Abstract
Background
Guidelines suggest that the serum carbohydrate antigen (CA19-9) level should be used when deciding on neoadjuvant treatment in patients with resectable and borderline resectable pancreatic ductal adenocarcinoma (hereafter referred to as pancreatic cancer). In patients with resectable pancreatic cancer, neoadjuvant therapy is advised when the CA19-9 level is ‘markedly elevated’. This study investigated the impact of baseline CA19-9 concentration on the treatment effect of neoadjuvant chemoradiotherapy (CRT) in patients with resectable and borderline resectable pancreatic cancers.
Methods
In this post hoc analysis, data were obtained from two RCTs that compared neoadjuvant CRT with upfront surgery in patients with resectable and borderline resectable pancreatic cancers. The effect of neoadjuvant treatment on overall survival was compared between patients with a serum CA19-9 level above or below 500 units/ml using the interaction test.
Results
Of 296 patients, 179 were eligible for analysis, 90 in the neoadjuvant CRT group and 89 in the upfront surgery group. Neoadjuvant CRT was associated with superior overall survival (HR 0.67, 95 per cent c.i. 0.48 to 0.94; P = 0.019). Among 127 patients (70, 9 per cent) with a low CA19-9 level, median overall survival was 23.5 months with neoadjuvant CRT and 16.3 months with upfront surgery (HR 0.63, 0.42 to 0.93). For 52 patients (29 per cent) with a high CA19-9 level, median overall survival was 15.5 months with neoadjuvant CRT and 12.9 months with upfront surgery (HR 0.82, 0.45 to 1.49). The interaction test for CA19-9 level exceeding 500 units/ml on the treatment effect of neoadjuvant CRT was not significant (P = 0.501).
Conclusion
Baseline serum CA19-9 level defined as either high or low has prognostic value, but was not associated with the treatment effect of neoadjuvant CRT in patients with resectable and borderline resectable pancreatic cancers, in contrast with current guideline advice.
In two RCTs, comparing neoadjuvant chemoradiotherapy (CRT) with upfront surgery in patients with resectable and borderline resectable pancreatic cancers, CRT was associated with better survival. There was no difference in treatment effect between patients with a baseline CA19-9 level higher or lower than 500 units/ml, meaning that neoadjuvant CRT should not be withheld because of a low CA19-9 concentration.
Introduction
Pancreatic ductal adenocarcinoma (pancreatic cancer) is known for its poor 5-year survival rate of 10 per cent1. Only 20 per cent of patients are eligible for resection. Neoadjuvant therapy is increasingly being used in patients with primary resectable and borderline resectable pancreatic cancers. Possible advantages of a neoadjuvant approach include better selection of patients for surgery, more microscopically complete (R0) resections, and improved overall survival (OS)2. It remains unknown whether all patients benefit from this approach.
Carbohydrate antigen 19-9 (CA19-9) is a sialylated Lewis blood group antigen, which was first described as a tumour marker in 19813. A raised CA19-9 level is a known prognostic biomarker for worse OS4. CA19-9 is mainly used to determine treatment response and recurrence in addition to imaging. The 2019 American Society of Clinical Oncology (ASCO)5 and 2022 National Comprehensive Cancer Network (NCCN)6 guidelines recommend upfront surgery for resectable pancreatic cancer, and neoadjuvant therapy for borderline resectable pancreatic cancer. In addition, these guidelines5,6 recommend consideration of neoadjuvant therapy for patients with resectable pancreatic cancer with ‘markedly elevated’ CA19-9 levels, without specifying a cut-off value. However, the predictive value of CA19-9 when selecting patients for neoadjuvant therapy has not been investigated.
Two multicentre RCTs7–9 recently reported superior OS with neoadjuvant chemoradiotherapy (CRT) compared with upfront resection in patients with resectable and borderline resectable pancreatic cancers. The aim of the present study was to investigate the predictive impact of baseline CA19-9 levels on the treatment effect of neoadjuvant CRT in patients with resectable and borderline resectable pancreatic cancers.
Methods
Patients
This was a post hoc analysis of two RCTs7,9 that compared neoadjuvant CRT with upfront surgery in patients with resectable pancreatic cancer and borderline resectable pancreatic cancer. Patients were included in the present analysis if the baseline serum CA19-9 level was available. Patients with a serum bilirubin concentration exceeding 5.85 mg/dl (100 µmol/l) at the time of CA19-9 measurement were excluded as obstructive jaundice may raise CA19-9 levels10. All patients provided written informed consent before participation in the trials.
Korean trial
In the trial reported by Jang et al.7 from Korea, 27 patients were randomized to receive neoadjuvant CRT and 23 to upfront surgery. Neoadjuvant CRT consisted of 45 Gy in 25 fractions and 9 Gy in 5 fractions (5 times a week for 6 weeks) combined with gemcitabine at 400 mg/m2 administered at the start of each week. In the upfront surgery group, patients received adjuvant CRT in the same doses as the neoadjuvant group. Patients in both groups received adjuvant gemcitabine at 1000 mg/m2 on days 1, 8, and 15 during four cycles with a duration of 4 weeks each.
Dutch PREOPANC trial
In the PREOPANC trial9, 119 patients were assigned to neoadjuvant CRT and 127 to upfront surgery. Neoadjuvant CRT consisted of three cycles of gemcitabine. The first and third cycles had a duration of 3 weeks, with gemcitabine on days 1 and 8 at 1000 mg/m2. The second cycle had a duration of 4 weeks, with gemcitabine on days 1, 8 and 15, combined with 36 Gy radiotherapy in 15 fractions (5 times a week for 3 weeks). After surgery, patients in the neoadjuvant CRT arm received four cycles of adjuvant gemcitabine at 1000 mg/m2 on days 1, 8, and 15 (4 weeks per cycle). Patients in the upfront surgery group received six cycles of adjuvant gemcitabine. Inclusion and exclusion criteria for both trials are summarized in Fig. 1, and the definitions used for resectable pancreatic cancer and borderline resectable pancreatic cancer are shown in Table 1.
Fig. 1.
Eligibility criteria in two randomized trials of neoadjuvant chemoradiotherapy in patients with (borderline) resectable pancreatic adenocarcinoma (pancreatic cancer)
*Chemotherapy, radiotherapy, immunotherapy. BRPC, borderline resectable pancreatic cancer; NCCN, National Comprehensive Cancer Network; PDAC, pancreatic ductal adenocarcinoma; RPC, resectable pancreatic cancer; DPCG, Dutch Pancreatic Cancer Group; LAPC, locally advanced pancreatic cancer.
Table 1.
Definitions of primary resectable and borderline resectable pancreatic adenocarcinoma: degrees of vascular contact according to National Comprehensive Cancer Network 2.2012 (Korean trial) versus Dutch Pancreatic Cancer Group (PREOPANC trial) criteria
| SMA | CA | CHA | SMV–PV | Comment | ||
|---|---|---|---|---|---|---|
| NCCN 2012 |
PR pancreatic cancer | No contact | No contact | No contact | No contact | – |
| BR pancreatic cancer | ≤180° contact | No contact | ≤90° contact* | Contact or narrowing or occlusion† | *No extension to CA †With possibility of allowing safe resection and replacement |
|
| DPCG 2012 |
PR pancreatic cancer | No contact | No contact | No contact | ≤90° contact | All four required |
| BR pancreatic cancer | ≤90° contact | ≤90° contact | ≤90° contact | ≤90–270° contact | Minimally one required and no venous occlusion | |
SMA, superior mesenteric artery; CA, coeliac axis; CHA, common hepatic artery; SMV, superior mesenteric vein; PV, portal vein; NCCN, National Comprehensive Cancer Network; PR, primary resectable; BR, borderline resectable; DPCG, Dutch Pancreatic Cancer Group.
Outcomes
The primary outcome was OS from the date of randomization. Secondary outcomes included resection rate, R0 resection rate, and N0 resection rate. A raised serum CA19-9 level was defined by a concentration exceeding 500 units/ml as this is a commonly used cut-off in the literature11,12. As a sensitivity analysis, cut-offs of 180 and 1000 units/ml3 were tested.
Statistical analysis
Continuous variables are presented as median (i.q.r.) and categorical variables as counts with percentages. Continuous variables were compared using Mann–Whitney U test, and categorical variables using χ2 test. OS was estimated using the Kaplan–Meier method and compared using the log rank test. The effect of a raised baseline CA19-9 level was investigated using Cox proportional hazards analysis. The interaction test was used to investigate whether the effect of neoadjuvant chemotherapy differed based on the CA19-9 level. P < 0.050 was considered to indicate statistical significance. Statistical analysis was undertaken using R software version 4.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients and baseline characteristics
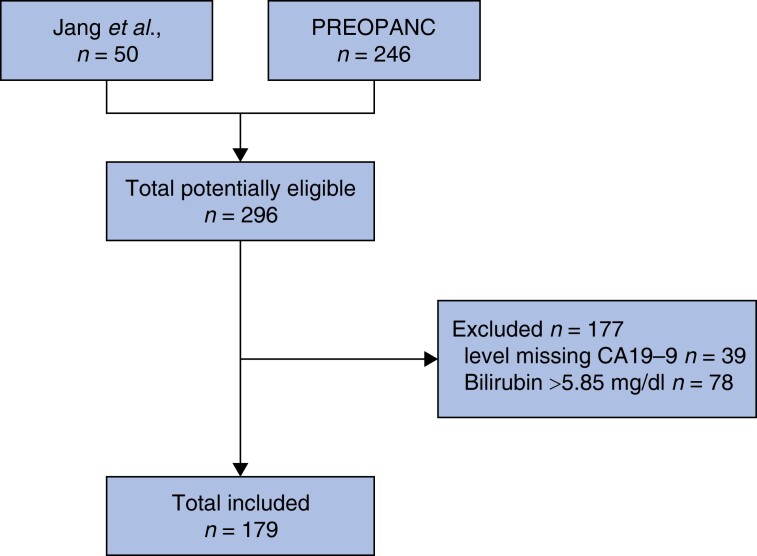
Of the 296 eligible patients, 39 had missing data on baseline CA19-9 and were excluded. The serum bilirubin concentration at the time of CA19-9 measurement was greater than 5.85 mg/dl (100 µmol/l) in 78 patients, resulting in the final inclusion of 179 patients (Fig. 2). Baseline characteristics are summarized in Table 2. The majority of patients (56 per cent) were staged as having borderline resectable pancreatic cancer, with 51 (57 per cent) in the neoadjuvant therapy group and 49 (55 per cent) in the upfront surgery group. In the neoadjuvant therapy group, 24 patients (27 per cent) had a CA19-9 level over 500 units/ml compared with 28 (31 per cent) in the upfront surgery group.
Fig. 2.
Study flow chart
CA19-9, carbohydrate antigen 19-9.
Table 2.
Baseline characteristics
| Neoadjuvant CRT (n = 90) | Upfront surgery (n = 89) | |
|---|---|---|
| Age (years), median (i.q.r.) | 63 (57–70) | 66 (60–72) |
| ≥ 65 | 40 (44) | 51 (57) |
| Sex ratio (M : F) | 44 : 46 | 46 : 43 |
| WHO performance status | ||
| 0 | 56 (58) | 48 (52) |
| 1 | 40 (42) | 43 (46) |
| 2 | 0 (0) | 2 (2) |
| Tumour location in pancreas | ||
| Head, n (%) | 77 (80) | 77 (83) |
| Body/tail, n (%) | 19 (20) | 16 (17) |
| Resectability status | ||
| Primary resectable, n (%) | 39 (43) | 40 (45) |
| Borderline resectable, n (%) | 51 (57) | 49 (55) |
| Baseline CA19-9 (units/ml) | ||
| ≤ 500, n (%) | 66 (73) | 61 (69) |
| >500, n (%) | 24 (27) | 28 (31) |
Values are n (%) unless indicated otherwise. CRT, chemoradiotherapy; CA19-9, carbohydrate antigen 19-9.
Survival
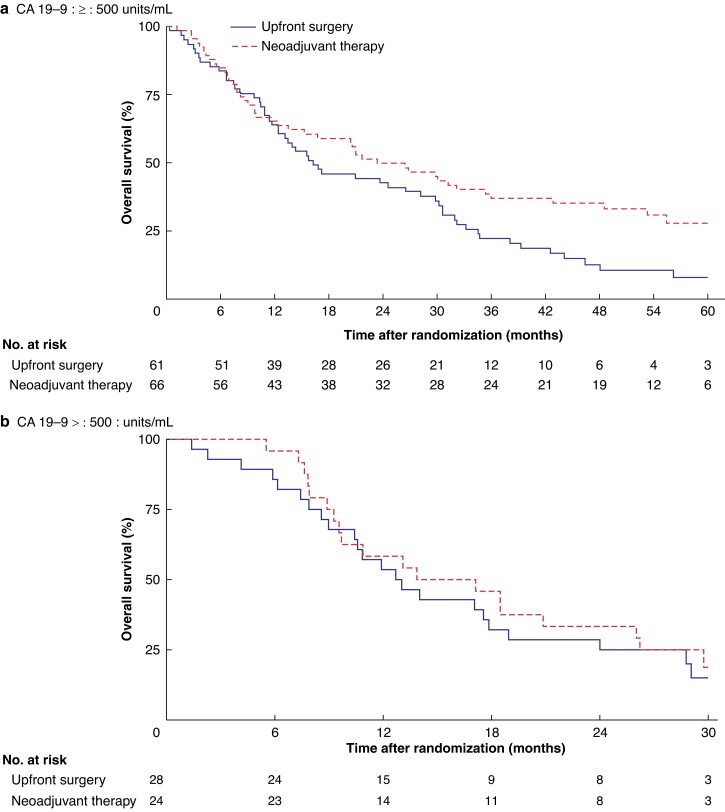
After a median follow-up of 55 months, patients in the neoadjuvant CRT group had a median OS of 20.9 months compared with 15.6 months in the upfront surgery group (HR 0.67, 95 per cent c.i. 0.48 to 0.94; P = 0.019). In patients with a low CA19-9 level (500 units/ml or less), median OS was 23.5 months with neoadjuvant CRT compared with 16.3 months with upfront surgery (HR 0.63, 0.42 to 0.93) (Fig. 3a). In patients with a high CA19-9 level (over 500 units/ml), median OS was 15.5 months with neoadjuvant CRT compared with 12.9 months with upfront surgery (HR 0.82, 0.45 to 1.49) (Fig. 3b). The interaction test did not detect an impact of CA19-9 on treatment effect of neoadjuvant CRT (P = 0.501). Using cut-off values for CA19-9 of 180 and 1000 units/ml also did not show a difference in treatment effect (P = 0.210 and P = 0.511 respectively) (Figs S1 and S2). In a subgroup analysis excluding 17 patients with a CA19-9 level below 5 units/ml (non-producers), no impact of CA19-9 on treatment effect was detected by an interaction test (P = 0.616) (Fig. S3).
Fig. 3.
Overall survival by treatment group according to carbohydrate antigen 19-9 level a 500 units/ml or less and b over 500 units/ml. CA19-9, carbohydrate antigen 19-9.
a P = 0.020, bP = 0.509 (log rank test).
Surgical resection
Among patients with a low CA19-9 level (500 units/ml or less), 44 of 66 patients (67 per cent) in the neoadjuvant CRT group underwent resection compared with 44 of 61 (72 per cent) in the upfront surgery group (P = 0.635) (Table 3). In patients with a high CA19-9 level (over 500 units/ml), 13 of 24 patients (54 per cent) in the neoadjuvant CRT group underwent resection compared with 24 of 28 (86 per cent) in the upfront surgery group (P = 0.084) (Table 3).
Table 3.
Impact of carbohydrate antigen 19-9 on treatment outcomes
| Neoadjuvant CRT (n = 90) | Upfront surgery (n = 89) | P¶ | P for interaction# | |
|---|---|---|---|---|
| Resection | 0.072 | |||
| CA19-9 ≤ 500 units/ml | 44 of 66 (67)* | 44 of 61 (72)‡ | 0.635 | |
| CA19-9 > 500 units/ml | 13 of 24 (54)† | 24 of 28 (86)§ | 0.084 | |
| R0 resection | 0.019 | |||
| CA19-9 ≤ 500 units/ml | 29 of 66 (44) | 21 of 61 (34) | 0.360 | |
| CA19-9 > 500 units/ml | 12 of 24 (50) | 8 of 28 (29) | 0.194 | |
| N0 resection | 0.983 | |||
| CA19-9 ≤ 500 units/ml | 33 of 66 (50) | 12 of 61 (20) | < 0.001 | |
| CA19-9 > 500 units/ml | 7 of 24 (29) | 3 of 28 (11) | 0.183 |
Values are n (%). Reasons for not undergoing resection: *progression before chemoradiotherapy (CRT) (7), progression during CRT (6), locally unresectable during surgery (1), metastatic disease found during surgery (7), medical decision (1); †progression before CRT (1), progression during CRT (4), locally unresectable during surgery (1), metastatic disease found during surgery (4), patient’s decision (1); ‡progression before surgery (1), locally unresectable during surgery (4), metastatic disease found during surgery (12). §patient’s decision (1), metastatic disease found during surgery (3). CA19-9, carbohydrate antigen 19-9. ¶χ2 test; #interaction test.
R0 resection
Pathological outcomes are shown in Table 3. In patients with a low CA19-9 level (500 units/ml or less), an R0 resection was reported in 29 of 66 patients (44 per cent) who had neoadjuvant CRT and 21 of 61 (34 per cent) in the upfront surgery group (P = 0.360). In patients with a high CA19-9 level (over 500 units/ml), an R0 resection was reported in 12 of 24 patients (50 per cent) and 8 of 28 (29 per cent) respectively (P = 0.194). The interaction test revealed a treatment effect of neoadjuvant CRT on R0 resection between high and low CA19-9 groups (P = 0.019).
N0 resection
Among patients with a low CA19-9 level (500 units/ml or less), 33 of 66 patients (50 per cent) with neoadjuvant CRT had an N0 resection compared with 12 of 61 (20 per cent) in the upfront surgery group (P < 0.001). In patients with a high CA19-9 level (over 500 units/ml), an N0 resection was obtained in 7 of 24 patients (29 per cent) in the neoadjuvant CRT group and in 3 of 28 (11 per cent) in the upfront surgery group (P = 0.183). The interaction test found no difference in treatment effect on N0 resection between high and low CA19-9 groups (P = 0.983).
Discussion
This patient-level post hoc analysis of two RCTs found no impact of baseline serum CA19-9 (cut-off 500 units/ml) on the treatment effect of neoadjuvant CRT in patients with resectable and borderline resectable pancreatic cancers. Overall, OS was better after neoadjuvant CRT compared with upfront resection (HR 0.67, 95 per cent c.i. 0.48 to 0.94)7,9. If anything, neoadjuvant CRT seemed to improve OS, especially in patients with a low CA19.9 level (less than 500 units/ml), but the interaction test was negative.
This is the first study to assess the impact of CA19.9 on neoadjuvant CRT in data from RCTs. Currently, CA19-9 is used for diagnosis, treatment evaluation, and prognostication in patients with pancreatic cancer. Several authors13,14, including the MD Anderson group, have long advocated for staging to go beyond tumour anatomy. In addition to anatomy, staging should consider biology (CA19-9 value) and patient condition (performance status). Several studies have found that a CA19-9 level above 500 units/ml in patients with resectable pancreatic cancer is associated with similar or worse OS than in patients with borderline resectable pancreatic cancer11. The NCCN6 and ASCO5 guidelines recommend considering neoadjuvant treatment in patients with resectable pancreatic cancer and ‘markedly elevated’ CA19-9 levels. The present study confirmed the strong association between a raised CA19-9 concentration and worse OS. However, a difference in treatment effect of neoadjuvant CRT associated with high or low baseline CA19-9 levels could not be demonstrated (Fig. 3). Although baseline serum CA19-9 level is a strong prognostic factor, in the present study it was not a predictive factor for the treatment effect of neoadjuvant treatment on OS.
The evidence for systemic therapy in patients with resectable and borderline resectable pancreatic cancers is shifting from purely adjuvant to perioperative/neoadjuvant administration. One of the problems of upfront surgery followed by adjuvant therapy is that about 40 per cent of patients do not receive chemotherapy after surgical resection15,16. A recent meta-analysis17 of seven RCTs found better OS with neoadjuvant therapy compared with upfront surgery followed by adjuvant therapy (pooled HR 0.66, 95 per cent c.i. 0.52 to 0.85). A limitation of this meta-analysis was that it did not identify studies that used adjuvant FOLFIRINOX (a combination of fluorouracil with leucovorin, oxaliplatin, and irinotecan). Several ongoing RCTs are comparing neoadjuvant with adjuvant FOLFIRINOX in patients with resectable pancreatic cancer: the Scandinavian NorPACT-1 (NCT02919787), the United States Alliance A021806 (NCT04340141), and the Dutch PREOPANC-3 (NCT04927780) trials. While the results of these trials are awaited, the NCCN guidelines6 recommend participation in clinical trials or upfront surgery followed by adjuvant FOLFIRINOX for patients with resectable pancreatic cancer. To allow proper subgroup analysis, future RCTs should stratify by baseline CA19-9 level.
Among patients with CA19-9 levels above 500 units/ml, the resection rate was lower in the neoadjuvant CRT group whereas OS was better. Neoadjuvant CRT avoided a (futile) resection in one in three patients, and was still associated with better OS. A treatment effect of baseline serum CA19-9 level was demonstrated only for R0 resection. The higher R0 rate after neoadjuvant CRT was most profound in patients with a high CA19-9 level. This treatment effect, however, did not translate into a treatment effect for OS. No such treatment effect of baseline CA19-9 concentration could be demonstrated for the association between neoadjuvant CRT and nodal status.
Some limitations should be taken into account when assessing the present findings. First, baseline CA19-9 values were missing for some patients in the PREOPANC trial. Consequently, the relatively small sample size for investigation of interactions in the present study may have resulted in a lack of statistical power to detect a small treatment effect. Second, baseline CA19-9 levels may have been influenced by residual cholestasis after excluding only patients with a bilirubin level of more than 5.85 mg/dl (100 µmol/l). Third, approximately 10 per cent of the population are Lewis antigen-negative and are unable to synthesize CA19-918. This is a well known problem that should be addressed in future prospective studies, for instance by taking other tumour markers such as carcinoembryonic antigen into account. Fourth, one might hypothesize that higher levels of CA19-9 represent a higher systemic tumour load and the presence of micrometastatic disease. Neoadjuvant gemcitabine-based CRT may be inadequate for these patients. The ESPAC-419 and PRODIGE-24/CCTG PA.620 RCTs have demonstrated superior OS with multiagent regimens compared with gemcitabine monotherapy in the adjuvant setting,. Future studies will have to assess whether the observations in the present analysis hold true in trials with FOLFIRINOX and other multiagent regimens.
Supplementary Material
Acknowledgements
D.D. and J.L.v.D. are joint first authors, and G.v.T., J.Y.J., M.G.B., and B.G.K., are joint senior authors, of this article.
Contributor Information
Deesje Doppenberg, Department of Surgery, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands; Cancer Centre Amsterdam, Amsterdam, the Netherlands; Department of Radiation Oncology, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands.
Jacob L van Dam, Department of Surgery, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Youngmin Han, Department of Surgery, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea.
Bert A Bonsing, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Olivier R Busch, Department of Surgery, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands; Cancer Centre Amsterdam, Amsterdam, the Netherlands.
Sebastiaan Festen, Department of Surgery, OLVG, Amsterdam, the Netherlands.
Erwin van der Harst, Department of Surgery, Maasstad Hospital, Rotterdam, the Netherlands.
Ignace H de Hingh, Department of Surgery, Catherina Hospital, Eindhoven, the Netherlands.
Marjolein Y V Homs, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Wooil Kwon, Department of Surgery, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea.
Mirang Lee, Department of Surgery, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea.
Daan J Lips, Department of Surgery, Medisch Spectrum Twente, Enschede, the Netherlands.
Vincent E de Meijer, Department of Surgery, University of Groningen and University Medical Centre Groningen, Groningen, the Netherlands.
I Quintus Molenaar, Department of Surgery, Regional Academic Cancer Centre Utrecht, University of Utrecht, Utrecht, the Netherlands.
Joost J Nuyttens, Department of Radiation Oncology, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Gijs A Patijn, Department of Surgery, Isala Oncology Centre, Zwolle, the Netherlands.
Stijn van Roessel, Department of Surgery, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands; Cancer Centre Amsterdam, Amsterdam, the Netherlands.
George P van der Schelling, Department of Surgery, Amphia Hospital, Breda, the Netherlands.
Mustafa Suker, Department of Surgery, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Eva Versteijne, Cancer Centre Amsterdam, Amsterdam, the Netherlands; Department of Radiation Oncology, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands.
Judith de Vos-Geelen, Department of Internal Medicine, Division of Medical Oncology, GROW, Maastricht University Medical Centre, Maastricht, the Netherlands.
Johanna W Wilmink, Cancer Centre Amsterdam, Amsterdam, the Netherlands; Department of Medical Oncology, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands.
Casper H J van Eijck, Department of Surgery, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Geertjan van Tienhoven, Cancer Centre Amsterdam, Amsterdam, the Netherlands; Department of Radiation Oncology, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands.
Jin-Young Jang, Department of Surgery, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea.
Marc G Besselink, Department of Surgery, Amsterdam UMC, Location University of Amsterdam, Amsterdam, the Netherlands; Cancer Centre Amsterdam, Amsterdam, the Netherlands.
Bas Groot Koerkamp, Department of Surgery, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Funding
The authors have no funding to declare.
Author contributions
Conceptualization DD;JD;MB;BG;, Methodology DD;JD;, Software n.a., Validation n.a., Formal analysis JD;DD;SR, Investigation DD;JD;YH;BB;OB;SF;IH;MH;WK;ML;DL;VM;IM;JN;GP;SR;GS;MS;EV;JV;JW;CE;GT;JY;MB;BG, Resources DD;JD;YH;BB;OB;SF;IH;MH;WK;ML;DL;VM;IM;JN;GP;SR;GS;MS;EV;JV;JW;CE;GT;JY;MB;BG, Data curation DD;JD;YH, Writing-Original draft DD;JD, Writing - Review&Editing DD;JD;YH;BB;OB;SF;IH;MH;WK;ML;DL;VM;IM;JN;GP;SR;GS;MS;EV;JV;JW;CE;GT;JY;MB;BG, Visualization DD;JD, Supervision MB;JY;BG, Project Administration DD, Funding Acquisition n.a.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
The data that support the findings of this study are available on reasonable request from the corresponding author.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33 [DOI] [PubMed] [Google Scholar]
- 2. Park W, Chawla A, O’Reilly EM. Pancreatic cancer: a review. JAMA 2021;326:851–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science 1981;212:53–55 [DOI] [PubMed] [Google Scholar]
- 4. Berger AC, Garcia M Jr, Hoffman JP, Regine WF, Abrams RA, Safran Het al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol 2008;26:5918–5922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khorana AA, McKernin SE, Berlin J, Hong TS, Maitra A, Moravek Cet al. Potentially curable pancreatic adenocarcinoma: ASCO clinical practice guideline update. J Clin Oncol 2019;37:2082–2088 [DOI] [PubMed] [Google Scholar]
- 6. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma (Version 1.2022).https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed 24 February 2022)
- 7. Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KHet al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg 2018;268:215–222 [DOI] [PubMed] [Google Scholar]
- 8. Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BAet al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol 2020;38:1763–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Versteijne E, van Dam JL, Suker M, Janssen QP, Groothuis K, Akkermans-Vogelaar JMet al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch randomized PREOPANC trial. J Clin Oncol 2022;40:1220–1230 [DOI] [PubMed] [Google Scholar]
- 10. Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol 2012;3:105–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anger F, Doring A, van Dam J, Lock JF, Klein I, Bittrich Met al. Impact of borderline resectability in pancreatic head cancer on patient survival: biology matters according to the new international consensus criteria. Ann Surg Oncol 2021;28:2325–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oba A, Del Chiaro M, Satoi S, Kim SW, Takahashi H, Yu Jet al. New criteria of resectability for pancreatic cancer: a position paper by the Japanese Society of Hepato-Biliary-Pancreatic Surgery (JSHBPS). J Hepatobiliary Pancreat Sci 2022;29:725–731 [DOI] [PubMed] [Google Scholar]
- 13. Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JBet al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833–846; discussion 46–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tzeng CW, Fleming JB, Lee JE, Xiao L, Pisters PW, Vauthey JNet al. Defined clinical classifications are associated with outcome of patients with anatomically resectable pancreatic adenocarcinoma treated with neoadjuvant therapy. Ann Surg Oncol 2012;19:2045–2053 [DOI] [PubMed] [Google Scholar]
- 15. Mackay TM, Smits FJ, Roos D, Bonsing BA, Bosscha K, Busch ORet al. The risk of not receiving adjuvant chemotherapy after resection of pancreatic ductal adenocarcinoma: a nationwide analysis. HPB (Oxford) 2020;22:233–240 [DOI] [PubMed] [Google Scholar]
- 16. Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MSet al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 2014;260:372–377 [DOI] [PubMed] [Google Scholar]
- 17. van Dam JL, Janssen QP, Besselink MG, Homs MYV, van Santvoort HC, van Tienhoven Get al. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomised controlled trials. Eur J Cancer 2022;160:140–149 [DOI] [PubMed] [Google Scholar]
- 18. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol 2007;33:266–270 [DOI] [PubMed] [Google Scholar]
- 19. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CMet al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011–1024 [DOI] [PubMed] [Google Scholar]
- 20. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JLet al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018;379:2395–2406 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author.