Abstract
To assess if calfactant reduces mortality among children with leukemia/lymphoma or after hematopoietic cell transplantation (HCT) with pediatric acute respiratory distress syndrome (PARDS), we conducted a multicenter, randomized, placebo-controlled, double-blinded trial in 17 pediatric intensive care units (PICUs) of tertiary care children’s hospitals. Patients ages 18 months to 25 years with leukemia/lymphoma or having undergone HCT who required invasive mechanical ventilation for bilateral lung disease with an oxygenation index (OI) > 10 and <37 were studied. Interventions used were intratracheal instillation of either calfactant or air placebo (1 or 2 doses). Forty-three subjects were enrolled between November 2010 and June 2015: 26 assigned to calfactant and 17 to placebo. There were no significant differences in the primary outcome, which was survival to PICU discharge (adjusted hazard ratio of mortality for calfactant versus placebo, 1.78; 95% confidence interval, .53 to 6.05; P = .35), OI, functional outcomes, or ventilator-free days, adjusting for risk strata and Pediatric Risk of Mortality (PRISM) score. Despite the risk-stratified randomization, more allogeneic HCT patients received calfactant (76% and 39%, respectively) due to low recruitment at various sites. This imbalance is important because independent of treatment arm and while adjusting for PRISM score, those with allogeneic HCT had a nonsignificant higher likelihood of death at PICU discharge (adjusted odds ratio, 3.02; 95% confidence interval, .76 to 12.06; P = .12). Overall, 86% of the patients who survived to PICU discharge also were successfully discharged from the hospital. These data do not support the use of calfactant among this high mortality group of pediatric leukemia/lymphoma and/or HCT patients with PARDS to increase survival. In spite of poor enrollment, allogeneic HCT patients with PARDS appeared to be characterized by higher mortality than even other high-risk immunosuppressed groups. Conducting research among these children is challenging but necessary, because survival to PICU discharge usually results in successful discharge to home.
Keywords: Pediatric acute respiratory distress syndrome, Acute lung injury, Surfactant, Hematopoietic stem cell transplantation, Leukemia/lymphoma
INTRODUCTION
The recently convened Pediatric Acute Lung Injury Consensus Conference [1,2], along with others [3–8], has highlighted differences in outcomes from pediatric acute respiratory distress syndrome (PARDS) in children with immunosuppressive diseases compared with those with a functional immune system. Historically, acute lung injury in these children has been associated with worse survival, particularly among the hematopoietic cell transplantation (HCT) population, with mortality rates of up to 60% [7–13]. Historically, these children have been excluded from interventional trials evaluating therapeutic interventions for respiratory failure [6]. However, given this unacceptably high mortality, the prevalence of respiratory failure among these children [7,14], their relatively large contribution to pediatric intensive care unit (PICU) admissions [12,13,15,16], and their significant decline in functional status after critical illness [17], prospective interventional research among this patient population is warranted.
Exogenous surfactant preparations have been successful in treating and preventing neonatal respiratory distress syndrome [18–20] and are now considered standard of care. However, efficacy in older children and adults has been variable [6,21–24]. A 21-center trial of intratracheal calfactant installation in children with PARDS demonstrated improved survival relative to placebo [6]. Moreover, a post-hoc analysis suggested a potential benefit of calfactant in immunocompromised children with acute respiratory failure characterized by an oxygenation index (OI) between 13 and 37 [25].
Encouraged by these results, a double-blinded, randomized, controlled, multicenter trial was designed to evaluate the efficacy of calfactant relative to placebo in reducing mortality in immunosuppressed children with leukemia/lymphoma or following HCT for any indication with PARDS.
METHODS
Patient Recruitment
Seventeen PICUs from the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network enrolled patients (see Appendix 1). The protocol was reviewed and approved by each institutional review board, and informed consent was obtained before randomization. The study was registered on ClinicalTrials.gov (NCT00999713).
Entry criteria included ages 18 months to 25 years, a diagnosis of leukemia/lymphoma or having undergone HCT for any indication, intubation and mechanical ventilation, radiographic evidence of bilateral lung disease, presence of an arterial catheter, and an OI > 10 and <37 (OI = FiO2 ⨯ mean airway pressure ⨯ 100 ÷ partial pressure of oxygen from an arterial blood gas) for 2 consecutive blood gases separated by at least 1 hour within 48 hours of endotracheal intubation (this was extended to 72 hours after 24 patients had been enrolled). The OI criteria [25] were intended to identify a patient population likely to experience a survival benefit by excluding those with minor lung injury expected to survive irrespective of therapy as well as those with such severe disease that survival was improbable. Exclusion criteria included chronic lung disease, status asthmaticus, brain death, uncorrected congenital heart disease, myocardial dysfunction, pulmonary edema from clinically evident cardiac failure (in the event that cardiac disease is suspected, this was excluded by echocardiography), severe neurologic injury, airway anomalies, or if care was electively limited. Additionally, potential subjects were excluded if they required the use of noninvasive continuous positive airway pressure or bilevel positive airway pressure ventilation for >168 hours (7 days) at any level or if they had received noninvasive ventilation for >72 hours with any of the following settings: FiO2 > .60, continuous positive airway pressure > 8 cm H2O, or intermittent positive airway pressure (IPAP) > 24 cm H2O.
Randomization was stratified by institution and the presence of an allogeneic HCT before study entry [10, 12, 15] in an effort to balance the risk of mortality between the 2 treatment groups (calfactant and placebo). Patients were recruited between November 2010 and June 2015.
Study Protocol
Subjects were randomized to receive either intratracheal installation of 2 doses of calfactant or air placebo. Initially, the protocol specified a calfactant concentration of 60 mg/mL of phospholipid in concert with the Calfactant in the Acute Respiratory Distress Syndrome (CARDS) study (ClinicalTrials.gov, NCT00682500) [26,27]. After 7 subjects had been enrolled and the CARDS trial completed, the calfactant concentration was changed back to the original concentration of 35 mg/mL phospholipid with US Food and Drug Administration (FDA) and institutional review board approvals [6]. The protocol mandated a second dose of calfactant be given 12 (±2) hours after the initial treatment only if the patient remained intubated and on mechanical ventilation with an OI > 10 or if the patient experienced at least a 25% decrease in OI. The volume of calfactant for a child weighing ≤ 10 kg was 3 mL/kg and 80 mL/m2 body surface area for children over 10 kg. Calfactant was initially administered in 2 equal aliquots but was changed to 4 aliquots as previously described [6], after a review of the CARDS trial data.
Each participating pharmacy was provided a randomization log. To maintain blinding the pharmacist delivered an opaque container to the PICU containing either the study drug or an air-filled syringe and handed it directly to the administrator of the intervention, a trained professional who was not involved in the daily care of the patient. The endotracheal tube was covered with opaque tape, and the intervention was completed behind a barrier, allowing physicians, investigators, and other healthcare personnel caring for the patient to remain blinded throughout the study.
In addition to the qualifying blood gases, arterial blood gases were obtained at baseline before intervention and at a minimum of 3 time points after the intervention (1, 4, and 12 hours). Investigators agreed to follow ventilator recommendations, which consisted of a low tidal volume/open lung strategy and permissive hypercapnia, with the goal of adequate oxygenation and ventilation. These recommendations were based on a previous PALISI network study of prone positioning [28].
Study Drug
Calfactant (provided by ONY Inc., Amherst, NY) is a modified natural calf lung surfactant approved by the FDA for neonatal respiratory distress syndrome. It is produced by saline lavage of calf lung and subsequent extraction of the phospholipids, neutral lipids, and hydrophobic apoproteins SP-B and SP-C.
Study Outcomes
The primary outcome was survival from PICU admission to PICU discharge. Secondary outcomes included changes in oxygenation after dosing, ventilator-free days (VFDs; the number of days alive and free of invasive mechanical ventilation at 28 days), proportion of patients requiring retreatment, and changes in Pediatric Outcome Performance Category (POPC) and Pediatric Cerebral Performance Category (PCPC) scores [29]. Given the established worse outcomes from respiratory failure among allogeneic HCT patients [10] and the ability of Pediatric Risk of Mortality (PRISM) scores to discern mortality [30], these covariates were controlled for when appropriate in analyses.
Adverse events around the time of drug or placebo administration were considered safety outcomes and included oxygen desaturation (<80% for >60 seconds), bronchospasm requiring bronchodilator therapy, air leak, hypotension, and/or bradycardia and any clinical change in the patient deemed significant or requiring additional therapy deemed related to the intervention. Serious adverse events, including death, were collected and reviewed by the independent Data Safety Monitoring Board.
Study Management
Sample size calculations were based on previous work [25] demonstrating that 33% of calfactant-treated patients died before PICU discharge as compared with 71% of placebo patients. Using selective OI entry criterion, stratifying based on the presence of allogeneic HCT, and accepting the 27% difference in mortality between the 2 treatment groups observed in the post-hoc analysis, it was estimated that 63 patients were required in each study arm to demonstrate an effect of calfactant on mortality with 85% power in this patient population at the .05 significance level. Therefore, a total sample size of 140 subjects (70 in each arm) was targeted based on an estimate that 10% of patients would withdraw or have missing data.
An interim analysis was planned after the first 70 patients were enrolled, with defined stopping rules for both efficacy and futility. Enrollment in the study was voluntarily halted for approximately 9 months after the CARDS study [26,27] was completed to determine the impact of the CARDS study data on the present protocol. After review of the CARDS study data, protocol modifications were proposed and approved by the FDA and institutional review boards before reopening the trial to subject accrual. These modifications involved reverting to the treatment protocol used in the original multicenter pediatric calfactant randomized controlled trial [6], which included using the less concentrated formulation of calfactant and administering the medication in 4 aliquots rather than 2. Because of poor enrollment after nearly 5 years of study, the interim analysis was conducted after the enrollment of only 43 patients. Based on that analysis the Data Safety Monitoring Board elected to terminate the study because of the poor enrollment and lack of evidence of efficacy.
Statistical Analysis
Cox proportional hazards regression was used to assess the differences between treatment groups for the primary clinical outcome of survival from PICU admission to PICU discharge while controlling for the risk strata and PRISM score measured at the time of PICU admission. A total of 20 patients died before discharge, 22 were censored (alive at the time of discharge), and 1 was excluded during analysis because of a missing PRISM score. The Cox proportional hazards assumption was assessed using the supremum test [31].
Binary logistic regression models, adjusting for risk strata and PRISM score at the time of PICU admission, were used to evaluate differences between groups for those requiring retreatment (eg, a second intervention after 12 hours) and those experiencing no change or experiencing improvement in their POPC/PCPC scores between PICU admission and hospital discharge. In evaluating the proportion requiring retreatment, 3 patients were excluded in assessment of this outcome, because 2 patients did not receive the first intervention (therefore making them ineligible to receive a second intervention) and 1 patient was missing a PRISM score. It is worth noting that all patients had the opportunity to be retreated, meaning that no deaths were experienced before the assessment for retreatment (within the 12-hour time window). The difference in POPC/PCPC scores was defined as the hospital discharge score minus the PICU admission score. Therefore, a difference of zero or less than zero was indicative of no change or an improvement, respectively.
A general linear model with correlated errors [32] was used to analyze the repeated measurements of OI over the 12 hours (ie, 0, 1, 4, and 12 hours) after the first and second interventions. A natural log transformation of the response was necessary to satisfy the normality assumption of the model; therefore, geometric means are reported. The interaction of the treatment group over time was assessed while controlling for risk strata.
Differences in the number of VFDs were analyzed using a zero-inflated Poisson model with a log link function due to a large number of zero VFDs. One patient was excluded because of a missing PRISM score. An unsuccessful extubation was defined as death before extubation or on ventilation for longer than 28 days. An extubation was only considered successful if the subject remained extubated for >48 hours. If a subject was not successfully extubated according to these criteria, they were classified as having zero VFDs. If a subject was successfully extubated, the number of VFDs was calculated as the difference between 28 days and the number of days the subject was on a ventilator. In 1 instance a subject was extubated and then reintubated. In this case the number of VFDs was calculated manually. The initiation point for ventilation was defined as the date and time of the preintervention qualifying OI. The stopping point was the date and time of extubation. All hypothesis tests were 2-sided, and a significance level of .05 was considered significant. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC), and figures were generated using R software (The R Foundation, Vienna, Austria).
RESULTS
Forty-three subjects were consented and randomized: 26 assigned to the calfactant group and 17 to the placebo group (Figure 1). Two subjects assigned to the calfactant group did not receive any treatment (1 died after randomization but before treatment secondary to pulmonary hemorrhage and 1 worsened and was withdrawn by the clinical team) but are included as per intention-to-treat planned analyses. Both groups met criteria for severe PARDS at study entry, with similar mean OI values between groups (mean for drug group, 21.9 [standard deviation, 12.2], versus mean for placebo group, 22.6 [standard deviation, 12.1]) (Table 1). Demographics, severity of illness, and cause of lung injury also appeared to be similarly distributed between the 2 treatment groups (Table 1). Of the 18 subjects who were stratified to the leukemia/lymphoma/autologous HCT group, 7 had undergone HCT.
Figure 1.

Patient enrollment and randomization. CONSORT flow diagram for the trial. ITT indicates intention to treat.
Table 1.
Demographics, Severity, and Diagnoses at Study Entry
| Calfactant Group (n = 26) | Placebo Group (n = 17) | |
|---|---|---|
| Mean age, yr (SD) | 12.1 (6.1) | 12.8 (5.4) |
| Mean weight, kg (SD) | 45.1 (23.3) | 50.1 (28.7) |
| Mean height, cm (SD) | 142.2 (33.6) | 146.2 (29.3) |
| Mean BSA, m2 (SD) | 1.3 (.5) | 1.4 (.5) |
| Sex | ||
| Male | 16 (61.5) | 12 (70.6) |
| Female | 10 (38.5) | 5 (29.4) |
| Race (if known) | ||
| American Indian/Alaska | 1 (4.4) | 0 (0) |
| Native | ||
| Black or African American | 5 (21.7) | 2 (12.5) |
| White | 16 (69.6) | 14 (87.5) |
| More than 1 race | 1 (4.4) | 0 (0) |
| Ethnicity (if known) | ||
| Hispanic or Latino | 7 (31.8) | 8 (50.0) |
| Not Hispanic or Latino | 15 (68.2) | 8 (50.0) |
| Severity of illness* | ||
| Mean PRISM score (SD) | 19.8 (7.5) | 16.1 (4.7) |
| Risk strata | ||
| Leukemia | ||
| Lymphoma | 7 (26.9) | 11 (64.7) |
| Autologous HCT | ||
| Allogeneic HCT | 19 (73.1) | 6 (35.3) |
| Mean OI (SD) | 21.9 (12.2) | 22.6 (12.1) |
| Mean PaO2 (SD) | 77.7 (35.0) | 75.1 (26.1) |
| Mean Ph (SD) | 7.34 (.09) | 7.31 (.11) |
| Mean PaCO2 (SD) | 51.4 (18.7) | 52.8 (11.8) |
| Mean oxygen saturation (SD) | 93.4 (5.3) | 94.4 (4.9) |
| POPC | ||
| Good | 7 (29.2) | 3 (18.8) |
| Mild disability | 6 (25.0) | 3 (18.8) |
| Moderate disability | 7 (29.2) | 7 (43.8) |
| Severe disability | 3 (12.5) | 3 (18.8) |
| Coma/vegetative state | 1 (4.2) | 0 (0) |
| PCPC | ||
| Normal | 15 (62.5) | 7 (43.8) |
| Mild disability | 5 (20.8) | 4 (25.0) |
| Moderate disability | 2 (8.3) | 2 (12.5) |
| Severe disability | 1 (4.2) | 3 (18.8) |
| Coma/vegetative state | 1 (4.2) | 0 (0) |
| Causes of PARDS | ||
| Pneumonia | 17 (65.4) | 12 (70.6) |
| Viral | 8 (30.8) | 2 (11.8) |
| Protozoa | 0 (0) | 0 (0) |
| Idiopathic syndrome | 0 (0) | 5 (29.4) |
| Fungal | 2 (7.7) | 2 (11.8) |
| Radiation | 1 (3.9) | 0 (0) |
| Bacterial | 6 (23.1) | 2 (11.8) |
| Aspiration | 0 (0) | 1 (5.9) |
| Pulmonary hemorrhage | 1 (3.9) | 4 (23.5) |
| Pulmonary graft-versus-host disease | 1 (3.9) | 1 (5.9) |
| BOOP | 0 (0) | 1 (5.9) |
| Other† | 12 (46.2) | 6 (35.3) |
Values are n (%) unless otherwise defined. SD indicates standard deviation; BSA, body surface area; BOOP, bronchiolitis obliterans organizing pneumonia.
Measures represent the preintervention arterial blood gas if available; otherwise, the arterial blood gas on PICU admission was used.
Other category includes sepsis, bacteremia, human herpesvirus-6 without pneumonia, multiorgan dysfunction syndrome, secondary to Ara-C related inflammatory response, and others.
After adjusting for risk strata and PRISM score, there was no difference in survival at the time of PICU discharge. Those who received calfactant were more likely (adjusted hazard ratio, 1.78; 95% confidence interval, .53 to 6.05; P = .35) (Table 2 and Figure 2) to experience death compared with the placebo group; however, this was not statistically significant. The median survival time for the calfactant group was 42 days, whereas the median survival time for the placebo group was 58 days (Figure 2).
Table 2.
Clinical Outcomes Comparing Differences between Those Subjects Treated with Calfactant and Those Who Received Placebo
| Clinical Outcomes | Effect Comparison (95% CI)* | P |
|---|---|---|
| Mortality (PICU discharge): HR† | 1.78 (.53–6.05) | .35 |
| First intervention (outcome = OI)‡ | ||
| Treatment × time interaction | — | .64 |
| Second intervention (outcome = OI)‡ | ||
| Treatment × time interaction | — | .52 |
| Deterioration in POPC score: OR† | .57 (.11–2.85) | .49 |
| Deterioration in PCPC score: OR† | .45 (.09–2.22) | .33 |
| Retreated (received the second intervention): OR† | .44 (.08–2.35) | .34 |
| Expected no. of VFDs: RR† | 1.11 (.88–1.41) | .37 |
| Unsuccessful extubation§: OR† | .58 (.12–2.77) | .50 |
HR indicates hazard ratio; OR, odds ratio; RR, rate ratio.
All effects compare the calfactant group with the placebo group.
Adjusted for risk strata + PRISM score.
Adjusted for risk strata.
Defined as free from mechanical ventilation for greater than 48 hours.
Figure 2.
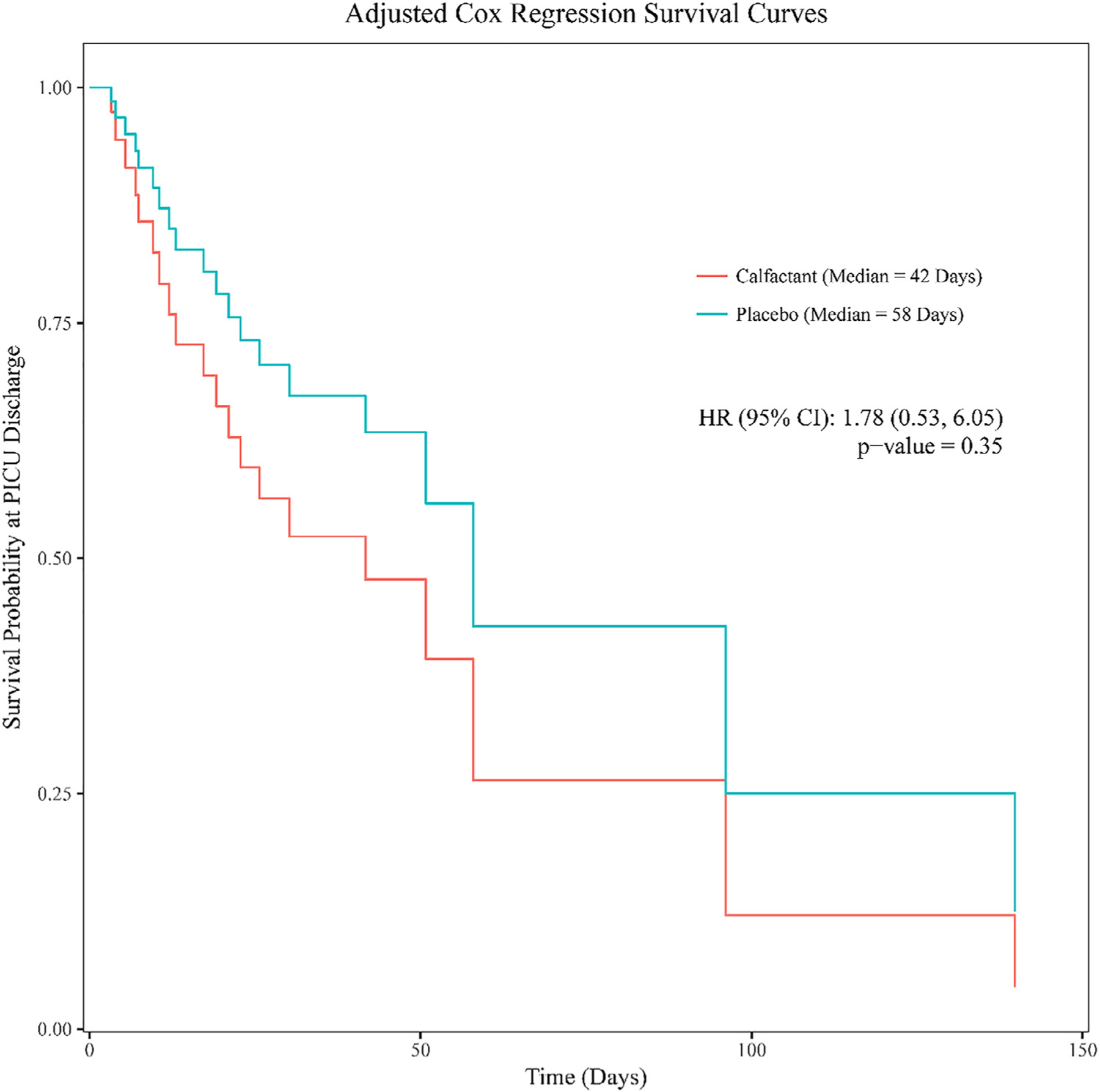
Adjusted Cox regression survival curves for the 2 treatment groups. The adjusted (risk strata and PRISM score) Cox regression survival curves for the 2 treatment groups from PICU admission to PICU discharge. There was no significant difference in survival between the 2 groups.
Despite stratifying based on risk strata, the unexpectedly small number of subjects recruited at each study site and the size of the randomization blocks resulted in a larger proportion of the 25 allogeneic HCT patients receiving calfactant versus placebo (76% and 39%, respectively) (Table 1). Allogeneic HCT patients tended to be characterized by higher mortality irrespective of treatment allocation. Fifteen the 25 (60%) allogeneic HCT patients died before PICU discharge as compared with only 6 of 18 (33%) of the leukemia/lymphoma and autologous HCT patients (x2 test P = .08). Even after adjusting for PRISM score, allogeneic HCT patients were 3 times as likely (adjusted odds ratio, 3.02; 95% confidence interval, .76 to 12.06; P = .12) to die before PICU discharge compared with the nontransplant and autologous HCT patients. Although this difference was not statistically significant, allogeneic HCT patients were characterized by a 5-fold increase in their odds of death at the time of hospital discharge, adjusting for PRISM score, as compared with the nontransplant and autologous HCT patients (adjusted odds ratio, 5.0; 95% confidence interval, 1.3 to 19.6; P = .02).
After adjusting for risk strata there was no significant difference in oxygenation as measured by the OI between the calfactant and placebo groups in the 12 hours after the intervention (Figure 3). Additionally, other secondary clinical outcomes, including change in POPC/PCPC, VFDs, and need for retreatment at 12 hours, were not different between calfactant and placebo subjects (Table 2). A total of 22 patients survived until PICU discharge, of which 19 (86%) were successfully discharged from the hospital. Ten of 25 (40%) allogeneic HCT patients survived to PICU discharge; 7 of those 10 survived to hospital discharge.
Figure 3.
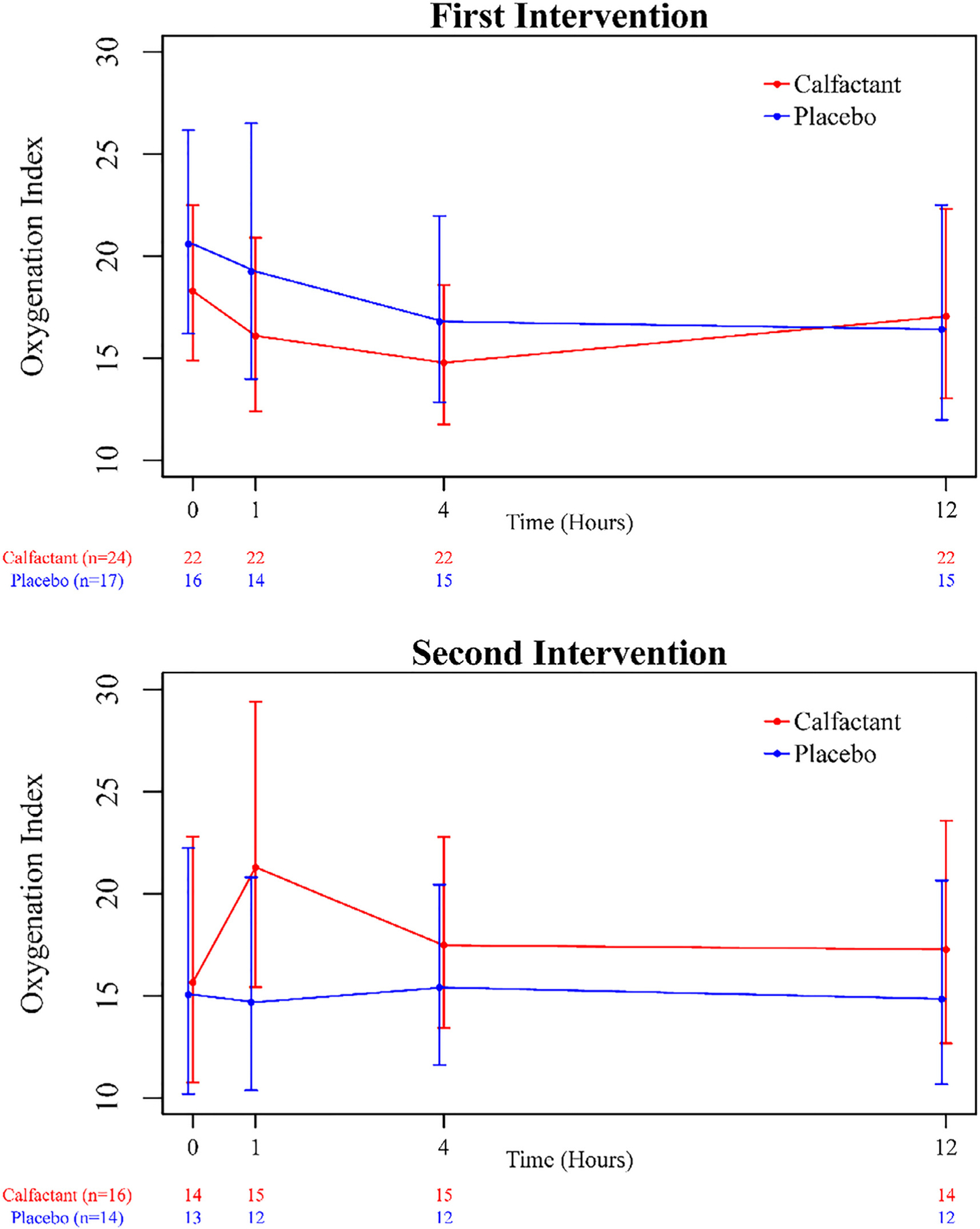
Comparison of the OI at assessed time points for the 2 treatment groups. The OI was measured over a 12-hour time period immediately after study intervention for both treatment groups, adjusting for risk strata. Points plotted represent geometric means and corresponding 95% confidence intervals at each data collection time point. There were no significant differences between the treatment groups with respect to the OI over time.
Nine peridosing events were reported: 3 episodes of hypotension (all calfactant), 5 episodes of desaturation (4 calfactant and 1 placebo), and 1 pneumomediastinum (calfactant). Twenty-five serious adverse events reported and reviewed by the Data Safety Monitoring Board. Only 2 were believed to be related to the study, and both occurred during dosing in the calfactant group. One was a severe episode of desaturation without bradycardia, and 1 was a severe hypotensive event requiring a fluid bolus and a single dose of epinephrine.
Three protocol violations were identified. One patient was older than the allowable age (calfactant), 1 patient had an initial OI higher than allowed (placebo), and 1 patient mistakenly did not receive a second intervention despite qualifying (calfactant).
DISCUSSION
Despite the limited enrollment, useful information on the care of this high-risk patient population can be gleaned from this study. First, in the context of the present study with the stated limitations of imbalanced enrollment, small sample size, and others, these data do not support a survival advantage with the use of intratracheal instillation of calfactant in this population of children. The overall comparison of mortality among treatment groups did not provide sufficient evidence in support of a benefit with calfactant therapy. The mortality rate of 74% at hospital discharge among the 19 calfactant-treated allogeneic HCT patients is quite comparable with the results of other contemporaneous studies of pediatric HCT patients with PARDS [9,11,33,34].
Second, allogeneic HCT patients with PARDS appear to have significantly worse outcomes compared with other immunosuppressed populations including leukemia/lymphoma and autologous HCT patients. These findings are consistent with previously published results [10,12,15]. Possible reasons include intensive conditioning regimens, baseline organ dysfunction, more aggressive malignant disease, and, perhaps most notably, a dysregulated (rather than simply suppressed) immune system. Although the data collection was limited and we do not have access to data related to the time from HCT to the onset of PARDS, this is an issue that is likely important in the design of future research. Regardless of the cause, this finding is most relevant to the design of future studies of PARDS enrolling children with cancer and/or who have undergone HCT.
Third, the study demonstrates that most children who survive their acute episode of PARDS survive to hospital discharge. In this study 22 patients survived until PICU discharge, of which 19 (86%) were successfully discharged from the hospital. With respect to the 25 allogeneic HCT patients, 10 (40%) survived until PICU discharge, 7 of which also survived to hospital discharge. These findings add to the growing body of literature suggesting that recovery from PARDS is likely to result in longer term survival in HCT patients [10,11,33,35]. Such data support aggressive treatment of PARDS in these children and affirm the need for further research to improve their acute outcomes. Moreover, longer term functional outcomes should be considered in any study of PARDS in immune-compromised children.
Fourth, this study illustrates the difficulty of performing effective research in this population. Given the dismal prognosis of PARDS in these children, effective study is needed to advance understanding and improve outcomes. However, conducting randomized controlled trials has proven to be challenging in pediatric critical care in general [36] and particularly difficult in the immunosuppressed population. A funded study of hemofiltration in pediatric HCT patients with PARDS was closed after enrolling only 16 subjects, despite 35 participating sites over a 2-year period (J.V. DiCarlo, personal communication University of Southern California, 2017). A study of etanercept in adult HCT patients with idiopathic pneumonia syndrome was terminated for poor accrual after enrolling only 34 of the targeted 120 subjects [35,37]. Similarly, a pediatric trial of etanercept in idiopathic pneumonia syndrome in children undergoing stem cell transplantation enrolled only 39 subjects over a 5-year period despite 22 participating sites [37]. Although that study was stopped prematurely when it satisfied an established stopping rule for efficacy, the authors concluded that they were unlikely to ever be able to perform the definitive phase III trial. However, etanercept is now used routinely in children undergoing stem cell transplantation with idiopathic pneumonia syndrome.
The challenges to research in this field are clear. First, the overall patient population is relatively small. The Center for International Blood and Marrow Transplant Research reported that between 2008 and 2014, 4408 children aged ≤ 18 years underwent a first allogeneic HCT and an additional 3076 received a first autologous transplant [38]. Accepting a 25% incidence of PARDS among pediatric HCT patients [7], there are less than 2000 available patients to be studied over a comparable time period. Consequently, identifying a sufficient number of patients for study requires a large number of centers, each of which is only likely to enroll a handful of patients. This situation contributed substantially to the unequal patient distribution in the current trial, which may have influenced the results obtained. For future multicenter designed studies looking to recruit this patient population (or other cohorts with rare diseases/known recruitment difficulty), we suggest randomizing with a fixed block size equal to the number of treatments and using as few strata as possible to account for primary confounders. Later, possible confounders (such as study site) can be accounted for by incorporating them as random effects in applicable models. This is a particularly ideal approach when there are a large number of sites with little recruitment. An additional approach, based on the fact that an open-label trial of etanercept [37] completed enrollment sooner than expected, whereas a similar randomized controlled trial in adults [35] took longer to complete, would be to use alternative methodology other than randomized placebo-controlled trials to attempt to overcome the issues related to randomization. With the lessons learned from this trial and with the recommendations above, it is feasible that this patient population can undergo further study. Moreover, the patient population is extremely heterogeneous, varying by etiology of the lung disease and the underlying diagnosis and other transplant-related factors, including the type of transplant, conditioning regimen, source of the transplant, and presence of graft-versus-host disease. Therefore, identifying a well-defined patient population of adequate size for studies of this condition is most difficult.
In addition to the limited patient pool, it is a challenge to secure mutual support and true equipoise from 2 discordant clinical services. Many of these patients will have been previously enrolled in a clinical trial led by the transplant service, and subsequent enrollment in another trial can complicate analysis of the initial study. In the current trial contemporaneous enrollment accounted for 25% of the patients who were eligible but not approached for enrollment. Collaboration between pediatric intensivists and oncologists in the early stages of future trial design has the potential to mitigate this concern, and the HSCT subgroup of the PALISI Network has taken this approach in recent protocol design.
Additionally, participating in research during a time of critical illness may be difficult for the parents of these children. They must agree to participate in a trial after being informed that there is a medication that may help their critically ill child, although its true efficacy remains to be determined; the medication appears to be relatively well tolerated, although the potential for adverse effect is not completely established and will be assessed as part of the trial; and there is no guarantee of who will receive the study medication. Although not formally studied, there were reports of parent and physician refusal to participate because of both reasons: They did not want to risk the chance of being randomized to placebo and wished to receive calfactant as an off-label clinical therapy, or they did not wish to undertake potential risks of calfactant without proven benefit. This issue was markedly similar to the study of hemofiltration in pediatric HCT patients referenced above (JV DiCarlo, personal communication University of Southern California, 2017). Furthermore, all this information is couched within the context that the primary purpose of the study is not to help their child (although that is a possibility of participation) but rather to best inform the use of this medication for future children. Impressively, despite this most difficult of situations, 60% of families that were approached consented to participate in the study.
In conclusion, this trial does not provide sufficient evidence in support of an overall survival benefit for calfactant in this population of immune-compromised children with PARDS. The results demonstrate that allogeneic HCT patients with lung injury tend to be a unique group characterized by higher mortality than other high-risk immunosuppressed groups. The trial not only highlights many of the difficulties associated with conducting research among these children but also reaffirms the need for such study, because short-term survival appears to correlate strongly with longer term survival, as most children discharged from the PICU are successfully discharged home.
ACKNOWLEDGMENTS
The CALIPSO study investigators thank all the staff members of the PICUs who participated in this study. The authors also thank the tireless volunteer work of our Data Safety and Monitoring Board members from Duke University: Raymond Barfield, MD, PhD, Ira M. Cheifetz, MD, Paul Martin, MD, PhD, and P. Brian Smith, MD. Additionally, this work could not be completed without the support of the Office of Orphan Products Development of the FDA and ONY, Inc. Finally, the authors thank Douglas Willson, MD, for his thoughtful review and critique of this manuscript.
Financial disclosure:
This project was funded by the FDA Office of Orphan Products Development Grant no. 5R01FD003410. The calfactant used in the study was provided by ONY Inc., Amherst, NY. The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR002014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding was received from Pennsylvania State University and Penn State/Hershey Medical Center.
Conflict of interest statement:
N.J.T. received funding from Therabron and CareFusion. S.P. received funding from the Methodist Hospital. S.J.G. received funding from Hackensack University Medical Center and Saint Barnabas Medical Center. R.F.T. received funding from Springer Publishing.
APPENDIX 1. CALIPSO STUDY INVESTIGATORS
Children’s Hospital Los Angeles (B. Markovitz, R. Morzov, K. Waters); Children’s Hospital & Medical Center, Omaha (E. Truemper, M. Dawson); Children’s Hospital of Philadelphia (J. Fitzgerald, J. Bush); Children’s Hospital of Pittsburgh of UPMC (S. Venkataraman); Children’s Hospital of Wisconsin-Medical College of Wisconsin, Department of Pediatrics, Division of Critical Care Medicine (J. McArthur, K. Woods); Division of Critical Care Medicine, St. Jude Children’s Research Hospital (R. Morrison, A. Norris); Helen DeVos Children’s Hospital/Spectrum Health (S. Rajasekaran, M. Duba); Indiana University School of Medicine/Riley Hospital for Children (C. Rowan, C. Rider); Hackensack University Medical Center, Joseph M. Sanzari Children’s Hospital (S. Gertz, J. Haugh); Maria Fareri Children’s Hospital, New York Medical College (A. Singh, S. Li, N. Ansari); Nationwide Children’s Hospital (M. Chase, T. Karsies); Penn State Hershey Children’s Hospital (N. Thomas, R. Tamburro, D. Spear); Phoenix Children’s Hospital (D. Tellez, A. LaBell, C. Dillon); Sainte-Justine Hospital, University of Montreal, Canada (P. Jouvet, M. Dumitrascu); Texas Children’s Hospital/Baylor College of Medicine (L. Loftis, N. Jaimon); UH Rainbow Babies and Children’s Hospital (R. Speicher, S. Bergant); Weill Cornell Medicine (S. Pon, C. Carlo).
REFERENCES
- 1.Jouvet P, Thomas NJ, Willson DF, et al. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khemani RG, Smith LS, Zimmerman JJ, et al. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015;16(5 Suppl 1):S23–40. [DOI] [PubMed] [Google Scholar]
- 3.Erickson S, Schibler A, Numa A, et al. Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8:317–323. [DOI] [PubMed] [Google Scholar]
- 4.Bindl L, Buderus S, Dahlem P, et al. Gender-based differences in children with sepsis and ARDS: the ESPNIC ARDS Database Group. Intensive Care Med. 2003;29:1770–1773. [DOI] [PubMed] [Google Scholar]
- 5.Hu X, Qian S, Xu F, et al. Incidence, management and mortality of acute hypoxemic respiratory failure and acute respiratory distress syndrome from a prospective study of Chinese paediatric intensive care network. Acta Paediatr 2010;99:715–721. [DOI] [PubMed] [Google Scholar]
- 6.Willson DF, Thomas NJ, Markovitz BP, et al. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293:470–476. [DOI] [PubMed] [Google Scholar]
- 7.Rowan CM, Nitu ME, Moser EAS, et al. Weight gain and supplemental O2: risk factors during the hematopoietic cell transplant admission in pediatric patients. Pediatr Blood Cancer. 2017;64. https://www.ncbi.nlm.nih.gov/pubmed/28439949. [DOI] [PubMed] [Google Scholar]
- 8.Spicer AC, Calfee CS, Zinter MS, et al. A simple and robust bedside model for mortality risk in pediatric patients with acute respiratory distress syndrome. Pediatr Crit Care Med. 2016;17:907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Gestel JP, Bollen CW, Bierings MB, et al. Survival in a recent cohort of mechanically ventilated pediatric allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2008;14: 1385–1393. [DOI] [PubMed] [Google Scholar]
- 10.Tamburro RF, Barfield RC, Shaffer ML, et al. Changes in outcomes (1996–2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care Med. 2008;9:270–277. [DOI] [PubMed] [Google Scholar]
- 11.Rowan CM, Gertz SJ, McArthur J, et al. Invasive mechanical ventilation and mortality in pediatric hematopoietic stem cell transplantation: a multicenter study. Pediatr Crit Care Med. 2016;17:294–302. [DOI] [PubMed] [Google Scholar]
- 12.Zinter MS, DuBois SG, Spicer A, et al. Pediatric cancer type predicts infection rate, need for critical care intervention, and mortality in the pediatric intensive care unit. Intensive Care Med. 2014;40:1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balit CR, Horan R, Dorofaeff T, et al. Pediatric hematopoietic stem cell transplant and intensive care: have things changed? Pediatr Crit Care Med. 2016;17:e109–e116. [DOI] [PubMed] [Google Scholar]
- 14.Kaya Z, Weiner DJ, Yilmaz D, et al. Lung function, pulmonary complications, and mortality after allogeneic blood and marrow transplantation in children. Biol Blood Marrow Transplant. 2009;15:817–826. [DOI] [PubMed] [Google Scholar]
- 15.Zinter MS, Dvorak CC, Spicer A, et al. New insights into multicenter PICU mortality among pediatric hematopoietic stem cell transplant patients. Crit Care Med. 2015;43:1986–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chima RS, Daniels RC, Kim MO, et al. Improved outcomes for stem cell transplant recipients requiring pediatric intensive care. Pediatr Crit Care Med. 2012;13:E336–E342. [DOI] [PubMed] [Google Scholar]
- 17.Zinter MS, Holubkov R, Steurer MA, et al. Pediatric hematopoietic cell transplant patients who survive critical illness frequently have significant but recoverable decline in functional status. Biol Blood Marrow Transplant 2018;24:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiwara T, Maeta H, Chida S, et al. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980;1:55–59. [DOI] [PubMed] [Google Scholar]
- 19.Kendig JW, Notter RH, Cox C, et al. A comparison of surfactant as immediate prophylaxis and as rescue therapy in newborns of less than 30 weeks’ gestation. N Engl J Med. 1991;324:865–871. [DOI] [PubMed] [Google Scholar]
- 20.Seger N, Soll R. Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database Syst Rev 2009;(2):C; D007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tibby SM, Hatherill M, Wright SM, et al. Exogenous surfactant supplementation in infants with respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1251–1256. [DOI] [PubMed] [Google Scholar]
- 22.Willson DF, Zaritsky A, Bauman LA, et al. Instillation of calf lung surfactant extract (calfactant) is beneficial in pediatric acute hypoxemic respiratory failure. Members of the Mid-Atlantic Pediatric Critical Care Network. Crit Care Med. 1999;27:188–195. [DOI] [PubMed] [Google Scholar]
- 23.Luchetti M, Ferrero F, Gallini C, et al. Multicenter, randomized, controlled study of porcine surfactant in severe respiratory syncytial virus-induced respiratory failure. Pediatr Crit Care Med. 2002;3:261–268. [DOI] [PubMed] [Google Scholar]
- 24.Luchetti M, Casiraghi G, Valsecchi R, et al. Porcine-derived surfactant treatment of severe bronchiolitis. Acta Anaesthesiol Scand. 1998;42:805–810. [DOI] [PubMed] [Google Scholar]
- 25.Tamburro RF, Thomas NJ, Pon S, et al. Post hoc analysis of calfactant use in immunocompromised children with acute lung injury: impact and feasibility of further clinical trials. Pediatr Crit Care Med. 2008;9:459–464. [DOI] [PubMed] [Google Scholar]
- 26.Willson DF, Truwit JD, Conaway MR, et al. The Adult Calfactant in Acute Respiratory Distress Syndrome trial. Chest 2015;148:356–364. [DOI] [PubMed] [Google Scholar]
- 27.Willson DF, Thomas NJ, Tamburro R, et al. Pediatric Calfactant In Acute Respiratory Distress Syndrome trial. Pediatr Crit Care Med 2013;14:657–665. [DOI] [PubMed] [Google Scholar]
- 28.Curley MA, Arnold JH, Thompson JE, et al. Clinical trial design—effect of prone positioning on clinical outcomes in infants and children with acute respiratory distress syndrome. J Crit Care. 2006;21:23–32. discussion 32–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiser DH, Tilford JM, Roberson PK. Relationship of illness severity and length of stay to functional outcomes in the pediatric intensive care unit: a multi-institutional study. Crit Care Med. 2000;28:1173–1179. [DOI] [PubMed] [Google Scholar]
- 30.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. [DOI] [PubMed] [Google Scholar]
- 31.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 32.Diggle PJ HP, Liang K, Zeger SL. Analysis of Longitudinal Data. Oxford, UK: Oxford University Press; 2002. [Google Scholar]
- 33.Duncan CN, Lehmann LE, Cheifetz IM, et al. Clinical outcomes of children receiving intensive cardiopulmonary support during hematopoietic stem cell transplant. Pediatr Crit Care Med. 2013;14:261–267. [DOI] [PubMed] [Google Scholar]
- 34.Lindell RB, Gertz SJ, Rowan CM, et al. High levels of morbidity and mortality among pediatric hematopoietic cell transplant recipients with severe sepsis: insights from the Sepsis PRevalence, OUtcomes, and Therapies International Point Prevalence study. Pediatr Crit Care Med 2017;18:1114–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanik GA, Horowitz MM, Weisdorf DJ, et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: Enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: Blood and Marrow Transplant Clinical Trials Network Protocol. Biol Blood Marrow Transplant. 2014;20:858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duffett M, Choong K, Hartling L, et al. Randomized controlled trials in pediatric critical care: a scoping review. Crit Care. 2013;17:R256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanik GA, Grupp SA, Pulsipher MA, et al. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A Joint Pediatric Blood and Marrow Transplant Consortium and Children’s Oncology Group Study (ASCT0521). Biol Blood Marrow Transplant. 2015;21:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khandelwal P, Millard HR, Thiel E, et al. Hematopoietic stem cell transplantation activity in pediatric cancer between 2008 and 2014 in the United States: a Center for International Blood and Marrow Transplant Research report. Biol Blood Marrow Transplant. 2017;23:1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]


