Abstract
The impact of apolipoprotein E (ApoE) isoforms on sporadic Alzheimer’s disease has long been studied; however, the influences of apolipoprotein E gene (APOE) on healthy and pathological human brains are not fully understood. ApoE exists as three common isoforms (ApoE2, ApoE3, and ApoE4), which differ in two amino acid residues. Traditionally, ApoE binds cholesterol and phospholipids and ApoE isoforms display different affinities for their receptors, lipids transport and distribution in the brain and periphery. The role of ApoE in the human depends on ApoE isoforms, brain regions, aging, and neural injury. APOE ε4 is the strongest genetic risk factor for sporadic Alzheimer’s disease, considering its role in influencing amyloid-beta metabolism. The exact mechanisms by which APOE gene variants may increase or decrease Alzheimer’s disease risk are not fully understood, but APOE was also known to affect directly and indirectly tau-mediated neurodegeneration, lipids metabolism, neurovascular unit, and microglial function. Consistent with the biological function of ApoE, ApoE4 isoform significantly altered signaling pathways associated with cholesterol homeostasis, transport, and myelination. Also, the rare protective APOE variants confirm that ApoE plays an important role in Alzheimer’s disease pathogenesis. The objectives of the present mini-review were to describe classical and new roles of various ApoE isoforms in Alzheimer’s disease pathophysiology beyond the deposition of amyloid-beta and to establish a functional link between APOE, brain function, and memory, from a molecular to a clinical level. APOE genotype also exerted a heterogeneous effect on clinical Alzheimer’s disease phenotype and its outcomes. Not only in learning and memory but also in neuropsychiatric symptoms that occur in a premorbid condition. Clarifying the relationships between Alzheimer’s disease-related pathology with neuropsychiatric symptoms, particularly suicidal ideation in Alzheimer’s disease patients, may be useful for elucidating also the underlying pathophysiological process and its prognosis. Also, the effects of anti-amyloid-beta drugs, recently approved for the treatment of Alzheimer’s disease, could be influenced by the APOE genotype.
Keywords: Alzheimer’s disease, amyloid-beta, apolipoprotein E, dementia, glymphatic transport, lipids, neuropsychiatric symptoms, neurovascular unit, tau protein
Introduction to the Role of Apolipoprotein E in Alzheimer’s Disease
Human apolipoprotein E (ApoE) is a 299-amino acid secreted glycoprotein binding cholesterol and phospholipids, codified by the apolipoprotein E gene (APOE) placed at locus 19q13.32, and with three common isoforms (ApoE2, ApoE3, and ApoE4) (Weisgraber, 1994). ApoE function in the central nervous system (CNS) pathophysiology does not impact only lipid and cholesterol transport and cell membrane lipid bilayer properties, but its other CNS functions are still unclear. There is increasing evidence for other functions of APOE through the involvement in other biological processes such as transcriptional regulation, mitochondrial metabolism, immune response, and responsiveness to dietary factors (Rueter et al., 2022).
The role of ApoE in CNS is focused on cholesterol transport during cell membrane reorganization, prompting synaptic restoration and dendritic remodeling (Leduc et al., 2010). Furthermore, a greater amount of ApoE is secreted from astrocytes under physiological circumstances; instead, activated microglia secreted it under pathological conditions; finally, under certain stress conditions, ApoE is also derived from vascular mural cells, from choroid plexus, and neurons (Poirier et al., 1995). APOE has been shown to affect lipids and cholesterol in several different cell types, especially microglia and astrocytes, but recently also at the oligodendrocyte level (Blanchard et al., 2022). The role of ApoE in modulating the immune system during brain inflammatory responses is well known and varies by its isoforms. Noteworthy, the expression of ApoE from neuronal cells is prompted during aging and injuries to accelerate neuronal repair and remodeling (Poirier et al., 1995).
Numerous studies from the early 90’s have shown the role of ApoE in Alzheimer’s disease (AD), driving the different AD-associated proteinopathies, with a slightly different equilibrium between neurobiology and neuroinflammation, until neurodegeneration (Liu et al., 2013). However, the exact mechanism by which APOE gene variants may increase/decrease AD risk was not fully understood, but the ApoE isoforms differently affected brain homeostasis. How ApoE isoforms that differ for only two amino acids residues at positions 112 and 158 (ApoE2: Cys112 and Cys158; ApoE3: Cys112 and Arg158; ApoE4: Arg112 and Arg158) have such profound effects on AD risk and other age-related dementias has stumped the field for decades. Compared to APOE ε3 carriers, being heterozygous carriers of the APOE ε4 allele leads to a 4-fold increased risk of developing AD, while being homozygous APOE ε4 carriers increases 12 times the risk. Having almost one APOE ε4 allele reduces the age of AD onset by 8 years. Conversely, heterozygous carriers of the APOE ε2 allele have a 40% lower AD risk and homozygous carriers have a further lower risk (Liu et al., 2013).
The role of this protein in normal and abnormal brain metabolism from the cellular and molecular level to the clinical phase has been defined by the genetic variability of ApoE, deriving from different alleles coding proteins with single amino acid substitutions. The differential relative risk in AD pathogenesis is determined by a gain or a loss of function of the three ApoE isoforms in activating neuronal signaling (ApoE4 > ApoE3 > ApoE2). ApoE2 is the most effective isoform when produced by injured neurons, in protecting cells from oxidative stress in vitro, while ApoE3 has a medium potency, and ApoE4 is the least effective (Mahley and Huang, 2012). Moreover, neurons may upregulate ApoE in disease conditions. In the ApoE4 context, this may result in neurotoxicity (Mahley and Huang, 2012), because the homeostatic roles of astrocytes and microglia can be disrupted by ApoE4 isoform in normal aging and AD (Hasel and Liddelow, 2021).
APOE ε2 genotype drives unique serum metabolome profiles. Distinct serum metabolite profiles were founded in APOE ε2 mice with dramatically upregulated lipid levels (including phospholipids and sphingolipids) compared to APOE ε3 and APOE ε4 mice, likely due to its reduced binding affinity to apoE receptors including the low-density lipoprotein receptor, which might consequently elevate apoE levels and lipids in the blood (Zhao et al., 2020). Along with the common ApoE2 variant, the ApoE rare protective variants may play an important role in the course and onset of AD and can teach us how to target ApoE as we seek effective ways to prevent and cure AD and other age-related dementias. An extremely rare isoform (ApoE3r), known as ApoE3-Jacksonville (ApoE3/Jac), was identified in 0.3% population carrier. This ApoE variant was shown to be protective against AD by limiting neuronal injuries and promoting lipid metabolism (Le Guen et al., 2022). An individual who had two copies of the ApoE3 Christchurch mutation is protected by autosomal dominant AD, due to a mechanism involving ApoE interaction with heparin, and affecting lipid metabolism (Arboleda-Velasquez et al., 2019). The different isoforms interact with each other by complex mechanisms considering that homozygosity for ApoE3 Christchurch may be more protective than homozygosity for ApoE2, and ApoE2 homozygotes might still have an exceptionally low risk of late-onset AD (Arboleda-Velasquez et al., 2019).
The large risk reductions reported suggest that protein chemistry and functional assays of these variants should be pursued, as they have the potential to guide drug development targeting ApoE. The fact that these rare variants carry mutations in different regions of APOE offers additional opportunities to explore how structural and related biochemical properties of ApoE impact its pathophysiology in aging and AD, beyond its role in amyloid-beta (Aβ) metabolism. The protective mechanism of rare variants remains unexplored but finding a single amino acid substitution that renders the APOE ε4 allele protective supports the idea that APOE ε4-specific treatments are worth exploring (Lo Vecchio et al., 2022). The objectives of the present mini-review were to describe classical and new roles of various ApoE isoforms in AD pathophysiology beyond the deposition of Aβ peptides and to establish a link between APOE, brain function, and cognition from a molecular to a clinical level.
Search Strategy
Studies cited in the present review article were retrieved performing separate searches in the US National Library of Medicine (PubMed), Medical Literature Analysis and Retrieval System Online (MEDLINE), EMBASE, Scopus, Ovid, and Google Scholar databases to find articles published from inception to October 10, 2022, enquiring any association between the exposure (ApoE isoforms) and the health-related outcome(s) (AD pathophysiology and cognition/cognitive function) using selected key words/descriptors, i.e., (apolipoprotein E[tiab]) OR (ApoE[tiab]) OR (isoforms[tiab]) AND (Alzheimer’s disease[tiab]) OR (AD[tiab]) OR (pathophysiology[tiab]) OR (cognition[tiab]) OR (cognitive function[tiab]). This search strategy was used in PubMed and MEDLINE and adapted to the other four electronic sources. No language limitation was introduced.
Two investigators (ML, FP) searched for papers, screened titles and abstracts of the retrieved articles separately and in duplicate, checked the complete texts, and selected records for inclusion.
Classic Theory of Apolipoprotein E in Amyloid-Beta Metabolism
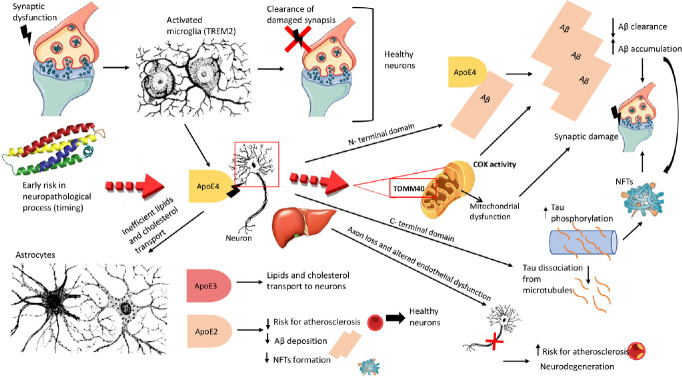
Although the APOE ε4 allele presence increased the risk for sporadic AD, the role of this allele is not itself a causal factor. Through complex mechanisms, involving genetic disequilibrium and expression, epigenetics, and cellular phase of AD, the APOE genotype can modulate the basic disease process (Mayeux et al., 1998). The mechanism linking the APOE genotype with the development of AD-type neuropathology is still unclear (Figure 1). Various ApoE isoforms trigger a chain of events, through biological signals, which activate specific pathways mediating different neuronal functions with influence on the systems that result in neuroplasticity and cognition (Poirier et al., 1995).
Figure 1.
Apolipoprotein E (ApoE)-mediated development of Alzheimer’s disease-type neuropathology.
Synapse dysfunction induces the activation of microglia, which removes damaged synapses in healthy neurons. Apolipoprotein E (APOE) alleles differentially impact glial responses. Triggering receptor expressed on myeloid cells 2 (TREM2) protein could be an important mediator. The inefficient transport of lipids and cholesterol from astrocytes, and microglia activation (including TREM2 activation) could be conditioned by the APOE ε4 allele. The stress and injury condition stimulate the neurons to release ApoE4, which with its N-terminal domain binds amyloid-beta (Aβ), promoting a reduction in clearance and accumulation of Aβ, leading to the formation of Aβ plaques and accentuating the synaptic damage. The C-terminal domain of ApoE can interact with neurofibrillary tangles (NFTs), which originate from tau hyperphosphorylation and successive dissociation of tau from microtubules provocating axon loss and neurodegeneration. This mechanism seems to be principally consequent to Aβ. ApoE4 isoform released by the central nervous system and peripherally by hepatocytes increases the risk for atherosclerosis, while ApoE2 and ApoE3 (intermedius) decrease this risk because of its protective effect in sporadic Alzheimer’s disease (decreases Aβ deposition and NFT formation). In addition, a dynamic mitochondrial dysfunction could be induced by the ApoE4 interaction with the translocase of the outer mitochondrial membrane 40 (TOMM40) gene on the outer mitochondrial membrane. Compared to noncarriers, APOE ε4 carriers have lower mitochondrial cytochrome oxidase (COX) activity in posterior cingulate cortex neurons, also without soluble or insoluble Aβ or tau pathology. This could be considered a very initial change that come first and prompted β-site amyloid-β precursor protein-cleaving enzyme (BACE1) activity and amyloid genesis. Created using Microsoft PowerPoint 2019.
The major role of ApoE isoforms in AD pathogenesis is played by differential effects on Aβ aggregation and clearance. ApoE isoforms determine the amount of Aβ pathology: ApoE4 > ApoE3 > ApoE2. Carriers of APOE ε4 with normal cognition showed a greater amount of Aβ and tau cerebral burden compared to APOE ε3 homozygotes; while regional tau burden or tau accumulation did not differ over time in APOE ε2 carriers with lower global Aβ load (Salvadó et al., 2021). Cholesterol synthesis and ApoE transport from astrocytes also regulate Aβ production in neuronal membranes. ApoE-mediated cholesterol uptake is essential for amyloid-β precursor protein processing and Aβ production (Wang et al., 2021a).
The effects of ApoE isoforms on Aβ aggregation and clearance are predominantly direct to the stabilization of synaptotoxic Aβ oligomers (Koffie et al., 2012) and to increase colocalization of Aβ oligomers with synapses (Hasel et al., 2021) in AD pathogenesis. In AD, strong clinical and preclinical evidence suggested that the main pathway by which the APOE ε4 allele drives AD risk was to promote earlier and more abundant Aβ deposition, likely by inhibiting Aβ clearance and accelerating Aβ aggregation and amyloid seeding (Yamazaki et al., 2019). While the APOE ε4 allele was linked to an increased risk of developing AD and to steeper cognitive declines after symptoms appear, these effects of the APOE ε4 allele on AD were seen only in the presence of AD pathology, specifically the characteristic accumulation of amyloid plaques in the brain or of tau protein neurofibrillary tangles inside of neurons.
Moreover, indirect effects are reported through microglial response and neuroinflammation: the third most important mechanism involved in AD after Aβ and neurofibrillary tangle pathology (Hasel and Liddelow, 2021). Soluble Aβ can be cleared from the brain through various mechanisms including enzymatic degradation, glial cell phagocytosis, transport across the blood-brain barrier (BBB), and glymphatic clearance. The dysfunction of the glymphatic system-mediated ApoE clearance was recently shown to be a main contributor to the intraneuronal accumulation of Aβ in the early pathological stages of AD (Feng et al., 2020). Although Aβ may perform important cellular functions, its endogenous accumulation is not sufficient to cause dementia.
New Apolipoprotein E-Mediated Mechanisms in Alzheimer’s Disease Pathogenesis
Although initial studies causally linked APOE with Aβ peptide aggregation and clearance, over the past 5 years, our understanding of APOE-mediated AD pathogenesis has expanded beyond Aβ peptide-centric mechanisms to tau/neurofibrillary tangle degeneration, microglia/astrocyte responses, and BBB disruption (Bu, 2022). ApoE affects tau pathogenesis, neuroinflammation, and tau-mediated neurodegeneration independent of Aβ pathology. ApoE4 exerts a “toxic” gain of function whereas the absence of ApoE4 is protective (Shi et al., 2017). Importantly, the removal of astrocytic ApoE4 decreased tau-induced synaptic loss and microglial phagocytosis of synaptic elements, suggesting a key role for astrocytic ApoE in synaptic degeneration (Wang et al., 2021b).
Microglia regulate cholesterol metabolism and contribute to learning and memory, in part, by promoting cholesterol elimination. Microglial survival is dependent on both the microglial-specific receptor triggering receptor expressed on myeloid cells 2 gene (TREM2) and ApoE-mediated transport of exogenous cholesterol (Li et al., 2022). Thus, the APOE ε4 allele might prime microglia towards a phagocytic and proinflammatory state through an APOE-TREM2 axis in normal aging as well as in AD (Hasel and Liddelow, 2021). In contrast to microglia, the influence of APOE alleles on the astrocyte transcriptome appears to be modest and suggests a dysregulation of lipid metabolism and the extracellular matrix. ApoE4 significantly altered signaling pathways associated with cholesterol homeostasis and transport. Fatty ApoE4 astrocytes have a reduced ability to clear toxic fatty acids from the extracellular milieu because ApoE4 reroutes them back to secretion (Lindner et al., 2022).
Furthermore, confirming these findings with histological and lipidomic analysis of the post-mortem human brain, induced pluripotent stem-cell-derived cells, and targeted-replacement mice, cholesterol is aberrantly deposited also in oligodendrocytes-myelinating cells that are responsible for insulating and promoting the electrical activity of neurons (Blanchard et al., 2022). Finally, ApoE4 expression differentially modulates regional neuronal lipid signatures, which may underlie the increased susceptibility of entorhinal cortex localized neurons to AD pathology (Miranda et al., 2022).
Recently, an ApoE cascade hypothesis was proposed emphasizing the structural and related biochemical differences among the three isoforms including lipidation, protein levels, receptor binding, and oligomerization as drivers of downstream effects at the cellular and phenotypical levels (Martens et al., 2022). The structural properties of the different ApoE isoforms receptor-binding, may account for the different AD risks, maybe with an early mechanism of action for the neuropathological process (Weisgraber, 1994). Also, the time of APOE interaction with the cellular phase of AD could be determinant. Different biological signals trigger specific pathways that lead to synaptic dysfunction and their massive loss: cAMP response element-binding protein, N-methyl-D-aspartate receptor-mediated protein synthesis, γ-aminobutyric acid-ergic inhibitory network, and mitochondrial dysfunctions (Rueter et al., 2022).
Furthermore, peripheral ApoE isoforms, produced primarily by hepatocytes and separated from those in the brain by the BBB, differentially impact AD pathogenesis and cognition. Plasma proteome profiling revealed ApoE isoform-dependent functional pathways highlighting cell adhesion, lipoprotein metabolism, and complement activation. A human-induced pluripotent stem cell-derived endothelial cell model summarized the plasma ApoE isoform-specific effect on endothelial integrity, further supporting a vascular-related mechanism (Liu et al., 2022). BBB breakdown contributes to cognitive decline in APOE ε4 carriers independently of AD pathology; ApoE4, but not ApoE3 activates the proinflammatory cyclophilin A-matrix metalloproteinase-9 pathway in the cerebral spinal fluid, which may lead to accelerated BBB breakdown causing neuronal and synaptic dysfunction (Montagne et al., 2020). APOE ε4 allele presence may accelerate advanced-stage vascular dysfunction, BBB breakdown, and neurodegeneration in AD mice via the CypA pathway in pericytes independently of Aβ (Montagne et al., 2021). As recently suggested, the association of orthostatic hypotension with brain lobar perivascular spaces among APOE ε4 carriers suggested that lobar perivascular spaces may be a marker for Aβ-associated small-vessel disease (Laveskog et al., 2020).
Moreover, the APOE ε4 allele could play a novel role in the premature shrinkage of meningeal lymphatic vessels (meningeal lymphosclerosis), leading to abnormal meningeal lymphatic functions (meningeal lymphedema), and, in turn, reduction in the clearance of Aβ and other macromolecules and inflammatory mediators, as well as immune cells, from the brain, exacerbation of AD manifestations, and progression of the disease (Mentis et al., 2021).
Recent Evidence on the Association of Apolipoprotein E with Alzheimer’s Disease: from Molecular to Clinical Level
From a biochemical/clinical level, memory consolidation is potentiated by ApoE-mediated epigenetic control in the brain. ApoE-mediated regulation in learning and memory displays an isoform-dependent effect, because ApoE4 is less efficient in suppressing neuronal cholesterol synthesis compared with ApoE3 due to the lower capacity of astrocytic ApoE4 to vector microRNAs that silence neuronal cholesterol biosynthesis (Li et al., 2022). Furthermore, carrying the APOE ε4 allele predicts future cognitive decline also in terms of olfactory dysfunction, considered a potential early signal of neurodegenerative processes in AD. Compared to non-carriers, APOE ε4 allele carriers showed a reduction in olfactory acuity and memory, and this olfactory loss could be explained by disrupted γ-aminobutyric acid signaling in the olfactory bulb (Olofsson et al., 2013).
Moreover, a critical challenge about the clinical phase of AD is that almost all patients with AD are affected also by neuropsychiatric symptoms (NPS) with depression, agitation, apathy, and psychosis being particularly common. However, the neuropathological basis for these symptoms is poorly understood. These AD endophenotypes may be correlated to AD pathophysiology. Clarifying the relationships between the AD-related pathology NPS of AD patients may be useful for elucidating also the underlying pathophysiological process and its prognosis. APOE genotype exerted a heterogeneous effect on clinical AD phenotype, also in NPS. Recently, the APOE ε4 allele and inflammation were shown to be detrimental factors also for suicidal ideation in later life (Lozupone et al., 2022). APOE genotype has been suggested underlying these AD endophenotypes. During the process of neurodegeneration, various brain cell types, such as astrocytes, microglia, and neurons, together with the neurovascular unit, develop distinct inflammatory phenotypes that impact their functions and could have characteristic transcriptomic fingerprints. Surprisingly, several potent anti-Aβ drugs accelerated the cognitive decline of AD and, in some cases, worsened NPS, also according to the APOE genotype (Panza et al., 2019a). Steady overproduction of Aβ in AD may represent an attempt of the brain to mitigate or repair neuronal damage/insult. Sudden reductions of brain Aβ levels with potent anti-Aβ drugs may worsen cognition and exacerbate NPS (Panza et al., 2019a).
Conclusions and Future Perspectives
A significant problem concerning the amyloid-centric explanation of AD is the contradictory relationship between biological phenotypes and clinical/behavioral characteristics of the disease. At a therapeutic level, the molecular interactions between ApoE and its targets, and with other pivotal biological substrates like amyloid-β precursor protein and its cleaving enzymes (α-, β-, and γ-secretase) were critically investigated (Panza et al., 2019b) for understanding also the potential cognitive and clinical consequences of the selective and precocious removal of Aβ. The association between AD and its neuropathological markers (e.g., amyloidosis, neuroinflammation, tauopathy, cerebral-amyloid angiopathy, etc.) has been recognized for some time, but misfolded proteins affect neuronal function in a still unknown way. Also, various speculations about the putative molecular mechanism of action or adverse drug reaction of several compounds under clinical testing include the association with cognitive and clinical benefits (aducanumab, lecanemab, and donanemab) (Panza et al., 2019b).
The complete absence of ApoE caused by a rare ablative APOE frameshift mutation may cause abnormal lipoprotein metabolism but normal visual, cognitive, neurological, and retinal function, with normal cerebral spinal fluid Aβ and tau protein levels and normal findings on brain magnetic resonance imaging (Mak et al., 2014). This observation has encouraged different approaches to interfere with the pathological role of ApoE in AD (Yamazaki et al., 2019). Regarding therapeutic interference with the physiological role of APOE, it is known that APOE genotype may suggest risk stratification, with APOE ε4 genotyping helping to guide treatment decisions. Amyloid-related imaging abnormalities are a common, dose-dependent effect of amyloid-targeting antibodies, strongly associated with the APOE ε4 allele. Going forward, if anti-amyloid antibodies become part of future treatment regimens for AD, knowledge of symptoms related to amyloid-related imaging abnormalities and an approach to management will be critical for treating clinicians (VandeVrede et al., 2020).
Meanwhile, neuron interactions, Aβ precursor protein processing, tau hyperphosphorylation, oxidative stress, neuroinflammation, protein synthesis, microbiota, and neurovascular mechanisms are the different areas of AD pathogenesis under discussion for understanding the contribution of the various ApoE isoforms to the disease. Not univocal interpretations are hypothesized for the specific ApoE functions inside the different brain cell types and the consequent modulation of the cellular phase of AD and age-related cognitive decline. A critical challenge is also that, only in the presence of AD pathology, APOE ε4 allele carriers are predisposed to an increased risk of developing AD and to a faster cognitive decline. With the aim of understanding the clinical phase of AD, it is important to study the time of APOE interaction with the cellular phase. Understanding how ApoE isoforms may affect AD pathophysiology represents a stimulating road for future research, also because ApoE is in a balanced equilibrium between the different isoforms and anti-ApoE therapeutics should be directed at the pathological isoforms without interfering with its physiological functions. It is not clear whether chronic treatment with anti-ApoE drugs will be limited by the physiological role of this important glycoprotein in cytoskeletal assembly and stability, mitochondrial integrity and function, and dendritic morphology and function.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: Not applicable.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Arboleda-Velasquez JF, Lopera F, O'Hare M, Delgado-Tirado S, Marino C, Chmielewska N, Saez-Torres KL, Amarnani D, Schultz AP, Sperling RA, Leyton-Cifuentes D, Chen K, Baena A, Aguillon D, Rios-Romenets S, Giraldo M, Guzmán-Vélez E, Norton DJ, Pardilla-Delgado E, Artola A, et al. Resistance to autosomal dominant Alzheimer's disease in an APOE3 Christchurch homozygote:a case report. Nat Med. (2019);25:1680–1683. doi: 10.1038/s41591-019-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchard JW, Akay LA, Davila-Velderrain J, von Maydell D, Mathys H, Davidson SM, Effenberger A, Chen CY, Maner-Smith K, Hajjar I, Ortlund EA, Bula M, Agbas E, Ng A, Jiang X, Kahn M, Blanco-Duque C, Lavoie N, Liu L, Reyes R, et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature. (2022);611:769–779. doi: 10.1038/s41586-022-05439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bu G. APOE targeting strategy in Alzheimer's disease:lessons learned from protective variants. Mol Neurodegener. (2022);17:51. doi: 10.1186/s13024-022-00556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng W, Zhang Y, Wang Z, Xu H, Wu T, Marshall C, Gao J, Xiao M. Microglia prevent beta-amyloid plaque formation in the early stage of an Alzheimer's disease mouse model with suppression of glymphatic clearance. Alzheimers Res Ther. (2020);12:125. doi: 10.1186/s13195-020-00688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasel P, Liddelow SA. Isoform-dependent APOE secretion modulates neuroinflammation. Nat Rev Neurol. (2021);17:265–266. doi: 10.1038/s41582-021-00483-y. [DOI] [PubMed] [Google Scholar]
- 6.Koffie RM, Hashimoto T, Tai HC, Kay KR, Serrano-Pozo A, Joyner D, Hou S, Kopeikina KJ, Frosch MP, Lee VM, Holtzman DM, Hyman BT, Spires-Jones TL. Apolipoprotein E4 effects in Alzheimer's disease are mediated by synaptotoxic oligomeric amyloid-β. Brain. (2012);135:2155–2168. doi: 10.1093/brain/aws127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laveskog A, Wang R, Vetrano DL, Bronge L, Wahlund LO, Qiu C. Associations of vascular risk factors and APOE genotype with perivascular spaces among community-dwelling older adults. J Am Heart Assoc. (2020);9:e015229. doi: 10.1161/JAHA.119.015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Guen Y, Belloy ME, Grenier-Boley B, de Rojas I, Castillo-Morales A, Jansen I, Nicolas A, Bellenguez C, Dalmasso C, Küçükali F, Eger SJ, Rasmussen KL, Thomassen JQ, Deleuze JF, He Z, Napolioni V, Amouyel P, Jessen F, Kehoe PG, van Duijn C, et al. Association of rare APOE missense variants V236E and R251G with risk of Alzheimer disease. JAMA Neurol. (2022);79:652–663. doi: 10.1001/jamaneurol.2022.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leduc V, Jasmin-Bélanger S, Poirier J. APOE and cholesterol homeostasis in Alzheimer's disease. Trends Mol Med. (2010);16:469–477. doi: 10.1016/j.molmed.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Li D, Zhang J, Liu Q. Brain cell type-specific cholesterol metabolism and implications for learning and memory. Trends Neurosci. (2022);45:401–414. doi: 10.1016/j.tins.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Lindner K, Beckenbauer K, van Ek LC, Titeca K, de Leeuw SM, Awwad K, Hanke F, Korepanova AV, Rybin V, van der Kam EL, Mohler EG, Tackenberg C, Lakics V, Gavin AC. Isoform- and cell-state-specific lipidation of ApoE in astrocytes. Cell Rep. (2022);38:110435. doi: 10.1016/j.celrep.2022.110435. [DOI] [PubMed] [Google Scholar]
- 12.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease:risk, mechanisms and therapy. Nat Rev Neurol. (2013);9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu CC, Zhao J, Fu Y, Inoue Y, Ren Y, Chen Y, Doss SV, Shue F, Jeevaratnam S, Bastea L, Wang N, Martens YA, Qiao W, Wang M, Zhao N, Jia L, Yamazaki Y, Yamazaki A, Rosenberg CL, Wang Z, et al. Peripheral apoE4 enhances Alzheimer's pathology and impairs cognition by compromising cerebrovascular function. Nat Neurosci. (2022);25:1020–1033. doi: 10.1038/s41593-022-01127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo Vecchio F, Bisceglia P, Imbimbo BP, Lozupone M, Latino RR, Resta E, Leone M, Solfrizzi V, Greco A, Daniele A, Watling M, Panza F, Seripa D. Are apolipoprotein E fragments a promising new therapeutic target for Alzheimer's disease? Ther Adv Chronic Dis. (2022);13:20406223221081605. doi: 10.1177/20406223221081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lozupone M, Donghia R, Sardone R, Mollica A, Berardino G, Lampignano L, Griseta C, Zupo R, Castellana F, Bortone I, Dibello V, Resta E, Stallone R, Seripa D, Daniele A, Solfrizzi V, Altamura M, Bellomo A, Panza F. Apolipoprotein E genotype, inflammatory biomarkers, and non-psychiatric multimorbidity contribute to the suicidal ideation phenotype in older age. The Salus in Apulia Study. J Affect Disord. (2022);319:202–212. doi: 10.1016/j.jad.2022.09.046. [DOI] [PubMed] [Google Scholar]
- 16.Mahley RW, Huang Y. Apolipoprotein e sets the stage:response to injury triggers neuropathology. Neuron. (2012);76:871–885. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mak AC, Pullinger CR, Tang LF, Wong JS, Deo RC, Schwarz JM, Gugliucci A, Movsesyan I, Ishida BY, Chu C, Poon A, Kim P, Stock EO, Schaefer EJ, Asztalos BF, Castellano JM, Wyss-Coray T, Duncan JL, Miller BL, Kane JP, et al. Effects of the absence of apolipoprotein E on lipoproteins, neurocognitive function, and retinal function. JAMA Neurol. (2014);71:1228–1236. doi: 10.1001/jamaneurol.2014.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martens YA, Zhao N, Liu CC, Kanekiyo T, Yang AJ, Goate AM, Kanekiyo T, Yang AJ, Goate AM, Holtzman DM, Bu G. ApoE cascade hypothesis in the pathogenesis of Alzheimer's disease and related dementias. Neuron. (2022);110:1304–1317. doi: 10.1016/j.neuron.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayeux R, Saunders AM, Shea S, Mirra S, Evans D, Roses AD, Hyman BT, Crain B, Tang MX, Phelps CH. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer's disease. Alzheimer's disease centers consortium on apolipoprotein E and Alzheimer's disease. N Engl J Med. (1998);338:506–511. doi: 10.1056/NEJM199802193380804. [DOI] [PubMed] [Google Scholar]
- 20.Mentis AA, Dardiotis E, Chrousos GP. Apolipoprotein E4 and meningeal lymphatics in Alzheimer disease:a conceptual framework. Mol Psychiatry. (2021);26:1075–1097. doi: 10.1038/s41380-020-0731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miranda AM, Ashok A, Chan RB, Zhou B, Xu Y, McIntire LB, Area-Gomez E, Di Paolo G, Duff KE, Oliveira TG, Nuriel T. Effects of APOE4 allelic dosage on lipidomic signatures in the entorhinal cortex of aged mice. Transl Psychiatry. (2022);12:129. doi: 10.1038/s41398-022-01881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, Pachicano M, Joe E, Nelson AR, D'Orazio LM, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Reiman EM, Caselli RJ, Chui HC, et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. (2020);581:71–76. doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montagne A, Nikolakopoulou AM, Huuskonen MT, Sagare AP, Lawson EJ, Lazic D, Rege SV, Grond A, Zuniga E, Barnes SR, Prince J, Sagare M, Hsu CJ, LaDu MJ, Jacobs RE, Zlokovic BV. APOE4 accelerates advanced-stage vascular and neurodegenerative disorder in old Alzheimer's mice via cyclophilin A independently of amyloid-β. Nat Aging. (2021);1:506–520. doi: 10.1038/s43587-021-00073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olofsson JK, Josefsson M, Ekström I, Wilson D, Nyberg L, Nordin S, Nordin Adolfsson A, Adolfsson R, Nilsson LG, Larsson M. Long-term episodic memory decline is associated with olfactory deficits only in carriers of ApoE-є4. Neuropsychologia. (2016);85:1–9. doi: 10.1016/j.neuropsychologia.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Panza F, Lozupone M, Bellomo A, Imbimbo BP. Do anti-amyloid-βdrugs affect neuropsychiatric status in Alzheimer's disease patients? Ageing Res Rev. (2019a);55:100948. doi: 10.1016/j.arr.2019.100948. [DOI] [PubMed] [Google Scholar]
- 26.Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Rev Neurol. (2019b);15:73–88. doi: 10.1038/s41582-018-0116-6. [DOI] [PubMed] [Google Scholar]
- 27.Poirier J, Minnich A, Davignon J. Apolipoprotein E, synaptic plasticity and Alzheimer's disease. Ann Med. (1995);27:663–670. doi: 10.3109/07853899509019253. [DOI] [PubMed] [Google Scholar]
- 28.Rueter J, Rimbach G, Huebbe P. Functional diversity of apolipoprotein E:from subcellular localization to mitochondrial function. Cell Mol Life Sci. (2022);79:499. doi: 10.1007/s00018-022-04516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvadó G, Grothe MJ, Groot C, Moscoso A, Schöll M, Gispert JD, Ossenkoppele R Alzheimer's Disease Neuroimaging Initiative. Differential associations of APOE-ε2 and APOE-ε4 alleles with PET-measured amyloid-βand tau deposition in older individuals without dementia. Eur J Nucl Med Mol Imaging. (2021);48:2212–2224. doi: 10.1007/s00259-021-05192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S, Grinberg LT, Rojas JC, Gallardo G, Wang K, Roh J, Robinson G, Finn MB, Jiang H, Sullivan PM, Baufeld C, Wood MW, Sutphen C, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. (2017);549:523–527. doi: 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VandeVrede L, Gibbs DM, Koestler M, La Joie R, Ljubenkov PA, Provost K, Soleimani-Meigooni D, Strom A, Tsoy E, Rabinovici GD, Boxer AL. Symptomatic amyloid-related imaging abnormalities in an APOE ε4/ε4 patient treated with aducanumab. Alzheimers Dement (Amst) (2020);12:e12101. doi: 10.1002/dad2.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Kulas JA, Wang C, Holtzman DM, Ferris HA, Hansen SB. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol (2021a) Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc Natl Acad Sci USA. 118:e2102191118. doi: 10.1073/pnas.2102191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, Xiong M, Gratuze M, Bao X, Shi Y, Andhey PS, Manis M, Schroeder C, Yin Z, Madore C, Butovsky O, Artyomov M, Ulrich JD, Holtzman DM. Selective removal of astrocytic APOE4 strongly protects against tau-mediated neurodegeneration and decreases synaptic phagocytosis by microglia. Neuron. (2021b);109:1657–1674. doi: 10.1016/j.neuron.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisgraber KH. Apolipoprotein E:structure-function relationships. In: Anfinsen CB, Edsall JT, Richards FM, Eisenberg DS, editors. Advances in protein chemistry. Cambridge: Elsevier; (1994). pp. 249–302. [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki Y, Zhao N, Caulfield TR, Liu CC, Bu G. Apolipoprotein E and Alzheimer disease:pathobiology and targeting strategies. Nat Rev Neurol. (2019);15:501–518. doi: 10.1038/s41582-019-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao N, Ren Y, Yamazaki Y, Qiao W, Li F, Felton LM, Mahmoudiandehkordi S, Kueider-Paisley A, Sonoustoun B, Arnold M, Shue F, Zheng J, Attrebi ON, Martens YA, Li Z, Bastea L, Meneses AD, Chen K, Thompson JW, St John-Williams L, et al. Alzheimer's risk factors age, APOE genotype, and sex drive distinct molecular pathways. Neuron. (2020);106:727–742. doi: 10.1016/j.neuron.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]