Abstract
Conventional therapies for acute myeloid leukemia (AML) are characterized by high rates of relapse, severe toxicities, and poor overall survival rates. Thus, the development of new therapeutic strategies is crucial for improving the survival and quality of life of AML patients. CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapy has been extremely successful in the treatment of B-cell acute lymphoid leukemia and several mature B-cell lymphomas. However, the use of CAR T-cell therapy for AML is currently prevented due to the lack of a myeloid equivalent to CD19, as currently known cell surface targets on leukemic blasts are also expressed on healthy hematopoietic stem and progenitor cells as well as their progeny. In addition, the immunosuppressive tumor microenvironment has a dampening effect on the antitumor activity of CAR-T cells. Here, we review the therapeutic challenges limiting the use of CAR T-cell therapy for AML and discuss promising novel strategies to overcome them.
INTRODUCTION
The principles of modern therapy for acute myeloid leukemia (AML) were established in the 1970s with the introduction of multimodal chemotherapeutic regimens involving the combination of cytarabine with short infusions of an anthracycline.1,2 Currently, patients are subjected to complex and risk-adapted protocols.3 In addition, in a considerable portion of patients, the only potentially curative treatment available is allogeneic hematopoietic stem cell transplantation (HSCT), which is fraught by high levels of short- and long-term toxicities. With advances in supportive care and risk stratification as well as refinements in the delivery of HSCT, treatment outcomes have gradually improved over the past 5 decades. Nevertheless, AML remains difficult to cure. The resistance of leukemic stem cells to chemotherapy remains a major problem and hampers the achievement of long-term remission.4,5 Thus, the need for new, effective therapeutic strategies that complement or replace existing ones remains undisputed.
Of immense current interest to accomplish this goal is immunotherapy, that is, stimulating the immune system to scavenge and attack cancer cells, including chimeric antigen receptor (CAR) T-cell therapy. Much of the enthusiasm for exploiting CAR-T cells in AML originates from the excellent efficacy and safety demonstrated in the treatment of B-cell acute lymphoblastic leukemia using CD19-directed CAR-T cells, without the risk of associated graft-versus-host disease associated with allogeneic HSCT.6,7 How best to translate the success of CAR T-cell therapy for B-cell malignancies to AML is a matter of intense investigation. Targeting lymphoid tumors with CD19-targeted CAR-T cells is effective and clinically applicable because of the near ubiquitous cell surface expression of CD19 on malignant B cells and, importantly, B cell aplasia and the resultant hypogammaglobulinemia are clinically benign and can be managed with infusions of intravenous immunoglobin. A similar target has not yet been identified in AML due to overlapping antigen expression between malignant and normal myeloid cells; resulting in myeloablation, which may be intolerable because of neutropenic infections and bleeding complications.8,9 In this review, we highlight the limitations in clinical translation as well as the most recent strategies to overcome these barriers, paving the way for CAR T-cell therapy as a safe and viable option for the treatment of AML patients.
CAR T-CELL THERAPY
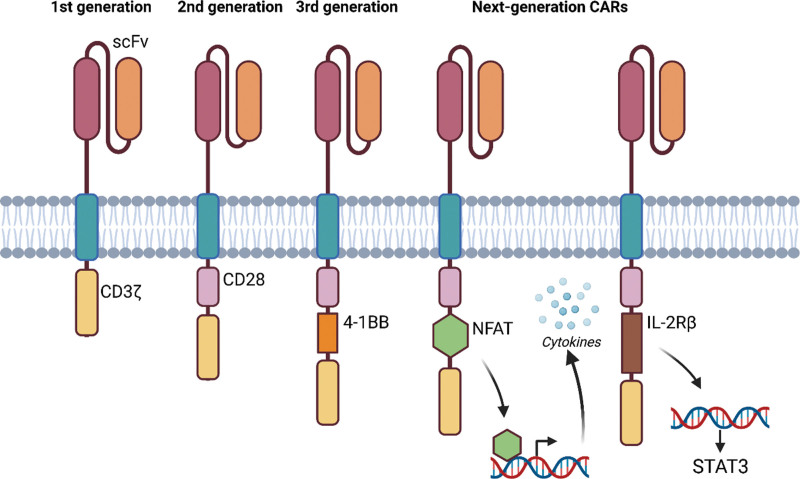
CAR T-cell therapy is a type of cellular immunotherapy that aims to redirect the cytotoxic activity of T lymphocytes toward specific antigens of cancer cells in a major histocompatibility complex-independent manner.10 Initially, CAR-T cells consisted of an antibody-derived single-chain variable fragment (scFv) extracellular antigen recognition domain linked to an intracellular CD3ζ signaling domain (Figure 1). These first-generation CAR-T cells showed inadequate therapeutic effects in early clinical trials mainly due to insufficient activation, expansion, and persistence of the modified T cells.11 The development of second-generation CAR-T cells, which included an additional costimulatory domain such as 4-1BB or CD28,12,13 solved many of the latter issues and pushed the clinical development of CAR T-cell therapy. Various CAR-T constructs have subsequently been developed in order to address limitations in efficacy, persistence and excessive toxicity, many of which are currently under evaluation in clinical trials. Third-generation CAR-T constructs harboring an additional costimulatory domain are under investigation (NCT01853631, NCT02132624, and NCT04014881).14 Better efficacy and persistence of CARs stimulated the development of other groups of next-generation CARs focusing on limiting toxicity. CARs were developed where a nuclear factor of activated T-cells (NFAT)-responsive promotor induces tumor-killing cytokine production upon activation (NCT01236573 and NCT03542799)14 or CAR-T cell clearance with specific agents such as Rituximab or rather allowing selective activation using rapamycin, EGFRt and others (eg, NCT03114670, NCT02159495, NCT04097301, NCT05105152, and NCT02028455). Finally, also genetically edited CAR-T cells for allogeneic use are being explored in clinical trials (NCT04106076 and NCT03190278), as well as CARs incorporating a truncated cytoplasmic domain of IL-2Rβ and STAT3 binding motif, inducing JAK-STAT pathway activation, which promotes their proliferation and prevents terminal differentiation. Several of these next-generation CARs will be discussed in the section below. For an extensive discussion about the optimization of chimeric constructs, we refer the reader to several excellent review articles published recently.15,16
Figure 1.
Design of the 4 generations CARs. CARs are composed of scFv, a transmembrane domain, and an intracellular signaling domain. The first-generation CARs only had an intracellular CD3ζ signaling domain (yellow). The second-generation CARs included an additional costimulatory domain, for example, CD28 (purple). The third-generation CARs added 2 costimulatory domains in the CAR design, for example, CD28 and 4-1BB (orange). The next-generation CARs introduced for example a transcription factor, for example, NFAT (green), for the production of tumor-killing cytokines upon CAR activation or added the intracellular domains of cytokine receptor for interleukin 2 (IL-2Rβ) (brown) with a binding site for the transcription factor STAT3. Created with BioRender.com. CARs = chimeric antigen receptors; NFAT = nuclear factor of activated T cells; scFvs = single-chain variable fragments.
The identification of a suitable target antigen is critical for the success of CAR T-cell immunotherapy. Ideally, the target has a high-density expression on all malignant cells, including underlying cancer stem cells and progenitor cells, and no or low expression on healthy tissues or expression only on nonvital tissues. Furthermore, the target antigen should be expressed homogeneously in the target population and should play a critical role in the differentiation and proliferation of malignant cells. Importantly, the antigen should not be expressed on activated T cells to obviate fratricide elimination, and it should have a low propensity for antigen downregulation to alleviate the risk of immune escape. Several AML CAR-T cell products are in the pipeline, and ongoing early clinical studies are exploring many AML-specific antigens (eg, CD33, CD123, CLL-1, CD70, and TIM-3) which have been discussed in depth by Schorr and Perna.17 Recently, a systematic review and meta-analysis addressed the feasibility and safety of CAR-T cells for AML; however, efficacy was limited compared to CD19 CAR-T cell therapy for ALL.18 Specific challenges limiting the current broader implementation of CAR T-cell therapy to treat AML as well as novel strategies to overcome them are discussed in detail below.
CHALLENGES AND NOVEL STRATEGIES
Safety
The clinical use of CAR T-cell therapy is limited by the associated toxicities of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). CRS is a systemic inflammatory response syndrome that presents with a range of clinical manifestations, from mild symptoms to severe multiorgan failure.19,20 It usually coincides with the peak of CAR T-cell expansion and cytokine production. The ICANS clinical spectrum ranges from reversible neurocognitive dysfunction to severe neurological disturbances, such as seizures and coma, and to rare but extremely serious cerebral edema.19,21 The onset of ICANS has been observed to occur between 2 days and 4 weeks after CAR T-cell infusion, and it often happens concomitantly with CRS. The molecular mechanisms driving CRS and ICANS are becoming clearer and point to an important role of the main proinflammatory cytokines, such as IL-1 and IL-6, which are primarily released by monocytes and macrophages.22,23 The risk factors for the development of these toxicities include the infusion of a high number of CAR-T cells as well as patient-related factors such as endothelial activation, preexisting thrombocytopenia, or neurological comorbidities. Nevertheless, it would be interesting to uncover molecular (eg, liquid biopsy) markers predicting the risk of CRS and/or ICANS.24 Models evaluating combinations of cytokine levels (eg, interferon gamma, IL-13, and macrophage inflammatory protein 1 alpha concentrations within 72 h of infusion) or cytokine levels and fever have shown promising results; however, they are often difficult to implement as measuring several of these cytokines is not readily available in many hospitals.25
The current guidelines for the management of CRS and ICANS vary between clinics but are typically comprised of supportive care and treatment with corticosteroids or tocilizumab, depending on the severity of the symptoms. Although early intervention with tocilizumab and dexamethasone has been shown to decrease the rate of severe CRS,26 it does not seem to have an impact on the incidence of neurotoxicity.27 Thus, acquiring control over the CAR-T cells once they are administered should be the main focus. Several promising strategies to do so include biodegradable CARs (mRNA electroporation), antibody-mediated depletion of CARs, CARs containing a suicide switch, and drug-inducible on/off switches.
mRNA electroporation
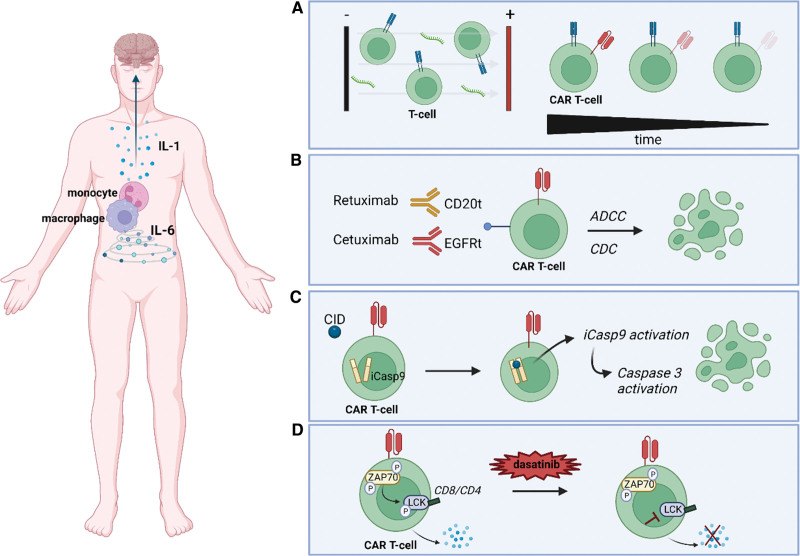
The nonviral transduction method of mRNA electroporation results in CAR-T cells with a self-limiting lifespan, thus avoiding the risk of persistent toxicity (Figure 2A). Various studies performed in vitro and in vivo have tested the safety of CAR T-cell therapy using mRNA electroporation. In 2017, an early phase I clinical trial was conducted to test the feasibility and short-term toxicity of serial infusions of anti-CD123 CAR-T cells manufactured via mRNA electroporation in patients with relapsed/refractory AML. This study determined the biological effect of these CAR-T cells as manifested by the presence of fever and CRS. However, no antitumor activity was demonstrated due to the lack of persistence of CAR-T cells (NCT02623582).28 Multiple infusions of mRNA-electroporated CAR-T cells are likely required to achieve remission, though this could potentially be compromised by the presence of anti-CAR antibodies, as illustrated in a clinical trial by Maus et al (NCT01355965).29 In this trial, 4 patients were treated with 3 time-separated infusion periods of a CAR-T product with the scFv region derived from a mouse antibody. The investigators clearly observed elevated levels of human anti-mouse IgG, which increased with exposure to anti-mesothelin mRNA CAR-T cells, and the occurrence of one period of anaphylaxis with highly elevated tryptase levels in one of the study subjects.
Figure 2.
Strategies to acquire control over the cytokine release of CAR-T cells. (A) mRNA electroporation, a nonviral transduction method resulting in CAR-T cells with transient activity to limit the risk of persistent toxicity. (B) ADCC or CDC of CAR-T cells containing a truncated CD20 (CD20t) or EGFRt molecule. (C) Suicide gene system consisting of iCasp9 activation after the administration of a CID, which will dimerize caspase 9 and subsequently activate the downstream executioner caspase 3, resulting in apoptosis of the CAR-T cell. (D) A temporary switch-off system using dasatinib to inhibit the phosphorylation of LCK, a key regulator for T-cell signaling. Created with BioRender.com. ADCC = antibody-dependent cellular cytotoxicity; CAR = chimeric antigen receptor; CDC = complement-dependent cytotoxicity; CID = chemical inducer of dimerization; EGFRt = epidermal growth factor receptor; iCasp9 = inducible caspase 9; LCK = lymphocyte-specific protein tyrosine kinase.
Permanent and temporary CAR-T inactivation strategies
In addition to limiting the half-life of the CARs using nonviral transfection methods, active elimination and active on/off switching options can be incorporated within the CAR design. A first option is the use of antibody-mediated depletion of CARs through antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. In the event of uncontrolled toxicity, genetically engineered CAR-T cells co-expressing truncated cell-surface protein can also be eliminated by clinically approved monoclonal antibodies (mAbs) (Figure 2B). A truncated CD20 molecule is often used to this end since it can be targeted by the well-characterized and approved mAb rituximab.30 To allow visualization of transduced T cells and efficient selection during generation, Philip et al developed a highly compact epitope, that is, RQR8, with a dual role. It contains a small portion of the CD34 molecule, allowing selection of transduced cells using an anti-CD34 mAb, and a portion of the CD20 molecule, which can be targeted by the mAb rituximab, thus provoking the suicide of T cells.31 This selection/safeguarding system has been successfully employed in preclinical studies developing anti-CD117 CAR-T cells.32 In addition to CD20, a truncated version of epidermal growth factor receptor (EGFR) has been used to target CAR-T cells. A preclinical study by Wang et al developed a truncated human EGFR (HuEGFRt) that is devoid of extracellular N-terminal ligand binding domains and intracellular receptor tyrosine kinase activity but retains the native amino acid sequence and an intact binding site for cetuximab, an antibody that targets extracellular domain III of EGFR, to investigate CAR-T cell ablation upon the administration of the commercially available anti-EGFR mAb cetuximab both in vitro and in vivo.33 This study showed that >50% of HuEGFRt+ CAR-T cells were eliminated in vitro within 1 hour of cetuximab administration and that these lymphocytes were depleted in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice at 4–6 days after daily intraperitoneal injection of 1 mg of cetuximab. The HuEGFRt-cetuximab suicide CAR product is under evaluation in several phase I clinical trials (NCT03114670, NCT02159495).
Similarly, suicide switches making use of intracellular signaling also have been developed. The herpes simplex virus-thymidine kinase (HSV-tk) suicide gene system and the activating antiviral agent ganciclovir have long been used as a method to eliminate transduced cells in the case of adverse events. Although this system has not been tried to treat AML to date, it has been used and demonstrated to be safe in preclinical models of lung and ovarian cancer.34 However, there are many drawbacks associated with this HSV-tk system. First, it can take up to 3 days to achieve a complete effect. Second, the highly immunogenic characteristics of the transduced cells can cause them to be rejected by the host’s immune system. Finally, ganciclovir is also used for the treatment of cytomegalovirus infections, meaning that the administration of this therapeutic agent inadvertently leads to activation of the suicide gene and the subsequent elimination of the transduced cells. An alternative suicide gene system is inducible caspase 9, which consists of a drug–dimer binding domain derived from the human FK506 binding protein fused to the intracellular part of the proapoptotic human caspase 9 protein (Figure 2C). The administration of the chemical inducer of dimerization drug AP1903 then causes cross-linking of the drug-binding domains, which in turn dimerizes caspase 9 and subsequently activates the downstream executioner caspase 3, resulting in cellular apoptosis.35 Warda et al have illustrated that this system would allow safe clinical trials targeting IL-1RAP-directed CAR-T cells in the treatment of AML.36
The rescue strategies discussed above are effective at controlling CAR-T cell-mediated toxicity; however, they also lead to the irreversible loss of CAR-T cells and their therapeutic potential. Since CAR-T cell-induced toxicity is frequently of a transient nature, it may suffice to place the modified T cells in a temporarily functional off-phase and revive them once the side effects have passed. In that respect, interfering with downstream T-cell receptor (TCR) signaling seems to be a logical way forward. Dasatinib, developed to inhibit the BCR::ABL fusion oncogene, is known to block the adenosine triphosphate binding sites of the lymphocyte-specific protein tyrosine kinase (LCK), which is a key regulator of initiation of TCR signaling, T-cell development, and T-cell homeostasis.37 Dasatinib interferes with the phosphorylation of LCK, thus inhibiting the phosphorylation of CD3-ζ and ZAP70 and subsequently limiting the induction of nuclear factor of activated T cells (Figure 2D). An in vitro study by Mestermann et al showed that dasatinib is able to pause CD4+ and CD8+ CAR-T cells in a stable functional off-state, even when encountering target cells.38 The administered dose could be titrated to achieve partial or complete inhibition of CAR-T cell function. The functional off-state did not affect the viability of CAR-T cells, and the removal of dasatinib rapidly unleashed the cells from their inactive state. It also has been demonstrated that dasatinib can be used as a pharmacological control switch in vivo. To this end, SCID/beige mice were injected with Raji lymphoma cells and treated with anti-CD19 CAR-T cells, which resulted in acute CRS. The administration of dasatinib at 3 hours after CAR-T cell infusion caused a significant decrease in cytokine levels; meanwhile, 48 hours later, 70% of tyrosine kinase inhibitor-treated mice were still alive as opposed to only 25% of the control mice.38 Remarkably, Zhang et al have revealed that pretreatment of CD19-directed CAR-T cells during their production process by dasatinib controls excessive activation and potential exhaustion/differentiation, which sometimes lead to loss of early CAR-T cells and tumor recurrence.39 However, no AML-specific preclinical data have been obtained to illustrate the potential of this approach.
In addition to strategies focused on adding drugs to inactivate CAR-T cells, Leung and colleagues40 developed dimerizing agent regulated immunoreceptor complex (DARIC) CARs, a split-receptor design segregating the antigen-binding and intracellular signaling subunits into 2 membrane-tethered polypeptides that dimerize in the presence of rapamycin. The antigen recognition subunit contains an the scFv fused to the FK506-binding protein and a CD4 transmembrane domain. The physically separated signaling subunit contains the FKBP-rapamycin binding domain from the human mTOR complex fused to the CD8α transmembrane domain, followed by the cytoplasmic signaling domains of 4-1BB and CD3ζ. This strategy has been shown to successfully control disease in xenograft models, using CD33 as target41 and, moreover, has illustrated that reactivation of CARs is possible following extended periods of rapamycin drug cessation. Temporal control provided by the DARIC architecture promises to enhance safety and potentially efficacy of CAR-T therapy for AML, for example by enabling hematopoietic recovery or providing T-cell rest. Currently, this project is under investigation in a clinical trial NCT05105152.42
ON-TARGET/OFF-TUMOR TOXICITY
On-target/off-tumor toxicity entails normal tissue devastation mediated by targeting antigens with shared expression on both tumor cells and healthy cells. Considering the potency of engineered T cells, toxicity on nonpathogenic tissues expressing low levels of the antigen can be detrimental. Potential strategies to avoid fatal side effects include neoantigen targeting, Boolean logic-gated CAR, affinity modulation, and a combination of CAR therapies with allogeneic HSCT using HSCs edited out for the CAR target antigen.
Neoantigen targeting
One way to avoid on-target/off-tumor toxicity is by broadening the spectrum of targetable antigens with neoantigens, which are generated by mutations in tumor cells and consequentially are only expressed in tumor cells (Figure 3A). Neoantigens are technically self-antigens, but the immune system can still be exploited to target them because of the absence of tolerance to these new extremely immunogenic mutant epitopes.43 Unfortunately, compared to other types of tumors such as malignant melanoma or non-small cell lung cancer, AML is amongst those malignancies with the lowest mutational burden, with a consequentially lower number of neoepitopes to be exploited for therapeutic targeting. Nonetheless, a few neoantigens have been described in AML.43 However, the expression of various neoantigens is most often restricted to the intracellular portion of the leukemic cell, thus making it impossible for classical CAR-T cells to target them. With the development of TCR-mimicking CARs, first toward well-known antigens such as WT1, it has become possible to attack intracellular antigens.44
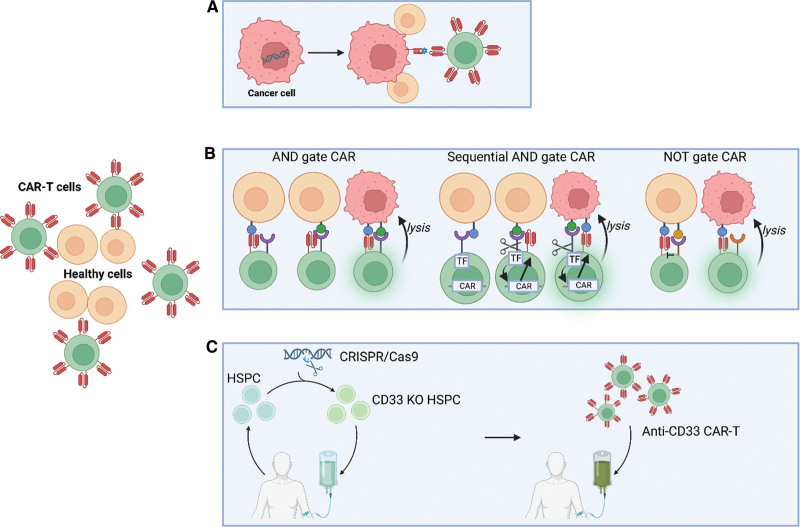
Figure 3.
Strategies to avoid on-target/off-tumor toxicity. (A) Targeting of neoantigens generated by mutations in tumor cells. (B) Boolean logic-gated CAR-T cells include AND gated CARs, sequential AND gated CARs, and NOT gated CARs. First, AND gated CARs consist of 2 separate CAR molecules of which the first provides only the activation signal and the second the necessary costimulation. Second, sequential AND gated CARs are placed under the control of an inducible promoter that can only be activated in response to signals mediated from a constitutively expressed synthetic Notch receptor (synNotch). Binding of the target antigen results in proteolytic cleavage of the Notch core region and the release of the transcription factor from the cell membrane, after which it translocates to the nucleus and drives the expression of an “effector” CAR. Third, NOT gated CARs use an inhibitory CAR (iCAR), where killing will only be obtained if target cells only engage with one of the receptors present on the CAR-T cells. C. Autologous CD33 knock-out in hematopoietic stem cell progenitors generates a hematopoietic system resistant to anti-CD33 CAR T-cell therapy. Created with BioRender.com. CAR = chimeric antigen receptor.
In AML, 2 types of neoantigens have been explored in the context of therapeutic targeting: mutation and aberrant splicing. The landmark study conducted by the Cancer Genome Atlas Research Network intensively examined the mutational composition of de novo AML and identified recurrent and significant mutations that contribute to leukemogenesis.45 Interestingly, about one-third of AML patients harbor a mutation in the nucleophosmin (NPM1) gene. A somatic mutation of exon 12 in this gene leads to a nuclear export signal and the localization of the mutant protein in the cytoplasm.46 Xie and colleagues have reported a human scFv, identified via yeast surface display, that specifically binds to the NPM1c epitope human leukocyte antigen (HLA)-A2 complex but not to HLA-A2 or to HLA-A2 loaded with control peptides. Both in vitro and in mice, CAR-T cells with the scFv exhibit potent cytotoxicity against NPM1c+HLA-A2+ leukemic cells and primary AML blasts, but not NPM1c–HLA-A2+ leukemia cells or HLA-A2– tumor cells. Therapies using NPM1c CAR-T cells for the treatment of NPM1c+HLA-A2+ AML may limit on-target/off-tumor toxicity and tumor resistance.47 Dysregulated splicing represents a second source of neoantigens if the splicing results in an alternative isoform that is distinguishable from its wild-type counterpart(s). A variant of neurogenic locus Notch homolog protein 2 (Notch2-Va) and a variant of Fms-related receptor tyrosine kinase 3 (FLT3-Va) can be found on the cell surface of AML blasts. DNA fragment analysis showed that Notch-Va and FLT3-Va were present in 73% and 50% of AML patients, respectively.48 However, none of these variants have been employed in the design of CAR-T preclinical products.
Boolean logic-gated CAR-T cells
Boolean logic-gated CAR-T cells are yet another method to avoid on-target/off-tumor toxicity. Several options are available, including AND-, OR-, and NOT-gated CARs, which are explained in more detail below and illustrated in Figure 3B. Using AND-gated CARs, a certain combination of antigens is required for CAR activation. Often, this dual CAR design consists of 2 separate CAR molecules with specificity for different antigens: the first CAR provides only the activation signal (the same as first-generation CARs) and the other CAR provides costimulation (similar to second-generation CARs but without an activating domain).49 As a result, optimal T-cell activation can only be achieved by the simultaneous recognition of 2 different antigens on the tumor cells. This strategy has been successfully employed in preclinical mouse models, which illustrate prolonged survival times for AML-bearing mice treated with AND-gated CARs targeting CLL1 and CD33 or AND-gated CARs targeting CLL1 and CD123.50 Unfortunately, a major drawback of these designs is the increased possibility for tumor escape due to the heterogeneity of antigen expression in AML. In addition, with the mentioned AND design, the possibility of generating a sufficient activation signal upon engagement of one antigen on normal tissues remains a concern. To overcome this, AND-gated CARs using sequential signaling have been developed. A second-generation CAR has been placed under the control of an inducible promoter that can only be activated in response to signals mediated from a constitutively expressed synthetic Notch receptor (synNotch).51,52 These synNotch receptors use the regulatory core region of the wild-type Notch receptor to link the extracellular antigen binding domain to an intracellular transcription factor. Binding of the target antigen results in proteolytic cleavage of the Notch core region and the release of the transcription factor from the cell membrane, after which it translocates to the nucleus and drives the expression of an “effector” CAR. This second-generation CAR is capable of driving T-cell activation and cell lysis in response to target cells expressing the appropriate target antigen.
Discrimination between malignant and healthy cells can also be achieved through a NOT-gated CAR approach using an inhibitory CAR (iCAR), whereby killing will only be obtained if target cells only engage with one of the receptors present on the CAR-T cells. The major hurdle in developing NOT-gated CARs is the identification of a target antigen that is abundantly expressed on healthy tissues yet absent on tumor cells. Recently, Richards and colleagues have illustrated the potential of this system by developing NOT-gated CD93+ CAR-T cells targeting AML cells.53 CD93 is a well-known molecule expressed in a fraction of AML patients; unfortunately, it is also expressed in endothelial cells. As a proof of principle, the authors designed human umbilical vein endothelial cells expressing CD19 and generated a CD93+CD19+ NOT-gated CAR, which was shown to attack only CD93-bearing AML cells. To identify targets that could be employed in a real-life situation, the authors performed single-cell RNA sequencing of 2 endothelial and 3 AML cell lines grown under both normal and cytokine-conditioned environments. They identified 232 candidate targets that could be employed to generated a NOT-based CAR targeting CD93, among which well-known endothelial cell surface markers, such as PECAM1 and TIE1, were identified.53 It will be interesting to see how such CARs that incorporate these targets perform in an in vivo situation.
Allogenic HSCT with HSCs edited out for the CAR target antigen
Kim et al hypothesized that a leukemia-specific antigen could be created by editing out CD33 from normal hematopoietic stem and progenitor cells (HSPCs), thereby generating a hematopoietic system resistant to CD33-targeted therapy and enabling specific targeting of AML with CAR-T cells (Figure 3C).54 Their study demonstrated that human CD34+ cells lacking CD33 (CRISPR/Cas9) differentiate and function normally in vitro and in NOD/SCID gamma mouse transplantation models. Moreover, autologous CD33-knockout (KO) HSPCs transplanted in rhesus macaques after full-body irradiation demonstrated long-term multilineage engraftment of gene-edited cells, with normal myeloid function. Finally, xenotransplantation of MOLM14 cells, an AML cell line model, in mice previously engrafted with control or CD33-KO HSPCs, revealed that upon treatment with CAR-T cells targeting CD33, rapid clearance of leukemia occurred, but only in CD33-KO HSPC-differentiated myeloid cells and HSPCs.54 These results were corroborated by 2 independent research teams.55,56 Humber et al generated cells expressing a short CD33 isoform through editing out exon 2 (targeted by the current CD33-based immunotherapeutics), a strategy expected to have fewer adverse effects compared to complete disruption of the CD33 locus.56 Most interestingly, Borot et al were able to demonstrate that the system also holds true when co-injecting leukemia cells and CD33-KO HSPCs simultaneously, mimicking a clinically relevant situation. Furthermore, they performed an allogeneic bone marrow transplant, which is potentially more practical in a clinical setting of AML.55
HETEROGENEITY AND ANTIGEN LOSS
The inter- and intrapatient genetic and phenotypic heterogeneity in AML limits the applicability of a universal CAR T-cell therapy. Additionally, targeting antigens with heterogenous expression is likely to result in relapse due to incomplete targeting and clonal selection as well as general loss of the tumor antigen, as has been illustrated in CD19+ CAR T-cell therapy.57 A potential solution is to attack multiple antigens, a strategy that entails several advantages: (1) a reduction of antigen escape-related disease persistence and relapse, (2) the ability to target low-antigen-density tumor cells that otherwise escape CAR T-cell therapy, and (3) the potential to induce more AML-specific cytotoxicity. Hazelton et al have reported the development of a multi-antigen anti-CD33/CD123/CLL1 CAR T-cell therapy. In vitro, the TanCAR-T cells behaved as an OR gated, that is, CAR-T cells can be activated in the presence of one of the AML tumor-associated antigens, and can generate antigen-specific cytolytic activity approximately equal to or greater than that of single antigen-targeting CAR-T cells.58 The feasibility, safety and efficacy of this approach is currently under clinical evaluation (NCT04010877). Similarly, Ghamari and colleagues have designed a bispecific CAR, allowing interaction with CD123 and folate receptor beta as well as better tumor control and enhanced signaling upon costimulation of both receptors.59 Also, He and colleagues developed CAR-T cells bispecific for CD13 and TIM3, both upregulated in AML leukemia cells, and showed that they could eradicate patient-derived AML with much reduced toxicity to human bone marrow cells and peripheral myeloid cells in mouse model because of absent expression of TIM3. In addition, they also developed a system, Sequentially Tumor-Selected Antibody and Antigen Retrieval (STAR), to more rapidly identify CAR-T cell compatible nanobodies and their associated antigens which would allow more quick expansion of the available choices of CAR-T cells by targeting previously unappreciated cell surface antigens/targets to develop potent cancer immunotherapy.60
THE IMMUNOSUPPRESSIVE TUMOR MICROENVIRONMENT
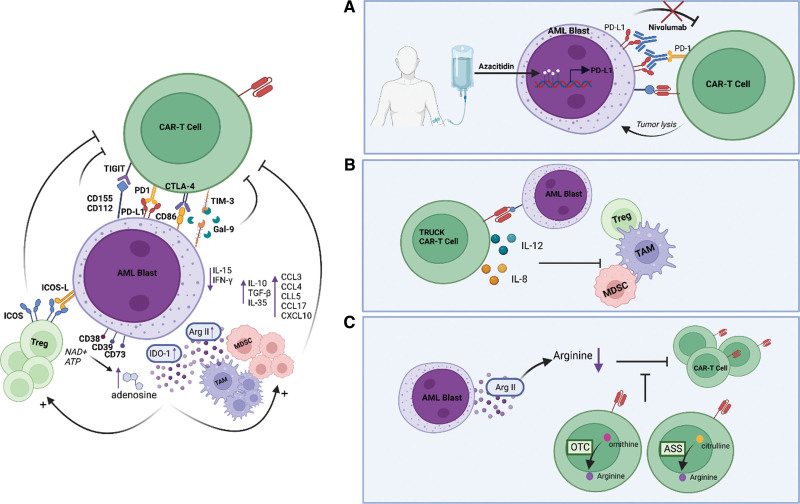
The activity of CARs against AML may also be limited by the immunosuppressive microenvironment that is created by AML. First, AML blasts can express a number of inhibitory ligands, including programmed death ligand 1 (PD-L1), B7-H3, and galectin 9.61,62 The interaction of PD-L1/PD-L2 with programmed cell death protein 1 (PD-1) on T cells initiates an intracellular signaling cascade that inhibits T-cell activation. In addition, PD-1 can be expressed on regulatory T cells (Tregs) attracted to the tumor microenvironment and can provide an activation signal for these immunosuppressive cells.63 Azacytidine, a hypomethylating agent used in front-line therapy for elderly patients with AML and higher-risk myelodysplastic syndromes who are often not eligible for HSCT, has been shown to dampen antitumor immunity by the upregulation of PD-L1. Therefore, clinical trials are investigating the therapeutic effect of the combination of nivolumab, a PD-1 immune checkpoint inhibitor, and azacytidine in adult patients with AML (NCT03092674 and NCT02775903) (Figure 4A). The results showed that full doses of azacytidine and nivolumab are tolerable and produce an encouraging response rate, and durable responses in relapsed AML patients with poor risk features were observed.64 The relevance of inhibiting the PD1/PD-L1 axis has clearly been demonstrated in solid tumor CAR-T trials.65 In the context of AML, PD-1 knockdown in CLL-1 CAR-T cells showed in vitro a stronger antileukemia effect and fewer side effects than CLL-1 CAR-T cells without PD-1 knockdown.66 Recently, a clinical study employing PD-1 silenced anti-CLL-1 CARs, allowed rescue of 2 AML patients who had failed multiple lines of therapy, including CD38 directed CAR-T therapy.67 Although limited evidence currently, these studies indicate the possible benefit of inhibiting the PD-1/PD-L1 axis in CAR-T-based treatment of AML.
Figure 4.
Strategies to overcome the immunosuppressive tumor microenvironment. (A) Nivolumab is used to counteract the upregulated expression of PD-L1 induced by azacytidine in primary treatment. (B) TRUCKs secrete cytokines like interleukin (IL)-8 and IL-12 to stimulate inflammation and inhibit immunosuppressive cells. (C). The insertion of expression cassettes from argininosuccinate synthase, ornithine transcarbamylase, or both enzymes into CAR-T cells allows them to express arginine themselves by the metabolization of ornithine and citrulline and thus adapt to their metabolic microenvironment and enhance their antitumor activity. Created with BioRender.com. CAR = chimeric antigen receptor; PD-L1 = programmed death ligand 1; TRUCKs = T cells redirected for universal cytokine-mediated killing.
Second, AML blasts are well-equipped to attract a number of immunosuppressive cells, including Tregs, myeloid-derived suppressor cells (MDSCs), dendritic cells, and tumor-associated macrophages (TAMs). The AML microenvironment favors the expansion of Tregs via the inducible T-cell costimulator ligand (ICOSL)/inducible T-cell costimulator (ICOS) interaction and the overexpression of indoleamine 2,3-dioxygenase (IDO).68 Several potential strategies are under development to avoid the inhibitory effects of Tregs on CAR-T cells. In a preclinical lymphoma study, the administration of IL-15 did not reverse or block Tregs, although it was sufficient to promote the proliferation of cytotoxic T lymphocytes (CTLs) relative to Tregs. These findings suggest that the administration of proinflammatory agents may interfere with the suppressive function or depletion of Tregs, resulting in enhanced function of CTLs.68 Moreover, it has been demonstrated that the integration of costimulatory domains in CAR-T cells shows superior resistance compared to CAR-T cells containing only the CD3 domain.69 Furthermore, increased levels of MDSCs are associated with the detection of minimal residual disease in adult AML patients.70 In the AML microenvironment, blasts and MDSCs steer the macrophages towards an M2 inhibitory phenotype. These TAMs produce soluble factors such as transforming growth factor beta (TGF-β), arginase, IL-10, and vascular endothelial growth factor, which can remodel the local matrix, enlarge the vasculature, and additionally inhibit T-cell function.71 Multiple studies in different TAM-bearing malignancies have associated an increased abundance of TAMs with poorer outcomes.72,73
Third, AML cells may secrete a range of immunosuppressive soluble factors. Most obviously, AML cells may secrete anti-inflammatory cytokines and chemokines. AML blasts also can stimulate monocytes to secrete the anti-inflammatory cytokine IL-10. In addition, the upregulation of ICOSL on AML blasts can provide costimulation through ICOS on Tregs to maintain a suppressive cell function with secretion of inhibitory cytokines such as IL-10 and TGF-β. Moreover, chemokines have been implicated in the trafficking of T cells to tumor sites. The serum chemokine profile in AML patients differs from that of healthy controls, including the levels of C-C motif chemokine ligand (CCL) 3, CCL4, CCL5, CCL17, and C-X-C motif chemokine ligand 10.74,75 Variations in systemic chemokine levels and chemokine receptor expression in patients with AML have been associated with their prognosis and treatment response.74,75 The cytokine levels in the tumor microenvironment not only impact the antitumor activity of CAR-T cells but also affect the safety profile. Multiple CAR T-cell strategies have been developed to modulate the cytokine milieu in the tumor microenvironment. First, T cells redirected for universal cytokine-mediated killing (TRUCKs) secrete cytokines such as IL-8 and IL-12 to stimulate inflammation and inhibit immunosuppressive cells and signals (Figure 4B). Second, CARs can be engineered to neutralize cytokines in order to reduce CRS/ICANS or to target pathophysiological signaling. Finally, CARs can also be engineered to coexpress an inverted cytokine receptor that can adapt variable signals in the tumor microenvironment.76
In addition to chemokines and cytokines, dysregulation of tryptophan, glutamine, adenosine, and arginine may impact the immunosupressivity of the environment. AML blasts may be the source of elevated levels of kynurenine as they express IDO, an enzyme responsible for the oxidation of tryptophan to N-formyl kynurenine, both constitutively and after exposure to interferon gamma.77 N-Formyl kynurenine inhibits the proliferative capacity and differentiation of CD8+ T cells, resulting in the conversion of CD4+ T cells to Tregs, which also boost the suppressive capacity. Moreover, the AML microenvironment is rich in glutamine, an amino acid that is able to inhibit T cells by contributing to T cell exhaustion at high concentrations. Inhibiting glutamine metabolism using L-asparaginase, a chemotherapeutic agent that also has glutaminase activity, has been shown to effectively treat AML.78 Third, the ectonucleotidases CD38, CD39, and CD73 on myeloid blasts metabolize adenosine triphosphate to adenosine, which results in inhibition of T cells via the A2A adenosine receptor.79 Targeting downstream adenosine metabolism signaling by blocking A2A adenosine receptors with pharmacological agents resulted in enhanced CAR T-cell therapy in solid tumors in a preclinical study, but its role in hematologic malignancies is less clear.80 Finally, AML blasts have been demonstrated to mediate a microenvironment with low levels of arginine via the expression of arginase II.81 The lack of arginine acts as a metabolic brake for T cells, as illustrated by a lower production of interferon gamma and an increased expression of checkpoint inhibitors, but it also steers the monocyte population toward a suppressive phenotype. Furthermore, inhibition of arginine metabolism has been shown to enhance the antitumor activity of anti-CD33 CAR-T cells in a preclinical AML study.82 However, it has been demonstrated that arginase and IDO inhibitors only have a slight efficacy in vivo.83,84 Additionally, T cells are the most sensitive to extracellular concentrations of arginine due to their low/absent expression of the arginine resynthesis pathway enzymes argininosuccinate synthase (ASS) and ornithine transcarbamylase (OTC) (Figure 4C). Therefore, Fultang and colleagues inserted the expression cassettes of ASS, OTC, or both enzymes into CAR-T cells, allowing them to adapt to their metabolic microenvironment and enhance their antitumor activity.85
MANUFACTURING CHALLENGES
With the current list prices of commercial CAR T-cell therapies being about EUR 300,00086 and the expected expansion of indications for CAR T-cell therapy, it is obvious that the financial burden on the healthcare system will increase substantially, with direct consequences on patient access. Importantly, as more CAR T-cell therapies gain approval, competition may lead to a price reduction. However, a more proactive attitude may be essential for equal patient access to these specialized products. To this end, specialized hospitals are exploring the possibility of making their own CAR T-cell treatments, which leaves the healthcare payers with only the manufacturing costs.87 Moreover, progress is being made in the development of allogeneic “universal” CAR T-cell therapies. These off-the-shelf CAR-T cells can be manufactured in batches instead of on-demand, resulting in economies of scale, which may lower the cost for the healthcare payers.88 Finally, the costs of associated care could also potentially be reduced through a reduction/shift in side effects with novel CAR T-cell therapies. Ultimately, it may become possible in the future to reprogram T cells in vivo, thereby avoiding ex-vivo manufacturing costs.
In addition to pricing, the lengthy production process of CAR-T cells currently limits the applicability of CAR T-cell therapy. Due to the time-consuming manufacturing process of these cells and the often fast-evolving disease status at eligibility for receiving them, approximately 10% of patients die before they can undergo treatment. Furthermore, the time that elapses between apheresis and reinfusion of modified T cells at the hospital, that is, the vein-to-vein time, is critical for patient outcomes. The current vein-to-vein time of 2–3 weeks is problematic and can affect CAR T-cell eligibility, especially in patients with rapid progression of disease.89 Therefore, a new platform, called FasT CAR-T, has been developed to decrease the manufacturing time to only 24 hours.90,91 In the conventional CAR-T manufacturing process, a patients’ T-cells are first activated using CD3 and/or CD28 antibodies, and then transduced by virus vector to express one or more CARs, followed by expansion ex vivo. This process typically takes 1–6 weeks. In contrast, the FasT CAR-T platform allows to concurrently activate and transduce resting T cells into a single activation-transduction step allowing to achieve next-day manufacturing.
OUTSTANDING QUESTIONS AND CONCLUSION
In conclusion, several preclinical and clinical studies have demonstrated the efficacy of CAR T-cell immunotherapy for AML and its potential to cause a paradigm shift in the therapeutic landscape of this complex disease. An important caveat of these observations is that patients with relapsed or refractory disease were the focal point of current studies; therefore, the obtained results may not simply be extrapolated to patients undergoing front-line therapies. As patient numbers included in AML CAR-T trials are currently low and will continue to be low, a registry that is used worldwide would definitely be an asset. This would allow recording of essential product formulations and administration, such as cell phenotype, transfection technique, and cell dosage. In addition, a better tracking system of the status and results of trials could be included.
Importantly, CAR T-cell therapy is a “living drug” treatment in which modified T cells are able to persist in patients for several years and can undergo sequential expansion, contraction, and re-expansion. With these features, CAR-T cells differ fundamentally from conventional pharmacologically active compounds that decay with a predictable half-life and have to be administered repeatedly to sustain the therapeutic effect. Therefore, the monitoring of patients who have undergone CAR T-cell therapy is extremely important, but this issue has only recently gained attention. Moreover, the use of flow cytometry to detect the CAR surface product or a recombinant biotinylated target protein as well as reverse transcription–quantitative polymerase chain reaction (qPCR) and digital droplet qPCR employing probes specific for the CAR genetic sequence has been evaluated in recent publications.92,93
In addition to identifying the optimal therapeutic target(s) for CAR T-cell therapy in AML patients, its exact positioning in the therapeutic scheme may be questioned. More specifically, will AML-CARs eventually serve as a destination therapy or rather serve as a bridge to transplantation? Also, what is the optimal duration for the presence of CAR-T cells? Furthermore, should CAR-T cells be eliminated prior to allogeneic HSCT to reduce the risk of rejection in AML patients? While developing CAR T-cell therapy for AML in preclinical models and early clinical trials, the field of acute lymphocytic leukemia might provide valuable information regarding these questions.
Finally, as the field of CAR-T cells has evolved for the treatment of acute lymphocytic leukemia, it has become clear that no magic bullet exists. Escape mechanisms involving the loss of the respective antigen by tumor cells as well as the heterogeneous population holding one or several tumor subpopulations that express the CAR target at low levels or not at all have illustrated the need for smart combinations of therapeutics and the design of multitarget CARs. Nevertheless, whether or not such options should become the standard is a matter of debate.
Altogether, much progress has already been made towards understanding the role of CARs in AML; however, further intensive research is needed to provoke a real paradigm shift in AML treatment. The proverbial CAR race has started and will hopefully improve and enrich the therapeutic armamentarium against AML.
AUTHOR CONTRIBUTIONS
JV, CD, RD, and TL drafted the manuscript. JV designed the figures. All authors critically revised the manuscript and approved the final version.
DISCLOSURES
The authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This work was supported in part by vzw Kinderkankerfonds (grant to TL) and the Olivia Fund (grant to BDM and TL).
Footnotes
JV, RD, and CD contributed equally as first authors.
REFERENCES
- 1.Thomas E, Buckner C, Banaji M, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49:511–533. [PubMed] [Google Scholar]
- 2.Eppinger-Helft M, Pavlovsky S, Suarez A, et al. Sequential therapy for induction and maintenance of remission in acute myeloblastic leukemia. Cancer. 1975;35:347–353. [DOI] [PubMed] [Google Scholar]
- 3.Zwaan CM, Kolb EA, Reinhardt D, et al. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J Clin Oncol. 2015;33:2949–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeijlemaker W, Grob T, Meijer R, et al. CD34+CD38- leukemic stem cell frequency to predict outcome in acute myeloid leukemia. Leukemia. 2019;33:1102–1112. [DOI] [PubMed] [Google Scholar]
- 5.Hanekamp D, Denys B, Kaspers GJL, et al. Leukaemic stem cell load at diagnosis predicts the development of relapse in young acute myeloid leukaemia patients. Br J Haematol. 2018;183:512–516. [DOI] [PubMed] [Google Scholar]
- 6.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey NV. Chimeric antigen receptor T cells for acute lymphoblastic leukemia. Am J Hematol. 2019;94:S24–S27. [DOI] [PubMed] [Google Scholar]
- 8.Cummins KD, Gill S. Will CAR T cell therapy have a role in AML? Promises and pitfalls. Semin Hematol. 2019;56:155–163. [DOI] [PubMed] [Google Scholar]
- 9.Mardiana S, Gill S. CAR T cells for acute myeloid leukemia: state of the art and future directions. Front Oncol. 2020;10:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.June CH, O’Connor RS, Kawalekar OU, et al. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. [DOI] [PubMed] [Google Scholar]
- 11.Brocker T. Chimeric Fv-ζ or Fv-ε receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood. 2000;96:1999–2001. [PubMed] [Google Scholar]
- 12.Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–75. [DOI] [PubMed] [Google Scholar]
- 13.Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. [DOI] [PubMed] [Google Scholar]
- 14.Tomasik J, Jasiński M, Basak GW. Next generations of CAR-T cells—new therapeutic opportunities in hematology? Front Immunol. 2022;13:1034707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 2020;21:e168–e178. [DOI] [PubMed] [Google Scholar]
- 16.Boettcher M, Joechner A, Li Z, et al. Development of CAR T cell therapy in children-a comprehensive overview. J Clin Med. 2022;11:2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schorr C, Perna F. Targets for chimeric antigen receptor T-cell therapy of acute myeloid leukemia. Front Immunol. 2022;13:1085978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahzad M, Nguyen A, Hussain A, et al. Outcomes with chimeric antigen receptor t-cell therapy in relapsed or refractory acute myeloid leukemia: a systematic review and meta-analysis. Front Immunol. 2023;14:1152457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris EC, Neelapu SS, Giavridis T, et al. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herr MM, Chen GL, Ross M, et al. Identification of neurotoxicity after chimeric antigen receptor (CAR) T cell infusion without deterioration in the immune effector cell-associated encephalopathy (ICE) score. Biol Blood Marrow Transplant. 2020;26:e271–e274. [DOI] [PubMed] [Google Scholar]
- 22.Wehrli M, Gallagher K, Chen Y-B, et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J ImmunoTher Cancer. 2022;10:e003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caimi PF, Pacheco Sanchez G, Sharma A, et al. Prophylactic tocilizumab prior to anti-CD19 CAR-T cell therapy for non-Hodgkin lymphoma. Front Immunol. 2021;12:745320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner RA, Ceppi F, Rivers J, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood. 2019;134:2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locke FL, Neelapu SS, Bartlett NL, et al. Preliminary results of prophylactic tocilizumab after axicabtageneciloleucel (axi-cel; KTE-C19) treatment for patients with refractory, aggressive non-Hodgkin lymphoma (NHL). Blood. 2017;130:1547–1547. [Google Scholar]
- 28.Cummins KD, Frey N, Nelson AM, et al. Treating relapsed/ refractory (RR) AML with biodegradable anti-CD123 CAR modified T cells. Blood. 2017;130:1359. [Google Scholar]
- 29.Maus MV, Haas AR, Beatty GL, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tasian SK, Kenderian SS, Shen F, et al. Optimized depletion of chimeric antigen receptor T cells in murine xenograft models of human acute myeloid leukemia. Blood. 2017;129:2395–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philip B, Kokalaki E, Mekkaoui L, et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood. 2014;124:1277–1287. [DOI] [PubMed] [Google Scholar]
- 32.Myburgh R, Kiefer JD, Russkamp NF, et al. Anti-human CD117 CAR T-cells efficiently eliminate healthy and malignant CD117-expressing hematopoietic cells. Leukemia. 2020;34:2688–2703. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, He F, He W, et al. A transgene-encoded truncated human epidermal growth factor receptor for depletion of anti- B-cell maturation antigen CAR-T cells. Cell Immunol. 2021;363:104342. [DOI] [PubMed] [Google Scholar]
- 34.Porcellini S, Asperti C, Corna S, et al. CAR T cells redirected to CD44v6 control tumor growth in lung and ovary adenocarcinoma bearing mice. Front Immunol. 2020;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straathof KC, Pulè MA, Yotnda P, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warda W, Da Rocha MN, Trad R, et al. Overcoming target epitope masking resistance that can occur on low-antigen-expresser AML blasts after IL-1RAP chimeric antigen receptor T cell therapy using the inducible caspase 9 suicide gene safety switch. Cancer Gene Ther. 2021;28:1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee KC, Ouwehand I, Giannini AL, et al. Lck is a key target of imatinib and dasatinib in T-cell activation. Leukemia. 2010;24:896–900. [DOI] [PubMed] [Google Scholar]
- 38.Mestermann K, Giavridis T, Weber J, et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med. 2019;11:eaau5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Hu Y, Shao M, et al. Dasatinib enhances anti-leukemia efficacy of chimeric antigen receptor T cells by inhibiting cell differentiation and exhaustion. J Hematol Oncol. 2021;14:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung W-H, Gay J, Martin U, et al. Sensitive and adaptable pharmacological control of CAR T cells through extracellular receptor dimerization. JCI Insight. 2019;5:e124430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Appelbaum J, Leung W-H, Martin U, et al. 100 Drug-regulatable engineered T cells eliminate CD33+ and CD33ΔE2+ AML. J ImmunoTher Cancer. 2020;8:A63-A63. [Google Scholar]
- 42.Cooper TM, Wu V, Wilson A, et al. Pediatric and young adult leukemia adoptive therapy (PLAT)-08: A phase 1 study of SC-DARIC33 in pediatric and young adults with relapsed or refractory CD33+ AML. J Clin Oncol. 2022;40:TPS7078–TPS7078. [Google Scholar]
- 43.Biernacki MA, Bleakley M. Neoantigens in hematologic malignancies. Front Immunol. 2020;11:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rafiq S, Purdon TJ, Daniyan AF, et al. Optimized T-cell receptor-mimic chimeric antigen receptor T cells directed toward the intracellular Wilms Tumor 1 antigen. Leukemia. 2017;31:1788–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinstein JN, Collisson EA, Mills GB, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45:1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greiner J, Ono Y, Hofmann S, et al. Mutated regions of nucleophosmin 1 elicit both CD4(+) and CD8(+) T-cell responses in patients with acute myeloid leukemia. Blood. 2012;120:1282–1289. [DOI] [PubMed] [Google Scholar]
- 47.Xie G, Ivica NA, Jia B, et al. CAR-T cells targeting a nucleophosmin neoepitope exhibit potent specific activity in mouse models of acute myeloid leukaemia. Nat Biomed Eng. 2021;5:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adamia S, Bar-Natan M, Haibe-Kains B, et al. NOTCH2 and FLT3 gene mis-splicings are common events in patients with acute myeloid leukemia (AML): new potential targets in AML. Blood. 2014;123:2816–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbott RC, Hughes-Parry HE, Jenkins MR. To go or not to go? Biological logic gating engineered T cells. J ImmunoTher Cancer. 2022;10:e004185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atilla PA, McKenna MK, Watanabe N, et al. Combinatorial antigen targeting strategies for acute leukemia: application in myeloid malignancy. Cytotherapy. 2022;24:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srivastava S, Salter AI, Liggitt D, et al. Logic-gated ROR1 chimeric antigen receptor expression rescues T cell-mediated toxicity to normal tissues and enables selective tumor targeting. Cancer Cell. 2019;35:489–503.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hyrenius-Wittsten A, Su Y, Park M, et al. SynNotch CAR circuits enhance solid tumor recognition and promote persistent antitumor activity in mouse models. Sci Transl Med. 2021;13:eabd8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richards RM, Zhao F, Freitas KA, et al. NOT-gated CD93 CAR T cells effectively target AML with minimized endothelial cross-reactivity. Blood Cancer Discov. 2021;2:648–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim MY, Yu K-R, Kenderian SS, et al. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T cell immunotherapy for acute myeloid leukemia. Cell. 2018;173:1439–1453.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borot F, Wang H, Ma Y, et al. Gene-edited stem cells enable CD33-directed immune therapy for myeloid malignancies. Proc Natl Acad Sci U S A. 2019;116:11978–11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Humbert O, Laszlo GS, Sichel S, et al. Engineering resistance to CD33-targeted immunotherapy in normal hematopoiesis by CRISPR/Cas9-deletion of CD33 exon 2. Leukemia. 2019;33:762–808. [DOI] [PubMed] [Google Scholar]
- 57.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16:372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hazelton W, Ghorashian S, Pule M. Nanobody based tri-specific chimeric antigen receptor to treat acute myeloid leukaemia. Blood. 2020;136:10–11. [Google Scholar]
- 59.Ghamari A, Pakzad P, Majd A, et al. Design and production an effective bispecific tandem chimeric antigen receptor on T cells against CD123 and folate receptor ß towards B-acute myeloid leukaemia blasts. Cell J Yakhteh 2021;23:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He X, Feng Z, Ma J, et al. Bispecific and split CAR T cells targeting CD13 and TIM3 eradicate acute myeloid leukemia. Blood. 2020;135:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang SH, Hwang HJ, Yoo JW, et al. Expression of immune checkpoint receptors on T-cells and their ligands on leukemia blasts in childhood acute leukemia. Anticancer Res. 2019;39:5531–5539. [DOI] [PubMed] [Google Scholar]
- 62.Dama P, Tang M, Fulton N, et al. Gal9/Tim-3 expression level is higher in AML patients who fail chemotherapy. J ImmunoTher Cancer. 2019;7:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong Y, Han Y, Huang Y, et al. PD-L1 is expressed and promotes the expansion of regulatory T cells in acute myeloid leukemia. Front Immunol. 2020;11:1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daver N, Basu S, Garcia-Manero G, et al. Phase IB/II study of nivolumab in combination with azacytidine (AZA) in patients (pts) with relapsed acute myeloid leukemia (AML). Blood. 2016;128:763–763.27354720 [Google Scholar]
- 65.McGowan E, Lin Q, Ma G, et al. PD-1 disrupted CAR-T cells in the treatment of solid tumors: Promises and challenges. Biomed Pharmacother. 2020;121:109625. [DOI] [PubMed] [Google Scholar]
- 66.Lin G, Zhang Y, Yu L, et al. Cytotoxic effect of CLL-1 CAR-T cell immunotherapy with PD-1 silencing on relapsed/refractory acute myeloid leukemia. Mol Med Rep. 2021;23:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma Y-J, Dai H-P, Cui Q-Y, et al. Successful application of PD-1 knockdown CLL-1 CAR-T therapy in two AML patients with post-transplant relapse and failure of anti-CD38 CAR-T cell treatment. Am J Cancer Res. 2022;12:615–621. [PMC free article] [PubMed] [Google Scholar]
- 68.Perna SK, De Angelis B, Pagliara D, et al. Interleukin 15 provides relief to CTLs from regulatory T cell-mediated inhibition: implications for adoptive T cell-based therapies for lymphoma. Clin Cancer Res. 2013;19:106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kegler A, Koristka S, Bergmann R, et al. T cells engrafted with a UniCAR 28/z outperform UniCAR BB/z-transduced T cells in the face of regulatory T cell-mediated immunosuppression. Oncoimmunology. 2019;8:e1621676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun H, Li Y, Zhang Z, et al. Increase in myeloid-derived suppressor cells (MDSCs) associated with minimal residual disease (MRD) detection in adult acute myeloid leukemia. Int J Hematol. 2015;102:579–586. [DOI] [PubMed] [Google Scholar]
- 71.Lamble AJ, Lind EF. Targeting the immune microenvironment in acute myeloid leukemia: a focus on T cell immunity. Front Oncol. 2018;8:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang H, Hu W-M, Xia Z-J, et al. High numbers of CD163+ tumor-associated macrophages correlate with poor prognosis in multiple myeloma patients receiving bortezomib-based regimens. J Cancer. 2019;10:3239–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan Z-X, Li L, Wang W, et al. Clinical efficacy and tumor microenvironment influence in a dose-escalation study of anti-CD19 chimeric antigen receptor T cells in refractory B-cell non-Hodgkin’s lymphoma. Clin Cancer Res. 2019;25:6995–7003. [DOI] [PubMed] [Google Scholar]
- 74.Olsnes AM, Motorin D, Ryningen A, et al. T lymphocyte chemotactic chemokines in acute myelogenous leukemia (AML): local release by native human AML blasts and systemic levels of CXCL10 (IP-10), CCL5 (RANTES) and CCL17 (TARC). Cancer Immunol Immunother CII. 2006;55:830–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merle M, Fischbacher D, Liepert A, et al. Serum chemokine-release profiles in AML-patients might contribute to predict the clinical course of the disease. Immunol Invest. 2020;49:365–385. [DOI] [PubMed] [Google Scholar]
- 76.Tokarew N, Ogonek J, Endres S, et al. Teaching an old dog new tricks: next-generation CAR T cells. Br J Cancer. 2019;120:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fukuno K, Hara T, Tsurumi H, et al. Expression of indoleamine 2,3-dioxygenase in leukemic cells indicates an unfavorable prognosis in acute myeloid leukemia patients with intermediate-risk cytogenetics. Leuk Lymphoma. 2015;56:1398–1405. [DOI] [PubMed] [Google Scholar]
- 78.Matre P, Velez J, Jacamo R, et al. Inhibiting glutaminase in acute myeloid leukemia: metabolic dependency of selected AML subtypes. Oncotarget. 2016;7:79722–79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaisitti T, Arruga F, Guerra G, et al. Ectonucleotidases in blood malignancies: a tale of surface markers and therapeutic targets. Front Immunol. 2019;10:2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beavis PA, Henderson MA, Giuffrida L, et al. Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J Clin Invest. 2017;127:929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mussai F, De Santo C, Abu-Dayyeh I, et al. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood. 2013;122:749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mussai F, Wheat R, Sarrou E, et al. Targeting the arginine metabolic brake enhances immunotherapy for leukaemia. Int J Cancer. 2019;145:2201–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muller AJ, Manfredi MG, Zakharia Y, et al. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol. 2019;41:41–48. [DOI] [PubMed] [Google Scholar]
- 84.Steggerda SM, Bennett MK, Chen J, et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J ImmunoTher Cancer. 2017;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fultang L, Booth S, Yogev O, et al. Metabolic engineering against the arginine microenvironment enhances CAR-T cell proliferation and therapeutic activity. Blood. 2020;136:1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heine R, Thielen FW, Koopmanschap M, et al. Health economic aspects of chimeric antigen receptor T-cell therapies for hematological cancers: present and future. HemaSphere. 2021;5:e524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palani HK, Arunachalam AK, Yasar M, et al. Decentralized manufacturing of anti CD19 CAR-T cells using CliniMACS Prodigy®: real-world experience and cost analysis in India. Bone Marrow Transplant. 2023;58:160–167. [DOI] [PubMed] [Google Scholar]
- 88.Maldonado-Pérez N, Tristán-Manzano M, Justicia-Lirio P, et al. Efficacy and safety of universal (TCRKO) ARI-0001 CAR-T cells for the treatment of B-cell lymphoma. Front Immunol. 2022;13:1011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang R, Li X, He Y, et al. Recent advances in CAR-T cell engineering. J Hematol Oncol. 2020;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang J, He J, Zhang X, et al. Next-day manufacture of a novel anti-CD19 CAR-T therapy for B-cell acute lymphoblastic leukemia: first-in-human clinical study. Blood Cancer J. 2022;12:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang C, He J, Liu L, et al. CD19-directed fast CART therapy for relapsed/refractory acute lymphoblastic leukemia: from bench to bedside. Blood. 2019;134:1340–1340. [Google Scholar]
- 92.Selim AG, Minson A, Blombery P, et al. CAR-T cell therapy: practical guide to routine laboratory monitoring. Pathology (Phila). 2021;53:408–415. [DOI] [PubMed] [Google Scholar]
- 93.Peinelt A, Bremm M, Kreyenberg H, et al. Monitoring of circulating CAR T cells: validation of a flow cytometric assay, cellular kinetics, and phenotype analysis following tisagenlecleucel. Front Immunol. 2022;13:830773. [DOI] [PMC free article] [PubMed] [Google Scholar]