Abstract
Background
Periodontitis has been recently defined as a dysbiotic disease resulting from imbalanced oral microbiota. The transition of microbial communities from commensal to periodontitis-associated ones likely requires colonization by specific pathogens, including Porphyromonas gingivalis. We previously reported an antagonistic relationship between Streptococcus cristatus and P. gingivalis and the role of S. cristatus in inhibition of the biofilm formation, invasion, and gingipain enzymatic activity of P. gingivalis. Given the importance of P. gingivalis as a keystone pathogen of polymicrobial communities, the determinants of P. gingivalis levels, its interaction with the core microbiota, and association with the pathogenic potential of the microbial communities need to be addressed.
Results
This present study intends to determine the role of S. cristatus in altering interactions of P. gingivalis with other oral bacteria in a complex context. We collected dental plaque samples from periodontitis patients and assigned them into two groups based on their ratios of S. cristatus and P. gingivalis. We then characterized microbial profiles of the dental plaque samples using shotgun metagenomic sequencing and subsequently compared oral microbial composition and functional capabilities between groups with high or low S. cristatus-P. gingivalis ratios. Taxonomic annotation showed significant differences in microbial compositions at both genus and species levels between the two groups. Notably, a higher microbial composition diversity was observed in the samples with low S. cristatus-P. gingivalis ratios. The antibiotic resistance gene profiles of the two groups are also distinct, with significantly increased diversity and abundance of antibiotic resistance genes in the dental plaque samples with low S. cristatus-P. gingivalis ratios, which likely lead to elevated virulence potential.
Conclusions
Overall, our work highlights the importance of S. cristatus-P. gingivalis ratios in influencing the virulence of the oral microbiome. Approaches to enhance S. cristatus-P. gingivalis ratios in oral microbial communities will be attractive for revising the dysbiotic oral microbiome.
Keywords: Porphyromonas gingivalis, periodontitis, oral microbiome, shotgun metagenomic sequencing
Introduction
Periodontitis is one of the most common diseases in humans, affecting ~ 42% of adults aged 30 years and older in the US. The prevalence of periodontitis is influenced by multiple factors including age, gender, socioeconomic status, and race/ethnicity (1). The oral microbiome includes hundreds of bacterial species and phylotypes and is considered an important contributor to the development of periodontitis (2, 3). Understanding the effects of individual bacterial species and their relationship with others in complex oral microbial communities is crucial for the identification of microbiological factors associated with susceptibility to and severity of periodontitis.
Porphyromonas gingivalis is known to play a vital role in the development of dysbiotic microbial communities and can disrupt host-microbial homeostasis and induce inflammatory responses by acting with other oral bacteria. Therefore, it is considered a keystone-pathogen of periodontitis (4, 5). We previously reported an antagonistic relationship between Streptococcus cristatus and P. gingivalis and identified S. cristatus ArcA and a streptococcal ArcA derived anti-P. gingivalis peptide (SAPP) that can effectively inhibit biofilm formation, invasion, and gingipain enzymatic activity of P. gingivalis (6-10). We also demonstrated using a mouse model that SAPP can reduce P. gingivalis-induced alveolar bone loss (11, 12) and showed using ex-vivo assays that it can eliminate P. gingivalis and other periodontitis-associated bacteria from the oral microbial communities (11, 13). Our clinical studies showed a negative correlation between the distributions of S. cristatus and P. gingivalis in dental plaques derived from periodontitis patients and a higher S. cristatus-P. gingivalis ratios in dental plaque samples collected from Caucasian Americans (CAs) periodontitis patients, compared to those from African Americans (AAs) and Hispanic Americans (HAs) patients (14, 15). However, it is unclear whether different S. cristatus-P. gingivalis ratios accompany particular oral microbial profiles and functions, and if S. cristatus-P. gingivalis ratios can be used as clinical parameters for predicting periodontitis progression.
The goal of this work is to identify similarities and differences shared among dental samples with relatively high or low S. cristatus-P. gingivalis ratios. Our overall hypothesis is that the ratio of S. cristatus-P. gingivalis may play a vital role in the pathogenic properties of the oral microbiome and contribute to the pathogenetic potential of the oral microbiota. Previously, using qPCR, levels of P. gingivalis, S. cristatus, and total bacteria in the dental plaque from periodontitis patients were identified, and S. cristatus-P. gingivalis ratio of each sample was calculated (14). In this study, we profiled microbial communities at the species level and characterized the functional and biological process of microbial communities using metagenome shotgun sequencing. By comparing abundance and diversity of microbial taxa in the dental plaque samples with relatively high S. cristatus-P. gingivalis ratios to those with the low ratios, our approach identified differential composition and abundance of oral microbial communities with different S. cristatus-P. gingivalis ratios. Additionally, we found increased diversity and abundance of antibiotic resistance genes in the samples with low ratios. Altogether, the comparative study revealed an involvement of S. cristatus and P. gingivalis ratios in regulating the pathogenicity of oral microbial communities.
Methods
Study cohorts.
The research protocol was approved by the Committee for the Protection of Human Subjects of the University of Texas Health Science Center at Houston (IRB number: HSC-DB-17-0636). Candidates were screened during routine dental visits in clinic at the School of Dentistry, University of Texas Health Science Center at Houston between 2017 and 2022. Individuals aged 21 –75 with self-reported ethnicity/race of AAs, CAs, or HAs were enrolled after the initial periodontal examination that included determination of plaque index (PI) and bleeding on probing (BOP) (16). Radiographs were taken during this screening phase to assess bone loss. The clinical oral examinations were performed by trained dental examiners who are faculty members of the School of Dentistry, University of Texas Health Science Center at Houston. The examiners are calibrated in diagnosis of periodontitis annually. All study participants were diagnosed with generalized periodontitis Stage II or III, regardless of their grading, based on the 2017 World Workshop classification (17, 18). The enrolled patients also met the following criteria: ≤4 tooth loss due to periodontitis, interdental CAL ≥ 3mm and PD ≥ 5 mm at two or more teeth in different quadrants, and radiographic bone loss ≥ 15%. Other criteria for study participation were 1) no scaling and root planning within the previous year or periodontal surgeries in the previous five years; 2) no antibiotic therapy in the previous six months; 3) not pregnant. Information on demographics and self-reported dental and medical histories of the participants were abstracted from the Electronic Health Record.
Dental plaque sample collection.
Dental plaque samples were collected by board certified periodontists using sterile paper points at baseline prior to any dental treatment and labelled with numbers according to sampling sequences. The paper points were placed in ≥ 5mm pockets in different quadrants for 1 minute and then immersed immediately in an Eppendorf tube with 0.5 ml of Tris-EDTA (TE) buffer (pH 7.5) (15). Oral bacteria were harvested by centrifugation and bacterial pellets were resuspended in 100 μl TE buffer.
Sequencing and quality control.
The samples were sent to Novogene Co. (Sacramento, CA) for metagenomic sequencing. Briefly, DNA extracted from human dental plaques was randomly sheared into short fragments. The obtained fragments were end repaired, A-tailed and further ligated with Illumina adapters. The fragments with adapters were then PCR amplified, size selected, and purified. Quality control of the library was conducted with Qubit (≥ 20 ng, ≥ 10ng/μl) and real-time PCR for quantification and bioanalyzer for size distribution detection. Quantified libraries were pooled and sequenced on an Illumina NovaSeq high throughput sequencer by Novogene Corporation, Inc., with paired-end sequencing length of 150bp and an output of ~ 6GB raw data per sample.
Data preprocessing.
The amount of raw data generated per sample is 6.276GB on average. To ensure accuracy and reliability of the subsequent data analysis, we trimmed all low-quality bases. We also discarded the reads which contain uncalled N nucleotide stretching over 10bp and reads which overlap with adapter for more than 15 bp. To minimalize host DNA contamination, we also discarded the raw reads mapping to the human reference genome using Bowtie2 (19). After quality control and host-exclusion filtering, the amount of clean data was reduced slightly to 6.275GB per sample, with 96.66% and 92.37% bases having PHRED quality scores greater than 20 and 30, respectively.
Gene prediction and abundance analysis.
Software MetaGeneMark (version 2.10) was used to predict Open Reading Frames (ORFs) from Scaftigs (≥ = 500bp) (20, 21). ORFs less than 100nt were discarded. The ORFs were then dereplicated using CD-HIT (22, 23) which was run in default settings (identity = 95%, coverage = 90%) to generate gene catalogues. To calculate gene quantity, clean raw data were mapped to the gene catalogue using Bowtie2 (parameters: -end-to-end, -sensitive, -I 200, -X 400). Gene abundance was calculated based on the total number of mapped reads and gene length , using the formula as follows:
where stands for number of mapping reads and stands for the length of gene. Downstream analyses were performed based on the abundance of gene catalogues.
Statistical analysis.
Statistical analyses were performed using scipy.stats in SciPy (version 1.4.1), an open-source Python library for scientific computing. Independent two sample t-tests were performed to compare the samples with high S. cristatus and P. gingivalis ratios to those with low ratios. The t-tests were two-sided, and an assumption of identical variances on the sample distributions was used. The threshold of statistical significance was set to p-value ≤ 0.05.
Results
Characteristics of the study cohort.
We previously investigated the oral microbial profiles of dental plaques derived from periodontitis patients with different racial/ethnic backgrounds and using qPCR measured the distribution and levels of several well-studied oral bacteria in their dental plaque samples. These included keystone pathogens, accessory pathogens, and pathobionts (24). To examine the role of P. gingivalis and its antagonistic species S. cristatus in the composition of the oral microbiota, we selected 14 samples with relatively low S. cristatus-P. gingivalis ratios (< 1) and 16 samples with the higher ratios (> 100) for metagenome shotgun sequencing. The general characteristics of the participants included in the present study is shown in Table 1. Significant differences in gender, age, BOP, PI, and number of teeth between the two groups were not observed (Table 1).
Table 1.
Characteristics of the study cohort.
| S. cristatus-P. gingivalis ratio | |||
|---|---|---|---|
| Characteristics | < 1 | > 100 | P – value |
| Gender (Male/Female) | 6/8 | 12/4 | 0.078 |
| Age (year; Mean ± SD) | 54.6 ± 13.3 | 64.1 ± 12.2 | 0.052 |
| BOP (%, Mean ± SD) a | 52.9 ± 22.3 | 44.6 ± 31.0 | 0.413 |
| PI (%, Mean ± SD) b | 69.1 ± 27.2 | 69.7 ± 31.5 | 0.958 |
| Tooth number (Mean ± SD) c | 26.7 ± 1.9 | 25.9 ± 1.8 | 0.232 |
BOP: Bleeding on probing
PI: Modified O'Leary plaque index
Tooth number is based on a total of 32.
Diversity and similarity of oral microbiota with different S. cristatus-P. gingivalis ratios.
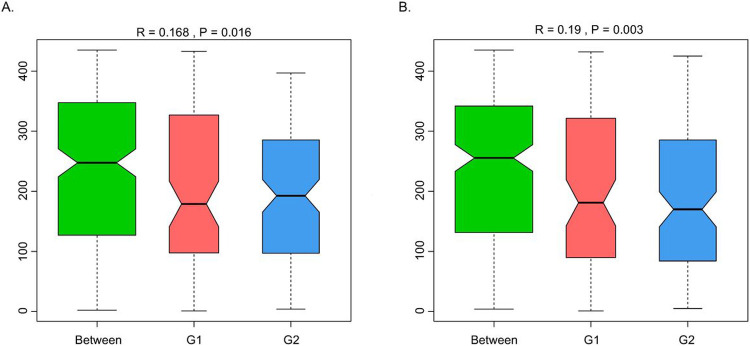
A total of 965,080 genes were identified using MetaGeneMark (20, 21). Figure 1 shows the mean and median number of non-redundant genes (238,472 and 216,072, respectively) in samples with lower S. cristatus-P. gingivalis ratio (< 1). While the mean and median of gene counts is 294,355 and 316,927, respectively, in samples with higher S. cristatus-P. gingivalis ratio (> 100). Although a statistically significant difference in numbers of the non-redundant genes was not reached between the groups with different S. cristatus-P. gingivalis ratios, significant abundance dissimilarities were found at bacterial genus and species levels between these groups using ANOSIM analysis, a non-parametric test based on ranked dissimilarity measure (Fig. 2). This suggests a higher similarity of gene abundance within each group than the similarity between the groups at the genus level (R = 0.168, p = 0.016) and the species level (R = 0.19, p = 0.003). Moreover, non-redundant genes were observed in both the groups as shown in Venn diagrams (Fig. 3A). Among the 965,080 non-redundant genes identified, 91,727 unique genes (9.50%) were detected in the group with low S. cristatus-P. gingivalis ratios (G1), while 113,466 (11.75%) were observed in the group with the high ratios (G2). Further, taxonomic annotations identified a total of 1484 microbial species in the cohort. Microbial taxa were found more diverse in the samples with low S. cristatus-P. gingivalis ratios. A total of 1201 species were identified in the group with low S. cristatus-P. gingivalis ratios, while only 630 species in the group with high S. cristatus-P. gingivalis ratios. Among the 1201 microbial taxa annotated in the group with low S. cristatus-P. gingivalis ratios, 854 group-specific species (58.6%) were found compared to 283 unique species (19.1 %) in the group with the high ratios. A total of 347 species annotated in both groups. These results indicate a more complex profile of microbial species in the group with low S. cristatus-P. gingivalis ratios (Fig. 3B).
Figure 1.
Comparison of non-redundant genes between samples with different S. cristatus-P. gingivalis ratios. The gene abundance was calculated based on the total number of mapped reads and gene lengths. Blue violin represents richness and abundance of non-redundant genes in the samples with lower S. cristatus-P. gingivalisratios (G1). Red violin represents richness and abundance of non-redundant genes in the samples with higher S. cristatus-P. gingivalisratios (G2).
Figure 2.
Analysis of dissimilarities between the groups with different S. cristatus-P. gingivalis ratios. ANOSIM test was used to compare the mean of ranked dissimilarities between group of samples with lower S. cristatus-P. gingivalisratios (G1) and group with a higher ratio (G2) at (A) genus level and (B) species level. The red and blue boxes stand for dissimilarities between samples within each group, whilst green box (Between) represents dissimilarities of pairs of samples from two different groups. Positive R values 0.168 and 0.19 and p-value<0.05 indicate that inter-group variation is statistically more significant than intra-group variation.
Figure 3.
Differences in genes and taxonomy between groups. (A) Total genes annotated in groups with low (G1) or high (G2) S. cristatus-P. gingivalisratios are presented in a Venn figure. Green and blue areas represent number of peculiar genes identified within each group, respectively, and an overlap area represents the number of common genes found in both groups. (B) Taxonomic difference between the groups at the species level.
We also conducted Principal Coordinates Analysis (PCoA), Principal Component Analysis (PCA), and Non-metric MultiDimensional Scaling (NMDS) analysis, an indirect gradient analysis approach that produces an ordination based on a distance matrix. Figure 4 provides the visualization of the results of PCoA, PCA, and NMDS analysis. In Fig. 4A and 4C, the distance between each pair of samples represents the dissimilarity between them. These two plots show clear distinction between the two groups with different S. cristatus-P. gingivalis ratios (G1 and G2), with samples within each group gathering closer together. The PCA plot in Fig. 4B also shows the overall separation between the two groups.
Figure 4.
Schematic of PCoA (A), PCA(B), and NMDS (C) plots. Data were analyzed at bacterial species level. Black dots represent samples with lower ratio of S. cristatus-P. gingivalis, and red ones denote samples with the higher ratios. In plots (A) and (C), the distance between each pair of samples represents the dissimilarity between them.
Abundance of common bacterial taxa.
Metastats analysis (25) was performed to detect differential abundance of common oral bacteria between two groups with different S. cristatus-P. gingivalis ratios. As shown in Fig. 5, three bacterial species, P. gingivalis, Treponema denticola, and Desulfomicrobium orale, out of the 12 most abundant taxa, were detected 56.08, 5.83, and 5.62-fold more abundant, respectively, in samples with low S. cristatus-P. gingivalis ratios, compared to the samples with high ratios. The other nine common bacterial taxa (Prevotella denticola, Alloprevotella tannerae, Actinomyces dentalis, Corynebacterium matruchotii, Bacteroidetes oral taxon 274, Prevotella nigrescens, Streptococcus gordonii, S. cristatus, and Campylobacter gracilis) were 1.80-fold to 6.77-fold more abundant in samples with the high ratios than those with the low ratios. In addition, the top 35 microbial taxa with differential abundance between groups are shown in an abundance heatmap (Fig. 6A). Among them, eleven species exhibit a higher abundance in the group with lower S. cristatus-P. gingivalis ratio, compared to the group with a higher ratio (Fig. 6A and B). These observations are consistent with previous studies of microbial profiles of dental plaques (26, 27), where a relatively higher abundance of P. gingivalis was found with elevated levels of T. denticola, Tannerella forsythia, Filifacter alocis in the group with low S. cristatus-P. gingivalis ratios, compared to those found in the group with high ratios. Other bacterial species found more abundance in the group with low ratios were Treponema lecithinolyticum, Treponema maltophilum, Treponema vincentii, Desulfobulbus oralis, Fretibacterium sp. OH1220_COT-178, Prevotella intermedia, and Lachnospiraeae bacterium oral taxon 500 (Fig. 6B). In contrast, species of Streptococcus and Actinomyces were dominated in the samples with the higher ratios, in which more than six-fold higher level of S. cristatus was discovered. Notably, hierarchical clustering algorithms showed that along with T. denticola, T. forsythia, another bacterial species, D. oralis, was often positively co-occurrent in the samples (Fig. 6A). While, S. cristatus was clustered with C. matruchotii, Actinomyces dentalis, and S. gordonii. Interestingly, S. cristatus was clustered with Caudoviricetes sp., bacteriophages with dsDNA genomes, which are highly prevalent in human gastrointestinal tract (28). These results revealed differential levels of microbial taxa in the samples with low or high S. cristatus-P. gingivalis ratios, respectively, suggesting that different core microbiotas are configured based on the S. cristatus-P. gingivalis ratios.
Figure 5.
Relative abundance of the top 12 bacterial species. The x and y axis of boxplots represent bacterial abundance and sample groups, respectively. The oral microbial richness and evenness were estimated using Metastats analysis, and the differences between groups with low (G1) or high (G2) ratios of S. cristatus-P. gingivalis were determined using a non-parametric t-test. Asterisk ‘*’ means P value between the two groups is smaller than 0.05, and double asterisks ‘**’ means P value is smaller than 0.01.
Figure 6.
Abundance heatmap based on the top 35 species derived from metagenomic sequencing data. (A) A heatmap of all 30 samples. X-axis represents the sample information; Y-axis represents species annotation; and the left side of heatmap is clustering tree of species. The value of the heatmap is standardized Z score. (B) A heatmap by groups.
Functional profile of oral microbiome with different S. cristatus-P. gingivalis ratios.
To determine functional diversity of bacterial communities with lower and higher S. cristatus-P. gingivalis ratios, we mapped function annotation against several functional databases such as Comprehensive Antibiotic Resistance Database (CARD) (29), Carbohydrate-Active enzymes Database (CAZy) (30), Kyoto Encyclopedia of Genes and Genomes (KEGG) (31), and Genealogy of Genes: Non-supervised Orthologous Groups (eggNOG) (32). As shown in Fig. 7A, sixty-nine antibiotic resistance genes were identified in the 30 dental plaque samples, with forty-eight of them found in all samples at different abundance levels. Twenty-one antibiotic resistant genes, such as multidrug efflux pump membrane fusion proteins (mdtA, B, F, and G), were unique to the group with low S. cristatus-P. gingivalis ratios, although with relatively low abundance. Percentage of antibiotic resistance genes in each sample was indicated in Fig. 7B, and absolute copies of antibiotic resistance genes were listed in Table 2. Among the top 30 antibiotic resistance genes annotated, six were significantly abundant in samples with lower S. cristatus-P. gingivalis ratios compared to those with higher ratios, while seven genes were more prevalent in the samples with higher ratios. The antibiotic resistance genes that exhibit remarkably differential abundance between two groups include Tem-116, catI, aph3-IIa, and aph3-IIIa with increased levels in the samples with the low S. cristatus-P. gingivalis ratios (Table 2). These results of differential richness of antibiotic resistance genes in the two groups indicate a potential regulatory mechanism contributing to the physiology of the oral microbiome.
Figure 7.
Identification of antibiotic resistance genes in the dental plaque samples. (A) The total number of antibiotic resistant genes identified in 30 samples was presented in a Venn diagram. Blue circle represents number of antibiotic resistant genes were detected in the group with low ratios, and green circle shows the genes in the group with high S. cristatus-P. gingivalis ratios. The unique genes are in area that is not overlapped. (B) Each stacked bar represents relatively abundance of antibiotic resistance genes in each sample.
Table 2.
Differential abundance of antibiotic resistance genes between groups with low or high S. cristatus-P. gingivalis ratios
| Antibiotic resistance genes | Gene function | Total of gene copies |
Fold changes |
p- value |
|
|---|---|---|---|---|---|
| G1 | G2 | G1/G2 | |||
| tem-116 | β-lactamase β-lactam antibiotics |
24527.77 | 42.29 | 580.03 | 0.045 |
| catI | Chloramphenicol acetyltransferase chloramphenicol | 16825.01 | 26.61 | 632.28 | 0.045 |
| aph3-IIa | Aminoglycoside phosphotransferase | 8841.63 | 10.39 | 851.09 | 0.045 |
| aph3-IIIa | Aminoglycoside-3'-Phosphotransferase kanamycin | 860.43 | 2.22 | 388.38 | 0.045 |
| vanT_gene_in_vanG_cluster | Resistance to vancomycin and teicoplanin type antibiotics | 114.94 | 149.04 | 0.77 | 0.164 |
| ermF | Member of RNA methyltransferase family | 26.46 | 45.72 | 0.58 | 0.349 |
| tetQ | Tetracycline-resistant ribosomal protection protein | 25.05 | 33.31 | 0.75 | 0.586 |
| patB | Associated with fluoroquinolone resistance | 14.38 | 44.64 | 0.32 | 0.023 |
| patA | 12.42 | 45.82 | 0.27 | 0.014 | |
| Escherichia coli_EF-Tu_mutants_conferring_resistance_to_Pulvomycin | Resistance to Pulvomycin | 7.54 | 14.66 | 0.51 | 0.399 |
| ErmB | A member of RNA methyltransferase family | 2.86 | 15.25 | 0.19 | 0.123 |
| vanW_gene_in_vanI_cluster | Resistance to vancomycin and teicoplanin type antibiotics | 31.52 | 50.32 | 0.63 | 0.031 |
| vanY_gene_in_vanM_cluster | 25.54 | 42.52 | 0.60 | 0.073 | |
| vanY_gene_in_vanG_cluster | Tetracycline-resistant ribosomal protection protein | 5.58 | 23.15 | 0.24 | 0.010 |
| tetM | 8.40 | 24.48 | 0.34 | 0.026 | |
| cfxA2 | Beta-lactamase | 17.17 | 26.61 | 0.65 | 0.271 |
| vanW_gene_in_vanB_cluster | Resistance to vancomycin and teicoplanin type antibiotics | 21.36 | 1.59 | 13.45 | 0.001 |
| pgpB | Phosphatidylglycerophosphatase | 22.91 | 0.05 | 441.68 | 0.000 |
| qacG | Resistance to benzalkonium chloride and ethidium bromide | 9.82 | 23.83 | 0.41 | 0.039 |
| Haemophilus_influenzae_PBP3_conferring_resistance_to_beta-lactam_antibiotics | Resistance to beta-lactam antibiotics | 2.92 | 6.37 | 0.46 | 0.320 |
| nimI | Nitroimidazole reductase | 6.83 | 10.44 | 0.65 | 0.425 |
| vanW_gene_in_vanG_cluster | Resistance to vancomycin and teicoplanin type antibiotics | 7.36 | 6.04 | 1.22 | 0.705 |
| tetW | A protein binds to the 30S ribosomal subunit | 1.03 | 5.68 | 0.18 | 0.061 |
| vanY_gene_in_vanB_cluster | Resistance to vancomycin and teicoplanin type antibiotics | 7.78 | 11.10 | 0.70 | 0.270 |
| mdeA | Multidrug efflux pump | 2.02 | 7.44 | 0.27 | 0.077 |
| tet32 | Tetracycline-resistant ribosomal protection protein | 5.87 | 6.40 | 0.92 | 0.807 |
| vanY_gene_in_vanF_cluster | Resistance to vancomycin and teicoplanin type antibiotics | 12.66 | 10.04 | 1.26 | 0.403 |
| Klebsiella pneumoniae_kpnH | Pumping of antibiotic out of a cell to confer resistance | 0.76 | 3.29 | 0.23 | 0.104 |
| nimJ | Deactivation of nitroimidazole antibiotics | 1.51 | 6.16 | 0.25 | 0.007 |
| adeF | Antibiotic efflux | 4.03 | 3.92 | 1.03 | 0.941 |
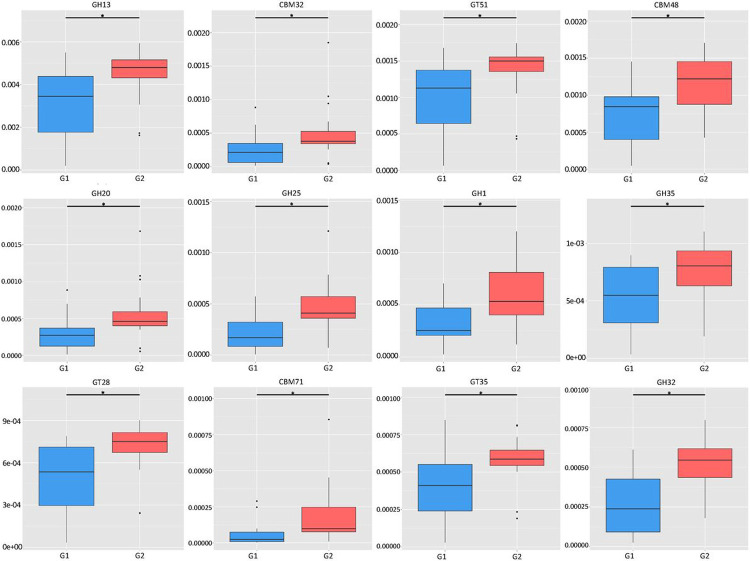
In the light of the observations on differential abundance of functional genes, we then investigated the molecular interaction and reaction networks using KEGG pathway databases and compared them between the samples with low or high ratios of S. cristatus-P. gingivalis. The results revealed that genes involved in human diseases-associated antimicrobial drug resistance were enriched in the samples with the low ratios (p = 0.0498) (Fig. 8). While genes encoding enzymes essential for carbohydrate metabolism were more abundant in the samples with high ratios (p = 0.0324), which was in agreement with the analyses on the CAZy database (Fig. 9). The functional profile of the oral microbiome showed significant variations in abundance of genes involved in carbohydrate processing between the groups with different S. cristatus-P. gingivalis ratios, including a reduction in some families of glycoside hydrolase, carbohydrate-binding module, and glycosyl transferase in the samples with low ratios of S. cristatus-P. gingivalis. Moreover, genes involved in metabolic pathways of amino acid (p = 0.040), energy (p = 0.0463), amino acid metabolism (p = 0.0403) and nucleotide (p = 0.0296) as well as glycan biosynthesis and metabolism (p = 0.0495) were significantly abundant in the group with high ratios (Fig. 8). However, there is no significant difference in pathways environmental information processing (signal transduction and membrane transport), genetic information processing (translation), metabolism of cofactors and vitamins, cofactors vitamins. These observations suggest a potential association of S. cristatus-P. gingivalis ratios with functional variation in the oral microbiome.
Figure 8.
The functional diversities of groups with low (G1) or high (G2) S. cristatus-P. gingivalis ratios. Metagenomic proteins were quantified by annotating metagenomic sequences with functions. Protein coding sequences were mapped against functional databases. Stacked bar represents proportion of relative functional abundance.
Figure 9.
Variation of abundance of carbohydrate-active enzymes in samples with different S. cristatus-P. gingivalis ratios. Box-plots represent richness and evenness of the top 12 genes encoding enzymes involved in bacterial metabolism. The y-axis of boxplots represents gene abundance. An asterisk refers significant difference in abundance of the enzymatic genes between two groups. Abbreviations: GH, Glycoside Hydrolase Family; CBM, Carbohydrate-Binding Module Family; and GT, Glycosyl Transferase Family.
Discussion
A series of studies in our laboratory has established a specific role of S. cristatus on violence potential of P. gingivalis and on formation and composition of oral microbial biofilms using qPCR (9, 10, 14, 15, 33, 34). Although negative correlation of S. cristatus-P. gingivalis has been identified in dental plaque samples derived from periodontitis patients, it is currently not clear how this relationship influences microbial communities as a whole. The study presented here provides a first glance on intraspecies interactions associated with different ratios of S. cristatus-P. gingivalis using metagenomic shotgun sequencing. Besides identification of over 1400 microbial taxa at the species level in plaque samples from 30 periodontitis patients, this approach revealed significant differences in abundance, diversity, and functions between two groups of dental plaque samples with high or low S. cristatus-P. gingivalis ratios, respectively. Earlier studies by Dewhirst et al. (35, 36) estimated, using 16S rRNA sequencing, there may be about 600 common taxa in the human oral microbiome. With powerful shotgun metagenomic sequencing here, we successfully identified over 1400 microbial species, including ones at a very low abundance, therefore, enabling future investigation of potential roles of low prevalent taxa in oral health and diseases.
Our study also revealed significant difference in abundances of common bacterial species between two groups with different S. cristatus-P. gingivalis ratios. Well-known periodontitis associated bacteria, including T. denticola, T. forsythia, and F. alocis, were much abundant in the samples with low S. cristatus and P. gingivalis ratios. Along with low S. cristatus and P. gingivalis ratios, we found a close correlation of abundance among P. gingivalis, T. forsythia, T. denticola, and D. oralis in the samples tested. The correlation is likely due to coaggregation between/among these bacterial species. The molecular mechanisms of coaggregation between P. gingivalis and T. denticola have been found to include interaction of P. gingivalis fimbrial protein and T. denticola dentilisin (37). Moreover, T. denticola dentilisin is also known responsible for coaggregation between T. denticola and T. forsythia (38). P. gingivalis Hgp44 may also involve in P. gingivalis adhesion to T. denticola, as a truncated Hgp446 fragment reduced the coaggregation of P. gingivalis and T. denticola (39). A similar observation reported by Zhu et al. indicated an essential role of P. gingivalisgingipains in synergistic polymicrobial biofilm formation of P. gingivalis and T. denticola (40). Unlike well studied pathogenicity of P. gingivalis, T. denticola, and T. forsythia, the role of D. oralis in pathogenesis of periodontitis is unclear. D. oralis, which was first isolated and purified from subgingival plaque samples from a periodontitis patient (41), is defined as a non-motile Gram negative and rod-shaped bacterium. Pathogenicity of D. oralis may rely on its ability to stimulate production of IL-1β, IFN-α, IFN-γ, MCP-1, IL-6, IL-8, and IL-1 by oral keratinocyte cells (41), suggesting that the organism plays a role in periodontal inflammation. Although there is no evidence that D. oralis physically interacts with P. gingivalis, T. forsythia, and/or T. denticola, our discovery of a cluster abundance of these four bacteria and increased levels of all four species in high ratios of S. cristatus-P. gingivalis indicates that D. oralis may cooperate with these bacteria as a core microbiota responsible for etiology and progression of periodontitis.
The shotgun metagenomic sequencing also allowed us to identify sixty-nine antibiotic resistance genes in dental plaques from the 30 periodontitis patients. Among these genes, forty-eight were found in both groups, and twenty-one only in the group with low S. cristatus-P. gingivalis ratios. Additionally, four out of five most abundant antibiotic resistance genes, tem-116, catI, aph3-IIa, and IIIa, were predominantly detected in the group with low S. cristatus-P. gingivalis ratios compared to its counterpart. The observation of more diverse and abundant genes in the group with the low ratios may result from the diversity of microorganisms in the samples in this group. Another explanation is that the low ratio of S. cristatus-P. gingivalis associated microbiome create an environment facilitating the transmission of resistance genes. Horizontal gene transfer is known as a major pathway for spreading antibiotic resistance gene, e.g., an outbreak of multidrug-resistant nosocomial pathogens caused by a transferable plasmid encoding SHV-12 extended-spectrum β-lactamase (TEM-116) (42). Our discovery of more diverse and abundance antibiotic resistance genes in the low ratio group implies the important role of S. cristatus-P. gingivalis ratios in controlling pathogenicity of the oral microbial communities.
As reported in several studies of 16S rRNA sequencing (43, 44), abundance and diversity of several bacterial genera shifted during periodontal health and disease transition. The dental plaque samples in this study were collected from patients with stage II and III periodontitis. Despite no significant difference in gender, age, tooth numbers, BOP (bleeding on probing) scores, and PI (plaque index) between two tested groups that were designated based on their S. cristatus-P. gingivalis ratios, significant differences in abundance and diversity of bacterial species as well as functional pathways between the two groups exist. We postulate that S. cristatus-P. gingivalis ratios associated increase in abundance and diversity of periodontitis- associated bacteria and antibiotic resistance genes accelerate progression of periodontitis and reduce response to periodontitis treatment. This notion is supported by our previous observations that the ratio of S. cristatus-P. gingivalis was significantly higher in Caucasians Americans (CAs) than in Africa Americans (AAs) and that better gains in clinical attachment levels were observed in CA periodontitis patients compared to those found in AAs after nonsurgical periodontal treatment (14, 45).
In conclusion, our work highlights the role of S. cristatus-P. gingivalis ratios in virulence potentials of the oral microbiome. Microbial communities with low S. cristatus-P. gingivalis ratios had an elevated level of several well-studied periodontitis-associated bacteria, decreased levels of streptococcus and actinomyces species, and much diversified microbial profiles and antibiotic resistance genes. Differential diversity and abundance of the oral microbiome are likely regulated by core bacteria in microbiota, including the ratios of S. cristatus-P. gingivalis, which may lead to functional changes of the oral microbiome. Therefore, approaches to enhance S. cristatus-P. gingivalis ratio will be attractive for rebuilding and maintaining healthy oral microbiome.
Acknowledgements:
The authors are grateful to all study participants for their contributions to this research. The authors thank the Meharry Office of Scientific Editing and Publications for editorial assistance. The authors also thank Krishna Kookal for abstracting clinical parameters from the Electronic Health Record at the School of Dentistry, University of Texas Health Science Center at Houston.
Funding:
The study was supported in part by grant U54MD007586 from the National Institute on Minority Health and Health Disparities, USA; grant 2117282 from the National Science Foundation, USA; and grant R16GM149359 from National Institute of General Medical Sciences, USA.
Footnotes
Competing interests: There are no conflicts of interest in connection with this article.
Ethics approval and Consent to participate: The research protocol was approved by the Committee for the Protection of Human Subjects of the University of Texas Health Science Center at Houston (IRB number: HSC-DB-17-0636). Consent forms were signed by all participants, before sample collection.
Consent for publication: We give our consent for the publication of the manuscript including all tables and figures.
Availability of data and materials: Any data and materials presented in the manuscript will be available upon request.
Contributor Information
Qingguo Wang, Meharry Medical College.
Bing-Yan Wang, University of Texas Health Science Center at Houston.
Siddharth Pratap, Meharry Medical College.
Hua Xie, Meharry Medical College.
References
- 1.Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. 2018. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc 149: 576–88 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. 2005. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43: 5721–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deo PN, Deshmukh R. 2019. Oral microbiome: Unveiling the fundamentals. J Oral Maxillofac Pathol 23: 122–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA. 2011. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10: 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajishengallis G, Lamont RJ. 2021. Polymicrobial communities in periodontal disease: Their quasi-organismal nature and dialogue with the host. Periodontol 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng-Hsuan Ho RJL, and Hua Xie. 2017. A novel peptidic inhibitor derived from Streptococcus cristatus ArcA attenuates virulence potential of Porphyromonas gingivalis. . Sci Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho MH, Lamont RJ, Xie H. 2017. Identification of Streptococcus cristatus peptides that repress expression of virulence genes in Porphyromonas gingivalis. Sci Rep 7: 1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho MH, Lamont RJ, Xie H. 2017. A novel peptidic inhibitor derived from Streptococcus cristatus ArcA attenuates virulence potential of Porphyromonas gingivalis. Sci Rep 7: 16217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Xie H. 2010. Role of arginine deiminase of Streptococcus cristatus in Porphyromonas gingivalis colonization. Antimicrob Agents Chemother 54: 4694–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie H, Lin X, Wang BY, Wu J, Lamont RJ. 2007. Identification of a signalling molecule involved in bacterial intergeneric communication. Microbiology 153: 3228–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho MH, Hasturk H, Young DF, Xie H. 2020. In vivo and ex vivo actions of a novel P. gingivalis inhibitor on multi-species biofilm, inflammatory response, and periodontal bone loss. Mol Oral Microbiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie H, Hong J, Sharma A, Wang BY. 2012. Streptococcus cristatus ArcA interferes with Porphyromonas gingivalis pathogenicity in mice. J Periodontal Res 47: 578–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho MH, Lamont RJ, Chazin WJ, Chen H, Young DF, Kumar P, Xie H. 2018. Characterization and development of SAPP as a specific peptidic inhibitor that targets Porphyromonas gingivalis. Mol Oral Microbiol 33: 430–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang BY, Lu T, Cai Q, Ho MH, Sheng S, Meng HW, Arsto L, Hong J, Xie H. 2021. Potential Microbiological Risk Factors Associated With Periodontitis and Periodontal Health Disparities. Front Cell Infect Microbiol 11: 789919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang BY, Wu J, Lamont RJ, Lin X, Xie H. 2009. Negative correlation of distributions of Streptococcus cristatus and Porphyromonas gingivalis in subgingival plaque. J Clin Microbiol 47: 3902–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman M, Takei H., Klokkevold H., & Carranza F. 2018. Newman and Carranza's Clinical Periodontology. [Google Scholar]
- 17.Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, Greenwell H, Herrera D, Kao RT, Kebschull M, Kinane DF, Kirkwood KL, Kocher T, Kornman KS, Kumar PS, Loos BG, Machtei E, Meng H, Mombelli A, Needleman I, Offenbacher S, Seymour GJ, Teles R, Tonetti MS. 2018. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 89 Suppl 1: S173–S82 [DOI] [PubMed] [Google Scholar]
- 18.Tonetti MS, Greenwell H, Kornman KS. 2018. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Clin Periodontol 45 Suppl 20: S149–S61 [DOI] [PubMed] [Google Scholar]
- 19.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu W, Lomsadze A, Borodovsky M. 2010. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res 38: e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh J, Byrd AL, Deming C, Conlan S, Program NCS, Kong HH, Segre JA. 2014. Biogeography and individuality shape function in the human skin metagenome. Nature 514: 59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Godzik A. 2006. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22: 1658–9 [DOI] [PubMed] [Google Scholar]
- 23.Fu L, Niu B, Zhu Z, Wu S, Li W. 2012. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28: 3150–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajishengallis G, Lamont RJ. 2016. Dancing with the Stars: How Choreographed Bacterial Interactions Dictate Nososymbiocity and Give Rise to Keystone Pathogens, Accessory Pathogens, and Pathobionts. Trends Microbiol 24: 477–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White JR, Nagarajan N, Pop M. 2009. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol 5: e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lafaurie GI, Castillo DM, Iniesta M, Sanz M, Gomez LA, Castillo Y, Pianeta R, Delgadillo NA, Neuta Y, Diaz-Baez D, Herrera D. 2023. Differential analysis of culturable and unculturable subgingival target microorganisms according to the stages of periodontitis. Clin Oral Investig 27: 3029–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aja E, Mangar M, Fletcher HM, Mishra A. 2021. Filifactor alocis: Recent Insights and Advances. J Dent Res 100: 790–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulyaeva A, Garmaeva S, Kurilshikov A, Vich Vila A, Riksen NP, Netea MG, Weersma RK, Fu J, Zhernakova A. 2022. Diversity and Ecology of Caudoviricetes Phages with Genome Terminal Repeats in Fecal Metagenomes from Four Dutch Cohorts. Viruses 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J. 2015. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 17: 690–703 [DOI] [PubMed] [Google Scholar]
- 30.Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. 2009. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res 37: D233–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanehisa M, Goto S, Hattori M, Aoki-Kinoshita KF, Itoh M, Kawashima S, Katayama T, Araki M, Hirakawa M. 2006. From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res 34: D354–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell S, Forslund K, Szklarczyk D, Trachana K, Roth A, Huerta-Cepas J, Gabaldon T, Rattei T, Creevey C, Kuhn M, Jensen LJ, von Mering C, Bork P. 2014. eggNOG v4.0: nested orthology inference across 3686 organisms. Nucleic Acids Res 42: D231–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie H, Cook GS, Costerton JW, Bruce G, Rose TM, Lamont RJ. 2000. Intergeneric communication in dental plaque biofilms. J Bacteriol 182: 7067–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang BY, Cao A, Ho MH, Wilus D, Sheng S, Meng HW, Guerra E, Hong J, Xie H. 2023. Identification of microbiological factors associated with periodontal health disparities. Front Cell Infect Microbiol 13: 1137067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol 192: 5002–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. 2001. Bacterial diversity in human subgingival plaque. J Bacteriol 183: 3770–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashimoto M, Ogawa S, Asai Y, Takai Y, Ogawa T. 2003. Binding of Porphyromonas gingivalis fimbriae to Treponema denticola dentilisin. FEMS Microbiol Lett 226: 267–71 [DOI] [PubMed] [Google Scholar]
- 38.Sano Y, Okamoto-Shibayama K, Tanaka K, Ito R, Shintani S, Yakushiji M, Ishihara K. 2014. Dentilisin involvement in coaggregation between Treponema denticola and Tannerella forsythia. Anaerobe 30: 45–50 [DOI] [PubMed] [Google Scholar]
- 39.Yoshikawa K, Kikuchi Y, Kokubu E, Imamura K, Saito A, Ishihara K. 2018. Identification of a specific domain of Porphyromonas gingivalis Hgp44 responsible for adhesion to Treponema denticola. Pathog Dis 76 [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Dashper SG, Chen YY, Crawford S, Slakeski N, Reynolds EC. 2013. Porphyromonas gingivalis and Treponema denticola synergistic polymicrobial biofilm development. PLoS One 8: e71727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cross KL, Chirania P, Xiong W, Beall CJ, Elkins JG, Giannone RJ, Griffen AL, Guss AM, Hettich RL, Joshi SS, Mokrzan EM, Martin RK, Zhulin IB, Leys EJ, Podar M. 2018. Insights into the Evolution of Host Association through the Isolation and Characterization of a Novel Human Periodontal Pathobiont, Desulfobulbus oralis. mBio 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naiemi NA, Duim B, Savelkoul PH, Spanjaard L, de Jonge E, Bart A, Vandenbroucke-Grauls CM, de Jong MD. 2005. Widespread transfer of resistance genes between bacterial species in an intensive care unit: implications for hospital epidemiology. J Clin Microbiol 43: 4862–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abusleme L, Hoare A, Hong BY, Diaz PI. 2021. Microbial signatures of health, gingivitis, and periodontitis. Periodontol 2000 86: 57–78 [DOI] [PubMed] [Google Scholar]
- 44.Nowicki EM, Shroff R, Singleton JA, Renaud DE, Wallace D, Drury J, Zirnheld J, Colleti B, Ellington AD, Lamont RJ, Scott DA, Whiteley M. 2018. Microbiota and Metatranscriptome Changes Accompanying the Onset of Gingivitis. MBio 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang BY, Burgardt G, Parthasarathy K, Ho DK, Weltman RL, Tribble GD, Hong J, Cron S, Xie H. 2023. Influences of race/ethnicity in periodontal treatment response and bacterial distribution, a cohort pilot study. Front Oral Health 4: 1212728. [DOI] [PMC free article] [PubMed] [Google Scholar]