Abstract
A novel panfungal PCR assay which detects the small-subunit rRNA gene sequence of the two major fungal organism groups was used to test whole-blood specimens obtained from a series of blood or bone marrow transplant recipients. The 580-bp PCR product was identified after amplification by panfungal primers and hybridization to a 245-bp digoxigenin-labeled probe. The lower limit of detection of the assay was approximately four organisms per milliliter of blood. Multiple whole-blood specimens from five patients without fungal infection or colonization had negative PCR results. Specimens from 11 infected patients had positive PCR results. Blood from three patients with pulmonary aspergillosis had positive PCR results: one patient’s blood specimen obtained in the week prior to the diagnosis of infection by a positive bronchoalveolar lavage fluid culture result was positive by PCR, and blood specimens obtained from two patients 1 to 2 days after lung biopsy and which were sterile by culture were positive by PCR. The blood of four patients with candidemia, three patients with mixed fungal infections, and one patient with fusariosis also had positive PCR signals. The panfungal PCR assay can detect multiple fungal genera and may be used as an adjunct to conventional methods for the detection of fungal infection or for describing the natural history of fungal infection. Further studies are needed to define the sensitivity and specificity of this assay for the diagnosis of fungal infection prior to the existence of other clinical or laboratory indications of invasive fungal infection.
The prevalence of fungal infections has increased in recent years due to an increasing population of immunocompromised patients, intensive immunosuppressive chemotherapy, increasing awareness of fungal infections, and the widespread use of broad-spectrum antibiotics and central venous catheters (2). Standard methods for the diagnosis of Candida and Aspergillus fungal infections include culture and histopathology, but these methods have limited sensitivity and specificity (11, 23, 46). For example, blood cultures are positive for fewer than 50% of patients with hepatosplenic candidiasis (46) and are rarely positive for patients with invasive aspergillosis (11, 49). In addition, cultures of bronchoalveolar lavage fluid are frequently negative for patients with pulmonary aspergillosis (23), and by the time that positive cultures are obtained, disease is usually advanced.
Rapid diagnostic strategies for fungal infections include detection of antibody, antigen, or DNA. Antibody detection in bone marrow transplant (BMT) patients is limited due to unpredictable humoral responses (53). Second-generation tests for the detection of Candida and Aspergillus antigens look promising but have not been compared with culture with samples from humans (20, 34).
A PCR assay for the detection of fungal nucleic acids may be the optimal diagnostic approach because it offers the potential of (i) being more sensitive than current culture-based methods, (ii) encompassing multiple fungal genera, and (iii) being applied to a variety of specimen types. The design of fungal primers that can detect the appropriate range of fungal organisms has remained a challenge to the development of PCR assays for fungi. Most studies of the application of PCR techniques to the detection of fungal DNA have used assays with either primers (6–9, 19, 22, 24, 33, 38, 45, 48) or probes (5, 7, 12, 15, 22, 24, 31, 40) targeted to gene sequences restricted to one genus or species of fungi in order to maintain specificity. However, since the incidence of individual infecting species, e.g., Candida (10%) (32, 47), Aspergillus (5 to 15%) (21, 32, 35, 39, 41, 49), and Fusarium (<2%) (16), is relatively low and may vary from center to center, a panfungal assay is desired. This universality of nucleic acid-based methods is not possible with antigen-, antibody-, or metabolite-based assays and is practical because rRNA gene sequences are relatively conserved among eukaryotic organisms (30) and specifically among members of the fungal kingdom, including the Aspergillus and Candida species, the dimorphic fungi, the agents of zygomycosis, and Pneumocystis (4).
We used sequence analysis to develop a PCR assay reactive to the DNA of medically important fungi but unreactive to bacterial or human DNA. The panfungal primers were optimized separately for Candida albicans and Aspergillus fumigatus, the major organisms responsible for invasive yeast and mold infections, respectively. The long probe was synthesized by PCR with an internal primer pair and multiply labeled with digoxigenin. The long probe detects organisms in several subdivisions of the fungal kingdom because its long length will anneal to any product with >85% sequence homology. The known sequences are at least 85% homologous. Primary detection of the fungal PCR product must include hybridization to a probe when human blood specimens are used because human DNA can obscure the detection of a weak fungal DNA signal on agarose gels. We evaluated the assay for its ability to detect fungi in the blood of patients with and without invasive fungal disease.
MATERIALS AND METHODS
Sequence analysis for the design of the primers and probe.
rRNA gene sequences from 42 organisms were accessed via the GenBank database and were aligned by using the Wisconsin Sequence Analysis Package (Genetics Computer Group, Madison, Wis.) (10). Multiple potential primer-binding sites for the panfungal primer pair were chosen by comparing regions of Aspergillus homologous with regions of the fungal group from the fungal kingdom with the most divergent DNA sequences, Mucor, and regions of Aspergillus incongruous with the human DNA sequence. The primer selection was optimized for melting temperature equivalence, lack of duplex, hairpin, or primer dimer formation, and internal stability by using OLIGO software (National Biosciences Inc., Plymouth, Minn.). Amplification conditions for A. fumigatus and C. albicans were optimized separately by a previously described method (3).
Subjects and collection of specimens.
Whole-blood specimens were collected from patients at the Fred Hutchinson Cancer Research Center in Seattle, Wash., placed in Vacutainers containing 1.5 ml of acid citrate dextrose (ACD) anticoagulant, and stored at 4°C. Written informed consent was obtained from all subjects by procedures approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center. Mouthwash specimens for fungal cultures were obtained on a weekly basis to determine colonization status. Blood cultures were performed with the BacT/Alert FAN aerobic culture bottle (Organon Teknika, Durham, N.C.) in combination with the Isolator aerobic culture tube (Wampole, Cranbury, N.J.). To obtain specimens from the days preceding infection, patients were asked to donate 6 ml of whole-blood specimens prospectively on a weekly basis. If the patient showed symptoms or signs relating to fungal disease, we attempted to collect specimens daily while the clinical workup for disease proceeded. Patients from whom prospective specimens were not collected were asked to consent to blood collection if a fungal infection occurred, even though specimens would postdate the onset of the fungal infection.
BMT recipients without clinical, radiographic, biopsy, or culture evidence of fungal infection were defined as patients with “no infection.” Recipients in whom fungus was detectable at superficial sites were considered “colonized” and were not tested further for this feasibility study. Specimens taken from patients with culture or histopathologic evidence of fungus from sterile tissue sites were considered patients with “invasive disease.” Patients with no specimens collected during a 2-week window at the time of documentation of fungal infection were not tested by the PCR assay, and the remaining specimens were discarded. Assay results for serial specimens from 10 patients that were tested in PCR runs with evidence of contamination in the negative extraction controls or the negative amplification controls were discarded.
DNA extraction from whole-blood specimens.
DNA extraction was performed in a laminar-air-flow biohazard cabinet (Envirco, Albuquerque, N.Mex.) as described previously (6, 12), with minor modifications as detailed below. The Vacutainer’s rubber stopper was carefully removed, and blood was aseptically transferred with a disposable pipette into a 50-ml centrifuge tube. Erythrocytes were lysed with 30 ml of erythrocyte lysis buffer (10 mM Tris [pH 7.6], 5 mM MgCl2, 10 mM NaCl) by incubation for 10 min on a tilting table at 37°C and were separated by centrifugation (10 min at 5,000 × g). The pellet was washed with 15 ml of erythrocyte lysis buffer, incubated again at 37°C for 10 min, and centrifuged (10 min at 5,000 × g). Leukocytes were then lysed by incubation for 2 h in a water bath at 65°C with 1 ml of leukocyte lysis buffer (10 mM Tris [pH 7.6], 10 mM EDTA [pH 8.0], 50 mM NaCl, 0.2% sodium dodecyl sulfate, 200 μg of proteinase K per ml). The remainder of the sample was repelleted by centrifugation (15 min at 5,000 × g).
Fungal cell walls were lysed by resuspending the pellet with 500 μl of lysis buffer (50 mM Tris [pH 7.6], 1 mM EDTA, 20% 2-mercaptoethanol), to which ∼3 U of lyticase (Sigma, St. Louis, Mo.) was added. The digestion mixture was allowed to incubate for 1 h at 37°C, and then the fungal nuclei were lysed by the addition of 50 μl of 10% sodium dodecyl sulfate, and then the nuclei were incubated at 65°C for 20 min. For protein precipitation, 200 μl of 5 M potassium acetate was added, and the mixture was incubated on ice for 25 min and then centrifuged for 10 min at 12,000 × g. The supernatant was transferred to a new tube, nucleic acids were precipitated with 600 μl of ice cold isopropyl alcohol, and the mixture was incubated at −20°C for 30 min and centrifuged at 4°C for 30 min at 12,000 × g. The pellet was washed with 600 μl of 70% ethanol. After an additional 15 min of cold centrifugation at 12,000 × g, the final pellet was suspended in 30 μl of sterile water (Baxter Healthcare Corporation, Deerfield, Ill.).
Extraction controls.
Negative controls included one tube of whole blood from a healthy volunteer for every four patient specimens. Positive controls were included in every extraction to verify extraction efficiency by spiking blood samples obtained from volunteers with approximately 150 CFU of A. fumigatus or C. albicans conidial or blastoconidial suspensions respectively, in a volume of 500 μl. To determine the actual numbers of CFU injected, a suspension of 100 μl containing ∼30 CFU was plated onto Sabouraud dextrose agar, and the plate was incubated for 72 h at 30°C. The stock cultures of our two most frequently used positive controls, C. albicans and A. fumigatus, were grown in bulk cultures once and were stored refrigerated in aliquots. Up to 100 aliquots were used from each of the bulk cultures to spike positive extraction controls for each batch of 16 samples.
PCR assay.
The measures used to avoid PCR assay contamination included the use of separate rooms and glassware supplies for PCR setup and products, aliquoted reagents, positive-displacement pipettes, aerosol-resistant tips, multiple negative controls, and low-copy-number positive controls (28). Five microliters of extracted DNA was amplified in a 50-μl reaction volume with PCR buffer (20 mM Tris [pH 8.0], 50 mM KCl) (Gibco BRL, Gaithersburg, Md.), 1.25 U of Taq polymerase (Gibco BRL), 100 μM deoxynucleoside triphosphates, 30 pmol of each primer, (forward primer 999 [5′-GATACCGTCGTAGTCTTA-3′] and reverse primer 1574c [5′-ATTCCTCGTTGAAGAGC-3′]), 2.2 mM MgCl2, and 50 μl of a mineral oil overlay (Sigma). The primers were synthesized at the Fred Hutchinson Cancer Research Center Biotechnology Laboratory, and reproducibility was monitored with low-copy-number positive controls. Amplification was performed in a model 480 Perkin-Elmer Cetus thermocycler (Perkin-Elmer Corporation, Norwalk, Conn.). After an initial denaturation at 94°C for 5 min, the cycling conditions were 35 cycles of 94°C for 30 s, 52°C for 1 min, and 72°C for 2 min, followed by a final elongation at 72°C for 7 min. Fifteen microliters of each PCR product was electrophoresed through a 2% agarose gel for 2 h at 100 V and 50 mA in 1× TBE (Tris-borate-EDTA) buffer, and the gel was stained with ethidium bromide. The DNA was transferred to a nylon membrane by Southern transfer overnight (44), followed by hybridization with the panfungal detection probe at 50°C for 1 h in 45% formamide–0.72 M NaCl.
Long panfungal probe.
The PCR-synthesized, digoxigenin-labeled, 245-bp probe was made with previously amplified A. fumigatus DNA as described previously for cytomegalovirus (13, 14), with minor modifications. The probe was synthesized with forward primer 1196 (5′-CGGGGAAACTCACCAG-3′) and reverse primer 1440c (5′-AAGGGCATCACAGACC-3′), which bind to regions internal to the 580-bp PCR amplicon. The reaction mixture contained dATP, dCTP, and dGTP each at a concentration of 100 μM, 65 μM dTTP, and 35 μM digoxigenin-11-dUTP.
The reaction was visualized by the use of alkaline phosphatase-labeled antidigoxigenin Fab fragments and the colorimetric reagents 5-bromo-4-chloro-3-indolyl-phosphate (0.4 mg/ml; Boehringer Mannheim, Indianapolis, Ind.) and 4-nitroblue tetrazolium chloride (0.19 mg/ml; Boehringer Mannheim). Southern hybridization was used as the primary method for the detection of all fungal PCR products from blood specimens because human DNA can obscure the detection of weak fungal DNA bands on agarose gels.
RESULTS
Assay specifications.
The lower limit of detection of the assay and the comparability of culture from the blood culture tube versus extraction from the PCR assay collection tube (ACD anticoagulant) were determined with 500-μl serial dilutions of A. fumigatus conidia. Wampole Isolator tubes containing 10 ml of blood from a volunteer were processed according to the manufacturer’s directions, and the pellet was plated onto two Sabourad dextrose agar plates. DNA was extracted from ACD tubes containing 7 ml of blood from a volunteer and was amplified by the PCR assay as described above; a 100-μl aliquot of each dilution was plated onto Sabourad dextrose agar. The amplification extinction point was found to be equal to 21 CFU/tube (2 CFU/ml) for the Isolator tube and 30 CFU/tube (4 CFU/ml) for the ACD tube. Additionally, the detection of DNA by PCR was tested with serial 10-fold dilutions of the purified 580-bp PCR template for both A. fumigatus and C. albicans. One copy, or 3 ag, was detectable. This equals one copy of the product. Therefore, the theoretical lower limit of detection of the PCR assay is 4 CFU/ml.
In addition to using A. fumigatus and C. albicans as positive controls, other stock fungi were isolated from patients, classified by a certified clinical microbiology laboratory, and amplified by the assay described above. They included Absidia, Acremonium, Alternaria, Aspergillus flavus, Aspergillus terreus, Aspergillus niger, Aspergillus nidulans, Bipolaris, Blastomyces dermatitidis, Candida glabrata, Candida parapsilosis, Candida krusei, Chaetomium, Chrysosporium, Cladosporium carrionii, Cryptococcus neoformans, Curvularia, Exophiala werneckii, Fusarium solani, Lecythophora, Malassezia furfur, Microsporum canis, Microsporum gypseum, Mucor indicus, Paecilomyces, Penicillium, Phoma, Pseudallescheria boydii, Rhizopus arrhizus, Rhodotorula rubra, Scopulariopsis, Sordaria macropoia, Sporothrix schenckii, Trichophyton rubrum, Ulocladium, Verticillium, Williopsis mrakii, and Zygorhyncus (data not shown). DNA was also detected from mycelia that grew on solid medium but that did not develop the fruiting structures necessary for identification (mycelia sterilia). The fungi which failed to be amplified included Aureobasidium pullulans, Cunninghamella, Drechslera, Fonsecaea pedrosoi, Rhizomucor pusillus, and Sepedonium. DNA was believed to have been successfully extracted from each of these nonamplifiable organisms because DNA was visualized on a 2% agarose gel after electrophoresis and because amplification was successful for four of the organisms when a different set of panfungal primers was used (12). Specificity was confirmed by nonamplification of DNA from Staphylococcus aureus, viridans group streptococci, coagulase-negative staphylococci, Pseudomonas aeruginosa, and Escherichia coli and of human DNA (data not shown). The DNA of Prototheca wickerhamii, an alga, was amplified. Fungal organisms added to the ACD tubes containing blood can be detected after storage at 4°C for up to 18 months.
Results of the optimized PCR assay with human blood specimens.
Specimens were collected from a total of ∼200 patients over a 1-year period. Forty blood and BMT recipients had no fungal disease or colonization, and multiple blood specimens from five of these patients, chosen as a convenience sample, demonstrated no PCR signal when they were tested in duplicate. For more than 100 patients, a yeast or mold was cultured from samples from superficial sites, but the patients were not thought to be invasively infected on clinical or other laboratory grounds. Specimens from these patients were not tested further. For 23 patients invasive fungal infections were documented by a positive culture result for a sample from a usually sterile site and/or by histopathologic appearance. Data for five patients were excluded because specimens were not collected at the point of infection. For six patients, the assay mixture was contaminated (sham controls were positive) during the testing of the patient’s specimens. Specimens from two patients with Aspergillus sinusitis were not tested. We present the results for four patients with candidemia, three patients with pulmonary aspergillosis, three patients with mixed fungal infections, and one patient with fusariosis. For each of these 11 patients with documented invasive fungal infection, PCR assay of whole-blood specimens was positive at least once in the course of the patient’s illness.
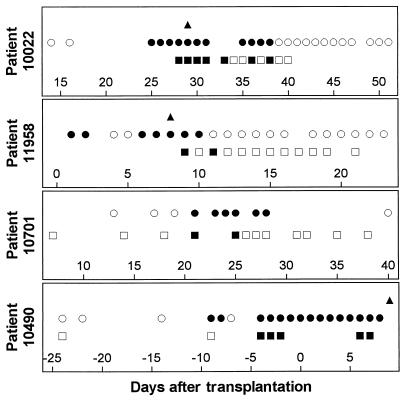
Serial whole-blood specimens from the four candidemic patients generally showed positive PCR signals on the days that the blood cultures were positive (Fig. 1). Control blood samples from volunteer donors tested in the same assay runs as specimens from infected patients remained negative. C. parapsilosis was repeatedly cultured from the blood of patient 10022 until the patient’s central venous catheter was removed. Patient 10701 developed C. glabrata fungemia just after the central venous catheter had been removed for persistent bacterial infection. For both patients, the blood culture was positive on 2 days when the PCR signal was negative, but this occurred toward the end of infection, after the patients had received significant amounts of antifungal therapy. Conversely, patient 11958, who also had C. parapsilosis fungemia, had a negative PCR signal on the last day that the blood culture was positive but a positive PCR signal on the following day. Whether this signal represents a true-positive result due to the sensitivity of the assay or a false-positive result is not known. Few specimens from days which predated the candidemias by less than 1 week were available for testing, and specimens were not always obtained on days when blood samples for culture were drawn. Patient 10490 developed fungemia caused by C. tropicalis, C. albicans, and a Candida organism whose species was not determined, which led to a fungus-related death 18 days after the first blood sample was positive by culture. PCR signals were found on all days that blood cultures were positive when blood specimens were available, except for the day of the onset of infection. Several possible explanations for this false-negative signal include sampling error, prolonged cold storage time, transposition of specimen tubes, or suboptimal yield from the DNA extraction.
FIG. 1.
Results for candidemic patients 10022 (C. parapsilosis), 11958 (C. parapsilosis), 10701 (C. glabrata), and 10490 (C. tropicalis, C. albicans, and a Candida strain whose species was not determined). Solid symbols indicate positive results; ▴, Hickman catheter tip culture; •, blood culture; and ▪, whole-blood PCR assay. Open symbols indicate negative results; ○, blood culture; □, whole-blood PCR assay.
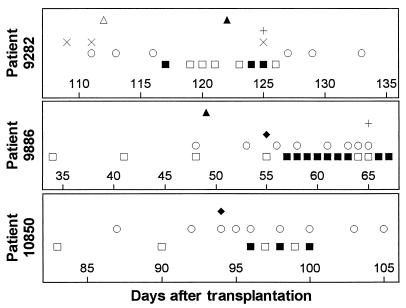
Specimens from three patients with pulmonary aspergillosis as the only fungal infection demonstrated occasional positive PCR results (Fig. 2). Two cultures of sputum from patient 9282 grew A. fumigatus, at which time a computed tomography (CT) scan of the chest did not reveal evidence of fungal disease. Two weeks later, two of four specimens postdating a follow-up CT scan with fungal-like nodules were positive by PCR. The patient died of invasive pulmonary aspergillosis 3 weeks after the diagnostic CT scan. Patients 9886 and 10850 developed pulmonary aspergillosis while being treated with steroids in the outpatient setting. For both patients, PCR of whole blood became positive after the results of the diagnostic lung biopsy were already available, and the patients died within 2 weeks of the diagnosis of infection by biopsy.
FIG. 2.
Results for patients with pulmonary aspergillosis. Infection in patient 9282 was diagnosed by bronchoscopy, 3 days after a CT scan demonstrated pulmonary nodules. A. fumigatus cultured from open lung biopsy specimens from patients 9886 and 10850. ▴, CT scan with nodules; ▵, CT scan without nodules; +, bronchoalveolar fluid culture positive; ×, sputum culture positive; ⧫, lung biopsy specimen positive; •, blood culture positive; ○, blood culture negative; ▪, PCR-positive blood; □, PCR-negative blood.
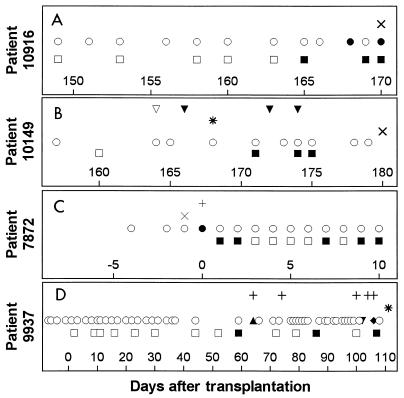
The blood of three patients with mixed infections and one patient with fusariosis also had positive panfungal PCR signals (Fig. 3). For these patients the panfungal PCR assay could diagnose the presence of fungal DNA but could not suggest prospectively the presence of a mixed infection. Patient 10916 had a positive PCR assay result 3 days before demonstrating Candida fungemia and 5 days before the autopsy, which demonstrated pulmonary Aspergillus, in addition to persistent Candida, and we do not know if the positive PCR signals preceded candidemia or followed the onset of aspergillosis. Patient 10149 had Pneumocystis carinii pneumonia, followed by the discovery of pulmonary aspergillosis at autopsy 2 weeks later. Three blood specimens had positive PCR signals prior to the demonstration of pulmonary aspergillosis at autopsy, but the PCR results were difficult to interpret in the setting of concurrent P. carinii pneumonia (P. carinii is an organism that is also theoretically detectable by the assay). The blood of patient 7872, who had fusariosis, had positive PCR signals on 5 of 10 days after the blood culture had turned negative. He died 12 days after the onset of the disseminated infection.
FIG. 3.
Clinical course for patients with mixed infections. Patient 10916 had multiorgan failure in the setting of difficult-to-control graft-versus-host disease. Infection with neither C. albicans nor A. fumigatus was suspected, and no antifungal therapy had been initiated prior to death. Patient 10149 had pulmonary infection with P. carinii followed by infection with A. fumigatus. Patient 7872 died of fusariosis. Patient 9937 died of disseminated yeast and mold infection after struggling for months with severe chronic graft-versus-host disease. (A to D) ▪, positive PCR result for blood; □, negative PCR result for blood; and ○, culture-negative blood specimen. (A) ×, lung sample culture positive for Aspergillus at autopsy; •, blood specimen culture positive for Torulopsis (Candida) glabrata. (B) ▿, normal chest radiograph; ▾, chest radiograph with infiltrates; ×, lung specimen culture positive for Aspergillus at autopsy; ∗, bronchoalveolar lavage fluid positive for P. carinii by silver stain. (C) +, sputum specimen culture positive for Fusarium; ×, skin biopsy specimen positive for hyphae; •, blood specimen culture positive for Fusarium. (D) +, blood specimen culture positive for a bacterial organism; ∗, cultures of organ specimens obtained at autopsy grew Torulopsis and sterile mycelia; ▴, blood culture grew a zygomycete; ▾, blood culture grew Prototheca; ⧫, blood culture grew Penicillium.
For patient 9937 two PCR signals could be interpreted as false-positive signals. This patient had chronic graft-versus-host disease and recurrent mucosal breakdown. While most of the patients described above served as their own controls and the specimens obtained weekly had negative PCR results until the days immediately surrounding the invasive fungal infection, patient 9937 was the only patient to demonstrate intermittent PCR signals positive for fungi before the definitive invasive infection which was found at autopsy. Among the specimens obtained weekly from this patient, 11 specimens were negative and 3 specimens were positive by PCR before his death. The first positive signal was on day 59 after transplantation, but a blood sample obtained on that day was negative by routine culture. He did have intermittent seeding of his blood by a variety of organisms, as demonstrated by blood cultures positive for a zygomycete, Klebsiella, and Serratia on day 64; the positive PCR result on day 59 could have been an early indication of the zygomycete. A gram-negative bacillus was cultured from the patient’s blood on day 74, Serratia was cultured on day 100, Prototheca was cultured on day 102, and Penicillium was cultured on day 106. C. glabrata and a nonviable mold (mycelia sterilia) were found in most autopsied organs on day 111. The two positive PCR signals which occurred on days 86 and 107, with a particularly strong signal for the specimen obtained on day 107 (4 days before what was in retrospect a fungus-related death), could have been false positive, could have been early indications of Prototheca or Penicillium infection, or could have represented transient fungemia not otherwise noted by blood cultures.
DISCUSSION
This report describes a novel panfungal PCR assay in a nonradioactive format which has a lower limit of detection of 4 CFU/ml of blood but which retains the ability to detect a broad range of fungi. The amplicon sequences predict that our probe will result in a panfungal assay. This prediction was confirmed by positive PCR results in tests with a myriad of organisms representative of many taxonomic genera. Specimens from BMT patients were chosen for testing by this assay because approximately 5% of these patients predictably develop fungal infections during the course of their transplant-associated neutropenia and other immunosuppression (1, 18, 49). However, the PCR method can also be applied to specimens from other patient populations, including immunocompromised patients with human immunodeficiency virus infection.
In order to screen for fungal infections in BMT patients at risk for fungal infection, an assay detecting multiple fungal genera with high sensitivity is needed. The genera which should be targeted by the primers include Candida, Aspergillus, and other less common but medically relevant fungi. Furthermore, the spectrum of organisms causing infection has changed from C. albicans to C. glabrata, Candida tropicalis, C. krusei, C. parapsilosis, and fluconazole-resistant strains of C. albicans since the introduction of fluconazole in 1992 as prophylaxis against fungal infections (17, 35, 36, 43, 50–52). A probe which is panfungal and nonradioactive can facilitate the screening process for detection of an unknown organism in a clinical diagnostic laboratory. Previous studies that used panfungal PCR assays are limited by the lack of a panfungal probe (12, 25, 40) or detect the panfungal probe in a radioactive format (29).
Six fungi did not react with the probe. We confirmed adequate cell wall lysis and DNA extraction by the presence of the usual smear of DNA when the DNA was run on an agarose gel. In most cases, we were also able to confirm adequate preparation by performing amplification with another set of panfungal primers (12). Interestingly, not all organisms detected by the assay with another set of panfungal primers were detectable by the assay with our primers, and vice versa. At present there are no published sequence data for some of the organisms (mostly zygomycetes), so we are uncertain about the percentage homology with the primer sequences. In one case, the gel was negative and both PCR assays were negative, so the DNA may have not been adequately extracted, possibly because of a poorly lysed cell wall, or inhibitors unique to the fungus may have been present.
Animal model studies support the utility of PCR for the detection of disseminated fungal disease. They have demonstrated that PCR assays can be as sensitive as blood cultures for the detection of Candida yet specific enough to remain negative for colonized animals (24, 48). Kan (24) inoculated 20 mice with C. albicans intravenously and then sacrificed the mice in groups of five mice each at 1- to 4-day intervals. Blood culture and PCR results were 100% concordant. Candida was inoculated into a thigh muscle of 20 additional mice, which led to the formation of local abscesses. All 20 mice were negative by both blood culture and PCR of blood before and for up to 15 days after the abscesses were established. van Deventer et al. (48) inoculated mice intravenously and then sacrificed the animals at 1, 6, 24, 48, 72, 96, or 144 h after inoculation. The PCR assay of blood was positive for 48 of 50 (96%) animals, while the blood culture was positive for 35 of 50 (70%) animals.
The results of previously published studies of PCR with patient blood specimens strengthen the results of these studies with animals. In one study in which Candida genus-specific primers were used, PCR signals were positive for 24 of 25 blood samples from 15 fungemic patients positive by culture (22). In another study which used panfungal PCR primers coupled to species-specific C. albicans or genus-specific Aspergillus hybridization probes, blood specimens tested PCR positive concurrently with positive blood cultures for four patients with candidemia and 0 to 8 days prior to the detection of pulmonary infiltrates in 13 patients with invasive Aspergillus (12). In that study, the rate of false positivity was 3%, because only 3 of 189 blood specimens from 100 subjects with febrile neutropenia (n = 29), fungal colonization (n = 36), or healthy volunteers (n = 35) were PCR positive. Thus, any detection of Aspergillus or Candida DNA in blood could be a harbinger of invasive disease, and Candida colonization does not appear to create false-positive PCR signals with blood specimens.
In our study, neither PCR nor blood culture proved to be uniformly more positive, and a negative blood culture on any given day did not necessarily imply that a patient was cured, because for our patients 11958, 10490, and 10916, days of positive cultures were interspersed with days of negative cultures. Conversely, patient 7872 with fusariosis had a PCR positivity rate of 50% when blood cultures were negative. The patient was receiving treatment against the organism during the 10-day sampling interval in question, and the treatment may have caused intermittent clearing of the organism from the bloodstream.
The possible false-negative PCR signals which occurred toward the end of the courses of candidemia for patients 11958 and 10701 could be the result of two types of sampling errors. The first is the specific time that the specimen for PCR was drawn from the patient in relation to the time that the blood sample for culture was drawn and when the daily dose of amphotericin B was administered. The specimen tubes were labeled only with the date on which the sample was drawn. The second possible reason is that the numbers of circulating organisms are lower toward the end of an infection. As the frequency of organisms approaches one detectable unit per assay, the assays will produce sporadic positive results, as delineated by the Poisson distribution. It is also possible that the lack of sensitivity of PCR can be attributed in part to the prolonged cold storage time in anticoagulant, an undetectable experimental error such as transposition of specimen tubes during the testing process, or the possibility that our procedure for lysing the organisms and extracting the DNA provided a suboptimal yield.
Contamination has been the main obstacle to the clinical application of PCR. Contamination occurs by airborne spore inoculation during the extraction process, by product carryover, and because of the presence of nonviable fungal spores found in reusable equipment, despite autoclaving. To minimize the risk of contamination by reusable equipment and maximize the fungal DNA yield from blood specimens containing small quantities of infecting fungus, we chose an enzymatic method of fungal cell wall disruption for the extraction procedure. β-1,3-Glucanase enzymes hydrolyze glucose polymers at β-1,3-glucan linkages in fungi, releasing laminaryipentaose and resulting in fungal spheroplasts, which are modified organisms that have partially lost the cell wall and that have increased osmotic sensitivity (37). Until the 1970s, snail gut enzyme was the prototype enzyme used for fungal cell wall lysis, but the preparation had variable activity from batch to batch (26, 27). β-1,3-Glucanase products include zymolyase (ICN Pharmaceuticals, Costa Mesa, Calif.), a natural β-1,3-glucanase purified from a submerged culture of Arthrobacter luteus in the fermentation of yeast (27), and lyticase (Sigma), a synthetic equivalent of β-1,3-glucanase (42). The use of lyticase avoids the impurities found in zymolyase, such as β-1,3-gluconase, protease, mannanase, amylase, xylanase, phosphatase, and trace DNase. We discarded the results for six patients (35%) due to contamination. To minimize the rate of contamination in the future, we will use the lyticase lysis enzyme, prealiquoted reagents, dedicated pipettes, aerosol-resistant tips, separate rooms for DNA extraction, amplification, and PCR product analysis, and negative amplification controls placed intermittently among the samples in each amplification assay.
We assessed the feasibility of using our panfungal PCR assay with a limited series of whole-blood specimens (6 ml each) obtained from several BMT patients with and without documented fungal infections. The limitation of the assay for the candidemic patients was the lack of specimens from before the onset of fungemia that could be used to prove whether the PCR assay can demonstrate disease before culture can. For our candidemic patients, the PCR assay signal did not persist beyond the time that the signal was found by the traditional means of proving infection. Among the patients with invasive pulmonary aspergillosis, only patient 9282 had positive PCR results more than 1 week prior to the diagnosis of infection. Future experiments will be directed at improving the specimen collection schedule and storage procedure and improving the real-time testing of specimens before we proceed with a larger prospective trial.
We do not yet know whether the assay can distinguish between colonization and infection. We excluded the data for colonized patients because the interpretation of the results was beyond the scope of the present study. Investigation of colonized patients would be a worthwhile effort in future studies.
In summary, we have developed a panfungal PCR assay which has a lower limit of detection of 4 CFU/ml and which is applicable to a broad range of fungi. When following previously described methods to avoid contamination, the DNA extraction procedure runs smoothly, although unpredictable episodes of contamination have occurred in our laboratory. When applied to blood specimens from patients infected with fungi, the assay shows promise as a future diagnostic tool. Further studies are needed to define the sensitivity and specificity of this assay for the diagnosis of fungal infection prior to other clinical or laboratory indications of invasive fungal infection. The thrust of this study was to show that this PCR assay may be used as an adjunct to conventional methods for the detection of fungal infection or describing the natural history of fungal infection. Our ultimate goal will be to evaluate the sensitivity and specificity of the assay results in predicting mold infection in larger numbers of serial specimens from immunosuppressed BMT patients.
ACKNOWLEDGMENTS
J.-A. van Burik is a recipient of a Clinician Scientist Award (K08 AI-01411) from the National Institutes of Health. All authors are supported by grants CA-18029 and CA-15704 from the National Institutes of Health. The Biocomputing Shared Resource at Fred Hutchinson Cancer Research Center is supported by P30 CA-15704.
We thank Pfizer Inc. (New York, New York) for an unrestricted research grant to support this study.
REFERENCES
- 1.Armitage J O. Bone marrow transplantation. N Engl J Med. 1994;330:827–838. doi: 10.1056/NEJM199403243301206. [DOI] [PubMed] [Google Scholar]
- 2.Beck-Sague C M, Jarvis W R the National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 3.Boleda M D, Briones P, Farrás J, Tyfield L, Pi R. Experimental design: a useful tool for PCR optimization. BioTechniques. 1996;21:134–140. doi: 10.2144/96211rr05. [DOI] [PubMed] [Google Scholar]
- 4.Bowman B H, Taylor J W, Brownlee A G, Lee J, Lu S-D, White T J. Molecular evolution of the fungi: relationship of the Basidiomycetes, Ascomycetes, and Chytridiomycetes. Mol Biol Evol. 1992;9:285–296. doi: 10.1093/oxfordjournals.molbev.a040720. [DOI] [PubMed] [Google Scholar]
- 5.Bretagne S, Costa J-M, Marmorat-Khuong A, Poron F, Cordonnier C, Vidaud M, Fleury-Feith J. Detection of Aspergillus species DNA in bronchoalveolar lavage samples by competitive PCR. J Clin Microbiol. 1995;33:1164–1168. doi: 10.1128/jcm.33.5.1164-1168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchman T G, Rossier M, Merz W G, Charache P. Detection of surgical pathogens by in vitro DNA amplification. Part I. Rapid identification of Candida albicans by in vitro amplification of a fungus-specific gene. Surgery. 1990;108:338–347. [PubMed] [Google Scholar]
- 7.Burgener-Kairuz P, Zuber J-P, Jaunin P, Buchman T G, Bille J, Rossier M. Rapid detection and identification of Candida albicans and Torulopsis (Candida) glabrata in clinical specimens by species-specific nested PCR amplification of a cytochrome P-450 lanosterol-α-demethylase (L1A1) gene fragment. J Clin Microbiol. 1994;32:1902–1907. doi: 10.1128/jcm.32.8.1902-1907.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chryssanthou E, Andersson B, Petrini B, Löfdahl S, Tollemar J. Detection of Candida albicans DNA in serum by polymerase chain reaction. Scand J Infect Dis. 1994;26:479–485. doi: 10.3109/00365549409008623. [DOI] [PubMed] [Google Scholar]
- 9.Couroux P R, Hussain Z, Rutledge F, Lannigan R, Ralph E D, Nancekivell B, Austin T W. Abstracts of the 96th General Meeting of the American Society of Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Polymerase chain reaction based method for the investigation of deep seated candidiasis, abstr. C-216; p. 39. [Google Scholar]
- 10.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duthie R, Denning D W. Aspergillus fungemia: report of two cases and review. Clin Infect Dis. 1995;20:598–605. doi: 10.1093/clinids/20.3.598. [DOI] [PubMed] [Google Scholar]
- 12.Einsele H, Hebart H, Roller G, Loeffler J, Rothenhoefer I, Mueller C, Bowden R, van Burik J, Engelhard D, Kanz L, Schumacher U. Detection and identification of fungal pathogens in blood by using molecular probes. J Clin Microbiol. 1997;35:1353–1360. doi: 10.1128/jcm.35.6.1353-1360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finckh U, Lingenfelter P A, Henne K W, Schmidt C A, Siegert W, Myerson D. In vitro amplification and digoxigenin labeling of single-stranded and double-stranded DNA probes for diagnostic in situ hybridization. In: Rolfs A, Schuller I, Finckh U, Weber-Rolfs I, editors. PCR: clinical diagnostics and research. Berlin, Germany: Springer-Verlag; 1992. pp. A37–A43. [Google Scholar]
- 14.Finckh U, Lingenfelter P A, Myerson D. Producing single stranded DNA probes with the Taq DNA polymerase: a high yield protocol. BioTechniques. 1991;10:35–39. [PubMed] [Google Scholar]
- 15.Fujita S, Lasker B A, Lott T J, Reiss E, Morrison C J. Microtitration plate enzyme immunoassay to detect PCR-amplified DNA from Candida species in blood. J Clin Microbiol. 1995;33:962–967. doi: 10.1128/jcm.33.4.962-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamis A, Gudnason T, Giebink G, Ramsay N. Disseminated infection with Fusarium in recipients of bone marrow transplants. Rev Infect Dis. 1991;13:1077–1088. doi: 10.1093/clinids/13.6.1077. [DOI] [PubMed] [Google Scholar]
- 17.Goodman J L, Winston D J, Greenfield R A, Chandrasekar P H, Fox B, Kaizer H, Shadduck R K, Shea T C, Stiff P, Friedman D J, Powderly W G, Silber J L, Horowitz H, Lichtin A, Wolff S N, Mangan K F, Silver S M, Weisdorf D, Ho W G, Gilbert G, Buell D. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845–851. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- 18.Goodrich J M, Reed E C, Mori M, Fisher L D, Skerrett S, Dandliker P S, Klis B, Counts G W, Meyers J D. Clinical features and analysis of risk factors for invasive candidal infection after marrow transplantation. J Infect Dis. 1991;164:731–740. doi: 10.1093/infdis/164.4.731. [DOI] [PubMed] [Google Scholar]
- 19.Holmes A R, Cannon R D, Shepherd M G, Jenkinson H F. Detection of Candida albicans and other yeasts in blood by PCR. J Clin Microbiol. 1994;32:228–231. doi: 10.1128/jcm.32.1.228-231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurst S F, McLaughlin D W, Reyes G, Reiss E, Morrison C J. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Development of an inhibition EIA to detect galactomannanemia/uria: comparison to the pastorex Aspergillus latex agglutination test (PALA) for the diagnosis of invasive aspergillosis, abstr. D43; p. 154. [Google Scholar]
- 21.Iwen P C, Reed E C, Armitage J O, Bierman P J, Kessinger A, Vose J M, Arneson M A, Winfield B A, Woods G L. Nosocomial invasive aspergillosis in lymphoma patients treated with bone marrow or peripheral stem cell transplants. Infect Control Hosp Epidemiol. 1993;14:131–139. doi: 10.1086/646698. [DOI] [PubMed] [Google Scholar]
- 22.Jordan J A. PCR identification of four medically important Candida species by using a single primer pair. J Clin Microbiol. 1994;32:2962–2967. doi: 10.1128/jcm.32.12.2962-2967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahn F W, Jones J M, England D M. The role of bronchoalveolar lavage in the diagnosis of invasive pulmonary Aspergillus. Am J Clin Pathol. 1986;86:518–523. doi: 10.1093/ajcp/86.4.518. [DOI] [PubMed] [Google Scholar]
- 24.Kan V L. Polymerase chain reaction for the diagnosis of candidemia. J Infect Dis. 1993;168:779–783. doi: 10.1093/infdis/168.3.779. [DOI] [PubMed] [Google Scholar]
- 25.Kappe R, Fauser C, Okeke C N, Maiwald M. Universal fungus-specific primer systems and group-specific hybridization oligonucleotides for 18S rDNA. Mycoses. 1996;39:1–6. doi: 10.1111/j.1439-0507.1996.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura K, Kaneko T, Yamamoto Y. Lysis of viable yeast cells by enzymes of Arthrobacter luteus. I. Isolation of lytic strain and studies on its lytic activity. J Gen Appl Microbiol. 1972;18:57–71. [Google Scholar]
- 27.Kitamura K, Kaneko T, Yamamoto Y. Lysis of viable yeast cells by enzymes of Arthrobacter luteus. II. Purification and properties of an enzyme, zymolyase, which lyses viable yeast cells. J Gen Appl Microbiol. 1974;20:323–344. [Google Scholar]
- 28.Kwok S, Higuchi R. Avoiding false positives with PCR. Nature. 1989;339:237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- 29.Makimura K, Murayama S Y, Yamaguchi H. Detection of a wide range of medically important fungi by the polymerase chain reaction. J Med Microbiol. 1994;40:358–364. doi: 10.1099/00222615-40-5-358. [DOI] [PubMed] [Google Scholar]
- 30.Medlin L, Elwood H J, Stickel S, Sogin M L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 31.Melchers W J G, Verweij P E, van den Hurk P, van Belkum A, De Pauw B E, Hoogkamp-Korstanje J A A, Meis J F G M. General primer-mediated PCR for detection of Aspergillus species. J Clin Microbiol. 1994;32:1710–1717. doi: 10.1128/jcm.32.7.1710-1717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyers, J. D. 1990. Fungal infections in bone marrow transplant patients. Semin. Oncol. 17(Suppl. 6):10–13. [PubMed]
- 33.Miyakawa Y, Mabuchi T, Fukazawa Y. New method for detection of Candida albicans in human blood by polymerase chain reaction. J Clin Microbiol. 1993;31:3344–3347. doi: 10.1128/jcm.31.12.3344-3347.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison C J, McLaughlin D W, Reiss E. Program and abstracts of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1993. Improved EIA detection of mannanuria and mannanemia for the diagnosis of disseminated candidiasis using serotype A and B reagents, abstr. 1106; p. 321. [Google Scholar]
- 35.Morrison V A, Haake R J, Weisdorf D J. Non-Candida fungal infections after bone marrow transplantation: risk factors and outcome. Am J Med. 1994;96:497–503. doi: 10.1016/0002-9343(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 36.Morrison V A, McGlave P B. Mucormycosis in the BMT population. Bone Marrow Transplant. 1993;11:383. [PubMed] [Google Scholar]
- 37.Pringle A, Forsdyke J, Rose A. Scanning electron microscope study of Saccharomyces cerevisiae spheroplast formation. J Bacteriol. 1979;140:289–293. doi: 10.1128/jb.140.1.289-293.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy L V, Kumar A, Kurup V P. Specific amplification of Aspergillus fumigatus DNA by polymerase chain reaction. Mol Cell Probes. 1993;7:121–126. doi: 10.1006/mcpr.1993.1016. [DOI] [PubMed] [Google Scholar]
- 39.Rousey S, Russler S, Gottlieb M, Ash R. Low-dose amphotericin B prophylaxis against invasive Aspergillus infections in allogeneic marrow transplantation. Am J Med. 1991;91:484–492. doi: 10.1016/0002-9343(91)90184-y. [DOI] [PubMed] [Google Scholar]
- 40.Sandhu G S, Kline B C, Stockman L, Roberts G D. Molecular probes for diagnosis of fungal infections. J Clin Microbiol. 1995;33:2913–2919. doi: 10.1128/jcm.33.11.2913-2919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saugier-Veber P, Devergie A, Sulahian A, Ribaud P, Traore F E. Epidemiology and diagnosis of invasive pulmonary aspergillosis in bone marrow transplant patients: results of a 5 year retrospective study. Bone Marrow Transplant. 1993;12:121–124. [PubMed] [Google Scholar]
- 42.Scott J, Schekman R. Lyticase: endoglucanase and protease activities that act together in yeast cell lysis. J Bacteriol. 1980;142:414–423. doi: 10.1128/jb.142.2.414-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slavin M A, Osborne B, Adams R, Levenstein M J, Schoch H G, Feldman A R, Meyers J D, Bowden R A. Efficacy and safety of fluconazole for fungal infections after marrow transplant—a prospective, randomized, double-blind study. J Infect Dis. 1995;171:1545–1552. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 44.Southern E. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 45.Spreadbury C, Holden D, Aufauvre-Brown A, Bainbridge B, Cohen J. Detection of Aspergillus fumigatus by polymerase chain reaction. J Clin Microbiol. 1993;31:615–621. doi: 10.1128/jcm.31.3.615-621.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thaler M, Pastakia B, Shawker T H, O’Leary T, Pizzo P A. Hepatic candidiasis in cancer patients: the evolving picture of the syndrome. Ann Intern Med. 1988;108:88–100. doi: 10.7326/0003-4819-108-1-88. [DOI] [PubMed] [Google Scholar]
- 47.Tollemar J, Ringden O, Bostrom L, Nilsson B, Sundberg B. Variables predicting deep fungal infections in bone marrow transplant recipients. Bone Marrow Transplant. 1989;4:635–641. [PubMed] [Google Scholar]
- 48.van Deventer A J M, Goessens W H F, van Belkum H J A, van Vliet E W M, Verbrugh H A. Improved detection of Candida albicans by PCR in blood of neutropenic mice with systemic candidiasis. J Clin Microbiol. 1995;33:625–628. doi: 10.1128/jcm.33.3.625-628.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wald A, Leisenring W, van Burik J, Bowden R A. Natural history of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–1466. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- 50.Wingard J R. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin Infect Dis. 1995;20:115–125. doi: 10.1093/clinids/20.1.115. [DOI] [PubMed] [Google Scholar]
- 51.Wingard J R, Merz W G, Rinaldi M G, Johnson T R, Karp J E, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991;324:1274–1277. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]
- 52.Wingard J R, Merz W G, Saral R. Candida tropicalis: a major pathogen in immunocompromised patients. Ann Intern Med. 1979;91:539–543. doi: 10.7326/0003-4819-91-4-539. [DOI] [PubMed] [Google Scholar]
- 53.Young R, Bennett J. Invasive aspergillosis. Absence of detectable antibody response. Am Rev Respir Dis. 1971;104:710–716. doi: 10.1164/arrd.1971.104.5.710. [DOI] [PubMed] [Google Scholar]