Abstract
Neurodegenerative disorders affect millions of people worldwide, and the prevalence of these disorders is only projected to rise as the number of people over 65 will drastically increase in the coming years. While therapies exist to aid in symptomatic relief, effective treatments that can stop or reverse the progress of each neurodegenerative disease are lacking. Recently, research on the role of extracellular vesicles as disease markers and therapeutics has been intensively studied. Exosomes, 30–150 nm in diameter, are one type of extracellular vesicles facilitating cell-to-cell communication. Exosomes are thought to play a role in disease propagation in a variety of neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Accordingly, the exosomes derived from the patients are an invaluable source of disease biomarkers. On the other hand, exosomes, especially those derived from stem cells, could serve as a therapeutic for these disorders, as seen by a rapid increase in clinical trials investigating the therapeutic efficacy of exosomes in different neurological diseases. This review summarizes the pathological burden and therapeutic approach of exosomes in neurodegenerative disorders. We also highlight how heat shock increases the yield of exosomes while still maintaining their therapeutic efficacy. Finally, this review concludes with outstanding questions that remain to be addressed in exosomal research.
Keywords: aging, Alzheimer’s disease, biomarker, drug, exosome, extracellular vesicle, neurodegenerative disease, Parkinson’s disease, therapy
Introduction
Neurodegenerative diseases encompass many disorders that impact the central nervous system and are associated with neuronal loss in specific brain regions and the accumulation of misfolded or aggregated proteins. While some proteins are found to be associated with specific neurodegenerative diseases, the pathogenesis for each disease is not fully understood. As a result, limited therapies and diagnostic tools are available for affected individuals. Most therapies are targeted at symptomatic relief without stopping or reversing the progression of the disease (Lamptey et al., 2022). Therefore, a fundamental understanding of the underlying pathophysiology of each neurodegenerative disorder and tests of additional therapeutic agents are essential to develop effective treatments that will provide hope for those impacted by these incurable diseases.
In recent years, interest in the role of extracellular vesicle (EV) research in neurodegenerative diseases has dramatically increased, as these vesicles have the potential to serve as biomarkers, therapies, and disease propagators depending on the sources of the vesicles (Yin et al., 2020; Mathew et al., 2021; Fan et al., 2022). All cell types release EVs into the extracellular environment under physiological or pathological conditions (Gurung et al., 2021). EVs have three major subtypes: apoptotic bodies, microvesicles, and exosomes (Figure 1). Apoptotic bodies range from 1000–5000 nm in diameter and are released from cells undergoing apoptosis. Microvesicles are 50–1000 nm in diameter and are released from the plasma membrane through clathrin-mediated shedding (Doyle and Wang, 2019). Microvesicles play a role in nerve regeneration, neuronal development, and synaptic activity (Lai and Breakefield, 2012). In addition, microvesicles have been shown to transmit signals throughout the brain (Porro et al., 2015). In a subset of neurodegenerative disorders, microvesicles have been shown to contain reduced concentrations of disease-associated proteins (Spitzer et al., 2019). Exosomes that arise from the endocytic pathway are 30–150 nm in diameter (Doyle and Wang, 2019). Apoptotic bodies contain the degraded products from cells undergoing apoptosis, and some studies highlight how exosomes can be derived from apoptotic bodies (Dieude et al., 2015). As exosomes facilitate intercellular communication, the role of exosomes derived from apoptotic bodies carrying biomolecules can then be engulfed by target cells. In neurodegenerative disorders, a cell undergoing apoptosis consists of dysfunctional mitochondria and other organelles that could then be taken to other cells by exosomes derived from apoptotic bodies to impact the function of the recipient cell (Figure 1). This review will focus on the therapeutic role of exosomes, as recent evidence suggests that stem cell-derived exosomes have therapeutic potential in neurodegenerative diseases. In contrast, the exosomes derived from diseased cells, including diseased neurons, have the pathogenic potential in the propagation of disease pathology.
Figure 1.
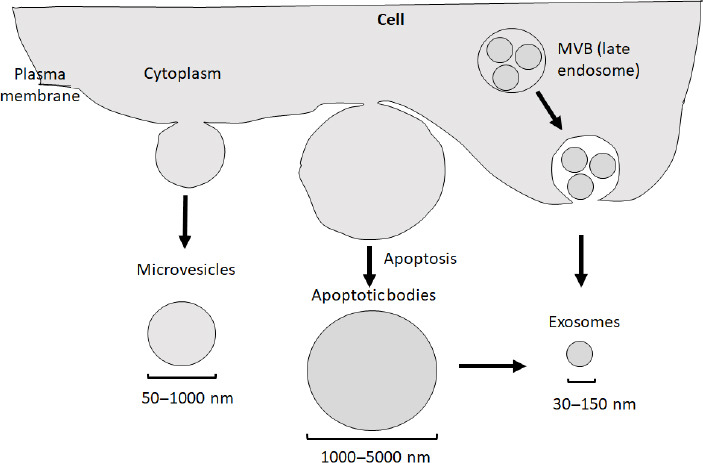
Three types of extracellular vesicles.
Based on their sizes and biogenesis, extracellular vesicles can be divided into three subtypes: apoptotic bodies (1000–5000 nm) generated during apoptosis (middle), microvesicles (50–1000 nm, left), and exosomes (30–150 nm, right). It is noted that in addition to being directly produced from a cell via the endocytic pathway, exosomes can also be generated from apoptotic bodies. Created with PowerPoint. MVB: Multivesicular body.
Search Strategy
The search was limited to studies published in PubMed in English, and the search period was from July 1983 to November 2022. The search keywords used are as follows: Exosomes in Neurodegenerative Disease/Disorder OR Exosomes OR Extracellular Vesicles in Neurodegenerative Disease OR Neurodegenerative Disorders OR Extracellular Vesicles OR Exosomes in Alzheimer’s Disease OR Pathological Burden of Exosomes in Neurodegenerative Disorders OR Stem Cells and Exosomes OR Exosomes in Parkinson’s Disease OR Exosomes as a Therapy OR Exosomes in Clinical Trials OR Mesenchymal Stem Cell-derived Exosomes OR Exosomes in Amyotrophic Lateral Sclerosis OR Exosome in Cell-to-Cell Communication.
Overview of Exosomal Biogenesis, Composition, and Function
Exosomes were first discovered in the 1980s when two labs published similar findings within a week of each other of how reticulocytes could endocytose gold-labeled transferrin and deliver it to the plasma membrane inside small vesicles (Harding et al., 1983; Pan et al., 1985). These small vesicles were later termed as exosomes (Johnstone et al., 1987). Initially, exosomes were thought to serve as the cell’s ‘garbage can’ where cells package their waste into these vesicles. Once these vesicles fused with the plasma membrane, their waste would be secreted into the extracellular space (Johnstone et al., 1991). The field of exosomal research has proliferated with studies of stem cell-derived exosomes conducted on the therapeutic potential and those derived from diseased cells in propagating the disease in various pathological conditions.
Exosomal biogenesis begins with the inward budding of the plasma membrane to generate an early endosome. Upon the early endosome maturation, the endosomes’ membrane invaginates to form intraluminal vesicles within multivesicular bodies. These multivesicular bodies have two fates: fusing with the lysosome to be degraded or fusing with the plasma membrane, where the intraluminal vesicles are released into the extracellular space as exosomes (Doyle and Wang, 2019; Figure 2). Once released into the extracellular space, exosomes play a role in cell-cell communication, thereby delivering their content to the recipient cells to alter the functions in the targeted cells (Ratajczak et al., 2006; Mathieu et al., 2019). Exosomes can interact with cells in different brain regions, suggesting their ability to have a widespread effect (Sterzenbach et al., 2017). The ability of exosomes to facilitate intercellular communication is a positive for their therapeutic potential; however, in disease states, this characteristic can cause further propagation of disease.
Figure 2.
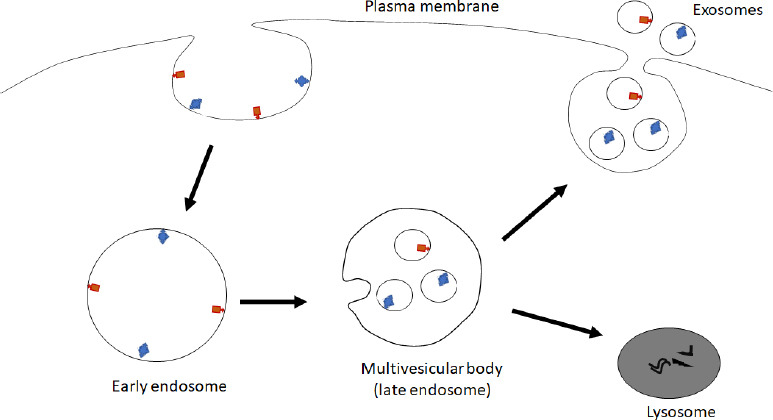
Biogenesis of exosomes.
Inward budding of plasma membrane leads to formation of the early endosome. Following invagination of the early endosome membrane and maturation, the early endosome becomes multivesicular body, a form of late endosome. The multivesicular body fuses either with a lysosome to digest the content of multivesicular body or with the plasma membrane to release the inside exosomes to the extracellular space. Created with PowerPoint.
Exosomes are secreted from all cell types in the brain: microglia, astrocytes, neurons, and neural progenitor cells (Pascua-Maestro et al., 2018; Jiang et al., 2020; Luong and Olson, 2021; Huber et al., 2022). Due to exosomes being a product of the endocytic pathway, their content reflects their donor cells, allowing them to reflect the real-time state of the parental cells. Exosomes contain nucleic acids, proteins, metabolites, and lipids within their vesicular structure. Over 9000 proteins, 3000 mRNA, and 1000 lipid entries are entered into ExoCarta (Mathivanan et al., 2012). Accordingly, exosomes can positively or negatively influence a recipient cell depending on the cargo they deliver.
Exosomes as Biomarkers for Neurodegenerative Disorders
Neurodegenerative disorders encompass a wide range of incurable neurological diseases with limited treatment options for impacted individuals (Lamptey et al., 2022). Although these disorders share a distinct feature of the aggregation of misfolded proteins, the aggregated proteins cause brain changes years before the clinical onset of symptoms, and current therapies cannot effectively control the accumulation of these aggregated proteins (Sweeney et al., 2017). Accumulating evidence supports that the exosomes derived from the neurons of neurodegenerative disease patients could amplify the progression of neurodegenerative disorders by delivering their contents to recipient cells (Sardar Sinha et al., 2018; Ruan et al., 2021). Delivering their content to recipient cells alters the recipient cells’ function (Gurung et al., 2021; Liu et al., 2021). Moreover, due to exosomal content reflecting their parental cell, exosomes make an attractive biomarker for clinical diagnostics. As the parental cell undergoes changes associated with the neurodegenerative disorder, the content of exosomes will reflect those changes. Exosomes can be easily isolated from patients in a non-invasive way from biological fluids, and any change in exosomal content can be monitored (Lin et al., 2015). Changes in exosomal content may serve as early biomarkers for diagnosing neurodegenerative diseases (Rastogi et al., 2021; Figure 3). Below we outline the pathological burden of exosomes and some studies using exosomes as biomarkers in three neurodegenerative disorders.
Figure 3.
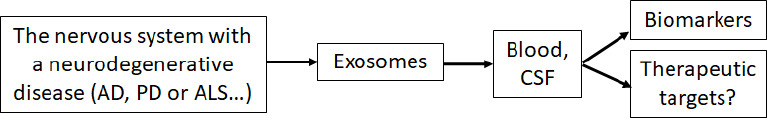
Exosomes serve as biomarkers for neurodegenerative diseases.
The cells in the nervous system with a neurodegenerative disease, such as AD, PD or ALS, secret exosomes carrying the disease-related molecules and enter the blood and CSF where the exosomes can be isolated to function as disease biomarkers or as therapeutic targets. Created with PowerPoint. AD: Alzheimer’s disease; ALS: amyotrophic lateral sclerosis; CSF: cerebrospinal fluid; PD: Parkinson’s disease.
Alzheimer’s disease
Alzheimer’s disease (AD) is the most common neurodegenerative disorder projected to impact an estimated 14 million people in the United States by 2050. AD is characterized by the accumulation of extracellular amyloid-β (Aβ) and intracellular accumulation of hyperphosphorylated tau. Unfortunately, current therapies for AD can only cause symptomatic relief at best, while a therapy that stops or reverses the progression of the disease is still lacking (No author listed, 2020).
The amyloid precursor protein (APP) can be cleaved by two pathways: non-amyloidogenic and amyloidogenic processing pathways. In the non-amyloidogenic pathway, APP is cleaved by α- and γ-secretase to produce C-terminal fragments and secreted form of APP. These products have been shown to have neuroprotective effects on the cell. In the amyloidogenic pathway, APP is cleaved by β- and γ-secretase to generate Aβ peptides of various lengths. A seminal paper by Rajendran et al. (2006) found that APP processing occurs in early endosomes, which yields Aβ peptides in multivesicular bodies. Aβ peptides can then be exported via exosomes. As APP processing occurs in the endosomal pathway, the cargo in AD-derived exosomes is significantly enriched in APP C-terminal fragments, BACE-1, γ-secretase, soluble APPβ, soluble APPα, and soluble Aβ-42 (Rajendran et al., 2006; Perez-Gonzalez et al., 2012; Goetzl et al., 2016).
Previous studies have shown that oligomeric Aβ is increased in exosomes isolated from AD brain samples. Further, intracellular oligomeric Aβ co-localizes with exosomes, and these exosomes facilitate the propagation of oligomeric Aβ between neurons (Sardar Sinha et al., 2018). Exosomes isolated from the bodily fluids of AD patients show an increase in Aβ1–42 (Abner et al., 2016). In addition, Alix and flotillin-1, two exosomal proteins, surround neuritic plaques in the brain (Rajendran et al., 2006). These findings strongly suggest that exosomes play a role in the propagation of Aβ in the brain; however, questions remain as to whether the cell getting rid of these proteins through the endocytic pathway is a protective mechanism.
Tau, a microtubule-associated protein, becomes hyperphosphorylated in AD. Exosomes can propagate hyperphosphorylated tau. For instance, exosomes isolated from patients with either mild cognitive impairment or an advanced stage of AD injected into healthy mice exhibited increased phosphorylated tau (Winston et al., 2016). In addition, AD EVs isolated from post-mortem brain samples contain more oligomeric tau compared to control samples. AD EVs also exhibited significantly higher neuronal uptake compared to control EVs, and the transfer of tau from the EVs to neurons was higher in AD EVs compared to control. AD EVs then propagate tau pathology in aged wild-type mice, and these EVs target GABAergic interneurons in the brains of these mice (Ruan et al., 2021). The propagation of the tau protein was significantly reduced when exosome secretion was inhibited, suggesting that exosomes are one mechanism mediating tau propagation in AD (Wang et al., 2017).
Along with neuronal tau propagation, microglia phagocytose human tau much more efficiently than neurons or astrocytes (Asai et al., 2015). Regardless of cell type, exosomes play an active role in tau propagation. Following injection of exosomes isolated from human induced pluripotent stem cells that express repeat domain of tau P301L and V337M mutations into wild-type mouse brains, tau inclusions (full-length and misfolded) were found in the mouse brains 2 months post-injection, suggesting that exosomes are sufficient to seed pathological forms of tau via long-distance propagation (Winston et al., 2019). This data strongly suggests exosomes play a key role in the disease propagation of tau in AD.
There is a correlation between Aβ1–42, phospho-T181-tau, and phosphor-S386-tau in exosomes with AD progress. Interestingly, the level of these proteins in exosomes could predict a patient having AD 10 years before clinical onset (Fiandaca et al., 2015). Furthermore, the levels of these proteins in exosomes can accurately predict the conversion of mild cognitive impairment to AD (Winston et al., 2016). Studies have shown that human bone marrow mesenchymal stem cell (MSC)-derived exosomes can alleviate the behavioral symptoms in an AD mouse model and decrease the plaque load (Cone et al., 2021). In addition, the inhibition of exosomal biogenesis has been shown to reduce plaque levels in mouse brains; however, the consequences of inhibiting exosomal secretion could have adverse effects on the cell (Asai et al., 2015), as inhibition of exosomal secretion in another neurodegenerative disease animal model was shown to exacerbate the related pathology (Iguchi et al., 2016).
Parkinson’s disease
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorder projected to impact 12 million people by 2040 (Dorsey et al., 2018). The disease is characterized by the loss of dopamine-producing neurons in the midbrain and the formation of Lewy bodies made up of the α-synuclein protein. Current therapeutics for treating PD can only improve symptoms without attenuating neuronal loss, as a cure for the disease is still lacking (Tysnes and Storstein, 2017).
Previous studies have shown that exosomes isolated from the serum of PD patients contain α-synuclein, and mild to late-stage PD patients contain the highest amounts of exosomal α-synuclein (Jiang et al., 2020). PD plasma-derived exosomes contained significantly higher amounts of oligomeric and Ser129 phosphorylated α-synuclein compared to control samples (Zheng et al., 2021). Furthermore, the level of α-synuclein in plasma neuronal exosomes isolated from patients with early-stage PD was significantly higher compared to healthy control individuals. In addition, 22 months later, patients with early-stage PD with longitudinally increased α-synuclein were at a higher risk for developing a progression of motor symptoms (Niu et al., 2020). These data suggest that α-synuclein in plasma neuronal exosomes is a biomarker for Parkinson’s disease development and progression.
Recently, the propagation of α-synuclein between cells was thought to be mediated at least partially by exosomes. In one study, exosomes provided an ideal environment for α-synuclein to aggregate (Grey et al., 2015). Most EV α-synuclein is attached to the outer membrane; however, it can also be detected inside the vesicle (Gustafsson et al., 2018). Mice treated with exosomes isolated from the serum of PD patients exhibited characteristics associated with PD, such as the degeneration of dopaminergic neurons and behavioral deficits (Han et al., 2019). Further support for the role of exosomes in mediating PD propagation came from the mice that were stereotaxically injected with exosomes derived from the plasma of PD patients and were taken up by microglial and transported to the cortex and substantia nigra (Xia et al., 2019). These findings provide strong evidence for the role of α-synuclein in PD and how exosomes can facilitate the propagation of α-synuclein in the brain. In the cerebrospinal fluid, lower levels of α-synuclein have been reported in PD patients compared to control individuals. In contrast, the plasma exosomal α-synuclein levels are significantly higher in PD patients (Chang et al., 2019), highlighting the role of exosomes in serving as a biomarker for diagnosing PD.
Amyotrophic lateral sclerosis
Amyotrophic Lateral Sclerosis (ALS) is a fatal neurodegenerative disorder projected to continually increase in prevalence in the coming years (Xu et al., 2020). ALS results in the loss of motor neurons causing individuals to experience muscle weakness, atrophy, and death after a few years of disease onset. Mutations in SOD1, an enzyme called Cu-Zn superoxide dismutase responsible for converting superoxide to hydrogen peroxide or oxygen, are attributed to causing ALS (Taylor et al., 2016). In addition, mutations in TARDBP that encode the protein TDP-43 function as a DNA/RNA binding protein have also been shown to be a hallmark of ALS pathophysiology (Suk and Rousseaux, 2020). While many studies have been conducted on the role of SOD1 and TARDBP in ALS, no therapy is available to stop or prevent the degeneration of motor neurons in these individuals.
In vitro studies suggest that exosomes facilitate the propagation of SOD1 (Gomes et al., 2007). For example, exosomes isolated from the brain and spinal cords of mice that overexpress mutant SOD1 had significantly more misfolded SOD1 on the vesicular surface compared to their non-transgenic littermates (Silverman et al., 2019). Furthermore, in ALS patients, plasma samples showed a significantly elevated exosomal TDP-43 ratio compared to baseline at 3- and 6-month follow-up and borderline significance at 12-month follow-up (Chen et al., 2020). Another study in corroboration of these findings found that TDP-43 (both C-terminal fragments and full length) increases in exosomes isolated from human sporadic ALS brains (Iguchi et al., 2016), indicating that exosomal TDP-43 might be a potential candidate for biomarkers for ALS.
Therapeutic Role of Stem Cell-Derived Exosomes in Treating Neurodegenerative Disorders
As mentioned above, the current treatments for neurodegenerative disorders are limited, and they can only provide symptomatic relief without stopping or reversing the progression of the disease (Lamptey et al., 2022). However, as a therapy for neurodegenerative disorders, stem cell-derived exosomes are gaining attention due to their favorable attributes, which are discussed in detail below.
Several recent reports have shown that MSC-derived exosomes exert a greater therapeutic effect over MSCs alone (Cai et al., 2020). Exosomes can be stored for a relatively long time without toxic preservatives (Sivanantham and Jin, 2022). Importantly, this avoids the risk of tumor formation, as exosomes do not divide. Exosomes can be administered intranasally or intravenously (Sun et al., 2022). The ability of new drugs to cross the blood-brain barrier is a concern for the development of new therapies for neurodegenerative disorders, as most small-molecule drugs cannot cross the blood-brain barrier. Exosomes can readily cross the BBB due to their hydrophobic nature and low solubility in water. Once in the brain, the properties of exosomes are still active, further enhancing their attractiveness as a therapy for neurodegenerative disorders. Exosomes also have a relatively long half-life and can be subjected to repeated systemic administration without evident toxicity, providing further support for their safety profile. In a condition of neurodegenerative disease, BBB is disrupted, while exosomes can repair the damaged BBB (Liu et al., 2020). In addition to their ability to target the brain, exosomes are biodegradable and exhibit low immunogenicity and toxicity upon systemic administration (Sun et al., 2022).
Due to exosomes being a single lipid bilayer membrane-enclosed vesicle, they make an attractive drug-delivery system. To enhance exosome-mediated therapeutic efficiency, many attempts tried to modify exosomes by genetic or chemical approaches. For instance, cells can be transfected with a specific miRNA, and exosomes isolated from these cells will overexpress this miRNA, which can cause gene silencing in target cells (Zhang et al., 2017). Interestingly, as exosomes can easily be isolated from patients, donor exosomes could be modified and then used to treat the exact same donor with less immunogenic and toxicity than manufactured exosomes (Yamamoto et al., 2019). As a result, engineering an individual’s exosomes to combat a disease could be beneficial, as the isolation and injection of exosomes are non-invasive.
Stem cell-derived exosomes in treating AD
Although many preclinical studies have demonstrated exosomes to be an ideal candidate for treating many neurodegenerative disorders, their ability as a therapeutic agent is drastically hindered by their limited secretion amount from stem cells. The yield of exosomes from culture media is usually less than 1 μg of exosomal protein from 1 mL of media. It has been reported that cells under stressful conditions will increase the number of intracellular multivesicular bodies and secrete more exosomes. One way to overcome the low yield from cultured cells is to heat-shock (HS) the cells before isolation. A recent study from our lab demonstrated that neural stem cells exposed to 42°C for 3 hours exhibit a significant increase in exosomal concentration and diameter compared to non-heat shock (NHS) cells. The concentration of HS-derived exosomes is 13 times higher than NHS-derived exosomes. The protein diversity of HS-derived exosomes is less than NHS-derived exosomes; however, the top two biological functions in HS-derived exosomes are negative regulations of apoptotic process and DNA damage, suggesting that these exosomes might have therapeutic efficacy (Figure 4). Importantly, the cells treated with HS-derived exosomes confer greater neuroprotection against not only hydrogen peroxide-induced cell death but also Aβ-induced neurotoxicity when compared to the non-HS-derived exosomes. Assessing Aβ-induced apoptosis revealed that HS-derived exosomes completely reversed Aβ -induced cell death and oxidative stress (Huber et al., 2022). This study shows promising results in utilizing HS to enhance the production of exosomes and alter their cargo content while maintaining the therapeutic efficacy against oxidative stress and amyloid-β induced neurotoxicity (Figure 4).
Figure 4.

Anti-apoptotic role of HS-induced exosomes derived from stem cells.
HS treatment of stem cells, such as neural stem cells, increases the yield of exosomes that have anti-apoptotic effect and prevent DNA damage following application. Created with PowerPoint. HS: Heat-shock.
Another hallmark of AD is neuroinflammation (Kinney et al., 2018). Recent reports suggest exosomes display anti-neuroinflammatory processes (Qian et al., 2021). Upon systemic injection of human umbilical cord MSC-derived exosomes in an AD mouse model, spatial learning and memory function were significantly improved compared to the control group in the Morris Water Maze. In addition to decreasing Aβ plaque load in exosome-treated mice, this study also showed a decrease in activated microglial, supporting the anti-neuroinflammatory role of exosomes in AD (Ding et al., 2018). Along with exosomes ability to alleviate neuroinflammation, exosomes also reduce oxidative stress, another hallmark of neurodegenerative disorders (Fan et al., 2022; Huber et al., 2022).
Stem cell-derived exosomes in treating PD
In PD, dopamine-loaded exosomes increase the amount of dopamine in the brain more than 15-fold. Administration of human umbilical cord MSCs to a mouse model of PD exhibited improved PD behavioral symptoms, decreased neuronal apoptosis, and increased amount of dopamine in the brain (Qu et al., 2018). As a result, this study highlighted how blood exosomes could be loaded with dopamine, cross the BBB, and deliver dopamine to the brain, including the substantia nigra and striatum, two areas implicated in PD.
In a progressive PD model, MSC-derived exosomes were shown to alleviate cognitive impairment, which is associated with altered neuron cholesterol metabolism (Xu et al., 2022). Additionally, bone marrow MSC-derived exosomes showed anti-inflammatory and antioxidative stress effects in a cell model of PD (Huang et al., 2022). Another interesting study is to prime MSCs with α-synuclein to determine whether the MSCs show altered neuroprotection in PD. Shin et al. (2022) pre-treated MSCs with α-synuclein and tested the effect of the pre-treated MSCs on autophagy and viability of dopaminergic neurons co-cultured with the MSCs. Their results revealed that priming MSCs with α-synuclein confers enhanced neuroprotection through increased stemness in the stem cells and upregulated autophagy in Parkinsonian models. Increasing studies will use modified MSC-derived exosomes due to their enhanced therapeutic efficacy and making exosomes more specific for the disease.
Pre-clinical studies of stem cell-derived exosomes in ALS
In ALS, exosomes isolated from adipose-derived stem cells ameliorate the aggregation of SOD1 and mitochondrial dysfunction. Furthermore, repeated injection of exosomes isolated from adipose-derived stem cells improved motor performance, diminished glial activation, and reached the lesion sites in an ALS mouse model (Bonafede et al., 2020). Treatment of a motoneuron cell line stably transfected with mutant SOD1(G93A) with exosomes isolated from adipose-derived stem cells reverse mitochondrial dysfunction (Calabria et al., 2019).
Clinical trials to test exosomes in neurodegenerative diseases
As mentioned previously, the preclinical use of exosomes has yielded promising results for treating various disorders. While there is no FDA-approved exosome product on the market, the number of clinical trials using exosome-based therapeutics is rapidly increasing. Importantly, several clinical trials are determining the therapeutic effects of stem cell-derived exosomes in a clinical setting. Using ClinicalTrials.gov, 82 clinical trials were identified, which applied exosomes to various diseases as of November 29, 2022 (advanced search: “Other terms”: keyword: “exosome”; exclusion criteria: “Phase”: “Not Applicable”). The majority of the trials are in Phases I and II (Figure 5). Respiratory Tract Diseases and Cancer were among the top conditions where exosomes are being tested in clinical trials. Currently, some clinical trials are underway to test the efficacy of exosomes in AD and PD.
Figure 5.
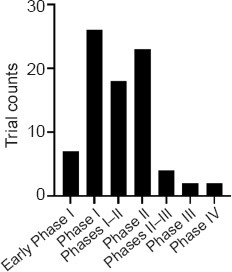
Clinical trials consisting of exosomes separated by phases.
As of November 29, 2022, 82 clinical trials have been conducted with exosomes (advanced search: “Other terms”: keyword: “exosome”; exclusion criteria: “Phase”: “Not Applicable”). Many of the trials are in Phases I and II. Created with GraphPad.
Future Perspectives
While exosomal research has taken off in the last decade, much remains to be known about these small extracellular vesicles that influence disease. For instance, increasing exosomal yield without impacting their therapeutic function is crucial for exosomes to succeed in future clinical trials. In addition, the cargo of exosomes is important to consider due to these vesicles reflecting their donor cells. One important consideration is why exosomes isolated from the same cell line exhibit diversity in diameter. Further, it is important to understand if the diversity in size influences the contents of exosomes. Future studies are necessary to know how different cargo of exosomes affects their therapeutic efficacy. Moreover, increasing the ability of exosomes to make it to the target organ is crucial to ensure that disease states receive the exosomes upon systemic administration. Finally, it is imperative to know how exosomes target specific tissue and how they are removed.
Conclusion
Exosomal research yields promising results for their role as a biomarker and therapy in neurodegenerative disorders. MSC-derived exosomes have been frequently used in various studies and clinical trials; however, exosomes derived from other types of stem cells, such as neural stem cells, appear also beneficial in treating neurodegenerative diseases in cell and animal models. Despite these studies, many questions remain: how does the heterogeneity of the isolated vesicles affect disease pathogenesis, and what are the long-term consequences of exosome administration? In addition, to improve the yield and specificity of exosomes, future research should address these issues in the treatment of various neurodegenerative diseases.
Footnotes
Funding: The work was supported by the National Institute on Aging of NIH (No. RF1AG072510 to HW), the National Institute of General Medical Sciences (NINGM) of NIH (No. P20GM103443 to HW via Dr. Victor Huber), the National Science Foundation (NSF) (No. DGE-1633213 to CCH via Dr. Brian Burrell), and the NIH/NIGMS (No. T32GM-136503 to CCH via Dr. Brian Burrell).
Conflicts of interest: The authors declare that there is no conflict of interest regarding the publication of this paper.
Data availability statement: Not applicable.
C-Editors: Zhao M, Liu WJ, Wang L; T-Editor: Jia Y
References
- 1.Abner EL, Jicha GA, Shaw LM, Trojanowski JQ, Goetzl EJ. Plasma neuronal exosomal levels of Alzheimer's disease biomarkers in normal aging. Ann Clin Transl Neurol. (2016);3:399–403. doi: 10.1002/acn3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kügler S, Ikezu T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. (2015);18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonafede R, Turano E, Scambi I, Busato A, Bontempi P, Virla F, Schiaffino L, Marzola P, Bonetti B, Mariotti R. ASC-exosomes ameliorate the disease progression in SOD1(G93A) murine model underlining their potential therapeutic use in human ALS. Int J Mol Sci. (2020);21:3651. doi: 10.3390/ijms21103651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai J, Wu J, Wang J, Li Y, Hu X, Luo S, Xiang D. Extracellular vesicles derived from different sources of mesenchymal stem cells:therapeutic effects and translational potential. Cell Biosci. (2020);10:69. doi: 10.1186/s13578-020-00427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabria E, Scambi I, Bonafede R, Schiaffino L, Peroni D, Potrich V, Capelli C, Schena F, Mariotti R. ASCs-exosomes recover coupling efficiency and mitochondrial membrane potential in an in vitro model of ALS. Front Neurosci. (2019);13:1070. doi: 10.3389/fnins.2019.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso S, Poon IKH. Apoptotic cell-derived extracellular vesicles:more than just debris. Front Immunol. (2018);9:1486. doi: 10.3389/fimmu.2018.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang CW, Yang SY, Yang CC, Chang CW, Wu YR. Plasma and serum alpha-synuclein as a biomarker of diagnosis in patients with Parkinson's disease. Front Neurol. (2019);10:1388. doi: 10.3389/fneur.2019.01388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen PC, Wu D, Hu CJ, Chen HY, Hsieh YC, Huang CC. Exosomal TAR DNA-binding protein-43 and neurofilaments in plasma of amyotrophic lateral sclerosis patients:A longitudinal follow-up study. J Neurol Sci. (2020);418:117070. doi: 10.1016/j.jns.2020.117070. [DOI] [PubMed] [Google Scholar]
- 9.Cone AS, Yuan X, Sun L, Duke LC, Vreones MP, Carrier AN, Kenyon SM, Carver SR, Benthem SD, Stimmell AC, Moseley SC, Hike D, Grant SC, Wilber AA, Olcese JM, Meckes DG., Jr Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer's disease-like phenotypes in a preclinical mouse model. Theranostics. (2021);11:8129–8142. doi: 10.7150/thno.62069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dieudé M, Bell C, Turgeon J, Beillevaire D, Pomerleau L, Yang B, Hamelin K, Qi S, Pallet N, Béland C, Dhahri W, Cailhier JF, Rousseau M, Duchez AC, Lévesque T, Lau A, Rondeau C, Gingras D, Muruve D, Rivard A, et al. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med. (2015);7:318ra200. doi: 10.1126/scitranslmed.aac9816. [DOI] [PubMed] [Google Scholar]
- 11.Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z, Wang Y, Lyu Y, Wang D, Xu L, Bi J, Yang H. Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer's disease. Neurochem Res. (2018);43:2165–2177. doi: 10.1007/s11064-018-2641-5. [DOI] [PubMed] [Google Scholar]
- 12.Dorsey ER, Sherer T, Okun MS, Bloem BR. The emerging evidence of the parkinson pandemic. J Parkinsons Dis. (2018);8:S3–S8. doi: 10.3233/JPD-181474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose and, methods for exosome isolation and analysis. Cells. (2019);8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan Y, Chen Z, Zhang M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J Transl Med. (2022);20:291. doi: 10.1186/s12967-022-03493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes:A case-control study. Alzheimers Dement. (2015);11:600–607.e1. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, Schwartz JB, Miller BL. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer's disease. FASEB J. (2016);30:3853–3859. doi: 10.1096/fj.201600756R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes C, Keller S, Altevogt P, Costa J. Evidence for secretion of Cu,Zn superoxide dismutase via exosomes from a cell model of amyotrophic lateral sclerosis. Neurosci Lett. (2007);428:43–46. doi: 10.1016/j.neulet.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Grey M, Dunning CJ, Gaspar R, Grey C, Brundin P, Sparr E, Linse S. Acceleration of alpha-synuclein aggregation by exosomes. J Biol Chem. (2015);290:2969–2982. doi: 10.1074/jbc.M114.585703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey:from biogenesis to uptake and intracellular signalling. Cell Commun Signal. (2021);19:47. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustafsson G, Lööv C, Persson E, Lázaro DF, Takeda S, Bergström J, Erlandsson A, Sehlin D, Balaj L, György B, Hallbeck M, Outeiro TF, Breakefield XO, Hyman BT, Ingelsson M. Secretion and uptake of alpha-synuclein via extracellular vesicles in cultured cells. Cell Mol Neurobiol. (2018);38:1539–1550. doi: 10.1007/s10571-018-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han C, Xiong N, Guo X, Huang J, Ma K, Liu L, Xia Y, Shen Y, Li J, Jiang H, Wang L, Guo S, Xu X, Zhang G, Liu J, Cao X, Zhang Z, Lin Z, Wang T. Exosomes from patients with Parkinson's disease are pathological in mice. J Mol Med (Berl) (2019);97:1329–1344. doi: 10.1007/s00109-019-01810-z. [DOI] [PubMed] [Google Scholar]
- 22.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. (1983);97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang D, Zhang M, Tan Z. Bone marrow stem cell-EXO-derived TSG-6 attenuates 1-methyl-4-phenylpyridinium+-induced neurotoxicity via the STAT3/miR-7/NEDD4/LRRK2 axis. J Neuropathol Exp Neurol. (2022);81:621–634. doi: 10.1093/jnen/nlac049. [DOI] [PubMed] [Google Scholar]
- 24.Huber CC, Callegari EA, Paez MD, Romanova S, Wang H. Heat shock-induced extracellular vesicles derived from neural stem cells confer marked neuroprotection against oxidative stress and amyloid-beta-caused neurotoxicity. Mol Neurobiol. (2022);59:7404–7412. doi: 10.1007/s12035-022-03055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iguchi Y, Eid L, Parent M, Soucy G, Bareil C, Riku Y, Kawai K, Takagi S, Yoshida M, Katsuno M, Sobue G, Julien JP. Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain. (2016);139:3187–3201. doi: 10.1093/brain/aww237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang C, Hopfner F, Katsikoudi A, Hein R, Catli C, Evetts S, Huang Y, Wang H, Ryder JW, Kuhlenbaeumer G, Deuschl G, Padovani A, Berg D, Borroni B, Hu MT, Davis JJ, Tofaris GK. Serum neuronal exosomes predict and differentiate Parkinson's disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. (2020);91:720–729. doi: 10.1136/jnnp-2019-322588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnstone RM, Mathew A, Mason AB, Teng K. Exosome formation during maturation of mammalian and avian reticulocytes:evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol. (1991);147:27–36. doi: 10.1002/jcp.1041470105. [DOI] [PubMed] [Google Scholar]
- 28.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. (1987);262:9412–9420. [PubMed] [Google Scholar]
- 29.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement (N Y) (2018);4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai CP, Breakefield XO. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol. (2012);3:228. doi: 10.3389/fphys.2012.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamptey RNL, Chaulagain B, Trivedi R, Gothwal A, Layek B, Singh J. A review of the common neurodegenerative disorders:current therapeutic approaches and the potential role of nanotherapeutics. Int J Mol Sci. (2022);23:1851. doi: 10.3390/ijms23031851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin J, Li J, Huang B, Liu J, Chen X, Chen XM, Xu YM, Huang LF, Wang XZ. Exosomes:novel biomarkers for clinical diagnosis. Scientific World Journal 2015. (2015):657086. doi: 10.1155/2015/657086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Huber CC, Wang H. Disrupted blood-brain barrier in 5×FAD mouse model of Alzheimer's disease can be mimicked and repaired in vitro with neural stem cell-derived exosomes. Biochem Biophys Res Commun. (2020) doi: 10.1016/j.bbrc.2020.02.074. S0006-291X(20)30342-9. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Subedi K, Baride A, Romanova S, Callegari E, Huber CC, Wang X, Wang H. Peripherally misfolded proteins exacerbate ischemic stroke-induced neuroinflammation and brain injury. J Neuroinflammation. (2021);18:29. doi: 10.1186/s12974-021-02081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luong N, Olson JK. Exosomes secreted by microglia during virus infection in the central nervous system activate an inflammatory response in bystander cells. Front Cell Dev Biol. (2021);9:661935. doi: 10.3389/fcell.2021.661935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynch C, Panagopoulou M, Gregory CD. Extracellular vesicles arising from apoptotic cells in tumors:roles in cancer pathogenesis and potential clinical applications. Front Immunol. (2017);8:1174. doi: 10.3389/fimmu.2017.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathew B, Mansuri MS, Williams KR, Nairn AC. Exosomes as emerging biomarker tools in neurodegenerative and neuropsychiatric disorders-a proteomics perspective. Brain Sci. (2021);11:258. doi: 10.3390/brainsci11020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. (2019);21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 39.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. (2012);40:D1241–1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niu M, Li Y, Li G, Zhou L, Luo N, Yao M, Kang W, Liu J. A longitudinal study on alpha-synuclein in plasma neuronal exosomes as a biomarker for Parkinson's disease development and progression. Eur J Neurol. (2020);27:967–974. doi: 10.1111/ene.14208. [DOI] [PubMed] [Google Scholar]
- 41.2020 Alzheimer's disease facts and figures. Alzheimers Dement. (2020) doi: 10.1002/alz.12068. No author listed. doi:10.1002/alz.12068. [DOI] [PubMed] [Google Scholar]
- 42.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. (1985);101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pascua-Maestro R, González E, Lillo C, Ganfornina MD, Falcón-Pérez JM, Sanchez D. Extracellular vesicles secreted by astroglial cells transport apolipoprotein D to neurons and mediate neuronal survival upon oxidative stress. Front Cell Neurosci. (2019);12:526. doi: 10.3389/fncel.2018.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem. (2012);287:43108–43115. doi: 10.1074/jbc.M112.404467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porro C, Trotta T, Panaro MA. Microvesicles in the brain:Biomarker, messenger or mediator? J Neuroimmunol. (2015);288:70–78. doi: 10.1016/j.jneuroim.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Qian X, An N, Ren Y, Yang C, Zhang X, Li L. Immunosuppressive effects of mesenchymal stem cells-derived exosomes. Stem Cell Rev Rep. (2021);17:411–427. doi: 10.1007/s12015-020-10040-7. [DOI] [PubMed] [Google Scholar]
- 47.Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, Fu Y, Yang S, Zhang Z, Zhang L, Sun X. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson's disease. J Control Release. (2018);287:156–166. doi: 10.1016/j.jconrel.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 48.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. (2006);103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rastogi S, Sharma V, Bharti PS, Rani K, Modi GP, Nikolajeff F, Kumar S. The evolving landscape of exosomes in neurodegenerative diseases:exosomes characteristics and a promising role in early diagnosis. Int J Mol Sci. (2021);22:440. doi: 10.3390/ijms22010440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors:evidence for horizontal transfer of mRNA and protein delivery. Leukemia. (2006);20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 51.Ruan Z, Pathak D, Venkatesan Kalavai S, Yoshii-Kitahara A, Muraoka S, Bhatt N, Takamatsu-Yukawa K, Hu J, Wang Y, Hersh S, Ericsson M, Gorantla S, Gendelman HE, Kayed R, Ikezu S, Luebke JI, Ikezu T. Alzheimer's disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain. (2021);144:288–309. doi: 10.1093/brain/awaa376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.ardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjö C, Larsson M, Lannfelt L, Ingelsson M, Hallbeck M. Alzheimer's disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. (2018);136:41–56. doi: 10.1007/s00401-018-1868-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shin JY, Kim DY, Lee J, Shin YJ, Kim YS, Lee PH. Priming mesenchymal stem cells with alpha-synuclein enhances neuroprotective properties through induction of autophagy in Parkinsonian models. Stem Cell Res Ther. (2022);13:483. doi: 10.1186/s13287-022-03139-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silverman JM, Christy D, Shyu CC, Moon KM, Fernando S, Gidden Z, Cowan CM, Ban Y, Stacey RG, Grad LI, McAlary L, Mackenzie IR, Foster LJ, Cashman NR. CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)(G93A) ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J Biol Chem. (2019);294:3744–3759. doi: 10.1074/jbc.RA118.004825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sivanantham A, Jin Y. Impact of storage conditions on EV Integrity/surface markers and cargos. Life (Basel) (2022);12:697. doi: 10.3390/life12050697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spitzer P, Mulzer LM, Oberstein TJ, Munoz LE, Lewczuk P, Kornhuber J, Herrmann M, Maler JM. Microvesicles from cerebrospinal fluid of patients with Alzheimer's disease display reduced concentrations of tau and APP protein. Sci Rep. (2019);9:7089. doi: 10.1038/s41598-019-43607-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sterzenbach U, Putz U, Low LH, Silke J, Tan SS, Howitt J. Engineered exosomes as vehicles for biologically active proteins. Mol Ther. (2017);25:1269–1278. doi: 10.1016/j.ymthe.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suk TR, Rousseaux MWC. The role of TDP-43 mislocalization in amyotrophic lateral sclerosis. Mol Neurodegener. (2020);15:45. doi: 10.1186/s13024-020-00397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun K, Zheng X, Jin H, Yu F, Zhao W. Exosomes as CNS drug delivery tools and their applications. Pharmaceutics. (2022);14:2252. doi: 10.3390/pharmaceutics14102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sweeney P, Park H, Baumann M, Dunlop J, Frydman J, Kopito R, McCampbell A, Leblanc G, Venkateswaran A, Nurmi A, Hodgson R. Protein misfolding in neurodegenerative diseases:implications and strategies. Transl Neurodegene. (2017);6:6. doi: 10.1186/s40035-017-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor JP, Brown RH, Jr, Cleveland DW. Decoding ALS:from genes to mechanism. Nature. (2016);539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tysnes OB, Storstein A. Epidemiology of Parkinson's disease. J Neural Transm (Vienna) (2017);124:901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Balaji V, Kaniyappan S, Krüger L, Irsen S, Tepper K, Chandupatla R, Maetzler W, Schneider A, Mandelkow E, Mandelkow EM. The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener. (2017);12:5. doi: 10.1186/s13024-016-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D, Masliah E, Rissman RA. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) (2016);3:63–72. doi: 10.1016/j.dadm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winston CN, Aulston B, Rockenstein EM, Adame A, Prikhodko O, Dave KN, Mishra P, Rissman RA, Yuan SH. Neuronal exosome-derived human tau is toxic to recipient mouse neurons in vivo. J Alzheimers Dis. (2019);67:541–553. doi: 10.3233/JAD-180776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xia Y, Zhang G, Han C, Ma K, Guo X, Wan F, Kou L, Yin S, Liu L, Huang J, Xiong N, Wang T. Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis. (2019);10:174. doi: 10.1038/s41419-019-1404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu L, Liu T, Liu L, Yao X, Chen L, Fan D, Zhan S, Wang S. Global variation in prevalence and incidence of amyotrophic lateral sclerosis:a systematic review and meta-analysis. J Neurol. (2020);267:944–953. doi: 10.1007/s00415-019-09652-y. [DOI] [PubMed] [Google Scholar]
- 68.Xu X, Li Z, Zuo H, Chen H, Gui Y. Mesenchymal stem cell-derived exosomes altered neuron cholesterol metabolism via Wnt5a-LRP1 axis and alleviated cognitive impairment in a progressive Parkinson's disease model. Neurosci Lett. (2022);787:136810. doi: 10.1016/j.neulet.2022.136810. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto M, Guo DH, Hernandez CM, Stranahan AM. Endothelial Adora2a activation promotes blood-brain barrier breakdown and cognitive impairment in mice with diet-induced insulin resistance. J Neurosci. (2019);39:4179–4192. doi: 10.1523/JNEUROSCI.2506-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin Q, Ji X, Lv R, Pei JJ, Du Y, Shen C, Hou X. Targetting exosomes as a new biomarker and therapeutic approach for Alzheimer's disease. Clin Interv Aging. (2020);15:195–205. doi: 10.2147/CIA.S240400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. (2017);312:L110–121. doi: 10.1152/ajplung.00423.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng H, Xie Z, Zhang X, Mao J, Wang M, Wei S, Fu Y, Zheng H, He Y, Chen H, Xu Y. Investigation of alpha-synuclein species in plasma exosomes and the oligomeric and phosphorylated alpha-synuclein as potential peripheral biomarker of Parkinson's disease. Neuroscience. (2021);469:79–90. doi: 10.1016/j.neuroscience.2021.06.033. [DOI] [PubMed] [Google Scholar]


