Abstract
Purpose
Surgery, multiagent systemic chemotherapy, and radiation are used for patients with orbital retinoblastoma but are associated with unacceptable short- and long-term toxicity (including death). We studied orbital and systemic exposure of topotecan in the swine model after ophthalmic artery chemosurgery (OAC) and intravenous (IV) delivery.
Methods
Landrace pigs (n = 3) underwent 30-minute OAC of topotecan (4 mg), and samples were serially obtained from the femoral artery and from a microdialysis probe inserted into the lateral rectus muscle sheath of the infused eye as a surrogate of the orbital irrigation. Animals were recovered, and, after a wash-out period, plasma and microdialysate samples from the contralateral eye were collected after a 30-minute IV infusion of topotecan (4 mg). Samples were quantified by high-performance liquid chromatography, and population pharmacokinetic analysis was conducted using MonolixSuite.
Results
After OAC, median topotecan exposure in the orbit was 5624 ng × h/mL (range 3922–12531) compared to 23 ng × h/mL (range 18–75) after IV infusion. Thus, topotecan exposure in the orbit was 218-fold (range 75–540) higher after OAC than after IV infusion despite comparable systemic exposure (AUCpl) between routes (AUCpl, OAC: 141 ng × h/mL [127–191] versus AUCpl, IV: 139 ng × h/mL [126–186]). OAC was more selective to target the orbit because the median (range) orbital-to-plasma exposure ratio was 44 (28–65) after OAC compared to 0.18 (0.13–0.40) after IV infusion.
Conclusions
OAC of topotecan resulted in higher orbital exposure than after IV infusion and was a more selective route for local drug delivery. Patients with orbital retinoblastoma may benefit from a multimodal treatment strategy including OAC therapy.
Keywords: ophthalmic artery chemosurgery, pharmacokinetics, retinoblastoma, orbital tumor
Extraocular retinoblastoma is a common presenting feature accounting for up to 50% of all cases in low- and middle-income countries where most children who have this cancer live.1,2 Unfortunately patients with direct orbital invasion of retinoblastoma can develop either (or both) central nervous system and bone marrow metastases.).3–8 Treatment is therefore directed at both the local (orbit) and distant sites with surgery, external beam radiation (EBRT), and multiagent systemic chemotherapy.9,10
Although this approach is often successful there are important short- and long-term toxicities, second malignancies, and lifelong cosmetic issues.3,4,9–12 Therefore there is an unmet need to establish intensified local delivery of chemotherapy that are equally as effective but with less toxicity.
Another challenging scenario is treatment of widespread metastatic disease and massive orbital involvement. Standard approaches include high-dose multiagent systemic chemotherapy, bone marrow transplantation, and EBRT. In addition to acute and long-term consequences including hearing problems, impaired growth, and toxic deaths, metastatic patients frequently present with a poor clinical status and rapid deterioration that hinders up-front intensive systemic chemotherapy.3,4,9–12 Hence, these patients are in particular need of highly efficient local therapies that exert a fast response to alleviate pain and prompt clinical recovery for subsequent treatment compliance.
A promising approach for orbital drug targeting is the selective administration of chemotherapy through the ophthalmic artery (OAC), which supplies blood to the eye globe, the optic nerve in its orbital segment, and the orbit via branches of this artery.13,14 Therefore OAC is expected to result in high chemotherapy exposure in the ocular tissues as previously reported in the swine model and also in the orbit.15
During the last decade, the advent of OAC changed the treatment paradigm for intraocular disease, resulting in drastic increments in the rate of eye cure and salvage.16–19 Nonetheless, only anecdotal reports of the use of OAC for extraocular disease are available. Rodriguez et al.20 reported on a metastatic patient with overt extraocular retinoblastoma treated with OAC as part of a multimodality treatment resulting in a marked reduction of the orbital tumor mass and partial radiological response. Another report provided evidence of the efficacy of OAC as monotherapy in a patient with extraocular relapse.21
To provide scientific evidence of the capacity of OAC to localize chemotherapy in the orbit, we characterized orbital and systemic pharmacokinetics of topotecan after OAC in pigs and compared to the exposure attained after intravenous (IV) infusion. To reduce the number of studied animals while obtaining the full description of the pharmacokinetic profile of topotecan in the orbit after OAC or IV infusion, we obtained serial microdialysis samples from the sheath of the lateral rectus muscle as a surrogate of the orbital blood concentration because this muscle is supplied with blood exclusively from the ophthalmic artery in the pig.
Methods
This study was conducted in compliance with the Statement for the Use of Animals in Ophthalmic and Vision Research of the Association for Research in Vision and Ophthalmology. IRB approval was granted by the Animal Care and Use Committee of Hospital de Pediatria J.P. Garrahan (protocol no. 1369).
Animal Procedures
Topotecan pharmacokinetics after OAC and IV infusion was studied in three Landrace pigs weighing 25 to 40 kg. Both eyes of each animal were used to reduce the total number of subjects in the study.
Each animal underwent two pharmacokinetic studies under a fixed treatment sequence. On both occasions, the animals were sedated, with anesthesia maintained under mechanical ventilation, and continuously monitored.15 For sedation, animals received intramuscularly 20 mg/kg ketamine, and intravenously 0.8 mg/kg midazolam with 0.1mg/kg acepromazine. Then, general anesthesia was induced with 2.2 mg/kg propofol and maintained and 2 µg/kg fentanyl with 0.1 mg/kg pancuronium. Vital signs were continuously monitored throughout the study, and body temperature was maintained with an external heat source forced-air unit.
In the anesthetized animal on the first pharmacokinetic study, a microdialysis probe was inserted into the lateral rectus sheath of the right eye after exposing the muscle and through an incision made with a 24-gauge Abbocath needle (Anhui Kangda Medical Products, Anhui, China). The site for probe insertion was chosen as in pigs, the lateral rectus muscle is exclusively supplied with blood from the external ophthalmic artery (that branches from the submaxillary artery, which, in turn, derives from the external carotid).22 Thus the concentration of topotecan in the interstitial fluid of the lateral rectus muscle was considered as a surrogate of the orbital blood concentration derived from the external ophthalmic artery. The probe was continuously exposed to PBS perfused at 1 µL/min using a microinfusion pump (KDS230; KD Scientific, Holliston, MA, USA). Then, under heparin anticoagulation, the right ophthalmic artery was catheterized.23 Briefly, after accessing the femoral artery by percutaneous puncture with ultrasound guidance, a 5-French vascular sheath (Johnson & Johnson, Cordis Corp., Miami Lakes, FL, USA) was placed, a 5-Fr guide catheter (Envoy guide catheter, 5F MPC; Johnson & Johnson, Cordis Corp.) was advanced into the common carotid artery, the external carotid artery, and thereafter the maxillary artery. The ophthalmic artery was super-selectively catheterized using a microcatheter (Marathon; Medtronic, Minneapolis, MN, USA) and a guidewire (Mirage; Medtronic). Angiographic series images were acquired to guide the procedure for the accurate position of the catheter at the ostium of the ophthalmic artery. Also, imaging of the orbital irrigation was obtained for comparative purposes of the orbital irrigation after enucleation. Once verified the accurate position of the microcatheter, 4 mg of topotecan in 30 mL of saline were infused into the ophthalmic artery over 30 minutes as performed in retinoblastoma patients.
A dose of 4 mg of topotecan was used in correspondence with our previous reports in which we showed that this dose allowed attaining detectable levels after OAC in pigs of similar weighs to the ones used in the present study.15 Otherwise, based on the size of the animal model and the associated volume of distribution, a lower dose of 1 mg, such as the one used in the clinical practice of OAC, would have yielded undetectable levels in the ocular tissues after the IV infusion. Still, despite higher than the dose used for OAC in patients, a dose of 4 mg is lower than the total systemic dose recommended for intravenous administration in schedules of 2 mg/m2/day administered in five days of two consecutive weeks.
Dialysates were collected at 30-minute intervals during topotecan administration and up to four hours after the start of the infusion, and the recovery of the probe was calculated as described elsewhere.15,24,25 Blood samples were collected from the femoral artery immediately before starting and at the end of the infusion and at 1, 1.5, 2.0, 2.5, 3.0, and 3.5 hours after administration. The animal was treated with antibiotics (60 mg/kg cephalexin two times daily) and analgesics (4 mg/kg/day caprofen and 0.04 mg/kg buprenorphine) following institutional protocols and was monitored for signs of distress, pain, or infection.
After four weeks, we inserted a microdialysis probe in the remaining eye as detailed before, infused topotecan 4 mg in the marginal ear vein, and microdialysates and plasma samples were collected at the same times described above. After the second pharmacokinetic study, the anesthetized animal was euthanized with potassium chloride (1 meq/kg).
All samples were stored at −80°C pending total topotecan quantification using a Waters e2965 HPLC system (Waters Corporation, Milford, MA, USA) equipped with a Waters 2475 fluorescence detector (Waters Corporation) and setting the chromatographic conditions as described elsewhere.15,24
Pharmacokinetic and Statistical Analysis
Topotecan interstitial and plasma pharmacokinetic data were analyzed by population pharmacokinetic modeling using MONOLIX Suite 2019R1 (Lixoft SAS, Antony, France). Model selection was performed based on the change in the objective function value, information criteria, the precision of the parameter estimates, and the goodness-of-fit plots. Differences in topotecan pharmacokinetics after OAC and IV infusion were assessed as a categorical covariate that could explain the variability in the drug transfer rate from the central to the muscle sheath compartment or the elimination from the interstitial space. The covariate was retained in the model if a decrease of at least 3.84 units was achieved in the objective function value (χ2 test, P < 0.05) and if the associated parameter was significantly different from zero (P < 0.05).
Individual orbital and plasma exposure (AUCo, AUCpl) were calculated using empirical Bayesian estimations with Simulx. The AUCo-to-AUCpl ratio was computed for each animal after topotecan infusion by each route of drug administration. Graphs were constructed with GraphPad Prism v8 (www.graphpad.com).
Results
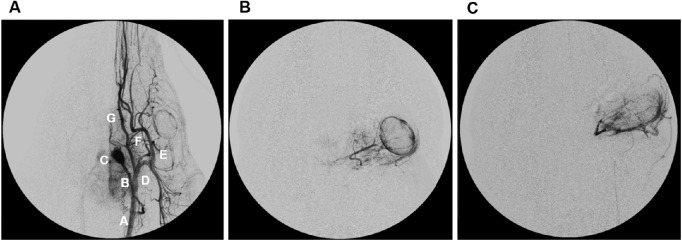
The swine cerebral vascular system of the left side is presented in Figure 1A. OAC was successfully performed in three pigs as shown in the angiographies of two representative animals depicted in Figures 1B and 1C.
Figure 1.
Representative angiographies. A. Arteriography of the left carotid trunk obtained as an anteroposterior projection. (A) common carotid, (B) ascending pharyngeal artery, (C) rete mirabilis, (D) external carotid artery, (E) internal maxillary artery, (F) ophthalmic artery, (G) lingual artery, (H) choroidal blush of the left eye. B and C: representative super-selective artreiographies of the left eye of pigs.
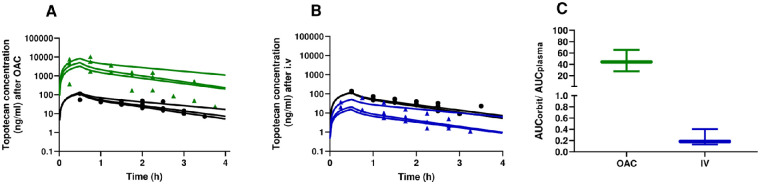
Topotecan concentration versus time profiles in the orbit and plasma after OAC are depicted in Figure 2A. A two-compartment model with linear elimination from the central compartment adequately described the data. Topotecan maximum concentration in both plasma and orbit was attained at the end of the OAC infusion. The median model-predicted maximum plasma concentration was 118.1 ng/mL (range 113.8–129.3). Four hours after the start of the infusion, the median topotecan plasma concentration was only 7.3 ng/mL (range 5.3–16.8). These values were far lower than those attained in the orbit of the animal after OAC administration because the median maximum and four-hour orbital concentration of topotecan was 5021.4 ng/mL (range 3268.9–8408.3) and 235.5 ng/mL (range 202.5–1102.7), respectively.
Figure 2.
Topotecan disposition after OAC and intravenous infusion. Orbital and plasma concentration versus time profiles after (A) OAC or (B) IV infusion of 4 mg of topotecan. Green triangles represent individual concentrations in the orbit after OAC whereas blue triangles after IV infusion. After both routes of topotecan administration, black circles represent individual concentrations in plasma and the lines the best predicted concentrations according to the pharmacokinetic model for each animal. (C) Orbital-to-plasma ratio of topotecan after OAC (green) and IV infusion (blue). Box and whiskers represent quartiles and range of data, respectively.
In terms of exposure computed as AUC, median topotecan exposure in the orbit (AUCo,OAC) and plasma (AUCpl,OAC) was 5624.3 ng × h/mL (range 3922.3–12530.7) and 141.2 ng × h/mL (range 126.9–191.4), respectively.
After IV infusion, maximum topotecan concentration in plasma was 117.4 ng/ml (range, 113.7–128.5) while four hours after the end of the infusion it was 6.9 ng/ml (range, 5.2–15.6), respectively. These were comparable values to the aforementioned levels attained after OAC. On the contrary, maximum topotecan orbital concentration after IV infusion was only 20.8 ng/mL (range 15.2–51.3) and notably decreased to only 1.0 ng/mL (range 0.89–6.30) after four hours. In correspondence, median systemic and orbital exposure resulted in AUCpl,iv of 138.7 ng × h/mL (range 126.5–186.4) and AUCo,iv of only 23.2 ng × h/mL (range 18.0–74.9), respectively. Orbital and plasma concentration versus time profiles after IV infusion are represented in Figure 2B.
Interestingly, the median (range) model-predicted topotecan concentrations in the orbit two hours after the start of the OAC and the IV infusion was 740 ng/mL (998.0–2757.6) and 4.1 ng/mL (3.4–16.2), respectively. Orbital topotecan concentrations predicted by the model were far higher than the concentrations that inhibit 90% of the cell growth (IC90) in Y79 (commercial) cells and in primary cell lines derived from the cerebrospinal fluid dissemination of two patients (HPG-CSF1 and HPG-CSF2) and from the massive orbital involvement of a metastatic patient (HPG-RBO1), all treated at our institution. For Y79, HPG-CSF1, HPG-CSF2, and HPG-RBO1 cells topotecan IC90 was 77.0 ng/mL, 411.0 ng/mL, 8.4 ng/mL, and 58.1 ng/mL, respectively.
Based on our model, the observed difference in topotecan exposure in the orbit was a consequence of a 157-fold higher rate of drug transfer from the blood to the interstitial fluid of the muscle sheath as a surrogate of the orbital blood flow from 0.0028h−1 to 0.44h−1 after OAC and IV infusion, respectively. All other parameters remained similar between routes of drug administration.
According to the attained AUC, topotecan OAC was a much more selective route to reach the orbit than the systemic circulation. In this sense, the median (range) orbital-to-plasma exposure ratio (AUCo/AUCpl) was 44.3 (27.8–65.5) compared to only 0.18 (0.13–0.4) after IV administration (Fig. 2C).
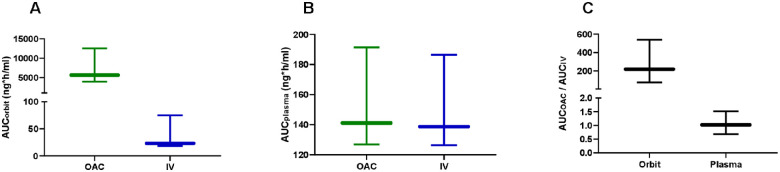
Comparing between local and systemic topotecan delivery, OAC resulted in significantly higher chemotherapy exposure in the orbit with respect to the IV infusion (P < 0.05, Fig. 3A). Conversely, systemic exposure remained comparable between routes of drug administration (P > 0.05, Fig. 3B). Importantly, the median (range) OAC-to-IV orbital exposure ratio was 218 (75–540), implying that OAC delivery allowed attaining about 200 times the orbital exposure found after IV infusion. Nonetheless, both routes of drug delivery resulted in similar systemic exposure with a median OAC-to-IV plasma exposure ratio of 1.1 (range 0.7–1.5, Fig. 3C).
Figure 3.
Topotecan exposure after OAC and intravenous infusion. Area under the concentration versus time profile of topotecan in the orbit (A) and in plasma (B), (C) OAC-to-IV exposure in the orbit and plasma. Box and whiskers represent quartiles and range of data, respectively.
Discussion
The present study describes for the first time the systemic and orbital disposition of topotecan after OAC in the swine model and compares with the results attained after IV infusion (currently used as the gold standard route of chemotherapy delivery for the treatment of orbital retinoblastoma). Our findings indicate that OAC results in significantly higher (×200) and sustained orbital exposure than that attained after the same dose of IV topotecan while attaining comparable systemic exposure of the chemotherapeutic agent between routes of administration.
The management of orbital retinoblastoma is still challenging as extraocular dissemination is associated with a greater risk of concurrent distant metastasis and few advances have been made in the development of treatment options.5,6,12 In low- and middle-income countries, mortality rates of these patients may be as high as 50 to 90% although some promising results were published for patients with extraocular disease limited to the orbit and/or preauricular nodes.1,2,5,7,8,26–28 On the other hand, metastatic patients with orbital disease usually present with a poor clinical status and rapid deterioration making it essential to provide treatments that exert a fast response and prevent local relapse. Thus intensive multimodal treatments are used showing important benefits compared to historical cohorts in terms of overall survival.9
Nonetheless, systemic chemotherapy results in normal tissues being exposed to unnecessary high concentrations of cytotoxic agents with the associated short- and long-term toxicity and even therapy-related deaths.9 Even at the cost of toxicity for exposing the whole body to unnecessary chemotherapy after IV administration, only a fraction of the dose actually reaches the orbit to exert the antitumor effect. Thus systemic chemotherapy would not be the route of choice for maximizing local drug delivery but it is still essential for targeting disseminated retinoblastoma. Importantly, a notable increase in the risk of second malignancies has been extensively documented in cases of hereditary retinoblastoma treated with radiotherapy or systemic chemotherapy. Moreover, even higher rates of second malignancies are reported when both EBRT and systemic chemotherapy are used together.11,29–33 Thus it is essential to maximize chemotherapy exposure in the orbit for consolidation treatment and fast local response and to minimize EBRT-associated morbidity and mortality.
In this scenario, we hypothesized that OAC could be an ideal route for maximizing chemotherapy delivery to the orbit. The rationale behind using OAC is that the ophthalmic artery is the main blood supply to this compartment34 and localizing high concentrations of chemotherapy may hinder tumor dissemination and trigger a rapid and massive antitumor response potentially avoiding consolidation with radiotherapy. Moreover, as previously reported also in the swine model, OAC is also an efficient and selective route for drug delivery to the optic nerve.15 Then, OAC could result in the dual benefit of orbital and optic nerve tumor control as shown in a case of metastatic disease with massive orbital involvement treated in whom neoadjuvant treatment including OAC chemotherapy in the orbit and CNS regions that resulted in complete clinical and partial radiological response.20
In this study, we conclusively show a significant increase in orbital exposure after OAC compared to the systemic infusion of topotecan. Notably, topotecan orbital exposure was about 200-fold higher than after IV infusion of an identical dose. This significant increase was explained by our model pharmacokinetic parameter estimates that showed a 157-fold higher rate of drug transfer from the blood to the orbit after OAC than after IV infusion. Despite this impressive difference in topotecan orbital exposure, systemic exposure remained similar between the two routes of drug delivery. Experience with OAC for intraocular retinoblastoma has shown that the total systemic exposure can be only 10% that of intravenous chemotherapy so if the orbital disease were comparably treated we would expect the systemic exposure to be decreased by 90% if OAC were used.24
As a surrogate of clinical response, the antitumor activity of topotecan can be measured as the drug concentration that inhibits 50% of the cell growth (IC50) in retinoblastoma cell cultures. As in the commercial cell line Y79 topotecan IC50 is 14 ng/mL,35 drug concentrations in the orbit were far above the concentration that kills retinoblastoma cells even after 4h of the OAC administration. On the contrary, this value of cytotoxicity was barely attained at the maximum concentration observed at the end of the IV infusion. Furthermore, topotecan OAC allowed to attaining concentrations in the orbit that were even greater than the IC90, a more stringent value that implies the 90% reduction of the viable cell culture, for at least two hours of the OAC in primary cell cultures derived from intraocular tumors and metastatic sites.
Another aspect to highlight is that in the animal model, topotecan orbital exposure was more than 40 times the value attained in plasma. In contrast, less than 20% of topotecan systemic exposure reached the orbit after IV infusion as the orbital-to-plasma exposure was 0.2 after IV infusion. Thus, in addition to resulting in higher exposure in the orbit, topotecan OAC was a more-selective route of drug delivery to this tissue compartment.
Based on the present findings, we propose that OAC could be included as part of a multimodal treatment strategy for orbital retinoblastoma, that could contribute to avoiding local relapse and CNS dissemination and prompting rapid clinical recovery for a better tolerance to intensive systemic chemotherapy. Nonetheless, several cycles of OAC may add systemic toxicity and cumulative doses could result in long-term toxicities that should be further explored.
We acknowledge some limitations of our data. The swine model was selected because the arterial supply to the orbit is similar to that of humans in terms of the ophthalmic artery as the main provision of blood supply to the orbit, optic nerve and the eye.22,36 Nonetheless, anatomical and physiological dissimilarities between species hinder direct translation to the clinics. Specifically, in pigs it is the external ophthalmic artery, branching from external carotid, that irrigates the orbit as a specular image to humans in whom the orbital blood supply is ultimately provided by the internal carotid that gives off the ophthalmic artery. Moreover, we did not directly collect samples from the orbital tissue but for continuous sampling for a richer pharmacokinetic analysis while reducing the number of animals used, we inserted a microdialysis probe in the sheath of the lateral rectus muscle as it is supplied with blood exclusively from the external ophthalmic artery.22 Then, the concentration of chemotherapy in the interstitial fluid of the lateral rectus muscle was a surrogate of the orbital blood concentration. Also, we acknowledge potential differences in the pharmacokinetics of drugs between normal and tumor bearing animals due to the disruption of the membrane barriers and the development of vessels that irrigate the tumor. Finally, we studied the disposition of topotecan, a hydrophilic drug with antitumor activity in retinoblastoma. However, other more lipophilic drugs may have distributed differently in the orbit and orbital fat (not assessed in the present study), and therefore further studies with other chemotherapeutic drugs should be performed.
Altogether, we show that OAC results in significantly higher topotecan orbital exposure compared to the gold-standard IV route of administration and is highly selective in targeting this compartment. These results provide a scientific rationale for the inclusion of OAC as part of a multimodal treatment in the management of orbital retinoblastoma.
Acknowledgments
The authors thank Gustavo Williams and Mariela Cabezas for their technical assistance with ophthalmic artery chemosurgery in the animals.
Supported by Agencia Nacional de Promoción Científica y Tecnológica (PICT 2016-1505), Fund for Ophthalmic Knowledge, NY, USA, Fundación Natalie Dafne Flexer de Ayuda al Niño con Cáncer.
Disclosure: F. Requejo, None; J. Opezzo, None; A. Vater, None; M. Asprea, None; E. Lagomarsino, None; C. Sampor, None; A. Fandiño, None; G. Chantada, None; J.H. Francis, None; D.H. Abramson, None; P. Schaiquevich, None
References
- 1. The Global Retinoblastoma Study Group. The global retinoblastoma outcome study: a prospective, cluster-based analysis of 4064 patients from 149 countries. Lancet Glob Health. 2022; 10(8): e1128–e1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Retinoblastoma Study Group, Didi Fabian I, Abdallah E, Abdullahi SU, et al.. Global retinoblastoma presentation and analysis by national income level. JAMA Oncol. 2020; 6: 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chantada G, Fandiño A, Casak S, Manzitti J, Raslawski E, Schvartzman E.. Treatment of overt extraocular retinoblastoma. Med Pediatr Oncol. 2003; 40: 158–161. [DOI] [PubMed] [Google Scholar]
- 4. Chantada GL, Guitter MR, Fandiño AC, et al.. Treatment results in patients with retinoblastoma and invasion to the cut end of the optic nerve. Pediatr Blood Cancer. 2009; 52: 218–222. [DOI] [PubMed] [Google Scholar]
- 5. Chawla B, Hasan F, Seth R, et al.. Multimodal therapy for Stage III retinoblastoma (international retinoblastoma staging system): a prospective comparative study. Ophthalmology. 2016; 123(9): 1933–1939. [DOI] [PubMed] [Google Scholar]
- 6. Honavar SG, Singh AD.. Management of advanced retinoblastoma. Ophthalmol Clin North Am. 2005; 18: 65–73. [DOI] [PubMed] [Google Scholar]
- 7. Honavar SG, Manjandavida FP, Reddy VAP.. Orbital retinoblastoma: an update. Indian J Ophthalmol. 2017; 65: 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Radhakrishnan V, Kashyap S, Pushker N, et al.. Outcome, pathologic findings, and compliance in orbital retinoblastoma (International Retinoblastoma Staging System stage III) treated with neoadjuvant chemotherapy: a prospective study. Ophthalmology. 2012; 119: 1470–1477. [DOI] [PubMed] [Google Scholar]
- 9. Dunkel IJ, Piao J, Chantada GL, et al.. Intensive multimodality therapy for extraocular retinoblastoma: a children's oncology group trial (ARET0321). J Clin Oncol. 2022; 40: 3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunkel IJ, Khakoo Y, Kernan NA, et al.. Intensive multimodality therapy for patients with stage 4a metastatic retinoblastoma. Pediatr Blood Cancer. 2010; 55: 55–59. [DOI] [PubMed] [Google Scholar]
- 11. Villanueva G, Sampor C, Moreno F, et al.. Subsequent malignant neoplasms in the pediatric age in retinoblastoma survivors in Argentina. Pediatr Blood Cancer. 2022; 69(8): e29710. [DOI] [PubMed] [Google Scholar]
- 12. Dunkel IJ, Chan HSL, Jubran R, et al.. High-dose chemotherapy with autologous hematopoietic stem cell rescue for stage 4B retinoblastoma. Pediatr Blood Cancer. 2010; 55: 149–152. [DOI] [PubMed] [Google Scholar]
- 13. Martins C, Costa e Silva IE, Campero A, et al.. Microsurgical anatomy of the orbit: the rule of seven. Anat Res Int. 2011; 2011: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rhoton AL. Cranial Anatomy and Surgical Approaches: Neurosurgery. Oxford, UK: Oxford University Press; 2008. [Google Scholar]
- 15. Taich P, Requejo F, Asprea M, et al.. Topotecan delivery to the optic nerve after ophthalmic artery chemosurgery. PLoS One. 2016; 11(3): 151343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abramson DH, Fabius AWM, Francis JH, et al.. Ophthalmic artery chemosurgery for eyes with advanced retinoblastoma. Ophthalmic Genet. 2017; 38: 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP.. A Phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma. Initial results. Ophthalmology. 2008; 115: 1398–1404. [DOI] [PubMed] [Google Scholar]
- 18. Schaiquevich P, Francis JH, Cancela MB, Carcaboso AM, Chantada GL, Abramson DH.. Treatment of retinoblastoma: what is the latest and what is the future. Front Oncol. 2022; 12: 822330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Francis JH, Levin AM, Zabor EC, Gobin YP, Abramson DH.. Ten-year experience with ophthalmic artery chemosurgery: ocular and recurrence-free survival. PLoS One. 2018; 13(5): e0197081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodriguez A, Zugbi S, Requejo F, et al.. Combined high-dose intra-arterial and intrathecal chemotherapy for the treatment of a case of extraocular retinoblastoma. Pediatr Blood Cancer. 2018; 65(12): e27385. [DOI] [PubMed] [Google Scholar]
- 21. Abramson DH, Gobin YP, Francis JH.. Orbital retinoblastoma treated with intra-arterial chemotherapy. Ophthalmology. 2021; 128: 1437. [DOI] [PubMed] [Google Scholar]
- 22. Simoens P. Morphologic study of the vasculature in the orbit and eyeball of the pig [doctoral dissertation]. Ghent, Belgium: State University of Ghent; 1985. [Google Scholar]
- 23. Reese AB, Hyman GA, Merriam GR, Forrest AW, Kligerman MM.. Treatment of retinoblastoma by radiation and triethylenemelamine. AMA Arch Ophthalmol. 1954; 53(4): 505–513. [DOI] [PubMed] [Google Scholar]
- 24. Schaiquevich P, Buitrago E, Ceciliano A, et al.. Pharmacokinetic analysis of topotecan after superselective ophthalmic artery infusion and periocular administration in a porcine model. Retina. 2012; 32: 387–395. [DOI] [PubMed] [Google Scholar]
- 25. Schaiquevich P, Buitrago E, Taich P, et al.. Pharmacokinetic analysis of melphalan after superselective ophthalmic artery infusion in preclinical models and retinoblastoma patients. Invest Ophthalmol Vis Sci. 2012; 53: 4205–4212. [DOI] [PubMed] [Google Scholar]
- 26. Dunkel IJ, Krailo MD, Chantada GL, et al.. Intensive multi-modality therapy for extra-ocular retinoblastoma (RB): a Children's Oncology Group (COG) trial (ARET0321). J Clin Oncol. 2017; 35: 10506–10506. [Google Scholar]
- 27. Gianotti Antoneli CB, Steinhorst F, Braga Ribeiro KDC, et al.. Extraocular retinoblastoma: a 13-year experience. Cancer. 2003; 98: 1292–1298. [DOI] [PubMed] [Google Scholar]
- 28. Aschero R, Torbidoni A, Sampor C, et al.. Minimally disseminated disease and outcome in overt orbital retinoblastoma. Pediatr Blood Cancer. 2019; 66(6): e27662. [DOI] [PubMed] [Google Scholar]
- 29. Abramson DH, Frank CM.. Second nonocular tumors in survivors of bilateral retinoblastoma: a possible age effect on radiation-related risk. Ophthalmology. 1998; 105: 573–580. [DOI] [PubMed] [Google Scholar]
- 30. Temming P, Arendt M, Viehmann A, et al.. Incidence of second cancers after radiotherapy and systemic chemotherapy in heritable retinoblastoma survivors: a report from the German reference center. Pediatr Blood Cancer. 2017; 64: 71–80. [DOI] [PubMed] [Google Scholar]
- 31. Wong JR, Morton LM, Tucker MA, et al.. Risk of subsequent malignant neoplasms in long-term hereditary retinoblastoma survivors after chemotherapy and radiotherapy. J Clin Oncol. 2014; 32: 3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gregersen PA, Gregersen PA, Olsen MH, et al.. Incidence and mortality of second primary cancers in Danish patients with retinoblastoma, 1943–2013. JAMA Netw Open. 2020; 3(10): e2022126–e2022126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marees T, Moll AC, Imhof SM, De Boer MR, Ringens PJ, Van Leeuwen FE.. Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J Natl Cancer Inst. 2008; 100: 1771–1779. [DOI] [PubMed] [Google Scholar]
- 34. Gray H, Henry VC. Gray's Anatomy : the anatomical basis of clinical practice. 41st ed. Philadelphia: Elsevier; 2015. [Google Scholar]
- 35. Winter U, Mena HA, Negrotto S, et al.. Schedule-dependent antiangiogenic and cytotoxic effects of chemotherapy on vascular endothelial and retinoblastoma cells. PLoS One. 2016; 11(7): e0160094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dondelinger RF, Ghysels MP, Brisbois D, et al.. Relevant radiological anatomy of the pig as a training model in interventional radiology. Eur Radiol. 1998; 8: 1254–1273. [DOI] [PubMed] [Google Scholar]