Abstract
Extracellular vesicles are released by all cell types and contain proteins, microRNAs, mRNAs, and other bioactive molecules. Extracellular vesicles play an important role in intercellular communication and in the modulation of the immune system and neuroinflammation. The cargo of extracellular vesicles (e.g., proteins and microRNAs) is altered in pathological situations. Extracellular vesicles contribute to the pathogenesis of many pathologies associated with sustained inflammation and neuroinflammation, including cancer, diabetes, hyperammonemia and hepatic encephalopathy, and other neurological and neurodegenerative diseases. Extracellular vesicles may cross the blood-brain barrier and transfer pathological signals from the periphery to the brain. This contributes to inducing neuroinflammation and cognitive and motor impairment in hyperammonemia and hepatic encephalopathy and in neurodegenerative diseases. The mechanisms involved are beginning to be understood. For example, increased tumor necrosis factor α in extracellular vesicles from plasma of hyperammonemic rats induces neuroinflammation and motor impairment when injected into normal rats. Identifying the mechanisms by which extracellular vesicles contribute to the pathogenesis of these diseases will help to develop new treatments and diagnostic tools for their easy and early detection. In contrast, extracellular vesicles from mesenchymal stem cells have therapeutic utility in many of the above pathologies, by reducing inflammation and neuroinflammation and improving cognitive and motor function. These extracellular vesicles recapitulate the beneficial effects of mesenchymal stem cells and have advantages as therapeutic tools: they are less immunogenic, may not differentiate to malignant cells, cross the blood-brain barrier, and may reach more easily target organs. Extracellular vesicles from mesenchymal stem cells have beneficial effects in models of ischemic brain injury, Alzheimer’s and Parkinson’s diseases, hyperammonemia, and hepatic encephalopathy. Extracellular vesicles from mesenchymal stem cells modulate the immune system, promoting the shift from a pro-inflammatory to an anti-inflammatory state. For example, extracellular vesicles from mesenchymal stem cells modulate the Th17/Treg balance, promoting the anti-inflammatory Treg. Extracellular vesicles from mesenchymal stem cells may also act directly in the brain to modulate microglia activation, promoting a shift from a pro-inflammatory to an anti-inflammatory state. This reduces neuroinflammation and improves cognitive and motor function. Two main components of extracellular vesicles from mesenchymal stem cells which contribute to these beneficial effects are transforming growth factor-β and miR-124. Identifying the mechanisms by which extracellular vesicles from mesenchymal stem cells induce the beneficial effects and the main molecules (e.g., proteins and mRNAs) involved may help to improve their therapeutic utility. The aims of this review are to summarize the knowledge of the pathological effects of extracellular vesicles in different pathologies, the therapeutic potential of extracellular vesicles from mesenchymal stem cells to recover cognitive and motor function and the molecular mechanisms for these beneficial effects on neurological function.
Keywords: extracellular vesicles, inflammation, cognitive function, mesenchymal stem cells, neurodegenerative diseases, neuroinflammation, therapy, transforming growth factor-β
Introduction
Extracellular vesicles (EVs) play a key role in intercellular communication (Yáñez-Mó et al., 2015). There is increasing evidence of their contribution to the development of different pathologies including cancer and neurological and neurodegenerative diseases (Yáñez-Mó et al., 2015; Théry et al., 2018). The analysis of the content of extracellular vesicles may become a very useful diagnostic tool in different pathologies. Although extracellular vesicles play a pathological role in many pathologies, some types of extracellular vesicles, i.e. those released by mesenchymal stem cells (MSCs), have therapeutic effects which may be beneficial in pathologies associated with sustained peripheral inflammation and neuroinflammation including neurological and neurodegenerative diseases (Börger et al., 2017; Harrell et al., 2019). We will summarize here the role of EVs in some pathologies, the therapeutic potential of EVs from MSCs, and some underlying mechanisms, focusing on new findings in the field that highlight the therapeutic potential of EVs from MSCs to reduce peripheral inflammation and neuroinflammation, restoring cognitive function in neurological diseases.
Literature Search Strategy
A computer-based online search of the PubMed database was performed to retrieve articles published until January 31, 2023. A combination of the following text words (MeSH terms) was used to maximize search specificity and sensitivity: “extracellular vesicles”, “mesenchymal stem cells”, “cognitive function”, “therapy”, “neurodegenerative diseases”, “inflammation”, “neuroinflammation”. The results were further screened by title and abstract, and studies focused on the role of EVs in pathology or EVs from MSCs as a therapy were reviewed. Studies without full text available were excluded. No language or study type restrictions were applied. Clinical trials were searched using the database hosted at https://clinicaltrials.gov (date: March 31, 2023). Search queries were “extracellular vesicles” and “mesenchymal stem cells”. Trials were then filtered by recruitment status, selecting those “completed” or “recruiting”.
Role of Extracellular Vesicles in Different Pathologies
The International Society of Extracellular Vesicles defines EVs as “particles naturally released from the cell that are delimited by a lipid bilayer and cannot replicate, i.e. do not contain a functional nucleus” (Théry et al., 2018). EVs are released by all the cells in the organism and are present in different body fluids, such as blood, urine, or cerebrospinal fluid (Yáñez-Mó et al., 2015). Initially, EVs were considered just as cellular garbage, a way to get rid of debris and unwanted compounds. However, some key reports changed this vision. In 1996, Raposo et al. discovered that exosomes derived from B lymphocytes contained MHC type II and were able to activate T cells. These data suggested a role for exosomes in antigen presentation. It was later discovered that EVs contain both mRNA and microRNA, which can be delivered to another cell and can be functional in this new location (Ratajczak et al., 2006; Valadi et al., 2007).
EVs play a key role in cell-to-cell communication, and are involved in a wide range of physiological and pathological functions. They contribute to the development and progression of different diseases, especially those with an inflammatory or autoimmune component, such as lupus erythematosus (Lee et al., 2016), diabetes (Guay et al., 2019) or atherosclerosis (Gao et al., 2016). EVs are also involved in the pathogenesis of liver diseases (Masyuk et al., 2013) and cancer progression and metastasis processes (Hannafon and Ding, 2013; Hoshino et al., 2015). EVs also play a key role in neurodegenerative diseases such as Alzheimer’s disease (Yuyama et al., 2014; Aguzzi and Rajendran, 2009), Parkinson’s disease (Grey et al., 2015; Jin et al., 2023), and multiple sclerosis (Kimura et al., 2018). Moreover, EVs may play an important role in the development of addiction to methamphetamine, cocaine, nicotine, opioid, and in alcohol use disorders (Odegaard et al., 2020) and also in other neurological pathologies, including those related to HIV as well as in neurological impairment associated to hyperammonemia and hepatic encephalopathy (Izquierdo-Altarejos et al., 2020, 2022; Odegaard et al., 2020).
EVs may act as mediators of neuroinflammation and may transmit pathological effects from the periphery to the brain (Gupta and Pulliam, 2014; Li et al., 2018; Izquierdo-Altarejos et al., 2020). Under pathological situations, the cargo of these EVs is altered and EVs may contain proteins and microRNAs that transmit the pro-inflammatory signals into the brain triggering neuroinflammation and mild cognitive and/or motor impairment. For example, the injection of EVs isolated from Parkinson’s disease patients into mice induces symptoms similar to those observed in Parkinson’s disease patients, such as activation of microglia, degeneration of dopaminergic neurons and movement disturbances (Han et al., 2019). EVs from patients with amyotrophic lateral sclerosis also contain pathological proteins that contribute to the spread of the pathology (Sproviero et al., 2018). Tsilioni and Theoharides (2018) reported that the amount of EVs is increased in the serum of children with autism spectrum disorder and these EVs are able to stimulate human microglia to secrete IL-1β, being a potential candidate to trigger inflammation in the brain of these children. Izquierdo-Altarejos et al. (2020) showed that EVs from the plasma of hyperammonemic rats, a model of minimal hepatic encephalopathy, induce neuroinflammation in the cerebellum and motor incoordination when injected to control rats (Figure 1). A further study showed that the content of TNFα is increased in the EVs from hyperammonemic rats and that TNFα plays a key role in the induction of neuroinflammation, which in turn impairs GABAergic neurotransmission in the cerebellum, leading to motor incoordination (Izquierdo-Altarejos et al., 2022).
Figure 1.
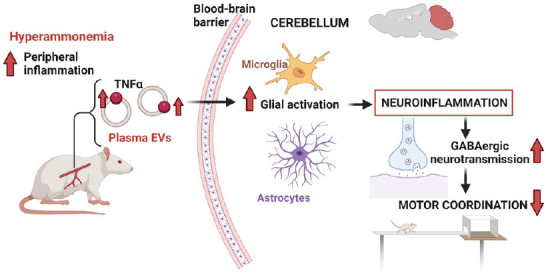
Example of pathological effects of extracellular vesicles.
Extracellular vesicles from plasma of hyperammonemic rats show increased content of tumor necrosis factor α (TNFα) which induces glial activation and neuroinflammation in the cerebellum when injected into normal rats, leading to enhanced GABAergic neurotransmission and motor incoordination. Created with BioRender.com. GABA: Gamma-aminobutyric acid.
A deep understanding of the implication of EVs in different pathologies and of the mechanisms involved may unveil new biomarkers for early and non-invasive detection of the pathologies and may allow the design of new therapeutic approaches for a variety of pathological conditions that curse with sustained inflammation and neuroinflammation.
EVs from different sources, especially those from MSCs, can also exert beneficial effects and are receiving an increasing interest to be used in therapy to treat different diseases, as we will discuss in the following sections.
Therapeutic Potential of Mesenchymal Stem Cells
MSCs have the potential to self-renew and to differentiate into several tissues of mesenchymal origin such as bone, fat, or cartilage and can be isolated from diverse sources including bone marrow, adipose tissue, or the umbilical cord among others (reviewed by Andrzejewska et al., 2019). MSCs can be easily and efficiently expanded in vitro since they can attach to the charged plastic of a culture dish and proliferate in the presence of growth factor-enriched medium for several passages keeping their stemness phenotype (Mellado-López et al., 2017). MSCs have demonstrated the capacity to preferentially migrate into the damaged tissue and homing ability. MSCs modify the local environment by releasing trophic factors and immunomodulatory signals that make them a very good source for cell therapy and tissue repair. Moreover, based on the reduced tumorigenic risk and the good immune tolerance in allogenic transplantation, the MSCs have been successfully translated to the clinical practice in a number of pathologies, including the treatment of patients with complete and chronic spinal cord injuries (Vaquero et al., 2017), of Crohn’s diseases ulcers (Lightner et al., 2023) or of osteoarthritis (Muñoz-Criado et al., 2017; Lamo-Espinosa et al., 2020).
The immunomodulatory function of the MSCs upon transplantation has been postulated as the most important mechanism for stimulating tissue repair by modulating the innate and adaptive immune responses. The ectopically added MSCs and the hosted tissue cross-talk and a good understanding became crucial to normalize the homeostasis of the injured tissue. The MSCs paracrine effect is conditioned by the injured microenvironment, and the secretome released by the MSCs also impacts the recipient by reducing the inflammatory responses, cell death, or fibrosis with the new vascular formation and tissue turnover (reviewed in Andrzejewska et al, 2019). In this process, there is a key role for the MSC-released cytokines, chemokines, and/or growth factors with angiogenic, proliferative, differentiating or cell protective capabilities. MSC can produce transforming growth factor β (TGFβ), insulin-like growth factor 1, stromal cell-derived factor, granulocyte-macrophage and granulocyte colony-stimulating factors, vascular, endothelial, hepatocyte, epidermal and fibroblast growth factors, nerve growth factor β or leukemia inhibitory factor; cytokines including the interleukins IL-1α, IL-1β, IL-2, IL-6, IL-7, IL-8, IL-10, IL-11, IL-12, IL-14, IL-15 and TNFα and the chemokines CXCL12, CCL2 and CCL5 as well as extracellular matrix proteins like fibronectin, laminin, collagen, and proteoglycans which alone or in combination, contribute to the microenvironment modulation (Andrzejewska et al., 2019). The modulation of neuroinflammation is considered the more relevant effect for the neuroprotective therapeutic application of the MSCs (Pischiutta et al., 2022; Muhammad et al., 2022). After traumatic brain injury, MSCs reduce the inflammatory infiltration with an associated decrease of pro-inflammatory cytokines such as IL-1β and increase of anti-inflammatory ones such as IL-10, correlating with improved sensorimotor function (Zhang et al., 2013). Ectopically transplanted MSCs reduce neuronal cell death by reducing the inflammatory cell reactivity, reactive oxidative species, and the pro-inflammatory cytokines such as IL-8 and IL-6 release in a cell-cell contact independent mechanism (Raffaghello et al., 2008). Since the secretome and the EVs produced by the MSCs can reproduce their above-described therapeutic effects, they have aroused great expectations for neuronal repair applications (Pischiutta et al., 2022; Soares et al., 2022).
Advantages of Mesenchymal Stem Cell-Derived Extracellular Vesicles versus Mesenchymal Stem Cells in Therapy
EVs released by MSCs provide a promising alternative to cell therapy, since they can recapitulate the beneficial effects provided by the parental MSCs (Lou et al., 2017; Bagno et al., 2018). MSC-derived EVs carry bioactive proteins, microRNAs, and functional lipids which act as mediators between MSCs and target cells (Heldring et al., 2015). EVs released from MSC have several advantages for clinical application compared to cell therapy:
1) Safety profile. While MSCs transplantation may be rejected by the host immune system (Badillo et al., 2007; Poncelet et al., 2007), MSC-derived EVs are less immunogenic (Ankrum et al., 2014; Liew et al., 2017). On the other hand, transplantation of MSCs carries the risk of the cells differentiating in an undesirable manner, with the possibility of transforming into malignant cells and forming tumors (Volarevic et al., 2018). EVs, lacking self-replicative capacity, do not have the potential to give rise to tumors.
2) Target tissues. Compared to MSCs, which are relatively large in size (30–60 μm in diameter), the small size of EVs allows them to migrate more efficiently to target organs or tissues, without becoming trapped, for example, in the pulmonary microvasculature, as is commonly the case with MSCs (Liew et al., 2017; Börger et al., 2017). Moreover, MSC-derived EVs express several adhesion molecules on their surface (CD29, CD44, and CD73) that favor their uptake in damaged and/or inflamed tissues (Harrell et al., 2019). For example, in a murine model of acute renal failure, EVs from MSCs were seen to accumulate mainly in the kidneys (Grange et al., 2014), while in a model of intracerebral hemorrhage, injected EVs were detected in the brain (Otero-Ortega et al., 2018). Another positive aspect to consider in the treatment of neurodegenerative diseases or diseases involving neuroinflammation is that EVs, unlike native MSCs, are able to cross the blood-brain barrier via transcytosis and exert direct effects in the brain (Chen et al., 2016; Otero-Ortega et al., 2018).
3) Versatility. One advantage of EVs is that they can be modified in multiple ways to increase their therapeutic potential. One strategy is to enrich them in a microRNA or protein that has a beneficial effect. For example, a Phase II clinical trial is investigating the effect of miR-124-enriched EVs from MSCs on neurovascular remodeling and functional recovery after acute ischaemic stroke (ClinicalTrials.gov: NCT03384433). Another strategy is to modify membrane proteins on the vesicle surface to increase their specificity for specific target tissues. Alvarez-Erviti et al. (2011) modified the N-terminal end of the membrane glycoprotein Lamp2B by adding the glycoprotein rabies virus glycoprotein (RVG), which binds specifically to the acetylcholine receptor, abundant in neurons, and transfected dendritic cells with the Lamp2B protein fused to RVG, so that the vesicles produced by these cells expressed RVG on the membrane. They then found that the modified EVs specifically targeted neurons in control mice, compared to unmodified EVs.
Beneficial Effects of Extracellular Vesicles from Mesenchymal Stem Cells on Neuroinflammation and Neurological and Neurodegenerative Diseases
EVs from MSCs attenuate neuroinflammation evoked by ischemic brain injury (Dabrowska et al., 2019; Go et al., 2019; Kang et al., 2019), perinatal brain injury (Thomi et al., 2019) and may also be useful in models of Alzheimer’s disease (Reza-Zaldivar et al., 2019), Parkinson’s disease (Vilaça-Faria et al., 2019) or multiple sclerosis (Hosseini Shamili et al., 2019). EVs from MSCs activated with interferon gamma (IFNγ) reduce neuroinflammation and demyelination and improve functional outcomes in a murine model of chronic experimental autoimmune encephalomyelitis (EAE) (Riazifar et al, 2019).
In experimental stroke, intravenous administration of EVs from bone marrow MSCs increases neurogenesis, neurite remodeling, and angiogenesis, improving animals’ functional recovery (Xin et al., 2013). Similar results were observed in a traumatic brain injury model, showing a reduction of inflammation and improved outcomes after the administration of EVs from bone marrow MSCs (Zhang et al., 2015). Injection of bone marrow MSC-derived EVs also reduced inflammation and promoted neuro-regeneration in a rat model of spinal cord injury (Han et al., 2015). Otero-Ortega et al. (2018) showed that injected EVs from adipose tissue MSCs reached the brain in a rat model of intracerebral hemorrhage and improved functional recovery. The results reported suggest that the EVs stimulate brain repair processes, as injected rats showed more axons, higher tract connectivity, and higher expression of oligodendrocyte formation markers. The same group has recently published the results of a pilot phase IIa clinical trial in patients with systemic stroke treated with EVs from MSCs, demonstrating the safety of the treatment and a trend towards improvement in the National Institutes of Health Stroke Scale scores in the treated patients compared with placebo (de Celis-Ruiz et al., 2022).
EVs from MSCs may be useful to improve cognitive function in patients with hyperammonemia and hepatic encephalopathy. Patients with liver cirrhosis show hyperammonemia and may show cognitive and motor impairment, known as hepatic encephalopathy, which is mediated by neuroinflammation (Felipo 2013; Cabrera-Pastor et al., 2019; Häussinger et al., 2022). Izquierdo-Altarejos et al. (2023) found that injection of EVs from adipose MSCs to hyperammonemic rats reduces neuroinflammation in the hippocampus and reverses the alterations in glutamatergic neurotransmission which lead to cognitive impairment, restoring different types of memory and learning. These effects were mainly attributed to the TGFβ in the extracellular vesicles. Promising studies suggest that these EVs could be a therapeutic agent for two of the most prevalent neurodegenerative diseases: Alzheimer’s and Parkinson’s diseases. MSCs and the secreted EVs have the potential to promote and protect neurogenesis, reduce Aβ depositions, and repress BACE1, relevant events for AD pathology. Reza-Zaldivar et al. (2019) observed that MSC-derived EVs enhance neurogenesis and restore cognitive function in a mouse model of Alzheimer’s disease established by injection of amyloid-beta 1–42 aggregates into the dentate gyrus. Wang and Yang (2021) found that EVs from bone marrow MSCs improved spatial learning and memory in transgenic APP/PS1 mice, reducing Aβ depositions by activating the sphingosine kinase/sphingosine-1-phosphate signaling pathway. Alvarez-Erviti et al. (2011) found that modified EVs are able to transfer a silencing RNA for the protease BACE1 to neurons in mice, reducing the expression of BACE1, which plays an essential role in Alzheimer’s disease by cleaving the amyloid precursor protein.
Regarding Parkinson’s disease, positive results using MSCs and their EVs have been reported. Chen et al. (2020a) found that EVs from human umbilical cord MSCs cross the blood-brain barrier in a rat model of Parkinson’s disease (6-hydroxydopamine injected rats), reduce dopaminergic neurons loss in the substantia nigra, increase dopamine levels in the striatum and improve apomorphine-induced rotation behavior. Other studies have used MSCs secretome, which includes EVs and other bioactive molecules secreted by MSCs, in preclinical models of Parkinson`s disease. Injection of the secretome released by bone marrow MSCs into a rat model of Parkinson’s disease rescue dopaminergic neurons in the substantia nigra and striatum and improves motor performance in the staircase test (Mendes-Pinheiro et al., 2019).
The above studies show that EVs from MSCs induce beneficial effects on cognitive and motor function in animal models of different pathologies. Understanding the underlying mechanisms may help to design better therapeutic approaches to be translated to clinical practice in humans.
There are few studies comparing the effect of EVs from MSCs of different tissue sources. Depending on the tissue of origin the MSCs and their derived EVs may have slightly different contents and cargo of proteins, mRNAs, and miRNAs which could result in slightly different properties and/or mechanisms of action. For example, it has been reported that EVs from adipose MSCs exert more potent angiogenic effects than EVs from bone marrow MSCs (Chance et al., 2020). Regarding the effects on the brain and cognitive function, most studies evaluate the effects of treatments with EVs from adipose, bone marrow, and umbilical cord-derived MSCs. All of them have beneficial effects, but it has not been studied if the effects or mechanisms may be different depending on the tissue of origin of EVs.
Recent research has started to unveil some of the mechanisms of the beneficial effects of EVs from MSCs, as discussed in the following sections. Although each pathology presents particular features and alterations, there is growing evidence that different neurological disorders share common pathways and mechanisms of action, most of them involving alterations in the immune system and neuroinflammation. EVs from MSCs could therefore modulate specific pathways depending on the pathology, but there is a major effect derived from their anti-inflammatory and immunomodulatory properties.
Mechanisms Underlying the Beneficial Effects of Extracellular Vesicles from Mesenchymal Stem Cells: Regenerative Properties
Nowadays great hopes are placed on the regenerative effects of cell transplantation. MSCs and their derived EVs have been extensively accredited for their tissue regeneration capabilities, with special success in wound healing. Wound healing is a well-orchestrated process that can be severely compromised under several pathological conditions, such as diabetes mellitus (Cao et al., 2017; reviewed by Nallakumarasamy et al., 2022). Treatment with MSC and/or their EVs has been shown to improve it significantly (Bailey et al., 2022). Indeed, the first approved clinical application in Europe for MSCs transplantation was based on their efficacy in accelerating healing in perianal fistulas of patients with Crohn´s disease (Panés et al., 2016; NCT01541579). The therapeutic effects of EVs treatment have been also extensively demonstrated after ischemic damage. For instance, after ischemic stroke and consequent brain damage, EVs administration reduces the infarct volume and rescues neuronal function in pre-clinical models (Zhao et al., 2023). The induction of tissue regeneration requires endogenous tissue remodeling, eventual cell replacement, and functional tissue rescue (Aguiar Koga et al., 2023). Tissue regeneration processes would involve apoptosis attenuation, preventing massive cell loss, induction of cell migration and cell reactivity, new blood vessel formation or repopulation of lost cells by boosting resident cells to proliferate or differentiate (Wu et al., 2018). Phagocytosis and removal of dead cells and pathological matrix deposition or any other pathogenic body would also contribute to restoring tissue homeostasis and accelerating the healing process. In addition, MSCs derived EVs defined cargos have been associated as responsible for rescuing damaged tissue and modulating the process of tissue regeneration and remodeling. For instance, high levels of 2′,3′-cyclic nucleotide 3′-phosphodiesterase enhance the potential of EVs to rescue from brain damage. Exosomal 2′,3′-cyclic nucleotide 3′-phosphodiesterase induces neurogenesis and neuritogenesis in the damaged hippocampus, by promoting beta3-tubulin polymerization and accumulating neuroprotective adenosine (Chen et al., 2020b).
Transplantation of stem cells or their secreted EVs has also generated great expectations for adult neuronal regeneration after traumatic injuries (Tsintou et al., 2021). To date, stem cell transplantation or administration of their secreted EVs is the most promising strategy for the regeneration of the damaged corticospinal tract, responsible for good motor control after spinal cord injury (Cheng et al., 2021). The locally delivered EVs within a hydrogel that increase the retention of the vesicles at the injury epicenter have been shown to promote neurogenesis and attenuation of the inhibitory scar formation (Cheng et al., 2021). By suppressing the macrophage infiltration and downregulation of pro-inflammatory factors such as TNFα and IL-1β (Luo et al., 2021) and induced angiogenesis (Mu et al., 2022), the loaded EVs promote significant neuronal activity recovery. In the acute and inflammatory early phase after spinal cord injury, the MSC-derived EVs locally injected in a hyaluronic-based hydrogel is able to modify the harmful injury microenvironment reducing the oxidative stress, ameliorating the secondary excitotoxicity (Li et al., 2020), reducing inflammatory infiltration, enhancing recruitment of endogenous neural precursor cells, and by the end, promoting neuronal regeneration and preservation of myelin-associated axons (Fan et al., 2022).
Mechanisms Underlying the Beneficial Effects of Extracellular Vesicles from Mesenchymal Stem Cells: Anti-Inflammatory Properties and Effects on the Peripheral Immune System
MSCs exert immunoregulatory properties, promoting an anti-inflammatory state and tissue repair. These cells can inhibit the proliferation and function of B lymphocytes, CD4+ and CD8+ T lymphocytes, NK cells, and mature dendritic cells, whilst inducing anti-inflammatory macrophages and T reg lymphocytes (Regmi et al., 2019). Therefore, the regulation of the peripheral immune system would be one of the main mechanisms of the therapeutic effects of MSCs and also of EVs released from MSCs.
Immunosuppressive effects of EVs from MSCs are mainly mediated by proteins and miRNAs contained in the vesicles. Although the mechanisms are not fully understood, several molecules have been identified in the EVs as responsible for their immunomodulatory effects. EVs from MSCs can contain FAS and TRAIL ligands, which induce apoptosis (Fathollahi et al., 2019); annexin1, which increases the sensitivity of activated T cells to apoptosis; PD-L1, which inhibits T cell activation by binding to PD-1 on the surface of the cells; or TGFβ, which produces anti-proliferative effects (Mokarizadeh et al., 2012). EVs can also carry different miRNAs that affect NF-κB, Wnt, PI3k/Akt, and HMGB1 signaling pathways and exert anti-inflammatory effects. EVs from umbilical cord MSCs overexpressing miR-126 reduced HMGB1, NLPR3 inflammasome, and NF-κB, thus reducing retinal inflammation in diabetic rats (Zhang et al., 2019). EVs from MSCs overexpressing miR-17-92 cluster negatively regulate PTEN expression and activate the PI3k/Akt/mTOR signaling pathway and inhibit GSK-3β activity (Xin et al., 2017). EVs from bone marrow MSCs expressing miR-92a-3p inhibit the Wnt pathway (Mao et al., 2018).
Several studies report that EVs from MSCs modulate the balance between pro-inflammatory T CD4+ Th17 and anti-inflammatory T CD4+ T reg in vivo in animal models of different pathologies (Figure 2). Riazifar et al. (2019) showed that IFNγ-activated bone marrow MSC-derived EVs reduced inflammation, demyelination, and CD4+ and CD8+ T cell infiltration in the spinal cord in an experimental mouse model of autoimmune encephalomyelitis. These EVs suppressed the proliferation of Th1 and Th17 lymphocytes (both pro-inflammatory) and induced Treg proliferation both in the spinal cord of treated rats and in vitro cultures.
Figure 2.

Therapeutic effects of extracellular vesicles from mesenchymal stem cells.
Extracellular vesicles (EVs) from mesenchymal stem cells modulate the immune system in different ways, including the modulation of the Th17/Treg ratio to reduce the pro-inflammatory Th17 and promote the anti-inflammatory Treg. In pathological situations associated with neuroinflammation, EVs from mesenchymal stem cells promote a shift of microglia from a pro-inflammatory to an anti-inflammatory form, reducing neuroinflammation and improving neurotransmission and cognitive function. See text for more details. Created with BioRender.com. TGFβ: Transforming growth factor-β.
Li et al. (2019a) observed that EVs from bone marrow MSCs reversed the increase in the Th17/Treg ratio in a model of aplastic anemia. The results of this study indicate that EVs promote the differentiation of CD4+ T cells into Treg through the interaction of sphingosine 1-phosphate, enriched in the vesicles, with sphingosine 1-phosphate receptor 1 on lymphocytes.
Moreover, Xie et al. (2019) identified miR-1246 as necessary to reduce the Th17/Treg ratio in a mouse model of liver ischemia. In this study, they observed that EVs from umbilical cord MSCs reduced the Th17/Treg ratio both in vivo and in vitro, promoting the secretion of anti-inflammatory cytokines such as TGFβ and IL-10, and that these effects did not occur if miR-1246 was inhibited. Based on the results of in vitro experiments, Xie et al. (2019) propose that miR-1246 acts by inhibiting glycoprotein 130, which belongs to the IL-6 receptor family, by decreasing the phosphorylation (and thus activity) of STAT3, a transcription factor essential for lymphocyte differentiation to Th17. A different study also demonstrates that EVs from umbilical cord MSCs reduce the total number of CD4+ T cells, regulate the proportion of Th17 and promote the expression of IL-17 and TGFβ in vitro, suggesting the potential of these EVs as a treatment for autoimmune diseases such as lupus erythematosus (Xie et al., 2022).
Franco da Cunha et al. (2020) investigated the effects of EVs from adipose MSCs on CD4+ T lymphocytes and the underlying mechanisms. They found that EVs were incorporated in vitro by activated CD4+ T cells and reduced the proliferation by around 50% in vitro and inhibited Th1 differentiation, decreasing the frequency of IFN-γ producing cells and increasing Foxp3-expressing cells This study shows that EVs from MSCs induce modifications in the miRNA profile, decreasing miR-23a-3p expression and increasing the expression of its target gene, TGFBR2, suggesting active participation of TGF-β pathway in this regulation. This pathway, in turn, may inactivate the mTOR pathway and, as a consequence, alter the metabolic profile of T cells treated with the EVs (Franco da Cunha et al., 2020).
Mechanisms Underlying the Beneficial Effects of Extracellular Vesicles from Mesenchymal Stem Cells: Direct Effects in the Brain (Modulation of Microglia Polarization)
The above data show that EVs from MSCs modulate peripheral inflammation reducing pro-inflammatory factors and increasing anti-inflammatory factors. These beneficial effects on peripheral inflammation may contribute to reducing the deleterious effects in the brain and improving neuroinflammation and cognitive and motor function in different pathologies, including neurological and neurodegenerative diseases as summarized above. However, this is not the only mechanism by which EVs from MSCs improve neuroinflammation and cognitive and motor function. These EVs may also induce beneficial effects directly in the brain.
In many pathological situations, including hyperammonemia and hepatic encephalopathy, cognitive and motor impairment are a consequence of altered neurotransmission which, in turn, is a consequence of neuroinflammation (Cabrera-Pastor et al., 2019; Häussinger et al., 2022). A key step in the induction of neuroinflammation is usually the activation of microglia, the central nervous system immune cells, which go from a resting state to a pro-inflammatory activated state. In this state, microglial cells release pro-inflammatory factors, alter neurotransmission, and may induce neurological alterations, neurotoxicity, and neurodegeneration (Block et al., 2007; Agusti et al., 2011; Hernández-Rabaza et al., 2016; Bartels et al., 2020; Shao et al., 2022). In response to different micro-environmental changes, microglia can polarize into either this pro-inflammatory phenotype or into an anti-inflammatory phenotype that releases anti-inflammatory mediators and induces anti-inflammatory and neuroprotective effects (Guo et al., 2022).
A main mechanism by which EVs from MSCs improve neuroinflammation and neurological function is by directly modulating the activation state of microglia, inducing a shift from the pro-inflammatory to the anti-inflammatory state (Figure 2). This effect is induced by EVs from MSCs when injected in rodent models in vivo (see below) and also when added ex vivo in hippocampal slices (Izquierdo-Altarejos et al., 2023) or in vitro in cultures of microglia (see below).
Li et al. (2017) administered EVs from human exfoliated deciduous teeth MSCs in a rat model of brain trauma and observed an improvement in the Basso-Beattie-Bresnahan motor function scale, injury reconstruction and a reduction in the pro-inflammatory microglia marker CD68 in rats treated with these EVs. This study also examined the effects of EVs from MSCs on microglial cultures stimulated by lipopolysaccharide (LPS). EVs reduced the levels of nitrite, TNFα, and IL-6, as well as the pro-inflammatory microglial marker CD68 and increased the levels of IL-10 and the anti-inflammatory microglial markers arginase 1 and CD206 (Li et al., 2017).
Li et al. (2019b) obtained similar results in a model of EAE, in which treatment with EVs from bone marrow MSCs reduced inflammation and demyelination of the central nervous system by regulating microglia polarization. Intravenous injection of EVs from MSCs improved neurological function in EAE rats and reduced the pro-inflammatory microglia marker CD68 and increased the anti-inflammatory microglia marker CD206 in the spinal cord. EVs also reduced the pro-inflammatory cytokines TNFα and IL-12 and increased the anti-inflammatory cytokines IL-10 and TGFβ in serum (Liu et al., 2019). These effects on microglia polarization were also corroborated in vitro in LPS-activated microglia cultures.
EVs from MSCs also induce a shift in microglia polarization from a pro-inflammatory to an anti-inflammatory state in the hippocampus of hyperammonemic rats. Hyperammonemia induces microglia activation and increases the hippocampal content of the pro-inflammatory IL-1β, TNFα, and IL-6 and reduces the anti-inflammatory IL-4, IL-10, and arginase 1. Treatment with EVs from MSCs reversed these changes, reducing IL-1β, TNFα, and IL-6 and increasing IL-4, IL-10, and arginase 1 (Izquierdo-Altarejos et al., 2023)
The polarization effects on microglia can be also relevant in Alzheimer’s disease. Kaniowska et al. (2022) reported that EVs from adipose MSCs are internalized by microglia cells in vitro and prevent the upregulation of inflammatory mediators such as TNFα and nitric oxide after stimulation with LPS and Aβ aggregates.
Mechanisms Underlying the Beneficial Effects of Extracellular Vesicles from Mesenchymal Stem Cells: Role of miR-124 and Transforming Growth Factor-β
Some studies have analyzed which components of the EVs from MSCs may be involved in their beneficial effects, including the induction of the microglia polarization from the pro-inflammatory to the anti-inflammatory state. Two main components that seem to promote this shift and contribute to the beneficial effects of EVs are the microRNA miR-124 and TGFβ.
Yang et al. (2019) analyzed the role of miR-124 contained in EVs from bone marrow MSCs in the polarization of microglia towards an anti-inflammatory state in a rat model of brain trauma. Intravenous administration of MSC-derived EVs enriched in miR-124 promoted the polarization of microglia towards an anti-inflammatory state in the hippocampus, decreasing the expression of the pro-inflammatory microglia marker CD32 and the levels of pro-inflammatory cytokines IL-1β, IL-6, and TNFα and increasing the expression of the anti-inflammatory microglia markers CD206 and Arginase 1, as well as the levels of anti-inflammatory cytokines IL-4, IL-10, and TGFβ. Furthermore, treatment with miR-124-enriched EVs from MSCs increased hippocampal neurogenesis and restored neurological function in rats with brain trauma, as assessed by the Morris water maze and neurological severity scale (Yang et al., 2019).
Previous studies showed that miR-124 promotes microglia polarization via the Toll-like receptor 4 (TLR4) signaling pathway (Yao et al., 2017; Periyasamy et al., 2018). Yang et al. (2019) investigated whether miR-124 contained in EVs from MSCs acts through this pathway. Upon binding of their ligand (i.e., LPS), TLR4 receptors can trigger intracellular myeloid differentiation primary response protein (MyD88)-dependent or MyD88-independent signaling cascades. The MyD88-dependent pathway involves the recruitment of TRAF6 factor and IRAKs kinases, which in turn result in the activation of TAK1 kinase. TAK1 phosphorylates the IKKβ complex, promoting the activation and translocation to the nucleus of the transcription factor NF-κB, which induces the transcription of pro-inflammatory genes such as IL-1β, IL-6, IL-18, and TNFα. Yang et al. (2019) found that treatment with miR-124-enriched EVs reduced the expression of TLR4, MyD88, interleukin 1 receptor associated kinase 1 (IRAK1), tumor necrosis factor receptor associated factor 6 (TRAF6), and the p65 subunit of NF-κB factor in both hippocampus of rats with brain trauma and LPS-stimulated microglia cultures, thus suggesting that miR-124 contained in EVs released by MSCs promotes microglia polarization by inhibiting the TLR4 pathway.
In addition to miR-124, other miRNAs may also modulate microglia polarization. Jiang et al. (2018) showed that EVs derived from adipose tissue MSCs reduced the lesion area, suppressing autophagy and promoting microglia polarization towards an anti-inflammatory state, in a mouse central cerebral artery occlusion infarction model, and that EVs enriched in miR-30d-5p had a greater anti-inflammatory effect.
Among the molecules that make up the MSCs secretome, the role of TGFβ, which regulates a wide variety of cellular processes and functions, is noteworthy (Eleuteri and Fierabracci, 2019).
TGFβ could act both at the level of the peripheral immune system and in the brain. Several studies support that TGFβ induces polarization of microglia towards an anti-inflammatory state. Zhou et al. (2012) observed that TGFβ induced alternative activation of microglia (anti-inflammatory state) by increasing the expression of YM1, arginase 1, and IL-4 receptor. This process was mediated by Smad2 phosphorylation and was inhibited if the TGFβ receptor was blocked. Spittau et al. (2013) propose that endogenous TGFβ promotes a quiescent state of microglia, preventing overexpression of the pro-inflammatory markers iNOS and IL-6. Noh et al. (2016) and Islam et al. (2018) showed that TGFβ exerts sustained anti-inflammatory effects in microglia: in LPS-stimulated microglia the transcription factor NF-κB translocates to the nucleus, increasing the production of pro-inflammatory factors. This was prevented in TGFβ-treated microglia.
EVs from MSCs contain TGFβ on their surfaces (Wada et al., 2010; Yu et al., 2013; Shelke et al., 2019). This TGFβ seems to mediate some of the beneficial effects of EVs. Exosomes expressing TGFβ in their membranes show a potent immunosuppressive activity and inhibit murine EAE, a model for multiple sclerosis (Yu et al., 2013). Exosomes derived from bone marrow MSCs reverse epithelial-mesenchymal transition via TGFβ/Smad pathway and promote the repair of damaged endometrium (Yao et al., 2019). TGFβ contained in EVs from MSCs modulates the immune system by regulating the Th1/Th2/Th17/Treg lymphocytes balance.
TGFβ contained in the EVs from adipose MSCs may also induce beneficial effects directly in the brain. Izquierdo-Altarejos et al. (2023) show that hyperammonemic rats have reduced TGFβ levels and decreased membrane expression of TGFβ receptors in the hippocampus. This leads to the activation of microglia and their polarization towards a pro-inflammatory state, as well as a reduction of the Smad7-IkBα pathway, which promotes translocation to the nucleus of NF-κB in neurons. Both microglia activation and NF-κB translocation increase IL-1β synthesis in microglia and neurons. This increase in IL-1β levels leads to IL-1 receptor activation, which in turn alters membrane expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptor subunits, inducing cognitive impairment.
EVs derived from MSCs injected into hyperammonemic rats reach the hippocampus and restore cognitive function. This improvement in cognitive function is mediated by TGFβ present on the surface of the EVs, which binds to TGFβ receptors on microglia and neurons. The TGFβ from the injected EVs leads to a shift in the phenotype of microglia from a pro-inflammatory to an anti-inflammatory state, reducing IL-1β production. In addition, TGFβ reduces the nuclear translocation of NF-κB in neurons by normalizing the Smad7-IkBα pathway. This also normalizes IL-1β production in neurons, contributing to the normalization of IL-1β levels in the hippocampus. As IL-1β levels are reduced, IL-1 receptor activation and changes in membrane expression of membrane expression of glutamatergic receptors subunits NR2B, GluA1 and GluA2 are reduced, restoring cognitive function. These effects are reproduced ex vivo by the addition of EVs from MSCs to hippocampal slices from hyperammonemic rats, indicating a direct effect of TGFβ in the EVs on the hippocampus (Izquierdo-Altarejos et al., 2023).
Therapy with Extracellular Vesicles from Mesenchymal Stem Cells: Challenges and Future Perspectives
The therapeutic potential of EVs from MSCs has been extensively demonstrated in multiple studies, as summarized in this review. These EVs represent a realistic option to treat a wide range of diseases that curse with peripheral or brain inflammation due to their regenerative, anti-inflammatory, and immunomodulatory properties.
Up to date, there are three clinical trials completed using EVs from MSCs as a therapy for coronavirus COVID-19 (https://clinicaltrials.gov, date: March 31, 2023). Two of them evaluate the treatment of coronavirus pneumonia (phase I, NCT04276987) and COVID-19-associated acute respiratory distress syndrome (phase II, NCT04493242). The third study evaluated tolerance to inhalation of EVs from MSCs in healthy volunteers (phase I, NCT04313647). Other ongoing studies which are now recruiting patients are evaluating the effects of treatment with EVs from MSCs in patients with ulcerative colitis, inflammatory bowel disease, Crohn’s disease, alopecia, ischemic stroke, pulmonary infection, and osteoarthritis.
However, therapy with EVs from MSCs is a relatively new field and presents some limitations and challenges to be addressed for their safe and effective clinical application. All steps in the isolation of EVs from MSCs should follow good manufacturing practices. The research studies performed, including clinical trials, employ different methodologies and experimental designs. Critical points to standardize are the source and culture conditions of MSCs, isolation protocol and quality control of EVs, storage conditions, dose, and administration route.
Conclusions
The studies summarized in this review show that EVs play an important role in the induction of many pathologies associated with sustained inflammation and neuroinflammation, including cancer, hyperammonemia and hepatic encephalopathy, and other neurological and neurodegenerative diseases. Identifying the mechanisms by which EVs contribute to the pathogenesis of these diseases and the transmission of pathologies from the periphery to the brain will help to develop new treatments for these pathologies and new diagnostic tools for their easy and early detection. In contrast, EVs from MSCs have a remarkable therapeutic utility in many of the above pathologies, by reducing inflammation, neuroinflammation and improving cognitive and motor function. Identifying the mechanisms by which EVs from MSC induce the beneficial effects and the main molecules (e.g., proteins and miRNAs) involved may help to improve the therapeutic utility of MSC-EVs.
Footnotes
Funding: This work was supported in part by the Ministerio de Ciencia e Innovación Spain (PID2020-113388RB-I00 to VF and PID2021-124359OB-I00 to VMM), Consellería Educación Generalitat Valenciana (CIPROM/2021/082 to VF), and co-funded with European Regional Development Funds (ERDF) to VF and VMM.
Conflicts of interest: The authors have no competing interests to disclose.
Data availability statement: Not applicable.
C-Editors: Zhao M, Liu WJ, Li CH; T-Editor: Jia Y
References
- 1.Aguiar Koga BA, Fernandes LA, Fratini P, Sogayar MC, Carreira ACO. Role of MSC-derived small extracellular vesicles in tissue repair and regeneration. Front Cell Dev Biol. (2023);10:1047094. doi: 10.3389/fcell.2022.1047094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agusti A, Cauli O, Rodrigo R, Llansola M, Hernández-Rabaza V, Felipo V. p38 MAP kinase is a therapeutic target for hepatic encephalopathy in rats with portacaval shunts. Gut. (2011);60:1572–1579. doi: 10.1136/gut.2010.236083. [DOI] [PubMed] [Google Scholar]
- 3.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. (2009);64:783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. (2011);29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 5.Andrzejewska A, Lukomska B, Janowski M. Concise review:mesenchymal stem cells:from roots to boost. Stem Cells. (2019);37:855–864. doi: 10.1002/stem.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells:immune evasive, not immune privileged. Nat Biotechnol. (2014);32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badillo AT, Beggs KJ, Javazon EH, Tebbets JC, Flake AW. Murine bone marrow stromal progenitor cells elicit an in vivo cellular and humoral alloimmune response. Biol Blood Marrow Transplant. (2007);13:412–422. doi: 10.1016/j.bbmt.2006.12.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagno L, Hatzistergos KE, Balkan W, Hare JM. Mesenchymal stem cell-based therapy for cardiovascular disease:progress and challenges. Mol Ther. (2018);26:1610–1623. doi: 10.1016/j.ymthe.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey AJM, Li H, Kirkham AM, Tieu A, Maganti HB, Shorr R, Fergusson DA, Lalu MM, Elomazzen H, Allan DS. MSC-derived extracellular vesicles to heal diabetic wounds:a systematic review and meta-analysis of preclinical animal studies. Stem Cell Rev Rep. (2022);18:968–979. doi: 10.1007/s12015-021-10164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartels T, De Schepper S, Hong S. Microglia modulate neurodegeneration in Alzheimer's and Parkinson's diseases. Science. (2020);370:66–69. doi: 10.1126/science.abb8587. [DOI] [PubMed] [Google Scholar]
- 11.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity:uncovering the molecular mechanisms. Nat Rev Neurosci. (2007);8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 12.Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci. (2017);18:1450. doi: 10.3390/ijms18071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabrera-Pastor A, Llansola M, Montoliu C, Malaguarnera M, Balzano T, Taoro-Gonzalez L, García-García R, Mangas-Losada A, Izquierdo-Altarejos P, Arenas YM, Leone P, Felipo V. Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy:Underlying mechanisms and therapeutic implications. Acta Physiol (Oxf) (2019);226:e13270. doi: 10.1111/apha.13270. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y, Gang X, Sun C, Wang G. Mesenchymal stem cells improve healing of diabetic foot ulcer. J Diabetes Res. (2017);2017:9328347. doi: 10.1155/2017/9328347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chance TC, Herzig MC, Christy BA, Delavan C, Rathbone CR, Cap AP, Bynum JA. Human mesenchymal stromal cell source and culture conditions influence extracellular vesicle angiogenic and metabolic effects on human endothelial cells in vitro. J Trauma Acute Care Surg. (2020);89:S100–108. doi: 10.1097/TA.0000000000002661. [DOI] [PubMed] [Google Scholar]
- 16.Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, Farhoodi HP, Zhang SX, Zimak J, Ségaliny A, Riazifar M, Pham V, Digman MA, Pone EJ, Zhao W. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng. (2016);9:509–529. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HX, Liang FC, Gu P, Xu BL, Xu HJ, Wang WT, Hou JY, Xie DX, Chai XQ, An SJ. Exosomes derived from mesenchymal stem cells repair a Parkinson's disease model by inducing autophagy. Cell Death Dis. (2020a);11:288. doi: 10.1038/s41419-020-2473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SY, Lin MC, Tsai JS, He PL, Luo WT, Chiu IM, Herschman HR, Li HJ. Exosomal 2',3'-CNP from mesenchymal stem cells promotes hippocampus CA1 neurogenesis/neuritogenesis and contributes to rescue of cognition/learning deficiencies of damaged brain. Stem Cells Transl Med. (2020b);9:499–517. doi: 10.1002/sctm.19-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng J, Chen Z, Liu C, Zhong M, Wang S, Sun Y, Wen H, Shu T. Bone mesenchymal stem cell-derived exosome-loaded injectable hydrogel for minimally invasive treatment of spinal cord injury. Nanomedicine (Lond) (2021);16:1567–1579. doi: 10.2217/nnm-2021-0025. [DOI] [PubMed] [Google Scholar]
- 20.Dabrowska S, Andrzejewska A, Strzemecki D, Muraca M, Janowski M, Lukomska B. Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats. J Neuroinflammation. (2019);16:216. doi: 10.1186/s12974-019-1602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Celis-Ruiz E, Fuentes B, Alonso de Leciñana M, Gutiérrez-Fernández M, Borobia AM, Gutiérrez-Zúñiga R, Ruiz-Ares G, Otero-Ortega L, Laso-García F, Gómez-de Frutos MC, Díez-Tejedor E. Final Results of Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells in Acute Ischemic Stroke (AMASCIS):A phase II, randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. Cell Transplant. (2022);31:9636897221083863. doi: 10.1177/09636897221083863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eleuteri S, Fierabracci A. Insights into the secretome of mesenchymal stem cells and its potential applications. Int J Mol Sci. (2019);20:4597. doi: 10.3390/ijms20184597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan L, Liu C, Chen X, Zheng L, Zou Y, Wen H, Guan P, Lu F, Luo Y, Tan G, Yu P, Chen D, Deng C, Sun Y, Zhou L, Ning C. Exosomes-loaded electroconductive hydrogel synergistically promotes tissue repair after spinal cord injury via immunoregulation and enhancement of myelinated axon growth. Adv Sci (Weinh) (2022);9:e2105586. doi: 10.1002/advs.202105586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fathollahi A, Hashemi SM, Haji Molla Hoseini M, Yeganeh F. In vitro analysis of immunomodulatory effects of mesenchymal stem cell- and tumor cell -derived exosomes on recall antigen-specific responses. Int Immunopharmacol. (2019);67:302–310. doi: 10.1016/j.intimp.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Felipo V. Hepatic encephalopathy:effects of liver failure on brain function. Nat Rev Neurosci. (2013);14:851–858. doi: 10.1038/nrn3587. [DOI] [PubMed] [Google Scholar]
- 26.Franco da Cunha F, Andrade-Oliveira V, Candido de Almeida D, Borges da Silva T, Naffah de Souza Breda C, Costa Cruz M, Faquim-Mauro EL, Antonio Cenedeze M, Ioshie Hiyane M, Pacheco-Silva A, Aparecida Cavinato R, Torrecilhas AC, Olsen Saraiva Câmara N. Extracellular vesicles isolated from mesenchymal stromal cells modulate CD4+T lymphocytes toward a regulatory profile. Cells. (2020);9:1059. doi: 10.3390/cells9041059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y, Zhu J, Ma L, Guo J, Shi H, Zou Y, Ge J. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-αmediated NF-κB pathway. J Cell Mol Med. (2016);20:2318–2327. doi: 10.1111/jcmm.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Go V, Bowley BGE, Pessina MA, Zhang ZG, Chopp M, Finklestein SP, Rosene DL, Medalla M, Buller B, Moore TL. Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. Geroscience. (2019);42:1–17. doi: 10.1007/s11357-019-00115-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, Camussi G. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med. (2014);33:1055–1063. doi: 10.3892/ijmm.2014.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grey M, Dunning CJ, Gaspar R, Grey C, Brundin P, Sparr E, Linse S. Acceleration of α-synuclein aggregation by exosomes. J Biol Chem. (2015);290:2969–2982. doi: 10.1074/jbc.M114.585703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guay C, Kruit JK, Rome S, Menoud V, Mulder NL, Jurdzinski A, Mancarella F, Sebastiani G, Donda A, Gonzalez BJ, Jandus C, Bouzakri K, Pinget M, Boitard C, Romero P, Dotta F, Regazzi R. Lymphocyte-derived exosomal microRNAs promote pancreatic βcell death and may contribute to type 1 diabetes development. Cell Metab. (2019);29:348–361.e6. doi: 10.1016/j.cmet.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Guo S, Wang H, Yin Y. Microglia polarization from M1 to M2 in neurodegenerative diseases. Front Aging Neurosci. (2022);14:815347. doi: 10.3389/fnagi.2022.815347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. J Neuroinflammation. (2014);11:68. doi: 10.1186/1742-2094-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han C, Xiong N, Guo X, Huang J, Ma K, Liu L, Xia Y, Shen Y, Li J, Jiang H, Wang L, Guo S, Xu X, Zhang G, Liu J, Cao X, Zhang Z, Lin Z, Wang T. Exosomes from patients with Parkinson's disease are pathological in mice. J Mol Med (Berl) (2019);97:1329–1344. doi: 10.1007/s00109-019-01810-z. [DOI] [PubMed] [Google Scholar]
- 35.Han D, Wu C, Xiong Q, Zhou L, Tian Y. Anti-inflammatory mechanism of bone marrow mesenchymal stem cell transplantation in rat model of spinal cord injury. Cell Biochem Biophys. (2015);71:1341–1347. doi: 10.1007/s12013-014-0354-1. [DOI] [PubMed] [Google Scholar]
- 36.Hannafon BN, Ding WQ. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci. (2013);14:14240–69. doi: 10.3390/ijms140714240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. (2019);8:1605. doi: 10.3390/cells8121605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Häussinger D, Dhiman RK, Felipo V, Görg B, Jalan R, Kircheis G, Merli M, Montagnese S, Romero-Gomez M, Schnitzler A, Taylor-Robinson SD, Vilstrup H. Hepatic encephalopathy. Nat Rev Dis Primers. (2022);8:43. doi: 10.1038/s41572-022-00366-6. [DOI] [PubMed] [Google Scholar]
- 39.Heldring N, Mager I, Wood MJ, Le Blanc K, Andaloussi SE. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum Gene Ther. (2015);26:506–517. doi: 10.1089/hum.2015.072. [DOI] [PubMed] [Google Scholar]
- 40.Hernández-Rabaza V, Cabrera-Pastor A, Taoro-González L, Malaguarnera M, Agustí A, Llansola M, Felipo V. Hyperammonemia induces glial activation, neuroinflammation and alters neurotransmitter receptors in hippocampus, impairing spatial learning:reversal by sulforaphane. J Neuroinflammation. (2016);13:41. doi: 10.1186/s12974-016-0505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, et al. Tumour exosome integrins determine organotropic metastasis. Nature. (2015);527:329–935. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosseini Shamili F, Alibolandi M, Rafatpanah H, Abnous K, Mahmoudi M, Kalantari M, Taghdisi SM, Ramezani M. Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J Control Release. (2019);299:149–164. doi: 10.1016/j.jconrel.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 43.Islam A, Choudhury ME, Kigami Y, Utsunomiya R, Matsumoto S, Watanabe H, Kumon Y, Kunieda T, Yano H, Tanaka J. Sustained anti-inflammatory effects of TGF-β1 on microglia/macrophages. Biochim Biophys Acta Mol Basis Dis. (2018);1864:721–734. doi: 10.1016/j.bbadis.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Izquierdo-Altarejos P, Cabrera-Pastor A, Gonzalez-King H, Montoliu C, Felipo V. Extracellular vesicles from hyperammonemic rats induce neuroinflammation and motor incoordination in control rats. Cells. (2020);9:572. doi: 10.3390/cells9030572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Izquierdo-Altarejos P, Martínez-García M, Felipo V. Extracellular vesicles from hyperammonemic rats induce neuroinflammation in cerebellum of normal rats:role of increased TNFαcontent. Front Immunol. (2022);13:921947. doi: 10.3389/fimmu.2022.921947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Izquierdo-Altarejos P, Cabrera-Pastor A, Martínez-García M, Sánchez-Huertas C, Hernández A, Moreno-Manzano V, Felipo V. Extracellular vesicles from mesenchymal stem cells reduce neuroinflammation in hippocampus and restore cognitive function in hyperammonemic rats. J Neuroinflammation. (2023);20:1. doi: 10.1186/s12974-022-02688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, Cai L, Hu R, Xu L, Li L. Exosomes from miR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem. (2018);47:864–878. doi: 10.1159/000490078. [DOI] [PubMed] [Google Scholar]
- 48.Jin Y, Wu R, Li L, Shen L, Gu Y, Sun C. Exosomes from inflamed macrophages promote the progression of Parkinson's disease by inducing neuroinflammation. Mol Neurobiol. (2023);60:1914–1928. doi: 10.1007/s12035-022-03179-6. [DOI] [PubMed] [Google Scholar]
- 49.Kang X, Zuo Z, Hong W, Tang H, Geng W. Progress of research on exosomes in the protection against ischemic brain injury. Front Neurosci. (2019);13:1149. doi: 10.3389/fnins.2019.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaniowska D, Wenk K, Rademacher P, Weiss R, Fabian C, Schulz I, Guthardt M, Lange F, Greiser S, Schmidt M, Braumann UD, Emmrich F, Koehl U, Jaimes Y. Extracellular vesicles of mesenchymal stromal cells can be taken up by microglial cells and partially prevent the stimulation induced by β-amyloid. Stem Cell Rev Rep. (2022);18:1113–1126. doi: 10.1007/s12015-021-10261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kimura K, Hohjoh H, Fukuoka M, Sato W, Oki S, Tomi C, Yamaguchi H, Kondo T, Takahashi R, Yamamura T. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat Commun. (2018);9:17. doi: 10.1038/s41467-017-02406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamo-Espinosa JM, Blanco JF, Sánchez M, Moreno V, Granero-Moltó F, Sánchez-Guijo F. Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. J Transl Med. (2020);18:356. doi: 10.1186/s12967-020-02530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JY, Park JK, Lee EY, Lee EB, Song YW. Circulating exosomes from patients with systemic lupus erythematosus induce an proinflammatory immune response. Arthritis Res Ther. (2016);18:264. doi: 10.1186/s13075-016-1159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li JJ, Wang B, Kodali MC, Chen C, Kim E, Patters BJ, Lan L, Kumar S, Wang X, Yue J, Liao FF. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J Neuroinflammation. (2018);15:8. doi: 10.1186/s12974-017-1038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li L, Zhang Y, Mu J, Chen J, Zhang C, Cao H, Gao J. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. (2020);20:4298–4305. doi: 10.1021/acs.nanolett.0c00929. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Yang YY, Ren JL, Xu F, Chen FM, Li A. Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Res Ther. (2017);8:198. doi: 10.1186/s13287-017-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Wang F, Guo R, Zhang Y, Chen D, Li X, Tian W, Xie X, Jiang Z. Exosomal sphingosine 1-phosphate secreted by mesenchymal stem cells regulated Treg/Th17 balance in aplastic anemia. IUBMB Life. (2019a);71:1284–1292. doi: 10.1002/iub.2035. [DOI] [PubMed] [Google Scholar]
- 58.Li Z, Liu F, He X, Yang X, Shan F, Feng J. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int Immunopharmacol. (2019b);67:268–280. doi: 10.1016/j.intimp.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Liew LC, Katsuda T, Gailhouste L, Nakagama H, Ochiya T. Mesenchymal stem cell-derived extracellular vesicles:a glimmer of hope in treating Alzheimer's disease. Int Immunol. (2017);29:11–19. doi: 10.1093/intimm/dxx002. [DOI] [PubMed] [Google Scholar]
- 60.Lightner AL, Reese J, Ream J, Nachand D, Jia X, Dadgar N, Steele SR, Hull T. A Phase IB/IIA study of ex vivo expanded allogeneic bone marrow derived mesenchymal stem cells for the treatment of perianal fistulizing Crohn's disease. Dis Colon Rectum. (2023) doi: 10.1097/DCR.0000000000002567. doi:10.1097/DCR.0000000000002567. [DOI] [PubMed] [Google Scholar]
- 61.Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. (2017);49:e346. doi: 10.1038/emm.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo Z, Peng W, Xu Y, Xie Y, Liu Y, Lu H, Cao Y, Hu J. Exosomal OTULIN from M2 macrophages promotes the recovery of spinal cord injuries via stimulating Wnt/β-catenin pathway-mediated vascular regeneration. Acta Biomater. (2021);136:519–532. doi: 10.1016/j.actbio.2021.09.026. [DOI] [PubMed] [Google Scholar]
- 63.Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, Liao W, Kang Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. (2018);9:247. doi: 10.1186/s13287-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masyuk AI, Masyuk TV, Larusso NF. Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. J Hepatol. (2013);59:621–625. doi: 10.1016/j.jhep.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mellado-López M, Griffeth RJ, Meseguer-Ripolles J, Cugat R, García M, Moreno-Manzano V. Plasma rich in growth factors induces cell proliferation, migration, differentiation, and cell survival of adipose-derived stem cells. Stem Cells Int. (2017);2017:5946527. doi: 10.1155/2017/5946527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mendes-Pinheiro B, Anjo SI, Manadas B, Da Silva JD, Marote A, Behie LA, Teixeira FG, Salgado AJ. Bone marrow mesenchymal stem cells'secretome exerts neuroprotective effects in a Parkinson's disease rat model. Front Bioeng Biotechnol. (2019);7:294. doi: 10.3389/fbioe.2019.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Dalir-Naghadeh B. Phenotypic modulation of auto-reactive cells by insertion of tolerogenic molecules via MSC-derived exosomes. Vet Res Forum. (2012);3(4):257–261. [PMC free article] [PubMed] [Google Scholar]
- 68.Mu J, Li L, Wu J, Huang T, Zhang Y, Cao J, Ma T, Chen J, Zhang C, Zhang X, Lu T, Kong X, Sun J, Gao J. Hypoxia-stimulated mesenchymal stem cell-derived exosomes loaded by adhesive hydrogel for effective angiogenic treatment of spinal cord injury. Biomater Sci. (2022);10:1803–1811. doi: 10.1039/d1bm01722e. [DOI] [PubMed] [Google Scholar]
- 69.Muhammad SA, Abbas AY, Imam MU, Saidu Y, Bilbis LS. Efficacy of stem cell secretome in the treatment of traumatic brain injury:A systematic review and meta-analysis of preclinical studies. Mol Neurobiol. (2022);59:2894–2909. doi: 10.1007/s12035-022-02759-w. [DOI] [PubMed] [Google Scholar]
- 70.Muñoz-Criado I, Meseguer-Ripolles J, Mellado-López M, Alastrue-Agudo A, Griffeth RJ, Forteza-Vila J, Cugat R, García M, Moreno-Manzano V. Human suprapatellar fat pad-derived mesenchymal stem cells induce chondrogenesis and cartilage repair in a model of severe osteoarthritis. Stem Cells Int 2017. (2017):4758930. doi: 10.1155/2017/4758930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nallakumarasamy A, Jeyaraman M, Maffulli N, Jeyaraman N, Suresh V, Ravichandran S, Gupta M, Potty AG, El-Amin SF, 3rd, Khanna M, Gupta A. Mesenchymal stromal cell-derived extracellular vesicles in wound healing. Life (Basel) (2022);12:1733. doi: 10.3390/life12111733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noh MY, Lim SM, Oh KW, Cho KA, Park J, Kim KS, Lee SJ, Kwon MS, Kim SH. Mesenchymal stem cells modulate the functional properties of microglia via TGF-βsecretion. Stem Cells Transl Med. (2016);5:1538–1549. doi: 10.5966/sctm.2015-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Odegaard KE, Chand S, Wheeler S, Tiwari S, Flores A, Hernandez J, Savine M, Gowen A, Pendyala G, Yelamanchili SV. Role of extracellular vesicles in substance abuse and HIV-related neurological pathologies. Int J Mol Sci. (2020);21:6765. doi: 10.3390/ijms21186765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Otero-Ortega L, Gómez de Frutos MC, Laso-García F, Rodríguez-Frutos B, Medina-Gutiérrez E, López JA, Vázquez J, Díez-Tejedor E, Gutiérrez-Fernández M. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J Cereb Blood Flow Metab. (2018);38:767–779. doi: 10.1177/0271678X17708917. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Leselbaum A, Danese S;ADMIRE CD Study Group Collaborators. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease:a phase 3 randomised, double-blind controlled trial. Lancet. (2016);388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 76.Periyasamy P, Liao K, Kook YH, Niu F, Callen SE, Guo ML, Buch S. Cocaine-mediated downregulation of miR-124 activates microglia by targeting KLF4 and TLR4 signaling. Mol Neurobiol. (2018);55:3196–3210. doi: 10.1007/s12035-017-0584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pischiutta F, Caruso E, Cavaleiro H, Salgado AJ, Loane DJ, Zanier ER. Mesenchymal stromal cell secretome for traumatic brain injury:Focus on immunomodulatory action. Exp Neurol. (2022);357:114199. doi: 10.1016/j.expneurol.2022.114199. [DOI] [PubMed] [Google Scholar]
- 78.Poncelet AJ, Vercruysse J, Saliez A, Gianello P. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation. (2007);83:783–790. doi: 10.1097/01.tp.0000258649.23081.a3. [DOI] [PubMed] [Google Scholar]
- 79.Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V. Human mesenchymal stem cells inhibit neutrophil apoptosis:a model for neutrophil preservation in the bone marrow niche. Stem Cells. (2008);26:151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 80.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. (1996);183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles:important and underappreciated mediators of cell-to-cell communication. Leukemia. (2006);20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 82.Regmi S, Pathak S, Kim JO, Yong CS, Jeong JH. Mesenchymal stem cell therapy for the treatment of inflammatory diseases:Challenges, opportunities, and future perspectives. Eur J Cell Biol. (2019);98:151041. doi: 10.1016/j.ejcb.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 83.Reza-Zaldivar EE, Hernández-Sapiéns MA, Gutiérrez-Mercado YK, Sandoval-Ávila S, Gomez-Pinedo U, Márquez-Aguirre AL, Vázquez-Méndez E, Padilla-Camberos E, Canales-Aguirre AA. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer's disease. Neural Regen Res. (2019);14:1626–1634. doi: 10.4103/1673-5374.255978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lässer C, Segaliny AI, McIntyre LL, Shelke GV, Hutchins E, Hamamoto A, Calle EN, Crescitelli R, Liao W, Pham V, Yin Y, Jayaraman J, Lakey JRT, Walsh CM, Van Keuren-Jensen K, Lotvall J, et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. (2019);13:6670–6688. doi: 10.1021/acsnano.9b01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shao F, Wang X, Wu H, Wu Q, Zhang J. Microglia and neuroinflammation:crucial pathological mechanisms in traumatic brain injury-induced neurodegeneration. Front Aging Neurosci. (2022);14:825086. doi: 10.3389/fnagi.2022.825086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shelke GV, Yin Y, Jang SC, Lässer C, Wennmalm S, Hoffmann HJ, Li L, Gho YS, Nilsson JA, Lötvall J. Endosomal signalling via exosome surface TGFβ-1. J Extracell Vesicles. (2019);8:1650458. doi: 10.1080/20013078.2019.1650458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soares MBP, Gonçalves RGJ, Vasques JF, da Silva-Junior AJ, Gubert F, Santos GC, de Santana TA, Almeida Sampaio GL, Silva DN, Dominici M, Mendez-Otero R. Current status of mesenchymal stem/stromal cells for treatment of neurological diseases. Front Mol Neurosci. (2022);15:883378. doi: 10.3389/fnmol.2022.883378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spittau B, Wullkopf L, Zhou X, Rilka J, Pfeifer D, Krieglstein K. Endogenous transforming growth factor-beta promotes quiescence of primary microglia in vitro. Glia. (2013);61:287–300. doi: 10.1002/glia.22435. [DOI] [PubMed] [Google Scholar]
- 89.Sproviero D, La Salvia S, Giannini M, Crippa V, Gagliardi S, Bernuzzi S, Diamanti L, Ceroni M, Pansarasa O, Poletti A, Cereda C. Pathological proteins are transported by extracellular vesicles of sporadic amyotrophic lateral sclerosis patients. Front Neurosci. (2018);12:487. doi: 10.3389/fnins.2018.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018):a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. (2018);7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomi G, Joerger-Messerli M, Haesler V, Muri L, Surbek D, Schoeberlein A. Intranasally administered exosomes from umbilical cord stem cells have preventive neuroprotective effects and contribute to functional recovery after perinatal brain injury. Cells. (2019);8:855. doi: 10.3390/cells8080855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsilioni I, Theoharides TC. Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial DNA, and stimulate human microglia to secrete IL-1β. J Neuroinflammation. (2018);15:239. doi: 10.1186/s12974-018-1275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsintou M, Dalamagkas K, Moore TL, Rathi Y, Kubicki M, Rosene DL, Makris N. The use of hydrogel-delivered extracellular vesicles in recovery of motor function in stroke:a testable experimental hypothesis for clinical translation including behavioral and neuroimaging assessment approaches. Neural Regen Res. (2021);16:605–613. doi: 10.4103/1673-5374.295269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. (2007);9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 95.Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Fernández C, Tapiador N, Sevilla M, Morejón C, Montilla J, Martínez F, Marín E, Bustamante S, Vázquez D, Carballido J, Rodríguez A, Martínez P, García C, Ovejero M, Fernández MV, et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy. (2017);19:349–359. doi: 10.1016/j.jcyt.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 96.Vilaça-Faria H, Salgado AJ, Teixeira FG. Mesenchymal stem cells-derived exosomes:a new possible therapeutic strategy for Parkinson's disease? Cells. (2019);8:118. doi: 10.3390/cells8020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. (2018);15:36–45. doi: 10.7150/ijms.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wada J, Onishi H, Suzuki H, Yamasaki A, Nagai S, Morisaki T, Katano M. Surface-bound TGF-beta1 on effusion-derived exosomes participates in maintenance of number and suppressive function of regulatory T-cells in malignant effusions. Anticancer Res. (2010);30:3747–3757. [PubMed] [Google Scholar]
- 99.Wang X, Yang G. Bone marrow mesenchymal stem cells-derived exosomes reduce Aβdeposition and improve cognitive function recovery in mice with Alzheimer's disease by activating sphingosine kinase/sphingosine-1-phosphate signaling pathway. Cell Biol Int. (2021);45:775–784. doi: 10.1002/cbin.11522. [DOI] [PubMed] [Google Scholar]
- 100.Wu P, Zhang B, Shi H, Qian H, Xu W. MSC-exosome:A novel cell-free therapy for cutaneous regeneration. Cytotherapy. (2018);20:291–301. doi: 10.1016/j.jcyt.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 101.Xie K, Liu L, Chen J, Liu F. Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life. (2019);71:2020–2030. doi: 10.1002/iub.2147. [DOI] [PubMed] [Google Scholar]
- 102.Xie M, Li C, She Z, Wu F, Mao J, Hun M, Luo S, Wan W, Tian J, Wen C. Human umbilical cord mesenchymal stem cells derived extracellular vesicles regulate acquired immune response of lupus mouse in vitro. Sci Rep. (2022);12:13101. doi: 10.1038/s41598-022-17331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. (2017);48:747–753. doi: 10.1161/STROKEAHA.116.015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. (2013);33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. (2015);4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang Y, Ye Y, Kong C, Su X, Zhang X, Bai W, He X. MiR-124 enriched exosomes promoted the M2 polarization of microglia and enhanced hippocampus neurogenesis after traumatic brain injury by inhibiting TLR4 pathway. Neurochem Res. (2019);44:811–828. doi: 10.1007/s11064-018-02714-z. [DOI] [PubMed] [Google Scholar]
- 107.Yao X, Liu S, Ding W, Yue P, Jiang Q, Zhao M, Hu F, Zhang H. TLR4 signal ablation attenuated neurological deficits by regulating microglial M1/M2 phenotype after traumatic brain injury in mice. J Neuroimmunol. (2017);310:38–45. doi: 10.1016/j.jneuroim.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 108.Yao Y, Chen R, Wang G, Zhang Y, Liu F. Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res Ther. (2019);10:225. doi: 10.1186/s13287-019-1332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu L, Yang F, Jiang L, Chen Y, Wang K, Xu F, Wei Y, Cao X, Wang J, Cai Z. Exosomes with membrane-associated TGF-β1 from gene-modified dendritic cells inhibit murine EAE independently of MHC restriction. Eur J Immunol. (2013);43:2461–2472. doi: 10.1002/eji.201243295. [DOI] [PubMed] [Google Scholar]
- 110.Yuyama K, Sun H, Usuki S, Sakai S, Hanamatsu H, Mioka T. A potential function for neuronal exosomes:sequestering intracerebral amyloid-βpeptide. FEBS Lett. (2015);589:84–88. doi: 10.1016/j.febslet.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 111.Zhang R, Liu Y, Yan K, Chen L, Chen XR, Li P, Chen FF, Jiang XD. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. (2013);10:106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang W, Wang Y, Kong Y. Exosomes derived from mesenchymal stem cells modulate miR-126 to ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1. Invest Ophthalmol Vis Sci. (2019);60:294–303. doi: 10.1167/iovs.18-25617. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. (2015);122:856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao J, Deng H, Xun C, Chen C, Hu Z, Ge L, Jiang Z. Therapeutic potential of stem cell extracellular vesicles for ischemic stroke in preclinical rodent models:a meta-analysis. Stem Cell Res Ther. (2023);14:62. doi: 10.1186/s13287-023-03270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou X, Spittau B, Krieglstein K. TGFβsignalling plays an important role in IL4-induced alternative activation of microglia. J Neuroinflammation. (2012);9:210. doi: 10.1186/1742-2094-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]


