Abstract
Lactate, a byproduct of glycolysis, was thought to be a metabolic waste until the discovery of the Warburg effect. Lactate not only functions as a metabolic substrate to provide energy but can also function as a signaling molecule to modulate cellular functions under pathophysiological conditions. The Astrocyte-Neuron Lactate Shuttle has clarified that lactate plays a pivotal role in the central nervous system. Moreover, protein lactylation highlights the novel role of lactate in regulating transcription, cellular functions, and disease development. This review summarizes the recent advances in lactate metabolism and its role in neurodegenerative diseases, thus providing optimal perspectives for future research.
Keywords: Alzheimer’s disease, Astrocyte-Neuron Lactate Shuttle, brain, central nervous system, glucose metabolism, glycolysis, neuroinflammation, Parkinson’s disease, protein lactylation, signaling molecule
Introduction
Although the brain accounts for only 2% of the human body weight, it consumes approximately 25% of the energy during rest (Castro et al., 2009). As new unexpected functions of lactate have been uncovered, the role of lactate in the central nervous system (CNS) is receiving increasing attention. Substantial evidence shows that lactate functions as but is not limited to: (1) an energy metabolism source (Stanley et al., 1985; Bergman et al., 1999), (2) substrate for gluconeogenesis (Stanley et al., 1988; Emhoff et al., 2013), (3) signaling molecule (Hashimoto et al., 2007; Brooks, 2009), (4) and donor for protein lactylation (Zhang et al., 2019a; Gaffney et al., 2020). The description of the Astrocyte-Neuron Lactate Shuttle (ANLS) clarified the importance of lactate in maintaining neuronal metabolism and neurotransmitter transmission (Suzuki et al., 2011). Moreover, lactate also participates in metabolic reprogramming (Pan et al., 2022b), neuroinflammation (Boland et al., 2018; Aldana, 2019), and neurodegenerative diseases (Le Douce et al., 2020; Schirinzi et al., 2020), including Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), amyotrophic lateral sclerosis, and Huntington’s disease (HD). Neurodegenerative diseases are characterized by cognitive and behavioral dysfunctions, which are caused by the gradual and progressive neuronal loss and functional decline (Wang et al., 2020). With the growth of aging population, the number of people affected by neurodegenerative diseases is increasing. Deciphering the pathogenesis and developing effective treatments for these neurodegenerative diseases are urgently needed (Xiao et al., 2021). This review summarizes the recent advances in the role and mechanism of lactate metabolism in neurodegenerative diseases and emphasizes the potential therapeutic strategy for these diseases by targeting lactate metabolism.
Search Strategy
Studies cited in this review were published from 1985 to 2022, with a predominant citation from 2011 to 2022. All studies cited here were searched on PubMed database using the following keywords: lactate, lactate metabolism, glucose metabolism, glycolysis, lactate shuttle, monocarboxylate transporter, neurodegenerative diseases, histone lactylation, non-histone lactylation, microglia, astrocyte, neuron, neuroinflammation, Alzheimer’s disease, Parkinson’s disease, tuberculous meningitis, Huntington’s disease, and multiple sclerosis as well as learning and memory.
Overview of Lactate Metabolism
Production and removal of lactate
D-lactate, L-lactate, and racemic DL-lactate are the three isomers of lactate that are frequently created; however, due to the asymmetry of the carbon atoms in humans, L-lactate is the predominant form (Li et al., 2022b). As a terminal metabolite of glycolysis, lactate is generally produced under hypoxic conditions (Brooks, 2009). The glucose transporter (Glut) mediates glucose transfer from the extracellular matrix into the cytoplasm. Normally, glucose is converted to pyruvate by a number of glycolytic enzymes that simultaneously produce two molecules of adenosine triphosphate (ATP) (Brooks, 1986). Under normoxia conditions, pyruvate is transported into the mitochondria for the tricarboxylic acid cycle and coupled oxidative phosphorylation (OXPHOS), which generates about 36 molecules of ATP. Under hypoxia conditions, OXPHOS is blocked and pyruvate is catalyzed by the enzyme lactate dehydrogenase A into lactate. The removal of lactate is reverse-catalyzed by lactate dehydrogenase B to produce pyruvate after the supply of oxygen is sufficient, which then enters the tricarboxylic acid cycle and is metabolized to produce water and carbon dioxide. However, even when there is enough oxygen available, tumor cells or many proliferative cells prefer to use glycolysis and create enormous amounts of lactate, which is known as the “Warburg effect”.
The brain is regarded as a net lactate generator when at rest (van Hall, 2010; Dienel, 2012). The lactate that the brain cells make is transported to the extracellular matrix through the cellular membrane and then enters the systemic circulation by way of the blood vessels or lymphatic system. When blood lactate levels increase, the brain becomes an organ of net lactate uptake and monocarboxylate transporters (MCTs) on the blood-brain barrier transport lactate from blood to the brain. As a result, brain cells take up more lactate, and during exercise, the brain can eliminate up to 11% of the body’s lactate (van Hall, 2010).
Lactate shuttle and MCTs in the central nervous system
Cell-cell lactate shuttle is the ability of lactate to shuttle from a highly glycolytic cell to an adjacent cell (Figure 1). The best-studied cell-cell lactate shuttle in the nervous system is named the ANLS (Suzuki et al., 2011). Glucose is taken up by astrocytes from the surrounding capillaries via glucose transporter 1. Glucose can then be stored as glycogen in astrocytes or undergo glycolysis to generate pyruvate. According to the ANLS, the lactate produced by glycolysis in astrocytes can be transported to the astrocyte-neuron intercellular space by MCT1 and MCT4, followed by neuronal uptake of the lactate via MCT2, which is required for synaptic formation and neurotransmitter transmission (Machler et al., 2016; Descalzi et al., 2019). Lactate is catalyzed to pyruvate and acetyl-CoA in neurons, which are utilized for generating a large amount of energy by OXPHOS along with regulating fatty acid synthesis to meet the needs of synaptic transmission and nerve excitability (Machler et al., 2016; Liu et al., 2017). Those studies have shown that the ANLS is disrupted in several neurological diseases, including AD, amyotrophic lateral sclerosis, and schizophrenia (Veloz Castillo et al., 2021).
Figure 1.
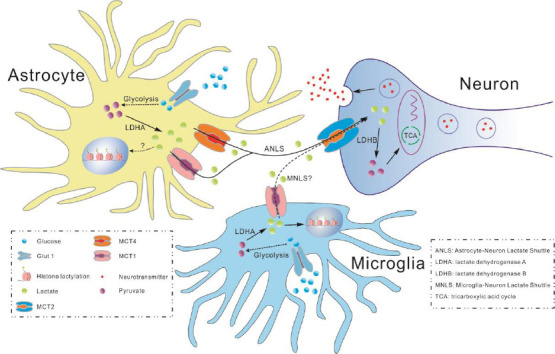
The role of lactate in the central nervous system.
Glucose transports into the cytoplasm is mediated by Glut1 and is converted to pyruvate by glycolytic enzymes in astrocytes. Under hypoxia conditions, pyruvate is catalyzed by LDHA into lactate that can be transported to the astrocyte-neuron intercellular space by MCT1 and MCT4, followed by neuronal uptake of the lactate via MCT2. Lactate is catalyzed to pyruvate by LDHB, which is utilized for generating energy by TCA cycle to meet the needs of synaptic transmission and nerve excitability. Whether histone lactylation is affected by lactate in astrocyte and microglia is waiting to be elucidated. Whether MNLS exists remains to be studied. Created with CorelDRAW 2018. ANLS: Astrocyte-Neuron Lactate Shuttle; LDHA: lactate dehydrogenase A; LDHB: lactate dehydrogenase B; MNLS: Microglia-Neuron Lactate Shuttle; TCA: tricarboxylic acid cycle.
There also exists an intracellular lactate shuttle (Butz et al., 2004; Brooks et al., 2022), which ensures that the lactate can be removed by the mitochondria (Brooks, 2002). In this shuttle, lactate oxidation to pyruvate is facilitated through the mitochondrial lactate oxidation complex, which is composed of mitochondrial MCT1, basigin (CD147), mitochondrial lactate dehydrogenase, and cytochrome oxidase (Hashimoto and Brooks, 2008). Consistently, MCT1, MCT2, and lactate dehydrogenase are reported to be located in the mitochondria and are associated with cytochrome oxidase in the neuronal mitochondria of the brain. Neuronal mitochondria contain mitochondrial lactate oxidation complex that has the potential to facilitate both the intracellular and cell-cell lactate shuttles in the rat brain (Hashimoto et al., 2008).
The shuttling system mediated by MCTs can also mediate the transportation of pyruvate and ketone bodies (Pierre and Pellerin, 2005). Since MCTs are lactate/proton symporters, the concentration gradient and protons decide the direction of the lactate transport. In addition, each type of MCT exhibits a specific cellular distribution in the brain, which can change with ontogenetic progression as well as different pathological states. MCT1 is highly expressed in most brain cell types during early postnatal development. In the adult brain, MCT1 is expressed mostly by endothelial cells, microglia (Kong et al., 2019), astrocytes, oligodendrocytes, ependymocytes, and choroid plexus cells (Murakami et al., 2021). MCT2 is expressed only by neurons and its expression increases with neuronal maturity and in the presence of some neurotransmitters (Pellerin et al., 1998). MCT4 is found exclusively in astrocytes (Bergersen et al., 2002; Rafiki et al., 2003). Interestingly, part of MCT2 immunoreactivity is located at postsynaptic sites, suggesting the particular role of MCT2 in synaptic transmission (Pierre and Pellerin, 2005).
A recent study showed that the PTEN/Akt pathway in endothelial cells can regulate the expression of MCT1 to enhance lactate transport across the brain endothelium, which is critical for lactate homeostasis, adult hippocampal neurogenesis, and cognitive function (Wang et al., 2019). Lactate, not pyruvate, can rescue memory impairment caused by the knockdown of MCT2 by promoting learning-induced mRNA translation and expression of Arc/Arg3.1 in both excitatory and inhibitory neurons, indicating that lactate is crucial because it fuels the neuronal reactions necessary for long-term memory (Descalzi et al., 2019). Furthermore, when blood lactate levels are over 4 mM because of exhaustive exercise or intravenous infusion of lactate, cognitive functions such as attention, working memory, and stress decline without sex differences (Coco et al., 2020). Insufficient astrocyte lactate supply may be an important cause of hypoxia or stroke-induced neurodegeneration (Yamagata, 2022). MCTs and lactate are necessary for long-term memory and cognitive function. Lactate metabolism disorder would result in the development of neurodegeneration (Descalzi et al., 2019; Wang et al., 2019; Coco et al., 2020; Yamagata, 2022).
Multifaceted Functions of Lactate
As a metabolic substrate, lactate can not only be catalyzed to pyruvate and serve as a crucial metabolic substrate but can also act as a gluconeogenic precursor to synthesize glucose to generate energy (Brooks, 2009). In addition to its metabolic functions, lactate can modulate transcription by epigenetic modification, which is called lactylation in macrophage and microglia (Zhang et al., 2019a; Gaffney et al., 2020; Pan et al., 2022b). Therefore, lactate plays a central role in both physiological and pathological contexts. In the CNS, cells can utilize lactate as a key energy source to meet the metabolic demand (Figure 1), in addition, it also acts as a signaling molecule to modulate cellular function. Thus, it may be an important regulator of cellular metabolic flexibility, which exerts modulatory effects on key cellular functions.
Signaling molecule
Lactate has a dual role in neurons and glia at different conditions. On the one hand, lactate is detrimental to CNS. For example, an increase in lactate intake by neurons was found to boost the generation of reactive oxygen species, enhanced mitochondrial energy metabolism and caused neurons to be in an oxidative state. Oxidative stress affected ATP synthesis, which increased reactive oxygen species production in mitochondria. This vicious cycle eventually caused axonal degeneration in the peripheral nervous system. (Jia et al., 2021). On the other hand, lactate is required for maintaining brain homeostasis. The G protein-coupled receptor 81 (GPR81) is highly expressed in the CNS and has a neuroprotective function. Lactate activates GPR81-mediated signaling and reduces excitatory damage, demonstrating that lactate may be involved in regulating whole-brain metabolism (Morland et al., 2015; Brown and Ganapathy, 2020; Laroche et al., 2021).
Mitochondrial antiviral-signaling could function as a lactate sensor, whose inactivation by direct lactate binding serves as a natural barrier to limit retinoic-acidinducible gene I-like receptors signaling activation for enabling type I interferon production (Zhang et al., 2019b). The C2 domain in cytosolic phospholipase A2 can interact with mitochondrial antiviral-signaling mitochondrial antiviral-signaling and disrupt mitochondrial antiviral-signaling-hexokinase 2 interactions in astrocytes, leading to decreased production of lactate to support neurons (Chao et al., 2019). N-methyl-D-aspartate receptors, which are glutamate receptors, are typical synaptic plasticity mediators. Lactate also stimulates N-methyl-D-aspartate receptors and downstream ERK1/2 signaling pathways, enhances the expression of Arc, c-Fos, and Zif268 implicated in neuronal plasticity and activity maintenance, and increases inward current flow and calcium inward flow brought on by glutamate and glycine (Yang et al., 2014; Veloz Castillo et al., 2021).
In addition, hippocampal injection of lactate prevents the inhibition of memory retention by 1,4-dideoxy-1,4-imino-D-arabinitol (Alberini et al., 2018). With memory impairment in a mouse model of AD, a study found that lactate level in the hippocampus and cerebral cortex declined (Lu et al., 2019). Interestingly, the lactate shuttle allows exercise-produced lactate to enter the hippocampus, where it activates sirtuin 1 to boost the expression of brain-derived neurotrophic factor, which is conducive to cognition, learning, and memory formation (El Hayek et al., 2019). Local application of L-lactate to the injured spinal cord was found to promote corticospinal tract axon regeneration, leading to behavioral recovery in adult mice (Li et al., 2020). Therefore, lactate could be a signaling molecule in the regulation of intrinsic signaling transduction and confers bidirectional function in CNS pathophysiology.
Protein lactylation
Recent studies have shown that lactate can mediate protein modification by lysine lactylation (alternatively named lysine lactoylation, Kla) (Zhang et al., 2019a). They described two varieties of Kla known as L-lactyllys (K [L-la]) and D-lactoyllys (K [D-la]). Moreover, L-lactly-CoA, an activated type of L-lactate, serves as histone K(L-la)’s substrate when an enzyme is in an active state (Zhang et al., 2019a), whereas under non-enzymatic conditions, lactyl-glutathione was transferred to protein lysine residues, generating a K (D-la) modification generally on non-histone proteins (Gaffney et al., 2020). At present, 26 histone lactylation modification sites have been identified in human cells and 18 histone lactylation modification sites have been identified in mouse cells (Zhang et al., 2019a).
Histone lysine lactylation
It has been reported that histone lactylation is closely related to the exogenous and endogenous lactate concentration (Zhang et al., 2019a). The majority of histone actylations involve so-called effectors, such as proteins termed writer and eraser that can add and subtract particular chromatin modifications, respectively (Liu et al., 2022). Coincidentally, studies have shown that p300, a classic histone acetyltransferase, together with its homolog CREB-binding protein, are potential histone Kla writer proteins (Zhang et al., 2019a; Yang et al., 2022). Besides, sirtuin 2, histone deacetylase 1, and histone deacetylase 3 are potential histone easer proteins that have the functions of delactylase (Dai et al., 2022; Moreno-Yruela et al., 2022; Zu et al., 2022).
Glycolysis is a key topic in the majority of recent investigations on histone lactylation. Histone lactylation is found throughout the brain and experiences broad modifications as the neural development (Dai et al., 2022). As a novel epigenetic modification regulated by cellular metabolism, histone lactylation modification explains the molecular mechanism of metabolic reprogramming and transcriptional reprogramming under pathological conditions (Zhang et al., 2019a; Pan et al., 2022b). However, there is a dearth of information on how histone lactylation affects neurological diseases. Our team has discovered that in an AD mouse model (5XFAD), H4K12la worsens microglia activation and functioning by affecting the glycolysis (Pan et al., 2022b). H3K18la is heavily implicated in transcriptome remodeling to support cell-fate transitions in the embryonic telencephalon and is closely associated with chromatin state and gene expression (Dai et al., 2022). Via histone H3K18la, macrophage aerobic glycolysis induces Arg1 expression and causes macrophages to enter a damage repair state (Zhang et al., 2019a). Aerobic glycolysis of tumor cells promotes tumor cell proliferation by upregulating YTHDF2 expression through H3K18la (Yu et al., 2021). Given the multiple pathophysiological effects of histone lactylation, it will be interesting to investigate the role and mechanism of histone lactylation in regulating neuronal and astrocytic functions during brain development and neurological pathogenesis.
Lactylation of non-histone proteins
Non-histone proteins were also found to be lactylated at lysine residues (Gaffney et al., 2020; Gao et al., 2020; Hagihara et al., 2021). Recently, a study demonstrated that neural excitement and behavior-related stimuli lead to the lactylation of 63 proteins in the mouse brain, accompanied by a change in the lactate level. These findings provide evidence for protein lactylation in the brain and its regulation by neural-activity-induced lactate production (Hagihara et al., 2021). In addition, methyltransferase-like 3 was also founded lactylation at g K281 and K345 that was essential for it to capture target RNA in the tumor-infiltrating myeloid cells (Xiong et al., 2022).
350 lactylated proteins were discovered by liquid chromatography-tandem mass spectrometry in HEK293T cells, the majority of which were glycolysis-related enzymes that affected the glycolytic production through negative feedback (Gaffney et al., 2020). Besides, 273 lysine lactylation was also found in 166 proteins, which were distributed in the nucleus (36%), mitochondria (27%), and cytoplasm (25%) in Botrytis cinerea (Gao et al., 2020). However, accurate Kla detection and restricted lactylation detection of non-histone proteins could not be performed by liquid chromatography-tandem mass spectrometry (Wu and Tao, 2022). Recently, Wan et al. (2022) identified the cyclic immonium ion that can be used to confidently identify novel Kla and modification sites, which revealed widespread lactylation beyond histones in the human proteome. They found that Kla proteins also existed in the cytoplasm and are mostly involved in glycolysis. For example, fructose bisphosphate aldolase A was lactylated on K147, which reduced its enzymatic activity. Interestingly, the modification site of dehydrogenase reductase 7, K321, was also found to be involved in methylation and ubiquitylation in previous studies, suggesting its potential importance (Wan et al., 2022; Wu and Tao, 2022).
Considering that protein lactylation might be a universal post-translational modification and plays an important role in the regulation of protein function, future studies to define specific protein(s) with lactylation and to investigate its role in different cell types will expand our understanding of the pathogenesis of neurodegenerative diseases that associated with lactate metabolism disorder.
Role of Lactate in Regulating Neuroinflammation
Neuroinflammation, an inflammatory response in the CNS, is mediated by the production of cytokines and chemokines, which are produced by resident CNS glia (microglia and astrocytes), endothelial cells, and peripherally derived immune cells (DiSabato et al., 2016). Generally, acute neuroinflammation is a defense against infection, injury, and toxins in the CNS. However, when the balance between pro-inflammatory and anti-inflammatory signals is disrupted, acute neuroinflammation will convert into chronic neuroinflammation (Ferreira et al., 2014; Kinney et al., 2018). Cytotoxic factors continuously activate the CNS glia, resulting in the release of pro-inflammatory signals (Grammas, 2011). Chronic neuroinflammation is an important pathological feature in neurological diseases, including AD (Holmes, 2013), PD (Tansey et al., 2022), amyotrophic lateral sclerosis (Appel et al., 2021), and MS (Ruiz et al., 2019).
There were multiple reports that neuroinflammation is regulated by microglial lactate metabolism (Woodburn et al., 2021). Microglia are predominantly fueled by OXPHOS in physiological conditions (Barros et al., 2018). However, in neurodegenerative diseases, pro-inflammatory microglia metabolically reprogram to aerobic glycolysis, with the mass production of lactate (Boland et al., 2018; Aldana, 2019). In turn, the lactate produced by microglia promotes the release of pro-inflammatory cytokines (Figure 2), such as tumor necrosis factor-alpha, interleukin (IL)-6, and interleukin-1β (IL-1β) (Andersson et al., 2005). It should be noted that under LPS-induced inflammation conditions, exogenous lactate may inhibit the secretion of tumor necrosis factor-alpha and IL-1β, along with the activation of phosphorylated nuclear factor kappa-B and NLRP3 inflammasome complex (NLRP3/ASC/caspase-1) (Liang et al., 2022).
Figure 2.
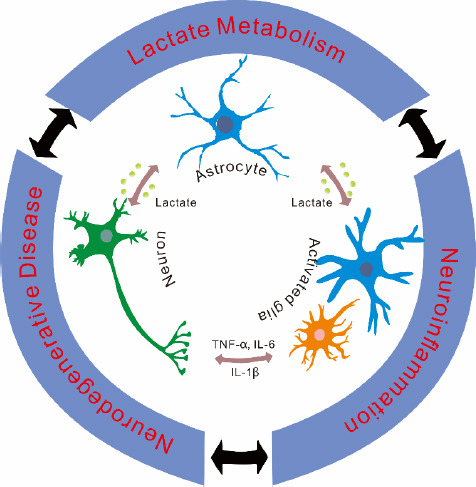
Relationship between lactate metabolism, neuroinflammation, and neurodegenerative diseases.
On one hand, the lactate produced by astrocyte provides energy to neuron by ANLS. On the other hand, the lactate would lead to activating glia that cause neuroinflammation. Moreover, activated glia secrete pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, which leads to neurodegenerative disease. ANLS: Astrocyte-Neuron Lactate Shuttle; IL-1β: interleukin-1β; IL-6: interleukin-6; TNF-α: tumor necrosis factor-alpha.
Lactate Metabolism in Neurodegenerative Diseases
Alzheimer’s disease
AD, a chronic neuroinflammatory disease, is characterized by the deposition of amyloid-β (Aβ) plaques and tau neurofibrillary tangles (Hardy and Selkoe, 2002; Huang and Mucke, 2012), along with progressive cognitive dysfunction and an alteration of aerobic glycolysis (Cunnane et al., 2020). Implementation of 2-Deoxy-2-[18F] fluoro-D-glucose positron emission tomography, a technique for the analysis of cerebral glucose metabolism, has revealed that glucose uptake is decreased in the brain of AD patients. Interestingly, it was reported that the lactate level is increased in the cerebrospinal fluid (CSF) of AD and mild cognitive impairment patients, suggesting that a glucose metabolism disorder is involved in the development and progression of AD (Liguori et al., 2016; Xiang et al., 2021). However, as mentioned above, microglia are in a state of glucose hypometabolism in physiological conditions, which may transform into glucose hypermetabolism in AD (Boland et al., 2018; Aldana, 2019; Xiang et al., 2021). These results suggest that microglial glucose metabolism disturbance may affect the lactate level, which further results in neuroinflammation in the cerebral environment in AD.
On the contrary, astrocytes and neurons were found to participate in significant glucose hypometabolism when exposed to Aβ oligomers (Tarczyluk et al., 2015). Besides, Glut1 is predominantly expressed on astrocytes and endothelial cells, while glucose transporter 3 is specifically expressed on neurons. Both Glut1 and glucose transporter 3 were found to be downregulated in a post-mortem analysis of AD brains (Simpson et al., 2007). Moreover, in a 3×Tg-AD model, resting lactate production via aerobic glycolysis was decreased in astrocytes (Le Douce et al., 2020). Due to faulty lactate shuttling from glial cells to neurons, dysmetabolism in the brain can cause degeneration similar to AD (Sun et al., 2020). In the APP/PS1 AD model mice, reduced expression of MCT1, MCT2, and MCT4 resulted in neuronal energy deficits due to disturbed lactate transport from glial cells to neurons (Zhang et al., 2018). Therefore, the low amount of lactate produced by astrocytes could affect the energy supply of neurons.
In the early onset stage in AD model mice, we found that sodium rutin, a small molecular compound, could improve microglial mitochondrial OXPHOS, promote tricarboxylic acid cycle, and inhibit glycolysis, thus significantly attenuating neuroinflammation and pathological symptoms and improving cognition in AD model mice (Pan et al., 2019). As mentioned before, our group discovered that histone lactylation is increased in the microglia. We identified a positive loop glycolysis/H4K12la/PKM2 pathway that exacerbates microglial activation and dysfunction in the context of AD. We showed that interruption of this loop by blocking PKM2 decreases the H4K12la level, which transcriptionally suppresses a set of glycolytic genes and thereby reduces lactate levels. This may be a potential therapeutic strategy for treating AD (Pan et al., 2022b). Consequently, focusing on compromised lactate metabolism may be a new AD intervention approach (Zhao and Xu, 2022). During the development of AD, the main cell types involved in the lactate metabolism disorder as well as the point, function, and regulatory mechanism of gene expression for lactate regulation are not clear. Therefore, more in-depth studies are required to investigate whether lactate metabolism may be an early intervention target for AD.
Parkinson’s disease
The dense substantia nigra exhibits a severe early deficiency of dopaminergic neurons, which is a hallmark of PD (Kalia and Lang, 2015). Major motor symptoms of PD include resting tremors, bradykinesia, stiffness, and postural instability, while the nonmotor symptoms include cognitive decline (Rocha et al., 2018; Dorsey et al., 2019; Xiao et al., 2021). It has been reported that lactate levels were abnormally elevated in the CSF of late-onset PD patients (Schirinzi et al., 2020). Upregulated hexokinase 2 and lactate dehydrogenase A with increased lactate levels promote the apoptosis of dopaminergic neurons, while inhibition of hexokinase 2 expression attenuates the apoptosis of abnormal neurons by downregulating lactate production and the AMPK/Akt/mTOR pathway in PD (Li et al., 2022a). Moreover, impaired glucose metabolism and reduced OXPHOS and ATP levels are also potential pathogenic factors in PD (Saxena, 2012; Cunnane et al., 2020). Terazosin enhances the enzymic activity of phosphoglycerate kinase 1, promoting glycolysis and increasing ATP levels (Chen et al., 2015). Both in vivo and vitro results suggested that terazosin has the potential of improving PD symptoms by enhancing glycolysis (Cai et al., 2019). These findings imply that targeting glucose metabolism may be an important PD therapeutic strategy.
Other neurodegenerative diseases
In addition to AD and PD, lactate metabolism dysfunction also participates in other neurodegenerative diseases, such as MS and HD. MS, a common chronic inflammatory disease in CNS, is now incurable (Reich et al., 2018). CSF lactate was higher in MS patients compared to controls (Albanese et al., 2016). Therefore, measuring CSF or serum lactate (Ghareghani et al., 2016) could be a low-cost and reliable laboratory test to gauge an MS patient’s response to treatment and the severity of their condition.
HD is an autosomal-dominant, progressive neurodegenerative disorder with a unique phenotype that includes chorea and dystonia, incoordination, cognitive impairment, and behavioral issues (Walker, 2007; Wyant et al., 2017). The research demonstrated that ascorbic acid’s inhibition of glucose transport results in the promotion of lactate absorption, but overexpressing glucose transporter 3 in HD cells totally restored lactate (Solís-Maldonado et al., 2018). These findings imply that lactate can be employed as a diagnostic marker and therapeutic target for MS and HD.
Treatment Strategies for Neurodegenerative Diseases by Targeting Lactate Metabolism
Most treatments for AD are pharmacological interventions, such as cholinesterase inhibitors (galantamine (Loy and Schneider, 2004)) and N-methyl-D-aspartate receptor blockers (memantine (Reisberg et al., 2003)). These drugs only partially relieve the symptoms in AD patients, and cannot delay the progression of AD (Tan et al., 2014). Moreover, one of the primary focuses for the creation of AD medications is neuroinflammation (Heneka et al., 2015). However, the activation of microglia can produce a variety of pro-inflammatory cytokines, such as IL-6, IL-1β, and so on; therefore, the prospect of targeting a single inflammatory factor in the treatment of AD is uncertain. In addition to drug intervention, studies have found that aerobic exercise can significantly improve the cognitive function of AD patients. Molecular mechanism studies have revealed that aerobic exercise can promote cerebral blood circulation and improve cerebral glucose metabolism (Vidoni et al., 2019; De la Rosa et al., 2020; Zhao and Xu, 2021). New research from our group demonstrates that intermittent fasting alters gut microbial composition with a large enrichment of probiotics like Lactobacillus, which improves cognitive skills and AD-related pathologies in a 5×FAD mouse model (Pan et al., 2022a).
Moreover, due to the lactate metabolic disorder in AD, targeting lactate metabolism may be a novel way to treat AD. We found that rutin sodium, a small molecular compound, significantly inhibited LPS-induced glycolysis in the microglia and promoted mitochondrial OXPHOS and ATP production, thus accelerating Aβ clearance. In vivo, it can significantly delay disease progression, reduce brain inflammation and Aβ plaque deposition, and improve the learning and memory function of AD model mice (Pan et al., 2019). Besides, by altering the production of lactate, the glycolysis/H4K12 lactylation/PKM2 positive feedback loop also relieved the symptoms and improved the cognition functions in an AD mouse model (Pan et al., 2022b).
Conclusion and Future Perspective
Lactate as a final metabolite of glycolysis is produced under anaerobic conditions. Recently, the role of lactate as a unique signaling molecule and lactylation as a novel post-translational modification of proteins have been studied in the physiological and pathological environment. Therefore, it is important to clarify how lactate affects the energy metabolism and the occurrence and progression of diseases in the CNS. Nowadays, lactate is used clinically to diagnose, predict, and assess the effectiveness of traumatic brain injury (Li et al., 2022b). Whether lactate can also be used for the diagnosis of neurological diseases needs to be elucidated.
In oxidative cancer cells, lactate exerts a beneficial effect by promoting v-ATPase-dependent lysosomal acidification and autophagy by lactate dehydrogenase B activity. Similarly, as the major phagocytes in the brain, microglia cells rely on lysosomes to maintain their phagocytic function (Brisson et al., 2016). In the early stages of AD, whether lactate produced by the microglia can affect lysosomes and subsequently affect the phagocytosis of Aβ plaques need to be further investigated.
Lactate accumulation and protein lactylation can regulate downstream gene expression, thereby determining cell-mediated inflammation, neurotrophy, synaptic pruning, neuronal injury, and other functional disorders. However, there are few studies on the disorders of lactate metabolism and lactylation of proteins in the CNS. Furthermore, it is unclear whether protein lactylation is an inevitable result of a high concentration of lactate accumulation or a fine regulation mode controlled by time and space. Is lactyl-CoA or lactyl-glutathione the direct substrate for protein lactylation? What enzyme catalyzes the formation of lactate to lactly-CoA and what are the “write”, “read”, and “erase” proteins that enable the modification of protein lactylation? Besides histones, what other proteins can be regulated by lactation modification? Future studies are urgently needed to address these questions.
Additional file: Open peer review reports 1 (90.7KB, pdf) and 2 (88.7KB, pdf) .
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, Nos. 82230042 and 81930029 (to ZY), U2004201 (to FG and RYP) and the China Postdoctoral Science Foundation, No. 2020M683748 (to RYP).
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: The data are available from the corresponding author on reasonable request.
Open peer reviewers: Kumar Aavula, Harvard Medical School, USA; Abhilash P. Appu, NIH:National Institutes of Health, USA.
P-Reviewers: Aavula K, Appu AP; C-Editors: Zhao M, Li JY, Qiu Y; T-Editor: Jia Y
References
- 1.Albanese M, Zagaglia S, Landi D, Boffa L, Nicoletti CG, Marciani MG, Mandolesi G, Marfia GA, Buttari F, Mori F, Centonze D. Cerebrospinal fluid lactate is associated with multiple sclerosis disease progression. J Neuroinflammation. (2016);13:36. doi: 10.1186/s12974-016-0502-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberini CM, Cruz E, Descalzi G, Bessieres B, Gao V. Astrocyte glycogen and lactate:new insights into learning and memory mechanisms. Glia. (2018);66:1244–1262. doi: 10.1002/glia.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldana BI. Microglia-specific metabolic changes in neurodegeneration. J Mol Biol. (2019);431:1830–1842. doi: 10.1016/j.jmb.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Andersson AK, Rönnbäck L, Hansson E. Lactate induces tumour necrosis factor-alpha, interleukin-6 and interleukin-1beta release in microglial- and astroglial-enriched primary cultures. J Neurochem. (2005);93:1327–1333. doi: 10.1111/j.1471-4159.2005.03132.x. [DOI] [PubMed] [Google Scholar]
- 5.Appel SH, Beers DR, Zhao W. Amyotrophic lateral sclerosis is a systemic disease:peripheral contributions to inflammation-mediated neurodegeneration. Curr Opin Neurol. (2021);34:765–772. doi: 10.1097/WCO.0000000000000983. [DOI] [PubMed] [Google Scholar]
- 6.Barros LF, Brown A, Swanson RA. Glia in brain energy metabolism:a perspective. Glia. (2018);66:1134–1137. doi: 10.1002/glia.23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergersen L, Rafiki A, Ottersen OP. Immunogold cytochemistry identifies specialized membrane domains for monocarboxylate transport in the central nervous system. Neurochem Res. (2002);27:89–96. doi: 10.1023/a:1014806723147. [DOI] [PubMed] [Google Scholar]
- 8.Bergman BC, Wolfel EE, Butterfield GE, Lopaschuk GD, Casazza GA, Horning MA, Brooks GA. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol (1985) (1999);87:1684–1696. doi: 10.1152/jappl.1999.87.5.1684. [DOI] [PubMed] [Google Scholar]
- 9.Boland B, Yu WH, Corti O, Mollereau B, Henriques A, Bezard E, Pastores GM, Rubinsztein DC, Nixon RA, Duchen MR, Mallucci GR, Kroemer G, Levine B, Eskelinen EL, Mochel F, Spedding M, Louis C, Martin OR, Millan MJ. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat Rev Drug Discov. (2018);17:660–688. doi: 10.1038/nrd.2018.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brisson L, Bański P, Sboarina M, Dethier C, Danhier P, Fontenille MJ, Van Hée VF, Vazeille T, Tardy M, Falces J, Bouzin C, Porporato PE, Frédérick R, Michiels C, Copetti T, Sonveaux P. Lactate dehydrogenase B controls lysosome activity and autophagy in cancer. Cancer Cell. (2016);30:418–431. doi: 10.1016/j.ccell.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Brooks GA. Lactate production under fully aerobic conditions:the lactate shuttle during rest and exercise. Fed Proc. (1986);45:2924–2929. [PubMed] [Google Scholar]
- 12.Brooks GA. Lactate shuttles in nature. Biochem Soc Trans. (2002);30:258–264. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 13.Brooks GA. Cell-cell and intracellular lactate shuttles. J Physiol. (2009);587:5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks GA, Curl CC, Leija RG, Osmond AD, Duong JJ, Arevalo JA. Tracing the lactate shuttle to the mitochondrial reticulum. Exp Mol Med. (2022);54:1332–1347. doi: 10.1038/s12276-022-00802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown TP, Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer:role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther. (2020);206:107451. doi: 10.1016/j.pharmthera.2019.107451. [DOI] [PubMed] [Google Scholar]
- 16.Butz CE, McClelland GB, Brooks GA. MCT1 confirmed in rat striated muscle mitochondria. J Appl Physiol (1985) (2004);97:1059–1066. doi: 10.1152/japplphysiol.00009.2004. [DOI] [PubMed] [Google Scholar]
- 17.Cai R, Zhang Y, Simmering JE, Schultz JL, Li Y, Fernandez-Carasa I, Consiglio A, Raya A, Polgreen PM, Narayanan NS, Yuan Y, Chen Z, Su W, Han Y, Zhao C, Gao L, Ji X, Welsh MJ, Liu L. Enhancing glycolysis attenuates Parkinson's disease progression in models and clinical databases. J Clin Invest. (2019);129:4539–4549. doi: 10.1172/JCI129987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro MA, Beltrán FA, Brauchi S, Concha II. A metabolic switch in brain:glucose and lactate metabolism modulation by ascorbic acid. J Neurochem. (2009);110:423–440. doi: 10.1111/j.1471-4159.2009.06151.x. [DOI] [PubMed] [Google Scholar]
- 19.Chao CC, Gutiérrez-Vázquez C, Rothhammer V, Mayo L, Wheeler MA, Tjon EC, Zandee SEJ, Blain M, de Lima KA, Takenaka MC, Avila-Pacheco J, Hewson P, Liu L, Sanmarco LM, Borucki DM, Lipof GZ, Trauger SA, Clish CB, Antel JP, Prat A, et al. Metabolic control of astrocyte pathogenic activity via cPLA2-MAVS. Cell. (2019);179:1483–1498.e1422. doi: 10.1016/j.cell.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Zhao C, Li X, Wang T, Li Y, Cao C, Ding Y, Dong M, Finci L, Wang JH, Li X, Liu L. Terazosin activates Pgk1 and Hsp90 to promote stress resistance. Nat Chem Biol. (2015);11:19–25. doi: 10.1038/nchembio.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coco M, Buscemi A, Ramaci T, Tusak M, Corrado DD, Perciavalle V, Maugeri G, Perciavalle V, Musumeci G. Influences of blood lactate levels on cognitive domains and physical health during a sports stress. Brief review Int J Environ Res Public Health. (2020);17:9043. doi: 10.3390/ijerph17239043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunnane SC, Trushina E, Morland C, Prigione A, Casadesus G, Andrews ZB, Beal MF, Bergersen LH, Brinton RD, de la Monte S, Eckert A, Harvey J, Jeggo R, Jhamandas JH, Kann O, la Cour CM, Martin WF, Mithieux G, Moreira PI, Murphy MP, et al. Brain energy rescue:an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov. (2020);19:609–633. doi: 10.1038/s41573-020-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai SK, Liu PP, Li X, Jiao LF, Teng ZQ, Liu CM. Dynamic profiling and functional interpretation of histone lysine crotonylation and lactylation during neural development. Development. (2022);149:dev200049. doi: 10.1242/dev.200049. [DOI] [PubMed] [Google Scholar]
- 24.De la Rosa A, Olaso-Gonzalez G, Arc-Chagnaud C, Millan F, Salvador-Pascual A, García-Lucerga C, Blasco-Lafarga C, Garcia-Dominguez E, Carretero A, Correas AG, Viña J, Gomez-Cabrera MC. Physical exercise in the prevention and treatment of Alzheimer's disease. J Sport Health Sci. (2020);9:394–404. doi: 10.1016/j.jshs.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Descalzi G, Gao V, Steinman MQ, Suzuki A, Alberini CM. Lactate from astrocytes fuels learning-induced mRNA translation in excitatory and inhibitory neurons. Commun Biol. (2019);2:247. doi: 10.1038/s42003-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dienel GA. Brain lactate metabolism:the discoveries and the controversies. J Cereb Blood Flow Metab. (2012);32:1107–1138. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiSabato DJ, Quan N, Godbout JP. Neuroinflammation:the devil is in the details. J Neurochem. (2016);139(Suppl 2):136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorsey ER, Elbaz A, Nichols E. Global, regional, and national burden of neurological disorders 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019);18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Hayek L, Khalifeh M, Zibara V, Abi Assaad R, Emmanuel N, Karnib N, El-Ghandour R, Nasrallah P, Bilen M, Ibrahim P, Younes J, Abou Haidar E, Barmo N, Jabre V, Stephan JS, Sleiman SF. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF) J Neurosci. (2019);39:2369–2382. doi: 10.1523/JNEUROSCI.1661-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emhoff CA, Messonnier LA, Horning MA, Fattor JA, Carlson TJ, Brooks GA. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J Appl Physiol (1985) (2013);114:297–306. doi: 10.1152/japplphysiol.01202.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira ST, Clarke JR, Bomfim TR, De Felice FG. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer's disease. Alzheimers Dement. (2014);10:S76–83. doi: 10.1016/j.jalz.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Gaffney DO, Jennings EQ, Anderson CC, Marentette JO, Shi T, Schou Oxvig AM, Streeter MD, Johannsen M, Spiegel DA, Chapman E, Roede JR, Galligan JJ. Non-enzymatic lysine lactoylation of glycolytic enzymes. Cell Chem Biol. (2020);27:206–213. doi: 10.1016/j.chembiol.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao M, Zhang N, Liang W. Systematic analysis of lysine lactylation in the plant fungal pathogen botrytis cinerea. Front Microbiol. (2020);11:594743. doi: 10.3389/fmicb.2020.594743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghareghani M, Ghanbari A, Dokoohaki S, Farhadi N, Hosseini SM, Mohammadi R, Sadeghi H. Methylprednisolone improves lactate metabolism through reduction of elevated serum lactate in rat model of multiple sclerosis. Biomed Pharmacother. (2016);84:1504–1509. doi: 10.1016/j.biopha.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 35.Grammas P. Neurovascular dysfunction, inflammation and endothelial activation:implications for the pathogenesis of Alzheimer's disease. J Neuroinflammation. (2011);8:26. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagihara H, Shoji H, Otabi H, Toyoda A, Katoh K, Namihira M, Miyakawa T. Protein lactylation induced by neural excitation. Cell Rep. (2021);37:109820. doi: 10.1016/j.celrep.2021.109820. [DOI] [PubMed] [Google Scholar]
- 37.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease:progress and problems on the road to therapeutics. Science. (2002);297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto T, Brooks GA. Mitochondrial lactate oxidation complex and an adaptive role for lactate production. Med Sci Sports Exerc. (2008);40:486–494. doi: 10.1249/MSS.0b013e31815fcb04. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells:activation of MCT1 and mitochondrial biogenesis. FASEB J. (2007);21:2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- 40.Hashimoto T, Hussien R, Cho HS, Kaufer D, Brooks GA. Evidence for the mitochondrial lactate oxidation complex in rat neurons:demonstration of an essential component of brain lactate shuttles. PLoS One. (2008);3:e2915. doi: 10.1371/journal.pone.0002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. (2015);14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmes C. Review:systemic inflammation and Alzheimer's disease. Neuropathol Appl Neurobiol. (2013);39:51–68. doi: 10.1111/j.1365-2990.2012.01307.x. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. (2012);148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia L, Liao M, Mou A, Zheng Q, Yang W, Yu Z, Cui Y, Xia X, Qin Y, Chen M, Xiao B. Rheb-regulated mitochondrial pyruvate metabolism of Schwann cells linked to axon stability. Dev Cell. (2021);56:2980–2994. doi: 10.1016/j.devcel.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Kalia LV, Lang AE. Parkinson's disease. Lancet. (2015);386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 46.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement (N Y) (2018);4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kong L, Wang Z, Liang X, Wang Y, Gao L, Ma C. Monocarboxylate transporter 1 promotes classical microglial activation and pro-inflammatory effect via 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3. J Neuroinflammation. (2019);16:240. doi: 10.1186/s12974-019-1648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laroche S, Stil A, Germain P, Cherif H, Chemtob S, Bouchard JF. Participation of L-lactate and its receptor HCAR1/GPR81 in neurovisual development. Cells. (2021);10:1640. doi: 10.3390/cells10071640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Douce J, Maugard M, Veran J, Matos M, Jégo P, Vigneron PA, Faivre E, Toussay X, Vandenberghe M, Balbastre Y, Piquet J, Guiot E, Tran NT, Taverna M, Marinesco S, Koyanagi A, Furuya S, Gaudin-Guérif M, Goutal S, Ghettas A, et al. Impairment of glycolysis-derived l-serine production in astrocytes contributes to cognitive deficits in Alzheimer's disease. Cell Metab. (2020);31:503–517. doi: 10.1016/j.cmet.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Li F, Sami A, Noristani HN, Slattery K, Qiu J, Groves T, Wang S, Veerasammy K, Chen YX, Morales J, Haynes P, Sehgal A, He Y, Li S, Song Y. Glial metabolic rewiring promotes axon regeneration and functional recovery in the central nervous system. Cell Metab. (2020);32:767–785. doi: 10.1016/j.cmet.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Chen L, Qin Q, Wang D, Zhao J, Gao H, Yuan X, Zhang J, Zou Y, Mao Z, Xiong Y, Min Z, Yan M, Wang CY, Xue Z. Upregulated hexokinase 2 expression induces the apoptosis of dopaminergic neurons by promoting lactate production in Parkinson's disease. Neurobiol Dis. (2022a);163:105605. doi: 10.1016/j.nbd.2021.105605. [DOI] [PubMed] [Google Scholar]
- 52.Li X, Yang Y, Zhang B, Lin X, Fu X, An Y, Zou Y, Wang JX, Wang Z, Yu T. Lactate metabolism in human health and disease. Signal Transduct Target Ther. (2022b);7:305. doi: 10.1038/s41392-022-01151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang L, Liu P, Deng Y, Li J, Zhao S. L-lactate inhibits lipopolysaccharide-induced inflammation of microglia in the hippocampus. Int J Neurosci. (2022) doi: 10.1080/00207454.2022.2084089. doi:10.1080/00207454.2022.2084089. [DOI] [PubMed] [Google Scholar]
- 54.Liguori C, Chiaravalloti A, Sancesario G, Stefani A, Sancesario GM, Mercuri NB, Schillaci O, Pierantozzi M. Cerebrospinal fluid lactate levels and brain [18F]FDG PET hypometabolism within the default mode network in Alzheimer's disease. Eur J Nucl Med Mol Imaging. (2016);43:2040–2049. doi: 10.1007/s00259-016-3417-2. [DOI] [PubMed] [Google Scholar]
- 55.Liu L, MacKenzie KR, Putluri N, Maletić-Savatić M, Bellen HJ. The glia-neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metab. (2017);26:719–737. doi: 10.1016/j.cmet.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu X, Zhang Y, Li W, Zhou X. Lactylation, an emerging hallmark of metabolic reprogramming:current progress and open challenges. Front Cell Dev Biol. (2022);10:972020. doi: 10.3389/fcell.2022.972020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loy C, Schneider L. Galantamine for Alzheimer's disease. Cochrane Database Syst Rev. (2004):CD001747. doi: 10.1002/14651858.CD001747.pub2. doi:10.1002/14651858.CD001747.pub2. [DOI] [PubMed] [Google Scholar]
- 58.Lu WT, Sun SQ, Li Y, Xu SY, Gan SW, Xu J, Qiu GP, Zhuo F, Huang SQ, Jiang XL, Huang J. Curcumin ameliorates memory deficits by enhancing lactate content and MCT2 expression in APP/PS1 transgenic mouse model of Alzheimer's disease. Anat Rec (Hoboken) (2019);302:332–338. doi: 10.1002/ar.23969. [DOI] [PubMed] [Google Scholar]
- 59.Machler P, Wyss MT, Elsayed M, Stobart J, Gutierrez R, von Faber-Castell A, Kaelin V, Zuend M, San Martin A, Romero-Gomez I, Baeza-Lehnert F, Lengacher S, Schneider BL, Aebischer P, Magistretti PJ, Barros LF, Weber B. In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab. (2016);23:94–102. doi: 10.1016/j.cmet.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 60.Moreno-Yruela C, Zhang D, Wei W, Bæk M, Liu W, Gao J, Danková D, Nielsen AL, Bolding JE, Yang L, Jameson ST, Wong J, Olsen CA, Zhao Y. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci Adv. (2022);8:eabi6696. doi: 10.1126/sciadv.abi6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morland C, Lauritzen KH, Puchades M, Holm-Hansen S, Andersson K, Gjedde A, Attramadal H, Storm-Mathisen J, Bergersen LH. The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1:expression and action in brain. J Neurosci Res. (2015);93:1045–1055. doi: 10.1002/jnr.23593. [DOI] [PubMed] [Google Scholar]
- 62.Murakami R, Chiba Y, Nishi N, Matsumoto K, Wakamatsu K, Yanase K, Uemura N, Nonaka W, Ueno M. Immunoreactivity of receptor and transporters for lactate located in astrocytes and epithelial cells of choroid plexus of human brain. Neurosci Lett. (2021);741:135479. doi: 10.1016/j.neulet.2020.135479. [DOI] [PubMed] [Google Scholar]
- 63.Pan RY, Ma J, Kong XX, Wang XF, Li SS, Qi XL, Yan YH, Cheng J, Liu Q, Jin W, Tan CH, Yuan Z. Sodium rutin ameliorates Alzheimer's disease-like pathology by enhancing microglial amyloid-βclearance. Sci Adv. (2019);5:eaau6328. doi: 10.1126/sciadv.aau6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan RY, Zhang J, Wang J, Wang Y, Li Z, Liao Y, Liao Y, Zhang C, Liu Z, Song L, Yu J, Yuan Z. Intermittent fasting protects against Alzheimer's disease in mice by altering metabolism through remodeling of the gut microbiota. Nat Aging. (2022a);2:1024–1039. doi: 10.1038/s43587-022-00311-y. [DOI] [PubMed] [Google Scholar]
- 65.Pan RY, He L, Zhang J, Liu X, Liao Y, Gao J, Liao Y, Yan Y, Li Q, Zhou X, Cheng J, Xing Q, Guan F, Zhang J, Sun L, Yuan Z. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer's disease. Cell Metab. (2022b);34:634–648. doi: 10.1016/j.cmet.2022.02.013. [DOI] [PubMed] [Google Scholar]
- 66.Pellerin L, Pellegri G, Martin JL, Magistretti PJ. Expression of monocarboxylate transporter mRNAs in mouse brain:support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc Natl Acad Sci U S A. (1998);95:3990–3995. doi: 10.1073/pnas.95.7.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system:distribution, regulation and function. J Neurochem. (2005);94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- 68.Rafiki A, Boulland JL, Halestrap AP, Ottersen OP, Bergersen L. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience. (2003);122:677–688. doi: 10.1016/j.neuroscience.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 69.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. (2018);378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. (2003);348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 71.Rocha EM, De Miranda B, Sanders LH. Alpha-synuclein:pathology, mitochondrial dysfunction and neuroinflammation in Parkinson's disease. Neurobiol Dis. (2018);109:249–257. doi: 10.1016/j.nbd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Ruiz F, Vigne S, Pot C. Resolution of inflammation during multiple sclerosis. Semin Immunopathol. (2019);41:711–726. doi: 10.1007/s00281-019-00765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saxena U. Bioenergetics failure in neurodegenerative diseases:back to the future. Expert Opin Ther Targets. (2012);16:351–354. doi: 10.1517/14728222.2012.664135. [DOI] [PubMed] [Google Scholar]
- 74.Schirinzi T, Di Lazzaro G, Sancesario GM, Summa S, Petrucci S, Colona VL, Bernardini S, Pierantozzi M, Stefani A, Mercuri NB, Pisani A. Young-onset and late-onset Parkinson's disease exhibit a different profile of fluid biomarkers and clinical features. Neurobiol Aging. (2020);90:119–124. doi: 10.1016/j.neurobiolaging.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 75.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism:the role of nutrient transporters. J Cereb Blood Flow Metab. (2007);27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solís-Maldonado M, Miró MP, Acuña AI, Covarrubias-Pinto A, Loaiza A, Mayorga G, Beltrán FA, Cepeda C, Levine MS, Concha II, Bátiz LF, Carrasco MA, Castro MA. Altered lactate metabolism in Huntington's disease is dependent on GLUT3 expression. CNS Neurosci Ther. (2018);24:343–352. doi: 10.1111/cns.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stanley WC, Gertz EW, Wisneski JA, Morris DL, Neese RA, Brooks GA. Systemic lactate kinetics during graded exercise in man. Am J Physiol. (1985);249:E595–602. doi: 10.1152/ajpendo.1985.249.6.E595. [DOI] [PubMed] [Google Scholar]
- 78.Stanley WC, Wisneski JA, Gertz EW, Neese RA, Brooks GA. Glucose and lactate interrelations during moderate-intensity exercise in humans. Metabolism. (1988);37:850–858. doi: 10.1016/0026-0495(88)90119-9. [DOI] [PubMed] [Google Scholar]
- 79.Sun Y, Wang Y, Chen ST, Chen YJ, Shen J, Yao WB, Gao XD, Chen S. Modulation of the astrocyte-neuron lactate shuttle system contributes to neuroprotective action of fibroblast growth factor 21. Theranostics. (2020);10:8430–8445. doi: 10.7150/thno.44370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. (2011);144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, Jiang T, Zhu XC, Tan L. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer's disease:a systematic review and meta-analysis. J Alzheimers Dis. (2014);41:615–631. doi: 10.3233/JAD-132690. [DOI] [PubMed] [Google Scholar]
- 82.Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V. Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol. (2022);22:657–673. doi: 10.1038/s41577-022-00684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tarczyluk MA, Nagel DA, Rhein Parri H, Tse EH, Brown JE, Coleman MD, Hill EJ. Amyloid β1-42 induces hypometabolism in human stem cell-derived neuron and astrocyte networks. J Cereb Blood Flow Metab. (2015);35:1348–1357. doi: 10.1038/jcbfm.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Hall G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol (Oxf) (2010);199:499–508. doi: 10.1111/j.1748-1716.2010.02122.x. [DOI] [PubMed] [Google Scholar]
- 85.Veloz Castillo MF, Magistretti PJ, Cali C. l-Lactate:food for thoughts, memory and behavior. Metabolites. (2021);11:548. doi: 10.3390/metabo11080548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vidoni ED, Perales J, Alshehri M, Giles AM, Siengsukon CF, Burns JM. Aerobic exercise sustains performance of instrumental activities of daily living in early-stage Alzheimer disease. J Geriatr Phys Ther. (2019);42:E129–134. doi: 10.1519/JPT.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walker FO. Huntington's disease. Lancet. (2007);369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 88.Wan N, Wang N, Yu S, Zhang H, Tang S, Wang D, Lu W, Li H, Delafield DG, Kong Y, Wang X, Shao C, Lv L, Wang G, Tan R, Wang N, Hao H, Ye H. Cyclic immonium ion of lactyllysine reveals widespread lactylation in the human proteome. Nat Methods. (2022);19:854–864. doi: 10.1038/s41592-022-01523-1. [DOI] [PubMed] [Google Scholar]
- 89.Wang J, Cui Y, Yu Z, Wang W, Cheng X, Ji W, Guo S, Zhou Q, Wu N, Chen Y, Chen Y, Song X, Jiang H, Wang Y, Lan Y, Zhou B, Mao L, Li J, Yang H, Guo W, et al. Brain endothelial cells maintain lactate homeostasis and control adult hippocampal neurogenesis. Cell Stem Cell. (2019);25:754–767. doi: 10.1016/j.stem.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 90.Wang J, Hu WW, Jiang Z, Feng MJ. Advances in treatment of neurodegenerative diseases:perspectives for combination of stem cells with neurotrophic factors. World J Stem Cells. (2020);12:323–338. doi: 10.4252/wjsc.v12.i5.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Woodburn SC, Bollinger JL, Wohleb ES. The semantics of microglia activation:neuroinflammation, homeostasis, and stress. J Neuroinflammation. (2021);18:258. doi: 10.1186/s12974-021-02309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu X, Tao WA. Uncovering ubiquitous protein lactylation. Nat Methods. (2022);19:793–794. doi: 10.1038/s41592-022-01536-w. [DOI] [PubMed] [Google Scholar]
- 93.Wyant KJ, Ridder AJ, Dayalu P. Huntington's disease-update on treatments. Curr Neurol Neurosci Rep. (2017);17:33. doi: 10.1007/s11910-017-0739-9. [DOI] [PubMed] [Google Scholar]
- 94.Xiang X, Wind K, Wiedemann T, Blume T, Shi Y, Briel N, Beyer L, Biechele G, Eckenweber F, Zatcepin A, Lammich S, Ribicic S, Tahirovic S, Willem M, Deussing M, Palleis C, Rauchmann BS, Gildehaus FJ, Lindner S, Spitz C, et al. Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases. Sci Transl Med. (2021);13 doi: 10.1126/scitranslmed.abe5640. eabe5640. [DOI] [PubMed] [Google Scholar]
- 95.Xiao Y, Wang SK, Zhang Y, Rostami A, Kenkare A, Casella G, Yuan ZQ, Li X. Role of extracellular vesicles in neurodegenerative diseases. Prog Neurobiol. (2021);201:102022. doi: 10.1016/j.pneurobio.2021.102022. [DOI] [PubMed] [Google Scholar]
- 96.Xiong J, He J, Zhu J, Pan J, Liao W, Ye H, Wang H, Song Y, Du Y, Cui B, Xue M, Zheng W, Kong X, Jiang K, Ding K, Lai L, Wang Q. Lactylation-driven METTL3-mediated RNA m(6)A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol Cell. (2022);82:1660–1677. doi: 10.1016/j.molcel.2022.02.033. [DOI] [PubMed] [Google Scholar]
- 97.Yamagata K. Lactate supply from astrocytes to neurons and its role in ischemic stroke-induced neurodegeneration. Neuroscience. (2022);481:219–231. doi: 10.1016/j.neuroscience.2021.11.035. [DOI] [PubMed] [Google Scholar]
- 98.Yang J, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I, Magistretti PJ. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A. (2014);111:12228–12233. doi: 10.1073/pnas.1322912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang K, Fan M, Wang X, Xu J, Wang Y, Tu F, Gill PS, Ha T, Liu L, Williams DL, Li C. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. (2022);29:133–146. doi: 10.1038/s41418-021-00841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu J, Chai P, Xie M, Ge S, Ruan J, Fan X, Jia R. Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. (2021);22:85. doi: 10.1186/s13059-021-02308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, Ding J, Czyz D, Hu R, Ye Z, He M, Zheng YG, Shuman HA, Dai L, Ren B, Roeder RG, et al. Metabolic regulation of gene expression by histone lactylation. Nature. (2019a);574:575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang M, Cheng X, Dang R, Zhang W, Zhang J, Yao Z. Lactate deficit in an Alzheimer disease mouse model:the relationship with neuronal damage. J Neuropathol Exp Neurol. (2018);77:1163–1176. doi: 10.1093/jnen/nly102. [DOI] [PubMed] [Google Scholar]
- 103.Zhang W, Wang G, Xu ZG, Tu H, Hu F, Dai J, Chang Y, Chen Y, Lu Y, Zeng H, Cai Z, Han F, Xu C, Jin G, Sun L, Pan BS, Lai SW, Hsu CC, Xu J, Chen ZZ, et al. Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell. (2019b);178:176–189. doi: 10.1016/j.cell.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao N, Xu B. The beneficial effect of exercise against Alzheimer's disease may result from improved brain glucose metabolism. Neurosci Lett. (2021);763:136182. doi: 10.1016/j.neulet.2021.136182. [DOI] [PubMed] [Google Scholar]
- 105.Zhao Y, Xu H. Microglial lactate metabolism as a potential therapeutic target for Alzheimer's disease. Mol Neurodegener. (2022);17:36. doi: 10.1186/s13024-022-00541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zu H, Li C, Dai C, Pan Y, Ding C, Sun H, Zhang X, Yao X, Zang J, Mo X. SIRT2 functions as a histone delactylase and inhibits the proliferation and migration of neuroblastoma cells. Cell Discov. (2022);8:54. doi: 10.1038/s41421-022-00398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


