Abstract
We describe a test which uses the ability of viable cells to reduce 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to detect resistance to a bactericidal drug, rifampin, in in vitro-cultured Mycobacterium tuberculosis. The assay shows a linear relationship between the number of viable bacteria and the ability to reduce MTT. Dead mycobacteria were unable to reduce MTT. Rifampin-sensitive M. bovis (BCG) and M. tuberculosis exposed to rifampin showed a rifampin concentration-dependent inhibition of the ability to reduce MTT, while the resistant strains were unaffected. The inhibition of MTT reduction after treatment with rifampin paralleled the reduction in the number of CFU. By using mixing experiments in which the population percentages of rifampin-sensitive and -resistant strains were varied, the assay could detect the presence of rifampin resistance in the mixture when at least 1% of the bacterial population was composed of drug-resistant strains. The assay is cheap, can be visually read, and requires less than 3 days to obtain susceptibility results. The total time required to obtain results, from the time sputum is received in the laboratory, is, in most cases, less than 4 to 5 weeks, which is the time required for primary culture of the bacteria. The MTT assay could, in combination with a test to detect resistance to isoniazid, be a cheap and rapid screening method for multidrug-resistant M. tuberculosis that is affordable even by low-income countries where tuberculosis is a major public health problem.
Tuberculosis (TB) remains a major public health problem around the world (2, 14, 17, 22, 28). This disease is estimated to account for 10 million deaths annually, making it the single most important cause of death from a single infectious agent (2, 28). The World Health Organization (WHO) promotes a standard package (passive case finding based on bacteriological examination of sputum, multidrug chemotherapy, and efficient and effective program management) for TB control (7, 30, 31). The spread of TB is expected to worsen as infection with the human immunodeficiency virus increases in many communities (14, 19, 20).
The main stay of TB treatment is a combination of rifampin and isoniazid (INH), and consequently, Mycobacterium tuberculosis strains resistant to at least both of these drugs are designated multidrug resistant (MDR) (15). The emergence of drug-resistant M. tuberculosis represents a major threat to TB control, and it has been shown that the major cause of drug-resistant TB is entirely human made, as it is a result of nonadherence to the recommended TB control strategies (5). Drug-resistant M. tuberculosis is found more frequently as acquired resistance in previously treated patients, and in some countries in Africa, previously treated patients represent as much as 13% of all detected cases of TB (4, 27). The WHO has estimated that as many as 50 million people may already be infected with drug-resistant strains of M. tuberculosis and that in some developing countries, primary resistance to one or more anti-TB drugs is as high as 15% (29).
Most low-income countries cannot afford to pay for the treatment of drug-resistant TB. Coupled with the fact that these countries are the ones that have the highest burden of HIV and TB infections, the spread of drug-resistant TB poses a real and major public health crisis. Prevention of the occurrence and spread of MDR TB is therefore a major priority of all TB control programs. With traditional techniques, it takes 6 to 12 weeks to obtain susceptibility results. This time lag poses a significant danger to patients, the community, and health-care workers. It is therefore important to develop techniques which will shorten the lag period. Recently acquired information on the genetics of drug resistance in mycobacteria has led to the development of rapid tests for detection of resistance to some of the drugs in a relatively short time (13, 24, 26). Unfortunately, most of these tests require heavy investment in equipment, training, and quality control, putting them out of the reach of many developing countries. Furthermore, their applicability in the context of a national control program remains to be validated. Relatively rapid tests based on the metabolic activity of M. tuberculosis, such as the BACTEC 460 system (23), require heavy investment in equipment and servicing. For a majority of national TB control programs, it will be extremely helpful to have a simple, cheap, and robust test that can, singly or in combination with another, similar test, detect MDR M. tuberculosis strains.
The yellow dye 3(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) is reduced by mitochondrial dehydrogenase in living cells to produce insoluble purple MTT formazan crystals (6, 8, 10, 16, 18, 21, 25) which, after solubilization, can be measured spectrophotometrically. This property has long been used to assess the viability of cells. In this study, we assessed the possibility of using MTT to detect the viability of M. tuberculosis after exposure to rifampin. This technique could form the basis of a very simple and cheap test for detection of drug-resistant M. tuberculosis strains.
MATERIALS AND METHODS
Bacterial strains.
Vaccine strain M. bovis BCG 390955B was purchased from Statens Serum Institut (Copenhagen, Denmark). M. tuberculosis S-01, S-07, S-29, and S-63 were selected from strains isolated from sputa of TB patients in Addis Ababa, Ethiopia. The susceptibility patterns of these isolates had previously been determined by using the BACTEC system (Becton-Dickinson, Sparks, Md.) (1). Standard rifampin-sensitive (H37Ra strain 35836) and -resistant (H37Rv strain 35838) strains were purchased from the American Type Culture Collection (Rockville, Md.).
Bacterial cultures.
BCG was cultured in 7H9 broth supplemented with 10% OADC (oleic acid-albumin-dextrose-catalase) enrichment (Difco Laboratories, Detroit, Mich.) and 0.05% Tween 80. M. tuberculosis strains were inoculated onto Löwenstein-Jensen medium and incubated for 3 to 4 weeks at 37°C, and then growing colonies were transferred to liquid 7H9 broth supplemented with 10% OADC. Cultures were further incubated for 10 to 15 days to allow the bacteria to reach the logarithmic phase of growth. Culture suspensions were disrupted in a water bath sonicator for 30 s. The concentration of the bacteria was determined by measuring the optical density (OD) of the bacterial suspension in a spectrophotometer (Beckman Instruments, Inc., Fullerton, Calif.) at a wavelength of 620 nm (OD of 1 at 620 nm equals 2 × 109 bacteria/ml).
Inactivation of bacteria.
Mycobacteria were inactivated by heat treatment at 91°C for 15 min in a boiling water bath or by treatment with a 5% sodium hypochlorite (NaOCl; household bleach) solution for 15 min at room temperature. Bacteria treated with NaOCL were washed three times in phosphate-buffered saline (PBS) before being resuspended in culture medium.
MTT assay.
The MTT assay was carried out as described by Mosmann (16), with some modifications. Briefly, each well of a flat-bottom microtiter plate received 40 μl of a bacterial suspension. Triplicate wells were used for each experimental condition. MTT (Sigma, St. Louis, Mo.) was dissolved in PBS (pH 7.2) to obtain a concentration of 5 mg/ml. Ten microliters of the MTT solution was then added to each culture well, and the plates were incubated for 4 h at 37°C. Fifty microliters of a lysing buffer containing 20% sodium dodecyl sulfate in 50% N1N-dimethylformamide (pH 4.7) (10) was then added to each well, and the plates were incubated overnight. Absorbance was measured with an automatic enzyme-linked immunosorbent assay reader (Flow Laboratories, Irvine, United Kingdom) at a wavelength of 570 nm. Wells that contained 7H9-OADC and were incubated with MTT and the lysing buffer served as blanks.
Microcolony counting.
Fifty microliters of a bacterial suspension was transferred to each well of a flat-bottom 96-well microtiter plate prefilled with 200 μl of 7H9 broth supplemented with OADC enrichment and 0.2% glycerol. Eight serial fivefold dilutions were made by transferring 50 μl of the next well after thorough mixing. Microcolonies were counted by using an inverted microscope in wells containing less than 100 colonies after 10 to 12 days of incubation at 37°C. Numbers of CFU per milliliter were calculated by multiplying the number of colonies by the dilution factor and the volume of the total sample in proportion to the 50-μl sample taken for setting up the microcolony culture.
Reduction of MTT in relation to viability of mycobacteria.
To examine the effect of rifampin treatment on the reduction of MTT and on the viability of the bacteria, M. bovis BCG was treated with various concentrations of rifampin for 48 or 72 h. After the incubation period, the MTT assay was performed as described above. Before determination of the number of CFU, the bacteria were washed three times in PBS, resuspended in culture medium, and plated for microcolony counting as described above. In both experiments, 5 × 107 bacilli were used.
Drug sensitivity tests.
Forty microliters of culture medium containing 5 × 107 bacteria was placed into each well of a microtiter plate, and then equal volumes of various rifampin concentrations diluted with culture medium were added. Each drug concentration was tested in triplicate. Plates were then incubated at 37°C for 48 or 72 h. After this incubation period, the MTT assay was carried out as described above.
Rifampin-resistant M. tuberculosis H37Rv (ATCC 35838) and rifampin-sensitive M. tuberculosis H37Ra (ATCC 35836) were used in the experiment in which MTT reduction at different bacterial concentrations was determined and in the experiment done to detect resistant bacteria in a mixed population. In the experiment with mixed populations, an increasing proportion (0 to 16%) of the resistant strain (ATCC 35838) was added to the sensitive strain while keeping the total number of bacteria per well constant at 109.
Presentation of data.
Spectrophotometric readings of the MTT assay for drug susceptibility testing are presented as relative OD units (RODU) derived by dividing the OD of cultures treated with rifampin by the OD of control cultures not treated with rifampin. Linear regression analysis was used to analyze the results.
RESULTS
Establishment and characteristics of the MTT assay.
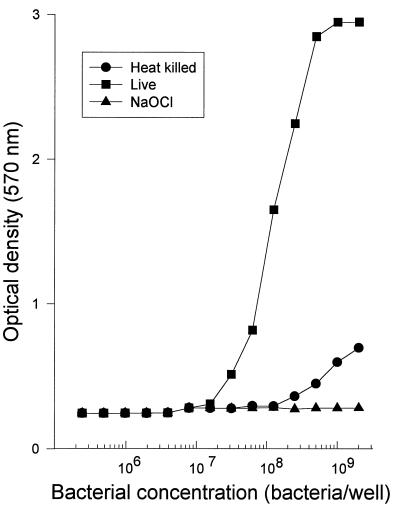
To establish the range of bacterial concentrations in which the reduction of MTT is reproducibly proportional to the number of viable bacteria, various concentrations of BCG were plated and the MTT assay was carried out as described in Materials and Methods. Figure 1 shows that there was a linear relationship between absorbance of the resultant formazan dye and the bacterial concentration (r = 0.924, P < 0.001). This relationship was even stronger when the bacterial concentration ranged from 2 × 107 to 1 × 109 bacteria per well (r = 0.984, P < 0.001). BCG that had been placed in a boiling water bath (91°C) for 15 min was unable to reduce the MTT dye except when high bacterial concentrations (109 BCG organisms/well) were used. BCG killed by incubation with a 5% solution of NaOCl for 15 min at room temperature did not reduce the MTT dye at any of the bacterial concentrations tested. Culture experiments showed that high concentrations of heat-inactivated BCG still yielded growth on culture, probably due to clumping of the bacteria, resulting in insufficient heat inactivation. BCG treated with NaOCl did not grow on culture. An OD of greater than 0.5 was thus taken as the value above which viable BCG could reliably be detected. ODs between 0.5 and 3.0 showed a strong linear relationship with the bacterial concentration (r = 0.984, P <0.001).
FIG. 1.
Reduction of MTT by M. bovis BCG in vitro is dependent on the concentration of the bacilli. Bacilli killed by treatment with 5% sodium hypochlorite for 15 min cannot reduce MTT. Heat killing (immersion in a boiling water bath at 91°C) was less efficient, especially at high bacterial concentrations.
Effect of rifampin on MTT reduction by BCG and M. tuberculosis.
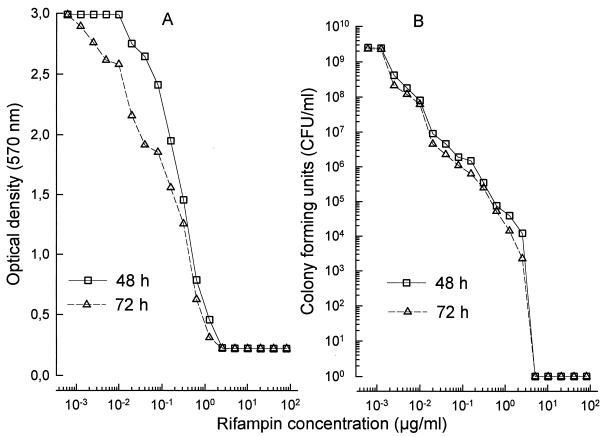
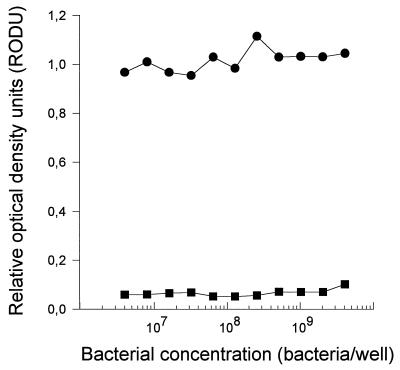
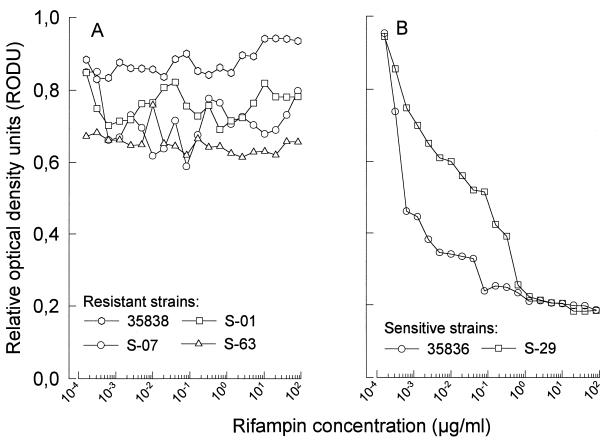
BCG which is sensitive to rifampin showed significant and rifampin dose-dependent inhibition of MTT reduction (Fig. 2A). This effect was seen as early as 48 h after incubation with rifampin (r = 0.949 and P < 0.001 for 48 h and r = 0.965 and P < 0.001 for 72 h of incubation with rifampin, respectively). With an OD of 0.5 as the cutoff point, Fig. 2A also shows that no viable BCG was detected by this assay in wells which were treated with rifampin at concentrations equal to or greater than 1.0 μg/ml. When rifampin was used at concentrations ranging from 0.01 to 1.0 μg/ml, there was a significant linear relationship between the drug concentration and the inhibition of MTT reduction (r = 0.975 and 0.980 for 48 and 72 h of incubation, respectively; P < 0.001 in both cases), showing that the MTT assay could be used to determine BCG susceptibility to rifampin at clinically relevant drug concentrations. To verify that this relationship was due to killing of BCG by the drug, similarly treated BCG was plated for a CFU assay. Figure 2B shows that there was a strong linear relationship between the number of CFU and the concentration of rifampin used (r = 0.950 and 0.953 and P < 0.001 for 48 and 72 h of incubation with rifampin, respectively). Figure 2B also shows that BCG treated with rifampin at 1 μg/ml did not produce any colonies, which supports our previous observation that BCG treated with this concentration of rifampin had an OD of <0.5 in the MTT assay, suggesting that it was not viable. Rifampin-sensitive (H37Ra [ATCC 35836]) and -resistant (H37Rv [ATCC 35838]) strains of M. tuberculosis were treated with rifampin at 1.0 μg/ml for 48 h before being assessed for the ability to reduce MTT. Figure 3 shows that as long as the bacterial concentration used was within the range of 5 × 106 to 2 × 109 bacterial/well, the ability of the rifampin-resistant strain to reduce MTT remained unchanged by treatment with rifampin. The rifampin-sensitive strain, on the other hand, was almost totally inhibited in its ability to reduce MTT, with <0.2 RODU. Various clinical isolates of M. tuberculosis were then tested for sensitivity to rifampin by using the MTT assay. The rifampin sensitivity patterns of these clinical isolates had previously been established by using the BACTEC 460 system (1). Figure 4 shows that rifampin-resistant strains (Fig. 4A) showed a pattern completely different from that of rifampin-sensitive strains (Fig. 4B). The ability of three rifampin-resistant strains (S-01, S-63, and ATCC 35838) to reduce MTT was not affected by incubation with rifampin, irrespective of the concentration of the drug used (r = 0.06, 0.68, and 0.87, respectively, P > 0.5 in all cases). One rifampin-resistant clinical isolate (S-07) showed weak but significant dose-dependent inhibition of its ability to reduce MTT (r = 0.455, P = 0.044). This observation suggests that the proportion of drug-resistant bacteria within the total population varied among the different isolates tested. All rifampin-resistant isolates had >0.5 RODU. Both rifampin-sensitive strains (S-29 and ATCC 35836) showed strong, dose-dependent inhibition of the ability to reduce MTT (r > 0.8 and P < 0.001 in both cases). Unlike the drug-resistant isolates, even including the one isolate which showed slight inhibition of its ability to reduce MTT in the presence of rifampin, the rifampin-sensitive isolates showed almost complete inhibition of the ability to reduce MTT (<0.2 RODU) when rifampin was used at concentrations of greater than 1 μg/ml. At this drug concentration, the RODU of the drug-resistant isolates were at least three times those of the rifampin-sensitive strains.
FIG. 2.
Effect of rifampin on the ability of M. bovis BCG to reduce MTT. BCG treated with various concentrations of rifampin for 48 or 72 h showed a drug dose-dependent inhibition of the ability to reduce MTT (A). This reduction in the ability of rifampin-treated bacilli to reduce MTT paralleled a reduction in the number of CFU when these bacilli were recultured (B). In both experiments, 5 × 107 bacilli were set up in culture, treated for 48 or 72 h with rifampin, and processed for the MTT or CFU assay as described in Materials and Methods.
FIG. 3.
Standard rifampin-resistant (•; H37Rv [ATCC 35838]) and rifampicin-sensitive (▪; H37Ra [ATCC 35836]) M. tuberculosis strains were set up in culture and treated with rifampin (1 μg/ml) for 48 h before being processed for the MTT reduction assay. Rifampin at 1 μg/ml was able to completely inhibit MTT reduction by the rifampin-sensitive M. tuberculosis strain, while it did not affect the rifampin-resistant strain.
FIG. 4.
Different MTT reduction patterns of rifampin-resistant (A) and -sensitive (B) M. tuberculosis isolates obtained after treatment with rifampin for 48 h. Bacilli (5 × 107) were set up in cultures containing rifampin and processed for MTT reduction. Drug-resistant isolates were not inhibited in the ability to reduce MTT, while drug-sensitive isolates showed a drug dose-dependent reduction in the ability to reduce MTT after treatment with rifampin. The sensitivity of the clinical isolates had previously been determined by using the BACTEC system. Strains 35838 and 35836 are rifampin-resistant and rifampin-sensitive reference strains, respectively.
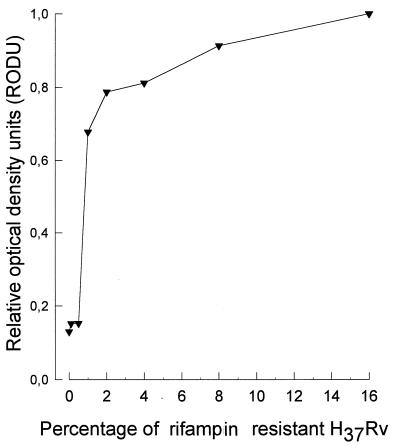
To determine whether the MTT assay could be used to detect small populations of rifampin-resistant M. tuberculosis in the presence of a large population of rifampin-sensitive M. tuberculosis, various proportions of the sensitive and resistant strains were mixed, incubated with rifampin at 1 μg/ml, and tested. The total number of bacilli per well was kept at 109. Figure 5 shows that as the proportion of a drug-resistant strain increased beyond 1% of the total bacterial population, there was a corresponding increase in the reduction of MTT (r = 0.736, P = 0.037). Populations of bacteria containing less than 1% drug-resistant strains were almost totally inhibited in the ability to reduce MTT, with <0.2 RODU, while populations containing a subpopulation of >1% rifampin-resistant bacteria had >0.5 RODU.
FIG. 5.
Detection of a rifampin-resistant subpopulation. Cultures containing various proportions of rifampin-resistant (H37Rv [ATCC 35838]) and rifampin-sensitive (H37Ra [ATCC 35836]) bacteria were set up in culture, treated with rifampin at 1 μg/ml, and processed for the MTT reduction assay. Bacterial populations containing a drug-resistant subpopulation of more than 1% could be detected in that their ability to reduce MTT was not inhibited by rifampin at 1 μg/ml. Further, the ability to continue to reduce MTT in the presence of rifampin increased with the proportion of the drug-resistant subpopulation.
DISCUSSION
Drug-resistant TB increases morbidity and the risk of mortality, as well as the costs associated with its management (12). In countries where human immunodeficiency virus infection is prevalent, there is a large pool of susceptible individuals who might be infected by and thus spread drug-resistant TB. Drug-resistant TB thus poses a great health risk to individual patients, as well as to the community at large. While the use of multidrug therapy, especially directly observed therapy, and a short-course regimen is a powerful tool against the emergence of drug-resistant TB, it will take many years before a majority of poor countries are able to implement these strategies. Nonadherence to these treatment strategies is the main cause of drug resistance in TB. Therefore, it is important to monitor drug sensitivity patterns as a part of national TB control programs in the community and to monitor individual patients with chronic TB after retreatment failure (5, 32). Understanding drug resistance patterns in a community is also of enormous epidemiological significance since it provides indicators, such as the existence and prevalence of primary and acquired drug resistance, which are useful in evaluating the quality of the anti-TB treatment provided to the community (5, 32).
Classical methods of detection of drug resistance in M. tuberculosis are expensive and time consuming, while the newer methods are expensive and most are not easily applicable in low-income countries. This has led to a situation in which very little information is available about the spread and pattern of drug resistance in these countries. In this paper, we describe a simple and cheap method that can be applied to detect rifampin resistance in M. tuberculosis. The method requires only one culture cycle to obtain sufficient amounts of M. tuberculosis for drug resistance testing. Our experience shows that only one loopful of bacteria taken directly from a primary culture is sufficient for performance of the MTT assay, and this should require only 3 to 4 weeks of in vitro culture, while the test itself takes a maximum of 72 h. The same method could, in principle, be used to detect resistance to other bactericidal drugs. However, we have had difficulties in standardizing the MTT assay to detect INH resistance since formazan production was high despite inhibition of mycobacterial proliferation (data not shown).
A colorimetric method using a dye, Alamar blue, for determining the MICs of antimicrobial agents for M. tuberculosis has recently been published (33). This method provides results which were in agreement with the agar proportion method in a relatively short period of time, ranging from 7 to 14 days. Gomez-Flores et al. (9) have also recently used the MTT method to determine the MIC for the M. avium-M. intracellulare complex in liquid medium. In both studies, the colorimetric methods were found to be rapid, quantitative, and cheap.
It has been reported that the addition of the electron coupling agent menadione (vitamin K3) increases the sensitivity of the basic MTT assay (8). We did not, however, observe any advantage over the basic model when we added menadione to our system (data not shown). Like MTT, 3-(4,5-dimethylthiazol-2-yl-5)-3carboxymethoxyphenyl)-2-(4-sulfonyl)-2H-tetra- zolium (MTS) is also reduced by mitochondrial dehydrogenases but unlike the MTT reduction product, that of MTS is water soluble and thus avoids the lysis and extraction steps required for MTT (1a). In our assays for rifampin resistance in M. tuberculosis, the use of MTS did not give consistent results. We also obtained inconsistent results when using 3,3-{1[(phenylamino)carbonyl]-3,4-tetrazolium}-bis-(4-methoxy- 6-nitro)benzene sulfonic acid hydrate with coenzyme Q instead of MTT (21).
One of the major problems with the standard methods of in vitro drug susceptibility testing in TB is standardization of culture media and culture conditions during the actual drug testing (3). Furthermore, most tests are very sensitive to the bacterial inoculum size used for drug testing (27). Our current method attempts to avoid both of these drawbacks by using a fixed, known concentration of a bacterial suspension, using a fixed concentration of rifampin (1 μg/ml), and measuring the viability of bacteria exposed to rifampin not during the growth period on Löwenstein-Jensen medium but for only 48 to 72 h in a defined medium (7H9 broth). For clinical isolates, which might have various proportions of rifampin-sensitive and -resistant subpopulations, our results suggest that 109 bacteria per well would be sufficient. The current method does not give the actual MBC of rifampin for each isolate but, rather, gives a pattern which is common to all rifampin-resistant isolates. This pattern differs markedly from that observed for drug-sensitive isolates in that the ability to reduce MTT, an indicator of viability, is retained in the presence of the drug in resistant strains while this is profoundly inhibited in drug-sensitive strains. Ideally, the method requires the inclusion of standard rifampin-sensitive and -resistant strains for each run so as to generate standard patterns. However, this is not an absolute requirement, since a comparison is only made between the test strain with and the test strain without rifampin. There may be in vitro differences in growth characteristics between rifampin-sensitive and -resistant strains (27), and this could account for the variations observed between clinical isolates in the ability to reduce MTT. It is also likely that the variations in the ability to reduce MTT that we observed between isolates could be due to differences in proportions of drug-sensitive and -resistant strains within the total population of mycobacteria obtained from a patient’s sputum culture. To equalize for strain differences in the ability to reduce MTT and emphasize the pattern obtained, we have used a relative value, the RODU. This approach does not negate the need to determine actual MICs for the isolates, but we believe that it offers all of the information necessary for patient management or mass drug sensitivity surveillance, in which cases the MICs are less important than knowing whether the isolate contains a population of drug-resistant bacteria whose size is clinically relevant. Our data suggest that a value of <0.2 RODU obtained when 109 bacilli per well are incubated with rifampin at 1 μg/ml for 48 h identifies rifampin-sensitive isolates while a value of >0.5 RODU strongly suggests the presence of a significantly large population of rifampin-resistant bacteria within the total bacterial population. It has been shown that a 1% population of resistant bacteria in a culture of M. tuberculosis is clinically relevant (11). We believe that our method would be useful in clinical and epidemiological investigations since the MTT assay was able to detect rifampin resistance if the proportion of resistant strains within the total population was equal to or greater than 1% (>0.5 RODU), although this requires the use of a higher bacterial concentration (109 bacteria per well).
For TB control purposes, it is most important to detect resistance to rifampin and INH. The majority of drug-resistant, non-MDR strains respond to the WHO-recommended retreatment regimen, whereas patients harboring strains resistant to both rifampin and INH require customized treatment based on the results of extended sensitivity testing. A simple method for detection of INH resistance could, in combination with the MTT assay, offer a cheap and rapid screening method for MDR TB strains. Based on the fact that INH at 4 μg/ml is within the range of achievable levels of this drug in serum, a simple assessment of catalase activity is useful in distinguishing strains for which the INH MIC is high (>16 μg/ml) from those for which the INH MIC is low (<4 μg/ml). Preliminary experiments indicate that assessment of catalase activity, used in conjunction with our MTT reduction method, could be a useful approach for the identification of MDR strains of M. tuberculosis.
The results obtained with the MTT reduction assay can be read visually because of the contrasting resultant colors. This is an advantage especially in mass surveys in that isolates which continue to reduce MTT in the presence of rifampin at 1 to 2 μg/ml can quickly be visually identified and processed for further detailed studies. The 96-well microtiter plate format enables screening of large numbers of samples in a relatively short period of time, but it has a laboratory safety disadvantage. Further studies on clinical material are needed to validate the applicability of the MTT assay for routine diagnostic purposes.
ACKNOWLEDGMENTS
The Armauer Hansen Research Institute is financially supported by the Norwegian, Swedish, and Ethiopian governments.
We thank Zufan Sisay and Assafa Wondimu for the illustrations.
REFERENCES
- 1.Abate, G., H. Miörner, O. Ahmed, and S. E. Hoffner. Int. J. Tuberc. Lung Dis., in press. [PubMed]
- 1a.Berg K, Zhai L, Chen M, Kharazmi A, Owen T C. The use of a water-soluble formazan complex to quantitate the cell number and mitochondrial function of Leishmania major promastigotes. Parasitol Res. 1994;80:235–291. doi: 10.1007/BF00932680. [DOI] [PubMed] [Google Scholar]
- 2.Bloom B R, Murray C J. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 3.Canetti G, Rist N, Grosset J. Primary drug resistance in tuberculosis. Am Rev Tuberc Pulm Dis. 1964;90:792–799. doi: 10.1164/arrd.1964.90.5.792. [DOI] [PubMed] [Google Scholar]
- 4.Carpels G, Fissete K, Limbana V, Van Deun A, Vandenbulcke W, Portaels F. Drug resistant tuberculosis in sub-saharan Africa: an estimation of incidence and cost for the year 2000. Tubercle Lung Dis. 1995;76:480–486. doi: 10.1016/0962-8479(95)90522-7. [DOI] [PubMed] [Google Scholar]
- 5.Chaulet P, Boulahal F, Grosset J. Surveillance of drug resistance for tuberculosis control: why and how. Tubercle Lung Dis. 1995;76:487–492. doi: 10.1016/0962-8479(95)90523-5. [DOI] [PubMed] [Google Scholar]
- 6.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 7.Enarson D A. The International Union Against Tuberculosis and Lung Disease model national tuberculosis programmes. Tubercle Lung Dis. 1995;76:95–99. doi: 10.1016/0962-8479(95)90548-0. [DOI] [PubMed] [Google Scholar]
- 8.Garn H, Krause H, Enzmann V, Drossler K. An improved MTT assay using the electron-coupling agent menadione. J Immunol Methods. 1994;168:253–261. doi: 10.1016/0022-1759(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Flores R, Gupta S, Tamez-Guerra R, Mehta R T. Determination of MICs for Mycobacterium avium-M. intracellulare complex in liquid medium by a colorimetric method. J Clin Microbiol. 1995;33:1842–1846. doi: 10.1128/jcm.33.7.1842-1846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen M B, Nielsen S E, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins J E, Wallace R J, Jr, Brown B A. Antibacterial susceptibility tests: mycobacteria. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1994. pp. 1138–1152. [Google Scholar]
- 12.Iseman M D. Treatment of multidrug-resistant tuberculosis. N Engl J Med. 1993;329:784–791. doi: 10.1056/NEJM199309093291108. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs W R, Jr, Barletta R G, Udani R, Chan J, Kalkut G, Sosne G, Kieser T, Sarkis G J, Hatfull G F, Bloom B R. Rapid assessment of drug susceptibilities of Mycobacterium tuberculosis by means of luciferase reporter phages. Science. 1993;260:819–822. doi: 10.1126/science.8484123. [DOI] [PubMed] [Google Scholar]
- 14.Kochi A. Focus on problems—tuberculosis: distribution, risk factors and mortality. Immunobiology. 1994;191:325–336. doi: 10.1016/S0171-2985(11)80437-7. [DOI] [PubMed] [Google Scholar]
- 15.Kochi A, Vareldzis B, Styblo K. Multidrug resistant tuberculosis and its control. Res Microbiol. 1993;144:104–110. doi: 10.1016/0923-2508(93)90023-u. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Murray C J, Styblo K, Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tubercle Lung Dis. 1990;65:6–24. [PubMed] [Google Scholar]
- 18.Peck R. A one-plate assay for macrophage bactericidal activity. J Immunol Methods. 1985;82:131–140. doi: 10.1016/0022-1759(85)90232-7. [DOI] [PubMed] [Google Scholar]
- 19.Richards S B, St Louis M E, Nieburg P, Coulibaly I M, Coulibaly D, Abouya L, Gayle H D, De Cock K M. Impact of the HIV epidemic on trends in tuberculosis in Abidjan, Côte d’Ivoire. Tubercle Lung Dis. 1995;76:11–16. doi: 10.1016/0962-8479(95)90572-3. [DOI] [PubMed] [Google Scholar]
- 20.Schulzer M, Fitzgerald J M, Enarson D A, Grzybowski S. An estimate of the future size of the tuberculosis problem in sub-saharan Africa resulting from HIV infection. Tubercle Lung Dis. 1992;73:52–81. doi: 10.1016/0962-8479(92)90080-4. [DOI] [PubMed] [Google Scholar]
- 21.Stevens M G, Olsen S C. Comparative analysis of using MTT and XTT in colorimetric assays for quantitating bovine neutrophil bactericidal activity. J Immunol Methods. 1993;157:225–231. doi: 10.1016/0022-1759(93)90091-k. [DOI] [PubMed] [Google Scholar]
- 22.Sudre P, ten Dam G, Kochi A. Tuberculosis: a global overview of the situation today. Bull W H O. 1992;70:149–159. [PMC free article] [PubMed] [Google Scholar]
- 23.Tarrand J J, Gröschel D H M. Evaluation of the BACTEC radiometric method for detection of 1% resistant populations of Mycobacterium tuberculosis. J Clin Microbiol. 1985;21:941–946. doi: 10.1128/jcm.21.6.941-946.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston M J, Matter L, Schopfer K, Bodmer T. Detection of rifampicin resistance mutations in Mycobacterium tuberculosis. Lancet. 1993;341:647–501. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- 25.Thom S M, Horobin R W, Seidler E, Barer M R. Factors affecting the selection and use of tetrazolium salts as cytochemical indicators of microbial viability and activity. J Appl Bacteriol. 1993;74:433–443. doi: 10.1111/j.1365-2672.1993.tb05151.x. [DOI] [PubMed] [Google Scholar]
- 26.van der Vliet G M E, Schepers P, Schukkink R A F, van Gemen B, Klatser P R. Assessment of mycobacterial viability by RNA amplification. Antimicrob Agents Chemother. 1994;38:1959–1965. doi: 10.1128/aac.38.9.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vareldis B P, Grosset J, de Kantor J, Croften J, Laszlo A, Felten M, Raviglione M C, Kochi A. Drug resistant tuberculosis: laboratory issues. World Health Organisation recommendations. Tubercle Lung Dis. 1994;75:1–7. doi: 10.1016/0962-8479(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 28.World Bank. World development report 1993: investing in health. Washington, D.C: World Bank; 1993. [Google Scholar]
- 29.World Health Organization. WHO surveillance of drug resistance in TB. WHO/TB/84.143. Geneva, Switzerland: World Health Organization; 1984. [Google Scholar]
- 30.World Health Organization. Treatment of tuberculosis: guidelines for national programmes. Geneva, Switzerland: World Health Organization; 1993. [Google Scholar]
- 31.World Health Organization Tuberculosis Programme. Framework for effective TB control. WHO/TB/94.179. Geneva, Switzerland: World Health Organization; 1994. [Google Scholar]
- 32.World Health Organization Tuberculosis Programme and International Union Against Tuberculosis and Lung Disease. Guidelines for surveillance of drug resistance in tuberculosis. WHO/TB/94.178. Geneva, Switzerland: World Health Organization; 1994. [Google Scholar]
- 33.Yajko D M, Madej J J, Lancaster M V, Sanders C A, Cawthon V L, Gee B, Babst A, Hadley W K. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J Clin Microbiol. 1995;33:2324–2327. doi: 10.1128/jcm.33.9.2324-2327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]