Abstract
Microsporidia are ancient, intracellular, eukaryotic protozoan parasites that form spores and that lack mitochondria. Currently, as many as eight species included under six genera are known to infect humans, mostly patients with AIDS. Among these, Enterocytozoon bieneusi, the agent of gastrointestinal (GI) disease, is the most frequently identified microsporidian in clinical laboratories in the United States. Encephalitozoon (Septata) intestinalis, the agent that causes a disseminated infection including infection of the GI tract, is the second most frequently identified microsporidian parasite. In spite of this, not many isolates of E. intestinalis have been established in culture. We describe here the continuous cultivation of eight isolates of E. intestinalis obtained from different samples including the urine, sputum, and duodenal aspirate or biopsy specimens from five AIDS patients originating from California, Colorado, and Georgia. The specific identification was made on the bases of ultrastructural, antigenic, and PCR analyses.
Microsporidia are ancient, intracellular, spore-forming, mitochondrion-lacking eukaryotic protozoan parasites (3). During the past decade, several genera (e.g., Encephalitozoon, Enterocytozoon, Nosema, Pleistophora, Septata, Trachipleistophora, and Vittaforma) of microsporidia have been identified as opportunistic pathogens of humans, especially patients with AIDS (25, 33). Although Enterocytozoon bieneusi is the most frequently identified microsporidium in patients with AIDS, infections due to Septata and Encephalitozoon are also being recognized frequently (3, 25, 33). E. bieneusi causes a gastrointestinal (GI) disease leading to diarrhea, whereas Encephalitozoon spp. (e.g., Encephalitozoon cuniculi and Encephalitozoon hellem) cause a disseminated microsporidiosis that does not involve the GI tract. In 1992, Orenstein et al. (22) described in AIDS patients with diarrhea an Encephalitozoon-like microsporidium that not only colonizes the intestinal epithelium but that also infects the gallbladder and parenchymal cells of the liver and bronchial epithelium. Cali et al. (2) established a new genus and a new species, Septata intestinalis, for this microsporidian parasite. However, on the basis of its antigenic and molecular relatedness to Encephalitozoon spp. it has been reclassified as Encephalitozoon intestinalis (13, 15). According to Schwartz and Bryan (25), E. intestinalis is the second most frequently identified microsporidial pathogen that causes a disseminated microsporidiosis, including infection of the GI tract. Reports of the isolation of E. intestinalis from different specimens, including urine, nasal mucosa, sputum, bronchoalveolar lavage fluid, and feces, have appeared in the recent literature (13, 14, 21, 28, 30). Several isolates of E. intestinalis have been cultured in vitro on mammalian cell lines, including monkey kidney (E6), human lung fibroblast (HLF), Madin-Darby canine kidney (MDCK), and rabbit kidney (RK 13) cells, as well as the intestinal cell lines HT-29, Caco-2, and I 047 (13). We report here on the continuous cultivation and the ultrastructural, immunofluorescence, Western blot, and PCR analyses of eight isolates of E. intestinalis. These isolates were established in culture from several different specimens, including urine, duodenal aspirate or biopsy, and sputum specimens, obtained from AIDS patients from different geographic locales within the United States and established on E6 and HLF cell lines.
MATERIALS AND METHODS
Patient specimens.
Urine, sputum, duodenal aspirate or biopsy, and serum specimens were obtained from five patients from California, Colorado, and Georgia, as indicated in Table 1. A fecal sample was also obtained from one patient (Table 1). Smears were made from all of these samples (except the serum) as well as the fecal sample that we had obtained previously from a patient infected with this parasite (30) and were stained with chromotrope by the procedure of Weber et al. (34) and with the quick-hot Gram-chromotrope by the procedure of Moura et al. (20).
TABLE 1.
Patient data, specimens obtained, and isolates established in culture
| Patient no.a | Age (yr) | Location | Samples obtained | Isolate name |
|---|---|---|---|---|
| 1 (controlb) | 26 | California | Urine, feces, saliva, nasal mucosa, serum | CDC:V297 |
| 2 | 32 | Georgia | Duodenal aspirate and biopsy specimen | CDC:V307 |
| 3 | 34 | Colorado | Urine, sputum, serum | CDC:V308c and CDC:V309d |
| 4 | 30 | California | Urine, sputum, serum | CDC:V314c and CDC:V315d |
| 5 | 25 | Colorado | Urine, sputum, serum | CDC:V324c and CDC:V325d |
All patients were males.
Data for this patient are from a previous report (30).
Isolated from urine.
Isolated from sputum.
The urine, sputum, and duodenal aspirate or biopsy specimens were also processed for culture as described previously (29, 30), with slight modifications. Briefly, urine samples were washed once with Hanks’ balanced salt solution (HBSS) and were inoculated into cell cultures. Sputum samples were treated with sputolysin and were processed as described previously (30). A duodenal aspirate was inoculated directly into cell culture, whereas the biopsy specimen was broken into small pieces by trituration, inoculated into cell cultures, and incubated at 37°C as described previously (31). After 24 h the culture medium from each flask was decanted into a centrifuge tube and mixed with an equal volume of HBSS, and the mixture was centrifuged at 2,000 × g at 4°C. The supernatants were aspirated and the sediment was inoculated back into the respective culture flasks containing fresh growth medium. Thereafter, the medium from each flask was removed twice a week for 4 weeks and centrifuged, and the sediments containing spores were inoculated into the respective flasks as described above to facilitate the rapid infection of the monolayers. The resultant cultures were designated CDC:V308, CDC:V309, CDC:V314, CDC:V315, CDC:V324, CDC:V325, and CDC:V307. Reference strains E. hellem CDC:0291:V013 (29, 32), E. cuniculi CDC:V282 (7), and E. intestinalis CDC:V297 (30) were also cultured as described previously.
Parasite harvest and purification.
The spores from each of the test isolates and the reference strains released into the culture supernatant were harvested separately by pooling the culture medium from several flasks and centrifuging the pooled medium at 2,000 × g for 20 min at 4°C. After two washes with phosphate-buffered saline (PBS), the remaining cells were disrupted by washing with PBS containing 0.3% Tween 20. An additional washing with PBS was done, and the spores were then purified by centrifugation with a Percoll gradient at 500 × g for 20 min at 4°C (29). The spores were collected from the pellet, washed twice with PBS, and stored at −20°C until use.
Electron microscopy.
Scanning electron microscopy was performed with a JEOL JSM 820 scanning electron microscope as described previously (29, 30). For transmission and scanning electron microscopy, E6 and HLF monolayers infected with microsporidia were treated with trypsin-EDTA as described elsewhere (30, 31), and the detached cell layers were washed with HBSS. After fixing in 2.5% glutaraldehyde buffered with cacodylate, the cells were postfixed in a 1% OsO4 solution, dehydrated in ethanol, embedded in Epon 812, and processed as described previously (29–32).
IIF test.
Spores of E. cuniculi, E. hellem, and E. intestinalis as well as spores of the seven test isolates were harvested from the culture supernatants and washed three times in HBSS before counting in a hemocytometer. They were then suspended in HBSS containing 1% Formalin to obtain 107 spores per ml and were then processed for the indirect immunofluorescence (IIF) test as described previously (30). The IIF test was also performed with smears of the patient’s fecal sample as well as with control fecal smears known to contain E. intestinalis spores. Polyclonal antibodies against E. hellem, E. cuniculi, and E. intestinalis made in rabbits were used in the IIF test as described previously (29, 30, 32).
SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting.
Proteins were extracted from purified spores of the three reference strains and of the test isolates, as well as from E6 cells, by suspending them in a sample buffer containing 2.5% sodium dodecyl sulfate (SDS) and 2.25 M urea and heating the mixture at 65°C for 15 min. Proteins extracted from approximately 108 spores or 1 μg of E6 cells were loaded onto each lane of gels with a 3% stacking gel and a 3 to 25% linear resolving gel, and the gels were subjected to electrophoresis. A constant current of 7 mA per gel was applied for 1 h, and subsequently, the current was increased to 12.5 mA per gel, with an upper voltage limit of 535 for 3.5 h. A discontinuous buffer system was used; the lower-chamber buffer and the resolving gel buffer solution contained 81.2 mM Tris–23 mM boric acid–1.35 mM EDTA (pH 8.9). The buffer solution in the upper chamber reservoir contained 41 mM Tris, 40 mM boric acid, and 0.1% (wt/vol) SDS. The stacking gel buffer solution contained 54 mM Tris. The separated proteins were either stained with silver (26) or electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, Mass.) as described previously (27). The protein contents, loaded onto gels that were subsequently used for the transfer of proteins to PVDF membranes, were increased to nearly three times that used for gels that were stained with silver. The membranes were then reacted with either a 1:500 dilution of rabbit anti-E. hellem or a 1:400 dilution of rabbit anti-E. intestinalis or anti-E. cuniculi serum or patient’s serum. The membranes were washed and then reacted with a 1:6,000 dilution of peroxidase-conjugated goat anti-rabbit immunoglobulin G (Cappel Laboratories, Westchester, Pa.) or a 1:1,000 dilution of peroxidase-conjugated goat anti-human immunoglobulin G. Hydrogen peroxide (3%) and diaminobenzidene (0.005%) were used as the substrate and the chromogen, respectively.
DNA extraction and PCR.
DNA was extracted from (i) a control (uninfected) E6 cell culture, (ii) E6 cell cultures infected with the different test isolates described earlier, (iii) an E6 cell culture infected with E. hellem CDC:0291:V213, (iv) an E6 cell culture infected with E. cuniculi CDC:V282, and (v) an E6 cell culture infected with E. intestinalis CDC:V297, as described previously (30–32). Nucleic acid from each sample was resuspended in 50 μl of distilled water and was amplified by PCR.
PCR.
PCR was performed by using three different diagnostic primer pairs, as follows: (i) primer pairs SINTF-SINTR, in which the forward diagnostic primer was SINTF1 (5′-TTTCGAGTGTAAAGGAGTCGA-3′), which was designed on the basis of the sequence from positions 362 to 382, and the reverse diagnostic primer was SINTR (5′-CCGTCCTCGTTCTCCTGCCCG-3′), which was based on the sequence of the nucleotides from positions 861 to 881 of the E. intestinalis small-subunit (SSU) rRNA sequence that amplifies an E. intestinalis-specific fragment of 520 bp encoding a region for E. intestinalis SSU rRNA, as described previously (6); (ii) primer pair EHEL-F–EHEL-R, which amplifies an E. hellem-specific fragment of 546 bp encoding a region for E. hellem SSU rRNA (32); and (iii) primer pair ECUN-F–ECUN-R, which amplifies a fragment of 549 bp encoding a region for E. cuniculi SSU rRNA (32). PCR was performed with the GenAmp kit (Perkin-Elmer Cetus, Norwalk, Conn.) according to the manufacturer’s instructions. A total of 35 PCR cycles were performed with denaturation, annealing, and elongation temperatures of 94°C, 55°C, and 72°C, respectively. The products of the amplification were resolved on a 2% agarose gel (SeaKem GTG; FMC BioProducts, Rockland, Maine) and were visualized for analysis by staining with ethidium bromide (6).
RESULTS
Clinical specimens.
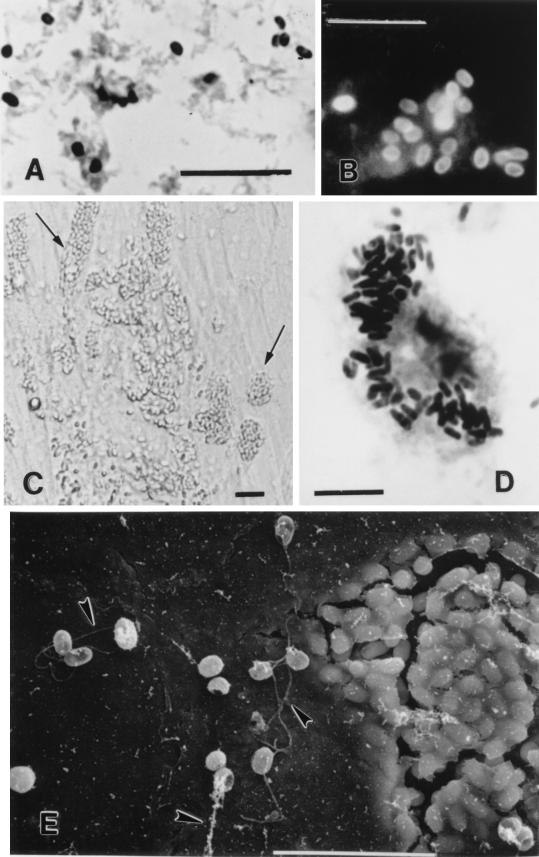
Chromotrope- and quick-hot Gram-chromotrope-stained smears of fecal, urine, and sputum samples from the patients being studied showed spores with the morphological features characteristic of microsporidia such as a vacuole, belt-like stripe, and gram-positive granules (20). Abundant spores were seen in all samples; the spores measured from 1.2 to 2.4 μm. The spores stained pinkish red and dark violet with the chromotrope and the quick-hot Gram-chromotrope stains, respectively (Fig. 1A). When smears made from these samples were reacted with the anti-E. intestinalis serum at a dilution of 1:400, the spores exhibited an apple-green fluorescence (Fig. 1B).
FIG. 1.
Optical and scanning electron microscopic images of microsporidia spores after treatment by various procedures. (A) Stool smear stained by the quick-hot Gram-chromotrope technique. Bar, 10 μm. (B) Stool smear from the same patient whose stool smear was used in panel A reacted with the anti-E. intestinalis serum. Bar, 10 μm. (C) Growth of E. intestinalis in cell culture. Note the host cells filled with spores (at the arrows). Differential interference contrast optics were used. Bar, 5 μm. (D) Smear of the culture supernatant from the same flask used for panel C but stained by the quick-hot Gram-chromotrope technique. Note the cell filled with darkly staining spores. Bar, 10 μm. (E) Scanning electron microscopic appearance of E. intestinalis from cell culture. Note the delicate thread-like polar tubules at the arrowheads. Bar, 10 μm.
Parasite growth and ultrastructure.
The E6 and HLF cell cultures inoculated with the samples from the patients showed no overt bacterial contamination, and foci of infected cells were seen after 4 to 8 weeks. Thereafter, the spores were continually released into the cultures. In the initial stages of culture and growth, the sedimented spores were reinoculated into the original culture flasks. By 2 to 4 months of continuous culture, the parasites had adapted well to culture conditions so that amplification of the cultures and regular harvest of spores for use in subsequent assays was possible. In all cultures large numbers of spores were found lying free in the supernatant. Additionally, many cells were found to be distended with spores (Fig. 1C and D). The spores appeared to be birefringent when examined with a microscope equipped with phase-contrast optics.
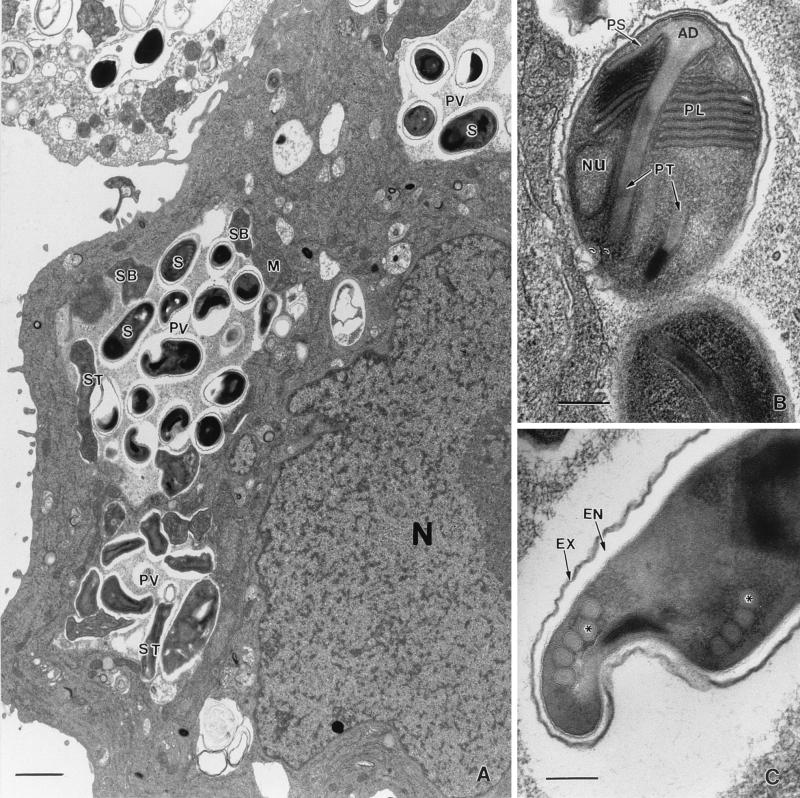
Scanning electron microscopic images of cells distended with spores appeared as though the spores were covered with a muslin-like cloth (Fig. 1E). By transmission electron microscopy all of the developing stages and spores from all isolates were found to be present within chambers of a septated, honeycomb-like parasitophorous vacuole, a feature typical of E. intestinalis (Fig. 2A). Developing stages consisted of meronts, some with two nuclei, which were always found attached to the membranous vacuolar space. Sporogonial stages consisted of mostly disporoblastic and a few tetrasporoblastic stages. Mature spores measured 1.2 to 2.4 μm by 0.9 to 1.2 μm and were smooth walled. The extruded polar tubules seen in many of the spores measured 15 to 22 μm in length. The spores had a thin electron-dense exospore, a thick electron-lucent endospore, and a thin cell membrane surrounding the spore contents. Although the spore contents were dense, the lamellar polaroplast could be discerned at one end and a vacuole could be discerned at the opposite end in some spores (Fig. 2B). Cross sections of four to seven coils of the polar tube were also seen in some spores from all five isolates (Fig. 2C). These morphological features were consistent with those of E. intestinalis.
FIG. 2.
Ultrastructure of E. intestinalis within host cells. (A) Infected E6 cell demonstrating the classical septated parasitophorous vacuole (PV), a characteristic feature of E. intestinalis, filled with spores (S). M, meront; SB, sporoblast; ST, sporont; N, host cell nucleus. Bar, 1 μm. (B) Spore demonstrating a lamellar polaroplast (PL), a polar tubule (PT) with the anchoring disk (AD), and a nucleus (Nu). PS, polar sac. Bar, 200 nm. (C) Spore with four to five turns of the polar tubule (at the asterisk). EX, exospore; EN, endospore. Bar, 200 nm.
IIF assay.
The spores present in the urine, sputum, and fecal smears, as well as those generated from cell cultures infected with the patient samples described above, reacted with the anti-E. intestinalis serum (30) to the same extent (>1:4,096) as the spores of strain CDC:V297, the positive control strain used for comparison, and produced a bright apple-green fluorescence. When spores from all cultures under study were treated with anti-E. cuniculi (7) and anti-E. hellem (29, 32) sera, only slight reactions were observed at a 1:200 dilution and none were observed at a 1:400 dilution, whereas the homologous reaction was recorded at a titer >1:4,096.
SDS-PAGE and immunoblotting.
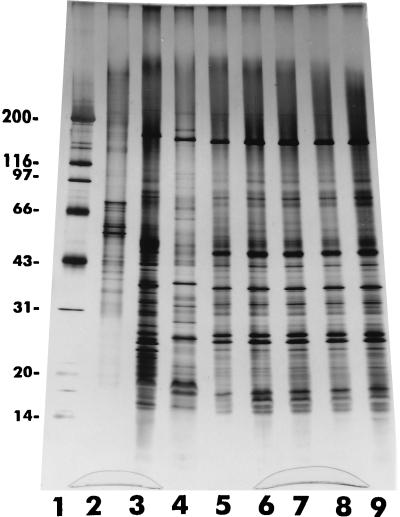
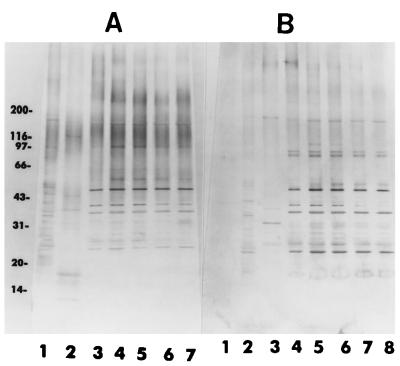
When the electrophoretically separated proteins extracted from the reference strains E. hellem, E. cuniculi, and E. intestinalis and the test isolates were stained with the silver reagent, they exhibited a complex pattern producing more than 50 bands ranging from 14 to 200 kDa. Even a cursory analysis of the patterns revealed, in spite of the complexity and a number of shared bands, characteristic banding patterns that could be easily visualized and that differentiated the three species of Encephalitozoon represented by the reference strains (Fig. 3). The protein banding patterns of the test isolates were almost identical to those of the reference strain (strain CDC:V297) of E. intestinalis (Fig. 3).
FIG. 3.
Silver-stained SDS-polyacrylamide gel showing remarkable similarities of the protein profiles of several isolates of E. intestinalis (lanes 4 to 9) with one another and differences from those of E. cuniculi (lane 2) and E. hellem (lane 3). Lane 4, CDC:V297; lane 5, CDC:V309; lane 6, CDC:V308; lane 7, CDC:V315; lane 8, CDC:V324; lane 9, CDC:V307. Lane 1 is the profile of E6 cells from an uninoculated culture. The numbers on the left are in kilodaltons.
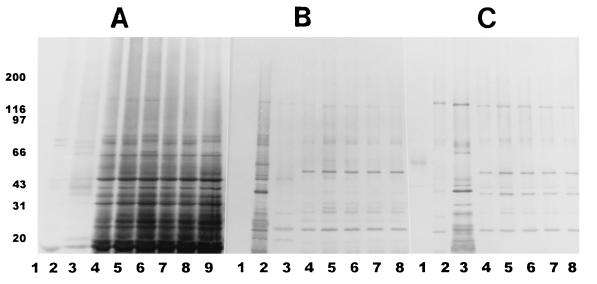
After the proteins were transferred to PVDF membranes and after reaction with the three rabbit polyclonal serum samples against E. intestinalis, E. hellem, and E. cuniculi, respectively, Western blot analysis of the separated proteins indicated that the test isolates belonged to E. intestinalis (Fig. 4A to C). In the homologous reaction, proteins from the reference strain and the test isolates reacted extensively with the anti-CDC:V297 serum, producing an almost similar pattern, with bands ranging from 16 to 160 kDa, and the proteins produced several darkly staining bands with approximate molecular masses of 78, 74, 64, 45, 42, 35, 32, 28, 25, 22, 19, and 16 kDa. Minor differences were noticed among the test isolates when they were compared with each other and with the reference isolate; for example, isolates CDC:V308 and CDC:V307 had an additional band at ∼66 kDa that were not seen in other isolates (Fig. 4A, lanes 6 and 9). Additionally, isolates CDC:V307 and CDC:308 lacked a band at ∼70 kDa. Several other bands of lesser intensity with molecular masses of between 20 and 31 kDa, between 45 and 66 kDa, and between 78 and 200 kDa were also seen. Although both E. hellem and E. cuniculi proteins reacted with the anti-E. intestinalis serum, the bands were few and weak and the banding patterns were very different from that for E. intestinalis (Fig. 4A). In the blots that were reacted with the anti-E. hellem serum, the homologous systems (e.g., E. hellem with the anti-E. hellem serum) produced many prominent bands; however, the bands that migrated at approximately 55, 45, 41, 29, 21, and 15 kDa were not shared by heterologous extracts (Fig. 4B). In the blots that were reacted with the anti-E. cuniculi serum, the homologous reaction (E. cuniculi with the anti-E. cuniculi serum) was strong, with bands at 155, 62, 44, 41, 39, 33, 26, 21, 18, and 16 kDa (Fig. 4C). E. intestinalis and the test isolates, however, reacted moderately with both antisera and produced a number of bands in the region between 20 and 160 kDa. The homologous reactions were much stronger and could be easily distinguished from the heterologous ones (Fig. 4A to C). The serum from one of the patients (patient A) showed clear reactivity with extracts from all four isolates as well as with E. intestinalis (Fig. 5A). Darkly staining bands migrated at approximately 170, 95, 75, 58, 53, 48, 40, 37, 30, and 28 kDa. The serum from this patient reacted minimally with the proteins of E. hellem and E. cuniculi. When the membrane was treated with serum from patient B, a fainter reaction was observed with homologous proteins; however, clear bands were distinguished at approximately 170, 86, 84, 50, 48, 40, 37, 30, 28, and 25 kDa. A number of lightly staining bands in the region of 26 to 32 kDa were also seen in reactions with sera from both patients. Minimal reaction was observed with heterologous proteins (Fig. 5B). When patient C’s serum was assayed, it also reacted with protein extracts and again showed a common pattern for proteins from all four isolates and E. intestinalis proteins, with the most prominents bands migrating at 50, 39, 37, and 29 kDa. A faint reaction was observed with E. hellem and E. cuniculi proteins (data not shown). The protein extract from E6 cells reacted minimally with all rabbit or human serum samples assayed, producing only a few, light bands in the region of 43 to 90 kDa (Fig. 4 and 5).
FIG. 4.
Western blot profiles of several E. intestinalis isolates (lanes 4 to 9) along with those of E. cuniculi and E. hellem. (A) Anti-E. intestinalis; (B) anti-E. hellem; (C) anti-E. cuniculi. Lanes 1, E6 cells; lanes 2, E. hellem; lanes 3, E. cuniculi; lanes 4, E. intestinalis CDC:V297; lanes 5, E. intestinalis CDC:V309; lanes 6, E. intestinalis CDC:V308; lanes 7, E. intestinalis CDC:V315; lanes 8, E. intestinalis CDC:V324; lane 9, E. intestinalis CDC:V307. Note that strain CDC:V307 was not included in the Western blot profiles in panels B and C. The numbers on the left are in kilodaltons.
FIG. 5.
Western blot profiles of the various microsporidial isolates after reactions with the sera from one patient (A; lane 1, E. hellem; lane 2, E. cuniculi; lanes 3 to 7, E. intestinalis) and a second patient (B; lane 1, E6 cells; lane 2, E. hellem; lane 3, E. cuniculi; lanes 4 to 8, E. intestinalis, as described in the legend to Fig. 4A and B). The numbers on the left are in kilodaltons.
PCR analysis.
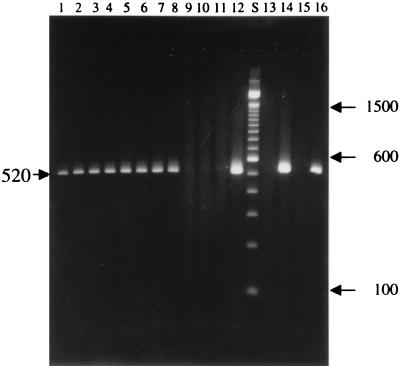
The three species-specific PCR primers targeting SSU rRNA-coding sequences selectively amplified fragments diagnostic for E. intestinalis, E. hellem, and E. cuniculi, respectively, with no background from mammalian cellular DNA being found. For example, DNA isolated from cell cultures infected with the test isolates (isolates CDC:V307, CDC:V308, CDC:V309, CDC:V314, CDC:V315, CDC:V324, and CDC:V325) and isolate CDC:V297 reacted with E. intestinalis-specific primers only and a diagnostic band of 520 bp was detected in the agarose gel (Fig. 6). PCR primers specific for E. hellem amplified only the DNA isolated from cell cultures infected with E. hellem and a diagnostic band of 546 bp was detected in the agarose gel, whereas PCR primers specific for E. cuniculi amplified only the DNA isolated from cell cultures infected with E. cuniculi, resulting in a diagnostic band of 549 bp (data not shown).
FIG. 6.
Agarose gel electrophoresis analysis of SSU rRNA fragments amplified by PCR with species-specific primers diagnostic for E. intestinalis (lanes 1 to 12), E. hellem (lanes 13 and 14), and E. cuniculi (lanes 15 and 16). Lanes 1 to 7, amplification of E. intestinalis isolates; lanes 8, 13, and 15, amplification of E. intestinalis CDC:V297; lane 9, amplification of uninfected E6 cells; lanes 10 and 14, amplification of E. hellem CDC:0291:V213; lanes 11 and 16, amplification of E. cuniculi; lane 12, amplification of the SSU rRNA cloned region of CDC:V297; lane S, a 100-bp molecular size marker. Numbers on the right and on the left of the gel are DNA fragment sizes (in base pairs).
DISCUSSION
E. bieneusi causes most of the microsporidial infections in patients with AIDS and is the most prevalent intestinal parasite of AIDS patients. It infects the small intestine and can spread into the hepatobiliary tree (4, 25, 33). However, recent reports indicate a gradual increase, albeit small, in the number of cases of disseminated microsporidiosis caused by E. intestinalis involving the kidneys, eyes, and the lungs, as well as the GI tracts, of people with AIDS. According to Schwartz and Bryan (25), infection with E. intestinalis is the second most prevalent microsporidial infection in AIDS patients. The two other species of Encephalitozoon (E. cuniculi and E. hellem) are also known to cause infections of the urogenital, respiratory, and ocular organs but not GI tract infections. Recent reports also indicate that E. cuniculi may also disseminate into the brain (19, 35).
Continuous in vitro cultivation of E. bieneusi has not yet been achieved, although several isolates of E. bieneusi have been cultured for short periods (31). However, a number of isolates of E. cuniculi, E. hellem, and E. intestinalis have been established in continuous culture in a variety of mammalian cell cultures, and the isolates have been compared with each other by antigenic and molecular biology-based analyses (8, 10–14, 16, 23, 24). On the basis of those studies, E. cuniculi isolates have been differentiated into three distinct types, e.g., murine, canine, and rabbit (12). On the other hand, E. hellem isolates cultured from samples from different sites of infected patients originating from different geographic locales have been shown to be similar on the basis of antigenic and molecular data (5, 23, 24). Several isolates of E. intestinalis have also been shown to be similar on the basis of antigenic and molecular data (13). Infection of animals and birds with E. cuniculi and E. hellem, respectively (1, 9, 16, 18), and infection of animals, including pigs and primates, with E. bieneusi (9, 17) have been reported. Recently, however, we have incontrovertible evidence that E. intestinalis also causes infection of animals, e.g., pigs, goats, and cows (1a). Many E. cuniculi isolates from animals have been established in culture, and efforts are under way to establish in culture other microsporidia from domestic animals.
Here we report the establishment in culture of eight isolates of E. intestinalis from five AIDS patients with disseminated microsporidiosis, including infection of the GI tract. All of these isolates readily adapted to culture conditions and grew rapidly and produced high yields of spores within 4 to 5 months. All of these isolates were identified as E. intestinalis on the basis of (i) their ultrastructural morphology, namely, the presence of the characteristic septated parasitophorous vacuole and di- and tetrasporous sporogony; (ii) their reactivity in the IIF assay with the rabbit anti-E. intestinalis CDC:V297 serum to the same extent as the type species; (iii) PCR analysis, when a 520-bp diagnostic fragment of the SSU rRNA-coding region of E. intestinalis was amplified from the DNA of the cell cultures infected with these isolates; and (iv) the similarity of the patterns of the proteins extracted from these isolates to the patterns exhibited by proteins extracted from the type species, as analyzed by SDS-PAGE and immunoblotting.
Although the strongest reactivity always occurred between the homologous systems when the antigenic profiles were analyzed in immunoblots with rabbit anti-E. hellem, anti-E. cuniculi, and anti-E. intestinalis sera, a certain amount of cross-reactivity between the three species also occurred, indicating that all these Encephalitozoon species share some common antigenic determinants. Although all isolates of E. intestinalis tested had very similar antigenic profiles by Western blotting, minor differences were noted in two of them (strains CDC:V307 and CDC:V308). It is possible that these differences are probably due to relative differences in the growth phases of the organisms, resulting in the expression of different proteins such as surface antigens or intracellular antigens. Since we had selected culture flasks containing parasites of approximately the same age and density, it is possible that these differences are perhaps indications of important variations between isolates of E. intestinalis. Further work with proteins derived from culture flasks of different ages as well as flasks with different parasite densities would provide answers to these questions. When serum samples from three patients were reacted in the immunoblot assay, they clearly reacted very strongly with antigens of the CDC:V297 isolate and those of the test E. intestinalis isolates and produced identical profiles. For serum samples from all patients, specific bands were seen at about 50, 48, 39, and 37 kDa. Slight cross-reactivity was also observed with heterologous proteins. It is noteworthy that the rabbit anti-E. intestinalis sera reacted with the separated antigens much more strongly and with more antigenic determinants over a wider range of molecular sizes than the sera from patients A and B. The immunized rabbits probably reacted to a host of antigens including nuclear, cytosol, and membrane antigens and hence produced bands with a wider range of molecular sizes. The patients, however, may have reacted more to the membrane antigens than to the cytosol antigens, hence the differences in the banding patterns. However, it should be remembered that these are AIDS patients, and the immune responses of these patients may be quite different from those of patients without AIDS. Nevertheless, AIDS patients infected with these microsporidia develop a measurable humoral response, and thus, the responses of our patients are in agreement with those described previously (10, 30).
As has been reported by other investigators (8, 13, 14, 16, 21, 23, 30, 32), in vitro cultivation of microsporidial organisms that infect humans and animals is invaluable for several reasons. For example, it facilitates (i) understanding of the biology of the parasite and the host-parasite relationships, (ii) the development of immunologic and molecular reagents for use in clinical diagnosis, (iii) the development of assays for screening newer and promising therapeutic agents, and (iv) the development of antigenic and molecular markers for isolates that may be useful in molecular epidemiology, particularly in tracing the sources of the causal agent, and thus that will be helpful in formulating preventive strategies.
ACKNOWLEDGMENTS
Carmen del Aguila was a recipient of a NATO senior fellowship; Gian Piero Croppo was a recipient of a fellowship from the Ministry of Health, Rome, Italy; Hercules Moura was a recipient of a fellowship from CNPq, Brazil; and Gordon J. Leitch was supported in part by Public Health Service grant RR03034.
We thank Randal Reves, Michael L. Wilson, and Betty Hummert for providing samples from patients.
REFERENCES
- 1.Black S S, Steinhort L A, Bertucci D C, Rogers L B, Didier E S. Encephalitozoon hellem in budgerigars. Vet Pathol. 1997;34:189–198. doi: 10.1177/030098589703400303. [DOI] [PubMed] [Google Scholar]
- 1a.Bornay-Llinares, F., et al. Unpublished data.
- 2.Cali A, Kotler D P, Orenstein J M. Septata intestinalis, n.g., n.sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J Protozool. 1993;40:101–112. doi: 10.1111/j.1550-7408.1993.tb04889.x. [DOI] [PubMed] [Google Scholar]
- 3.Canning E U, Lom J, Dykova I. The microsporidia of vertebrates. New York, N.Y: Academic Press, Inc.; 1986. [Google Scholar]
- 4.Coyle C M, Wittner M, Kotler D P, Noyer C, Orenstein J M, Tanowitz H B, Weiss L M. Prevalence of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon (Septata) intestinalis among patients with AIDS-related diarrhea: determination by polymerase chain reaction to the microsporidian small-subunit rRNA gene. Clin Infect Dis. 1996;23:1002–1006. doi: 10.1093/clinids/23.5.1002. [DOI] [PubMed] [Google Scholar]
- 5.Croppo G P, Leitch G J, Wallace S, Visvesvara G S. Immunofluorescence and Western blot analysis of microsporidia using anti-Encephalitozoon hellem immunoglobulin G monoclonal antibodies. J Eukaryot Microbiol. 1994;41:31S. [PubMed] [Google Scholar]
- 6.Da Silva A, Slemenda S B, Visvesvara G S, Schwartz D A, Wilcox C M, Wallace S, Pieniazek N J. Detection of Septata intestinalis (Microsporidia) Cali et al. 1993 using polymerase chain reaction primers targeting the small subunit ribosomal RNA coding region. Mol Diagn. 1997;2:47–52. doi: 10.1054/MODI00200047. [DOI] [PubMed] [Google Scholar]
- 7.DeGroote M A, Visvesvara G S, Wilson M L, Pieniazek N J, Slemenda S B, Da Silva A J, Leitch G J, Bryan R T, Reves R. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J Infect Dis. 1995;171:1375–1378. doi: 10.1093/infdis/171.5.1375. [DOI] [PubMed] [Google Scholar]
- 8.Deplazes P, Mathis A, Baumgartner R, Tanner I, Weber R. Immunologic and molecular characteristics of Encephalitozoon-like microsporidia isolated from humans and rabbits indicate that Encephalitozoon cuniculi is a zoonotic parasite. Clin Infect Dis. 1996;22:557–559. doi: 10.1093/clinids/22.3.557. [DOI] [PubMed] [Google Scholar]
- 9.Deplazes P, Mathis A, Muller C, Weber R. Molecular epidemiology of Encephalitozoon cuniculi and first detection of Enterocytozoon bieneusi in fecal samples of pigs. J Eukaryot Microbiol. 1996;43:93S. doi: 10.1111/j.1550-7408.1996.tb05018.x. [DOI] [PubMed] [Google Scholar]
- 10.Didier E S, Didier P J, Friedberg D N, Stenson S M, Orenstein J M, Yee R W, Tio F O, Davis R M, Vossbrinck C, Millichamp N, Shadduck J A. Isolation and characterization of a new human microsporidian, Encephalitozoon hellem (n.sp.), from three AIDS patients with keratoconjunctivitis. J Infect Dis. 1991;163:617–621. doi: 10.1093/infdis/163.3.617. [DOI] [PubMed] [Google Scholar]
- 11.Didier E S, Visvesvara G S, Baker M D, Rogers L B, Bertucci D C, DeGrotte M A, Vossbrinck C R. A microsporidian isolated from an AIDS patient corresponds to Encephalitozoon cuniculi III, originally isolated from domestic dogs. J Clin Microbiol. 1996;34:2835–2837. doi: 10.1128/jcm.34.11.2835-2837.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Didier E S, Vossbrinck C R, Baker M D, Rogers L B, Bertucci D C, Shadduck J A. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology. 1995;111:411–422. doi: 10.1017/s0031182000065914. [DOI] [PubMed] [Google Scholar]
- 13.Didier E S, Rogers L B, Orenstein J M, Baker M D, Vosbrinck C R, van Gool T, Hartskeerl R, Soave R, Beaudet L M. Characterization of Encephalitozoon (Septata) intestinalis isolates cultured from nasal mucosa and bronchoalveolar lavage fluids of two AIDS patients. J Eukaryot Microbiol. 1996;43:34–43. doi: 10.1111/j.1550-7408.1996.tb02470.x. [DOI] [PubMed] [Google Scholar]
- 14.Doultree J C, Maerz A L, Ryan N J, Baiird R W, Wright E, Crowe S M, Marshall J A. In vitro growth of the microsporidian Septata intestinalis from an AIDS patient with disseminated illness. J Clin Microbiol. 1995;33:463–470. doi: 10.1128/jcm.33.2.463-470.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartskeerl R A, van Gool T, Schuitema A R J, Didier E S, Terpstra W J. Genetic and immunological characterization of the microsporidian Septata intestinalis Cali, Kotler and Orenstein, 1993: reclassifcation to Encephalitozoon intestinalis. Parasitology. 1995;110:277–285. doi: 10.1017/s0031182000080860. [DOI] [PubMed] [Google Scholar]
- 16.Hollister W S, Canning E U, Colbourn N I, Aarons E J. Encephalitozoon cuniculi isolated from the urine of an AIDS patient, which differs from canine and murine isolates. J Eukaryot Microbiol. 1995;42:367–372. doi: 10.1111/j.1550-7408.1995.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 17.Mansfield K G, Carville A, Shvetz D, Mackey J, Tzipori S, Lackner A A. Identification of an Enterocytozoon bieneusi-like microsporidian parasite in simian-immunodeficiency-virus-inoculated macaques with hepatobiliary disease. Am J Pathol. 1997;150:1395–1405. [PMC free article] [PubMed] [Google Scholar]
- 18.Mathis A, Michel M, Kuster H, Muller C, Weber R, Deplazes P. Two Encephalitozoon cuniculi strains of human origin are infectious to rabbits. Parasitology. 1997;114:29–35. doi: 10.1017/s0031182096008177. [DOI] [PubMed] [Google Scholar]
- 19.Mertens R B, Didier E S, Fishbein M C, Bertucci D C, Rogers L B, Orenstein J M. Encephalitozoon cuniculi microsporidiosis: infection of the brain, heart, kidneys, trachea, adrenal glands, and urinary bladder in a patient with AIDS. Modern Pathol. 1997;10:68–77. [PubMed] [Google Scholar]
- 20.Moura H, Schwartz D A, Bornay-Llinares F, Sodré F C, Wallace S, Visvesvara G S. A new and improved “quick-hot Gram-chromotrope” technique that differentially stains microsporidian spores in clinical samples, including paraffin-embedded tissue sections. Arch Pathol Lab Med. 1997;121:888–893. [PubMed] [Google Scholar]
- 21.Ombrouck C, Desportes-Lavage I, Achbarou A, Gentilini M. Specific detection of the microsporidia Encephalitozoon intestinalis in AIDS patients. C R Acad Sci Paris. 1996;319:39–43. [PubMed] [Google Scholar]
- 22.Orenstein J M, Dietrich D T, Cotler D P. Systemic dissemination by a newly recognized intestinal microsoridia species in AIDS. AIDS. 1992;6:1143–1150. doi: 10.1097/00002030-199210000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Scaglia M, Sacchi L, Gatti S, Bernuzzi A M, de Piceis Polver P, Piacentini I, Concia E, Croppo G P, da Silva A J, Pieniazek N J, Slemenda S B, Wallace S, Leitch G J, Visvesvara G S. Isolation and identification of Encephalitozoon hellem from an Italian AIDS patient with disseminated microsporidiosis. APMIS. 1994;102:817–827. [PubMed] [Google Scholar]
- 24.Scaglia M, Sacchi L, Croppo G P, da Silva A J, Gatti S, Corona S, Orani A, Bernuzzi A M, Pieniazek N J, Slemenda S B, Wallace S, Visvesvara G S. Pulmonary microsporidiosis due to Encephalitozoon hellem in a patient with AIDS. J Infect. 1997;34:119–126. doi: 10.1016/s0163-4453(97)92414-2. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz D A, Bryan R T. Microsporidia. In: Horsburgh C R, Nelson A M, editors. Emerging infections: clinical and pathologic update. Washington, D.C: American Society for Microbiology; 1997. pp. 61–93. [Google Scholar]
- 26.Tsang V C W, Hancock K, Maddison S E, Beatty A L, Moss D M. Demonstration of species-specific and cross reactive components of the adult microsomal antigens from Schistosoma mansoni and S. japonicum (MAMA and JAMA) J Immunol. 1984;132:2607–2613. [PubMed] [Google Scholar]
- 27.Tsang V C W, Hancock K, Wilson M, Parmer D F, Whaley S D, McDougal J S, Kennedy S. Immunology series no. 15 procedural guide. Atlanta, Ga: Centers for Disease Control; 1986. Enzyme-linked immunoelectrotransfer blot technique (Western blot) for human T-lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-II/LAV) antibodies. [Google Scholar]
- 28.van Gool T, Canning E U, Gilis H, van den Bergh Weerman M A, Eeftinck-Schattenkirk J K M, Dankert J. Septata intestinalis frequently isolated from stool of AIDS patients with a new cultivation method. Parasitology. 1994;109:281–289. doi: 10.1017/s0031182000078318. [DOI] [PubMed] [Google Scholar]
- 29.Visvesvara G S, Leitch G J, Moura H, Wallace S, Weber R, Bryan R T. Culture, electron microscopy, and immunoblot studies on a microsporidian parasite isolated from the urine of a patient with AIDS. J Protozool. 1991;38:105S–111S. [PubMed] [Google Scholar]
- 30.Visvesvara G S, da Silva A J, Croppo G P, Pieniazek N J, Leitch G J, Ferguson D, de Moura H, Wallace S, Slemenda S B, Tyrrell I, Moore D F, Meador J. In vitro culture and serologic and molecular identification of Septata intestinalis isolated from urine of a patient with AIDS. J Clin Microbiol. 1995;33:930–936. doi: 10.1128/jcm.33.4.930-936.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visvesvara G S, Leitch G J, Pieniazek N J, Da Silva A J, Wallace S, Slemenda S B, Weber R, Schwartz D A, Gorelkin L, Mel Wilcox C, Bryan R T. Short-term in vitro culture and molecular analysis of the microsporidian, Enterocytozoon bieneusi. J Eukaryot Microbiol. 1995;42:506–510. doi: 10.1111/j.1550-7408.1995.tb05896.x. [DOI] [PubMed] [Google Scholar]
- 32.Visvesvara G S, Leitch G J, Da Silva A J, Croppo G P, Moura H, Wallace S, Slemenda S B, Schwartz D A, Moss D M, Bryan R T, Pieniazek N J. Polyclonal and monoclonal antibody and PCR-amplified small-subunit RNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J Clin Microbiol. 1994;32:2760–2768. doi: 10.1128/jcm.32.11.2760-2768.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber R, Bryan R T, Schwartz D A, Owen R L. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–461. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber R, Bryan R T, Owen R L, Wilcox C M, Gorelkin L, Visvesvara G S. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N Engl J Med. 1992;326:161–166. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- 35.Weber R, Deplazes P, Flepp M, Mathis A, Baumann R, Sauer B, Kuster H, Lüthy R. Cerebral microsporidiosis due to Encephalitozoon cuniculi in a patient with human immunodeficiency virus syndrome. N Engl J Med. 1997;336:474–478. doi: 10.1056/NEJM199702133360704. [DOI] [PubMed] [Google Scholar]