Abstract
Patient: Male, 69-year-old
Final Diagnosis: Corneal perforation
Symptoms: Decrease of vision
Clinical Procedure: —
Specialty: Ophthalmology
Objective:
Rare disease
Background:
Immune checkpoint inhibitors (ICIs) targeting the programmed cell death protein 1 (PD-1), or its ligand PD-L1, are the mainstay treatment for several metastatic malignant conditions. ICIs are associated with multiple toxic adverse events affecting various organs, known collectively as immune-related adverse events (irAEs). Dry eye, uveitis, ocular myasthenia, and cicatrizing conjunctivitis are well-recognized ocular irAEs associated with ICIs.
Case Report:
We present a case of 69-year-old man who presented with paracentral, punch-out corneal perforation in the left eye, associated with bilateral severe ocular surface disease 3 weeks after receiving the second dose of atezolizumab-bevacizumab combination therapy for the treatment of unresectable hepatocellular carcinoma. Corneal gluing using cyanoacrylate glue was performed along with bandage contact lens application and temporary tarsorrhaphy to seal the corneal perforation and improve the ocular surface. On the subsequent follow-ups, the corneal glue was unstable and dislodged. Thus, penetrating keratoplasty was performed to salvage the globe along with holding the combination therapy. At the 8-month follow-up, the graft remained clear, and the ocular surface improved substantially in both eyes.
Conclusions:
Ocular irAEs associated with immune-modulating agents can lead to vision-threatening complications. Therefore, communications between oncologists and ophthalmologists in a multidisciplinary team would be of utmost importance for early detection and timely management of any ocular-related adverse events associated with the use of immunotherapy agents.
Keywords: Atezolizumab, Bevacizumab, Corneal Perforation, Drug-Related Side Effects and Adverse Reactions
Background
Atezolizumab is a humanized IgG1 monoclonal antibody of the immune checkpoint inhibitory class that selectively targets programmed cell death 1 (PD-1) and programmed death ligand 1 (PD-L1) to prevent binding between the PD-1 T-cell receptor and cancer cell ligands PD-L1 and 2, thus preventing T-cell suppression and upregulating T-cell mediated immune-response and destruction of neoplastic cells [1]. Bevacizumab is a humanized immunoglobulin G monoclonal antibody that targets vascular endothelial growth factor (VEGF) to prevent VEGF-mediated angiogenesis and tumor growth, which has been linked to the development and progression of several neoplastic conditions [2,3]. Both medications have been approved either as a single agent or in combination with other chemotherapeutic agents for the treatment of various neo-plastic entities, including unresectable or metastatic hepato-cellular carcinoma [4].
Immune checkpoint inhibitors (ICIs) have been associated with several toxic adverse events affecting various organs, known collectively as immune-related adverse events (irAEs) [5]. Dry eye, uveitis, ocular myasthenia, and cicatrizing conjunctivitis are well-recognized ocular irAEs associated with ICIs [6,7]. Here we report a case of unilateral corneal perforation in a patient with unresectable hepatocellular carcinoma treated by a combination of atezolizumab and bevacizumab.
Case Report
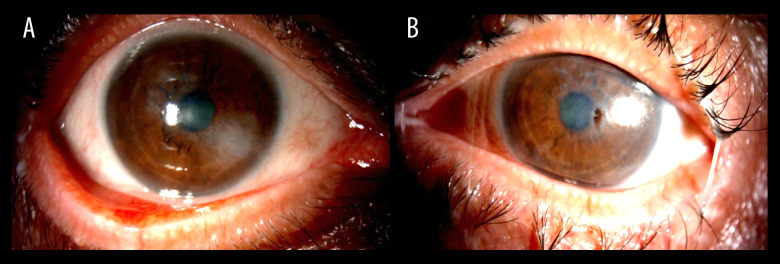
A 69-year-old man presented to our Emergency Department with a 1-week history of gradual decrease in vision and tearing in the left eye. He denied any previous ocular history apart from senile cataract. His best corrected visual acuity measured 20/60 and 20/200 in the right and left eye, respectively. Slit-lamp examination of the right eye demonstrated severe ocular surface disease manifested as diffuse superficial punctate keratopathy, filamentary keratitis, and corneal haze (Figure 1A), while left eye examination showed a paracentral, punched out, sterile corneal perforation measuring 1.0×0.5 mm in size, with a shallow anterior chamber (Figure 1B). Corneal gluing using cyanoacrylate glue was performed along with bandage contact lens application and temporary tarsorrhaphy to seal the corneal perforation and improve the ocular surface. The patient was started on a fourth-generation topical fluoroquinolone (moxifloxacin) 4 times daily in the left eye and preservative-free lubricant drops every 2 h in both eyes. The clinical picture was suspicious for autoimmune-related corneal perforation (Sjögren syndrome), although the patient’s age, sex, and absence of systemic symptoms rendered this diagnosis unlikely. Systemic laboratory workup revealed a high rheumatoid factor titer and thrombocytopenia, which added a diagnostic dilemma. On further questioning, the patient revealed that he was diagnosed with hepatocellular carcinoma elsewhere and received 2 cycles of atezolizumab-bevacizumab combination therapy (intravenous [i.v.] infusion of atezolizumab at a dose of 1200 mg and i.v. infusion of bevacizumab at a dose of 15 mg/kg, every 21 days). The patient reported that the ocular symptoms started 3 weeks after he received the second cycle. The ocular condition was discussed with the treating oncologist, and the combination therapy was put on hold. On the subsequent follow-ups, the corneal glue was unstable and dislodged. Subsequently, therapeutic penetrating keratoplasty was performed (Figure 2). Given the poor ocular surface in the right eye, temporary tarsorrhaphy was planned to be performed. However, the ocular surface of both eyes improved significantly during the off-period of the combination therapy. The patient was instructed to apply frequent preservative-free lubricant drops and gel in both eyes in addition to topical 1% prednisolone acetate 4 times a day in the left eye. The graft remained clear at the 8-month follow-up.
Figure 1.
(A) Slit-lamp clinical photo of the right eye showing severe ocular surface disease. (B) Slit-lamp clinical photo of the left eye showing a paracentral, punched out, sterile corneal perforation.
Figure 2.
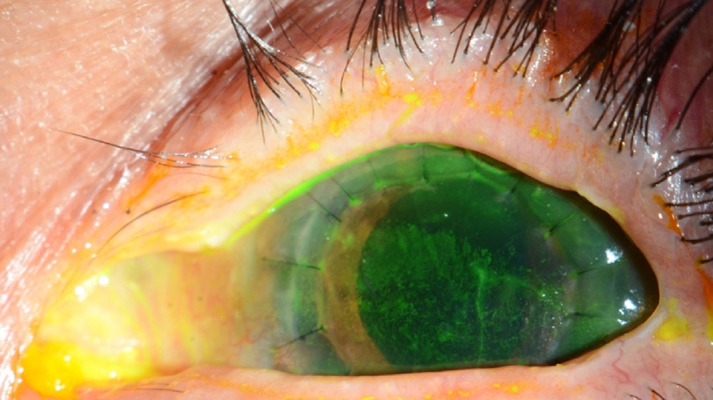
Slit-lamp clinical photo of the left eye post penetrating keratoplasty.
Discussion
Atezolizumab is a monoclonal anti-PD-L1 antibody that works by exerting antitumor effects by removing inhibition of effector T cells through blocking PD-1/PD-L1 signaling [8]. Over-activating the immune system can induce a toxic immune response in various organs and tissues, known as irAEs [5]. A recent meta-analysis showed that irAEs occur in 66% of patients treated with PD-1 and PD-L1 inhibitors [9]. Moreover, several recent reports estimated an incidence of 1% to 4% of ocular irAEs associated with ICIs, with dry eye disease and uveitis being most common [7,10–12]. To the best of our knowledge, this is the first case report describing a case of corneal perforation secondary to severe dryness in a patient treated with atezolizumab-bevacizumab combination therapy.
Ocular irAEs have been rarely reported with atezolizumab infusion. Oh reported a case of bilateral autoimmune keratitis occurring 2 months after starting atezolizumab for the treatment of metastatic bladder cancer [13]. Bitton et al described a case of bilateral cicatrizing conjunctivitis with significant superficial punctate keratitis, inferior fornix shortening, and tarsal conjunctival fibrosis after 10 infusions of atezolizumab for the treatment of parotid adenocarcinoma [14]. Aschauer et al reported a case of unilateral severe granulomatous conjunctival inflammation mimicking conjunctival metastasis after 4 cycles of atezolizumab for the treatment of metastatic non-small-cell lung cancer [15]. Venkat et al reported a case of bilateral superior limbic keratitis and posterior uveitis associated with macular edema occurring 5 months after starting atezolizumab for the treatment of advanced squamous cell carcinoma of the lung [16]. Mito et al described a case of bilateral anterior uveitis occurring 3 weeks after the first cycle of atezolizumab for the treatment of advanced non-small-cell lung cancer [17]. In a retrospective study, Fang et al reported 3 cases of unspecified eye inflammation and a single case of uveitis [6].
Bevacizumab is a recombinant humanized monoclonal antibody against VEGF, which has been used in conjunction with chemotherapy and immunotherapy agents for the treatment of multiple neoplastic conditions [18]. Since hepatocellular carcinoma is classified as an immune-excluded tumor, bevacizumab is used in combination with anti-PD1 inhibitors mainly to enhance T-cell trafficking and infiltration into the tumor bed; thus, converting hepatocellular carcinoma from immune-excluded to an inflamed tumor vulnerable to be attacked by activated T cells [19]. Few reports have documented ocular adverse effects related to the use of bevacizumab systematically. Biswas et al reported a case of unilateral optic neuritis occurring 11 days after the first cycle of bevacizumab for the treatment of cutaneous melanoma [20]. Sherman et al reported 6 cases of severe optic neuropathy in patients treated with bevacizumab for glioblastoma [21]. Table 1 summarizes the previously reported cases of ocular adverse effects in relation to the same chemotherapeutic agents.
Table 1.
Summary of previously reported cases of ocular adverse effects in relation to the same chemotherapeutic agents.
| Author | Ocular surface adverse effect | Chemotherapeutic agent/etiology | Onset |
|---|---|---|---|
| Oh [13] |
|
Atezolizumab/bladder cancer | Two months after initiating the treatment |
| Bitton et al [14] |
|
Atezolizumab/parotid adenocarcinoma | After 10 cycles |
| Aschauer et al [15] |
|
Atezolizumab/metastatic non-small-cell lung cancer | After 4 cycles |
| Venkat et al [6] |
|
Atezolizumab/advanced squamous cell carcinoma of the lung. | Five months after initiating the treatment |
| Mito et al [17] |
|
Atezolizumab/non-small-cell lung cancer | Three weeks after the first cycle of atezolizumab |
| Biswas et al [20] |
|
Bevacizumab/cutaneous melanoma | Occurring 11 days after the first cycle of bevacizumab |
| Sherman et al [21] |
|
Bevacizumab/glioblastoma | 3–18 months after initiating the treatment |
Our patient presented with a corneal perforation occurring 3 weeks after receiving the second cycle of atezolizumab-bevacizumab combination therapy for unresectable hepatocellular carcinoma. Both eyes had severe ocular surface disease with marked superficial punctate keratitis and filamentary keratitis, indicating an immune-related process. Although our patient had a high titer of rheumatoid factor, the patient’s age, sex, and lack of systemic symptoms do not support the diagnosis of Sjögren syndrome. In fact, elevated titers of rheumatoid factor can be detected in the serum of 10% to 26% of patients with malignant diseases [22,23]. Moreover, several studies showed a higher cancer mortality and tumor recurrence risk among patients with positive rheumatoid factor titer than in patients with negative rheumatoid factor titer [24–26]. Although corneal gluing was attempted along with temporary tarsorrhaphy to seal the perforation, the patient eventually needed therapeutic penetrating keratoplasty to salvage the globe, along with holding the combination therapy.
Conclusions
We report a case of unilateral corneal perforation in a patient with unresectable hepatocellular carcinoma after administration of 2 cycles of atezolizumab-bevacizumab combination therapy. Ocular irAEs associated with immune-modulating agents can lead to vision-threatening complications. Therefore, communication between oncologists and ophthalmologists in a multidisciplinary team would be of utmost importance for early detection and timely management of any ocular-related adverse events associated with the use of immunotherapy agents.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finn RS, Bentley G, Britten CD, et al. Targeting vascular endothelial growth factor with the monoclonal antibody bevacizumab inhibits human hepatocellular carcinoma cells growing in an orthotopic mouse model. Liver Int. 2009;29(2):284–90. doi: 10.1111/j.1478-3231.2008.01762.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333(2):328–35. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 4.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Prim. 2020;6(1):38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319–22. doi: 10.1016/j.joco.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalvin LA, Shields CL, Orloff M, et al. Checkpoint inhibitor immune therapy: Systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063–78. doi: 10.1097/IAE.0000000000002181. [DOI] [PubMed] [Google Scholar]
- 8.Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–39. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008–19. doi: 10.1001/jamaoncol.2019.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park RB, Jain S, Han H, Park J. Ocular surface disease associated with immune checkpoint inhibitor therapy. Ocul Surf. 2021;20:115–29. doi: 10.1016/j.jtos.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, Wei X. Ocular immune-related adverse events associated with immune checkpoint inhibitors in lung cancer. Front Immunol. 2021;12:3399. doi: 10.3389/fimmu.2021.701951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Rahman O, Oweira H, Petrausch U, et al. Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: A systematic review. Expert Rev Anticancer Ther. 2017;17(4):387–94. doi: 10.1080/14737140.2017.1296765. [DOI] [PubMed] [Google Scholar]
- 13.Oh JY. Autoimmune keratitis after atezolizumab treatment. N Engl J Med. 2020;383(15):1468. doi: 10.1056/NEJMicm1910925. [DOI] [PubMed] [Google Scholar]
- 14.Bitton K, Michot JM, Barreau E, et al. Prevalence and clinical patterns of ocular complications associated with Anti-PD-1/PD-L1 anticancer immunotherapy. Am J Ophthalmol. 2019;202:109–17. doi: 10.1016/j.ajo.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Aschauer J, Donner R, Lammer J, Schmidinger G. Atezolizumab induced immune-related adverse event mimicking conjunctival metastatic disease. Am J Ophthalmol Case Rep. 2022;26:101489. doi: 10.1016/j.ajoc.2022.101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkat AG, Arepalli S, Sharma S, et al. Local therapy for cancer therapy-associated uveitis: a case series and review of the literature. Br J Ophthalmol. 2020;104(5):703–11. doi: 10.1136/bjophthalmol-2019-314403. [DOI] [PubMed] [Google Scholar]
- 17.Mito T, Takeda S, Motono N, Sasaki H. Atezolizumab-induced bilateral anterior uveitis: A case report. Am J Ophthalmol Case Rep. 2021;24:101205. doi: 10.1016/j.ajoc.2021.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keating GM. Bevacizumab: A review of its use in advanced cancer. Drugs. 2014;74(16):1891–925. doi: 10.1007/s40265-014-0302-9. [DOI] [PubMed] [Google Scholar]
- 19.Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Biswas S, Wrigley J, East C, et al. A randomised trial evaluating bevacizumab as adjuvant therapy following resection of AJCC stage IIB, IIC and III cutaneous melanoma: An update. Ecancermedicalscience. 2008;2:108. doi: 10.3332/ecancer.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman JH, Aregawi DG, Lai A, et al. Optic neuropathy in patients with glioblastoma receiving bevacizumab. Neurology. 2009;73(22):1924. doi: 10.1212/WNL.0b013e3181c3fd00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ugolini A, Zizzari IG, Ceccarelli F, et al. IgM-Rheumatoid factor confers primary resistance to anti-PD-1 immunotherapies in NSCLC patients by reducing CD137+T-cells. EBioMedicine. 2020;62:103098. doi: 10.1016/j.ebiom.2020.103098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurri L, Perttala Y. Observations on non-specific Waaler-Rose and latex reactions in cancer patients. Ann Med Intern Fenn. 1965;54(4):181–83. [PubMed] [Google Scholar]
- 24.Gupta NP, Malaviya AN, Singh SM. Rheumatoid factor: Correlation with recurrence in transitional cell carcinoma of the bladder. J Urol. 1979;121(4):417–18. doi: 10.1016/s0022-5347(17)56803-2. [DOI] [PubMed] [Google Scholar]
- 25.Ajeganova S, Humphreys JH, Verheul MK, et al. Anticitrullinated protein antibodies and rheumatoid factor are associated with increased mortality but with different causes of death in patients with rheumatoid arthritis: A longitudinal study in three European cohorts. Ann Rheum Dis. 2016;75(11):1924–32. doi: 10.1136/annrheumdis-2015-208579. [DOI] [PubMed] [Google Scholar]
- 26.Ahn JK, Hwang J, Chang Y, Ryu S. Rheumatoid factor positivity in-creases all-cause and cancer mortality: A cohort study. Rheumatol Int. 2017;37(7):1135–43. doi: 10.1007/s00296-017-3738-x. [DOI] [PubMed] [Google Scholar]