Figure 2.
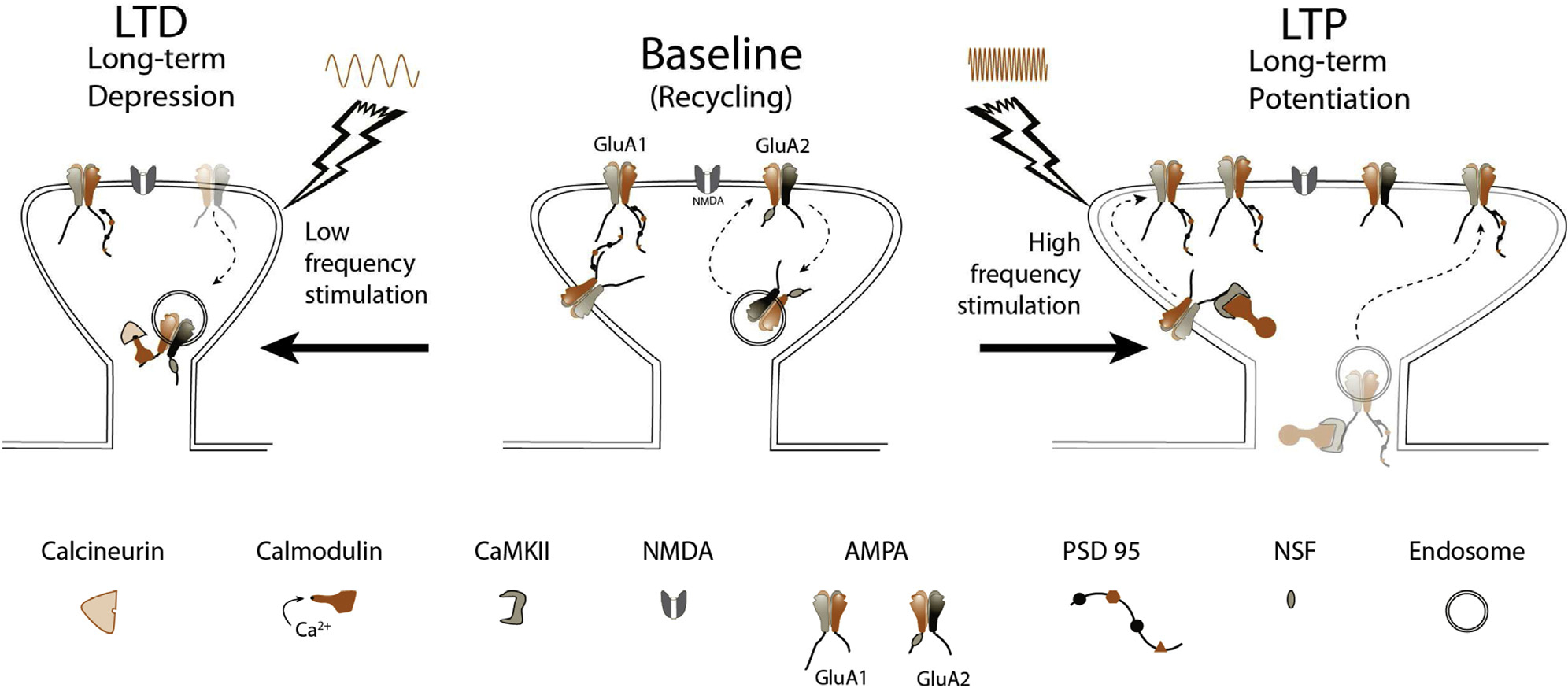
Synaptic plasticity. Center: At baseline, synaptic NMDA and AMPA receptor numbers are stable, with GluA2-subtype AMPA receptors recycling constitutively, dependent on NSF. Left: Following low-frequency stimulation, chronic low levels of calcium enter through the NMDA receptor and bind calmodulin. Calcineurin has the highest affinity for Ca2+/CaM, beating out CaMKII, and causing dephosphorylation of the AMPA receptor, and subsequent removal from the synapse. The result is a smaller spine and decreased synaptic strength. Right: High-frequency stimulation causes AMPA receptor depolarization, consequent ejection of the Mg2+ plug, and opening of the Ca2+-permeable NMDA receptor channel. A flood of Ca2+ leads to acutely elevated concentrations of Ca2+/CaM complexes, allowing interactions with CaMKII to dominate, ultimately causing phosphorylation and delivery of GluA1 to the synapse through translocation along the membrane (initial 20 minutes), or direct exocytosis and insertion in the synapse. Scaffolding proteins like PSD-95 accompany this insertion. Spine size grows, and synaptic strength is increased.
