Abstract
Taurine is a sulfur-containing, semi-essential amino acid that occurs naturally in the body. It alternates between inflammation and oxidative stress-mediated injury in various disease models. As part of its limiting functions, taurine also modulates endoplasmic reticulum stress, Ca2+ homeostasis, and neuronal activity at the molecular level. Taurine effectively protects against a number of neurological disorders, including stroke, epilepsy, cerebral ischemia, memory dysfunction, and spinal cord injury. Although various therapies are available, effective management of these disorders remains a global challenge. Approximately 30 million people are affected worldwide. The design of taurine formation could lead to potential drugs/supplements for the health maintenance and treatment of central nervous system disorders. The general neuroprotective effects of taurine and the various possible underlying mechanisms are discussed in this review. This article is a good resource for understanding the general effects of taurine on various diseases. Given the strong evidence for the neuropharmacological efficacy of taurine in various experimental paradigms, it is concluded that this molecule should be considered and further investigated as a potential candidate for neurotherapeutics, with emphasis on mechanism and clinical studies to determine efficacy.
Keywords: antioxidant, epilepsy, γ-amino butyric acid, neurodegenerative disorders, neuroprotection, oxidative stress, spinal cord injury, taurine
Introduction
Taurine, also known as 2-aminoethanesulfonic acid, is an amino acid found in nerve and muscle tissue (Jong et al., 2021; Figure 1). Taurine, which is found in meat and seafood, is widely distributed in the diet. It is endogenously produced from cysteine and catalyzed by cysteine dioxygenase and cysteine sulfinate decarboxylase. The biosynthesis of taurine occurs in the liver, and the main substrate is methionine or cysteine, in which the cysteine dioxygenase and cysteine sulfinate decarboxylase enzymes exert their action during the biosynthesis of taurine. Cysteine dioxygenase first converts cysteine to cysteine sulfonic acid by oxidation, which is then converted to hypotaurine. Taurine is excreted in two ways: first, in the form of urine, which is excreted via the kidney, and second, in the form of taurine conjugated to bile acid, which is excreted in the feces (Baliou et al., 2020).
Figure 1.
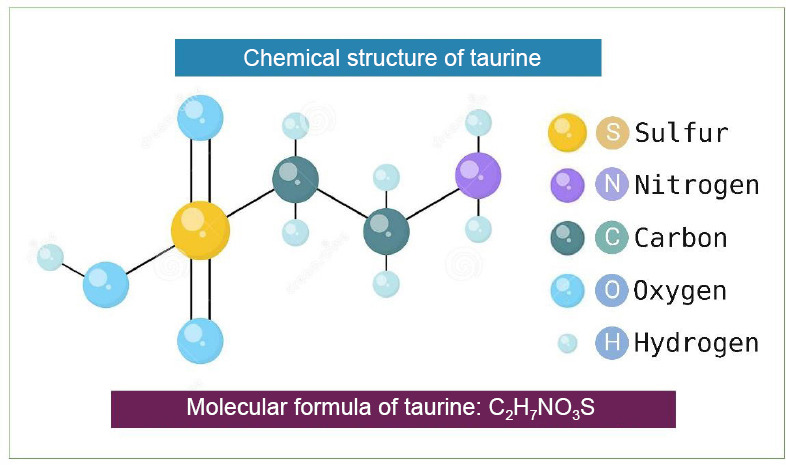
Chemical structure and a molecular formula of taurine (2-aminoethanesulfonic acid).
Created with BioRender.com.
Taurine is an inhibitory neurotransmitter that acts as an agonist at γ-amino butyric acid (GABA) and glycine receptors, modulates Ca2+ influx, and is an intracellular secondary messenger (Liu et al., 2022). It also contributes to homeostasis by modulating glutamatergic signaling and preventing excitotoxicity and oxidative stress. Pharmacological actions of taurine include membrane stabilization, cytoprotective and anti-inflammatory effects, regulation of intracellular calcium ions, and regulation of neurotransmitter levels (Adedara et al., 2017). Taurine has low water solubility, is naturally acidic, and has a pKa value of 1.5 (Jakaria et al., 2019). Taurine is a necessary nutrient for cats and foxes (Schaffer and Kim, 2018). Consumption of taurine lowers hypertension and hypercholesterolemia, and taurine supplements lower body mass index and inflammatory markers in obese women, according to a World Health Association study (Schaffer and Kim, 2018). This article discusses the use of taurine in the treatment of various neurological diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), cerebral ischemia, memory dysfunction, depression, anxiety, spinal cord injury (SCI), traumatic brain injury (TBI), and epilepsy. In the various disease models, it has the effect of reducing the injury caused by oxidative stress. In addition, taurine can modulate, at the molecular level, endoplasmic reticulum (ER) stress, calcium (Ca2+) homeostasis, and neuronal activity which is discussed in detail in the following sections of this article.
Search Strategy
Several electronic databases, including SCOPUS, PubMed, Web of Science, and Google Scholar, were used to search for published material. The Medical Subject Headings (MeSH) keywords were used in the search, including “neurodegenerative diseases and taurine”, “taurine and neuroprotection”, “taurine and neurological disorders”, “taurine for brain”, and “taurine and physiology”. The collected bibliographies were also searched for related literature on in vivo and in vitro research in which taurine was used to target at least one mediator of neurological diseases.
Therapeutic Potential of Taurine
Taurine has the potential to be used to treat a variety of human diseases, including stroke, neurodegenerative diseases such as AD and PD, epilepsy, and others. It reduces Ca2+ and increases B-cell lymphoma 2/Bcl-2-associated death promoter (BCL-2/BAD) ratio and suppresses ER stress. The effect of taurine in stroke counteracts the effects of glutamate (Rubio-Casillas et al., 2023). Taurine increases the expression of UUG-dependent protein ND6, a subunit of complex-1. Taurine-associated expression of ND6 can activate the functions of mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes, such as enhancing complex-1 activity, decreasing lactic acidosis, and increasing oxygen consumption. Thus, this functional respiratory chain causes the inhibition of reactive oxygen species (ROS) and an increase in adenosine triphosphate production (Schaffer and Kim, 2018; Jong et al., 2021). In AD, taurine reduces the formation of intracellular Ca2+ and ROS via Aβ1–42 (Su et al., 2014). In PD, taurine decreases mitochondrial complex-1 and respiratory complex activity, causes an increase in nicotinamide adenine dinucleotide hydrogen, and inhibits nicotinamide adenine dinucleotide hydrogen dehydrogenase. In patients with hypertension, it lowers oxidative stress levels and improves endothelial function (Katakawa et al., 2016). It has the ability to inhibit the action of norepinephrine and angiotensin and decreases myocardial performance by increasing afterload pressure and causing ventricular remodeling in patients with congestive heart failure (Gutiérrez-Castañeda et al., 2022). In the traumatic brain and spinal cord injuries, taurine has been shown to increase superoxide dismutase activity and glutathione levels while reducing malondialdehyde and lactic acid levels in injured tissues (Lucas et al., 2006). In addition, taurine is also found in proinflammatory cells such as polymorphonuclear leukocytes. Administration of taurine has been reported to relieve asthmatic symptoms and protect the lungs from oxidative stress (Chen et al., 2021). Taurine is able to reverse the generation of ROS caused by high glucose in diabetic neuropathy. It has been observed that taurine has a protective role in excitotoxicity that can be induced by glutamate, and therefore, helps in preventing neuronal depolarization in epilepsy (Junyent et al., 2009). Taurine has the potential to be used as a therapeutic agent in a variety of diseases, as shown in Figure 1.
Some recent evidence has suggested that retinal degeneration is related to increasing oxidative stress and production of ROS. Taurine deficiency is also responsible for triggering retinal dysfunction and is involved in antioxidant defense. Cellular transport of taurine is provided by the taurine transporter (TauT) in the plasma membrane (Ansar et al., 2020). In a recent study, the TauT knockout mouse (TauT–/–) model was used to investigate the role of taurine in retinal dysfunction. The results showed that 98% of taurine uptake was decreased and low taurine content was detected in all tissues as well as in fibroblasts. Myofibril necrosis in muscles and decreased exercise capacity lead to muscle contractile sarcopenia (Merckx and De Paepe, 2022). TauT–/– mice were found to have 74% decreased taurine levels, and severe and progressive retinal degeneration was reported in 12-day-old TauT–/– mice. Thus, it is concluded that taurine is an important factor in the development and maintenance of normal retinal function and morphology.
Taurine plays many physiological roles and its level is maintained in the liver and other tissues by endogenous synthesis and exogenous supplementation, but its level is decreased in a diseased liver. Several xenobiotics that cause liver damage are catabolized by NADPH-dependent cytochrome P450 2E1, which is expressed in the pericentral region of the liver, and taurine is considered a good option for the treatment of cytochrome P450 2E1-associated pericentral damage in the liver (Miyazaki and Matsuzaki, 2014). In a recent study by Hua et al. (2023), taurine was found to inhibit proliferation and apoptosis in cervical cancer (SiHa cells) and upregulate the expression of p73, p53, p53 upregulated modulator of apoptosis, caspase-3 protein and also promote the phosphorylation of ja-associated protein (Li et al., 2019a; Okano et al., 2023). It also helps maintain lipid peroxidation of renal tubule epithelial cells and glomerular mesangial cells exposed to high glucose or hypoxic conditions. Taurine is a new option for the treatment of diabetes because it lowers insulin levels and HBA1c levels, as shown in a meta-analysis study (Tao et al., 2022; Figure 2).
Figure 2.
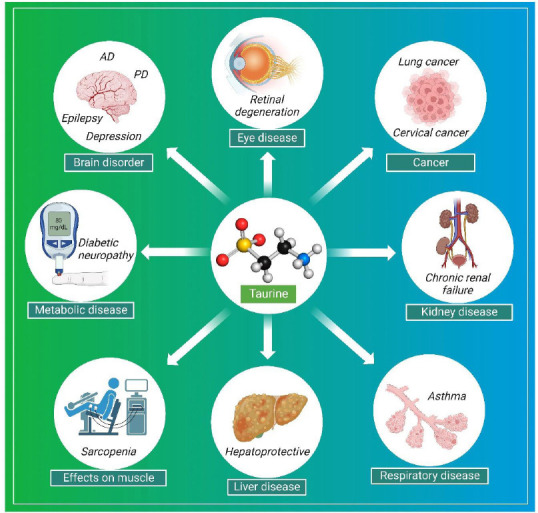
Pharmacological effects of taurine in various diseases including, neurological disorders, ophthalmic disease, renal disease, cancers, metabolic diseases such as mitochondrial encephalopathy, lactic acidosis, stroke-like episodes (MELAS), and diabetic neuropathy.
Created with BioRender.com. AD: Alzheimer’s disease; PD: Parkinson’s disease.
Alzheimer’s disease and the therapeutic effect of taurine
The neuroprotective effects of taurine have been shown to protect against various forms of dementia. Beresewicz-Haller et al. (2023) investigated the ability of taurine to improve cognition in a transgenic mouse model of AD. They administered taurine orally through drinking water to amyloid precursor protein (APP)/PS1 transgenic mice for six weeks. The results of the experiment showed that taurine treatment can improve the cognitive performance of the APP/PS1 mice without affecting their behavior in the Y-maze or passive avoidance test (Chen et al., 2019). In a transgenic AD mouse model, it has been shown that taurine has the ability to improve cognitive abilities. The insoluble fraction of amyloid beta (Aβ) in the cortex of APP/PS1 mice decreased when taurine was added, suggesting that it may help reduce cognitive impairment and Aβ-induced damage, as shown in Additional Table 1 (Kim et al., 2014). Taurine has been shown to block the neurotoxic effects of Aβ on rat hippocampal and cortical neurons in culture. Taurine supplementation may also protect central neurons from excitotoxicity caused by high concentrations of glutamate extracellularly. The neuroprotective effects of taurine are abolished by picrotoxin, an antagonist of GABA-A receptors. Neuronal death caused by Aβ in rat hippocampal and cortical areas can be prevented by GABA and muscimol, an agonist of the GABA-A receptor (Fontana et al., 2020). The use of taurine in the treatment of AD may be beneficial because of its ability to modulate GABA-A receptors and protect neurons from Aβ toxicity in AD-affected regions of the mammalian brain and from excitotoxicity (Paula-Lima et al., 2005). Taurine shows a beneficial role in AD by improving cognitive function in oligomeric Aβ mice. It restores acetylcholinesterase and acetylcholine transferase activities and inactivates microglia-dependent neuroinflammation to bypass dopaminergic neurons (Fontana et al., 2020).
Additional Table 1.
The different disease models and their findings at appropriate dose, route and duration of administration
| Disorder | Model / System | In vivo/ In vitro | Dose, route and time | Significant findings | Reference |
|---|---|---|---|---|---|
| Alzheimer’s disease | |||||
| Adult APP/PS1/ Mouse Model | In vivo | Taurine (1000 mg/kg/day), orally, 6 weeks | Improves cognitive impairment by inhibition of Aβ, determined by measurement of glial fibrillary acid protein | Kim et al., 2014 | |
| Transgenic mouse models of AD | In vivo | The gaseous physiological modulation (H2S) | Suppresses brain Aβ production and reduces tau phosphorylation | McCarty et al., 2019 | |
| FA x 5AD transgenic mice (2 months of age) | In vivo | Taurine (1000 mg/kg/day), orally | Increases brain glutamate receptor type 5 (MGLUR5) uptake and increases cerebral blood flow | Oh et al., 2020 | |
| Adult male Wister rats | In vivo | Dose: 250, 50 & 100 mg/kg/day), 2 weeks | Increases phosphorylated taurine protein levels in the prefrontal cortex and cerebellum | Jahanshahi et al., 2021 | |
| Parkinson’s disease | |||||
| Paraquat and maneb induced PD model | In vivo | Taurine (150 mg/kg), i.p., 6 weeks | Ameliorates progressive dopaminergic neurodegeneration by inactivating microglia-mediated neuroinflammation protects dopaminergic neurons, and also attenuates P+M-induced alpha-synuclein oligomerization in mice and suppresses the m1 microglia inflammatory response and microglia depletion. | Che et al., 2018 | |
| Mouse model | In vivo | PQ (intraperitoneal injection) | Inhibition of the PI3K/AKT/P pathway in the midbrain of PD mice by reducing damage to DAc neurons | Wang et al., 2022 | |
| 6-OHDA in rat striatum was used to model PD-like behavior | In vivo | Injection | Protective effects of caffeine and taurine on the 6-hydroxy- dopamine-induced rat model | Abuirmeileh et al., 2021 | |
| PQ-induced Parkinson’s mice model | In vivo | PQ (Percutaneous) | Degeneration of substantia nigra neurons in the hippocampus by inhibiting microglial cell activation and inflammatory factor release | Tian et al., 2020 | |
| Cerebral ischemia | |||||
| White rabbits (I/R) induced compartment syndrome in rabbit limbic model | In vivo | Taurine (1 g/kg) or normal saline IV infusion | Decreases malondialdehyde levels in muscle slices incubated with hydrogen peroxide and xanthine oxidase. Taurine treatment inhibits I/R-induced compartment syndrome by attenuating I/R- induced oxidative stress injury. | Wang et al., 2005 | |
| Sprague Dawley rats’ model or middle cerebral artery occlusion (MCAO) | In vivo | Dose: 250 mg/kg, i.p., single dose | Improves neurological function and decreases cerebrospinal fluid content and infarct volume. Overexpression of 12/15 lox, p38 MAPK, Cpla2, tumor necrosis factor-alpha, interleukin-1β, and interleukin-6 decreased in the tau group. | Xu et al., 2023 | |
| Male Sprague Dawley rat model | In vivo | Taurine (250 mg/kg), i.p. | Lower apoptosis indices with higher Bcl-2/Bax ratio, indicating that taurine prevents intestinal mucosal damage and inhibits intestinal epithelial cell apoptosis after intestinal IR in a rat. | Sukhotnik et al., 2016 | |
| Traumatic brain injury | |||||
| SD rats by a fluid percussion device | In vivo | Taurine (200 mg/kg), IV once daily for 7 days | Improves CBF of injured ipsilateral and contralateral cortex 30 minutes after injury. The effect of taurine improves CBF, alleviates edema, intracranial pressure (ICP) elevation, and other brain dysfunction, and also improves the hypercoagulable state | Wang et al., 2016 | |
| Sprague Dawley rats | In vivo | Taurine (200 mg/kg), IV for 7 days | Mitigates brain damage after TBI by reducing elevated levels of astrocyte activation, edema, and pro-inflammatory cytokines. | Su et al., 2014 | |
| Anxiety | |||||
| Adult short fin Zebrafish (novel tank and dark test) | In vivo | Taurine (42, 10, and 400 mg/kg), 6 minutes | Prevents anxiety-like behaviors, carbonylation of proteins, and stimulation of cortisol, resulting in a significant increase in shuttling and time spent in the illuminated compartment but no decrease in locomotor activity. | Murakami and Furuse, 2010 | |
| Mice | In vivo | Taurine supplementation (22.5 m mol/kg), beta alanine supplemented (22.5 mmol/kg diet) | Beta-alanine increases BDNF levels in the hippocampus when combined with beta-alanine in the cerebral cortex and hippocampus. The anxiolytic effect is shown by a taurine supplement diet, beta-alanine. | Hernández- Benítez et al., 2010 | |
| Memory dysfunction | |||||
| Male Wistar rats | In vivo | Taurine: 400, 800 mg/kg/day | Inhibits the damage caused by AI. Taurine’s ability to stabilize, support, and promote cell membranes is the basis for its ability to counteract the effects of Al. | Chen et al., 2020 | |
| Mice model (RS) | In vivo | Taurine: 200 mg/kg Four weeks | It significantly reduces the levels of interleukin-1 beta in the hippocampus of mice treated with taurine. | Nakajima et al., 2010 | |
| Spinal cord injury | |||||
| Male albino Wistar rats (180-210 g, 3- 4 months old) | In vivo | Taurine 100 mg/kg + ascorbic acid 100 mg/kg (continued daily for 45 consecutive days) | Taurine + ascorbic acid treatment reduces caspase-3 and p53 expression. In addition, it restores altered antioxidant markers and lipid peroxidation to near normal levels. | Wu and Prentice, 2010 | |
| Female (C57 black /6 mice model (8-10 weeks of age) (weight-18-20 g) | In vivo | Dose: 25, 80, 250, and 800 mg/kg within 30 minutes, i.p. | Significantly decreases interleukin-6 and MPO levels in a dose-dependent manner and substantially reduces phosphorylation of STAT3 and expression of Cox-2 after SCI compared to controls. | Chaiwong et al., 2021 | |
| Depression | |||||
| Male SPW Wistar rats | In vivo | Taurine: 200 mg/kg or 500 mg/kg for 28 days | Increases body weight in chronic unpredictable mild stress (CUMS) rats and reverses depression-like behavior. It also restores the levels of hormones and neurotransmitters. However, taurine failed to reverse the levels of inflammatory factors and upregulate the expression of a neurotrophic factor in the hippocampus of depressed rats. | Huang et al., 2020 | |
| Epilepsy | |||||
| Male NMRI strain mice | In vivo | Taurine (150 mg/kg dosage), i.p., 12 hours | Reduction or complete absence of seizures with simultaneous reduction or even disappearance of cellular and molecular effects. | Hassanein et al., 2021 | |
| Adult male Wistar rats | In vivo | Taurine (200 mg/kg), IV once daily for 7 days Anesthetized with methane (1.5 mg/kg), i.p. | Increase chloride conductance. Activated glycine receptor and Y-aminobutyric acid receptor. | Wang et al., 2016 | |
| Male FVB/NJ mice | In vivo | Subcutaneous injection, 0.05% taurine solution in drinking water for 4 weeks | Taurine-fed mice resistant to picrotoxin-induces seizures, increase latency, decrease seizure occurrence, and reduce the mortality rate. | Su et al., 2014 | |
| Male FVB/NJ mice | In vivo | Taurine (43 mg/kg) | Taurine-fed mice resistant to picrotoxin-induced seizures have a longer latency period, a lower incidence of seizures, and a lower mortality rate | Mezzomo et al., 2016 | |
AD: Alzheimer’s disease; APP: amyloid precursor protein; Aβ: amyloid-beta; BDNF: brain-derived neurotrophic factor; Cox-2: cyclo-oxygenase 2; CUMS: chronic unpredictable mild stress; ICP: intracranial pressure; i.p.: intraperitoneally; MCAO: middle cerebral artery occlusion; MPO: myeloperoxidase; PD: Parkinson’s disease; PI3K: phosphoinositide 3- kinases; PQ: percutaneous; STAT3: signal transducer and activator of transcription 3.
AD is a neurodegenerative disease characterized by specific neuropathological changes. AD is the most common cause of dementia, leading to memory loss and slowing cognitive functions. APP is a transmembrane glycoprotein and it has a large extracellular domain. The APP undergoes proteolysis to generate the Aβ peptide, which typically contains between 39 and 43 residues. In a study involving subchronic exposure to manganese, taurine was shown to improve memory deficits and learning disabilities. The activities of acetylcholinesterase and acetylcholine transferase, which are regulated in both the streptozocin- and manganese-induced AD models, are restored to normal by taurine. In addition, it protects neurons in the chick retina from the neurotoxicity caused by Aβ and glutamate receptor agonists (Jakaria et al., 2019). Taurine not only prevents the effects of paraquat- and maneb intoxicants but also induces polarization of microglia to the M1 state and secretion of proinflammatory mediators. P47 phagocyte oxidase and nuclear factor kappa B (NF-κB) are also involved in initiating and maintaining the inflammatory responses of M1 microglia, which is concentrated around the amyloid plaques in AD. Taurine can also inhibit the activation of NADPH oxidase by acting on both P47 and NF-κB which leads to the inactivation of microglia-dependent neuroinflammation to bypass dopaminergic neuroprotection with the help of taurine (Jakaria et al., 2019). In another experiment, Wistar rats were administered various doses of taurine either before or after the injection of scopolamine. These doses ranged from 25, 50, and 100 mg/kg. Administration of scopolamine to rats via intraperitoneal injection resulted in an increase in phosphorylated tau proteins in the cerebellum and prefrontal cortex. It was found that the phosphorylated form of tau significantly decreased after pretreatment with taurine. At high doses (100 mg/kg per day), taurine is able to reduce the amount of phosphorylated tau protein in the cerebellum. At low doses (25 and 50 mg/kg per day), taurine had no effect on the amount of tau protein in the cerebellum or prefrontal cortex. As shown in Additional Table 1, the administration of taurine can improve cognitive impairment in patients with AD (Jahanshahi et al., 2021). Another research focused on the neuroprotective properties of taurine and its effects. The effects of taurine on mice with AD were studied using functional molecular imaging. To study glutamate changes, taurine was administered to transgenic mice at 2 months of age when they showed signs of amyloid deposition. Mice participating in the study were started on taurine administration at 2 months of age to test its therapeutic potential at an early stage of AD. In such a model of AD, taurine supplementation increased brain uptake of metabotropic glutamate receptor subtype 5 but did not reduce amyloid pathology. The increased concentration of metabotropic glutamate receptor subtype 5 uptake was the indicator of increased cerebral blood flow, which facilitated the recovery of the glutamate system in AD. In AD, taurine treatment may help the glutamate system recovery (Oh et al., 2020). It has been observed in an experiment that Aβ1–42 treatment, pretreatment with taurine increased the levels of Aβ1–42, inhibited the opening of (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) MPTP, and reversed mitochondrial dysfunction. A decrease in intracellular Ca2+ and ROS generation was also observed after pretreatment with taurine. In addition, the expression of sirtuin 1 (SIRT1) in SK-N-SH cells was restored to normal levels after treatment with taurine. SIRT1 is activated by taurine, which in turn prevents mitochondrial dysfunction triggered by Aβ1–42 (Barangi et al., 2023). SIRT1 regulates many genes involved in metabolic pathways, and many SIRT1 activators play important roles in the prevention of various diseases. Activation of SIRT1 plays an important role in suppressing impaired autophagy (Biel et al., 2016). In mammals, there are seven types of SIRTs with different infections, of which SIRT1 and SIRT2 play important roles in neurodegenerative diseases. SIRT1 binds to different transcription factors such as peroxisome proliferator-activated receptor alpha, peroxisome proliferator-activated receptor γ coactivator or 1α, and liver X receptor in the liver, tau in the brain, peroxisome proliferator-activated receptor γ in white adipose tissue (Donmez, 2012). SIRT1 expression is protective against Aβ-plaque formation. In a recent study of the APPswe/PS1dE9 mouse model showing plaque formation at 3 months of age, SIRT1-overexpressed mice were crossed with SIRT1 knockout mice. The knockout mice showed overexpression of SIRT1, which is responsible for reducing Aβ production and Aβ plaques. Thus, it is clear from these results that SIRT1 is a good option for modulating Aβ production, and that taurine has the potential to be used for the treatment of AD (Louzada et al., 2004; Paula-Lima et al., 2005; Donmez and Outeiro, 2013).
Parkinson’s disease and the therapeutic effect of taurine
PD is a progressive neurodegenerative disorder of the pars compacta substantia nigra. Patients suffering from PD has symptoms such as rigidity, resting tremor, postural instability, and bradykinesia (Kulthinee et al., 2019). This is due to the loss of nigrostriatal dopaminergic neurons, which causes oxidative stress, neuroinflammation, mitochondrial complex 1 dysfunction, and impaired adenosine triphosphate production (Castro-Caldas et al., 2012; Doğanyiğit et al., 2022). Motor impairments cause akinesia, bradykinesia, hypokinesia, resting tremor, stiffness in arms, legs, and trunk, imbalance, incoordination, and bilateral vocal cord paralysis (Lotankar et al., 2017). Hyposmia, visual disturbances, depression, anxiety, orthostatic hypotension, and sleep disturbances, such as rapid eye movement sleep, are non-motor features of PD. Some neurotransmitters, such as ACh, glutamate, and GABA, are involved in PD. Dopamine and ACh act as postsynaptic excitatory neurotransmitters via muscarinic, nicotinic, and D1/D2 receptors (Lotankar et al., 2017). PD causes neuronal death, mitochondrial dysfunction, increased ROS levels, activation of cell death, and inflammatory responses (Castro-Caldas et al., 2012).
Taurine was shown to reduce dopaminergic neurodegeneration in mice intoxicated with paraquat and maneb, resulting in motor deficits. Taurine inhibited microglial activation and M1 polarization in paraquat and maneb treatments (Additional Table 1), as well as the expression of proinflammatory factors in microglia (Che et al., 2018). Taurine inhibits the membrane translocation of the cystolic subunit P47-phagocyte oxidase and NF-κB, both of which are involved in the induction and maintenance of microglial inflammation. Microglial inflammation is associated with neurodegenerative processes in PD. Taurine reduces the inflammation of microglia triggered by paraquat and maneb (Ma et al., 2022). Activation of microglia and innate immune cells in the brain is an indicator of activation of the inflammatory process (Che et al., 2018). Considering all the protective effects of taurine in the treatment of PD, it may be a promising agent for future studies and clinical trials in patients with PD and other neurodegenerative diseases.
Taurine in the management of cerebral ischemia
Cerebral ischemia is a major cause of death and disability in humans. Ischemia causes neuronal damage by triggering numerous biochemical reactions that lead to the release of excitatory amino acids and activation of the N-methyl-D-aspartate (NMDA) receptor, which opens ion channels and causes excessive Ca2+ influx, and stimulates enzymatic processes, resulting in irreversible neuronal damage. Most deaths worldwide are caused by ischemic cerebrovascular disease, which is characterized by neuronal damage and decreased functioning of a specific part of the brain where oxygen is not properly delivered by the blood (Seol et al., 2021). Massive induction of Na+ and water due to the presence of Na+, K+, and ATPase in the cell membrane, resulting in edema in neurons. Oxidative stress is caused by an imbalance between antioxidants and pro-oxidants. Membrane depolarization is caused by metabolic failure, and Ca2+ enters the cell through voltage-sensitive channels (Rafiee et al., 2022). Further albumin, estrogen, and erythropoietin are among the growth factors that are released due to focal cerebral ischemia. The adverse effects of cerebral ischemia include glutamate release, intracellular Ca2+ storage, disruption of cellular homeostasis, and structural damage to brain tissue (Sun and Xu, 2008). An unknown consequence of focal cerebral ischemia is the accumulation of Ca2+ in neuronal cells, which may lead to cellular damage in the infarcted region (Datta et al., 2020). Following middle cerebral artery occlusion, treatment with taurine increases calpastatin levels while decreasing the activities of m-calpain and caspase-3. Taurine is thought to inhibit apoptotic cell death pathways mediated by m-calpain and caspase-3 by inhibiting the expression of caspase-3 (Sun and Xu, 2008). Taurine activates GABA receptors and strychnine-sensitive glycine receptors, both of which have neuroprotective properties. Despite this, it is unknown whether taurine can inhibit caspase-3 through GABA. Taurine reduces brain water content and improves neurological function. It also reduces the expression of some factors such as 12/15 lipoxygenase, mitogen-activated protein kinase, phospholipase A2, and tumor necrosis factor alpha (Zhang et al., 2017). Taurine inhibits neuronal transmission and modulates neurotransmission via GABA-A and glycine receptors (Menzie et al., 2013). Taurine inhibits Na2+ and Ca2+ exchangers, N-type voltage-gated calcium channels, and prevents the release of Ca2+ from the ER by maintaining intracellular Ca2+ homeostasis (Menzie et al., 2013). Overall, the results of several studies give us evidence that taurine may have the ability to reduce ischemia by increasing calpastatin levels and decreasing the expression of m-calpain and caspase-3.
Role of taurine in epilepsy and seizure management
Epilepsy is a neurological disorder that affects people’s quality of life and health and is characterized by spontaneously recurring seizures (Pandey et al., 2020). Seizures are common in epilepsy, but not all seizures are caused by epilepsy or a drug-induced seizure. There are 65 million people worldwide who have epilepsy. Misconceptions, social stigma, discrimination, and the stress of living with an unpredictable chronic disease can lead to a loss of autonomy in daily living activities for people with epilepsy. Epilepsy is characterized by a persistent tendency to seizures, which has neurobiological, cognitive, psychological, and social consequences. Epilepsy is caused by an imbalance of excitatory and inhibitory neurotransmitters in the neuronal cells. Taurine is an abundant amino acid in the brain that acts as an inhibitory neuromodulator, causing hyperpolarization, inhibiting neuron firing, and altering amino acid concentrations (Oja and Saransaari, 2022). Administration of taurine suppresses seizures induced by all stimulants, including opioids, picrotoxin, and others. GABA is the dominant inhibitory neurotransmitter in the brain and contributes to the control of neuroexcitability and the prevention of hyperexcitability and seizures (L’Amoreaux et al., 2010). As shown in Additional Table 1, taurine acts as an agonist of the GABA-A receptor and increases chloride influx into postsynaptic neurons to prevent hyperexcitability through hyperpolarization (Junyent et al., 2009). Glycine, an inhibitory neurotransmitter, acts similarly to GABA by activating chloride conductance and hyperpolarizing neurons. It also has inhibitory effects on the glycine receptor, as the binding of taurine to this receptor results in eliciting chloride currents and suppressing neuronal activity (Wu et al., 2008). Due to its interactions with the GABAergic and glutamatergic systems, taurine has anticonvulsant properties as well as antioxidant properties. Taurine affects Cl channels via various interactions with GABA-A receptors, in the glutamatergic and GABAergic systems. Taurine has a very similar molecular structure to GABA, and its effects are largely mediated through GABA-A receptors (Song et al., 2012) Taurine acts as an inhibitor in the brain by causing hyperpolarization in the hippocampus, inhibition of neuron firing, and stabilization of the cell membrane (Del Olmo et al., 2000). Taurine protects neurons from glutamate-induced neurotoxicity by preventing glutamate-induced membrane depolarization, excitotoxicity, mitochondrial energy loss, calpain activation, Bcl-2 reduction, and apoptosis (Ye et al., 2013). It can prevent apoptosis triggered by glutamate by preventing downstream events such as a glutamate-induced increase in intracellular calcium (Chan et al., 2014). Taurine (100 mg/kg, intraperitoneally) increased the expression of the NMDA GluN2b subunit but not the GluN1 subunit in the rat frontal cortex. Reduction of calcium influx and protein stimulation also reduced the expression of GluR2-induced NADP oxidases. Taurine is considered an antioxidant that reduces oxidative stress. In a study, taurine was found to protect neurons from NMDA-induced injury while inhibiting the production of ROS, especially superoxide anions. Taurine has anticonvulsant effects by decreasing glutaminergic system activity and oxidative stress and increasing GABAergic system activity (Hrncic et al., 2018).
Potential of taurine for the treatment of depression
Depression is a dysthymic disorder that causes a variety of symptoms such as persistent depression, somatic symptoms, cognitive impairment, intellectual ability, and mental retardation. Chronic stress in adolescence causes changes in the endocrine and nervous systems. It affects both the central nervous system and the endocrine system, causing neurobiological, neurochemical, and morphological changes in stress-prone brain regions such as the hippocampus, which is responsible for learning, emotions, and memory. Hippocampal dysfunction includes loss of neurons, the release of hormones, and other symptoms. Some antidepressants such as tricyclic antidepressants, monoamine oxidase inhibitors, and selective serotonin reuptake inhibitors have a therapeutic effect on symptoms related to depression and are commonly administrated. However, antidepressants also have some disadvantages, such as the slow onset of action, low response rates, and drug resistance. It has been observed that pre-administration of taurine leads to changes in depression-like behavior (Liu et al., 2019; Stefanello et al., 2021). As shown in Additional Table 1, taurine appears to be beneficial in preventing depression-like behaviors. Pre-administration of taurine reduced the levels of 5-hydroxytryptamine, dopamine, noradrenaline, glutamate, and corticosterone in depressed rats, as shown in Additional Table 1. In depression, taurine prevents dysregulation of hormones and neurotransmitters, reduces sucrose absorption, and prevents memory loss and anxiety (Fontana et al., 2020). In a recent study, a chronic unpredictable mild stress-induced depression model was established and the effect of taurine on chronic unpredictable mild stress rats and glutamate-damaged neural stem cells was investigated. Ki67-positive cells were significantly more abundantly expressed in taurine-treated rats, and apoptosis in the dentate gyrus region of chronic unpredictable mild stress rats was significantly reduced. In the in vitro study, cell viability, Brdu+, β-tubulin III+, and glial fibrillary acidic protein (GFAP)+ cells were more highly expressed, while the apoptosis rate was lower in the taurine-treated group compared to the glutamate-treated cells. Finally, from the studies and experiments, it was concluded that taurine has a protective effect and improves cell survival, proliferation, maturation, and differentiation of neural stem cells. These effects are achieved by activating the expression of BDNF/ERK/CREB signaling pathways, and the apoptosis-inhibitory effect of taurine is due to its antioxidant effect (Lee et al., 2020). Taurine is an important amino acid found in the brain and other tissues and is required for brain development. In a recent study, microdialysis and metabolomic analysis revealed that taurine content in the extracellular fluid of the cerebral medial prefrontal cortex was greatly reduced due to depression caused by chronic social defeat stress. They used taurine as a complementary therapy to treat depression and found that supplementation restored immobility time in the tail suspension test and also improved social avoidance behavior in chronic social defeat stress mice. The expression of NMDA subunit 2A, which is a very important synaptic receptor, was greatly improved in the medial prefrontal cortex of chronic social defeat stress mice after taurine supplementation. Finally, taurine was found to have neuroprotective effects against depression and against loss of dendritic spines in cortical neurons (Zhu et al., 2022). Taurine reduces the probability of depression occurrence through the expression of BDNF, ERK, CREB, and NMDA, suggesting that taurine has good efficacy in the treatment of depression.
Taurine administration for anxiety disorder
Anxiety disorders are the most common psychiatric diagnosis, affecting 10% to 30% of the population. A serious anxiety disorder can have a negative impact on a person’s quality of life. Benzodiazepines have long been used to treat anxiety disorders, sedation, muscle relaxation, and amnesia. In a previous study, taurine supplementation increased distance traveled, movement speed, and time spent in the entire zone. Taurine injection decreased anxiety in the elevated arm maze, whereas taurine supplementation increased anxiety. This suggests that taurine affects anxiety and locomotion. Second, chronic taurine had the opposite effect compared to acutely administered taurine. Anxiety in zebrafish, an animal model used to study various psychiatric disorders, can be measured by observing their response to novelty and their habituation to a brightly lit environment. The authors examined whether acute treatment altered the anxiety-like behavior of zebrafish in both the novel tank and the light-dark test. In the novel tank and the light-dark test, fish were treated individually with taurine (42; 150; 400 mg/L). The behavior of the fish was observed and analyzed for 6 minutes. On the other hand, in the control group, the fish were kept in native pool water. These results suggest that taurine has an anxiety-relieving effect in zebrafish by increasing shuttling and time spent in the lighted compartment, but not by significantly reducing locomotor activity. In addition, the 150 mg/L taurine group experienced a significant reduction in risk assessment episodes (Mezzomo et al., 2016). In a study, brain metabolites and behavioral changes were compared between mice fed taurine (22.5 mmol/kg diet) and beta-alanine (22.5 mmol/kg diet) and mice fed a control diet. Beta-alanine-fed animals were more active than control and taurine-fed animals. As a result, dwell time and inputs to open arms increased in the Elevated Plus Maze Test. Taurine supplementation increased arginine levels in the hypothalamus, but beta-alanine supplementation decreased 5-hydroxyindoleacetic acid and increased carnosine levels in the cerebral cortex and hypothalamus. The results suggest that dietary taurine supplementation has an antidepressant effect, while beta-alanine has an anxiolytic effect. It also shows that taurine has anxiety-relieving properties. Taurine can be delivered to the brain by the intranasal route, resulting in an antianxiety effect. Taurine may have anxiety-relieving effects by inhibiting strychnine-induced convulsions via the strychnine-sensitive glycine receptor (Jung and Kim, 2019). In another experiment, the zebrafish are exposed to water (control) and treated with taurine (42, 150, and 400 mg/L) and ethanol (0.25 v/v) and 1 percent (v/v) in novelty test and light/dark test, respectively. Taurine at 42 mg/L and 400 mg/L positively modulate the effect of ethanol, while ethanol at 0.25 percent and taurine at 140 mg/L produce a U-shaped anxiolytic. When 1% ethanol was added to taurine, all concentrations prevented locomotion impairment and anxiety-like behavior. The main effect of taurine and ethanol is an anxiety-like response and exploration. Depending on the alcohol concentration, taurine modulates ethanol-evoked anxiety and anxiogenic behavior in different ways (Fontana et al., 2020). In a recent study, taurine was found to enter cells via the taurine transporter (TauT). Therefore, taurine knockout mice were studied and showed lower body weight, exercise intolerance, and behavioral defects, and also showed lower anxiety-like behavior, suggesting that taurine plays an important role in behavioral function (Watanabe et al., 2022). A recent study by Cruz et al. (2022) examined the effects of taurine at doses of 150 and 1000 ppm in a lead-induced anxiety model. The Elevated Plus Maze Test was used to assess the response to taurine. The results indicate that lead exposure induces anxiety-like behavior, but treatment with taurine significantly reduces anxiety-like behavior. However, the neurobiological mechanism is still unknown (Cruz et al., 2022). Therefore, further research is needed to evaluate the further mechanistic findings on taurine in relation to neurobehavioral and associated neurobiological functions.
Role of taurine on TBI
TBI can be defined as any change in brain function or other evidence of brain pathology caused by an external agent. TBIs account for a significant portion of the total number of epileptic seizures and pose a significant public health threat. An inflammatory response in TBI is characterized by activation of glia, recruitment of neutrophils, upregulation of adhesion molecules, and release of cytokines (Werner, 2007). When someone suffers a traumatic brain injury, they may suffer from neurological deficits, diffuse brain edema, and reactive astrogliosis, the severity of which depends on the extent of the injury (Laird et al., 2008). TBI is composed of two different types of injuries: primary injury (also referred to as mechanical injury) and secondary injury (delayed non-mechanical injury). Primary brain injuries can be either focal or diffuse injuries to the brain parenchyma. This depends on the force of the impact that caused the injury. Damage to neurons and blood vessels can interact to produce secondary brain injury, which can take the form of protein degradation, excitotoxicity, production of oxygen-free radicals, apoptosis, inflammation, and ischemia. When GFAP-IL-6 transgenic mice suffer brain damage, a significant amount of cytokines, including IL-6, are released into the bloodstream. In addition, the level of IL-8, known to play an important role in secondary brain injury, has been found to be elevated in the colony-stimulating factor of patients with traumatic brain injury (Ziebell and Morganti-Kossmann, 2010). After TBI, IL-1 is directly involved and Tehranian et al. (2002) found that central injection of IL-1ra showed a reduction in TBI after a certain period, between 15 and 30 minutes, and suppressed the production of IL-1 (Tehranian et al., 2002; Lucas et al., 2006). In the early stages of TBI, there is a reduction in cerebral blood flow, resulting in swelling of the brain and an increase in intracranial pressure. This leads to a decrease in metabolic activity and blood flow within the cerebral cortex (Jaggi et al., 1990; Soustiel and Sviri, 2007). According to the research findings, the main regulatory paradigms are the following: cerebral autoregulation, flow-metabolism coupling, and neurogenic regulation (Mahadevan and Asokan, 2011; Miyazaki et al., 2022). Taurine, a neuroprotective agent, has been shown to have a number of different inhibitory mechanisms, each of which has the potential to reduce the risk of traumatic brain injury (Vahdat et al., 2021). Some of these mechanisms include inhibition of cytotoxicity, prevention of apoptosis and oxidative stress, and reduction of edema and inflammation. After seven days of treatment with taurine in the injured brain cortex on both the ipsilateral and contralateral sides, the researchers discovered a significant increase in cerebral blood flow. It is possible that the synergistic effects of taurine improve cerebral blood flow while relieving edema, increased intracranial pressure, and other brain problems. Because coagulopathy is so common in TBI and has such a major impact on prognosis, it is important to diagnose and treat it as soon as possible (Roşca et al., 2013). By analyzing the parameters of the coagulation profile, Wang et al. (2016) investigated the effect of taurine on the hypercoagulability associated with TBI. It was concluded that taurine may protect against the hypercoagulable state in TBI, as the taurine-treated group showed a significant prolongation of reaction time, suggesting that it may exert an effect similar to anticoagulants in improving hypercoagulability (Wang et al., 2016). Rats injured by moderate lateral fluid percussion received either intravenous taurine (200 mg/kg) or saline immediately after injury or daily for seven days. Functional outcomes were assessed using the Modified Neurological Severity Scale, and brain GFAP levels were monitored using immunofluorescence. Using Luminex xMAP technology, the concentrations of IL-6 cytokine in the damaged cerebral cortex after TBI on day 1 till the 7th day after the accident were examined (Irvine and David Clark, 2018). Supplementation with taurine in the seven days after a TBI significantly decreased GFAP accumulation as well as the water content in the penumbral region. On day 1, the levels of interleukin-1β and growth-related oncogene (GRO/KC) were significantly lower in the taurine group than in the TBI group. In the taurine group, the levels of RANTES protein increased (Nakajima et al., 2010). Taurine significantly decreased the levels of 17 cytokines over 1 week. These cytokines included eotaxin, colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor. On the other hand, taurine significantly increased the levels of macrophage inflammatory protein-1 alpha. By decreasing edema, astrocyte activation, and pro-inflammatory cytokines, taurine reduces the extent of damage inflicted on the brain after TBI. One study examined the effects of taurine on neuronal ultrastructure and P2X purinoceptor 7 (P2X7) expression in rats that had sustained TBI after the experiment was conducted (Su et al., 2014). In the parietal cortex and hippocampal regions, the expression of P2X7 receptor proteins was significantly higher in the TBI groups than in the sham-operated groups. This was true for both the groups with TBI plus low-dose taurine and the groups with TBI plus high-dose taurine and found a significant decrease in the number of P2X7-positive cells in the TBI group compared with the other group (Bondarenko et al., 2018). Taurine administration following TBI was found to have a neuroprotective effect mediated by a decrease in the expression of P2X7 receptor proteins in the parietal cortex and hippocampus (Li et al., 2012). Based on the above findings, taurine serves as a potential agent for the treatment of TBI.
Memory dysfunction and taurine
Memory is the ability to take in, store, and then reconstruct information presented to you either externally or by yourself. Memory dysfunction can be caused by a variety of neuropathological conditions that affect the distributed neural network of multiple dissociable memory systems in the human brain. Memory dysfunction, or degenerative myelopathy, refers to a variety of neurological conditions that can disrupt normal brain function. A previous study investigated the neuroprotective effects of taurine in relation to aluminum-induced neurological disorders in rats. Treatment with taurine (400 to 800 milligrams per kilogram per day) significantly improved brain dysfunction. It was found that the levels of c-GABA and tau were significantly reduced, while the levels of aspartate and glutamate were significantly increased. Administration of taurine partially reversed the exacerbated changes and acted as a neuroprotective agent against learning and memory impairment and neurotransmitter dysfunction (Hernández-Benítez et al., 2010). The ability of taurine, in addition to its ability to stabilize, support and promote cell membranes, is at the core of its ability to counteract memory dysfunction. In addition, research has shown that taurine’s antioxidant and anti-inflammatory properties directly benefit cells by preventing the formation of lipid peroxidation, which is caused by free radicals and impairs learning and memory. This is achieved through the ability of taurine to inhibit lipid peroxidation (El-Sayed et al., 2011). Aluminum administration resulted in an increase in aspartate, glycine, and GABA levels but a decrease in taurine, GABA, and GABA levels. In the developing central nervous system, taurine acts as a neuroprotective agent against glutamate-induced excitotoxicity (El-Rahman, 2003). Through its antagonistic action, taurine causes a reversal of the increased excitatory amino acid activity in the central nervous system, enhances inhibition of neurotransmission via GABA-A and glycine receptors, and protects against excitotoxicity (Wu et al., 2008). According to these findings, taurine may provide some protection against memory loss, learning disabilities, and neurotransmitter dysfunction caused by aluminum. It may also stimulate growth and development, stabilize cell membranes, and prevent the significant damage caused by aluminum (Wenting et al., 2014). Further in another study, the effects of taurine on behavioral deficits as well as neuronal damage caused by repeated experiments in mice was observed. Adult mice exposed to restricted stress (RS) over 4 weeks developed dementia, depression-like behavior, anhedonia, oxidative-nitrosative stress, dysregulation of pain, and neuroinflammation. Taurine has been shown to protect against impairments in learning and memory by reducing inflammation and oxidative stress in the hippocampus of rats exposed to RS. In mice exposed to RS, overexpression of the caspase-3 and NF-κB proteins was prevented by the administration of taurine at a dose of 200 mg/kg. The neuroprotective properties of taurine have been shown to be influenced by a variety of mechanisms. These mechanisms include ROS, disruption of the hypothalamic-pituitary-adrenal axis, and dysregulation of cholinergic function. According to the results of some studies, ROS and pro-inflammatory cytokines may contribute to behavioral disorder. Administration of taurine to mice exposed to RS prevented the development of spatial learning deficits. In a previous study, it was discovered that taurine significantly reduced the levels of IL-1 in the hippocampus of mice treated with taurine. As a result, neuronal cell death and oxidative stress caused by RS were inhibited. For this reason, taurine showed neuroprotective potential and improved memory dysfunction (Jangra et al., 2020). Using cell models, it was found that taurine could rescue potentiation in the hippocampus. One of the main causes of hepatic encephalopathy is hyperammonemia, which impairs long-term synaptic potentiation in the hippocampus, the brain region that controls learning and memory. Taurine has an effect on the paradigm by altering long-term synaptic potentiation properties in mouse hippocampal slices with taurine from hyperammonemic rats and by acute exposure of rat hippocampal slices to ammonia. The antioxidant taurine halts ammonia-induced cell swelling and accumulation of cyclic guanosine monophosphate under hyperammonemic conditions. In another study, taurine was found to improve mitochondrial function by coactivating GABA-A and inhibitory glycine receptors under hyperammonic conditions. The research also suggests that taurine may be able to restore hippocampal plasticity by improving mitochondrial function (Chepkova et al., 2006).
Protective role of taurine on SCI
Taurine has a protective effect on the pharmacology and pathophysiology of SCI. According to the reports, there were about 15–40 cases of SCI per million people annually. The highest rate of SCI was found in patients older than 50 years, and these patients were predisposed to SCI due to pre-existing spondylosis that was the result of relatively low-energy trauma (Kim et al., 2017). The classification of an SCI as primary or secondary is determined by the timing of the trauma and subsequent pathologic conditions. A primary injury occurs immediately and cannot be reversed, whereas a secondary injury occurs after a primary injury (Liu et al., 2022). T cells, macrophages, and astrocytes are all involved in the inflammatory process, which leads either directly or indirectly to the activation of cellular and hormonal inflammatory mechanisms, which in turn causes necrosis, apoptosis, free radical production, and an increase in blood-brain barrier permeability (Kim et al., 2017). Although SCI is a serious condition that can lead to long-term disability, there are currently a limited number of treatment options for this condition. Traumatic spinal cord injury occurs when an initial physical injury is followed by a secondary injury that progresses over time. SCI causes apoptosis in the neurons and oligodendrocytes of the spinal cord, leads to axonal degeneration and demyelination, which in turn cause damage to the spinal cord. Previous research reported significant levels of inflammation and free radical formation in the injured area (Barreiro-Iglesias et al., 2019; Li et al., 2019b). In addition, these injuries are associated with significant levels of inflammation and free radical formation in the injured area (Chen et al., 2020). There is growing evidence that acute SCI is associated with early acute hemorrhagic necrosis as well as endothelial damage, a significant reduction in microcirculation, and major infarcts at the site of injury. Acute SCI triggers the migration of neutrophils to necrotic areas. Taurine is a non-proteinogenic amino acid that serves as an essential component in brain assembly. In studies on rat models induced with SCI, the combination of ascorbic acid and taurine was discovered to have a synergistic effect (Chen et al., 2020). When the rat models induced with SCI were treated with taurine and ascorbic acid, this resulted in a decrease in the expression of caspase-3, BAX, Bcl-2, and p53. Taurine and ascorbic acid were found to have a protective role against SCI. Accordingly, apoptotic and inflammatory markers of oxidative stress were found to be significantly reduced in the treatment group receiving taurine and ascorbic acid (Chen et al., 2020). SCI patients treated with taurine experienced dose-dependent decreases in levels of IL-6 and myeloperoxidase, as well as decreases in STAT3 and Cox-2 phosphorylation expression. Neutrophil numbers were found to be lower, particularly in the subarachnoid space, and the number of cells undergoing secondary apoptosis was also lower. In another study, taurine was shown to have an anti-inflammatory effect against SCI. It is also thought to play a protective role against secondary damage, giving it significant therapeutic potential (Hrncic et al., 2018). Evidence suggests that significant amounts of taurine are present in the central nervous system, suggesting that active transport occurs between cellular and extracellular spaces. The use of taurine analogs has led to better results. For example, the taurine derivative NCT has been shown to be superior to taurine at a dose of 200–400 μM in terms of its therapeutic efficacy (Gupta et al., 2006).
According to the findings of Nakajima et al., (2010) taurine chloramine provides protection against chlorinated oxidants and inhibits the toxicity of chlorinated oxidants by binding nitric oxide, prostaglandin E-2, and IL-6. This may be due, at least in part, to a reduction in the activity of the transcriptional regulators NF-κB and Activator Protein-1. The ability of taurine to regulate intracellular Ca2+ flux and its antioxidant potential make it useful for the prevention of endothelial dysfunction and cell death (Nakajima et al., 2010). After birth, the concentration of taurine in the brain of a newborn decreases to 30 percent from its initial level of 70 percent in the developing brain (Oja and Saransaari, 2022). Taurine plays an important role in a variety of neurological processes, including neuroprotection, modulation of excitable neurons, and learning and memory functions. The fact that taurine was shown to be an inhibitory neurotransmitter in the spinal cord in a previous study supports the hypothesis that this chemical is responsible for the antiepileptic effect (Gupta et al., 2006). Further, the neuroprotective mechanisms of taurine in various neurological diseases are depicted in Figure 3.
Figure 3.
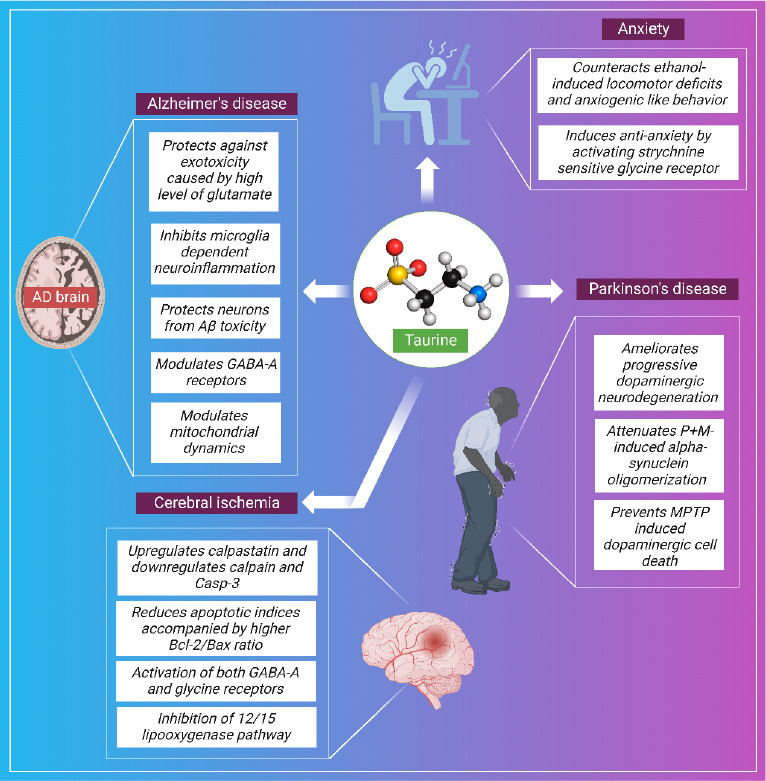
Schematic representation of general neuroprotective mechanisms of taurine.
Created with BioRender.com. AD: Alzheimer’s disease; Aβ: amyloid beta; GABA-A: γ-aminobutyric acid type A; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Concluding Remarks
Based on the above findings from previous studies, we can conclude that taurine is involved in a variety of functions in organisms and is necessary for the maintenance of cellular homeostasis. Taurine has an effect that usually starts slowly but increases in intensity over time. Taurine, also known as 2-aminoethanesulfonic acid, is an endogenous metabolite found in high concentrations in various tissues. It is a sulfur-containing amino acid produced from cysteine. It is excreted without going through the metabolic process. This article addresses the use of taurine in the treatment of various neurodegenerative diseases such as AD, PD, cerebral ischemia, memory impairment, depression, anxiety, SCI, TBI, and epilepsy. In the various disease models, it has the effect of reducing the damage caused by oxidative stress. In addition, at the molecular level, taurine can modulate ER stress, Ca2+ homeostasis, and neuronal activity. When taurine is taken by patients with AD, it inhibits neurotoxicity caused by Aβ and glutamate receptor agonists while activating GABA receptors. By inhibiting microglia-mediated neuroinflammation, taurine may slow the progression of dopaminergic neurodegeneration in PD. Taurine has been shown to reduce the incidence of seizures; therefore, its use in the treatment of epilepsy may be of great importance. In cerebral ischemia, taurine reduces the severity of the disease by preventing the activation of caspase-3 and m-apoptotic cell death pathways and increasing calpastatin levels. Meanwhile, adverse reactions to commonly prescribed medications may prompt people to seek safer forms of alternative and complementary medicine, which in turn may prompt people to look into the use of taurine for neuroprotective purposes. In addition, this article provides a general introduction to taurine and reviews the pharmacological applications of this therapeutic compound.
From the evidence compiled in this review article, it can be inferred that taurine has the potential to be used to treat a variety of neurological disorders. Taurine, a naturally occurring molecule in the human body, has been shown to have the ability to modulate ER stress, oxidative stress, Ca2+ homeostasis, production of ROS, and other related processes. Therefore, it has the potential to serve as a suitable replacement drug for the treatment of neurological disorders. However, before taurine can be developed into a drug, further studies of its toxicity must first be conducted. More research needs to be done to determine the potential signaling targets of this molecule in various neurological diseases. This review has summarized all the latest evidence on the use of taurine as a drug molecule for the treatment of neurodegenerative diseases, including all known molecular mechanisms of taurine’s effect on neuronal function, which can undoubtedly allow for a better understanding of the precise pharmacological effects that taurine exerts on the various organs of the body.
Additional file:
Additional Table 1: The different disease models and their findings at appropriate dose, route and duration of administration.
Acknowledgments:
The authors would like to acknowledge the support provided by their respective institutions throughout the writing process.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: All data relevant to the study are included in the article or uploaded as Additional files.
C-Editors: Zhao M, Sun Y, Qiu Y; T-Editor: Jia Y
References
- 1.Abuirmeileh AN, Abuhamdah SM, Ashraf A, Alzoubi KH. Protective effect of caffeine and/or taurine on the 6-hydroxydopamine-induced rat model of Parkinson's disease:behavioral and neurochemical evidence. Restor Neurol Neurosci. (2021);39:149–157. doi: 10.3233/RNN-201131. [DOI] [PubMed] [Google Scholar]
- 2.Adedara IA, Abolaji AO, Idris UF, Olabiyi BF, Onibiyo EM, Ojuade TD, Farombi EO. Neuroprotective influence of taurine on fluoride-induced biochemical and behavioral deficits in rats. Chem Biol Interact. (2017);261:1–10. doi: 10.1016/j.cbi.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Ansar M, Ranza E, Shetty M, Paracha SA, Azam M, Kern I, Iwaszkiewicz J, Farooq O, Pournaras CJ, Malcles A, Kecik M, Rivolta C, Muzaffar W, Qurban A, Ali L, Aggoun Y, Santoni FA, Makrythanasis P, Ahmed J, Qamar R, et al. Taurine treatment of retinal degeneration and cardiomyopathy in a consanguineous family with SLC6A6 taurine transporter deficiency. Hum Mol Genet. (2020);29:618–623. doi: 10.1093/hmg/ddz303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baliou S, Kyriakopoulos AM, Goulielmaki M, Panayiotidis MI, Spandidos DA, Zoumpourlis V. Significance of taurine transporter (TauT) in homeostasis and its layers of regulation (review) Mol Med Rep. (2020);22:2163–2173. doi: 10.3892/mmr.2020.11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barangi S, Hayes AW, Karimi G. The role of lncRNAs/miRNAs/Sirt1 axis in myocardial and cerebral injury. Cell Cycle. (2023):1–12. doi: 10.1080/15384101.2023.2172265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreiro-Iglesias A, Sobrido-Cameán D, Fernández-López B, Pereiro N, Lafuente A, Rodicio MC. Taurine promotes axonal regeneration after a complete spinal cord injury in lampreys. IBRO Rep. (2019);6:S507–508. doi: 10.1089/neu.2019.6604. [DOI] [PubMed] [Google Scholar]
- 7.Beresewicz-Haller M. Hippocampal region-specific endogenous neuroprotection as an approach in the search for new neuroprotective strategies in ischemic stroke. Fiction or fact?Neurochem Int. (2023);162 doi: 10.1016/j.neuint.2022.105455. doi:10.1016/j.neuint.2022.105455. [DOI] [PubMed] [Google Scholar]
- 8.Biel TG, Lee S, Flores-Toro JA, Dean JW, Go KL, Lee MH, Law BK, Law ME, Dunn WA, Jr, Zendejas I, Behrns KE, Kim JS. Sirtuin 1 suppresses mitochondrial dysfunction of ischemic mouse livers in a mitofusin 2-dependent manner. Cell Death Differ. (2016);23:279–290. doi: 10.1038/cdd.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bondarenko LB, Gorchakova NO, Golembiovska OI, Galkin OY. Promising new fixed combination for the treatment of diseases of the hepatobiliar system:Substantiation of pharmacotherapeutic properties and pharmaceutical quality profile. Regul Mech Biosyst. (2018);9:23–40. [Google Scholar]
- 10.Castro-Caldas M, Carvalho AN, Rodrigues E, Henderson CJ, Wolf CR, Rodrigues CMP, Gama MJ. Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson's disease. Mol Neurobiol. (2012);46:475–486. doi: 10.1007/s12035-012-8295-4. [DOI] [PubMed] [Google Scholar]
- 11.Chaiwong S, Chatturong U, Chanasong R, Deetud W, To-On K, Puntheeranurak S, Chulikorn E, Kajsongkram T, Raksanoh V, Chinda K, Limpeanchob N, Trisat K, Somran J, Nuengchamnong N, Prajumwong P, Chootip K. Dried mulberry fruit ameliorates cardiovascular and liver histopathological changes in high-fat diet-induced hyperlipidemic mice. J Tradit Complement Med. (2021);11:356–368. doi: 10.1016/j.jtcme.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan CY, Singh I, Magnuson H, Zohaib M, Bakshi KP, Le François B, Anazco-Ayala A, Lee EJ, Tom A, YeeMon K, Ragnauth A, Friedman E, Banerjee SP. Taurine targets the GluN2b-containing NMDA receptor subtype. Adv Exp Med Biol. (2015);803:531–544. doi: 10.1007/978-3-319-15126-7_43. [DOI] [PubMed] [Google Scholar]
- 13.Chan CY, Sun HS, Shah SM, Agovic MS, Friedman E, Banerjee SP. Modes of direct modulation by taurine of the glutamate NMDA receptor in rat cortex. Eur J Pharmacol. (2014);728:167–175. doi: 10.1016/j.ejphar.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Che Y, Hou L, Sun F, Zhang C, Liu X, Piao F, Zhang D, Li H, Wang Q. Taurine protects dopaminergic neurons in a mouse Parkinson's disease model through inhibition of microglial M1 polarization. Cell Death Dis. (2018);9:435. doi: 10.1038/s41419-018-0468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Xia S, He J, Lu G, Xie Z, Han H. Roles of taurine in cognitive function of physiology, pathologies and toxication. Life Sci. (2019);231:116584. doi: 10.1016/j.lfs.2019.116584. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Yang Q, Ma X. Synergistic effect of ascorbic acid and taurine in the treatment of a spinal cord injury-induced model in rats. 3 Biotech. (2020);10:50. doi: 10.1007/s13205-019-2032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Xue X, Cai J, Jia L, Sun B, Zhao W. Protective effect of taurine on sepsis-induced lung injury via inhibiting the p38/MAPK signaling pathway. Mol Med Rep. (2021);24:653. doi: 10.3892/mmr.2021.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chepkova AN, Sergeeva OA, Haas HL. Taurine rescues hippocampal long-term potentiation from ammonia-induced impairment. Neurobiol Dis. (2006);23:512–521. doi: 10.1016/j.nbd.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Cruz GB, Vasquez MA, Cabañas E, Joseph JN, Skeen JC, Lynch KP, Ahmed I, Khairi EB, Bonitto JR, Clarke EG, Rubi S, Hameed N, Kaur S, Mathew N, Dacius TF, Jose TJ, Handford G, Wolfe S, Feher A, Tidwell K, et al. Developmental lead exposure in rats causes sex-dependent changes in neurobiological and anxiety-like behaviors that are improved by taurine co-treatment. Adv Exp Med Biol. (2022);1370:461–479. doi: 10.1007/978-3-030-93337-1_43. [DOI] [PubMed] [Google Scholar]
- 20.Datta A, Sarmah D, Mounica L, Kaur H, Kesharwani R, Verma G, Veeresh P, Kotian V, Kalia K, Borah A, Wang X, Dave KR, Yavagal DR, Bhattacharya P. Cell death pathways in ischemic stroke and targeted pharmacotherapy. Transl Stroke Res. (2020);11:1185–1202. doi: 10.1007/s12975-020-00806-z. [DOI] [PubMed] [Google Scholar]
- 21.del Olmo N, Bustamante J, del Río RM, Solís JM. Taurine activates GABAA but not GABAB receptors in rat hippocampal CA1 area. Brain Res. (2000);864:298–307. doi: 10.1016/s0006-8993(00)02211-3. [DOI] [PubMed] [Google Scholar]
- 22.Doğanyiğit Z, Erbakan K, Akyuz E, Polat AK, Arulsamy A, Shaikh MF. The role of neuroinflammatory mediators in the pathogenesis of traumatic brain injury:a narrative review. ACS Chem Neurosci. (2022);13:1835–1848. doi: 10.1021/acschemneuro.2c00196. [DOI] [PubMed] [Google Scholar]
- 23.Donmez G. The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol Sci. (2012);33:494–501. doi: 10.1016/j.tips.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Donmez G, Outeiro TF. SIRT1 and SIRT2:emerging targets in neurodegeneration. EMBO Mol Med. (2013);5:344–352. doi: 10.1002/emmm.201302451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Idrissi A, Boukarrou L, Heany W, Malliaros G, Sangdee C, Neuwirth L. Effects of taurine on anxiety-like and locomotor behavior of mice. Adv Exp Med Biol. (2009);643:207–215. doi: 10.1007/978-0-387-75681-3_21. [DOI] [PubMed] [Google Scholar]
- 26.Elhussiny MZ, Tran PV, Tsuru Y, Haraguchi S, Gilbert ER, Cline MA, Bungo T, Furuse M, Chowdhury VS. Central taurine attenuates hyperthermia and isolation stress behaviors augmented by corticotropin-releasing factor with modifying brain amino acid metabolism in neonatal chicks. Metabolites. (2022);12:83. doi: 10.3390/metabo12010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Rahman SS. Neuropathology of aluminum toxicity in rats (glutamate and GABA impairment) Pharmacol Res. (2003);47:189–194. doi: 10.1016/s1043-6618(02)00336-5. [DOI] [PubMed] [Google Scholar]
- 28.El-Sayed WM, Al-Kahtani MA, Abdel-Moneim AM. Prophylactic and therapeutic effects of taurine against aluminum-induced acute hepatotoxicity in mice. J Hazard Mater. (2011);192:880–886. doi: 10.1016/j.jhazmat.2011.05.100. [DOI] [PubMed] [Google Scholar]
- 29.Fontana BD, Cleal M, Parker MO. Female adult zebrafish (Danio rerio) show higher levels of anxiety-like behavior than males, but do not differ in learning and memory capacity. Eur J Neurosci. (2020);52:2604–2613. doi: 10.1111/ejn.14588. [DOI] [PubMed] [Google Scholar]
- 30.Gupta R, Seki Y, Yosida J. Role of Taurine in Spinal Cord Injury. Curr Neurovasc Res. (2006);3:225–235. doi: 10.2174/156720206778018776. [DOI] [PubMed] [Google Scholar]
- 31.Gutiérrez-Castañeda NE, González-Corona J, Griego E, Galván EJ, Ochoa-de la Paz LD. Taurine promotes differentiation and maturation of neural stem/progenitor cells from the subventricular zone via activation of GABAA receptors. Neurochem Res. (2023) doi: 10.1007/s11064-023-03883-2. doi:10.1007/s11064-023-03883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassanein OH, Ahmed-Farid OA, Shehata AM, El Elaimy IA, Bayomy MFF, Ibrahim HM. Taurine as an adjuvant to valproic acid modulates the epileptic seizures and improves the brain aminergic system in PTZ-kindled rats. Curr Top Pept Protein Res. (2021);22:91–99. [Google Scholar]
- 33.Hernández-Benítez R, Pasantes-Morales H, Torres Saldaña I, Ramos-Mandujano G. Taurine stimulates proliferation of mice embryonic cultured neural progenitor cells. J Neurosci Res. (2010);88:1673–1681. doi: 10.1002/jnr.22328. [DOI] [PubMed] [Google Scholar]
- 34.Hrncic D, Rasic-Markovic A, Macut D, Mladenovic D, Susic V, Djuric D, Stanojlovic O. Sulfur –containing amino acids in seizures:current state of the art. Curr Med Chem. (2018);25:378–390. doi: 10.2174/0929867324666170609090613. [DOI] [PubMed] [Google Scholar]
- 35.Huang X, Li W, You B, Tang W, Gan T, Feng C, Li C, Yang R. Serum metabonomic study on the antidepressant-like effects of ellagic acid in a chronic unpredictable mild stress-induced mouse model. J Agric Food Chem. (2020);68:9546–9556. doi: 10.1021/acs.jafc.0c02895. [DOI] [PubMed] [Google Scholar]
- 36.Irvine KA, Clark JD. Chronic pain after traumatic brain injury:pathophysiology and pain mechanisms. Pain Med. (2018);19:1315–1333. doi: 10.1093/pm/pnx153. [DOI] [PubMed] [Google Scholar]
- 37.Jaggi JL, Obrist WD, Gennarelli TA, Langfitt TW. Relationship of early cerebral blood flow and metabolism to outcome in acute head injury. J Neurosurg. (1990);72:176–182. doi: 10.3171/jns.1990.72.2.0176. [DOI] [PubMed] [Google Scholar]
- 38.Jahanshahi M, Nikmahzar E, Gorgani S. Taurine can decrease phosphorylated tau protein levels in alzheimer's model rats'brains. Kathmandu Univ Med J. (2021);19:200–204. [PubMed] [Google Scholar]
- 39.Jakaria M, Azam S, Haque ME, Jo SH, Uddin MS, Kim IS, Choi DK. Taurine and its analogs in neurological disorders:Focus on therapeutic potential and molecular mechanisms. Redox Biol. (2019);24:101223. doi: 10.1016/j.redox.2019.101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jangra A, Rajput P, Dwivedi DK, Lahkar M. Amelioration of repeated restraint stress-induced behavioral deficits and hippocampal anomalies with taurine treatment in mice. Neurochem Res. (2020);45:731–740. doi: 10.1007/s11064-019-02945-8. [DOI] [PubMed] [Google Scholar]
- 41.Jong CJ, Sandal P, Schaffer SW. The role of taurine in mitochondria health:More than just an antioxidant. Molecules. (2021);26:4913. doi: 10.3390/molecules26164913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung JH, Kim SJ. Anxiolytic action of taurine via intranasal administration in mice. Biomol Ther. (2019);27:450–456. doi: 10.4062/biomolther.2018.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Junyent F, Utrera J, Romero R, Pallàs M, Camins A, Duque D, Auladell C. Prevention of epilepsy by taurine treatments in mice experimental model. J Neurosci Res. (2009);87:1500–1508. doi: 10.1002/jnr.21950. [DOI] [PubMed] [Google Scholar]
- 44.Katakawa M, Fukuda N, Tsunemi A, Mori M, Maruyama T, Matsumoto T, Abe M, Yamori Y. Taurine and magnesium supplementation enhances the function of endothelial progenitor cells through antioxidation in healthy men and spontaneously hypertensive rats. Hypertens Res. (2016);39:848–856. doi: 10.1038/hr.2016.86. [DOI] [PubMed] [Google Scholar]
- 45.Kim HY, Kim HV, Yoon JH, Kang BR, Cho SM, Lee S, Kim JY, Kim JW, Cho Y, Woo J, Kim Y. Taurine in drinking water recovers learning and memory in the adult APP/PS1 mouse model of Alzheimer's disease. Sci Rep. (2014);4:7467. doi: 10.1038/srep07467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YH, Ha KY, Kim SI. Spinal cord injury and related clinical trials. Clin Orthop Surg. (2017);9:1–9. doi: 10.4055/cios.2017.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulthinee S, Wyss JM, Roysommuti S. Taurine supplementation inhibits cardiac and systemic renin-angiotensin system overactivity after cardiac ischemia/reperfusion in adult female rats perinatally depleted of taurine followed by high sugar intake. Adv Exp Med Biol. (2019);1155:101–112. doi: 10.1007/978-981-13-8023-5_9. [DOI] [PubMed] [Google Scholar]
- 48.Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. (2008);16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- 49.L'Amoreaux WJ, Marsillo A, El Idrissi A. Pharmacological characterization of GABAA receptors in taurine-fed mice. J Biomed Sci. (2010);17:S14. doi: 10.1186/1423-0127-17-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee WJ, Lee GH, Hur J, Lee HG, Kim E, Won JP, Cho Y, Choi MJ, Seo HG. Taurine and ginsenoside Rf induce BDNF expression in SH-SY5Y cells:a potential role of BDNF in corticosterone-triggered cellular damage. Molecules. (2020);25:2819. doi: 10.3390/molecules25122819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H, Ruan WJ, Liu LQ, Wan HF, Yang XH, Zhu WF, Yu LH, Zhang XL, Wan FS. Impact of taurine on the proliferation and apoptosis of human cervical carcinoma cells and its mechanism. Chin Med J. (2019a);132:948–956. doi: 10.1097/CM9.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li K, Shi X, Luo M, Inam-U-Llah, Wu P, Zhang M, Zhang C, Li Q, Wang Y, Piao F. Taurine protects against myelin damage of sciatic nerve in diabetic peripheral neuropathy rats by controlling apoptosis of schwann cells via NGF/Akt/GSK3βpathway. Exp Cell Res. (2019b);383:111557. doi: 10.1016/j.yexcr.2019.111557. [DOI] [PubMed] [Google Scholar]
- 53.Li XJ, Li S, Li XQ, Wei LY, Li DL. Effects of taurine on the ultrastructure and P2X7 receptor expression in brain following traumatic brain injury in rats. Chin J Physiol. (2012);28:301–303. [PubMed] [Google Scholar]
- 54.Liu C, He P, Guo Y, Tian Q, Wang J, Wang G, Zhang Z, Li M. Taurine attenuates neuronal ferroptosis by regulating GABAB/AKT/GSK3β/β-catenin pathway after subarachnoid hemorrhage. Free Radic Biol Med. (2022);193:795–807. doi: 10.1016/j.freeradbiomed.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Liu X, Zheng X, Du G, Li Z, Qin X. Brain metabonomics study of the antidepressant-like effect of Xiaoyaosan on the CUMS-depression rats by 1H NMR analysis. J Ethnopharmacol. (2019);235:141–154. doi: 10.1016/j.jep.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 56.Lotankar S, Prabhavalkar KS, Bhatt LK. Biomarkers for Parkinson's disease:recent advancement. Neurosci Bull. (2017);33:585–597. doi: 10.1007/s12264-017-0183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Louzada PR, Paula Lima AC, Mendonca-Silva DL, Noël F, De Mello FG, Ferreira ST. Taurine prevents the neurotoxicity of β-amyloid and glutamate receptor agonists:activation of GABA receptors and possible implications for Alzheimer's disease and other neurological disorders. FASEB J. (2004);18:511–518. doi: 10.1096/fj.03-0739com. [DOI] [PubMed] [Google Scholar]
- 58.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. (2006);147:S232–240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Y, Zhang Y, Li R, Deng S, Qin Q, Ran C, Hao Y, Zhang J, Zhu L. Mechanism of taurine reducing inflammation and organ injury in sepsis mice. Cell Immunol. (2022);375:104503. doi: 10.1016/j.cellimm.2022.104503. [DOI] [PubMed] [Google Scholar]
- 60.Mahadevan V, Asokan A. Regulation of cerebral blood flow. Postgrad Topics Anesth. (2011) doi:10.5005/jp/books/11286_7. [Google Scholar]
- 61.McCarty MF, O'Keefe JH, DiNicolantonio JJ. A diet rich in taurine, cysteine, folate, B12 and betaine may lessen risk for Alzheimer's disease by boosting brain synthesis of hydrogen sulfide. Med Hypotheses. (2019);132:109356. doi: 10.1016/j.mehy.2019.109356. [DOI] [PubMed] [Google Scholar]
- 62.Menzie J, Prentice H, Wu JY. Neuroprotective mechanisms of taurine against ischemic stroke. Brain Sci. (2013);3:877–907. doi: 10.3390/brainsci3020877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merckx C, De Paepe B. The role of taurine in skeletal muscle functioning and its potential as a supportive treatment for duchenne muscular dystrophy. Metabolites. (2022);12:193. doi: 10.3390/metabo12020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mezzomo NJ, Silveira A, Giuliani GS, Quadros VA, Rosemberg DB. The role of taurine on anxiety-like behaviors in zebrafish:a comparative study using the novel tank and the light-dark tasks. Neurosci Lett. (2016);613:19–24. doi: 10.1016/j.neulet.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 65.Miyazaki T, Ito T, Baseggio Conrado A, Murakami S. Editorial for special issue on “regulation and effect of taurine on metabolism”. Metabolites. (2022);12:795. doi: 10.3390/metabo12090795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyazaki T, Matsuzaki Y. Taurine and liver diseases:a focus on the heterogeneous protective properties of taurine. Amino Acids. (2014);46:101–110. doi: 10.1007/s00726-012-1381-0. [DOI] [PubMed] [Google Scholar]
- 67.Oh SJ, Lee HJ, Jeong YJ, Nam KR, Kang KJ, Han SJ, Lee KC, Lee YJ, Choi JY. Evaluation of the neuroprotective effect of taurine in Alzheimer's disease using functional molecular imaging. Sci Rep. (2020);10:15551. doi: 10.1038/s41598-020-72755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oja SS, Saransaari P. Taurine and the brain. Adv Exp Med Biol. (2022);1370:325–331. doi: 10.1007/978-3-030-93337-1_31. [DOI] [PubMed] [Google Scholar]
- 69.Pandey DK, Dasgupta R, Levy J, Wang H, Serafini A, Habibi M, Song W, Shafer PO, Loeb JA. Enhancing epilepsy self-management and quality of life for adults with epilepsy with varying social and educational backgrounds using PAUSE to learn your epilepsy. Epilepsy Behav. (2020);111:107228. doi: 10.1016/j.yebeh.2020.107228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paula-Lima AC, De Felice FG, Brito-Moreira J, Ferreira ST. Activation of GABAA receptors by taurine and muscimol blocks the neurotoxicity of β-amyloid in rat hippocampal and cortical neurons. Neuropharmacology. (2005);49:1140–1148. doi: 10.1016/j.neuropharm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 71.Rafiee Z, García-Serrano AM, Duarte JMN. Taurine supplementation as a neuroprotective strategy upon brain dysfunction in metabolic syndrome and diabetes. Nutrients. (2022);14:1292. doi: 10.3390/nu14061292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roşca AE, Badiu C, Uscătescu V, Stoian I, Mirică R, Braga RI, Pavel B, Zăgrean L. Influence of chronic administration of anabolic androgenic steroids and taurine on haemostasis profile in rats. Blood Coagul Fibrinolysis. (2013);24:256–260. doi: 10.1097/MBC.0b013e32835b7611. [DOI] [PubMed] [Google Scholar]
- 73.Rubio-Casillas A, Redwan EM, Uversky VN. On the potential therapeutic roles of taurine in autism spectrum disorder. Neuroglia. (2023);4:1–14. [Google Scholar]
- 74.Schaffer S, Kim HW. Effects and mechanisms of taurine as a therapeutic agent. Biomol Ther. (2018);26:225–241. doi: 10.4062/biomolther.2017.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seol SI, Kim HJ, Choi EB, Kang IS, Lee HK, Lee JK, Kim C. Taurine protects against postischemic brain injury via the antioxidant activity of taurine chloramine. Antioxidants. (2021);10:372. doi: 10.3390/antiox10030372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song NY, Shi HB, Li CY, Yin SK. Interaction between taurine and GABAA/glycine receptors in neurons of the rat anteroventral cochlear nucleus. Brain Res. (2012);1472:1–10. doi: 10.1016/j.brainres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Soustiel JF, Sviri GE. Monitoring of cerebral metabolism:Non-ischemic impairment of oxidative metabolism following severe traumatic brain injury. Neurol Res. (2007);29:654–660. doi: 10.1179/016164107X240017. [DOI] [PubMed] [Google Scholar]
- 78.Stefanello FV, Müller TE, Franscescon F, Quadros VA, Souza TP, Canzian J, Leitemperger J, Loro VL, Rosemberg DB. Taurine modulates behavioral effects of intermittent ethanol exposure without changing brain monoamine oxidase activity in zebrafish: Attenuation of shoal- and anxiety-like responses, and abolishment of memory acquisition deficit. Pharmacol Biochem Behav. (2021);209:173256. doi: 10.1016/j.pbb.2021.173256. [DOI] [PubMed] [Google Scholar]
- 79.Su Y, Fan W, Ma Z, Wen X, Wang W, Wu Q, Huang H. Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience. (2014);266:56–65. doi: 10.1016/j.neuroscience.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 80.Sukhotnik I, Aranovich I, Ben Shahar Y, Bitterman N, Pollak Y, Berkowitz D, Chepurov D, Coran AG, Bitterman A. Effect of taurine on intestinal recovery following intestinal ischemia-reperfusion injury in a rat. Pediatr Surg Int. (2016);32:161–168. doi: 10.1007/s00383-015-3828-3. [DOI] [PubMed] [Google Scholar]
- 81.Sun M, Xu C. Neuroprotective mechanism of taurine due to up-regulating calpastatin and down-regulating calpain and caspase-3 during focal cerebral ischemia. Cell Mol Neurobiol. (2008);28:593–611. doi: 10.1007/s10571-007-9183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tao X, Zhang Z, Yang Z, Rao B. The effects of taurine supplementation on diabetes mellitus in humans:A systematic review and meta-analysis. Food Chem. (2022);4:100106. doi: 10.1016/j.fochms.2022.100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tehranian R, Andell-Jonsson S, Beni SM, Yatsiv I, Shohami E, Bartfai T, Lundkvist J, Iverfeldt K. Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J Neurotrauma. (2002);19:939–951. doi: 10.1089/089771502320317096. [DOI] [PubMed] [Google Scholar]
- 84.Tian T, Zhang BY, Wang KD, Zhang BF, Huang M. Protective effects of taurine on neurons and microglia in Parkinson's disease-like mouse model induced by paraquat. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. (2020);38:801–808. doi: 10.3760/cma.j.cn121094-20200121-00036. [DOI] [PubMed] [Google Scholar]
- 85.Vahdat M, Hosseini SA, Soltani F, Cheraghian B, Namjoonia M. The effects of taurine supplementation on inflammatory markers and clinical outcomes in patients with traumatic brain injury:a double-blind randomized controlled trial. Nutr J. (2021);20:53. doi: 10.1186/s12937-021-00712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang JX, Li Y, Zhang LK, Zhao J, Pang YZ, Tang CS, Zhang J. Taurine inhibits ischemia/reperfusion-induced compartment syndrome in rabbits. Acta Pharmacol Sin. (2005);26:821–827. doi: 10.1111/j.1745-7254.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 87.Wang K, Zhang B, Tian T, Zhang B, Shi G, Zhang C, Li G, Huang M. Taurine protects dopaminergic neurons in paraquat-induced Parkinson's disease mouse model through PI3K/Akt signaling pathways. Amino Acids. (2022);54:1–11. doi: 10.1007/s00726-021-03104-6. [DOI] [PubMed] [Google Scholar]
- 88.Wang Q, Fan W, Cai Y, Wu Q, Mo L, Huang Z, Huang H. Protective effects of taurine in traumatic brain injury via mitochondria and cerebral blood flow. Amino Acids. (2016);48:2169–2177. doi: 10.1007/s00726-016-2244-x. [DOI] [PubMed] [Google Scholar]
- 89.Watanabe M, Ito T, Fukuda A. Effects of taurine depletion on body weight and mouse behavior during development. Metabolites. (2022);12:631. doi: 10.3390/metabo12070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wenting L, Ping L, Haitao J, Meng Q, Xiaofei R. Therapeutic effect of taurine against aluminum-induced impairment on learning, memory and brain neurotransmitters in rats. Neurol Sci. (2014);35:1579–1584. doi: 10.1007/s10072-014-1801-x. [DOI] [PubMed] [Google Scholar]
- 91.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. (2007);99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 92.Wu GF, Ren S, Tang RY, Xu C, Zhou JQ, Lin SM, Feng Y, Yang QH, Hu JM, Yang JC. Antidepressant effect of taurine in chronic unpredictable mild stress-induced depressive rats. Sci Rep. (2017);7:4989. doi: 10.1038/s41598-017-05051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu J, Kohno T, Georgiev SK, Ikoma M, Ishii H, Petrenko AB, Baba H. Taurine activates glycine and γ-aminobutyric acid a receptors in rat substantia gelatinosa neurons. Neuroreport. (2008);19:333–337. doi: 10.1097/WNR.0b013e3282f50c90. [DOI] [PubMed] [Google Scholar]
- 94.Xu M, Che L, Gao K, Wang L, Yang X, Wen X, Li M, Jiang Z. Taurine alleviates oxidative stress in porcine mammary epithelial cells by stimulating the Nrf2-MAPK signaling pathway. Food Sci Nutr. (2023) doi: 10.1002/fsn3.3203. doi:10.1002/fsn3.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ye HB, Shi HB, Yin SK. Mechanisms underlying taurine protection against glutamate-induced neurotoxicity. Can J Neurol Sci. (2013);40:628–634. doi: 10.1017/s0317167100014840. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Z, Yu R, Cao L. Neuroprotection of taurine through inhibition of 12/15 lipoxygenase pathway in cerebral ischemia of rats. Neurol Res. (2017);39:453–458. doi: 10.1080/01616412.2017.1297906. [DOI] [PubMed] [Google Scholar]
- 97.Zhu Y, Wang R, Fan Z, Luo D, Cai G, Li X, Han J, Zhuo L, Zhang L, Zhang H, Li Y, Wu S. Taurine alleviates chronic social defeat stress-induced depression by protecting cortical neurons from dendritic spine loss. Cell Mol Neurobiol. (2022);43:827–840. doi: 10.1007/s10571-022-01218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. (2010);7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


