Abstract
Background Avoidance of sternotomy while preserving complete revascularization remains challenging in multivessel coronary disease. Technical issues and in-hospital outcomes of total coronary revascularization via a small left anterior thoracotomy (TCRAT) in nonselected patients with multivessel disease are reported.
Methods From November 2019 to September 2021, coronary artery bypass grafting via left anterior minithoracotomy on cardiopulmonary bypass and cardioplegic cardiac arrest was performed in 102 patients (92 males; 67 ± 10 [42–87] years). Slings were placed around ascending aorta, left pulmonary veins, and inferior vena cava for exposure of lateral and inferior ventricular wall. All patients had multivessel coronary disease (three-vessel disease: n = 72; two-vessel disease: n = 30; left main stenosis: n = 44). We included patients at old age (> 80 years, 14.7%), with severe left ventricular dysfunction (ejection fraction < 30%, 6.9%), massive obesity (body mass index > 35, 11.6%), and at increased risk (EuroSCORE II > 4, 15.7%).
Results Left internal thoracic artery ( n = 101), radial artery ( n = 83), and saphenous vein ( n = 39) grafts were used for total (61.8%) or multiple (19.6%) arterial grafting. A total of 323 distal anastomoses (3.2 ± 0.7 [2–5] per patient) were performed to revascularize left anterior descending (100%), circumflex (91.2%), and right coronary artery (67.7%). Complete revascularization was achieved in 95.1%. In-hospital mortality was 2.9%, stroke rate was 1.0%, myocardial infarction rate was 2.9%, and repeat revascularization rate was 2.0%.
Conclusion This novel surgical technique allows complete coronary revascularization in the broad majority of multivessel disease patients without sternotomy. TCRAT can be introduced into clinical routine safely. Long-term results remain to be investigated.
Keywords: coronary artery bypass grafting, CABG, minimally invasive cardiac surgery, minithoracotomy, cardiac, myocardial infarction
Introduction
Coronary artery bypass grafting (CABG) remains the most robust form of myocardial revascularization in multivessel coronary disease. 1 Consequently, CABG is recommended as first-line treatment in complex multivessel coronary disease. 2 A sternotomy approach is used for the overwhelming majority of surgical revascularizations worldwide. In Germany, almost all CABG operations (98%) were performed via a complete midline sternotomy. 3
However, conventional CABG with full midline sternotomy is a very invasive treatment. It is associated with significantly reduced limited physical activity, quality of life, and also treatment satisfaction during the first 6 months after the procedure when compared with percutaneous coronary intervention (PCI). 4 Chronically, poststernotomy patients not uncommonly experience decreased physical functioning, 5 and some patients still report thoracic pain even 1 year after surgery. 6
The thoracic invasiveness of CABG has not decreased more or less since its introduction more than 50 years ago. Although robotic endoscopic CABG significantly reduces invasiveness of the operation, this technique has been performed only in a very few specialized centers in a small number of very selected patients, 7 8 probably due to technical complexity and enormous infrastructural prerequisites. Similarly, minimally invasive cardiac surgery (MICS) CABG has been introduced to enable revascularization in multivessel disease through a small lateral thoracic incision by combining special retractors, special cardiac apical positioners, and special epicardial stabilizers. 9 Again, only a few groups worldwide adopted this technically challenging operation on a routine basis, and only a very small fraction of patients underwent MICS CABG so far. 8 At present, no technique systematically eliminated sternotomy while preserving key principles of complete revascularization as well as wide applicability for the broad majority of patients. 8
Babliak et al 10 proposed a new operative approach via a small left anterior thoracotomy, based on long proven cardiac surgery concepts for revascularization in multivessel coronary disease combined with technical aspects known from minimally invasive valve surgery, and named the procedure “total coronary revascularization via left anterior thoracotomy (TCRAT).” We introduced this minimally invasive technique to our clinical routine and recently described the technical details. 11 This report presents perioperative data as well as in-hospital outcomes of our initial series of patients.
Methods
Patients
Between November 2019 and September 2021, we operated 102 nonselected consecutive patients with multivessel coronary disease via left anterior minithoracotomy using cardiopulmonary bypass (CPB) and cardioplegic arrest. Baseline parameters of patients are given in Table 1 . All patients were scheduled after heart team discussion 12 including a recommendation on which coronary arteries should be grafted according to the guidelines for elective or urgent isolated surgical revascularization. 2
Table 1. Baseline and cardiovascular parameters.
| Baseline parameters | |
| Number of patients | 102 |
| Age (y) | 67.3 ± 10.1 (42–87) |
| Male | 90.2% |
| BMI (kg/m 2 ) | 28.6 ± 4.3 (19–42) |
| Diabetes mellitus | 37.3% |
| EuroSCORE II | 2.8 ± 3.7 (0.6–22) |
| Cardiovascular parameters | |
| Left ventricular ejection fraction (%) | 50.3 ± 9.0 (18–65) |
| Two-vessel disease | 29.4% |
| Three-vessel disease | 70.6% |
| Left main stenosis > 50% | 43.1% |
| NSTEMI | 23.5% |
Abbreviations: BMI, body mass index; NSTEMI, non-ST-elevation myocardial infarction.
Note: Data are presented as mean ± standard deviation (minimum–maximum) or percentage.
Emergency patients (meaning same day catheterization and operation), patients with significant atheromatous disease of the ascending aorta, and patients undergoing reoperation were not included in this study.
Data are part of our internal assurance documentation and were retrospectively extracted from patient records and presented as mean (±standard deviation) or number.
Postoperative myocardial infarction was defined according to the Society for Cardiovascular Angiography and Interventions criteria as an increase in creatine kinase (CK-MB) levels within 48 hours after the procedure up to 10 times the local laboratory upper limit of normal (ULN) or to five times the ULN with newly occurring Q-waves in two contiguous leads or a new persistent left bundle branch block (LBBB), or, in the absence of CK-MB determinations, an increase in cTn levels up to 70 times the local laboratory ULN, or 35 times the ULN with new pathological Q-waves in two contiguous leads or a new persistent LBBB. 13 14
Pneumonia was defined according to the S3 guideline of the German Society for Anaesthesiology and Intensive Care Medicine, the German Society for Infectious Diseases, the German Society for Hygiene and Microbiology, the German Respiratory Society and the Paul-Ehrlich-Society for Chemotherapy, the German Radiological Society, and the Society for Virology. 15 Pneumonia is present, when the main criterion (pneumonic infiltrates in X-ray images) and two of three secondary criteria (leukocytes more than 10,000 or less than 1,000, fever of 38.3°C or higher, putrid secretion) were met.
In this study population, we included patients older than 80 years (14.7%) as well as obese patients (body mass index >35, 11.6%), patients with severe ventricular dysfunction with an ejection fraction (EF) less than 30% (6.9%) and patients with an increased EuroSCORE II above 4 (15.7%). All patients had multivessel disease with or without left main stem stenosis ( Table 1 ).
Ethical Standards
The study has been approved by the local ethics committee (no. 2021-2621-evBO) and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Preoperative Evaluation
In addition to standard preoperative examinations, all patients received a computed tomography (CT) scan to evaluate the course of the aorta and the arteries supplying the brain. On the basis of these findings, any existing atherosclerotic disease was clarified with regard to intraoperative manipulation and the planned cross-clamping of the ascending aorta and the definition of the perfusion strategy (transaxillary or transfemoral) on CPB. In addition, severe stenosis of the right subclavian artery or anomalies of the aortic arch could be excluded or diagnosed.
The usability of radial artery (RA) was preoperatively clarified by clinical examination (Allen's test) and with Doppler ultrasound to rule out anatomical or circulatory disorders and sclerosis.
Anesthesia
For induction and maintenance of anesthesia standard cardiac anesthesia techniques (intravenous sufentanil 0.5 μg/kg/h, etomidate 0.25 mg/kg, pancuronium 0.1 mg/kg, sevoflurane, propofol 3 mg/kg/h) were used, and invasive monitoring was performed with standard arterial and venous lines. All patients had intraoperative transesophageal echocardiography to help position guidewires for femoral arterial and venous cannulation as well as to monitor cardiac function.
Surgical Technique
We described the surgical technique in detail recently. 11 Patients were operated in a supine position. Minimal invasive harvesting of RA was performed using an endoscopic reusable retractor (Bisleri Model, Karl Storz, Tuttlingen, Germany) and a bipolar radiofrequency vessel sealing system (LigaSure; Medtronic, Minneapolis, Minnesota, United States) via a 2-cm distal incision of the forearm. Saphenous vein graft (SVG) harvest was performed using small skin incisions in an atraumatic fashion under direct surgical vision. Grafts were stored in a preservation solution using iron chelators (TiPROTEC; Dr. Franz Köhler Chemie GmbH, Bensheim, Germany).
A retractor (Small Thoracotomy Retractor; Delacroix-Chevalier, Paris, France) was inserted in the chest via a minithoracotomy performed far anteriorly in the fourth intercostal space (incision length 8–10 cm). Left internal mammary artery (LIMA) was identified and severed. Using a special retractor (MIDAccess IMA Retractor; Delacroix-Chevalier), LIMA was harvested under direct surgical view beyond the origin of the left mammary vein.
Peripheral arterial cannulation was performed according to findings of preoperative CT scan via right femoral artery (17/19/21 Fr Bio-Medicus; Medtronic) in 18 patients or via right axillary artery (16/18 Fr OptiSite Arterial Perfusion Cannula; Edwards Lifesciences, Irvine, California, United States) in 84 patients. To expose the artery, a small cut-down was performed; 400 IU/kg heparin were administered intravenously prior insertion of cannulas. The venous cannula (23 Fr Bio-Medicus; Medtronic) was inserted percutaneously via the femoral vein. In case of body surface area greater than 2.0 m 2 , an additional venous cannula was inserted in the jugular vein (15/17 Fr Bio-Medicus; Medtronic) to enhance the venous return. Additionally, a vacuum-assisted venous return was used to optimize the heart decompression. During CPB, patients were kept normothermic.
The pericardium was opened longitudinally from the apex to the ascending aorta and to the sides. The ascending aorta was dissected from the pulmonary trunk and encircled with a tape. This makes it possible to expose the ascending aorta in the direction of thoracotomy to place a small cannula (7 Fr MTAR; Medtronic) for application of antegrade blood cardioplegia and venting the left ventricle during cardiac arrest.
Through a separate small skin incision in the anterior axillary line at the level of the second intercostal space, a transthoracic aortic clamp (ValveGate DeBakey; Geister, Plymouth, United States) was introduced. Pulling the aortic tape, the aorta was moved toward the thoracotomy and aortic cross-clamp performed under direct vision. Cardiac arrest was induced with infusion of cold blood cardioplegia (Dr. Franz Köhler Chemie GmbH) and maintained with intermittent cold reinfusion every 15 minutes ( Fig. 1 ).
Fig. 1.
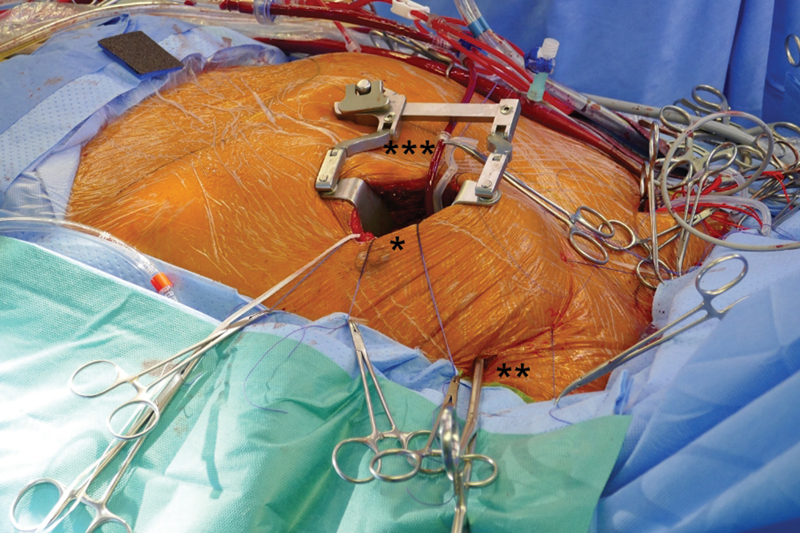
Surgical approach. * Minithoracotomy; ** transthoracic aortic clamp; *** cardioplegia line.
After cardiac arrest and decompression of the heart, left pulmonary veins and inferior vena cava were encircled with separate tapes. Pulling the tapes applied around the large intrapericardial vessels rotation and exposure of the heart enabled to reach all territories of the left ventricle by reducing the distance between skin incision and coronary arteries to less than 8 cm at maximum. This traction maneuver enabled adequate access to all coronary targets, especially the right coronary artery (RCA) and circumflex (Cx) territories at the lateral and inferior ventricular wall ( Fig. 2 ).
Fig. 2.
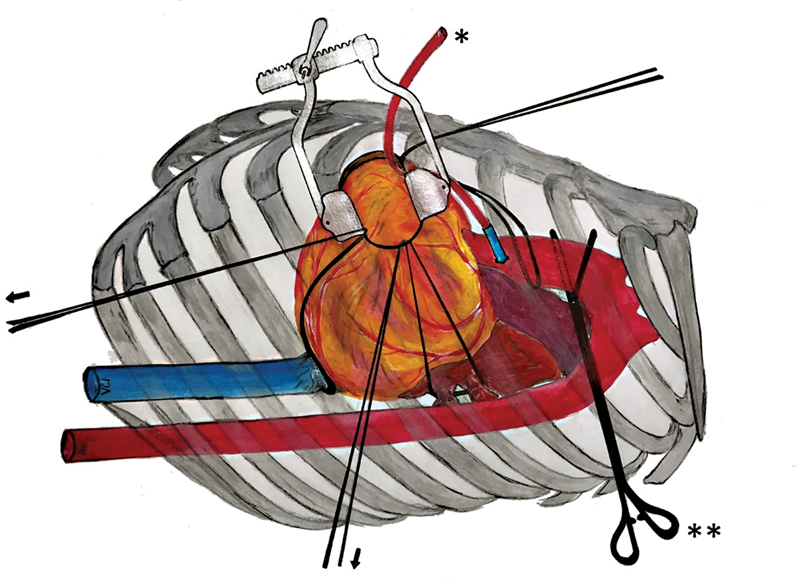
Traction maneuver: stable exposition with slings around great vessels. * Cardioplegia line; ** transthoracic aortic clamp; → slings around ascending aorta, left pulmonary veins, and inferior vena cava.
Coronary anastomoses were performed by using conventional anastomotic techniques of running 8–0 polypropylene sutures and usual coronary instruments. Starting with the most distal anastomosis (branches of the RCA or distal Cx) in an end-to-side fashion, the more proximal targets were performed in sequential technique side to side ( Figs. 3 and 4 ). LIMA was anastomosed as in situ graft to the left anterior descending (LAD). RA or SVG was either anastomosed to the LIMA as composite T-graft or Y-graft or to the ascending aorta ( Fig. 5 ). This anastomosis was also accomplished during cardiac arrest. All grafts were checked using transit time flow measurement (QuickFit TTFM; Medistim, Deisenhofen, Germany).
Fig. 3.
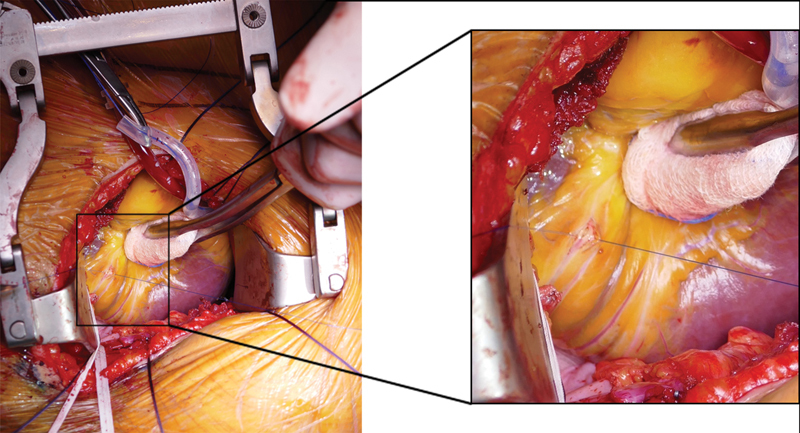
Exposition of posterior descending artery (PDA) of the right coronary artery. Right picture shows a magnification of the exposition of the PDA.
Fig. 4.
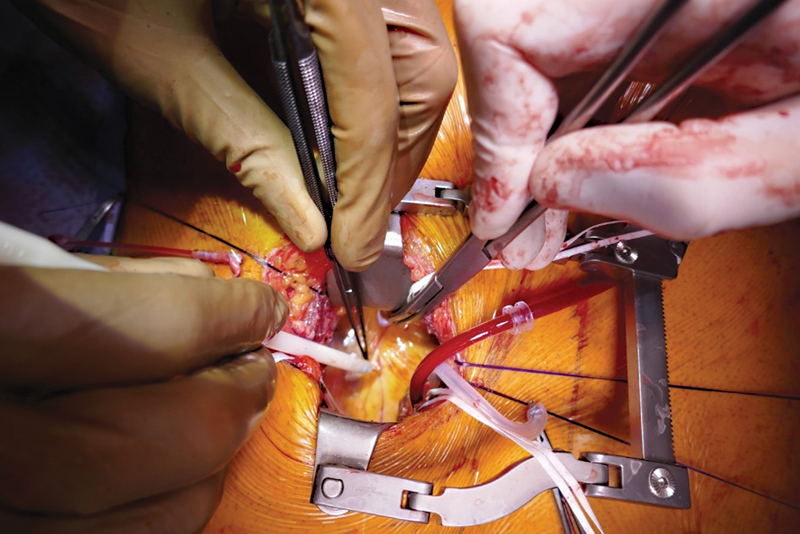
Surgical view, operation with standard coronary instruments.
Fig. 5.
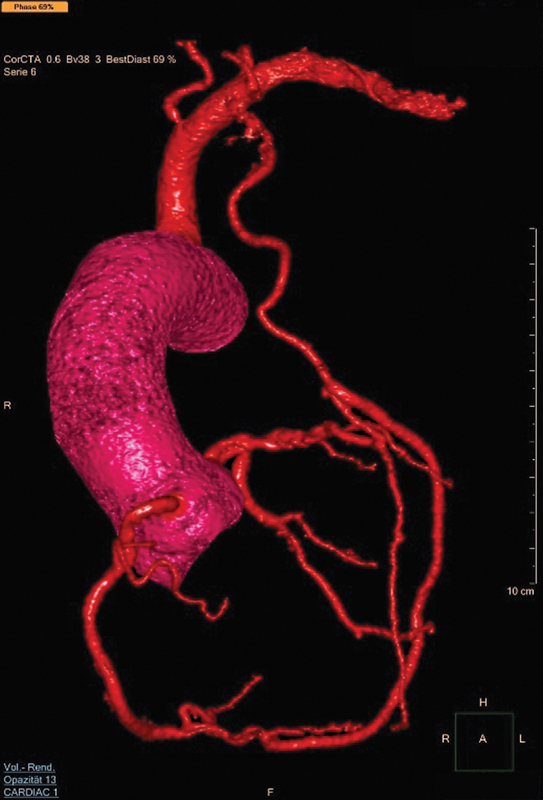
Computed tomography scan after complete arterial revascularization. (Left internal mammary artery on left anterior descending, T-graft with radial artery sequential on circumflex artery and posterior descending artery.)
Results
Grafts used were LIMA in 99.0%, RA in 81.4%, and SVG in 38.2%. This resulted in total arterial revascularization in 61.8% and multiple arterial revascularization in 19.6%. On average, 3.2 anastomoses were performed per patient, with a minimum of 2 and a maximum of 5 anastomoses. The LAD territory was grafted in all patients, Cx territory in 91.2% and the RCA in 67.7% of patients ( Table 2 ). Complete revascularization as preoperatively intended was achieved in 95.1% of patients.
Table 2. Operative data.
| Conduits | |
| Left internal mammary artery | 99.0% |
| Radial artery | 81.4% |
| Saphenous vein graft | 38.2% |
| Revascularization territories | |
| Left anterior descending | 100.0% |
| Ramus circumflexus | 91.2% |
| Right coronary artery | 67.7% |
| Number of distal anastomoses | 3.2 ± 0.7 (2–5) |
Note: Data are presented as mean ± standard deviation (minimum–maximum) or percentage.
Duration of aortic cross-clamping, CPB, and total operation was increased compared with CABG via sternotomy in our institution ( Table 3 ). However, this did not result in prolonged length of stay in intensive care unit (ICU) or hospital ( Table 4 ).
Table 3. Operation times.
| Length (min) of | |
| CPB | 173 (±43) |
| Aortic cross-clamp | 106 (±32) |
| Operation | 349 (±68) |
Abbreviation: CPB, cardiopulmonary bypass.
Note: Data are presented as mean ± standard deviation.
Table 4. Outcome parameters.
| Time on ICU | |
| 1 d | 68% |
| 2–4 d | 27% |
| > 4 d | 5% |
| In-hospital stay | |
| ≤7 d | 42% |
| 8–10 d | 31% |
| 11–20 d | 23% |
| > 20 d | 4% |
Abbreviation: ICU, intensive care unit.
Note: Data are presented as percentage.
Hospital mortality was 2.9%, with two patients dying from noncardiac complications (pneumonia, bowel obstruction). Myocardial infarction rate was 2.9%, and repeat revascularization rate was 2.0%. One patient suffered a perioperative stroke with minor clinical impairment. Other perioperative complications are also given in Table 5 .
Table 5. MACCE and other complications.
| MACCE | |
| In-hospital mortality | 2.9% |
| Myocardial infarction | 2.9% |
| Repeat revascularization (PCI) | 2.0% |
| Stroke | 1.0% |
| Other complications | |
| Revision due to bleeding | 8.8% |
| Delir | 15.7% |
| Pneumonia | 1.0% |
| New onset of atrial fibrillation | 16.7% |
| Superficial wound infection | 2.9% |
Abbreviations: MACCE, major adverse cardiac and cerebrovascular events; PCI, percutaneous coronary intervention.
Note: Data are presented as percentage.
In five out of nine patients undergoing rethoracotomy because of suspected active bleeding, no active surgical bleeding was detected. In two out of the remaining four patients with active surgical bleeding, a sternotomy during the revision procedure had to be performed.
Discussion
The beneficial lasting effect of CABG in treating stable coronary multivessel disease has been suggested to be based on the pathophysiological concept of “surgical collateralization,” 16 meaning that bypassing diseased proximal coronary artery segments with a vascular conduit prevents future myocardial infarctions and thereby prolongs life. The most important surgical issues for securing this long-term success comprise a combination of complete anatomical revascularization, the precise construction of coronary anastomoses, and the longevity of conduits. 2
A midline sternotomy enables an optimal exposure to all regions of the left ventricle as well as the evaluation of each epicardial coronary artery containing a proximal stenosis by direct external inspection and by palpation for a suitable distal target site. Therefore, this approach in combination with CPB and cardiac arrest has become the standard procedure for the overwhelming majority of CABG procedures worldwide within the past 50 years. 17
On the contrary, the surgical trauma related to conventional CABG has often been associated with damage to physical and mental integrity of the patient, at least early after the operation. 4 Most cardiac surgeons have accepted this as inevitable, but many patients (and cardiologists) disagree and prefer less invasive alternatives such as PCI, even at the cost of late harm, and regrettable often not concordant with guideline recommendations. 18 In this context, the midline sternal split seems to be most prohibitive for patients who increasingly ask for a therapeutic approach that leaves the sternum intact. 3
CPB and cardiac arrest are essential prerequisites in the presented TCRAT technique. 10 11 We used a peripheral CPB cannulation strategy and transthoracic aortic cross-clamping, a technique adapted from minimally invasive mitral valve surgery, to achieve an unrestricted direct vision of the operation field through a small thoracic incision. Transthoracic cross-clamping from the left side was possible by pulling the ascending aorta with an encircling tape to the left. With the heart arrested and emptied, the working space inside the pericardium becomes much larger while hemodynamics remain stable, thus allowing to encircle the great vessels inside the pericardium with tapes.
Compared with a more lateral approach used in MICS CABG, 9 19 20 21 the thoracic incision in TCRAT is located more anteriorly, a technical detail that is important because distance from the incision to both aorta as well as coronary target sites becomes shorter. In combination with the traction maneuver, a stable exposure of all regions including lateral and inferior wall at a maximal distance of about 8 cm to the thoracic incision can be established. As a result, direct examination of the coronary artery target sites even by palpation and the use of common surgical instruments for coronary anastomoses become possible. The goal of the operation is to allow the surgeon to perform, within the patients' closed chest, a revascularization configuration equivalent to that of a regular CABG operation with highest surgical precision and safety.
To achieve a complete anatomical revascularization in patients with multivessel disease, all coronary arteries with a diameter exceeding 1.5 mm and a luminal reduction of 50% or more should be grafted. 2 Therefore, the number of grafts performed in surgical revascularization of patients with multivessel disease often is considered as an indirect parameter for the completeness of revascularization. 22 The average number of grafts performed in the present study was 3.2, which compares well to the average number of 3.1 grafts reported for conventional CABG in the recent German Heart Surgery Report 2020. 23 However, it clearly exceeds the average number of 2.4 grafts in patients operated off-pump reported in the same registry 23 as well as the average number of grafts reported in MICS CABG. 9 19 21
It has been consistently shown that arterial grafts exhibit lower long-term occlusion rates compared with venous grafts. 24 Therefore, beside the use of a LIMA graft to the LAD, the additional use of a second arterial graft instead of venous grafts is recommended in recent revascularization guidelines. 2 Such a bypass material strategy has been applied in the present study with 62% of total arterial grafting and another 20% of multiple arterial grafting. However, right internal mammary artery or venous grafts may be used as well, just as standard proximal aortic anastomosing techniques can be used. 10
Long-term benefit from surgical compared with interventional myocardial revascularization is most pronounced in multivessel disease patients with diffuse coronary disease (high Syntax score), diabetes mellitus, and ischemic cardiomyopathy (low left ventricular EF). 2 In addition, nowadays, CABG patients often suffer from high comorbidity and surgical risk. While especially such patients often were regarded as not well suited for minimally invasive techniques, 8 21 we included patients with diffuse coronary disease requiring complex coronary surgery, with very low EF, and with high surgical risk. Also, we do not regard massive obesity or compromised lung function as contraindications for the presented TCRAT technique. Which patients may benefit most from TCRAT has to be investigated in further studies.
It may be of concern that duration of the operation was clearly longer than that known from routine CABG. This is partly attributed to the smaller surgical incision which increases the technical complexity to a certain degree, and moreover, to the need to carry out some surgical steps in sequence and not simultaneously, for example, harvesting of RA and mammary artery. However, this did not translate into an increased length of ICU or hospital stay in comparison to that of our routine CABG patients. Additionally, gaining more experience, we believe to shorten the procedure distinctly as it has been shown for newly introduced surgical techniques previously. 21
Aortic manipulation and CPB, especially when performed with peripheral arterial cannulation and retrograde perfusion, may be associated with an increased stroke rate. To minimize this risk, we routinely performed a preoperative CT scan to identify patients with atherosclerotic disease. 25 In case of atherosclerosis distal to the ascending aorta, a more central cannulation via the right subclavian artery was preferred. After occurrence of a stroke in a patient perfused from femoral arterial cannulation, we changed to a strictly central cannulation via the right subclavian artery and did not observe any stroke in the consecutive series of patients. In case of evidence of atherosclerotic disease of the ascending aorta, alternative no-touch aortic CABG techniques should be preferred. Whether such algorithm is effective in minimizing stroke risk remains to be investigated in further studies; however, the 1% stroke rate observed in our initial series is within the range of standard CABG or even below. 2
The main advantage of the TCRAT technique, 10 11 when compared with regular standard CABG, obviously results from the avoidance of sternotomy. The risks of superficial or deep sternal wound infections, mediastinitis, as well as sternal instability are completely eliminated. Enhanced thoracic stability, reduced wound size, and reduced wound infection risk associated with a minimally invasive thoracotomy approach have already been shown to result in accelerated early recovery and return to normal physical activity in different cardiac surgery procedures. 26 27 Currently, an ongoing multicenter randomized controlled trial compares the quality of life and recovery between nonsternotomy MICS CABG and sternotomy CABG. 28 Nevertheless, respecting sternal integrity might considerably improve both patients and physician's acceptance of surgical myocardial revascularization.
Conclusion
In conclusion, the nonsternotomy TCRAT approach allows complete coronary revascularization in the broad majority of multivessel disease patients with favorable outcomes, and it can be introduced into clinical routine safely. Long-term results remain to be investigated.
Authors' Contribution
S.A. and C.S. contributed with the study design, the collection of data, as well as in its analysis and later in the writing of the manuscript.
Conflict of Interest None declared.
Both authors contributed equally to the article .
Erratum: The article has been updated as per Erratum published on March 24, 2023. DOI of the Erratum is 10.1055/s-0043-1768041.
References
- 1.Sipahi I, Akay M H, Dagdelen S, Blitz A, Alhan C. Coronary artery bypass grafting vs percutaneous coronary intervention and long-term mortality and morbidity in multivessel disease: meta-analysis of randomized clinical trials of the arterial grafting and stenting era. JAMA Intern Med. 2014;174(02):223–230. doi: 10.1001/jamainternmed.2013.12844. [DOI] [PubMed] [Google Scholar]
- 2.ESC Scientific Document Group Neumann F-J, Sousa-Uva M, Ahlsson Aet al. 2018 ESC/EACTS guidelines on myocardial revascularization Eur Heart J 2019400287–165.30165437 [Google Scholar]
- 3.Doenst T, Diab M, Sponholz C, Bauer M, Färber G. The opportunities and limitations of minimally invasive cardiac surgery. Dtsch Arztebl Int. 2017;114(46):777–784. doi: 10.3238/arztebl.2017.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen D J, Van Hout B, Serruys P W et al. Quality of life after PCI with drug-eluting stents or coronary-artery bypass surgery. N Engl J Med. 2011;364(11):1016–1026. doi: 10.1056/NEJMoa1001508. [DOI] [PubMed] [Google Scholar]
- 5.Järvinen O, Saarinen T, Julkunen J, Huhtala H, Tarkka M R. Changes in health-related quality of life and functional capacity following coronary artery bypass graft surgery. Eur J Cardiothorac Surg. 2003;24(05):750–756. doi: 10.1016/s1010-7940(03)00413-5. [DOI] [PubMed] [Google Scholar]
- 6.Lahtinen P, Kokki H, Hynynen M. Pain after cardiac surgery: a prospective cohort study of 1-year incidence and intensity. Anesthesiology. 2006;105(04):794–800. doi: 10.1097/00000542-200610000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Bonaros N, Schachner T, Lehr E et al. Five hundred cases of robotic totally endoscopic coronary artery bypass grafting: predictors of success and safety. Ann Thorac Surg. 2013;95(03):803–812. doi: 10.1016/j.athoracsur.2012.09.071. [DOI] [PubMed] [Google Scholar]
- 8.Bonatti J, Wallner S, Crailsheim I, Grabenwöger M, Winkler B. Minimally invasive and robotic coronary artery bypass grafting-a 25-year review. J Thorac Dis. 2021;13(03):1922–1944. doi: 10.21037/jtd-20-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGinn J T, Jr, Usman S, Lapierre H, Pothula V R, Mesana T G, Ruel M.Minimally invasive coronary artery bypass grafting: dual-center experience in 450 consecutive patients Circulation 2009120(11, Suppl):S78–S84. [DOI] [PubMed] [Google Scholar]
- 10.Babliak O, Demianenko V, Melnyk Y, Revenko K, Pidgayna L, Stohov O. Complete coronary revascularization via left anterior thoracotomy. Innovations (Phila) 2019;14(04):330–341. doi: 10.1177/1556984519849126. [DOI] [PubMed] [Google Scholar]
- 11.Dörge H, Sellin C, Belmenai A, Asch S, Eggebrecht H, Schächinger V. Novel concept of routine total arterial coronary bypass grafting through a left anterior approach avoiding sternotomy. Heart Vessels. 2022;37(08):1299–1304. doi: 10.1007/s00380-022-02034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonzel T, Schächinger V, Dörge H. Description of a Heart Team approach to coronary revascularization and its beneficial long-term effect on clinical events after PCI. Clin Res Cardiol. 2016;105(05):388–400. doi: 10.1007/s00392-015-0932-2. [DOI] [PubMed] [Google Scholar]
- 13.Moussa I D, Klein L W, Shah B et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI) J Am Coll Cardiol. 2013;62(17):1563–1570. doi: 10.1016/j.jacc.2013.08.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Thygesen K, Alpert J S, Jaffe A S et al. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138(20):e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 15.Unter Mitwirkung der folgenden Wissenschaftlichen Fachgesellschaften und Institutionen: Deutsche Gesellschaft für Chirurgie Deutsche Gesellschaft für Innere Medizin e. V. Deutsche Gesellschaft für Internistische Intensivmedizin und Notfallmedizin Deutsche Sepsis-Gesellschaft e. V. und Robert Koch-Institut Dalhoff K, Abele-Horn M, Andreas Set al. Epidemiologie, Diagnostik und Therapie erwachsener Patienten mit nosokomialer Pneumonie – Update 2017 Pneumologie 2018720115–63.29341032 [Google Scholar]
- 16.Doenst T, Haverich A, Serruys P et al. PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J Am Coll Cardiol. 2019;73(08):964–976. doi: 10.1016/j.jacc.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 17.Head S J, Milojevic M, Taggart D P, Puskas J D. Current practice of state-of-the-art surgical coronary revascularization. Circulation. 2017;136(14):1331–1345. doi: 10.1161/CIRCULATIONAHA.116.022572. [DOI] [PubMed] [Google Scholar]
- 18.Hannan E L, Samadashvili Z, Cozzens K et al. Changes in percutaneous coronary interventions deemed “inappropriate” by appropriate use criteria. J Am Coll Cardiol. 2017;69(10):1234–1242. doi: 10.1016/j.jacc.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 19.Diab M, Färber G, Sponholz C et al. Coronary artery bypass grafting using bilateral internal thoracic arteries through a left-sided minithoracotomy: a single-center starting experience. Thorac Cardiovasc Surg. 2019;67(06):437–443. doi: 10.1055/s-0038-1670632. [DOI] [PubMed] [Google Scholar]
- 20.Nambiar P, Kumar S, Mittal C M, Saksena K. Minimally invasive coronary artery bypass grafting with bilateral internal thoracic arteries: will this be the future? J Thorac Cardiovasc Surg. 2018;155(01):190–197. doi: 10.1016/j.jtcvs.2017.07.088. [DOI] [PubMed] [Google Scholar]
- 21.Davierwala P M, Verevkin A, Sgouropoulou S et al. Minimally invasive coronary bypass surgery with bilateral internal thoracic arteries: early outcomes and angiographic patency. J Thorac Cardiovasc Surg. 2021;162(04):1109–1.119E7. doi: 10.1016/j.jtcvs.2019.12.136. [DOI] [PubMed] [Google Scholar]
- 22.Chikwe J, Lee T, Itagaki S, Adams D H, Egorova N N. Long-term outcomes after off-pump versus on-pump coronary artery bypass grafting by experienced surgeons. J Am Coll Cardiol. 2018;72(13):1478–1486. doi: 10.1016/j.jacc.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 23.Beckmann A, Meyer R, Lewandowski J, Markewitz A, Gummert J. German Heart Surgery Report 2020: the annual updated registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg. 2021;69(04):294–307. doi: 10.1055/s-0041-1730374. [DOI] [PubMed] [Google Scholar]
- 24.RADIAL Investigators . Gaudino M, Benedetto U, Fremes S et al. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N Engl J Med. 2018;378(22):2069–2077. doi: 10.1056/NEJMoa1716026. [DOI] [PubMed] [Google Scholar]
- 25.Merlo A, Chen K, Deo S, Markowitz A. Does routine preoperative computed tomography imaging provide clinical utility in patients undergoing primary cardiac surgery? Interact Cardiovasc Thorac Surg. 2017;25(04):659–662. doi: 10.1093/icvts/ivx098. [DOI] [PubMed] [Google Scholar]
- 26.Cohn L H, Adams D H, Couper G Set al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair Ann Surg 199722604421–426., discussion 427–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGee E, Jr, Danter M, Strueber M et al. Evaluation of a lateral thoracotomy implant approach for a centrifugal-flow left ventricular assist device: the LATERAL clinical trial. J Heart Lung Transplant. 2019;38(04):344–351. doi: 10.1016/j.healun.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Guo M H, Wells G A, Glineur D et al. Minimally Invasive coronary surgery compared to STernotomy coronary artery bypass grafting: the MIST trial. Contemp Clin Trials. 2019;78:140–145. doi: 10.1016/j.cct.2019.01.006. [DOI] [PubMed] [Google Scholar]


