Abstract
Objective:
Long-term intracranial electroencephalography (iEEG) in freely behaving animals provides valuable electrophysiological information and when correlated with animal behavior is useful for investigating brain function.
Approach:
Here we develop and validate an automated iEEG-based sleep-wake classifier for canines using expert sleep labels derived from simultaneous video, accelerometery, scalp EEG and iEEG monitoring. The video, scalp EEG, and accelerometery recordings were manually scored by a board-certified sleep expert into sleep-wake state categories: Awake, rapid-eye-movement (REM) sleep, and three non-REM sleep categories (NREM 1, 2, 3). The expert labels were used to train, validate, and test a fully automated iEEG sleep-wake classifier in freely behaving canines.
Main results:
The iEEG-based classifier achieved an overall classification accuracy of 0.878 ± 0.055 and a Cohen’s Kappa score of 0.786 ± 0.090. Subsequently, we used the automated iEEG-based classifier to investigate sleep over multiple weeks in freely behaving canines. The results show that the dogs spend a significant amount of the day sleeping, but the characteristics of daytime nap sleep differ from night-time sleep in three key characteristics: During the day, there are fewer NREM sleep cycles (10.81 ± 2.34 cycles per day vs. 22.39 ± 3.88 cycles per night; p<0.001), shorter NREM cycle durations (13.83 ± 8.50 mins per day vs. 15.09 ± 8.55 mins per night; p<0.001), and dogs spend a greater proportion of sleep time in NREM sleep and less time in REM sleep compared to night-time sleep (NREM 0.88 ± 0.09, REM 0.12 ± 0.09 per day vs. NREM 0.80 ± 0.08, REM 0.20 ± 0.08 per night; p<0.001).
Significance:
These results support the feasibility and accuracy of automated iEEG sleep-wake classifiers for canine behavior investigations.
Keywords: sleep classification, implantable devices for sensing and stimulation, intracranial EEG, canine
1. Introduction
Naturally occurring canine epilepsy is the most common neurological disorder in dogs [1] and shares many features with human epilepsy [2]. Dogs share an evolutionary history with humans, and are a promising model for studying behavior, sleep and epilepsy [3–5]. Moreover, dogs are large enough to accommodate implantable neural stimulation and sensing (INSS) devices designed for humans. Dogs with epilepsy have proven valuable in developing human INSS devices [6–12] and the same devices hold promise as a new canine therapy [13].
Electrical deep brain stimulation using implantable neural stimulators is a proven treatment in human drug resistant focal epilepsy [14–17]. Novel INSS devices capable of continuously streaming intracranial electroencephalography (iEEG) signals enable tracking brain activity and correlating iEEG with normal and pathological behavior [6,7,18,19].
Seizure risk and epileptiform brain activity are known to follow a circadian pattern and to be related to sleep [6,20–22]. However, a deeper understanding of the relationship between epilepsy, sleep dynamics, and seizure occurrence is needed to develop the next generation of adaptive stimulation therapies for canines [13] and humans [6,7].
Kremen et al. previously demonstrated that iEEG could be used to differentiate sleep-wake states in humans [23,24]. Subsequently, we developed a fully automated sleep-wake classifier using iEEG signals recorded with a novel INSS device in humans [18]. The automated iEEG sleep-wake classifier was trained, validated, and tested using expert sleep annotations from polysomnography (PSG). Here we apply a similar approach to dogs replacing PSG with manual sleep scoring using continuous video, scalp EEG, and accelerometry. Reliable sleep classifiers for analysis of long-term iEEG data recorded with INSS devices in dogs will facilitate future research on the relationship between sleep and epilepsy and serve as a translational platform for developing electrical brain stimulation protocols.
2. Methods
This study aimed to develop, validate, and test an automated iEEG sleep-wake state classifier using chronic iEEG recordings. Three canines (Canis familiaris - Beagle) (D1-3) were previously implanted with the investigational Medtronic Summit RC+S™ device capable of continuous iEEG data streaming, accelerometry and electrical stimulation [6,7,18,25–27].
Here, we recorded up to three consecutive nights of simultaneous video, accelerometry, scalp and iEEG for each subject. The continuous video, accelerometry, and scalp EEG signals were used to manually score each 30-second segment of the recordings according to standard human sleep-wake state categories: awake, rapid-eye-movement (REM) sleep and three non-REM (NREM1,2,3) sleep stages [28]. The gold-standard expert annotations were utilized to develop an automated, subject specific, iEEG sleep-wake state classifier for each subject. The classifiers were subsequently deployed for automated sleep-wake classification using the long-term chronic iEEG recordings in freely behaving dogs to investigate sleep patterns. The study scheme is shown in Figure 1.
Figure 1. Video-EEG and the data flow diagram.
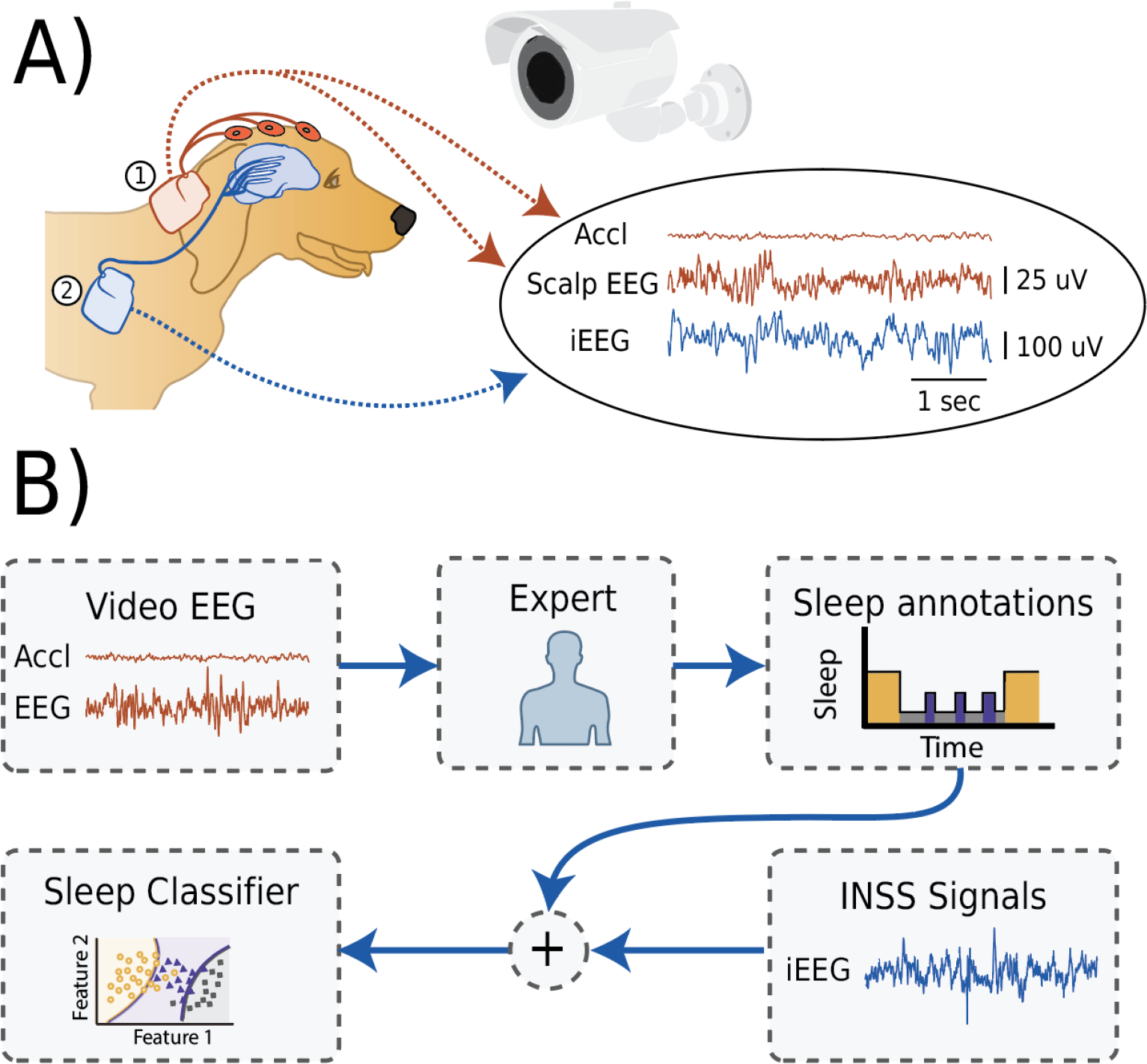
A) An illustration of a simultaneous video, accelerometry (Accl), scalp electroencephalography (EEG) and intracranial EEG (iEEG). 1) The external telemetry device (the investigational Medtronic Summit RC+S™) taped in place on the dorsal cervical area was utilized to acquire scalp EEG and accelerometry signals. 2) The iEEG signal was acquired using an implanted implantable neural sensing and stimulation (INSS) device (the investigational Medtronic Summit RC+S™. B) Block scheme of data flow during the experiment. The video, Accl, and scalp EEG are used to manually score the record into awake and sleep categories (awake, rapid-eye-movement (REM) sleep and three non-REM 1,2,3 sleep stages). The expert behavioral state annotations were used together with the iEEG signals to train and test an automated sleep classifier.
2.1. Ethical statement
The animal research took place at Mayo Clinic, Rochester, MN, under IACUC protocols “Chronic Wireless Electrophysiology and Modulation in Epileptic Dogs”.
2.2. Animals
Three adult female intact beagles (D1-3; 10.5 ± 1.0 kg; 4, 5 & 12 years) were housed on a 12/12 light cycle and fed approximately 2 cups Lab Diet 5L18 daily with water provided ad libitum. Animals were housed in temperature-controlled rooms with elevated floors that met all size, material, and sanitation requirements according to the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act [29]. Animals were provided mats and daily enrichment via assorted treats, chew toys, and human interaction. Animals were socially housed, except during the scalp EEG monitoring. Animals were assessed daily by a team of veterinarians and technicians.
2.3. Imaging
Magnetization-prepared rapid gradient echo (MP-RAGE) and Fast Gray matter acquisition T1 inversion recovery (FGATIR) sequences were used given the optimized grey-white matter contrast. These sequences are frequently used for human pre-operative electrode targeting and allows direct visualization of the mammillothalamic tract and direct targeting of the anterior nucleus of thalamus [30,31].
2.4. Implantable neural stimulation and sensing (INSR) device
The investigational Medtronic Summit RC+S™ is a four-lead device with 4 contacts on each lead (16 total contacts). The device is capable of 16-channel electrical stimulation and continuous sensing and wireless streaming of 4-bipolar iEEG channels and accelerometry. The sampling rate for the iEEG signals is selectable between 250 Hz, 500 Hz or 1000 Hz. The INSS device requires daily charging for ~0.5–1.5 hours to stream data for 24 hours depending on the sampling frequency. The iEEG and scalp EEG were filtered using hardware bandpass filters with cutoff frequencies of 0.8 Hz and 100 Hz. A sampling rate of 250 Hz was used for iEEG and scalp EEG signals, while accelerometry signals were sampled at 32 Hz.
2.5. Implant surgery
Canines were implanted with four leads (4 contacts on each lead; 16 total contacts) and the investigational Medtronic Summit RC+S™ device [25]. The implantation surgery was performed under anesthesia using a custom-made stereotactic frame for lead targeting. Each dog had 4 leads surgically implanted. Two dogs (D1 & D2) were implanted with the 4 leads targeting the bilateral subdural space over the brain convexity (Medtronic 3391) and one dog (D3) had two leads targeting bilateral hippocampus (Medtronic 3387) and two leads targeting bilateral anterior nucleus of the thalamus (ANT) (Medtronic 3389).The leads (Medtronic 3391, 3387 and 3389) are 1.27mm diameter and have 4 macroelectrodes with different electrode contact sizes (length: 3mm, 1.5mm and 1.5mm and spacing: 4mm, 1.5mm, 0.5 mm, respectively). These macroelectrodes were used to record local field potentials (bandwidth:1 – 100 Hz, sampling rate: 250 or 500 Hz).
The hippocampus and ANT targeting and implant trajectories were determined using a pre-operative 3.0T MRI scan (MP-RAGE and FGATIR) and stereotactic software Compass™ Stereotactic Systems [30–32]. The subdural electrodes were placed in the anterior (rostral) direction spanning the frontal and parietal lobes. The brain targets for all electrode leads were selected in accordance with other ongoing research studies.
Electrode leads were inserted via burr holes drilled into the skull and secured with metal anchors and bone screws. The electrode tails were tunneled to the implanted device that was placed in a pocket created behind the canine’s right scapula. Subsequently, the canines underwent a post-op x-ray CT scan which was then co-registered with the pre-operative MRI scan to verify the targeting accuracy (Analyze 12.0, BIR, Mayo Foundation) (Figure 2) [6–9,25].
Figure 2.
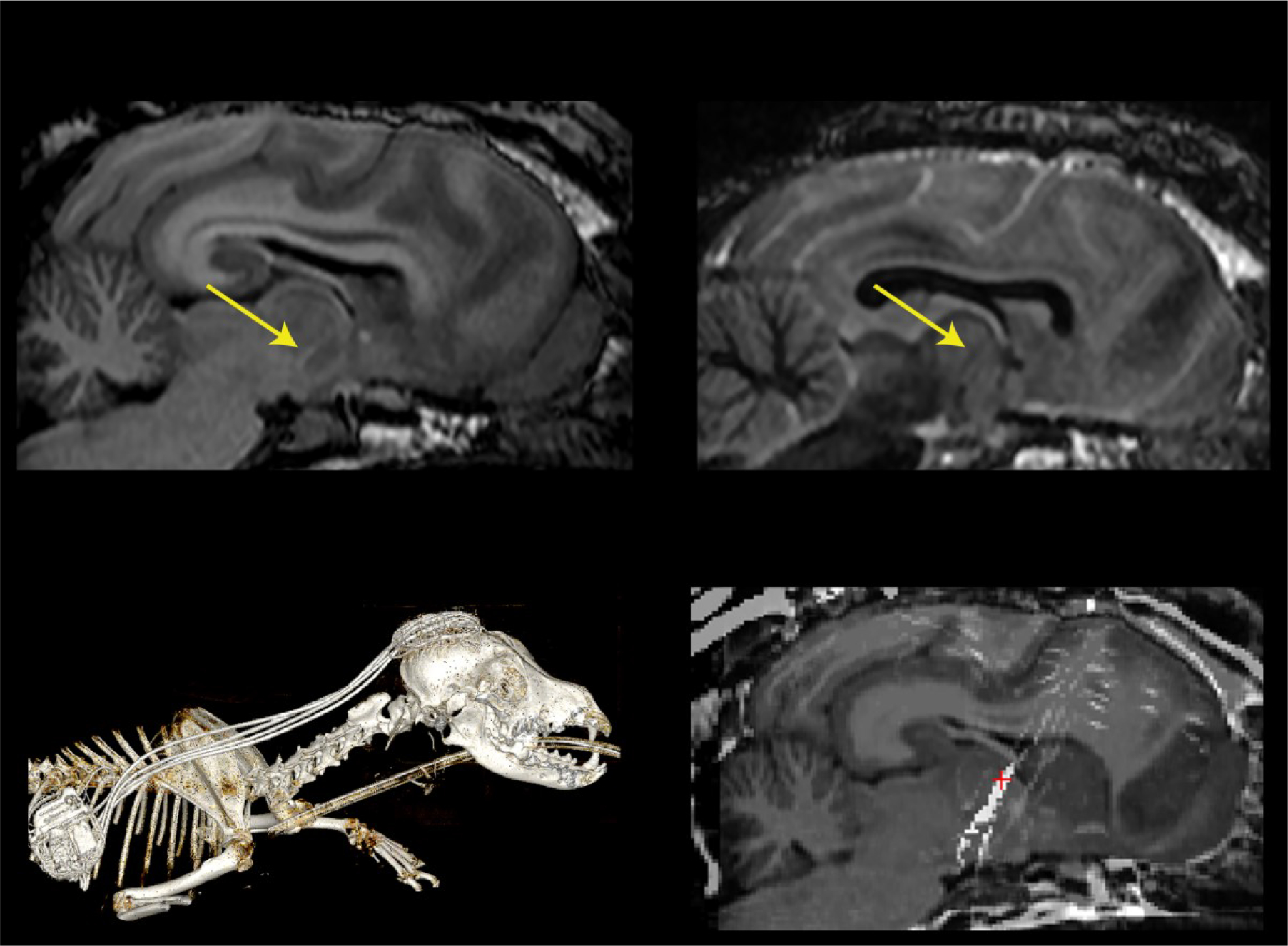
An illustrative 3 Tesla Magnetic Resonance (MR) and Computed tomography (CT) imaging of subject D3 with implanted additional ANT electrodes. A) MP RAGE and B) FGATIR MR sequences were used to plan electrode implant trajectory. Yellow arrows show distinct mammillothalamic tract used to target the ANT. C) CT imaging of fully implanted system. D) Co-registration of CT & MR
2.6. Scalp and intracranial EEG
The scalp-EEG was collected using a second investigational Medtronic Summit RC+S™ device taped to the dog’s shoulder (Figure 1) and will be referred to as the telemetry device hereafter. Scalp EEG included two-channel bipolar recordings (Fz-Cz, Fz-Oz) using three gold-cup scalp electrodes (Fz, Cz, Oz). As a post-processing step, the recorded bipolar channels Fz-Cz and Fz-Oz were subtracted providing another bipolar derivation Cz-Oz.
To apply the scalp-EEG electrode the dogs were sedated using Dexmedetomidine (0.005 – 0.01 mg/kg IM, Morphine 0.5 mg/kg IM) and Isoflurane 2% via mask used briefly (< 5 min) to maintain sedation (no reversal needed), for placement of the scalp EEG electrodes and the telemetry device. A small region of the head was shaved, and 3 gold-cup EEG electrodes (Genuine Grass 10 mm Gold Disc Electrodes) were placed on the anteroposterior midline spanning from the forehead to the occiput, avoiding the massive musculature on the dog’s head. The electrodes were placed using collodion solution. Approximately 10 mm wide and 50 mm long single-layer-gauze strip soaked with a collodion solution was placed around each electrode to ensure better electrode adhesion to the skin. After the electrode placement, the cup electrodes were filled with a conductive gel to decrease electrode-skin impedance.
The conductive gel filled electrodes were covered by antimicrobial incise drape (3M Ioban) to reduce gel evaporation. The telemetry device was taped on the dorsal cervical spine area (C2 & C3) using an antimicrobial incise drape so the dog could wear the telemetry device (like a scarf) in a safe location (beyond the dog’s reach) where it is unlikely to be damaged. The EEG electrodes and the telemetry device were additionally protected using a recovery cone collar. The dog wore the recovery cone collar for a one-day adaptation period prior to the experiment. The sampling rate for scalp EEG was 250 Hz and 32 Hz for the accelerometry signals. The signals were continuously streamed to Epilepsy Assistant Personalized Device (EPAD) tablet [25,26].
The gold cup electrodes were refilled with a conductive gel every night. During the conductive gel refill, the skin under the electrodes was gently scratched using the gel application syringe to remove the conductive gel film created by evaporation. The electrical impedance measured between the electrodes ranged from 400 Ω to 1,500 Ω. The impedance increased during the night up to 5,000 Ω due to the conductive gel evaporation. The iEEG recordings used 4 bipolar pair derivations with one bipolar pair from each of the 4 implanted leads as previously described [7, 25]. Recording bipolar derivations were selected based on visual review of the post implant imaging of the electrode leads and the iEEG recordings after surgery.
2.7. Video EEG monitoring
In humans, the manual scoring of PSG recordings is the gold standard for behavioral state classification (awake, REM and NREM). Here we utilized video, scalp-EEG, and accelerometry as an alternative to PSG. The video scalp-EEG monitoring was performed simultaneously with iEEG and accelerometry data streaming from the implanted INSS device. An IP camera (1080p, 30 frames per second Amcrest Inc.) with infrared night vision was used for the video recording.
The computer time of both recording workstations, (iEEG and video) were synchronized with the Network Time Protocol server before the start of each overnight recording.
The simultaneous continuous video, iEEG, scalp EEG, and accelerometry were collected for up to three consecutive nights depending on the canine’s tolerance of the telemetry device and scalp EEG electrodes. The electrical impedance of the scalp EEG electrodes was periodically measured, and data quality assessed every 30–60 minutes. The experiment was conducted in a research kennel with lights controlled by an automatic timer with a dark period between 7 PM and 7 AM.
2.8. Manual sleep scoring
A board-certified sleep expert manually scored each 30-second epoch of the video, scalp EEG, and accelerometery recording into: awake, rapid-eye-movement sleep (REM) and three non-REM sleep stages (NREM1,2 and 3). The classification rules used to score the video-EEG recordings were derived from human sleep scoring rules (American Association of Sleep Medicine 2012 [33]) similar to prior canine studies [34].
The video-EEG visualization and sleep scoring was facilitated using a research software tool CyberPSG (Certicon a.s.). As a preprocessing step, the EEG signals were bandpass filtered between 0.3 Hz and 75 Hz using sixth-order zero-phase Butterworth filters.
Wakefulness (awake) was determined using the video and accelerometry data and by the presence of eye blinks visualized in the anterior scalp EEG lead derivations (Fz-Cz, Fz-Oz), accompanied by distinct muscle artifacts (Figure 3). The NREM1 sleep stage is the lightest sleep and is a transition state between wakefulness and deeper sleep stages. The NREM1 sleep stage was determined by a decrease in muscle activity and visible attenuation of alpha rhythm (8–12 Hz) in the posterior EEG lead derivations (Cz-Oz, Fz-Oz) accompanied by no movement and slow roving eye movements. The NREM2 sleep stage was scored when low-frequency delta activity (0.5 – 4 Hz) was present accompanied by K-complexes or sleep spindles (Figure 3). The NREM3 state was scored when high-voltage (>50 μV), delta (0.5 – 4 Hz) activity on scalp EEG was present in at least 20 % (6 sec) of the data epoch. Due to a difference between amplitudes of dog scalp EEG and human scalp EEG, we used a criterion of 50 μV peak-peak amplitude of delta waves (compared to standard 75μV in human PSG). Rapid eye movements in frontal electrodes and low tonic muscle tone with intermittent phasic muscle activity visible in scalp EEG and accelerometry data was used to identify REM sleep. Notably, REM sleep is more frequent in dogs compared to humans and dogs often perform running leg movements during REM. Only segments with clear manual annotations were utilized for the sleep classifier development.
Figure 3. Representative examples of the collected data for the dog D2.
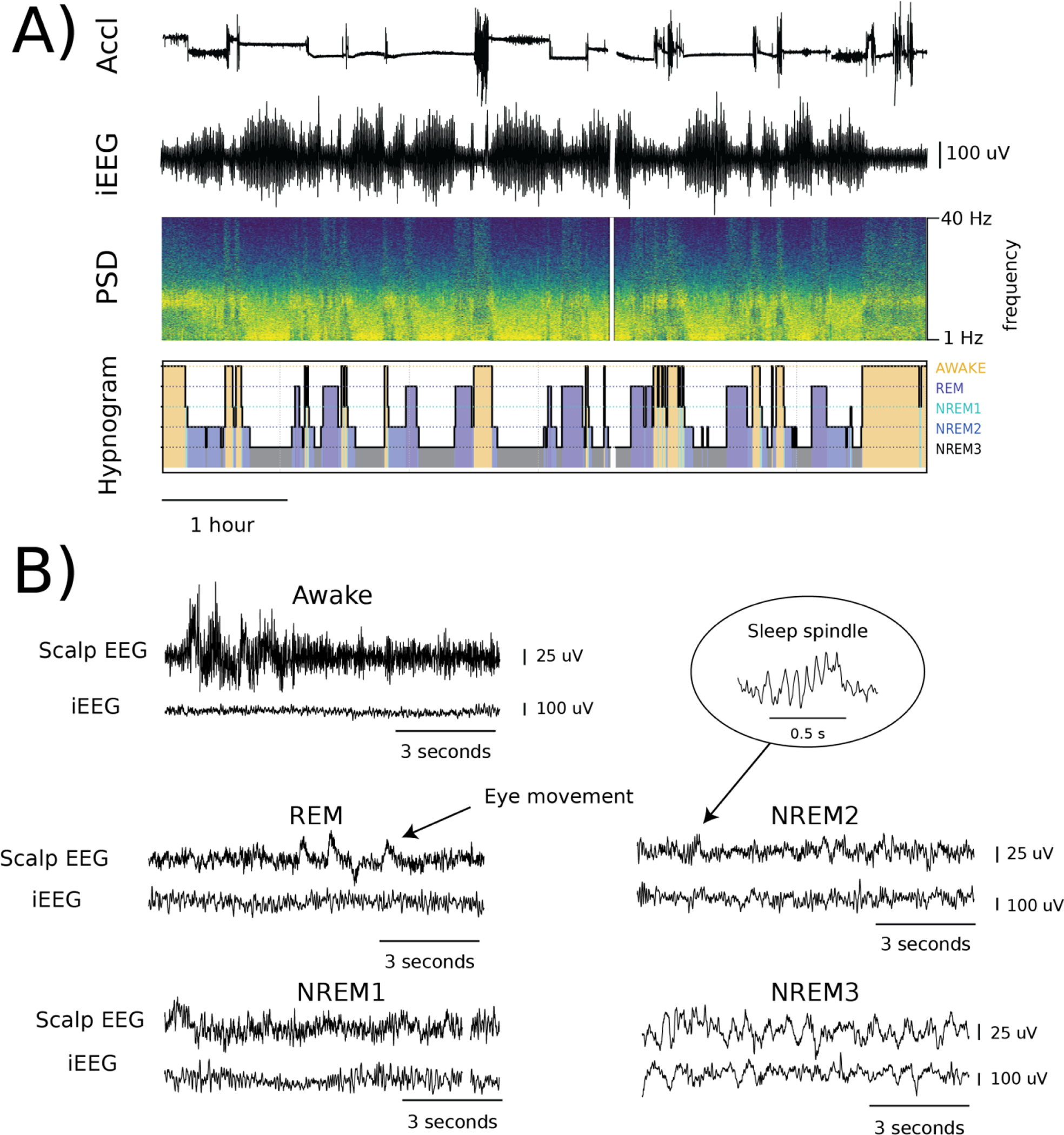
A) Accelerometry and intracranial electroencephalography (iEEG) data both recorded by the implantable neural stimulator with corresponding iEEG power spectrum density (PSD) and a hypnogram created by manual review. B) Representative samples of corresponding scalp electroencephalography (EEG) (Fz-Oz) and iEEG signals for all sleep categories: Awake, REM, and non-REM (NREM) 1,2,3. White spaces in the A) PSD and B) NREM1 are examples of data drops caused by disruptions in the wireless data streaming.
2.9. Automated sleep classification
We developed an automated sleep classification algorithm using Naïve Bayes (NB) for each subject separately, to automatically create sleep labels for longitudinal iEEG signals.
The automated sleep classifiers were created using 5,023 30-second segments (D1 – 772; D2 – 2,635; D3 – 1,616) labeled by a human expert with data rate >80% and distributed between Awake: 1,405, NREM: 2,929 and REM: 689. The data rate threshold was selected to account for short data drops caused by lost packets in wireless data streaming. The percentage distribution of datapoints between sleep categories was similar for all subjects. We developed a subject-specific (individualized) sleep staging classifier for each subject.
The classification models were trained using the first 80% of iEEG data for each respective sleep state. The remaining 20% of data was used for testing. For instance, if there were 100 epochs recorded during the experiment for each category, the first 80 epochs for each category would be used for training the models, and the last 20 epochs would be used for testing.
In this way, we tested the NB classifier on future data simulating a real-life use case where the ML model is trained on past data and then prospectively deployed on newly acquired data. The testing was performed for each subject separately. We used all available iEEG traces for classification for each subject.
We developed the methodology and created a toolbox for iEEG pre-processing, feature extraction, and sleep-wake classification [18]. The sleep-wake classification NB model uses power in band (PIB) features extracted from 30-second iEEG data segments. The NB classifier utilizes a statistical distribution of a training dataset to predict the category likelihood for new unseen samples. Subsequently, each classified segment is assigned into the behavioral state category with the highest likelihood.
The PIB features used for this study were: 0.5 – 4 Hz; 4 – 9 Hz; 9 – 14 Hz; 14 – 20 Hz; 20 – 30 Hz; 30 – 60 Hz; 60 – 80 Hz. The model was trained to classify canine behavioral state: Awake, REM, and non-REM.
To evaluate the classification performance of the classifier we report classification accuracy, F1 score and Cohen’s Kappa score. Classification scores are reported in the format of average ± standard deviation.
The classifiers were retrospectively and prospectively deployed to analyze sleep patterns for all three canines using continuous iEEG recordings.
2.10. Long-term sleep classification
To evaluate long-term sleep dynamics, we investigated data generated by the automated sleep classifiers over multiple weeks. During this long-term phase of the study the automated NB classifier was applied to the continuous iEEG data from freely behaving canines. The long-term data were tiled into 24-hour segments and only the segments with data rates higher than 0.9 were utilized to analyze the sleep dynamics. In total, we analyzed fifty-seven 24-hour segments (D1 – 11; D2 – 27; D3 – 19) with data rate >90%. The data rate threshold was selected to identify days with more than 10% of data missing for various reasons including, discharged INSS device, or the dog being out of range of the recording tablet.
We characterized how the following sleep features vary in time:
time spent sleeping per monitoring period
time spent in NREM sleep
time spent in REM sleep
NREM proportion relative to time spent sleeping (REM & NREM)
REM proportion relative to time spent sleeping (REM & NREM)
number and duration of NREM cycles (episodes) > 5 minutes per monitoring period
To evaluate the significance of the individual features, we utilized a non-parametric two-sided Mann-Whitney test with Bonferroni correction for multiple observations.
A sleep cycle was defined as a period from the beginning of a NREM sleep stage to the beginning of the next awake or end of REM. Sleep periods shorter than 5 minutes were not considered a sleep cycle.
2.8. Data and code availability
Codes can be found on a public GitHub repository https://github.com/mselair/best_toolbox. Data is available from the corresponding author upon a reasonable request.
3. Results
We developed and validated a framework for video, accelerometry and neurophysiology data acquisition and manual sleep-wake state annotation (Awake, NREM1,2,3, and REM sleep) in canines (Figure 3). Using this framework, we recorded 7 total nights of simultaneous video, scalp EEG, accelerometry and iEEG (4 channels) in three canines (D1 – 1 night; D2 – 3 nights; D3 – 3 nights). The recording was terminated after the first night for the subject D1 because two of the three scalp EEG electrodes detached. No further complications occurred during the experiment. We recorded on average 4.91 ± 1.51 hours of sleep per monitoring night.
The video scalp EEG and accelerometry recordings were used to create expert sleep labels. The amount of annotated data totals 52.12 hours (D1 – 6.85; D2 – 23.59; D3 – 21.71) Awake - 31.45 %; NREM1 – 2.62 %; NREM2 – 20.29 %; NREM3 – 32.85 %; REM - 12.78 %). Detail information about all annotated sleep stages during the monitoring is provided in Table 1.
Table 1.
A number of 30-second epochs of collected and annotated video-EEG data. Sleep was manually scored into the following sleep categories: Awake, rapid-eye-movement (REM) sleep, and three non-REM categories (NREM1-3).
| Nights | Awake | NREM1 | NREM2 | NREM3 | REM | All | |
|---|---|---|---|---|---|---|---|
| D1 | 1 | 395 | 15 | 104 | 219 | 89 | 822 |
| D2 | 3 | 541 | 90 | 716 | 1086 | 398 | 2831 |
| D3 | 3 | 1032 | 59 | 450 | 751 | 313 | 2605 |
| Overall | 7 | 1968 | 164 | 1270 | 2056 | 800 | 6258 |
The automated iEEG sleep classifiers achieved high classification performance reaching average Accuracy 0.878 ± 0.055, F1 score 0.816 ± 0.091 and Cohen’s Kappa score 0.786 ± 0.90 (Table 2).
Table 2.
Classification performance for each subject. Scores including Accuracy, F1-score, and Cohen’s Kappa score.
| Subject | Accuracy | F1 | Kappa |
|---|---|---|---|
| D1 | 0.839 | 0.724 | 0.723 |
| D2 | 0.956 | 0.940 | 0.914 |
| D3 | 0.839 | 0.785 | 0.723 |
| Overall | 0.878 ± .055 | 0.816 ± .091 | 0.786 ± .090 |
The automated sleep classifiers were deployed to multiple months of data recorded from freely behaving canines implanted with INSS devices. We selected 57 days with high 24-hour data rates (0.94 ± 0.02) to analyze long-term canine sleep dynamics.
We quantified canine sleep (NREM + REM) during 24-hour periods and during respective day and night periods (7 PM Lights Off – 7AM Lights On). The dogs slept 10.90 ± 1.47 (median 11.18) hours per 24-hours (Table 3).
Table 3.
Sleep dynamic during Video EEG (human expert labels) and long-term chronic INSS monitoring created by an automated sleep classifier using chronic iEEG data.
| Sleep Feature | Video EEG | Per night (7pm - 7am) | Per day (7am - 7pm) | Per 24-hours |
|---|---|---|---|---|
| Datarate [-] | 0.92 ± 0.3 | 0.97 ± 0.03 | 0.90 ± 0.04 | 0.94 ± 0.02 |
| Time spent sleeping [hrs] | 4.91 ± 1.51 | 7.63 ± 1.01 | 3.28 ± 0.85 | 10.90 ± 1.47 |
| Non-REM [hrs] | 3.96 ± 1.36 | 6.05 ± 0.57 | 2.87 ± 0.68 | 8.92 ± 0.92 |
| REM [hours] | 0.95 ± 0.29 | 1.58 ± 0.72 | 0.41 ± 0.41 | 1.99 ± 1.04 |
| Non-REM relative | 0.80 ± 0.04 | 0.80 ± 0.08 | 0.88 ± 0.09 | 0.83 ± 0.07 |
| REM relative | 0.20 ± 0.04 | 0.20 ± 0.08 | 0.12 ± 0.08 | 0.18 ± 0.07 |
| Non-REM cycles [n] | 15.29 ± 4.27 | 22.39 ± 3.88 | 10.81 ± 2.34 | 33.12 ± 4.20 |
| Non-REM episode duration [mins] | 13.53 ± 7.10 | 15.09 ± 8.55 | 13.83 ± 8.50 | 14.77 ± 8.63 |
The duration of sleep time was reduced during collection of the gold-standard data (video, scalp EEG, accelerometry) used for training and testing of behavioral state classifications (p<0.001) compared to full night sleep during INSS monitoring.
The dogs slept 7.63 ± 1.01 hours per night with 22.39 ± 3.88 NREM episodes with average duration of 15.09 ± 8.55 minutes. The proportion of sleep spent in NREM sleep during the night was 0.80 ± 0.08. During the day the dogs slept 2.87 ± 0.68 hours and the number of NREM episodes decreased to 10.81 ± 2.34 per 12 hours (p<0.001). Similarly, the duration of the NREM episodes decreased to 13.83 ± 8.50 minutes (p<0.001) and the proportion of sleep spent in NREM during the day increased to 0.88 ± 0.09 (p<0.001)
4. Discussion
Here we describe the methodology for collecting video, scalp EEG, iEEG and accelerometry for expert manual behavioral state classifications and development of an automated canine sleep state iEEG classifier.
The scalp EEG was collected using three electrodes that provided adequate fronto-occipital coverage for assessing sleep and wakefulness brain states. The scalp-EEG showed good eye movements, blinking artifacts, alpha activity, and sleep spindles (Figure 3). We also observed EMG artifacts on the scalp electrodes from the large canine temporalis muscles. We successfully provided a viable signal for expert sleep scoring using only three electrodes. This approach is similar to that employed in humans, except with a more limited number of electrodes given the canine skull size.
We show that the external instrumentation (scalp-EEG & recovery cone) reduced canine sleep time. This is likely related to discomfort associated with the scalp EEG system and the recovery collar that must be used to keep the dog from scratching and dislodging the scalp electrodes and external device. Interestingly, the amount of NREM and REM sleep normalized to account for the total sleep time is similar between Video scalp EEG and long-term INSS monitoring. This is evident from the comparable percentages of NREM and REM sleep during both experiments (Video scalp EEG: 0.80 ± 0.04; INSS monitoring: 0.80 ± 0.08, p=0.8).We argue the current system using a small wireless wearable device for collection of scalp EEG and accelerometry has advantages compared to previously developed canine PSG system using wired electrodes that tether the animal to larger recording devices [35]. Using a small wireless device offers several advantages, including the elimination of the long wires connecting electrodes to the recording device. This allows for unrestricted movement of the dog during the experiment, and enhances the durability of the surface electrodes, which can last for up to three consecutive nights. Additionally, it eliminates the need for invasive needle electrodes.
Daytime naps in canines show shorter NREM cycles compared to night-time sleep. This suggests the dog is in lighter sleep during the day.
5. Conclusion
We developed and validated an automated iEEG based behavioral state classifier using expert labeled data from non-invasive physiological signals (video, scalp-EEG, and accelerometry) in freely behaving canines implanted with an INSS device. The longterm sleep characteristics were obtained with the automated classification using only iEEG. The dogs cycle through many more NREM-REM cycles per night (22.39 ± 3.88) than humans (5 sleep cycles per night) as noted in previous studies [36,37]. Furthermore, the dogs frequently nap during the day, but have different sleep characteristics during the daytime naps with fewer NREM-REM cycles and longer NREM durations.
The system developed here for automated sleep state classification using an implanted INSS device enables research into long-term canine sleep and should prove useful for investigating the complex relationship between sleep, epilepsy, and therapeutic electrical brain stimulation.
Acknowledgements
This work was supported by NIH Brain Initiative UH2&3 NS095495 Neurophysiologically-Based Brain State Tracking & Modulation in Focal Epilepsy, R01- NS92882 Reliable Seizure Prediction Using Physiological Signals and Machine Learning, DARPA HR0011- 20-2-0028 Manipulating and Optimizing Brain Rhythms for Enhancement of Sleep (Morpheus). Medtronic provided the investigational Medtronic Summit RC+S™ devices. V K was partially supported by institutional funding of Czech Technical University in Prague, Czech Republic. Certicon a.s. provided Cyber PSG viewer for research purposes. FM appreciates the help and effort of his Ph.D. supervisor Pavel Jurak.
Footnotes
Conflict of interest
GW, BB, VK, and JVG, are named inventor for intellectual property developed at Mayo Clinic and licensed to Cadence Neuroscience Inc. GW has licensed intellectual property developed at Mayo Clinic to NeuroOne, Inc. GW and NG areinvestigators for the Medtronic Deep Brain Stimulation Therapy for Epilepsy Post-Approval Study (EPAS). VK consults for Certicon a.s. FM received salary support from Cadence Neuroscience Inc. IB has received compensation from an internship with Cadence Neuroscience Inc. for work unrelated to the current publication. Mayo Clinic has received research support and consulting fees on behalf of GW and BB from UNEEG, NeuroOne Inc., Epiminder, and Medtronic Plc.
References
- [1].Heske L, Nødtvedt A, Jäderlund KH, Berendt M and Egenvall A 2014. A cohort study of epilepsy among 665,000 insured dogs: Incidence, mortality and survival after diagnosis Vet. J [DOI] [PubMed] [Google Scholar]
- [2].Berendt M, Høgenhaven H, Flagstad A and Dam M 1999. Electroencephalography in dogs with epilepsy: Similarities between human and canine findings Acta Neurol. Scand [DOI] [PubMed] [Google Scholar]
- [3].Bunford N, Andics A, Kis A, Miklósi Á and Gácsi M 2017. Canis familiaris As a Model for Non-Invasive Comparative Neuroscience Trends Neurosci [DOI] [PubMed] [Google Scholar]
- [4].Hare B, Brown M, Williamson C and Tomasello M 2002. The domestication of social cognition in dogs Science (80-.) [DOI] [PubMed] [Google Scholar]
- [5].Tang R, Noh H, Wang D, Sigurdsson S, Swofford R, Perloski M, Duxbury M, Patterson EE, Albright J, Castelhano M, Auton A, Boyko AR, Feng G, Lindblad-Toh K and Karlsson EK 2014. Candidate genes and functional noncoding variants identified in a canine model of obsessive-compulsive disorder Genome Biol 15 R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gregg NM, Sladky V, Nejedly P, Mivalt F, Kim I, Balzekas I, Sturges BK, Crowe C, Patterson EE, Van Gompel J J, Lundstrom BN, Leyde K, Denison TJ, Brinkmann BH, Kremen V and Worrell GA 2021. Thalamic deep brain stimulation modulates cycles of seizure risk in epilepsy Sci. Rep 11 24250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sladky V, Nejedly P, Mivalt F, Brinkmann BH, Kim I, St. Louis EK, Gregg NM, Lundstrom BN, Crowe CM, Attia TP, Crepeau D, Balzekas I, Marks VS, Wheeler LP, Cimbalnik J, Cook M, Janca R, Sturges BK, Leyde K, Miller KJ, Van Gompel JJ, Denison T, Worrell GA and Kremen V 2022. Distributed brain co-processor for tracking spikes, seizures and behavior during electrical brain stimulation Brain Commun [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brinkmann BH, Wagenaar J, Abbot D, Adkins P, Bosshard SC, Chen M, Tieng QM, He J, Muñoz-Almaraz FJ, Botella-Rocamora P, Pardo J, Zamora-Martinez F, Hills M, Wu W, Korshunova I, Cukierski W, Vite C, Patterson EE, Litt B and Worrell GA 2016. Crowdsourcing reproducible seizure forecasting in human and canine epilepsy Brain 139 1713–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Davis KA, Sturges BK, Vite CH, Ruedebusch V, Worrell G, Gardner AB, Leyde K, Sheffield WD and Litt B 2011. A novel implanted device to wirelessly record and analyze continuous intracranial canine EEG Epilepsy Res 96 116–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Baldassano SN, Brinkmann BH, Ung H, Blevins T, Conrad EC, Leyde K, Cook MJ, Khambhati AN, Wagenaar JB, Worrell GA and Litt B 2017. Crowdsourcing seizure detection: Algorithm development and validation on human implanted device recordings Brain 140 1680–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nejedly P, Kremen V, Sladky V, Nasseri M, Guragain H, Klimes P, Cimbalnik J, Varatharajah Y, Brinkmann BH and Worrell GA 2019. Deep-learning for seizure forecasting in canines with epilepsy J. Neural Eng 16 036031. [DOI] [PubMed] [Google Scholar]
- [12].Pal Attia T, Crepeau D, Kremen V, Nasseri M, Guragain H, Steele SW, Sladky V, Nejedly P, Mivalt F, Herron JA, Stead M, Denison T, Worrell GA and Brinkmann BH 2021. Epilepsy Personal Assistant Device—A Mobile Platform for Brain State, Dense Behavioral and Physiology Tracking and Controlling Adaptive Stimulation Front. Neurol 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zamora M, Meller S, Kajin F, Sermon JJ, Toth R, Benjaber M, Dijk D-J, Bogacz R, Worrell GA, Valentin A, Duchet B, Volk HA and Denison T 2021. Case Report: Embedding “Digital Chronotherapy” Into Medical Devices—A Canine Validation for Controlling Status Epilepticus Through Multi-Scale Rhythmic Brain Stimulation Front. Neurosci 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nair DR, Laxer KD, Weber PB, Murro AM, Park YD, Barkley GL, Smith BJ, Gwinn RP, Doherty MJ, Noe KH, Zimmerman RS, Bergey GK, Anderson WS, Heck C, Liu CY, Lee RW, Sadler T, Duckrow RB, Hirsch LJ, Wharen RE, Tatum W, Srinivasan S, McKhann GM, Agostini MA, Alexopoulos AV., Jobst BC, Roberts DW, Salanova V, Witt TC, Cash SS, Cole AJ, Worrell GA, Lundstrom BN, Edwards JC, Halford JJ, Spencer DC, Ernst L, Skidmore CT, Sperling MR, Miller I, Geller EB, Berg MJ, Fessler AJ, Rutecki P, Goldman AM, Mizrahi EM, Gross RE, Shields DC, Schwartz TH, Labar DR, Fountain NB, Elias WJ, Olejniczak PW, Villemarette-Pittman NR, Eisenschenk S, Roper SN, Boggs JG, Courtney TA, Sun FT, Seale CG, Miller KL, Skarpaas TL and Morrell MJ 2020. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy Neurology 95 e1244–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Babu Krishnamurthy K, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R and Graves N 2010. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy Epilepsia 51 899–908 [DOI] [PubMed] [Google Scholar]
- [16].Salanova V, Sperling MR, Gross RE, Irwin CP, Vollhaber JA, Giftakis JE and Fisher RS 2021. The SANTÉ study at 10 years of follow-up: Effectiveness, safety, and sudden unexpected death in epilepsy Epilepsia 62 1306–17 [DOI] [PubMed] [Google Scholar]
- [17].Morrell MJ 2011. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy Neurology 77 1295–304 [DOI] [PubMed] [Google Scholar]
- [18].Mivalt F, Kremen V, Sladky V, Balzekas I, Nejedly P, Gregg NM, Lundstrom BN, Lepkova K, Pridalova T, Brinkmann BH, Jurak P, Van Gompel J J, Miller K, Denison T, St. Louis EK and Worrell GA 2022. Electrical brain stimulation and continuous behavioral state tracking in ambulatory humans J. Neural Eng 19 016019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gregg NM, Nasseri M, Kremen V, Patterson EE, Sturges BK, Denison TJ, Brinkmann BH and Worrell GA 2020. Circadian and multiday seizure periodicities, and seizure clusters in canine epilepsy Brain Commun 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dell KL, Payne DE, Kremen V, Maturana MI, Gerla V, Nejedly P, Worrell GA, Lenka L, Mivalt F, Boston RC, Brinkmann BH, D’Souza W, Burkitt AN, Grayden DB, Kuhlmann L, Freestone DR and Cook MJ 2021. Seizure likelihood varies with day-to-day variations in sleep duration in patients with refractory focal epilepsy: A longitudinal electroencephalography investigation EClinicalMedicine [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hofstra W Ae and de Weerd AW 2009. The circadian rhythm and its interaction with human epilepsy: A review of literature Sleep Med. Rev [DOI] [PubMed] [Google Scholar]
- [22].Durazzo TS, Spencer SS, Duckrow RB, Novotny EJ, Spencer DD and Zaveri HP 2008. Temporal distributions of seizure occurrence from various epileptogenic regions Neurology [DOI] [PubMed] [Google Scholar]
- [23].Kremen V, Duque JJ, Brinkmann BH, Berry BM, Kucewicz MT, Khadjevand F, Van Gompel J, Stead M, St Louis E K and Worrell GA 2017. Behavioral state classification in epileptic brain using intracranial electrophysiology J. Neural Eng 14 026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kremen V, Brinkmann BH, Van Gompel J J, Stead M, St Louis E K and Worrell GA 2019. Automated unsupervised behavioral state classification using intracranial electrophysiology J. Neural Eng 16 026004. [DOI] [PubMed] [Google Scholar]
- [25].Kremen V, Brinkmann BH, Kim I, Guragain H, Nasseri M, Magee AL, Pal Attia T, Nejedly P, Sladky V, Nelson N, Chang S-Y, Herron JA, Adamski T, Baldassano S, Cimbalnik J, Vasoli V, Fehrmann E, Chouinard T, Patterson EE, Litt B, Stead M, Van Gompel J, Sturges BK, Jo HJ, Crowe CM, Denison T and Worrell GA 2018. Integrating Brain Implants With Local and Distributed Computing Devices: A Next Generation Epilepsy Management System IEEE J. Transl. Eng. Heal. Med 6 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pal Attia T, Crepeau D, Kremen V, Nasseri M, Guragain H, Steele SW, Sladky V, Nejedly P, Mivalt F, Herron J, Stead M, Denison T, Worrell GA and Brinkmann BH 2021. Epilepsy Personal Assistant Device -A Mobile Platform for Brain State, Dense Behavioral and Physiology Tracking and Controlling Adaptive Stimulation Front. Neurol [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mivalt F, Sladky V, Balzekas I, Pridalova T, Miller KJ, van Gompel J, Denison T, Brinkmann BH, Kremen V and Worrell GA 2022. Deep Generative Networks for Algorithm Development in Implantable Neural Technology 2022 IEEE International Conference on Systems, Man, and Cybernetics (SMC) (IEEE) pp 1736–41 [Google Scholar]
- [28].Iber C, Ancoli-Israel S, Chesson A and Quan SF 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification J. Clin. Sleep Med [Google Scholar]
- [29].Albus U 2012. Guide for the Care and Use of Laboratory Animals (8th edn) Lab. Anim [Google Scholar]
- [30].Grewal SS, Middlebrooks EH, Kaufmann TJ, Stead M, Lundstrom BN, Worrell GA, Lin C, Baydin S and Van Gompel J J 2018. Fast gray matter acquisition T1 inversion recovery MRI to delineate the mammillothalamic tract for preoperative direct targeting of the anterior nucleus of the thalamus for deep brain stimulation in epilepsy Neurosurg. Focus 45 E6. [DOI] [PubMed] [Google Scholar]
- [31].Sudhyadhom A, Haq IU, Foote KD, Okun MS and Bova FJ 2009. A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: The Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR) Neuroimage [DOI] [PubMed] [Google Scholar]
- [32].Brant-Zawadzki M, Gillan GD and Nitz WR 1992. MP RAGE: a three-dimensional, T1-weighted, gradient-echo sequence--initial experience in the brain. Radiology 182 769–75 [DOI] [PubMed] [Google Scholar]
- [33].Silber MH 2012. Staging sleep Sleep Med. Clin 7 487–96 [Google Scholar]
- [34].Wauquier A, Verheyen JL, Van Den Broeck WAE and Janssen PAJ 1979. Visual and computer-based analysis of 24 h sleep-waking patterns in the dog Electroencephalogr. Clin. Neurophysiol 46 33–48 [DOI] [PubMed] [Google Scholar]
- [35].Kis A, Szakadát S, Kovács E, Gácsi M, Simor P, Gombos F, Topál J, Miklósi Á and Bódizs R 2014. Development of a non-invasive polysomnography technique for dogs (Canis familiaris) Physiol. Behav [DOI] [PubMed] [Google Scholar]
- [36].Bódizs R, Kis A, Gácsi M and Topál J 2020. Sleep in the dog: comparative, behavioral and translational relevance Curr. Opin. Behav. Sci 33 25–33 [Google Scholar]
- [37].Adams GJ and Johnson KG 1993. Sleep-wake cycles and other night-time behaviours of the domestic dog Canis familiaris Appl. Anim. Behav. Sci 36 233–48 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Codes can be found on a public GitHub repository https://github.com/mselair/best_toolbox. Data is available from the corresponding author upon a reasonable request.


