Abstract
Background
Atherosclerosis is considered the most common cause of morbidity and mortality worldwide. Athermanous plaque formation is pathognomonic of atherosclerosis. The main feature of atherosclerosis is the formation of plaque, which is inseparable from endothelial cells, vascular smooth muscle cells, and macrophages. MicroRNAs, a small highly conserved noncoding ribonucleic acid (RNA) molecule, have multiple biological functions, such as regulating gene transcription, silencing target gene expression, and affecting protein translation. MicroRNAs also have various pharmacological activities, such as regulating cell proliferation, apoptosis, and metabolic processes. It is noteworthy that many studies in recent years have also proved that microRNAs play a role in atherosclerosis.
Methods
To summarize the functions of microRNAs in atherosclerosis, we reviewed all relevant articles published in the PubMed database before June 2022, with keywords “atherosclerosis,” “microRNA,” “endothelial cells,” “vascular smooth muscle cells,” “macrophages,” and “cholesterol homeostasis,” briefly summarized a series of research progress on the function of microRNAs in endothelial cells, vascular smooth muscle cells, and macrophages and atherosclerosis. Results and Conclusion. In general, the expression levels of some microRNAs changed significantly in different stages of atherosclerosis pathogenesis; therefore, MicroRNAs may become new diagnostic biomarkers for atherosclerosis. In addition, microRNAs are also involved in the regulation of core processes such as endothelial dysfunction, plaque formation and stabilization, and cholesterol metabolism, which also suggests the great potential of microRNAs as a therapeutic target.
1. Introduction
According to statistics, as of 2019, an average of nearly 18 million people died of cardiovascular diseases every year, accounting for 32% of the global death toll. Atherosclerosis (AS) is the leading cause of death from cardiovascular diseases worldwide [1]. AS refers to a type of chronic blood disease in which a large amount of fat or cholesterol deposits on the walls of blood vessels and arteries, forming atherosclerotic plaques, resulting in reduced blood flow and blocked blood vessels. There are no obvious symptoms in the early stage of AS, but when the late symptoms do appear, the blood flow in the lumen is narrowed, and the blood flow is reduced, causing myocardial ischemia and hypoxia, and in severe cases, myocardial infarction, arrhythmia, and even sudden death [2]. The pathological mechanism of AS is complex, starting from the damaged intima, and the formation of plaques is also in the intima. In the early stage of the lesion, low-density lipoprotein (LDL) particles accumulate in the intima and are oxidized to oxidized LDL, causing an inflammatory response. Subsequently, proinflammatory monocytes bind to adhesion factors of endothelial cells, promoting the migration of monocytes to the arterial wall, where monocytes mature into macrophages in the intima. In the intima, macrophages transform into foam cells by engulfing lipoprotein particles, which further collect fat, cholesterol, and other substances. In the middle and late stages of the disease, the smooth muscle cells located in the media also enter the intima under the action of the medium produced by the aggregation of leukocytes, and proliferate, and accumulate in the arterial wall. As fat, cholesterol, and large amounts of cellular debris accumulate in the intima, plaques eventually form, which resemble “porridge.” With the increase of the plaque, some plaques are unstable and detached or are too large to split, causing blood vessel blockage and thrombus formation, which is also the main reason for the high mortality rate of AS [3]. Furthermore, the developmental, progression, and formation of clinically relevant atherosclerotic plaques consist of endothelial cells, vascular smooth muscle cells, and macrophages [4]. In short, vascular smooth muscle cells accumulate and aggregate in susceptible sites, resulting in dense cell arrangement and intima thickening, promoting endothelial cell activation and producing platelet-derived growth factor. With the stimulation of a large number of cytokines, the production of lipoproteins, proteoglycans, and fibronectin is promoted. As the retained lipoproteins are taken up by macrophages and vascular smooth muscle cells, lipid uptake and foam cell formation are enhanced, thereby accelerating the progression of the lesion [5].
MicroRNAs, also known as miRNAs or miRs, were first discovered in 1993 in Caenorhabditis elegans (C. elegans) [6, 7]. MicroRNAs are a class of small noncoding ribonucleic acid (RNA) regulatory molecules with a length of about 18–25 nucleotides, widely present in mammalian cells and body fluids, and involved in posttranscriptional regulation of gene expression and protein translation [8, 9]. Most mature microRNAs start from the initial primary transcription product primary microRNA (pri-miRNA), and pri-miRNA is cleaved by RNase III Drosha enzyme in the nucleus to become a single-stranded RNA precursor microRNA (pre-miRNA), and then the pre-miRNA is transported to the cytoplasm, where it is further processed into mature miRNA by RNase III Dicer [10]. Since the discovery of microRNAs, a variety of complex cellular events involved in it have been reported one after another [11]. For example, embryonic development [12], cell proliferation and apoptosis [13], and immune response [14], etc. At the same time, miRNAs are also involved in the regulation of many diseases, such as cancer [15], central nervous system diseases [16], infectious diseases [17], and diabetes [18]. Furthermore, microRNAs are required for the normal development of the cardiovascular system. MicroRNAs affect the development of AS by regulating the proliferation, adhesion, and endothelial dysfunction of endothelial cells, intervening in the accumulation of cholesterol mediated by macrophages and the formation of foam cells, and regulating the proliferation, migration, and inflammatory response of vascular smooth muscle cells (Figure 1) [7–9, 19–21].
Figure 1.
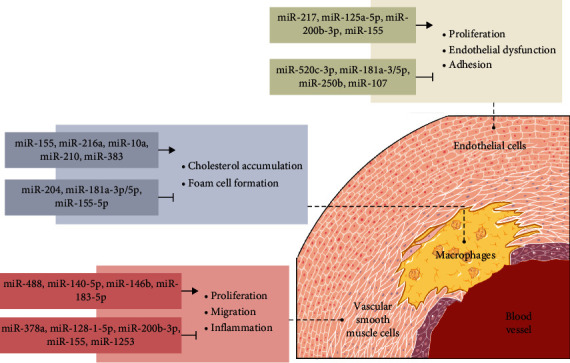
Regulation of microRNAs in different cells in AS.
Here, we systematically summarize the regulation mechanism of microRNAs on endothelial cells, vascular smooth muscle cells, macrophages, and cholesterol balance in the pathological process of AS and elaborate on the potential of microRNAs as clinical diagnostic biomarkers of AS, as well as future challenges, to provide a reference for researchers exploring related fields.
2. MicroRNAs Regulate Endothelial Cells
The abnormal function of endothelial cells is the initial stage of AS. Accumulating evidence indicates that endothelial dysfunction triggers an inflammatory response that drives the occurrence, development, and even rupture of atherosclerotic plaques and ultimately promotes the pathological development of AS [19, 22]. Some microRNAs have positive effects on improving AS. For instance, miR-520c-3p inhibits endothelial damage to attenuate AS by regulating vascular endothelial cell proliferation, apoptosis, and adhesion [23]. MiR-181a-5p, miR-181a-3p, and miR-250b have been proven to inhibit NF-κB activation to prevent the occurrence of vascular inflammation, thereby slowing the progression of AS [24, 25]. Furthermore, miR-107 protects vascular endothelial cells against injury by inhibiting endoplasmic reticulum stress [26]. All in all, in endothelial cells, microRNAs mainly slow the progression of AS by inhibiting inflammatory responses, endoplasmic reticulum stress, or reducing endothelial cell damage. Conversely, some microRNAs promote the processes of AS. To give two examples of exacerbating AS, miR-217 has been shown to promote endothelial dysfunction by regulating the endothelial signaling center to trigger eNOS signaling, thereby aggravating AS [27]. MiR-125a-5p, miR-200b-3p, and miR-155 are thought to cause pyroptosis, apoptosis, and autophagy of endothelial cells, respectively, resulting in endothelial injury (Table 1) [19, 28, 29].
Table 1.
The effects and mechanisms of microRNAs in regulating endothelial cells.
| MicroRNAs | Effects | Mechanisms | References |
|---|---|---|---|
| miR-125a-5p | Promoted oxLDL-induced vascular endothelial cells pyroptosis | Inhibited TET2 expression, resulting in DNA methylation, mitochondrial dysfunction, increasing ROS production, and activated NF-κB that induces activation of inflammasome and maturation, the release of proinflammatory cytokines | [28] |
|
| |||
| miR-181a-5p | Blocked blood vessel inflammation | Inhibited NF-κB signaling pathway by targeting TAB2 protein | [24] |
|
| |||
| miR-181a-3p | Blocked blood vessel inflammation | Inhibited NF-κB signaling pathway by targeting NEMO protein | [24] |
|
| |||
| miR-217 | Promoted endothelial dysfunction | Regulated an endothelial signaling hub and downregulated a network of eNOS activators | [27] |
|
| |||
| miR-200b-3p | Promoted endothelial cells apoptosis | Promoted oxidative stress-induced cell apoptosis by targeting HDAC4 | [29] |
|
| |||
| miR-107 | Inhibited inflammatory response and endoplasmic reticulum stress of vascular endothelial cells | Regulated notch 1 signaling pathway | [26] |
|
| |||
| miR-520c-3p | Inhibited vascular endothelium dysfunction | Regulated AKT and NF-κB signaling pathway by targeting RELA protein | [23] |
|
| |||
| miR-250b | Inhibited endothelial cells inflammation | Downregulated NF-κB p65-ICAM1/VCAM1 axis | [25] |
|
| |||
| miR-155 | Promoted endothelial cells autophagic activity | Inhibited Rheb-mediated mTOR/P70S6kinase/4EBP signaling pathway | [19] |
OxLDL, oxidized low-density lipoprotein; TET2, tet methylcytidine dioxygenase 2; NF-κB, nuclear factor κ-B; TAB2, mitogen-activated protein kinase 7-interacting protein 2; NEMO, NF-κ-B essential modulator; eNOS, endothelial nitric oxide synthase; HDAC4, histone deacetylase 4; RELA, nuclear factor NF-κ-B p65 subunit; ICAM1, intercellular adhesion molecule; VCAM1, vascular cell adhesion protein 1; mTOR, the mammalian target of rapamycin; 4EBP, translation initiation factor 4E-binding protein 1.
3. MicroRNAs Regulate Vascular Smooth Muscle Cells
Vascular smooth muscle cells reside in the medial layer of the arterial wall and are responsible for regulating vascular tone [30]. In AS, vascular smooth muscle cells migrate from the medial to the intima to proliferate and deposit in large numbers, further invading the plaque, causing the plaque to be unstable and prone to fall off, causing blood vessel blockage [21]. MicroRNAs have also been found to be involved in the transfer from endothelial cells to vascular smooth muscle cells, inhibiting apoptosis and inflammatory response or promoting cell proliferation and migration (Table 2) [31].
Table 2.
The effects and mechanisms of microRNAs in regulating vascular smooth muscle cells.
| MicroRNAs | Effects | Mechanisms | References |
|---|---|---|---|
| miR-192-5p | Inhibited cell proliferation and migration | Increased ATG7 expression | [32] |
|
| |||
| miR-183-5p | Promoted cell proliferation and migration | / | [39] |
|
| |||
| miR-17-5p | Inhibited cell proliferation and apoptosis | Increased SIRT7 expression | [35] |
|
| |||
| miR-378a | Inhibited cell proliferation, migration, and inflammation | Increased the expression IGF1 and TLR8 by PGC1α/NRF1/miR-378a regulatory axis | [36] |
|
| |||
| miR-214-3p | Eliminated cell proliferation, migration, and inflammation | Upregulated FOXO1 expression | [33] |
|
| |||
| miR-128-1-5p | Inhibited cell apoptosis and inflammation | Negatively regulated RMRP or Gadd45g | [37] |
|
| |||
| miR-326-3p | Inhibited cell viability, cell cycle distribution, and migration capacity | Inhibited VAMP3 expression | [42] |
|
| |||
| miR-1253 | Inhibited proliferation and promotion of apoptosis | Inhibited FOXF1 expression | [41] |
|
| |||
| miR-146b | Inhibited cell proliferation and migration | Upregulated Bag1 and MMP16 | [34] |
|
| |||
| miR-140-5p | Promoted cell viability, migration, and invasion | Reduced ROBO4 gene expression | [40] |
|
| |||
| miR-488 | Promoted the proliferation and migrated ability | / | [38] |
ATG7, autophagy related 7; SIRT7, sirtuin 7; IGF1, insulin-like growth factors 1; TLR8, Toll-like receptor 8; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1-α; NRF1, nuclear respiratory factor 1; FOXO1, forkhead box O1; RMRP, RNA component of mitochondrial RNA processing endoribonuclease; Gadd45g, growth arrest and DNA damage inducible gamma; VAMP3, vesicle associated membrane protein 3; FOXF1, forkhead box F1; Bag 1, bag cochaperone 1; MMP16, matrix metallopeptidase 16; ROBO4, roundabout guidance receptor 4.
Some microRNAs inhibit the proliferation and migration of vascular smooth muscle cells and slow down the progression of AS. For example, miR-192-5p targets the expression of ATG7 and regulates autophagy [32]. MiR-214-3p acts by downregulating the expression of FOXO1 [33]. MiR-146b exerts its effect by downregulating the expression of Bag1 and MMP16 [34]. In addition, some microRNAs can also inhibit apoptosis and inflammatory responses, thereby alleviating AS. For instance, miR-17-5p has the effect of enhancing cell proliferation and repairing wounds and reduces apoptosis by upregulating SIRT7 expression and inhibiting p53 activation [35]. MiR-378a targeting IGF1 and TLR8 significantly inhibits inflammatory response [36]. MiR-128-1-5p inhibits the expression of inflammatory factors and apoptotic proteins by regulating the RMRP/miR-128-1-5P/Gadd45g signaling pathway [37]. Conversely, some microRNAs play the opposite role and accelerate AS deterioration. For example, both miR-183-5p and miR-488 can promote cell proliferation and migration by targeting myocyte enhancer factor 2C (MEF2B) [38, 39]. MiR-140-5p increases cell viability and promotes cell invasion by reducing the ROBO4 expression [40]. MiR-1253 binds to FOXF1 to inhibit its proliferation, thereby promoting apoptosis [41].
4. MicroRNAs Regulate Macrophages
Macrophages are an important part of the human immune system and can phagocytose and clear cell debris, dead cells, and pathogens in vivo [43]. During AS, on the one hand, macrophages are responsible for processing a large amount of cholesterol and triglycerides, and simultaneously help to clear some inflammatory substances [44]. On the other hand, once LDL enters, cholesterol accumulates in the blood vessel wall, and macrophages absorb the cholesterol oxidized by their free radicals, eventually turning into foam cells, which are conducive to plaque formation [45]. Recently, the roles of microRNAs in macrophages have been focused on, affecting the pathological process of AS by regulating inflammatory response, cholesterol metabolism, and foam cell formation (Table 3) [21, 45]. MiR-204 downregulates NFATc3 expression and prevents the formation of foam cells and AS [46]. MiR-181a-3p/5p and miR-155-5p attenuate inflammatory responses, and delay plaque formation, thereby slowing AS [24, 47]. Some microRNAs prevent AS by promoting mitochondrial oxidative metabolism, reducing ROS production and necroptosis, and improving cell survival, such as miR-10a [48], miR-210 [49], and miR-383 [49]. In addition, miR-368a promotes reverse cholesterol transport through the CD47-SIRPα axis and hinders AS progression [50]. However, miR-155 activates the NLRP3 inflammasome by regulating the ERK1/2 pathway and aggravates AS [51]. MiR-216a activates telomerase by regulating the Smad3/NF-κB pathway and promotes AS development [50].
Table 3.
The effects and mechanisms of microRNAs in regulating macrophages.
| MicroRNAs | Effects | Mechanisms | References |
|---|---|---|---|
| miR-204 | Prevented foam cell formation | Upregulated NFATc3 to reduce SR-A and CD36 levels | [46, 52] |
|
| |||
| miR-181a-3p/5p | Delayed plaque formation | Reduced proinflammatory gene expression and macrophage infiltration | [24] |
|
| |||
| miR-155 | Activated NLRP3 inflammasome | Blocking the ERK1/2 pathway | [51] |
|
| |||
| miR-10a | Promoted mitochondrial oxidative metabolism in macrophages | Promoted Dicer/miR-10a-dependent metabolic reprograming | [48] |
|
| |||
| miR-210 | Reduced ROS production and necroptosis | Upregulated the HIF-1α level | [49] |
|
| |||
| miR-383 | Reduced energy consumption and increased cell survival | Blocked the targeting of Parg protein | [49] |
|
| |||
| miR-155-5p | Mitigated vascular inflammation | Stimulated CTRP12 production | [47] |
|
| |||
| miR-368a | Promoted reverse cholesterol transport | Reduced SIRPA expression | [50] |
|
| |||
| miR-216a | Activated telomerase | Inhibited the Smad3/NF-κB signaling pathway | [53] |
NFATc3, nuclear factor of activated T cells 3; SR-A, the class A macrophage scavenger receptors; CD36, fatty acid translocase; NLRP3, NLR family pyrin domain containing 3; ERK1/2, extracellular signal-regulated protein kinase1/2; HIF-1α, hypoxia-inducible factor 1-α; CTRP12, C1q tumor necrosis factor-related protein 12; SIRPA, signal regulatory protein alpha; Smad3, suppressor of mothers against decapentaplegic 3; NF-κB, nuclear factor κB.
5. MicroRNAs Regulate Cholesterol Homeostasis
Cholesterol homeostasis is a key to lipid accumulation in atherosclerotic plaques and increases the risk and exacerbation of AS once the balance of cholesterol is disrupted [54]. In recent years, microRNAs have played key regulatory roles in lipid homeostasis and cholesterol homeostasis involved in AS development (Table 4) [44]. For example, miR-210-3p inhibited NF-κB activation, reducing lipid accumulation and inflammatory responses [55]. MiR-34a, miR-33-5p, and miR-21 inhibit the development of AS by reducing intestinal cholesterol, regulating cholesterol efflux, and preventing foam cell formation, respectively [54, 56, 57]. In addition, miR-33a/b promotes lipid droplet accumulation by inhibiting apoptosis and accelerates AS [58]. So far, the role of microRNA in cholesterol homeostasis in AS needs more research support.
Table 4.
The effects and mechanisms of microRNAs in regulating cholesterol homeostasis.
| MicroRNAs | Effects | Mechanisms | References |
|---|---|---|---|
| miR-210-3p | Reduced lipid accumulation and inflammatory response | Inhibited IGF2/IGF2R to inhibit CD36 and NF-κB expressions | [55] |
|
| |||
| miR-34a | Reduced intestinal cholesterol or fat absorption | Inhibited CYP7A1 and CYP8B1 | [54] |
|
| |||
| miR-33a/b | Promoted lipid droplet accumulation | Inhibited apoptotic cell clearance via an autophagy-dependent mechanism | [58–62] |
|
| |||
| miR-33-5p | Regulated cholesterol efflux | Regulated the miR-33-5p/ABCA1/CS axis | [57] |
|
| |||
| miR-21 | Influenced foam cell formation | Promoted p38-CHOP and JNK signaling pathway | [56] |
IGF2, insulin-like growth factor 2; IGF2R, insulin-like growth factor 2 receptor; CD36, fatty acid translocase; NF-κB, nuclear factor κB; CYP7A1, cholesterol 7a-hydroxylase; CYP8B1, sterol-12a hydroxylase; ABCA1, ATP-binding cassette transporter A1; CS, citrate synthase; p38-CHOP, p38- C/EBP homologous protein; JNK, c-Jun N-terminal kinase.
6. MicroRNAs Therapeutic Potential for AS
As summarized above, numerous studies have demonstrated the roles of microRNAs in regulating various pathological mechanisms in AS. MicroRNAs play important roles in the dysregulation that affects endothelial integrity, the function of vascular smooth muscle cells, macrophage, and cellular cholesterol homeostasis, which drives the initiation and growth of an atherosclerotic plaque [63]. In recent years, more studies have investigated the potential of microRNAs as therapeutic targets or biomarkers in AS (Table 5) [64]. For instance, hsa-miR-654-5p and hsa-miR-409-3p are the potentially critical biomarkers for AS patients [65]. Low expression of miR-211-5p and miR-675-3p are associated with the poor prognosis of AS [66, 67]. Low expression of miR-191-3p, miR-933, and miR-425-3p are related to the peripheral circulation of patients with lipid metabolism disorders, mainly LDL [68]. Moreover, dysregulation of microRNAs has a role in vascular aging [69]. Although there are few clinical studies of microRNAs for AS treatment, a variety of microRNAs have been found to reduce atherosclerosis in preclinical animal models, and some of these microRNAs have entered clinical studies in other diseases. For example, miR-494 is used to treat ischemic stroke (NCT03577093). miR-33 for the treatment of metabolic syndrome (NCT02606812) and heart failure (NCT02997462). miR-44 for the treatment of intracranial atherosclerosis (NCT03208166). miR-210 for the treatment of angina (NCT05374694). miR-155 for the treatment of bladder cancer (NCT03591367). miR-181 for the treatment of psoriasis (NCT05683769). miR-29 for the treatment of shoulder and neck pain (NCT02534558). These pieces of evidence fully illustrate the feasibility of microRNA therapy.
Table 5.
MicroRNAs as clinical biomarkers with therapeutic potential in the AS.
| MicroRNAs | Cellular process | Cell type | Down- or upregulated | References |
|---|---|---|---|---|
| miR-654-3/5p | Apoptosis and inflammatory response | Endothelial cells | ↓ | [13, 77, 78] |
|
| ||||
| miR-409-3p | Senescence | Endothelial cells | ↑ | [13, 79] |
|
| ||||
| miR-933 | Oxidative stress and inflammatory response | Endothelial cells | ↑ | [16, 80] |
|
| ||||
| miR-122 | Plaque stabilization | Endothelial cells | ↑ | [81, 82] |
|
| ||||
| miR-92 | Cholesterol buildup, inflammatory response | Endothelial cells | ↓ | [83] |
| Foam cell formation | Macrophages | [84] | ||
|
| ||||
| miR-211-5p | Inflammatory response | Macrophages | ↓ | [14, 85] |
|
| ||||
| miR-675-3p | Adipogenesis and glucose metabolic | Macrophages | ↓ | [15, 86, 87] |
|
| ||||
| miR-16 | Inflammatory response | Macrophages | ↓ | [88, 89] |
|
| ||||
| miR-155 | Foam cell formation and cholesterol efflux | Macrophages | ↓ | [90] |
|
| ||||
| miR-191-3p | Platelet activation and fibrous cap thinning | Smooth muscle cells | ↓ | [16, 63, 91] |
|
| ||||
| miR-425-3/5p | Migration, phenotypic transformation, and proliferation | Vascular smooth muscle cells | ↓ | [16, 92] |
|
| ||||
| miR-34 | Vascular aging and inflammatory response | Vascular smooth muscle cells and endothelial cells | ↑ | [93, 94] |
| Inflammatory response | Endothelial cells | [95] | ||
|
| ||||
| miR-29 | Vascular endothelial injury | Endothelial cells | ↑ | [96] |
| Proliferation and migration | Vascular smooth muscle cells and endothelial cells | [97] | ||
|
| ||||
| miR-21 | Plaques vulnerability | Macrophages | ↓ | [98] |
| Proliferation and migration | Vascular smooth muscle cells | [99] | ||
Although there are currently no microRNA drugs approved for the treatment of AS, drug candidates are in clinical development and clinical trials. Candidate drugs for microRNA therapy are mostly concentrated in antisense oligonucleotides (anti-miRs), microRNA mimics, and microRNA inhibitors. Among them, anti-miRs are the reverse complementary sequences of mature microRNAs, which bind to endogenous microRNAs and inactivate them through steric blocking, thereby regulating the function of microRNAs [70, 71], such as miR-494, miR-33, miR-712, and miR-114. MicroRNA mimics are synthetic double-stranded microRNAs fragments that regulate the post-translational function of microRNAs by specifically binding to target genes and inhibiting their transcription and translation [72], such as miR-210, miR-125a-5p, miR-29a-3p, miR -115, and miR-181a-3/5p. In addition, miRNA inhibitors are designed to have the reverse complementary strand of the target gene, and microRNAs affect the normal function of miRNA inhibitors by binding to the target site [73], such as miR-29 and miR-24-3p. Encouragingly, a handful of microRNA drug candidates have recently entered clinical trials. For example, MRG-110, as an antisense oligonucleotide, has entered phase I clinical trials (NCT03603431) to control angiogenesis and myocardial ischemia by targeting and inhibiting miR-92a [74]. Except for MRG-110, MRG-201 targets miR-29b to regulate the synthesis of extracellular matrix and has entered phase II clinical trials for fibrosis (NCT03601052) [75]. Furthermore, CDR132L improves cardiac systolic and diastolic functions by targeting miR-132 and has completed phase I clinical trials for the treatment of heart failure (NCT04045405) [76]. We summarized these microRNAs with AS clinical therapeutic potential in Table 6, and we also expect these microRNAs to achieve positive results in the clinical treatment of AS.
Table 6.
MicroRNAs therapeutic strategies in AS.
| Strategy | MicroRNA | Target genes | Effects in AS | Clinical status | Reference |
|---|---|---|---|---|---|
| Antisense oligonucleotide (anti-miRs) | miR-494 | Mef2A | Promoted plaque stabilization | Preclinical | [100] |
| miR-33 | ABCA1 | Decreased lipid accumulation | Preclinical | [101] | |
| miR-712 | TIMP3 | Decreased endothelial inflammation | Preclinical | [102] | |
| miR-144 | ABCA1/ABCG1 | Regulated cholesterol metabolism and endothelial dysfunction | Preclinical | [103, 104] | |
| miR-92a | miR-92a | Regulated angiogenesis and ischemia | Phase Ⅰ | [74] | |
| miR-29b | miR-29b | Regulated extracellular matrix synthesis and fibrosis | Phase Ⅱ | [75] | |
| miR-132 | miR-132 | Improved cardiac function | Phase Ⅰ | [76] | |
|
| |||||
| microRNA mimics | miR-210 | IGF2 | Attenuated lipid accumulation and inflammation | Recruiting | [55, 105, 106] |
| miR-125a-5p | Ninjurin 1 | Attenuated vascular dysfunction | Preclinical | [107] | |
| CCL4 | Decreased ox-LDL | [108] | |||
| miR-29a-3p | TNFRSF1A | Suppressed proliferation, migration, and invasion of VSMCs | Preclinical | [109] | |
| miR-155 | NLRP3 | Attenuated inflammatory response | Preclinical | [7, 110] | |
| miR-181a-3p | NEMO | Inhibited vascular inflammation | Preclinical | [24, 111] | |
| miR-181a-5p | TAB2 | ||||
|
| |||||
| microRNA inhibitors | miR-29 | LYPLA1 | Promoted endothelial function | Preclinical | [112] |
| CDC7 | Regulated VSMCs proliferation and migration | [97] | |||
| miR-24-3p | Bcl2L11 | Prevented cell growth of VSMCs | Preclinical | [113] | |
| Impα3 | Inhibited the proliferation and migration of endothelial cells | [114] | |||
Mef2A, myocyte enhancer factor 2A; ABCA1, ATP-binding cassette transporter A1; TIMP3, TIMP metallopeptidase inhibitor 3; ABCG1, ATP-binding cassette subfamily G member 1; IGF2, insulin like growth factor 2; Ninjurin 1, nerve injury-induced protein 1; CCL4, chemokine C-C-motif ligand 4; TNFRSF1A, tumor necrosis factor receptor superfamily member 1A; NLRP3, NLR family pyrin domain containing 3; NEMO, NF-κ-B essential modulator; TAB2, TGF-beta activated kinase 1-binding protein 2; LYPLA1, lysophospholipase 1; CDC7, cell division cycle 7; Bcl2L11, BCL-2 like protein 11; Impα3, importin-α3; VSMCs, vascular smooth muscle cells; ox-LDL, oxidizied low-density lipoprotein.
7. Discussion
As mentioned earlier, AS, as the most common cause of high morbidity and mortality in the world, is viewed as the result of four major steps, including (1) the initiation of endothelial cells activation and inflammation; (2) the promotion of intimal lipoprotein deposition, retention, modification, and foam cell formation; (3) the progression of complex plaques by plaque growth, enlargement of the necrotic core, fibrosis, thrombosis, and remodeling; (4) the precipitation of acute events such as myocardial infarction, unstable angina, ventricular fibrillation, or sudden coronary death [5]. As we have seen, microRNAs play indispensable roles in various stages of AS progression. Interestingly, the regulatory effects of most microRNAs can slow down the development of AS. For example, miR-520c-3p, miR-181a-5p, miR-181a-3p, miR-250b, and miR-107 have been shown to regulate endothelial cell proliferation, injury, or inflammatory responses, thereby reducing endothelial dysfunction [24, 27–29]. miR-204, miR-181a-3p/5p, miR-155-5p, miR-10a, miR-210, miR-383, miR-368a, miR-210-3p, miR-34a, miR-33-5p and miR-21 delays plaque formation by regulating cholesterol metabolism, avoiding conversion of macrophages into foam cells [32, 33, 35–37, 39]. miR-204, miR-181a-3p/5p, miR-155-5p, miR-10a, miR-210, miR-383, miR-368a, miR-210-3p, miR-34a, miR-33-5p, and miR-21 delays plaque formation by regulating cholesterol metabolism and avoiding conversion of macrophages into foam cells [24, 45, 48, 49, 51, 54, 55, 59, 60]. On the contrary, there are also a small number of microRNAs that can worsen the process of AS. For example, miR-217, miR-125a-5p, miR-200b-3p, and miR-155 cause damage to endothelial cells [20, 23, 25, 26]. miR-183-5p, miR-488, miR-140-5p, and miR-1253 accelerate intravascular plaque shedding [34, 40–42]. miR-155, miR-216a, and miR-33a/b promote cholesterol accumulation and accelerate foam cell and plaque formation [47, 49, 58]. Therefore, microRNA is very likely to be used as a potential biomarker in the clinical diagnosis of AS in addition to routine blood tests and imaging tests.
However, the role of microRNAs in AS is still being explored, and many questions still need to be answered to deepen our understanding. For example, the entry of exogenous microRNA may interfere with the normal regulatory mechanism in cells and cause side effects, adverse reactions, or immune responses [115–117], which may limit the therapeutic effect of microRNA and its safety issues. Additionally, since AS involves multiple cell types and signaling pathways, how to ensure that microRNAs target specific target cells or tissues to avoid affecting normal cells or tissues cannot be ignored. In this regard, many developers have tried using different delivery systems to improve the biodegradation and targeting of microRNAs in vivo [118]. For instance, Liu et al. [46] use nanodiamonds as delivery vehicles for microRNAs and implant them into induced pluripotent stem cells to promote the differentiation of induced pluripotent stem cells into cardiomyocytes and enhance the ability of damaged cardiomyocytes to recover cardiac function. Lolli et al. [119] use fibrin/hyaluronic acid (F/H) hydrogel to encapsulate microRNAs and implant subcutaneous damaged cartilage tissue and enhances the cartilage repair and regeneration function of endogenous cells. Based on nanoparticles and hydrogels, Li et al. [121] developed a new delivery system in which nanocarriers were encapsulated in injectable hydrogels, using this delivery system to deliver microRNAs to promote angiogenesis while reducing inflammation and effectively reduce the infarct size after myocardial infarction [122]. In addition, exosomes are another novel delivery system for microRNAs [123], which can improve the uptake of microRNAs by cells while promoting angiogenesis and wound healing [124]. However, the complex nanocarrier encapsulation process will inevitably cause off-target effects of microRNAs in vivo [125]. The charge properties of microRNAs affect their rate of release from hydrogels [120]. Treatment methods such as sonication and incubation with permeabilizers may cause exosomes to reorganize or deform and destroy the integrity of exosomes, thereby affecting the delivery efficiency of microRNAs [105, 124]. The solution to these problems is still a major research focus in the development of microRNAs delivery vectors in the future. Nonetheless, microRNAs are an exciting area of research because of their unique regulatory mechanisms and therapeutic potential in AS. It is hoped that continued clinical research and technological advances will shed light on these unresolved questions and advance the practical application of microRNAs as therapeutics for cardiovascular diseases.
8. Conclusions
MicroRNAs play important roles in AS development. We elucidate and summarize the recent studies on microRNAs regulation of the functions of endothelial cells, vascular smooth muscle cells, macrophages, and cholesterol metabolism in AS. MicroRNAs have the potential to be a novel diagnostic biomarker and therapeutic targets for atherosclerosis in the future.
Acknowledgments
Authors would like to appreciate all authors who participated in this study. This work was supported by the Macau Science and Technology Development fund (FDCT 0007/2019/AKP, 0021/2020/AGJ, 0011/2020/A1); the National Natural Science Foundation of China (Nos. 81973320); the Key Technology R&D Program of Science and Technology Commission Foundation of Tianjin (20YFZCSY00460); and the Technology Base and Talents Special Program of Guangxi Province (Guike-AD20238024).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
L.Z. prepared the manuscript and drew charts. S.S. and Z.Y. drew charts. S.R. reviewed the manuscript. All authors have read and approved the final manuscript.
References
- 1.Fan J., Watanabe T. Atherosclerosis: known and unknown. Pathology International . 2022;72(3):151–160. doi: 10.1111/pin.13202. [DOI] [PubMed] [Google Scholar]
- 2.Herrington W., Lacey B., Sherliker P., Armitage J., Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circulation Research . 2016;118(4):535–546. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 3.Libby P., Buring J. E., Badimon L., et al. Atherosclerosis. Nature Reviews Disease Primers . 2019;5 doi: 10.1038/s41572-019-0106-z.56 [DOI] [PubMed] [Google Scholar]
- 4.Fasolo F., Di Gregoli K., Maegdefessel L., Johnson J. L. Non-coding RNAs in cardiovascular cell biology and atherosclerosis. Cardiovascular Research . 2019;115(12):1732–1756. doi: 10.1093/cvr/cvz203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannarino E., Pirro M. Molecular biology of atherosclerosis. Clinical Cases in Mineral Bone Metabolism . 2008;5(1):57–62. [PMC free article] [PubMed] [Google Scholar]
- 6.Lee R. C., Feinbaum R. L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell . 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 7.Fitzsimons S., Oggero S., Bruen R., et al. MicroRNA-155 is decreased during atherosclerosis regression and is increased in urinary extracellular vesicles during atherosclerosis progression. Frontiers in Immunology . 2020;11 doi: 10.3389/fimmu.2020.576516.576516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raitoharju E., Oksala N., Lehtimäki T. MicroRNAs in the atherosclerotic plaque. Clinical Chemistry . 2013;59(12):1708–1721. doi: 10.1373/clinchem.2013.204917. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan S., Selvan S. T., Archunan G., Gulyas B., Padmanabhan P. MicroRNAs-the next generation therapeutic targets in human diseases. Theranostics . 2013;3(12):930–942. doi: 10.7150/thno.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Frontiers in Endocrinology . 2018;9 doi: 10.3389/fendo.2018.00402.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabekkodu S. P., Shukla V., Varghese V. K., D’Souza J., Chakrabarty S., Satyamoorthy K. Clustered miRNAs and their role in biological functions and diseases. Biological Reviews . 2018;93(4):1955–1986. doi: 10.1111/brv.12428. [DOI] [PubMed] [Google Scholar]
- 12.Gross N., Kropp J., Khatib H. MicroRNA signaling in embryo development. Biology . 2017;6(3) doi: 10.3390/biology6030034.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu P., Wu Q., Lu D., et al. A systematic study of critical miRNAs on cells proliferation and apoptosis by the shortest path. BMC Bioinformatics . 2020;21 doi: 10.1186/s12859-020-03732-x.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y., Cui S., Wang Y., Xu R. The extensive regulation of microRNA in immune thrombocytopenia. Clinical and Applied Thrombosis/Hemostasis . 2022;28:1–19. doi: 10.1177/10760296221093595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syeda Z. A., Langden S. S. S., Munkhzul C., Lee M., Song S. J. Regulatory mechanism of microRNA expression in cancer. International Journal of Molecular Sciences . 2020;21(5) doi: 10.3390/ijms21051723.1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y., Hou K., Ji T., et al. The role of exosomal microRNAs in central nervous system diseases. Molecular and Cellular Biochemistry . 2021;476:2111–2124. doi: 10.1007/s11010-021-04053-0. [DOI] [PubMed] [Google Scholar]
- 17.Tribolet L., Kerr E., Cowled C., et al. MicroRNA biomarkers for infectious diseases: from basic research to biosensing. Frontiers in Microbiology . 2020;11 doi: 10.3389/fmicb.2020.01197.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angelescu M. A., Andronic O., Dima S. O., et al. miRNAs as biomarkers in diabetes: moving towards precision medicine. International Journal of Molecular Sciences . 2022;23(21) doi: 10.3390/ijms232112843.12843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv J., Yang L., Guo R., Shi Y., Zhang Z., Ye J. Ox-LDL-induced microRNA-155 promotes autophagy in human endothelial cells via repressing the Rheb/mTOR pathway. Cellular Physiology and Biochemistry . 2017;43(4):1436–1448. doi: 10.1159/000481875. [DOI] [PubMed] [Google Scholar]
- 20.Chen L., Zheng S.-Y., Yang C.-Q., Ma B.-M., Jiang D. MiR-155-5p inhibits the proliferation and migration of VSMCs and HUVECs in atherosclerosis by targeting AKT1. European Review for Medical and Pharmacological Sciences . 2019;23(5):2223–2233. doi: 10.26355/eurrev_201903_17270. [DOI] [PubMed] [Google Scholar]
- 21.Citrin K. M., Fernández-Hernando C., Suárez Y. MicroRNA regulation of cholesterol metabolism. Annals of the New York Academy of Sciences . 2021;1495(1):55–77. doi: 10.1111/nyas.14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., Liu X., Bai X., et al. Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. Journal of Pineal Research . 2018;64(2) doi: 10.1111/jpi.12449.e12449 [DOI] [PubMed] [Google Scholar]
- 23.Jiao Y., Zhao D., Gao F., et al. MicroRNA-520c-3p suppresses vascular endothelium dysfunction by targeting RELA and regulating the AKT and NF-κB signaling pathways. Journal of Physiology and Biochemistry . 2021;77:47–61. doi: 10.1007/s13105-020-00779-5. [DOI] [PubMed] [Google Scholar]
- 24.Su Y., Yuan J., Zhang F., et al. MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death & Disease . 2019;10 doi: 10.1038/s41419-019-1599-9.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang B., Yang H., Lu X., et al. MiR-520b inhibits endothelial activation by targeting NF-κB p65-VCAM1 axis. Biochemical Pharmacology . 2021;188 doi: 10.1016/j.bcp.2021.114540.114540 [DOI] [PubMed] [Google Scholar]
- 26.Gao Z.-F., Ji X.-L., Gu J., Wang X.-Y., Ding L., Zhang H. microRNA-107 protects against inflammation and endoplasmic reticulum stress of vascular endothelial cells via KRT1-dependent notch signaling pathway in a mouse model of coronary atherosclerosis. Journal of Cellular Physiology . 2019;234(7):12029–12041. doi: 10.1002/jcp.27864. [DOI] [PubMed] [Google Scholar]
- 27.de Yébenes V. G., Briones A. M., Martos-Folgado I., et al. Aging-associated miR-217 aggravates atherosclerosis and promotes cardiovascular dysfunction. Arteriosclerosis, Thrombosis, and Vascular Biology . 2020;40(10):2408–2424. doi: 10.1161/ATVBAHA.120.314333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhaolin Z., Jiaojiao C., Peng W., et al. OxLDL induces vascular endothelial cell pyroptosis through miR-125a-5p/TET2 pathway. Journal of Cellular Physiology . 2019;234(5):7475–7491. doi: 10.1002/jcp.27509. [DOI] [PubMed] [Google Scholar]
- 29.Zhang F., Cheng N., Du J., Zhang H., Zhang C. MicroRNA-200b-3p promotes endothelial cell apoptosis by targeting HDAC4 in atherosclerosis. BMC Cardiovascular Disorders . 2021;21 doi: 10.1186/s12872-021-01980-0.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girona J., Rosales R., Saavedra P., Masana L., Vallvé J.-C. Palmitate decreases migration and proliferation and increases oxidative stress and inflammation in smooth muscle cells: role of the Nrf2 signaling pathway. American Journal of Physiology-Cell Physiology . 2019;316(6):C888–C897. doi: 10.1152/ajpcell.00293.2018. [DOI] [PubMed] [Google Scholar]
- 31.Hergenreider E., Heydt S., Tréguer K., et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nature Cell Biology . 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L., Wang B., Sun L., Sun B., Li Y. Association of miR-192-5p with atherosclerosis and its effect on proliferation and migration of vascular smooth muscle cells. Molecular Biotechnology . 2021;63:1244–1251. doi: 10.1007/s12033-021-00376-x. [DOI] [PubMed] [Google Scholar]
- 33.Li X., Li L., Dong X., Ding J., Ma H., Han W. Circ_GRN promotes the proliferation, migration, and inflammation of vascular smooth muscle cells in atherosclerosis through miR-214-3p/FOXO1 axis. Journal of Cardiovascular Pharmacology . 2021;77(4):470–479. doi: 10.1097/FJC.0000000000000982. [DOI] [PubMed] [Google Scholar]
- 34.Sun D., Xiang G., Wang J., et al. miRNA 146b-5p protects against atherosclerosis by inhibiting vascular smooth muscle cell proliferation and migration. Epigenomics . 2020;12(24):2189–2204. doi: 10.2217/epi-2020-0155. [DOI] [PubMed] [Google Scholar]
- 35.Wang H., He F., Liang B., et al. p53-dependent LincRNA-p21 protects against proliferation and anti-apoptosis of vascular smooth muscle cells in atherosclerosis by upregulating SIRT7 via microRNA-17-5p. Journal of Cardiovascular Translational Research . 2021;14:426–440. doi: 10.1007/s12265-020-10074-9. [DOI] [PubMed] [Google Scholar]
- 36.Chong H., Wei Z., Na M., et al. The PGC-1α/NRF1/miR-378a axis protects vascular smooth muscle cells from FFA-induced proliferation, migration and inflammation in atherosclerosis. Atherosclerosis . 2020;297:136–145. doi: 10.1016/j.atherosclerosis.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 37.An J.-H., Chen Z.-Y., Ma Q.-L., Li Y.-B., Shi F.-W. Liraglutide improves atherosclerosis by regulating long non-coding RNA RMRP/miR-128-1-5P/Gadd45g axis. European Review for Medical and Pharmacological Sciences . 2020;24(5):2725–2737. doi: 10.26355/eurrev_202003_20545. [DOI] [PubMed] [Google Scholar]
- 38.Li Z., Xu C., Sun D. MicroRNA-488 serves as a diagnostic marker for atherosclerosis and regulates the biological behavior of vascular smooth muscle cells. Bioengineered . 2021;12(1):4092–4099. doi: 10.1080/21655979.2021.1953212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun B., Shan Z., Sun G., Wang X. Micro-RNA-183-5p acts as a potential diagnostic biomarker for atherosclerosis and regulates the growth of vascular smooth muscle cell. Journal of the Chinese Medical Association . 2021;84(1):33–37. doi: 10.1097/JCMA.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 40.Luo Y., Li Y., Peng H., Zhao Y. miR-140-5p regulates vascular smooth muscle cell viability, migration and apoptosis by targeting ROBO4 gene expression in atherosclerosis. Molecular Medicine Reports . 2021;23(3) doi: 10.3892/etm.2017.5355.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y.-Q., Xu Z.-M., Wang X.-L., et al. LncRNA FOXC2-AS1 regulated proliferation and apoptosis of vascular smooth muscle cell through targeting miR-1253/FOXF1 axis in atherosclerosis. European Review for Medical and Pharmacological Sciences . 2020;24(6):3302–3314. doi: 10.26355/eurrev_202003_20698. [DOI] [PubMed] [Google Scholar]
- 42.Li R., Jiang Q., Zheng Y. Circ_0002984 induces proliferation, migration and inflammation response of VSMCs induced by ox-LDL through miR-326-3p/VAMP3 axis in atherosclerosis. Journal of Cellular and Molecular Medicine . 2021;25(16):8028–8038. doi: 10.1111/jcmm.16734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kourtzelis I., Hajishengallis G., Chavakis T. Phagocytosis of apoptotic cells in resolution of inflammation. Frontiers in Immunology . 2020;11 doi: 10.3389/fimmu.2020.00553.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan J., Horng T. Lipid metabolism in regulation of macrophage functions. Trends in cell biology . 2020;30(12):979–989. doi: 10.1016/j.tcb.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Chistiakov D. A., Melnichenko A. A., Myasoedova V. A., Grechko A. V., Orekhov A. N. Mechanisms of foam cell formation in atherosclerosis. Journal of Molecular Medicine . 2017;95:1153–1165. doi: 10.1007/s00109-017-1575-8. [DOI] [PubMed] [Google Scholar]
- 46.Liu X., Guo J.-W., Lin X.-C., et al. Macrophage NFATc3 prevents foam cell formation and atherosclerosis: evidence and mechanisms. European Heart Journal . 2021;42(47):4847–4861. doi: 10.1093/eurheartj/ehab660. [DOI] [PubMed] [Google Scholar]
- 47.Wang G., Chen J.-J., Deng W.-Y., Ren K., Yin S.-H., Yu X.-H. CTRP12 ameliorates atherosclerosis by promoting cholesterol efflux and inhibiting inflammatory response via the miR-155-5p/LXRα pathway. Cell Death & Disease . 2021;12 doi: 10.1038/s41419-021-03544-8.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei Y., Corbalán-Campos J., Gurung R., et al. Dicer in macrophages prevents atherosclerosis by promoting mitochondrial oxidative metabolism. Circulation . 2018;138(18):2007–2020. doi: 10.1161/CIRCULATIONAHA.117.031589. [DOI] [PubMed] [Google Scholar]
- 49.Karshovska E., Wei Y., Subramanian P., et al. HIF-1α (hypoxia-inducible factor-1α) promotes macrophage necroptosis by regulating miR-210 and miR-383. Arteriosclerosis, Thrombosis, and Vascular Biology . 2020;40(3):583–596. doi: 10.1161/ATVBAHA.119.313290. [DOI] [PubMed] [Google Scholar]
- 50.Chen W., Li X., Wang J., Song N., Zhu A., Jia L. miR-378a modulates macrophage phagocytosis and differentiation through targeting CD47-SIRPα axis in atherosclerosis. Scandinavian Journal of Immunology . 2019;90(1) doi: 10.1111/sji.12766.e12766 [DOI] [PubMed] [Google Scholar]
- 51.Yin R., Zhu X., Wang J., et al. MicroRNA-155 promotes the ox-LDL-induced activation of NLRP3 inflammasomes via the ERK1/2 pathway in THP-1 macrophages and aggravates atherosclerosis in ApoE−/− mice. Annals of Palliative Medicine . 2019;8(5):676–689. doi: 10.21037/apm.2019.10.11. [DOI] [PubMed] [Google Scholar]
- 52.Zhu J., Liu B., Wang Z., et al. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics . 2019;9(23):6901–6919. doi: 10.7150/thno.37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang S., Li J., Chen Y., et al. MicroRNA-216a promotes M1 macrophages polarization and atherosclerosis progression by activating telomerase via the Smad3/NF-κB pathway. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease . 2019;1865(7):1772–1781. doi: 10.1016/j.bbadis.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 54.Xu Y., Xu Y., Zhu Y., et al. Macrophage miR-34a is a key regulator of cholesterol efflux and atherosclerosis. Molecular Therapy . 2020;28(1):202–216. doi: 10.1016/j.ymthe.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiao X.-R., Wang L., Liu M., Tian Y., Chen T. MiR-210-3p attenuates lipid accumulation and inflammation in atherosclerosis by repressing IGF2. Bioscience, Biotechnology, and Biochemistry . 2020;84(2):321–329. doi: 10.1080/09168451.2019.1685370. [DOI] [PubMed] [Google Scholar]
- 56.Canfrán-Duque A., Rotllan N., Zhang X., et al. Macrophage deficiency of miR-21 promotes apoptosis, plaque necrosis, and vascular inflammation during atherogenesis. EMBO Molecular Medicine . 2017;9(9):1244–1262. doi: 10.15252/emmm.201607492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie Q., Peng J., Guo Y., Li F. MicroRNA-33-5p inhibits cholesterol efflux in vascular endothelial cells by regulating citrate synthase and ATP-binding cassette transporter A1. BMC Cardiovascular Disorders . 2021;21 doi: 10.1186/s12872-021-02228-7.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ouimet M., Ediriweera H., Afonso M. S., et al. microRNA-33 regulates macrophage autophagy in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology . 2017;37(6):1058–1067. doi: 10.1161/ATVBAHA.116.308916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Price N. L., Rotllan N., Canfrán-Duque A., et al. Genetic dissection of the impact of miR-33a and miR-33b during the progression of atherosclerosis. Cell Reports . 2017;21(5):1317–1330. doi: 10.1016/j.celrep.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hussain M. M., Goldberg I. J. Human microRNA-33b promotes atherosclerosis in Apoe−/− mice. Arteriosclerosis, Thrombosis, Vascular Biology . 2018;38(10):2272–2275. doi: 10.1161/ATVBAHA.118.311617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishino T., Horie T., Baba O., et al. SREBF1/MicroRNA-33b axis exhibits potent effect on unstable atherosclerotic plaque formation in vivo. Arteriosclerosis, Thrombosis, Vascular Biology . 2018;38(10):2460–2473. doi: 10.1161/ATVBAHA.118.311409. [DOI] [PubMed] [Google Scholar]
- 62.Koyama S., Horie T., Nishino T., et al. Identification of differential roles of microRNA-33a and-33b during atherosclerosis progression with genetically modified mice. Journal of the American Heart Association . 2019;8(13) doi: 10.1161/JAHA.119.012609.e012609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Churov A., Summerhill V., Grechko A., Orekhova V., Orekhov A. MicroRNAs as potential biomarkers in atherosclerosis. International Journal of Molecular Sciences . 2019;20(22) doi: 10.3390/ijms20225547.5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He B., Zhao Z., Cai Q., et al. miRNA-based biomarkers, therapies, and resistance in cancer. International Journal of Biological Sciences . 2020;16(14):2628–2647. doi: 10.7150/ijbs.47203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han X., Wang H., Li Y., Liu L., Gao S. A 2 miRNAs-based signature for the diagnosis of atherosclerosis. BMC Cardiovascular Disorders . 2021;21 doi: 10.1186/s12872-021-01960-4.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teng P. P., Liu Y., Zhang M., Ji W. Diagnostic and prognostic significance of serum miR-18a-5p in patients with atherosclerosis. Clinical and Applied Thrombosis/Hemostasis . 2021;27:1–6. doi: 10.1177/10760296211050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y., Wang H., Xia Y. The expression of miR-211-5p in atherosclerosis and its influence on diagnosis and prognosis. BMC Cardiovascular Disorders . 2021;21 doi: 10.1186/s12872-021-02187-z.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J., Chen Z., Wang Y., et al. Several circulating miRNAs related to hyperlipidemia and atherosclerotic cardiovascular diseases. Lipids in Health and Disease . 2019;18 doi: 10.1186/s12944-019-1046-z.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kiss T., Giles C. B., Tarantini S., et al. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. GeroScience . 2019;41:419–439. doi: 10.1007/s11357-019-00095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lima J. F., Cerqueira L., Figueiredo C., Oliveira C., Azevedo N. F. Anti-miRNA oligonucleotides: a comprehensive guide for design. RNA Biology . 2018;15(3):338–352. doi: 10.1080/15476286.2018.1445959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saenz-Pipaon G., Dichek D. A. Targeting and delivery of microRNA-targeting antisense oligonucleotides in cardiovascular diseases. Atherosclerosis . 2023;374:44–54. doi: 10.1016/j.atherosclerosis.2022.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang P., Zhou Y., Richards A. M. Effective tools for RNA-derived therapeutics: siRNA interference or miRNA mimicry. Theranostics . 2021;11(18):8771–8796. doi: 10.7150/thno.62642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang L., Chen H.-Y., Hao N.-B., et al. microRNA inhibitors: natural and artificial sequestration of microRNA. Cancer Letters . 2017;407:139–147. doi: 10.1016/j.canlet.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 74.Peng D.-W., Lan C.-L., Dong L.-Q., et al. Anti-angiogenic properties of microRNA-29a in preclinical ocular models. Proceedings of the National Academy of Sciences . 2022;119(45) doi: 10.1073/pnas.2204795119.e2204795119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chioccioli M., Roy S., Newell R., et al. A lung targeted miR-29 mimic as a therapy for pulmonary fibrosis. eBioMedicine . 2022;85 doi: 10.1016/j.ebiom.2022.104304.104304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Täubel J., Hauke W., Rump S., et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. European Heart Journal . 2021;42(2):178–188. doi: 10.1093/eurheartj/ehaa898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang X., Yin R., Shi H., et al. LncRNA ZFAS1 confers inflammatory responses and reduces cholesterol efflux in atherosclerosis through regulating miR-654-3p-ADAM10/RAB22A axis. International Journal of Cardiology . 2020;315:72–80. doi: 10.1016/j.ijcard.2020.03.056. [DOI] [PubMed] [Google Scholar]
- 78.Ni J., Huang Z., Wang D. LncRNA TP73-AS1 promotes oxidized low-density lipoprotein-induced apoptosis of endothelial cells in atherosclerosis by targeting the miR-654-3p/AKT3 axis. Cellular & Molecular Biology Letters . 2021;26 doi: 10.1186/s11658-021-00264-x.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee Y.-N., Wu Y.-J., Lee H.-I., et al. Hsa-miR-409-3p regulates endothelial progenitor senescence via PP2A-P38 and is a potential ageing marker in humans. Journal of Cellular and Molecular Medicine . 2023;27(5):687–700. doi: 10.1111/jcmm.17691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dias I. H. K., Brown C. L., Shabir K., Polidori M. C., Griffiths H. R., Mecocci P. miRNA 933 expression by endothelial cells is increased by 27-hydroxycholesterol and is more prevalent in plasma from dementia patients. Journal of Alzheimer’s Disease . 2018;64(3):1009–1017. doi: 10.3233/JAD-180201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh S., de Ronde M. W. J., Kok M. G. M., et al. MiR-223-3p and miR-122-5p as circulating biomarkers for plaque instability. Open Heart . 2020;7(1) doi: 10.1136/openhrt-2019-001223.e001223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu X., Du X., Yang Y., et al. Inhibition of miR-122 reduced atherosclerotic lesion formation by regulating NPAS3-mediated endothelial to mesenchymal transition. Life Sciences . 2021;265 doi: 10.1016/j.lfs.2020.118816.118816 [DOI] [PubMed] [Google Scholar]
- 83.Griñán R., Escolà-Gil J. C., Julve J., Benítez S., Rotllan N. Epigenetic regulation by microRNAs in hyperhomocysteinemia-accelerated atherosclerosis. International Journal of Molecular Sciences . 2022;23(20) doi: 10.3390/ijms232012452.12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao F., Chen X., Xu B., et al. Inhibition of microRNA-92 alleviates atherogenesis by regulation of macrophage polarization through targeting KLF4. Journal of Cardiology . 2022;79(3):432–438. doi: 10.1016/j.jjcc.2021.10.015. [DOI] [PubMed] [Google Scholar]
- 85.Ye J., Wu Y., Guo R., et al. miR-221 alleviates the ox-LDL-induced macrophage inflammatory response via the inhibition of DNMT3b-mediated NCoR promoter methylation. Mediators of Inflammation . 2019;2019:15. doi: 10.1155/2019/4530534.4530534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y., Xiao L., Li J., et al. MicroRNA profiling of diabetic atherosclerosis in a rat model. European Journal of Medical Research . 2018;23 doi: 10.1186/s40001-018-0354-5.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hildebrandt A., Kirchner B., Meidert A. S., et al. Detection of atherosclerosis by small RNA-sequencing analysis of extracellular vesicle enriched serum samples. Frontiers in Cell and Developmental Biology . 2021;9 doi: 10.3389/fcell.2021.729061.729061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang M., Li J., Cai J., et al. Overexpression of microRNA-16 alleviates atherosclerosis by inhibition of inflammatory pathways. BioMed Research International . 2020;2020:12. doi: 10.1155/2020/8504238.8504238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen F., Li J., She J., Chen T., Yuan Z. Exosomal microRNA-16-5p from macrophage exacerbates atherosclerosis via modulating mothers against decapentaplegic homolog 7. Microvascular Research . 2022;142 doi: 10.1016/j.mvr.2022.104368.104368 [DOI] [PubMed] [Google Scholar]
- 90.Rachmawati E., Sargowo D., Rohman M. S., Widodo N., Kalsum U. miR-155-5p predictive role to decelerate foam cell atherosclerosis through CD36, VAV3, and SOCS1 pathway. Non-Coding RNA Research . 2021;6(2):59–69. doi: 10.1016/j.ncrna.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pordzik J., Pisarz K., De Rosa S., et al. The potential role of platelet-related microRNAs in the development of cardiovascular events in high-risk populations, including diabetic patients: a review. Frontiers in Endocrinology . 2018;9 doi: 10.3389/fendo.2018.00074.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wei W., Zhou Y. J., Shen J. L., et al. The compatibility of alisma and atractylodes affects the biological behaviours of VSMCs by inhibiting the miR-128-5p/p21 gene. Evidence-Based Complementary and Alternative Medicine . 2022;2022:13. doi: 10.1155/2022/7617258.7617258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gatsiou A., Georgiopoulos G., Vlachogiannis N. I., et al. Additive contribution of microRNA-34a/b/c to human arterial ageing and atherosclerosis. Atherosclerosis . 2021;327:49–58. doi: 10.1016/j.atherosclerosis.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 94.Raucci A., Macrì F., Castiglione S., Badi I., Vinci M. C., Zuccolo E. MicroRNA-34a: the bad guy in age-related vascular diseases. Cellular and Molecular Life Sciences . 2021;78:7355–7378. doi: 10.1007/s00018-021-03979-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hua C.-C., Liu X.-M., Liang L.-R., Wang L.-F., Zhong J.-C. Targeting the microRNA-34a as a novel therapeutic strategy for cardiovascular diseases. Frontiers in Cardiovascular Medicine . 2022;8 doi: 10.3389/fcvm.2021.784044.784044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.An L., Gao L., Ning M., et al. Correlation between decreased plasma miR-29a and vascular endothelial injury induced by hyperlipidemia. Herz . 2023;48:301–308. doi: 10.1007/s00059-022-05121-x. [DOI] [PubMed] [Google Scholar]
- 97.Ma Q., Zhang J., Zhang M., et al. MicroRNA-29b targeting of cell division cycle 7-related protein kinase (CDC7) regulated vascular smooth muscle cell (VSMC) proliferation and migration. Annals of Translational Medicine . 2020;8(22) doi: 10.21037/atm-20-6856.1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barwari T., Rienks M., Mayr M. MicroRNA-21 and the vulnerability of atherosclerotic plaques. Molecular Therapy . 2018;26(4):938–940. doi: 10.1016/j.ymthe.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun P., Tang L.-N., Li G.-Z., et al. Effects of MiR-21 on the proliferation and migration of vascular smooth muscle cells in rats with atherosclerosis via the Akt/ERK signaling pathway. European Review for Medical and Pharmacological Sciences . 2019;23(5):2216–2222. doi: 10.26355/eurrev_201903_17269. [DOI] [PubMed] [Google Scholar]
- 100.van Ingen E., Foks A. C., Kröner M. J., et al. Antisense oligonucleotide inhibition of microRNA-494 halts atherosclerotic plaque progression and promotes plaque stabilization. Molecular Therapy-Nucleic Acids . 2019;18:638–649. doi: 10.1016/j.omtn.2019.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X., Rotllan N., Canfrán-Duque A., et al. Targeted suppression of miRNA-33 using pHLIP improves atherosclerosis regression. Circulation Research . 2022;131(1):77–90. doi: 10.1161/CIRCRESAHA.121.320296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dosta P., Tamargo I., Ramos V., et al. Delivery of anti-microRNA-712 to inflamed endothelial cells using poly(β-amino ester) nanoparticles conjugated with VCAM-1 targeting peptide. Advanced Healthcare Materials . 2021;10(15) doi: 10.1002/adhm.202001894.2001894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng J., Cheng A., Clifford B. L., et al. MicroRNA-144 silencing protects against atherosclerosis in male, but not female mice. Arteriosclerosis, Thrombosis, and Vascular Biology . 2020;40(2):412–425. doi: 10.1161/ATVBAHA.119.313633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang H., Peng L., Wang Y., et al. Extracellular vesicle-derived miR-144 as a novel mechanism for chronic intermittent hypoxia-induced endothelial dysfunction. Theranostics . 2022;12(9):4237–4249. doi: 10.7150/thno.69035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang H., Wu J., Wu J., et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. Journal of Nanobiotechnology . 2019;17 doi: 10.1186/s12951-019-0461-7.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Comariţa I. K., Vîlcu A., Constantin A., et al. Therapeutic potential of stem cell-derived extracellular vesicles on atherosclerosis-induced vascular dysfunction and its key molecular players. Frontiers in Cell and Developmental Biology . 2022;10 doi: 10.3389/fcell.2022.817180.817180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hwang S. J., Ahn B. J., Shin M.-W., et al. miR-125a-5p attenuates macrophage-mediated vascular dysfunction by targeting Ninjurin1. Cell Death & Differentiation . 2022;29:1199–1210. doi: 10.1038/s41418-021-00911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J., Wu Q., Yu J., Cao X., Xu Z. miR-125a-5p inhibits the expression of NLRP3 by targeting CCL4 in human vascular smooth muscle cells treated with ox-LDL. Experimental and Therapeutic Medicine . 2019;18(3):1645–1652. doi: 10.3892/etm.2019.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.You L., Chen H., Xu L., Li X. Overexpression of miR-29a-3p suppresses proliferation, migration, and invasion of vascular smooth muscle cells in atherosclerosis via targeting TNFRSF1A. BioMed Research International . 2020;2020:15. doi: 10.1155/2020/9627974.9627974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peng Q., Yin R., Zhu X., et al. miR-155 activates the NLRP3 inflammasome by regulating the MEK/ERK/NF-κB pathway in carotid atherosclerotic plaques in ApoE−/− mice. Journal of Physiology and Biochemistry . 2022;78:365–375. doi: 10.1007/s13105-022-00871-y. [DOI] [PubMed] [Google Scholar]
- 111.Wang Y., Cen A., Yang Y., et al. miR-181a, delivered by hypoxic PTC-secreted exosomes, inhibits DACT2 by downregulating MLL3, leading to YAP-VEGF-mediated angiogenesis. Molecular Therapy-Nucleic Acids . 2021;24:610–621. doi: 10.1016/j.omtn.2021.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jensen D. M., Han P., Mangala L. S., et al. Broad-acting therapeutic effects of miR-29b-chitosan on hypertension and diabetic complications. Molecular Therapy . 2022;30(11):3462–3476. doi: 10.1016/j.ymthe.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang H., Xue S., Feng Y., Shen J., Zhao J. MicroRNA-24-3p inhibition prevents cell growth of vascular smooth muscle cells by targeting Bcl-2-like protein 11. Experimental and Therapeutic Medicine . 2020;19(4):2467–2474. doi: 10.3892/etm.2020.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zheng Y., Li Y., Liu G., Qi X., Cao X. MicroRNA-24 inhibits the proliferation and migration of endothelial cells in patients with atherosclerosis by targeting importin-α3 and regulating inflammatory responses. Experimental Therapeutic Medicine . 2018;15(1):338–344. doi: 10.3892/etm.2017.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jia Y., Wei Y. Modulators of microRNA function in the immune system. International Journal of Molecular Sciences . 2020;21(7) doi: 10.3390/ijms21072357.2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boštjančič E., Večerić-Haler Ž., Kojc N. The role of immune-related miRNAs in the pathology of kidney transplantation. Biomolecules . 2021;11(8) doi: 10.3390/biom11081198.1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shah P., Agbor-Enoh S., Bagchi P., et al. Circulating microRNAs in cellular and antibody-mediated heart transplant rejection. The Journal of Heart and Lung Transplantation . 2022;41(10):1401–1413. doi: 10.1016/j.healun.2022.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dasgupta I., Chatterjee A. Recent advances in miRNA delivery systems. Methods and Protocols . 2021;4(1) doi: 10.3390/mps4010010.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lolli A., Sivasubramaniyan K., Vainieri M. L., et al. Hydrogel-based delivery of antimiR-221 enhances cartilage regeneration by endogenous cells. Journal of Controlled Release . 2019;309:220–230. doi: 10.1016/j.jconrel.2019.07.040. [DOI] [PubMed] [Google Scholar]
- 120.Zhu J., Yang S., Qi Y., et al. Stem cell–homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model. Science Advances . 2022;8(13) doi: 10.1126/sciadv.abk0011.eabk0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Y., Chen X., Jin R., et al. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Science Advances . 2021;7(9) doi: 10.1126/sciadv.abd6740.eabd6740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bheri S., Davis M. E. Nanoparticle–hydrogel system for post-myocardial infarction delivery of microRNA. ACS Nano . 2019;13(9):9702–9706. doi: 10.1021/acsnano.9b05716. [DOI] [PubMed] [Google Scholar]
- 123.Yamayoshi A. Development of novel drug delivery system targeting exosomal microRNA. YAKUGAKU ZASSHI . 2020;140(5):625–631. doi: 10.1248/yakushi.19-00218-3. [DOI] [PubMed] [Google Scholar]
- 124.Yan C., Chen J., Wang C., et al. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Delivery . 2022;29(1):214–228. doi: 10.1080/10717544.2021.2023699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee S. W. L., Paoletti C., Campisi M., et al. MicroRNA delivery through nanoparticles. Journal of Controlled Release . 2019;313:80–95. doi: 10.1016/j.jconrel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


