Figure 2.
Mean IOP and the mean number of medications at preoperative and postoperative time points through 24 months in the (A) mild, (B) moderate, and (C) advanced glaucoma subgroups. (p values represent the change in IOP from baseline to each postoperative time point).
Table 2.
Average Percentage Reduction in IOP for the Overall Cohort, and Mild, Moderate, and Advanced Glaucoma Subgroups
| Mean Change from Baseline | ||||
|---|---|---|---|---|
| Overall (N = 171) | Mild (N = 56) | Moderate (N = 52) | Advanced (N = 63) | |
| Day 1 | −7.9% | −6.8% | −14.1% | −4.3% |
| Week 1 | −15.9% | −12.0% | −14.7% | −20.3% |
| Month 1 | −16.3% | −16.9% | −14.5% | −17.3% |
| Month 3 | −23.3% | −20.1% | −20.6% | −28.5% |
| Month 6 | −21.1% | −14.1% | −19.7% | −28.2% |
| Month 12 | −16.9% | −8.8% | −17.3% | −23.6% |
| Month 24 | −18.2% | −11.1% | −16.2% | −26.9% |
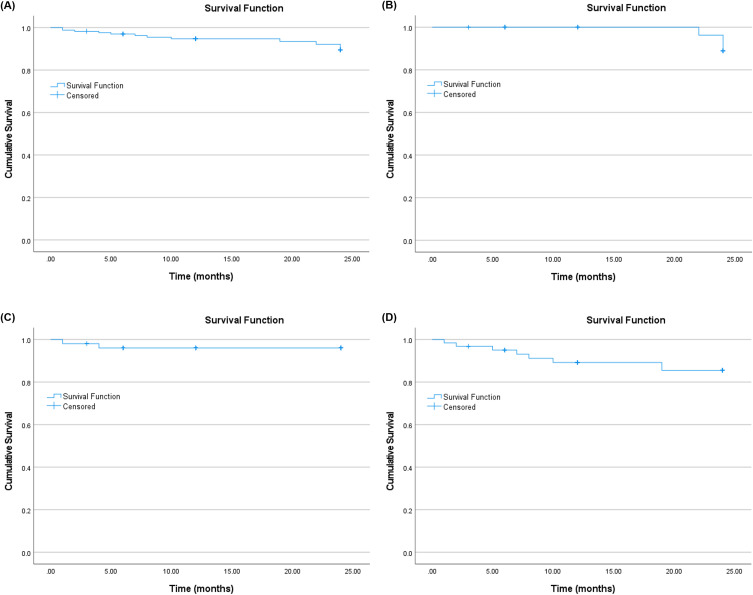
Figure 3A–D shows the Kaplan–Meier curve of the survival probability of the study procedure for the Month 24 follow-up for the overall, and mild, moderate, and advanced subgroups. Upon accounting for censoring events (N=12), the cumulative probability of surgical success, as defined by the percentage of eyes that did not require SSI, was 93.0% (N=159/171) overall with a mean survival time of 22.9 months. The corresponding values in the mild, moderate, and advanced glaucoma subgroups were 94.6%, 96.2%, and 88.9%, respectively, with a mean survival time of 23.9, 23.1, and 21.8 months, respectively.
Figure 3.
The Kaplan–Meier curve of the survival probability of the study procedure up to 24 months follow-up for the (A) overall cohort, (B) mild, (C) moderate, and (D) advanced glaucoma subgroups.
Incidence of Adverse Events
In the present study, an IOP spike (>10 mmHg) was recorded in 4.7% of the eyes, hypotony (<6 mmHg) developed in 1.2% of the eyes, one due to cyclodialysis cleft (0.6%) and the other without any pathology (numerical hypotony,0.6%). These resolved spontaneously, while the numerical hypotony resolved in a week, cyclodialysis cleft resolved at 5 months. Hyphema (>1mm in size) developed in 3.5% of the eyes All AEs were transient and self-resolved without any sequalae.
Discussion
The present real-world study was aimed at evaluating the safety and effectiveness of the OMNI-assisted sequential canaloplasty and trabeculotomy performed in combination with phacoemulsification in all stages of OAG. To date, this is the largest study reporting the outcomes of the OMNI surgical system. In the real world, glaucoma treatment is individualized to the patient’s clinical profile regardless of baseline IOP. The treatment goals depend on the patient’s age (life expectancy), extent of structural nerve fiber loss, family history, pachymetry, and disease stage based on visual field loss. Medication reduction may be a primary endpoint in some patients, and significant IOP reduction may be the goal in others. Ultimately, the main objective is to perform a safe, minimally invasive glaucoma procedure, which would ideally prevent glaucomatous progression and need for further SSI. As such, in the author’s clinical practice, the target IOP is individualized for each patient and patients underwent the OMNI procedure regardless of baseline IOP, if there was evidence of structural and functional changes consistent with glaucoma.
The juxtacanalicular TM and the inner wall of SC have been identified as the primary sites of abnormal flow resistance in OAG. The rise in IOP usually results from an increase in the resistance to aqueous humor outflow through the TM. Subsequent elevation of IOP may then cause the SC to collapse and the TM to compress, promoting a vicious cycle of progressively increasing IOP. Another potential point of resistance is at the site of the collector channels and its ostia. The inner-wall of SC can potentially herniate into the collector channel ostia, contributing to further outflow resistance.15 Addressing all three of these points of resistance is an appealing and intuitive solution. The OMNI surgical system targets these three potential sources of resistance by facilitating sequential canaloplasty and trabeculotomy. Canaloplasty addresses outflow resistance from both SC and the distal collector channels, while trabeculotomy addresses the increased outflow resistance within the TM.
In the present study, the use of the OMNI surgical system yielded a significant reduction in IOP, and medication use in cataract patients with OAG irrespective of disease stage. When stratified by glaucoma stage, the postoperative mean IOP was found to be in the range of low to mid-teens in all subgroups; however, with a higher mean number of medications in eyes with moderate/advanced glaucoma.
The mean IOP reduction in the present study (which included eyes with mild to advanced glaucoma) closely matches two similar previous studies evaluating the outcomes of the OMNI system combined with cataract surgery in eyes with mild-to-moderate OAG.10,13 The mean baseline IOP in these two studies (16.4 mmHg in Hirsch et al study and 17.1 mmHg in Gallardo et al) was similar to the present study (17.2 mmHg).10,13 The mean IOP reduction at postoperative 6 to 12 months ranged between 9% and 12% in the Hirsch et al study and 9% and 11% in Gallardo et al study,10,13 which was similar to that found in the present study (9% to 15%). This study is able to demonstrate that, in addition to mild-to-moderate glaucoma, the OMNI surgical system works well for patients with advanced glaucoma as well. When stratified by disease stage, the mean IOP reduction between postoperative 6 and 12 months ranged between 2% and 9% in mild, 10% and 16% in moderate and 15% and 20% in advanced glaucoma subgroups in the present study.
In several previous studies with the OMNI procedure, the mean baseline IOP of the included eyes was >20 mmHg.16–20 In these studies, the percent reduction in IOP at postoperative 12 months, ranging between ~29% and 40%, was higher than in the present study. Such a high reduction may be attributed to higher baseline IOP, that has been documented to be predictive of greater IOP reduction after a MIGS procedure.21 Given the present study outcomes and the results of the previous studies with the OMNI procedure, it is reasonable to assume that the OMNI system is able to predictably deliver postoperative mean IOP in the low to mid-teens, irrespective of the disease stage.
The 24-month cumulative probability of surgical success was 93.0% with a mean survival time of 22.9 months. In the authors’ experience, glaucoma patients (especially moderate to advanced stage) undergoing cataract surgery are at increased risk of glaucoma progression and are likely to require further surgical intervention for glaucoma control. In the present study, combined canaloplasty/trabeculotomy and cataract surgery in patients with mild, moderate, and advanced-stage glaucoma had a low 24-month incidence of SSI corresponding to 5.4% (mild), 3.8% (moderate) and 11.1% (advanced). These study findings provide real-world evidence that coupling OMNI-assisted canaloplasty/trabeculotomy with cataract extraction even in patients with moderate/advanced stage glaucoma has a low risk of SSI. Fellow glaucoma surgeons may use this approach to manage glaucoma patients with coexisting cataract.
With the OMNI surgical system, the extent of trabeculotomy and canaloplasty can be tailored to each patient’s diagnosis and treatment plan. In the present study, we performed a minimum of 180° of canaloplasty and a minimum of 90° of trabeculotomy. Depending on the stage of the disease, 360° of canaloplasty with 180° of trabeculotomy or some other combination can be chosen.
In addition to good efficacy in IOP control and reduction in the number of medications, the OMNI procedure showed a good safety profile with few mild and transient AEs observed in the present study. Postoperative hyphema is one of the most common complications of glaucoma surgery. Generally, hyphema is a short-term, self-limiting phenomenon that seldom requires additional intervention. Of note, some degree of hyphema after trabeculotomy is common and is expected when the TM is cut. In the present study, the incidence of clinically significant hyphema was found to be 3.5%, which is consistent with the previous reports following the OMNI surgical system.6,10,11,13,16,17 The incidence of IOP spikes (4.7%) was also low. Although rare, inadvertent cyclodialysis clefts can occur during MIGS procedures.22 In the present study, intraoperative cyclodialysis cleft, followed by hypotony occurred in one patient and resolved spontaneously.
The retrospective study design and lack of a control group can be considered as potential limitations of the present study. Despite these challenges, this study provides real-world evidence from a large cohort of patients to suggest adequate safety and efficacy of combined ab interno canaloplasty and trabeculotomy at the time of cataract surgery in eyes with mild to advanced OAG.
Conclusion
To conclude, canaloplasty and trabeculotomy performed using the OMNI surgical system at the time of phacoemulsification effectively reduced IOP and IOP-lowering medications in our study population of cataract patients with mild to advanced OAG for up to 24 months postoperatively. No permanent serious safety issues were identified.
Acknowledgments
The authors would like to thank Zeke Bourgeois, BS, for his contributions to this study.
Raman Bedi, MD critically reviewed the manuscript. IrisARC – Analytics, Research & Consulting (Chandigarh, India) provided statistical and editorial assistance in the preparation of the manuscript.
The abstract of this paper was presented at the 2023 ASCRS annual meeting (May 5–8, 2023 San Diego, CA) as a conference talk with interim findings. The paper’s abstract was not published.
Funding Statement
The present study is an Investigator Initiated Trial funded by SightSciences Inc.
Abbreviations
OAG, open-angle glaucoma; TM, trabecular meshwork; IOP, intraocular pressure; SC, Schlemm’s canal; MIGS, minimally invasive glaucoma surgeries; IOL, intraocular lens; CMS, Center for Medicare and Medicaid Services; SWAP, short-wavelength automated perimetry; FDT, frequency doubling technology perimetry; SSI, secondary surgical interventions; AE, adverse events; POAG, primary open-angle glaucoma.
Disclosure
AY is a consultant to SightSciences Inc. KD and QA have no conflicts of interest to disclose.
References
- 1.Dickerson JE Jr, Brown RH. Circumferential canal surgery: a brief history. Curr Opin Ophthalmol. 2020;31(2):139–146. doi: 10.1097/ICU.0000000000000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allingham RR, de Kater AW, Ethier CR. Schlemm’s canal and primary open angle glaucoma: correlation between Schlemm’s canal dimensions and outflow facility. Exp Eye Res. 1996;62(1):101–109. doi: 10.1006/exer.1996.0012 [DOI] [PubMed] [Google Scholar]
- 3.Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49(12):5346–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69(6):783–801. doi: 10.1001/archopht.1963.00960040789022 [DOI] [PubMed] [Google Scholar]
- 5.Chen DZ, Sng CCA. Safety and efficacy of microinvasive glaucoma surgery. J Ophthalmol. 2017;2017:3182935. doi: 10.1155/2017/3182935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallardo MJ, Sarkisian Jr SR Jr, Vold SD, et al. Canaloplasty and trabeculotomy combined with phacoemulsification in open-angle glaucoma: interim results from the GEMINI Study. Clin Ophthalmol. 2021;15:481–489. doi: 10.2147/OPTH.S296740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM, Virgili G. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi: 10.1371/journal.pone.0183142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillunat KR, Spoerl E, Terai N, Pillunat LE. Effect of selective laser trabeculoplasty on ocular haemodynamics in primary open-angle glaucoma. Acta Ophthalmol. 2017;95(4):374–377. doi: 10.1111/aos.13360 [DOI] [PubMed] [Google Scholar]
- 9.Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206. doi: 10.2147/OPTH.S80490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch L, Cotliar J, Vold S, et al. Canaloplasty and trabeculotomy ab interno with the OMNI system combined with cataract surgery in open-angle glaucoma: 12-month outcomes from the ROMEO study. J Cataract Refract Surg. 2021;47(7):907–915. doi: 10.1097/j.jcrs.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 11.Vold SD, Williamson BK, Hirsch L, et al. Canaloplasty and trabeculotomy with the OMNI system in pseudophakic patients with open-angle glaucoma: the ROMEO Study. Ophthalmol Glaucoma. 2021;4(2):173–181. doi: 10.1016/j.ogla.2020.10.001 [DOI] [PubMed] [Google Scholar]
- 12.Brown RH, Tsegaw S, Dhamdhere K, Lynch MG. Viscodilation of Schlemm canal and trabeculotomy combined with cataract surgery for reducing intraocular pressure in open-angle glaucoma. J Cataract Refract Surg. 2020;46(4):644–645. doi: 10.1097/j.jcrs.0000000000000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallardo MJ, Pyfer MF, Vold SD, et al. Canaloplasty and trabeculotomy combined with phacoemulsification for glaucoma: 12-month results of the GEMINI Study. Clin Ophthalmol. 2022;16:1225–1234. doi: 10.2147/OPTH.S362932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee DJ, Lau S, Sozeri YG. Glaucoma staging and progression risk factors. Review of Ophthalmology; 2022. Available from: https://www.reviewofophthalmology.com/article/glaucoma-staging-and-progression-risk-factors. Accessed May 12, 2023.
- 15.Gong H, Swain DL. The histopathological changes in the trabecular outflow pathway and their possible effects on aqueous outflow in eyes with primary open-angle glaucoma. In: Knepper PA, Samples JR, editors. Glaucoma Research and Clinical Advances: 2016 to 2018. Amsterdam, The Netherlands: Kugler Publications; 2016:17–40. [Google Scholar]
- 16.Murphy Iii JT, Terveen DC, Aminlari AE, Dhamdhere K, Dickerson Jr JE; Group RS. A multicenter 12-month retrospective evaluation of canaloplasty and trabeculotomy in patients with open-angle glaucoma: the ROMEO 2 Study. Clin Ophthalmol. 2022;16:3043–3052. doi: 10.2147/OPTH.S384105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toneatto G, Zeppieri M, Papa V, et al. 360° Ab-interno schlemm’s canal viscodilation with OMNI viscosurgical systems for open-angle glaucoma—midterm results. J Clin Med. 2022;11(1):259. doi: 10.3390/jcm11010259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klabe K, Kaymak H. Standalone trabeculotomy and viscodilation of Schlemm’s canal and collector channels in open-angle glaucoma using the OMNI surgical system: 24-month outcomes. Clin Ophthalmol. 2021;15:3121. doi: 10.2147/OPTH.S325394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes T, Traynor M. Clinical results of Ab interno canaloplasty in patients with open-angle glaucoma. Clin Ophthalmol. 2020;14:3641–3650. doi: 10.2147/OPTH.S275087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabska-Liberek I, Duda P, Rogowska M, et al. 12-month interim results of a prospective study of patients with mild to moderate open-angle glaucoma undergoing combined viscodilation of Schlemm’s canal and collector channels and 360 trabeculotomy as a standalone procedure or combined with cataract surgery. Eur J Ophthalmol. 2022;32(1):309–315. doi: 10.1177/1120672121998234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan NE, Tracer N, Terraciano A, Parikh HA, Panarelli JF, Radcliffe NM. Comparison of safety and efficacy between Ab interno and Ab externo approaches to XEN gel stent placement. Clin Ophthalmol. 2021;15:299–305. doi: 10.2147/OPTH.S292007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berk TA, An JA, Ahmed IIK. Inadvertent cyclodialysis cleft and hypotony following Ab-interno trabeculotomy using the trabectome device requiring surgical repair. J Glaucoma. 2017;26(8):742–746. doi: 10.1097/IJG.0000000000000719 [DOI] [PubMed] [Google Scholar]