Abstract
Spinal cord injury is a serious disease of the central nervous system involving irreversible nerve injury and various organ system injuries. At present, no effective clinical treatment exists. As one of the artificial hibernation techniques, mild hypothermia has preliminarily confirmed its clinical effect on spinal cord injury. However, its technical defects and barriers, along with serious clinical side effects, restrict its clinical application for spinal cord injury. Artificial hibernation is a future-oriented disruptive technology for human life support. It involves endogenous hibernation inducers and hibernation-related central neuromodulation that activate particular neurons, reduce the central constant temperature setting point, disrupt the normal constant body temperature, make the body “adapt” to the external cold environment, and reduce the physiological resistance to cold stimulation. Thus, studying the artificial hibernation mechanism may help develop new treatment strategies more suitable for clinical use than the cooling method of mild hypothermia technology. This review introduces artificial hibernation technologies, including mild hypothermia technology, hibernation inducers, and hibernation-related central neuromodulation technology. It summarizes the relevant research on hypothermia and hibernation for organ and nerve protection. These studies show that artificial hibernation technologies have therapeutic significance on nerve injury after spinal cord injury through inflammatory inhibition, immunosuppression, oxidative defense, and possible central protection. It also promotes the repair and protection of respiratory and digestive, cardiovascular, locomotor, urinary, and endocrine systems. This review provides new insights for the clinical treatment of nerve and multiple organ protection after spinal cord injury thanks to artificial hibernation. At present, artificial hibernation technology is not mature, and research faces various challenges. Nevertheless, the effort is worthwhile for the future development of medicine.
Keywords: artificial hibernation, central thermostatic-resistant regulation, hypothermia, multi-system protection, neuroprotection, organ protection, spinal cord injury, synthetic torpor
Introduction
Spinal cord injury (SCI) (Ahuja et al., 2017) is a serious disease of the central nervous system (CNS), leading to abnormal motor, sensory, and autonomic nervous function; it is one of the leading factors of disability and death in young people. The core medical challenges of SCI lie in the extremely low regeneration and repair ability of the CNS, secondary inflammatory damage, and the formation of neuroma during the repair process. The main SCI treatment strategies include surgical decompression and pharmacological therapy to improve the patients’ symptoms. However, these methods do not restore the function of the injured spinal cord, and neuroprotection remains a challenging part of the treatment. In the clinical treatment of SCI, neuroprotective strategies are crucial, and organ and systemic protective measures are equally indispensable. In the long term, SCI can lead to multiple organ dysfunction (Thietje et al., 2021), impede disease recovery, reduce the quality of life of patients, and even induce systemic inflammatory response syndrome, eventually leading to organ failure and endangering the health and life of patients (Sun et al., 2016). Neurological and multi-organ protective therapeutic measures after SCI are important challenges that clinical SCI treatment research needs to address.
In recent years, artificial hibernation (also called synthetic torpor (Cerri et al., 2021)) has gradually become a hot topic in medical research. It relies on drugs and physical cooling to reduce the core body temperature, metabolic rate, and physiological activities, thereby producing torpor, inducing hypothermia, improving microcirculation, and slowing down cell damage and other processes to protect the body (Tarahovsky et al., 2017). Some studies have confirmed that low temperatures protect nerves and organs (Ransom et al., 2022; Shin et al., 2022). Basic studies have shown that mild hypothermia can provide neuroprotection in rat spinal cord tissue after SCI by inhibiting apoptosis and autophagy (Seo et al., 2015), inhibiting the Toll-like receptor 4/nuclear factor-κB pathway, and promoting microglia M2 polarization, thereby reducing SCI-induced injury and inflammation (Fu et al., 2022). Artificial hibernation techniques include mild controlled hypothermia, the use of hibernation inducers, and hibernation-related central neuromodulation. Among them, the mild hypothermia technique is relatively mature. It has achieved certain effects in an SCI clinical study (Hansebout and Hansebout, 2014) and is applicable in the neuroprosthetic treatment of acute and subacute phases of SCI. A meta-analysis of clinical studies in Korea on the effects of hypothermia on acute spinal cord injury (Shin et al., 2022) has shown that 55.8% of 103 SCI patients treated with hypothermia showed neurological improvement. However, there are also inevitable side effects. Ransom et al. (2022) and Lee et al. (2017) found that treating SCI with hypothermia can cause complications such as pulmonary dysfunction, venous thrombosis, wound infection, arrhythmia, and respiratory complications. Therefore, there is an urgent clinical need for a treatment strategy that can maintain hypothermia for therapeutic purposes, such as neurological and organ protection, without causing serious side effects. Endogenous hibernation inducers and hibernation-related central neuromodulation technology may replace mild hypothermia technology to achieve more convenient and comprehensive hibernation-like states with less toxic side effects, and fundamentally resolve the opposition between the external hypothermia treatment and the body’s physiological thermogenic resistance from the CNS. Although the use of endogenous hibernation inducers and hibernation-related central neuromodulation technology in SCI have not been reported, they can provide new ideas for medical research on nerve and organ protection after SCI. Jinka et al. (2015) found that non-hibernating rats had a reduced core body temperature after an artificial hibernation intervention (intraperitoneal injection of CHA (N6-cyclohexyladenosine) combined with a low-temperature environment). This reduction lasted for 24 hours, and the rats had less neuronal cell damage after rewarming than the control rats. Some researchers used hibernation-related central neuromodulation techniques to identify specific neurons and neural pathways that can induce a dormant state in non-hibernating rodents, thereby protecting neural function (Song et al., 2016; Hrvatin et al., 2020; Takahashi et al., 2020). In addition, the extraordinary phenomenon of organ and systemic protection in animals during hibernation provides new therapeutic ideas for organ protection after SCI (Hadj-Moussa and Storey, 2019). Artificial hibernation after SCI may directly protect organs by suppressing the inflammatory response of the organism (Gundersen, et al., 2001), regulating metabolic inhibition, delaying organ damage, and reducing infection susceptibility.
Thus, the application of artificial hibernation for neuroprotection and organ protection after SCI has unique potential advantages. This review summarizes the barriers to treating SCI with hypothermia and expounds the advantages of treating SCI with artificial hibernation by analyzing the protective effects of this technique on neurological and organ systems after SCI and its possible mechanisms, so as to expand horizons for research on neurological and organ protection after clinical treatment of SCI.
Search Strategy
In September 2022, We searched the PubMed database for articles published from 2000 to 2022 (mid-September) using the terms “hibernation” OR “torpor” OR “hypothermia therapy” OR “hypothermy” OR “hypothermia”; “spinal cord injury” OR “SCI” OR “spinal cord trauma”. Supplementary search databases were CNKI, Wanfang, VIP, Web of science, and Embase. Further screening is done by reading literature titles and abstracts. Besides, after reading the literature in detail, we added two references (Frerichs et al., 1994; Drew et al., 1999). On January 18, 2023, we used the additional search terms based on the previous ones: “organ” OR “kidney” OR “renal” OR “renal injury” OR “urinary system”, “melatonin”, “endocrine”. Further screening was performed by reading abstracts and titles.
Barriers to the Application of Hypothermia in Spinal Cord Injury
Studies (Ransom et al., 2022; Shin et al., 2022) have shown that hypothermia alone or combined with other treatments has a certain recovery effect on SCI. However, it also causes many complications and side effects. First of all, in the cardiovascular system, hypothermia can cause vasospasm and contraction of the blood vessels (Darwazeh and Yan, 2013). With the continuous decrease in temperature, cardiac output and central venous pressure increase, leading to arrhythmia and low blood pressure. In severe cases, ventricular fibrillation or even cardiac arrest may occur (Darwazeh and Yan, 2013; Hantson and Duprez, 2017). Second, in the blood coagulation system, hypothermia can reduce platelet count, cause the dysfunction of coagulation and inhibit coagulation cascade reactions, inhibit thrombin synthesis, leading to bleeding or thrombosis (Darwazeh and Yan, 2013; Wang et al., 2022). In addition, hypothermia can lead to electrolyte disturbance, which increases the pH value of the blood by increasing the solubility of CO2 in the blood, thereby decreasing blood concentrations of K+ (hypokalemia) and Mg2+ (hypomagnesemia) (Al-Nashash and All, 2022). Patients undergoing hypothermia may also show signs of insulin resistance (hyperglycemia) and hypoglycemia during rewarming (Ruetzler and Kurz, 2018; Al-Nashash and All, 2022). More importantly, hypothermia also leads to reduced gastrointestinal motility, impaired nutrient absorption, and varying degrees of pathological damage to internal organs, increasing the risk of serious infections (Wang and Han, 2014; Ruetzler and Kurz, 2018). All these complications directly or indirectly hinder the regeneration of nerves to some extent. In conclusion, cryotherapy is highly effective for motor, sensory, and neurological functions in SCI, but inevitably has many side effects. Therefore, new strategies and treatment optimizations are urgently needed.
Introduction to the Application of Artificial Hibernation Technologies
Current research techniques that can achieve artificial hibernation include mild hypothermia technology, hibernation inducers, and hibernation-related central neuromodulation. Mild hypothermia technology is widely studied and has been used in clinical treatments. Hibernation inducers and hibernation-related central neuromodulation are now under active research (Zhang et al., 2022). Endogenous hibernation inducers and hibernation-related central neuromodulation directly act on the body’s central temperature regulation system, reduce the body temperature setting point, make the body “adapt” to the external low-temperature environment (Chi et al., 2018; Takahashi et al., 2020), and reduce the core body temperature by increasing heat dissipation and reducing heat production. Unlike mild hypothermia technology, they can adaptively suppress thermogenic behaviors, do not cause fierce shiver and strong physiological resistance.
Mild hypothermia technology
Mild hypothermia technology is a mature artificial hibernation technique. Mild hypothermia technology consists of physical cooling combined with sedatives, central nervous system inhibitors, or muscle relaxants, and reduces the human core body temperature to 28–35°C. Mild hypothermia has been used for neuroprotection (Csernyus et al., 2020), cardiac surgery, and cardiopulmonary resuscitation (Wang et al., 2021), and to treat traumatic brain injury (Carney et al., 2017; Jiang et al., 2019), SCI (Ransom et al., 2022; Shin et al., 2022), neonatal necrotizing enterocolitis (Gonçalves-Ferri et al., 2021), and neonatal encephalopathy (Kariholu et al., 2020; Shipley et al., 2022). The American Brain Trauma Foundation 2016 guidelines recommended mild hypothermia therapy for traumatic brain injury as Class IIB. Arrich et al. (2009) evaluated the efficacy of mild hypothermia in the treatment of cardiac arrest and found that mild hypothermia (32–34°C) maintained for more than 24 hours effectively improved the survival rate and nervous system outcome of cardiac arrest patients. The study by Jiang et al. (2019) showed that long-term mild hypothermia (> 5 days) protected brain tissue more effectively than short-term mild hypothermia (< 48 hours) did. Common clinical techniques include body surface cooling and, to a lesser extent, intravascular cooling. Physical cooling with an ice cap machine and ice blanket machine is simple but slow. Intravascular cooling is achieved by the intravenous infusion of a hypothermic fluid (0–10°C) for a short time. However, the rapid infusion of large amounts of hypothermic fluid to maintain a low core body temperature may cause cardiovascular instability (Dietrich et al., 2011). In addition, the intravascular catheter used for intravascular cooling is an efficient, controllable, and long-lasting but invasive tool (De Fazio et al., 2019). The cooling is achieved through the heat exchange between the coolant in the catheter and the blood in the femoral vein, which requires accurate operation techniques, and the cost/risk/benefit should be considered. To inhibit muscle tremor during cooling and improve human tolerance to cold stimulation, the above techniques are often used in combination with anesthetic sedatives and hibernation mixtures. However, drug-induced cooling causes addiction, drug resistance, and toxic side effects (Sessler, 2009) and is, therefore, not suitable for long-term use. Besides, mild hypothermia therapy requires much highly experienced medical staff to deal with the many thorny complications. Finally, this method also has technical constraints, and its process needs to be further improved to solve practical problems.
Hibernation inducers
Hibernation inducers are a kind of substance that can reduce body temperature and induce a hypothermic state by slowing down metabolism, inhibiting thermogenesis, and inducing sedation. There are two hibernation inducer categories based on the drug source.
Endogenous hibernation inducers are found in the serum of hibernating animals; they can decrease body temperature and metabolism and produce a spontaneous torpor state similar to natural hibernation. For example, Seitz et al. (2012) showed that the body temperature of rats decreased rapidly within 1 hour after the inhalation of H2S at 21°C. After 6 hours, the core body temperature dropped to 35°C, and the activity decreased. Another example is adenosine-5′-monophosphate, which plays an important role in the regulation of adenosine A1 receptors in hibernation (Muzzi et al., 2013). Frare et al. (2019) showed that the adenosine A1 receptor agonist CHA induced hibernation in non-hibernating rats and decreased their core body temperatures to 29.3°C and 35.6°C at ambient temperatures of 16°C and 25°C, respectively, after 4 hours (Jinka et al., 2015). Other examples include natural thyroxine derivatives (Huang et al., 2022), enkephalins (δ-opioid ligands) (Wolf et al., 2018), and 2-deoxy-D-glucose (Chi et al., 2018). Endogenous hibernation inducers are currently at the early research stages, and their targets and mechanisms of action remain unclear. Additionally, dimethyl sulfoxide (a commonly used solubilizer for CHA) has its own toxicity, and long-term injections cause adverse reactions (Macala and Hayslett, 2002; Ikeda et al., 2018). Besides, safe and effective inducer doses and rescue measures for hazards caused by toxic doses also need to be studied, such as the use of 8-sulfophenyltheophylline to counteract bradycardia triggered by the binding of CHA to cardiac adenosine A1 receptors (Cerri et al., 2021). Finally, screening studies of hibernation inducers suitable for large mammals and primates need to be conducted.
Synthetic hibernation inducers are widely used in the clinic. Some psychotropic drugs acting on the CNS (Tarahovsky et al., 2017) and anesthetic sedatives can induce hypothermia, such as phenothiazines (van Marum et al., 2007). In particular, chlorpromazine and promethazine have been used in clinical practice as the main constituent drugs of hibernation-inducing combinations. They increase the hypothermic effect by blocking α2-adrenergic receptors and altering vasodilatory regulation. Additionally, pentobarbital reduces brain temperature and core body temperature by inhibiting brain metabolic activity (Kiyatkin and Brown, 2005). Thanks to its biophysical properties, such as high thermal conductivity, helium is also a cooling substance. The inhalation of a mixture of cryogenic helium and oxygen causes a heat exchange with the pulmonary vasculature, rapidly reducing the core body temperature (Yin et al., 2022). The combined application of synthetic hibernation inducers and mild hypothermia technology can enhance cooling efficiency and depth, albeit not without risks (Lee et al., 2017). Notably, hypothermia triggered by synthetic hibernation inducers can trigger physiological cold defenses such as violent shivering, reduced blood pressure, and bradycardia, which can be detrimental to treatment (Zonnenberg et al., 2017). Besides, the ability of synthetic hibernation inducers (mostly antipsychotic drugs) to induce hypothermia can vary in patients with concomitant diseases. For example, patients with underlying diseases such as metabolic disease, hypothyroidism, or organic brain disease are more prone to hypothermia (Kreuzer et al., 2012). Thus, these inducers can endanger or even be lethal to these patients.
Hibernation-related central neuromodulation
Central neuromodulation technology uses electrical, magnetic, optical, acoustic, or chemical means to excite, inhibit or regulate signal transmission in specific brain regions, neurons, or neural networks. Current research often uses advanced techniques such as optogenetics or chemogenetics to precisely modulate brain regions or neurons in the central neural network that control thermogenesis and/or control energy metabolism to achieve a central autonomic regulation of hypothermic and hypometabolic hibernation. Takahashi et al. (2020) used optogenetics to activate Q neurons in the anteroventral periventricular nucleus, the medial preoptic area brain region, and the dorsomedial hypothalamic nucleus brain region and successfully induced a hibernation-like state in rodents. These results indicate that Q neurons play an important regulatory role for inducing a hibernation state in non-hibernating animals. Song et al. (2016) used chemogenetics and found that activating transient receptor potential melastatin-like subfamily member 2 (Trpm2) neurons in the preoptic area of the hypothalamus using clozapine-N-oxide continuously decreased body temperature. The hypothalamus is a key regulator of body temperature and metabolism. Hrvatin et al. (2020) used a chemogenetic tool, the Gq-DREADD receptor (Gq-coupled designer receptors exclusively activated by designer drugs) to selectively stimulate Gq-DREADD-injected neurons in the hypothalamus with clozapine-N-oxide. They found that the anterior and ventral portion of the medial and lateral preoptic area (avMLPA) was the key region for inducing torpor. This study revealed the key role that Adcyap1 neurons of the avMLPA played in body temperature regulation. The brain regions and nuclei involved in the induction of hypothermia are rich and diverse, and the mechanisms of action involved are complex. As an invasive neuromodulation technique, it requires a high level of operation and a demanding surgical environment to prevent infection, and may come with foreign body rejection and neurological damage caused by the incision. Besides, the use of central neuromodulation techniques to induce hypothermia may be time-limited; they are safe and effective in the short term, but long-term invasive interventions produce unpredictable and even irreversible damage to the nerves and brain. The risk/benefit ratio may gradually increase with time. The development of hibernation-related central neuromodulation techniques (optogenetic- or chemogenetic-based) is currently limited to laboratory studies, with notable differences between animal models and human studies.
Advantages and Mechanisms of Artificial Hibernation in the Treatment of Spinal Cord Injury
Mechanism of the artificial hibernation-induced neuroprotection after SCI
Inflammation inhibition and neuroprotection in hypothermia
Artificial hibernation technology combined with a low ambient temperature can reduce the body temperature of homeothermic animals to below 37°C (Wang and Han, 2014). Han et al. (2015) pointed out that a hypothermia intervention can reduce the levels of inflammatory factors, such as interleukin-1β, interleukin-6, and tumor necrosis factor-α, reduce transforming growth factor-β2 levels and reduce the production of interleukin-10. Hypothermia can protect cells and nerves by relieving the local inflammatory response after SCI and inhibiting the expression of apoptosis factors and axon growth inhibitors. Using a rat model of SCI, Xu et al. (2016) found that a mild hypothermia intervention inhibited the RhoA/ROCK signal pathway, reduced intracellular inhibitory signal transduction, and promoted nerve tissue growth and axonal regeneration. Li et al. (2022) found that hypothermia reduced caspase-3 expression, inhibited apoptosis signal transduction in neurons and glial cells, and induced neuroprotection after SCI. In addition, hypothermia can protect neural stem cells and bone marrow mesenchymal stem cells. Martin et al. (2021) showed that hypothermia inhibited the interaction between cyclophilin D and the adenine nucleotide translocator, reduced the intracellular accumulation of Ca2+, reduced the number of mitochondrial permeability transition pores, reduced mitochondrial apoptosis, and increased small ubiquitin-related modifier 1 (SUMO1) conjugated proteins. Studies have confirmed that mild hypothermia promotes SUMOylation and maintains the stemness of neural stem cells, thus improving hypoxia tolerance (Cai et al., 2022). SUMOylation may be an important protective mechanism for bone marrow mesenchymal stem cells survival under adverse conditions (Liu et al., 2017), which may be closely related to hypothermia-induced neuroprotection and the improvement of nerve cell resistance to adverse environments after SCI. Besides, Jin et al. (2015) suggested that hypothermia may induce neuroprotection by enhancing the autophagy of damaged cells. Moreover, in vitro experiments confirmed that hypothermia promoted axonal growth (Schmitt et al., 2010) and upregulated genes involved in synaptic organization in a rat model of traumatic brain injury (Feng et al., 2010; Figure 1 and Table 1). This shows that hypothermia has not only an indirect protective effect on nerves but also a direct repair effect.
Figure 1.
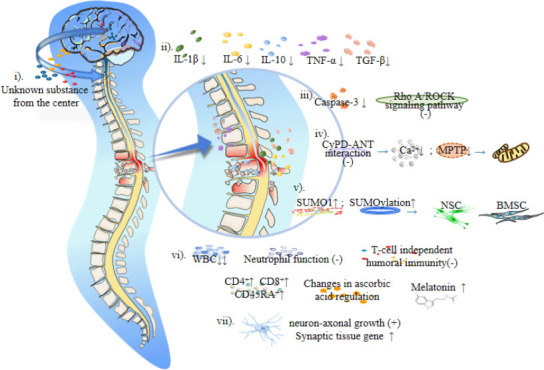
Mechanism of the artificial hibernation-induced neuroprotection after spinal cord injury.
Artificial hibernation may protect the nerves after spinal cord injury through the following aspects: i) Some unknown substances produced by the brain may directly protect the nerves during central thermostatic-resistant regulation. ii) Hypothermia inhibits the inflammatory reaction, reducing nerve cell damage. iii) Hypothermia can effectively reduce the transduction of intracellular inhibition signals and apoptosis signals, iv) protect mitochondria from apoptosis, v) maintain the stemness of neural stem cells and improve the tolerance of BMSC under adverse conditions, and vi) establish immunosuppressive and oxidative defense systems to reduce the injury caused by tissue reperfusion. vii) In vitro experimental studies showed that low temperatures can promote the growth of nerve axons and upregulate synaptic tissue genes. Created with Microsoft PowerPoint 2019. ANT: Adenine nucleotide translocator; BMSC: bone marrow mesenchymal stem cells; CD4+: CD4-positive T-lymphocytes; CD45RA+: CD45 naive/resting T-lymphocytes; CD8+: CD8-positive T-lymphocytes; CyPD: Cyclophilin-D; IL-10: interleukin-10; IL-1β: interleukin-1β; IL-6: interleukin-6; MPTP: mitochondrial permeability transition pore; NSC: neural stem cells; SUMO1: sumoylated-1 conjugated proteins; TGF-β: transforming growth factor-β; TNF-α: tumor necrosis factor-α; WBC: white blood cell.
Table 1.
The mechanisms underlying artificial hibernation as a treatment for SCI
| Mechanism of artificial hibernation-induced neuroprotection after spinal cord injury | Inflammation inhibition and neuroprotection in hypothermia | 1. Decreased levels of inflammatory factors |
| 2. Inhibition of apoptosis signal transduction and reduction of the expression of axon growth inhibitory factors | ||
| 3. Improvement of the tolerance of neural stem cells and bone marrow mesenchymal stem cells | ||
| 4. Maintenance of the mitochondrial function | ||
| 5. Increased autophagy of damaged cells | ||
| 6. Upregulation of synaptic tissue genes | ||
| Establishment of immunosuppression and oxidation defense systems during hibernation | 1. Inhibition of neutrophil function | |
| 2. The ability of adaptive regulation of immune cells | ||
| 3. Regulation of ascorbic acid levels | ||
| 4. Strong inhibition of the production of oxygen radicals | ||
| 5. Increased antioxidant levels | ||
| Role of central thermostatic-resistant regulation on nerves | 1. Reducing the constant temperature point leads the body to adapt to the external low temperature and reduces the damage of cold stress to the nerves | |
| 2. Release of central-related neuroprotective substances | ||
| Artificial hibernation-induced protection of body systems after spinal cord injury | Respiratory and digestive system | 1. Intestinal tract: changes in microorganisms and increased levels of lymphocytes and pro-inflammatory factors forming a strong immune barrier protection and preventing bacterial blood translocation from damaging the liver and lung |
| 2. Liver: increased lactate dehydrogenase release enhancing liver tolerance; increased H2S levels reducing oxygen consumption and metabolism | ||
| 3. Lung: reduced lung ventilation; inhibited inflammatory reaction | ||
| Cardiovascular system | 1. Intracellular Ca2+ homeostasis enhances the resistance to ventricular fibrillation | |
| 2. Promotion of intermolecular signal transduction maintaining systolic power | ||
| 3. Blocking of inflammation-related receptors, slowing down the damage caused by abnormal autonomic reflex | ||
| Locomotor system | 1. Formation of intracellular Ca2+ homeostasis | |
| 2. Reduction of interleukin-6 levels | ||
| 3. Maintenance of the function of skeletal muscle cells | ||
| Urinary system | 1. Significant reduction of renal fibrosis | |
| 2. Promotion of adenosine-5’-triphosphate recovery | ||
| 3. Maintenance of renal tubular function | ||
| Endocrine system | Increased melatonin levels can alleviate damage caused by ischemia-reperfusion and oxidation |
Establishment of immunosuppression and an oxidation defense system during hibernation
Zhou et al. (2001) showed that hibernation exerts neuroprotection by significantly inhibiting macrophage infiltration, axonal swelling, and oxidative stress. This may be related to immunosuppression and oxidative defense. Considering that, during hibernation, the white blood cells of Arctic ground squirrels decreased to 10% of the normal level (Frerichs et al., 1994), Drew et al. (2001) proposed that leukopenia and immunosuppression can inhibit the inflammatory response induced by tissue reperfusion after rewarming. Reitsema et al. (2021) recently found that hamsters at the early stage of awakening had circulating neutrophils with severely limited function, which may be the key to the hibernation-awakening transition without damage to organs and tissues. Hibernation-induced immunosuppression may provide a period of “inflammatory quiescence” and delay the process of injury, which is of great significance for the protection of organs and tissues in the environment of the “inflammatory storm.” Bouma et al. (2013) showed that the T-cell independent humoral immunity of thirteen-lined ground squirrels was suppressed during hibernation, and the blood levels of CD4-positive, CD8-positive, and CD45-naive/resting T-lymphocytes increased significantly. These results suggest that hibernating animals can adaptively regulate immune cells. Besides, Drew et al. (1999) found that hibernating animals had high levels of ascorbic acid in the plasma and cerebrospinal fluid, while Henry et al. (2007) observed low total ascorbic acid levels in hibernating animals, suggesting a regulatory change or different concentrations of ascorbic acid in tissues. Moreover, hibernating cells seem to be able to autonomously adapt to cold stress, maintain mitochondrial function and adenosine triphosphate production capacity, and strongly limit oxygen free radical production (Giroud et al., 2021). In addition, melatonin levels are elevated during arousal periods during hibernation, and melatonin is an antioxidant that improves oxidative defenses and mitigates ischemia/reperfusion-induced oxidative damage (Tan et al., 2005; Figure 1 and Table 1). These results suggest that the establishment of antioxidant defenses during hibernation is an important part of the physiological protection of neural tissues during hibernation.
Neuroprotective mechanism of central counter thermoregulation
In addition to the neuroprotective mechanism of artificial hibernation described above, we speculate that a direct protective effect of the central system on nerves may also exist.
Artificial hibernation may disrupt body thermoregulation, forcing the body to adaptively adjust the central thermostatic point through the perception of the external low-temperature environment and re-establish a central thermostatic system with a lower temperature point or even a central variable temperature system, finally forming a central thermostatic-resistant regulation. This system allows the body to “adapt” to the external low-temperature environment by reducing physiological activities and vital signs, thereby protecting the organs and tissues from low-temperature damage, and reducing the nerve damage caused by cold stress injury. The transition period is characterized by an increase in heat dissipation, a decrease in heat production, and a slowdown of metabolism, finally decreasing body temperature (Song et al., 2016; Takahashi et al., 2020). We speculate that, under central thermostatic-resistant regulation conditions, the brain produces central-related neuroprotective substances that directly affect nerve cells and play a role in protection and repair. Henry et al. (2007) measured the concentrations of 18 kinds of biological substances in the brain of hibernating ground squirrels. They observed significant level changes and a balance between recovery and decrease. Moreover, significantly increased levels of several anti-apoptotic proteins and phosphorylation in the brain of hibernating ground squirrels (Giroud et al., 2021), including B-cell lymphoma 2, B-cell lymphoma-extra-large, Bax-inhibitor 1, and myeloid cell leukemia sequence 1, were beneficial for the brain and played a role in neuroprotection. Gonzalez-Riano et al. (2019) studied metabolic changes in brain tissue during hibernation for the first time and revealed significant differences in 337 metabolites; they concluded that these metabolites played a key role in hibernation regulation. This result suggests that the changes caused by hibernation cannot be caused entirely by hypothermia, and there may be a more complex central mechanism. Researchers in this field have always considered that hibernation exerted neuroprotection through a central mechanism. In recent years, research on the mechanism of central regulation of hypothermia has advanced. In 2016, Song et al. discovered hypothermia induced by trpm-2 neurons and pathways. In 2020, Takahashi et al. discovered the important role of Q nerve in Q-neuron-induced hypometabolism. During the same year, Hrvatin et al. (2020) revealed the key role of Adcyap1 neurons of the avMLPA brain region in the regulation of body temperature, and Zhang et al. (2020) found that medial preoptic area neurons play an important role in thermoregulation and metabolism (Figure 1 and Table 1). This is a good beginning for the in-depth study of central regulatory neuroprotective mechanisms, and different neuroprotective mechanisms and neuroprotective agents may be found through different central temperature regulation mechanisms in the future.
Protective effect of artificial hibernation on the body after SCI
Respiratory and digestive system
During hibernation, the intestinal immune system changes greatly, the intestinal microbiome changes, the levels of lymphocytes in the mucosal epithelium and lamina propria increase significantly, and the levels of the proinflammatory cytokines interferon-γ, tumor necrosis factor-α, and interleukin-10 increase, forming a strong immune barrier to protect hibernators from intestinal microorganisms and inhibit potentially destructive inflammation (Kurtz et al., 2021). The strong protective effect on the intestinal barrier can prevent the bacterial translocation caused by SCI (Myers et al., 2019), and thus protect the liver and decrease the occurrence of systemic inflammatory response syndrome and multiple organ dysfunction syndrome. Santora et al. (2010) found that mild hypothermia had a cytoprotective effect in a mesenteric ischemia/reperfusion injury model and provided protection to distant organs, giving priority to the regulation of the ischemia/reperfusion injury activation transcriptome in the lung. This result indicated that the protective mechanism of mild hypothermia on the intestinal tract can not only reduce local metabolite levels and inhibit inflammatory development, but also involve remote regulation and protection between organs. The lung, as the main target of SCI-induced acute inflammation, is most vulnerable to inflammation (Sun et al., 2016). Hypothermia can reduce the systemic inflammatory response, thus slow down pulmonary inflammatory damage after SCI. Interestingly, the inhalation of H2S during mechanical ventilation may achieve the dual therapeutic effect of independent lung protection and hypothermia-induced protection (Faller et al., 2010). In addition, during hibernation, the liver can inhibit mitochondrial respiration through H2S (Jensen et al., 2021) and reduce oxygen consumption, pulmonary ventilation, heart rate, and metabolic rate, which may have a protective effect on the lung. Moreover, hypothermia can increase the release of lactate dehydrogenase in animal liver (Alva et al., 2018), which increases the tolerance of this organ to adverse environments, thus protecting it against inflammatory environmental damage after traumatic spinal cord injury (Sun et al., 2016). For details, see Figure 2 and Table 1.
Figure 2.
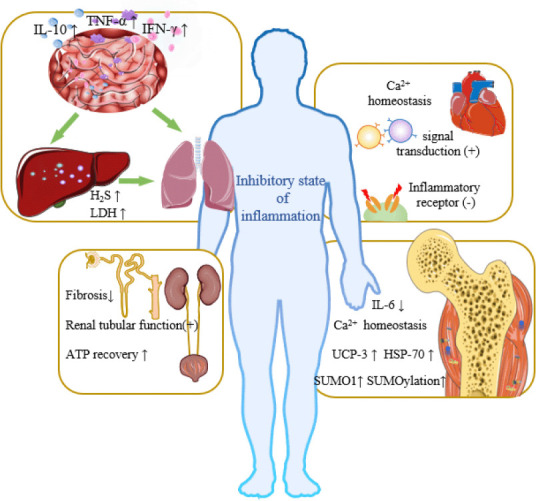
Artificial hibernation-induced protection of organs.
Under artificial hibernation conditions, the body adopts an inflammatory inhibition state, reducing the harm caused by inflammation and protecting body organs and skeletal muscles. i) Respiratory and digestive system: there is an interactive relationship between the intestine, liver, and lung. During hibernation, intestinal microorganisms change and the levels of lymphocytes and pro-inflammatory factors increase, forming a strong immune barrier protection, preventing bacterial translocation into the blood after SCI, thus protecting the liver and fragile lungs. In addition, the release of lactate dehydrogenase in the liver is increased, which improves liver tolerance Besides, the increase of H2S levels can reduce the body’s oxygen consumption and lung ventilation, further protecting the lung. ii) Under hibernation conditions, intracellular Ca2+ homeostasis enhances heart’s resistance to ventricular fibrillation. Hyperthermia can promote intermolecular signal transduction, thus maintaining contractile power. It can also slow down the cardiovascular damage caused by abnormal autonomic nerve reflex after SCI by blocking inflammation-related receptors. iii) Locomotor system: Ca2+ homeostasis is an important mechanism for maintaining the normal function of muscles under hibernation conditions, and hypothermia can actively protect the function and morphology of skeletal muscle cells. iv) Urinary system: artificial hibernation can reduce the damage caused by renal ischemia and reperfusion, and protect renal function and tissue structure. Created with Microsoft PowerPoint 2019. ATP: Adenosine 5′ triphosphate; HSP-70: heat shock protein-70; IFN-γ: interferon γ; IL-10: interleukin-10; IL-6: interleukin-6; LDH: lactate dehydrogenase; SUMO1: sumoylated-1 conjugated proteins; TNF-α: tumor necrosis factor-α; UCP-3: uncoupling protein-3.
Cardiovascular system
A survey in Germany (Thietje et al., 2021) showed that cardiovascular disease is the main cause of individual death in patients with SCI. Hibernating animals have a strong heart protection ability (Yang et al., 2021), which may provide a reference for heart protection after SCI. Hibernating mammals can stabilize intracellular Ca2+, which grants them better protection against ventricular fibrillation induced by pharmacological or pathological conditions than other mammals (Li et al., 2011). A study by Yang et al. (2021) suggested that cardiomyin-mediated junctophilin-2/caveolin-3 upregulation tightened the transversal tubule-sarcoplasmic reticulum junction, promoted the efficiency of intermolecular signal transduction between L-type Ca2+ channels and ryanodine receptors, and maintained cardiac systolic power. At the same time, the risk of calcium overload during hibernation is avoided. In addition, anti-inflammatory therapy is a potential treatment for cardiovascular dysfunction after injury (Parvin et al., 2021). Artificial hibernation may reduce the degree of abnormal autonomic reflex after SCI by blocking inflammation-related receptors and alleviate the high degree of cardiovascular system instability caused by the destruction of autonomic nervous pathways (Phillips and Krassioukov, 2015). For details, see Figure 2 and Table 1.
Locomotor system
Long-term SCI can cause osteoporosis (Shams et al.,2021) and neurogenic heterotopic ossification (Torossian et al., 2017), accompanied by different degrees of muscle atrophy. Black bears experience complete hibernation for 130 days, but their muscle strength is reduced by only 23%, they keep their number of skeletal muscle cells, maintain their size was, and experience no muscle dystrophy (Harlow et al., 2001). The research team of Mitsunori Miyazaki of Hokkaido University in Japan found that human skeletal muscle cells cultured in hibernating bear serum had significantly higher total protein content than cells cultured in regular medium (Miyazaki et al., 2022). Some studies have shown that the intracellular Ca2+ balance during hibernation is an important mechanism to inhibit apoptosis and prevent muscle atrophy in hibernating animals (Zhang et al., 2019; Wang et al., 2020). In addition, when a muscle is injured, denervation activates fibrous adipogenic progenitor cells, which is characterized by the continuous activation of STAT3 (signal transducer and activator of transcription 3) and increased interleukin-6 secretion, which promotes muscle atrophy and fibrosis. Fibrous adipogenic progenitor cells with abnormal activation of STAT3/interleukin-6 signal transduction were also found in a mouse model of SCI (Madaro et al., 2018). Besides, hypothermia lowers interleukin-6 levels, and muscle protection may be associated with this. An animal study showed that hypothermia can significantly upregulate the acylation levels of heat shock protein-70, uncoupling protein-3 and SUMOylation, and reduce the interaction of the mitochondrial permeability transition pore-related proteins in the skeletal muscle (Martin et al., 2021), indicating that hypothermia can protect the skeletal muscle. For details, see Figure 2 and Table 1.
Urinary system
A recent study found that artificial hibernation in a mouse model of renal ischemia/reperfusion reduced serum creatinine level, inhibited macrophage infiltration, slowed down renal tubular apoptosis, maintained mitochondrial function, significantly reduced renal fibrosis, and protected renal function and tissue structure (Schleef et al., 2022). In addition, another animal study showed that artificial hibernation promoted the recovery of adenosine-5′-triphosphate under ischemia conditions and reduced fibrosis; this study also confirmed that hypothermia protected renal tissues after ischemia/reperfusion injury (Yamamoto et al., 2020). A clinical study found that mild hyperthermia reduced the risk of acute renal injury and significantly reduced creatinine and cystatin C levels in patients who had undergone cardiopulmonary resuscitation. Although the effect varies according to the age of patients, artificial hibernation protects renal function (Hasslacher et al., 2018). These studies suggest that artificial hibernation can alleviate renal injury and protect the urinary system after SCI. For details, see Figure 2 and Table 1.
Endocrine system
The endocrine system plays a key role in hibernation by releasing hormones that regulate physiological activities and control metabolism and body temperature. Endogenous hibernation-inducing substances produced during hibernation may have therapeutic use for patients with SCI. For example, one of the main sites of action of the pineal hormone melatonin is the nodal part of the pituitary gland, a regulator of pituitary endocrine function (Castle-Miller et al., 2017). This hormone decreases metabolism, promotes sleep, and is elevated during hibernation arousal. Schiaveto-de-Souza et al. (2013) found that the intraperitoneal injection of melatonin in SCI model rats alleviated oxidative damage, reduced inflammation, and exerted neuroprotective effects. Tan et al. (2005) suggested that a transient elevation of melatonin during ischemia/reperfusion episodes reduces ischemia/reperfusion-induced oxidative damage. For details, see Figure 2 and Table 1.
Limitations
This review summarizes the relevant research on the protective effect of hypothermia and hibernation on organs and nerves but has some limitations. The protective effect of artificial hibernation on nerve and multi-organ systems after SCI and its related mechanism are speculative. Although controlled hypothermia and artificial hibernation both reduce body temperature, they have essential differences. Controlled hypothermia uses physical and/or pharmacological cooling methods to force body temperature reduction and triggers the body’s resistance to cold stimulation. Meanwhile, artificial hibernation reduces the central constant temperature setting point, disrupting the normal constant temperature state maintained by the body, making the body “adapt” to the external cold environment, and reducing the physiological resistance caused by cold stimulation. Therefore, they cannot act through completely equal mechanisms. In addition, artificial hibernation technology is still in the basic research stage,and the mechanism of the torpor state is not clear, and high-quality basic research reports are scarce. Next, the results obtained through animal experimental research are different from those of human research. The exact effectiveness of mild hypothermia in the treatment of SCI needs to be verified by numerous clinical studies. Moving from animal research to human application requires a long time, huge investments, and relentless scientific research.
Conclusion
Artificial hibernation protects nerve cells by reducing body temperature, inhibiting the inflammatory reaction, inducing an immunosuppressive state, and forming an oxidation defense system; it may also produce central-related neuroprotective substances directly acting on injured nerves. In addition, it promotes the repair and protection of the respiratory, digestive, cardiovascular, locomotor, urinary, and endocrine systems. In conclusion, artificial hibernation technology is a promising therapeutic tool for the protection and treatment of nerves and organs after SCI.
Perspective
Artificial hibernation protects nerves and organs and makes the body enter a state of torpor, greatly reducing the pathological damage caused by cold stimulation stress. It is a promising tool for the clinical treatment of SCI. In the future, scientists are expected to discover and produce endogenous hibernation-inducing substances suitable for humans by mastering the mechanisms of hibernation, in order to realize human hibernation.Moreover, methods that can prevent human diseases can be developed by studying the protective mechanism of animals’ nerves, organs, and systems under hibernation. Besides, it is essential to identify the brain regions and nerve nuclei involved in hypothermia induction, thus discover different neuroprotective mechanisms through different central temperature-regulating mechanisms to develop diverse neuroprotective measures. Artificial hibernation has great potential application value and scientific significance for nerve regeneration and repair and organ protection treatment after SCI. At present, artificial hibernation is still in the primary research stage, and the hibernation initiation mechanism in hibernating and non-hibernating animals remains unclear. After artificial hibernation intervention, it is not clear how central regulation directly affects the development of diseases and how the neuro-immune-endocrine system regulates biological processes under artificial hibernation. Although major challenges remain, it is worth exploring artificial hibernation for the treatment of SCI. Achieving a safer, deeper, and longer-lasting torpor state and SCI treatment through artificial hibernation still require much scientific and clinical research.
Footnotes
Funding: This work was supported by the Key Projects of the National Natural Science Foundation of China, No. 11932013 (to XC); Key Military Logistics Research Projects, No. BWJ21J002 (to XC); the Key projects of the Special Zone for National Defence Innovation, No. 21-163-12-ZT-006-002-13 (to XC); the National Nature Science Foundation of China, No. 82272255 (to XC); the National Defense Science and Technology Outstanding Youth Science Fund Program, No. 2021-JCJQ-ZQ-035 (to XC); the Scientific Research Innovation Team Project of Armed Police Characteristic Medical Center, No. KYCXTD0104 (to ZL); the National Natural Science Foundation of China Youth Fund, No. 82004467 (to BC).
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: Not applicable.
C-Editor: Zhao M; S-Editor: Li CH; C-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers. (2017);3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 2.Al-Nashash H, All AH. Neuroprotective role of hypothermia in acute spinal cord injury. Biomedicines. (2022);10:104. doi: 10.3390/biomedicines10010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alva N, Bardallo RG, Basanta D, Palomeque J, Carbonell T. Preconditioning-like properties of short-term hypothermia in isolated perfused rat liver (IPRL) system. Int J Mol Sci. (2018);19:1023. doi: 10.3390/ijms19041023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arrich J, Holzer M, Herkner H, Müllner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. (2009):CD004128. doi: 10.1002/14651858.CD004128.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Bouma HR, Henning RH, Kroese FG, Carey HV. Hibernation is associated with depression of T-cell independent humoral immune responses in the 13-lined ground squirrel. Dev Comp Immunol. (2013);39:154–160. doi: 10.1016/j.dci.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Cai H, Ma X, Lu D, Chen L, Bian X, Zhang N, Tang W, Liu X, Li Z. Mild hypothermia promotes ischemic tolerance and survival of neural stem cell grafts by enhancing global SUMOylation. Oxid Med Cell Longev. (2022);2022:6503504. doi: 10.1155/2022/6503504. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. (2017);80:6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 8.Castle-Miller J, Bates DO, Tortonese DJ. Mechanisms regulating angiogenesis underlie seasonal control of pituitary function. Proc Natl Acad Sci U S A. (2017);114:E2514–2523. doi: 10.1073/pnas.1618917114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerri M, Hitrec T, Luppi M, Amici R. Be cool to be far:Exploiting hibernation for space exploration. Neurosci Biobehav Rev. (2021);128:218–232. doi: 10.1016/j.neubiorev.2021.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Chi QS, Li XJ, Wang DH. 2-Deoxy-D-glucose, not mercaptoacetate, induces a reversible reduction of body temperature in male desert hamsters (Phodopus roborovskii) J Therm Biol. (2018);71:189–194. doi: 10.1016/j.jtherbio.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Csernyus B, SzabóÁ, Zátonyi A, Hodován R, Lázár C, Fekete Z, Erőss L, Pongrácz A. Recent antiepileptic and neuroprotective applications of brain cooling. Seizure. (2020);82:80–90. doi: 10.1016/j.seizure.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Darwazeh R, Yan Y. Mild hypothermia as a treatment for central nervous system injuries:Positive or negative effects. Neural Regen Res. (2013);8:2677–2686. doi: 10.3969/j.issn.1673-5374.2013.28.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Fazio C, Skrifvars MB, Søreide E, Creteur J, Grejs AM, Kjærgaard J, Laitio T, Nee J, Kirkegaard H, Taccone FS. Intravascular versus surface cooling for targeted temperature management after out-of-hospital cardiac arrest:an analysis of the TTH48 trial. Crit Care. (2019);23:61. doi: 10.1186/s13054-019-2335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich WD, Levi AD, Wang M, Green BA. Hypothermic treatment for acute spinal cord injury. Neurotherapeutics. (2011);8:229–239. doi: 10.1007/s13311-011-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drew KL, Osborne PG, Frerichs KU, Hu Y, Koren RE, Hallenbeck JM, Rice ME. Ascorbate and glutathione regulation in hibernating ground squirrels. Brain Res. (1999);851(1-2):1–8. doi: 10.1016/s0006-8993(99)01969-1. [DOI] [PubMed] [Google Scholar]
- 16.Faller S, Ryter SW, Choi AM, Loop T, Schmidt R, Hoetzel A. Inhaled hydrogen sulfide protects against ventilator-induced lung injury. Anesthesiology. (2010);113:104–115. doi: 10.1097/ALN.0b013e3181de7107. [DOI] [PubMed] [Google Scholar]
- 17.Feng JF, Zhang KM, Jiang JY, Gao GY, Fu X, Liang YM. Effect of therapeutic mild hypothermia on the genomics of the hippocampus after moderate traumatic brain injury in rats. Neurosurgery. (2010);67:730–742. doi: 10.1227/01.NEU.0000378023.81727.6E. [DOI] [PubMed] [Google Scholar]
- 18.Frare C, Jenkins ME, McClure KM, Drew KL. Seasonal decrease in thermogenesis and increase in vasoconstriction explain seasonal response to N(6) -cyclohexyladenosine-induced hibernation in the Arctic ground squirrel (Urocitellus parryii) J Neurochem. (2019);151:316–335. doi: 10.1111/jnc.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. J Cereb Blood Flow Metab. (1994);14:193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]
- 20.Fu D, Chen C, He L, Li J, Li A. Protective effect of mild hypothermia on spinal cord ischemia-induced delayed paralysis and spinal cord injury. Neurochem Res. (2022);47:1212–1225. doi: 10.1007/s11064-021-03515-7. [DOI] [PubMed] [Google Scholar]
- 21.Giroud S, Habold C, Nespolo RF, Mejías C, Terrien J, Logan SM, Henning RH, Storey KB. The torpid state:recent advances in metabolic adaptations and protective mechanisms. Front Physiol. (2021);11:623665. doi: 10.3389/fphys.2020.623665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Riano C, León-Espinosa G, Regalado-Reyes M, García A, DeFelipe J, Barbas C. Metabolomic study of hibernating syrian hamster brains:in search of neuroprotective agents. J Proteome Res. (2019);18:1175–1190. doi: 10.1021/acs.jproteome.8b00816. [DOI] [PubMed] [Google Scholar]
- 23.Gonçalves-Ferri WA, Ferreira CHF, Couto LCA, Souza TR, de Castro Peres T, Carmona F, Aragon DC, Crott G, Mussi-Pinhata MM, Junior JSC, Roosch A, Neto LS. Low technology, mild controlled hypothermia for necrotizing enterocolitis treatment:an initiative to improve healthcare to preterm neonates. Eur J Pediatr. (2021);180:3161–3170. doi: 10.1007/s00431-021-04014-1. [DOI] [PubMed] [Google Scholar]
- 24.Gundersen Y, Vaagenes P, Pharo A, Valø ET, Opstad PK. Moderate hypothermia blunts the inflammatory response and reduces organ injury after acute haemorrhage. Acta Anaesthesiol Scand. (2001);45:994–1001. doi: 10.1034/j.1399-6576.2001.450812.x. [DOI] [PubMed] [Google Scholar]
- 25.Hadj-Moussa H, Storey KB. Bringing nature back:using hibernation to reboot organ preservation. FEBS J. (2019);286:1094–1100. doi: 10.1111/febs.14683. [DOI] [PubMed] [Google Scholar]
- 26.Han Z, Liu X, Luo Y, Ji X. Therapeutic hypothermia for stroke:Where to go. Exp Neurol. (2015);272:67–77. doi: 10.1016/j.expneurol.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Hansebout RR, Hansebout CR. Local cooling for traumatic spinal cord injury:outcomes in 20 patients and review of the literature. J Neurosurg Spine. (2014);20:550–561. doi: 10.3171/2014.2.SPINE13318. [DOI] [PubMed] [Google Scholar]
- 28.Hantson P, Duprez T. Hypothermia with extreme bradycardia following spinal cord infarction of septic origin. Case Rep Neurol Med. (2017);2017:1351549. doi: 10.1155/2017/1351549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harlow HJ, Lohuis T, Beck TD, Iaizzo PA. Muscle strength in overwintering bears. Nature. (2001);409:997. doi: 10.1038/35059165. [DOI] [PubMed] [Google Scholar]
- 30.Hasslacher J, Barbieri F, Harler U, Ulmer H, Forni LG, Bellmann R, Joannidis M. Acute kidney injury and mild therapeutic hypothermia in patients after cardiopulmonary resuscitation - a post hoc analysis of a prospective observational trial. Crit Care. (2018);22:154. doi: 10.1186/s13054-018-2061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry PG, Russeth KP, Tkac I, Drewes LR, Andrews MT, Gruetter R. Brain energy metabolism and neurotransmission at near-freezing temperatures:in vivo (1)H MRS study of a hibernating mammal. J Neurochem. (2007);101:1505–1515. doi: 10.1111/j.1471-4159.2007.04514.x. [DOI] [PubMed] [Google Scholar]
- 32.Hrvatin S, Sun S, Wilcox OF, Yao H, Lavin-Peter AJ, Cicconet M, Assad EG, Palmer ME, Aronson S, Banks AS, Griffith EC, Greenberg ME. Neurons that regulate mouse torpor. Nature. (2020);583:115–121. doi: 10.1038/s41586-020-2387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang S, Liu L, Tang X, Xie S, Li X, Kang X, Zhu S. Research progress on the role of hormones in ischemic stroke. Front Immunol. (2022);13:1062977. doi: 10.3389/fimmu.2022.1062977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda K, Ohto H, Okuyama Y, Yamada-Fujiwara M, Kanamori H, Fujiwara SI, Muroi K, Mori T, Kasama K, Iseki T, Nagamura-Inoue T, Fujii N, Ashida T, Kameda K, Kanda J, Hirose A, Takahashi T, Nagai K, Minakawa K, Tanosaki R. Adverse events associated with infusion of hematopoietic stem cell products:a prospective and multicenter surveillance study. Transfus Med Rev. (2018) doi: 10.1016/j.tmrv.2018.05.005. S0887-7963(18)30023-3. [DOI] [PubMed] [Google Scholar]
- 35.Jensen BS, Pardue S, Duffy B, Kevil CG, Staples JF, Fago A. Suppression of mitochondrial respiration by hydrogen sulfide in hibernating 13-lined ground squirrels. Free Radic Biol Med. (2021);169:181–186. doi: 10.1016/j.freeradbiomed.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang JY, Gao GY, Feng JF, Mao Q, Chen LG, Yang XF, Liu JF, Wang YH, Qiu BH, Huang XJ. Traumatic brain injury in China. Lancet Neurol. (2019);18:286–295. doi: 10.1016/S1474-4422(18)30469-1. [DOI] [PubMed] [Google Scholar]
- 37.Jin Y, Lin Y, Feng JF, Jia F, Gao GY, Jiang JY. Moderate hypothermia significantly decreases hippocampal cell death involving autophagy pathway after moderate traumatic brain injury. J Neurotrauma. (2015);32:1090–1100. doi: 10.1089/neu.2014.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jinka TR, Combs VM, Drew KL. Translating drug-induced hibernation to therapeutic hypothermia. ACS Chem Neurosci. (2015);6:899–904. doi: 10.1021/acschemneuro.5b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kariholu U, Montaldo P, Markati T, Lally PJ, Pryce R, Teiserskas J, Liow N, Oliveira V, Soe A, Shankaran S, Thayyil S. Therapeutic hypothermia for mild neonatal encephalopathy:a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2020);105:225–228. doi: 10.1136/archdischild-2018-315711. [DOI] [PubMed] [Google Scholar]
- 40.Kiyatkin EA, Brown PL. Brain and body temperature homeostasis during sodium pentobarbital anesthesia with and without body warming in rats. Physiol Behav. (2005);84:563–570. doi: 10.1016/j.physbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Kreuzer P, Landgrebe M, Wittmann M, Schecklmann M, Poeppl TB, Hajak G, Langguth B. Hypothermia associated with antipsychotic drug use:a clinical case series and review of current literature. J Clin Pharmaco. (2012);52:1090–1097. doi: 10.1177/0091270011409233. [DOI] [PubMed] [Google Scholar]
- 42.Kurtz CC, Otis JP, Regan MD, Carey HV. How the gut and liver hibernate. Comp Biochem Physiol A Mol Integr Physiol. (2021);253:110875. doi: 10.1016/j.cbpa.2020.110875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JH, Zhang J, Yu SP. Neuroprotective mechanisms and translational potential of therapeutic hypothermia in the treatment of ischemic stroke. Neural Regen Res. (2017);12:341–350. doi: 10.4103/1673-5374.202915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li N, Chau CYC, Liu J, Yao M, Kiang KMY, Zhu Z, Zhang P, Cheng H, Leung GKK. Postcooling but not precooling benefits motor recovery by suppressing cell death after surgical spinal cord injury in rats. World Neurosurg. (2022);159:e356–364. doi: 10.1016/j.wneu.2021.12.049. [DOI] [PubMed] [Google Scholar]
- 45.Li XC, Wei L, Zhang GQ, Bai ZL, Hu YY, Zhou P, Bai SH, Chai Z, Lakatta EG, Hao XM, Wang SQ. Ca2+cycling in heart cells from ground squirrels:adaptive strategies for intracellular Ca2+homeostasis. PLoS One. (2011);6:e24787. doi: 10.1371/journal.pone.0024787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X, Ren W, Jiang Z, Su Z, Ma X, Li Y, Jiang R, Zhang J, Yang X. Hypothermia inhibits the proliferation of bone marrow-derived mesenchymal stem cells and increases tolerance to hypoxia by enhancing SUMOylation. Int J Mol Med. (2017);40:1631–1638. doi: 10.3892/ijmm.2017.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macala LJ, Hayslett JP. Basolateral and apical A1 adenosine receptors mediate sodium transport in cultured renal epithelial (A6) cells. Am J Physiol Renal Physiol. (2002);283:F1216–1225. doi: 10.1152/ajprenal.00085.2002. [DOI] [PubMed] [Google Scholar]
- 48.Madaro L, Passafaro M, Sala D, Etxaniz U, Lugarini F, Proietti D, Alfonsi MV, Nicoletti C, Gatto S, De Bardi M, Rojas-García R, Giordani L, Marinelli S, Pagliarini V, Sette C, Sacco A, Puri PL. Denervation-activated STAT3-IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nat Cell Biol. (2018);20:917–927. doi: 10.1038/s41556-018-0151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin LJ, Niedzwiecki MV, Wong M. Chronic intermittent mild whole-body hypothermia is therapeutic in a mouse model of ALS. Cells. (2021);10:320. doi: 10.3390/cells10020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyazaki M, Shimozuru M, Tsubota T. Supplementing cultured human myotubes with hibernating bear serum results in increased protein content by modulating Akt/FOXO3a signaling. PLoS One. (2022);17:e0263085. doi: 10.1371/journal.pone.0263085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muzzi M, Blasi F, Masi A, Coppi E, Traini C, Felici R, Pittelli M, Cavone L, Pugliese AM, Moroni F, Chiarugi A. Neurological basis of AMP-dependent thermoregulation and its relevance to central and peripheral hyperthermia. J Cereb Blood Flow Metab. (2013);33:183–190. doi: 10.1038/jcbfm.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myers SA, Gobejishvili L, Saraswat Ohri S, Garrett Wilson C, Andres KR, Riegler AS, Donde H, Joshi-Barve S, Barve S, Whittemore SR. Following spinal cord injury, PDE4B drives an acute, local inflammatory response and a chronic, systemic response exacerbated by gut dysbiosis and endotoxemia. Neurobiol Dis. (2019);124:353–363. doi: 10.1016/j.nbd.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parvin S, Williams CR, Jarrett SA, Garraway SM. Spinal cord injury increases pro-inflammatory cytokine expression in kidney at acute and sub-chronic stages. Inflammation. (2021);44:2346–2361. doi: 10.1007/s10753-021-01507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phillips AA, Krassioukov AV. Contemporary cardiovascular concerns after spinal cord injury:mechanisms, maladaptations, and management. J Neurotrauma. (2015);32:1927–1942. doi: 10.1089/neu.2015.3903. [DOI] [PubMed] [Google Scholar]
- 55.Ransom SC, Brown NJ, Pennington ZA, Lakomkin N, Mikula AL, Bydon M, Elder BD. Hypothermia therapy for traumatic spinal cord injury:an updated review. J Clin Med. (2022);11:1585. doi: 10.3390/jcm11061585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reitsema VA, Oosterhof MM, Henning RH, Bouma HR. Phase specific suppression of neutrophil function in hibernating Syrian hamster. Dev Comp Immunol. (2021);119:104024. doi: 10.1016/j.dci.2021.104024. [DOI] [PubMed] [Google Scholar]
- 57.Ruetzler K, Kurz A. Consequences of perioperative hypothermia. Handb Clin Neurol. (2018);157:687–697. doi: 10.1016/B978-0-444-64074-1.00041-0. [DOI] [PubMed] [Google Scholar]
- 58.Santora RJ, Lie ML, Grigoryev DN, Nasir O, Moore FA, Hassoun HT. Therapeutic distant organ effects of regional hypothermia during mesenteric ischemia-reperfusion injury. J Vasc Surg. (2010);52:1003–1014. doi: 10.1016/j.jvs.2010.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schiaveto-de-Souza A, da-Silva CA, Defino HL, Del Bel EA. Effect of melatonin on the functional recovery from experimental traumatic compression of the spinal cord. Braz J Med Biol Res. (2013);46:348–358. doi: 10.1590/1414-431X20132322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schleef M, Gonnot F, Pillot B, Leon C, Chanon S, Vieille-Marchiset A, Rabeyrin M, Bidaux G, Guebre-Egziabher F, Juillard L, Baetz D, Lemoine S. Mild therapeutic hypothermia protects from acute and chronic renal ischemia-reperfusion injury in mice by mitigated mitochondrial dysfunction and modulation of local and systemic inflammation. Int J Mol Sci. (2022);23:9229. doi: 10.3390/ijms23169229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmitt KR, Boato F, Diestel A, Hechler D, Kruglov A, Berger F, Hendrix S. Hypothermia-induced neurite outgrowth is mediated by tumor necrosis factor-alpha. Brain Pathol. (2010);20:771–779. doi: 10.1111/j.1750-3639.2009.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seitz DH, Fröba JS, Niesler U, Palmer A, Veltkamp HA, Braumüller ST, Wagner F, Wagner K, Bäder S, Wachter U, Calzia E, Radermacher P, Huber-Lang MS, Zhou S, Gebhard F, Knöferl MW. Inhaled hydrogen sulfide induces suspended animation, but does not alter the inflammatory response after blunt chest trauma. Shock. (2012);37:197–204. doi: 10.1097/SHK.0b013e31823f19a0. [DOI] [PubMed] [Google Scholar]
- 63.Seo JY, Kim YH, Kim JW, Kim SI, Ha KY. Effects of therapeutic hypothermia on apoptosis and autophagy after spinal cord injury in rats. Spine (Phila Pa 1976) (2015);40:883–890. doi: 10.1097/BRS.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 64.Sessler DI. Thermoregulatory defense mechanisms. Crit Care Med. (2009);37(7 Suppl):S203–210. doi: 10.1097/CCM.0b013e3181aa5568. [DOI] [PubMed] [Google Scholar]
- 65.Shams R, Drasites KP, Zaman V, Matzelle D, Shields DC, Garner DP, Sole CJ, Haque A, Banik NL. The pathophysiology of osteoporosis after spinal cord injury. Int J Mol Sci. (2021);22:3057. doi: 10.3390/ijms22063057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin HK, Park JH, Roh SW, Jeon SR. Meta-analysis on the effect of hypothermia in acute spinal cord injury. Neurospine. (2022);19:748–756. doi: 10.14245/ns.2244444.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shipley L, Mistry A, Sharkey D. Outcomes of neonatal hypoxic-ischaemic encephalopathy in centres with and without active therapeutic hypothermia:a nationwide propensity score-matched analysis. Arch Dis Child Fetal Neonatal Ed. (2022);107:6–12. doi: 10.1136/archdischild-2020-320966. [DOI] [PubMed] [Google Scholar]
- 68.Song K, Wang H, Kamm GB, Pohle J, Reis FC, Heppenstall P, Wende H, Siemens J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science. (2016);353:1393–1398. doi: 10.1126/science.aaf7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun X, Jones ZB, Chen XM, Zhou L, So KF, Ren Y. Multiple organ dysfunction and systemic inflammation after spinal cord injury:a complex relationship. J Neuroinflammation. (2016);13:260. doi: 10.1186/s12974-016-0736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi TM, Sunagawa GA, Soya S, Abe M, Sakurai K, Ishikawa K, Yanagisawa M, Hama H, Hasegawa E, Miyawaki A, Sakimura K, Takahashi M, Sakurai T. A discrete neuronal circuit induces a hibernation-like state in rodents. Nature. (2020);583:109–114. doi: 10.1038/s41586-020-2163-6. [DOI] [PubMed] [Google Scholar]
- 71.Tan DX, Manchester LC, Sainz RM, Mayo JC, León J, Reiter RJ. Physiological ischemia/reperfusion phenomena and their relation to endogenous melatonin production:a hypothesis. Endocrine. (2005);27:149–158. doi: 10.1385/endo:27:2:149. [DOI] [PubMed] [Google Scholar]
- 72.Tarahovsky YS, Fadeeva IS, Komelina NP, Khrenov MO, Zakharova NM. Antipsychotic inductors of brain hypothermia and torpor-like states:perspectives of application. Psychopharmacology (Berl) (2017);234:173–184. doi: 10.1007/s00213-016-4496-2. [DOI] [PubMed] [Google Scholar]
- 73.Thietje R, Kowald B, Böthig R, Schulz AP, Northmann M, Rau Y, Hirschfeld S. Long-term survival and causes of death in patients below the age of 60 with traumatic spinal cord injury in Germany. J Clin Med. (2021);11:26. doi: 10.3390/jcm11010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torossian F, Guerton B, Anginot A, Alexander KA, Desterke C, Soave S, Tseng HW, Arouche N, Boutin L, Kulina I, Salga M, Jose B, Pettit AR, Clay D, Rochet N, Vlachos E, Genet G, Debaud C, Denormandie P, Genet F, et al. Macrophage-derived oncostatin M contributes to human and mouse neurogenic heterotopic ossifications. JCI Insight. (2017);2:e96034. doi: 10.1172/jci.insight.96034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Marum RJ, Wegewijs MA, Loonen AJ, Beers E. Hypothermia following antipsychotic drug use. Eur J Clin Pharmacol. (2007);63:627–631. doi: 10.1007/s00228-007-0294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang HS, Han JS. Research progress on combat trauma treatment in cold regions. Mil Med Res. (2014);1:8. doi: 10.1186/2054-9369-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X, Li M, Yang Z, Li H, Wang Y, Tang W, Wu Y, Xiao P, Jiang S, Shi Q, Lu Y, Li H. Comparison of the protective effect of different mild therapeutic hypothermia temperatures on intestinal injury after cardiopulmonary resuscitation in rats. Shock. (2021);56:450–460. doi: 10.1097/SHK.0000000000001745. [DOI] [PubMed] [Google Scholar]
- 78.Wang XH, Jiang W, Zhang SY, Nie BB, Zheng Y, Yan F, Lei JF, Wang TL. Hypothermia selectively protects the anterior forebrain mesocircuit during global cerebral ischemia. Neural Regen Res. (2022);17:1512–1517. doi: 10.4103/1673-5374.330616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Z, Zhang J, Ma XF, Chang H, Peng X, Xu SH, Wang HP, Gao YF. A temporal examination of cytoplasmic Ca2+levels, sarcoplasmic reticulum Ca2+levels, and Ca2+-handling-related proteins in different skeletal muscles of hibernating daurian ground squirrels. Front Physiol. (2020);11:562080. doi: 10.3389/fphys.2020.562080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolf A, Lusczek ER, Beilman GJ. Hibernation-based approaches in the treatment of hemorrhagic shock. Shock. (2018);50:14–23. doi: 10.1097/SHK.0000000000001094. [DOI] [PubMed] [Google Scholar]
- 81.Xu X, Li N, Zhu L, Zhou Y, Cheng H. Beneficial effects of local profound hypothermia and the possible mechanism after experimental spinal cord injury in rats. J Spinal Cord Med. (2016);39:220–228. doi: 10.1179/2045772315Y.0000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamamoto S, Yamamoto M, Nakamura J, Mii A, Yamamoto S, Takahashi M, Kaneko K, Uchino E, Sato Y, Fukuma S, Imamura H, Matsuda M, Yanagita M. Spatiotemporal ATP dynamics during AKI predict renal prognosis. J Am Soc Nephrol. (2020);31:2855–2869. doi: 10.1681/ASN.2020050580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang L, Li RC, Xiang B, Li YC, Wang LP, Guo YB, Liang JH, Wang XT, Hou T, Xing X, Zhou ZQ, Ye H, Feng RQ, Lakatta EG, Chai Z, Wang SQ. Transcriptional regulation of intermolecular Ca2+signaling in hibernating ground squirrel cardiomyocytes:The myocardin-junctophilin axis. Proc Natl Acad Sci U S A. (2021);118:e2025333118. doi: 10.1073/pnas.2025333118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yin H, Chen Z, Zhao H, Huang H, Liu W. Noble gas and neuroprotection:From bench to bedside. Front Pharmacol. (2022);13:1028688. doi: 10.3389/fphar.2022.1028688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang J, Li X, Ismail F, Xu S, Wang Z, Peng X, Yang C, Chang H, Wang H, Gao Y. Priority strategy of intracellular Ca2+homeostasis in skeletal muscle fibers during the multiple stresses of hibernation. Cells. (2019);9:42. doi: 10.3390/cells9010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Z, Reis FMCV, He Y, Park JW, DiVittorio JR, Sivakumar N, van Veen JE, Maesta-Pereira S, Shum M, Nichols I, Massa MG, Anderson S, Paul K, Liesa M, Ajijola OA, Xu Y, Adhikari A, Correa SM. Estrogen-sensitive medial preoptic area neurons coordinate torpor in mice. Nat Commun. (2020);11:6378. doi: 10.1038/s41467-020-20050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Z, Shan L, Wang Y, Li W, Jiang M, Liang F, Feng S, Lu Z, Wang H, Dai J. Primate preoptic neurons drive hypothermia and cold defense. Innovation (Camb) (2022);4:100358. doi: 10.1016/j.xinn.2022.100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou F, Zhu X, Castellani RJ, Stimmelmayr R, Perry G, Smith MA, Drew KL. Hibernation, a model of neuroprotection. Am J Pathol. (2001);158:2145–2151. doi: 10.1016/S0002-9440(10)64686-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zonnenberg C, Bueno-de-Mesquita JM, Ramlal D, Blom JD. Hypothermia due to antipsychotic medication:a systematic review. Front Psychiatry. (2017);8:165. doi: 10.3389/fpsyt.2017.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]


