Abstract
Background and Objective
Cancer drug costs have increased considerably within healthcare systems, but many drugs lack quality-of-life (QoL) and overall survival (OS) data at the time of reimbursement approval. This study aimed to review the extent of subsequent literature documenting improvements in OS and QoL for cancer drug indications where no such evidence existed at the time of reimbursement approval.
Methods
Drug indications with claims of added therapeutical value but a lack of evidence on OS and QoL that were reimbursed between 2010 and 2020 in Sweden were included for review. Searches were conducted in PubMed and ClinicalTrial.gov for randomized controlled trials examining OS and QoL.
Results
Of the 22 included drug indications, seven were found to have at least one trial with conclusive evidence of improvements in OS or QoL after a mean follow-up of 6.6 years. The remaining 15 drug indications either lacked subsequent randomized controlled trial data on OS or QoL (n = 6) or showed no statistically significant improvements (n = 9). Only one drug demonstrated evidence of improvement in both OS and QoL for its indication.
Conclusions
A considerable share of reimbursed cancer drug indications continue to lack evidence of improvement in both OS and QoL. With limited healthcare resources and an increasing cancer burden, third-party payers have strong incentives to require additional post-reimbursement data to confirm any improvements in OS and QoL.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40261-023-01285-4.
Key Points
| Many cancer drugs have limited overall survival and quality-of-life data at the time of reimbursement, questioning their value for money. This study systematically reviewed the subsequent evidence of cancer drugs following reimbursement approval in Sweden. |
| Sixty-eight percent of reimbursements continue to have limited evidence of improvement in overall survival and quality of life after a mean follow-up of 6.6 years from reimbursement. A lack of published evidence on the effects on quality of life was observed and 42% of included trials did not report on quality-of-life assessments. |
| Given the limited resources of healthcare systems and the increasing burden of cancer, there is a need to discuss the requirements informing reimbursement approvals and to continue to monitor reimbursed cancer drugs. |
Introduction
Increasing costs of cancer drugs have spurred considerable debate and concern across many healthcare systems [1, 2]. In Europe, the cost of cancer drugs tripled between 2005 and 2018, and cancer drug expenditures amounted to 32 billion Euros in 2018, constituting a third of the total direct healthcare costs of cancer [3]. Larger increases have occurred in the USA, and global spending is expected to grow further [4]. High costs and prices may be motivated by substantial clinical benefits, but a growing literature has questioned the evidence supporting many newly approved cancer drugs, including their effects on overall survival (OS) and quality of life (QoL). There is also a documented lack of association between cancer drug prices and their clinical benefits [5–10].
In most European countries, the accessibility of cancer drugs after a market authorization depends on reimbursement decisions by public and social health insurers. As health expenditures increase and resources are finite, ensuring that funds are spent on effective drugs with clear value for money is vital for healthcare systems’ long-term sustainability and effectiveness [11]. While several outcomes are considered in assessing the benefits of cancer drug treatments, such as progression-free survival, the primary objective for patients and health policymakers is typically to improve OS and/or QoL [12–14]. Evidence on intermediate or surrogate outcomes can generate useful information and be a partial goal of treatment in certain settings. However, the implications and ability to predict effects on OS or QoL remain debated [15, 16]. For many cancer drugs, evidence of efficacy on OS and QoL has been limited and surrounded by uncertainties due to unvalidated surrogates [16, 17], the use of single-arm trials, and the scarcity of long-term outcomes at the time of market entry [7, 15, 18–21].
In a previous study of the clinical and cost-effectiveness evidence underlying reimbursement assessments in Sweden [22], we observed a lack of conclusive evidence of improvements in OS and QOL at the time of reimbursement in around 50% of reimbursed cancer drug indications, and suggestions of increased reliance on single-arm studies and surrogate outcomes compared with previous studies. Similar limitations have been reported for different regulatory and reimbursement decisions in Canada, China, and Europe [23–26], and in an overview of accelerated approvals in the USA over the last decades, only 20% of authorized drugs had shown improvements in OS in confirmatory trials [22].
Given the basis of health technology assessment processes and economic evaluations, limited evidence of comparative effectiveness at the time of reimbursement decision causes considerable uncertainty for the economic assessment and modeling of cancer drugs’ cost effectiveness. To ensure efficient resource use and value for money of implemented therapies, it is essential to assess the extent of longer term data post-introduction that would facilitate updated cost-effectiveness and value assessments. In this study, we aimed to fill this gap by reviewing the post-reimbursement OS and QoL evidence for all cancer drug indications approved for reimbursement in 2010–20 in Sweden, with limited evidence on OS and QoL at the time of decision making.
Methods
Context and Identification of the Study Cohort
The Swedish Health Technology Assessment Agency, the Dental and Pharmaceutical Benefits Agency (TLV), is responsible for assessing and making reimbursement decisions for prescription drugs. The decision process is based on the principles of clinical effectiveness, disease severity, need, and cost effectiveness. Following a drug’s market authorization, the pharmaceutical company initiates the reimbursement application that presents the clinical and cost-effectiveness evidence of the drug and proposes a price. The TLV assesses the application and suggests modifications in the economic modeling and assumptions [27]. The reimbursement decision is made by the TLV’s Board of Pharmaceutical Benefits, constituted by independent professionals from sectors of academia and healthcare and patient organizations. Assessments and decisions are made for each specific drug-indication combination.
In a previous review of the clinical and cost-effectiveness evidence used in reimbursement applications, we identified all cancer drug indications seeking reimbursement and claiming an added clinical value between 2010 and 2020 in Sweden [22]. Briefly, 60 drug indications were included in the initial review of which 46 were granted full or restricted reimbursement. Within this cohort (n = 46), we identified drugs with limited evidence of improvements in OS and QoL for their reimbursed indication based on decision dossiers and assessments made by the TLV. We defined limited evidence as having clinical evidence based on single-arm trials or comparative studies (pivotal phase 3 studies and in lack of these, phase 2 studies) with imprecise (non-significant, p ≥ 0.05) estimates of differences in OS and QoL at the time of the reimbursement. In total, 20 drugs concerning 22 specific indications were identified to have limited evidence of improvements in OS or QoL at the time of reimbursement and were included for a follow-up (Table S1 of the Electronic Supplementary Material [ESM]). Two of the included drugs were reimbursed for two indications each. These were regarded as individual observations and were reviewed separately.
Search Strategy and Selection Criteria
PubMed was used for a systematic search of post-reimbursement evidence on OS and QoL for each drug indication between 17 December, 2021 and 24 January, 2022. The search terms “active substance name OR drug brand name AND cancer form AND Cochrane Highly Sensitive Search Strategy for identifying randomized trials” were used for all drugs based on the specified indications for which reimbursement had been approved. The search strategy was developed in consultation with university librarians and included synonyms from the National Institutes of Health cancer dictionary and the library of Medical Subject Heading terms. The search strategy was formed to capture studies published 1 year prior to the reimbursement decision up to the date of the search. Thus, we ensure the inclusion of study results published just prior to and during the reimbursement process, which may not have been included in the decision dossiers handled by TLV.
To be included, studies had to report OS or QoL results from a randomized controlled trial (RCT). Population eligibility criteria were also set for each drug indication based on the characteristics of the concerned patient group specified in the TLV approvals (Table S1 of the ESM). Studies without OS or QoL as an endpoint, commentaries, pharmacokinetic modeling studies, single-arm trials, dose-response trials, and reviews were excluded. Only papers in English and Swedish were eligible.
All authors took part in the screening and selection of studies based on pre-specified inclusion and exclusion criteria and data extraction templates. The screening, selection, and data extraction were conducted independently by at least two reviewers for each drug indication. Disagreements were resolved through discussions among all authors. In addition to PubMed, National Institutes of Health trial registry ClinicalTrials was searched for all drug indications using the search terms active substance name OR drug brand name AND cancer form. These searches were conducted between 12 and 23 September, 2022, and screened using the same inclusion criteria. The initial PubMed search was additionally updated between 24 and 26 October, 2022. For a complete specification of the search strategies for each drug indication and the screening process of the updated search, see Tables S2–S21 and Fig. S1 of the ESM, respectively.
Data Extraction
All included reports were used to extract data on clinical efficacy outcomes for each drug indication. Whenever applicable, separate articles of cross-over adjustments with changes in a trial’s primary results were considered for sensitivity analyses. Data were extracted on trial characteristics and efficacy outcomes, including trial phase, follow-up time, blinding status, sample size, eligibility criteria, hazard ratios (HRs), number of events, and median survival in months for the assessment of OS. For QoL, all instrument(s) used, completion rates, and between-group differences in global QoL domains or summary scores were extracted. If more than one instrument was used to assess QoL, results for all instruments were extracted.
Statistical Analysis
Improvements in OS were defined as having a statistically significant (p < 0.05) positive difference in median OS or reduced HR of all-cause death (95% confidence interval of the HR not exceeding the null value). For QoL, an improvement was defined as having a statistically significant positive between-group difference in global QoL or summary scores compared to the control treatment in at least one of the included instruments. Trial characteristics and results for all trials included in the review were analyzed and presented descriptively. To assess the available post-reimbursement evidence, data were synthesized based on the specific drug indications and characteristics of concerned patient groups specified by the TLV in the reimbursement approvals (Table S1 of the ESM). A summary of the evidence based on the most recently published article of the main study population (i.e. not subgroups) on OS and QoL from each trial was synthesized and presented descriptively for each specific drug indication. For trials including the drug of interest as a control against a new agent, the results of HRs on OS were inverted for presentation (n = 9). A drug indication was regarded as having shown evidence of improvements in the post-reimbursement period if improvements in OS or QoL were found in at least one trial. Additionally, fixed-effects inverse variance meta-analyses of the effects on OS were performed as a sensitivity analysis of the conclusions of post-reimbursement evidence for drug indications with multiple trials (n = 7).
Ethics
This study was based on publicly accessible literature and did not involve individual patient information. Therefore, no institutional review was required.
Results
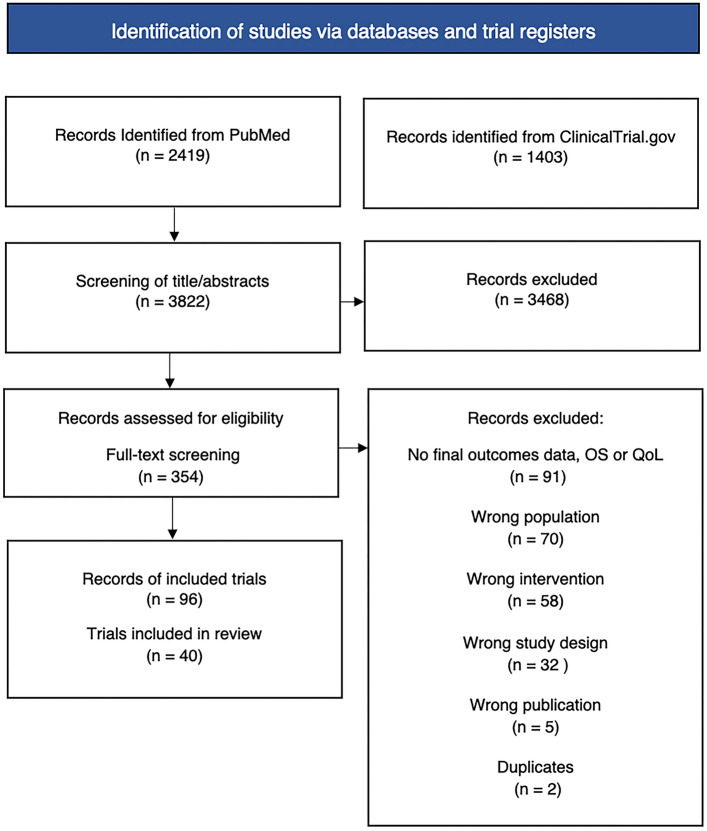
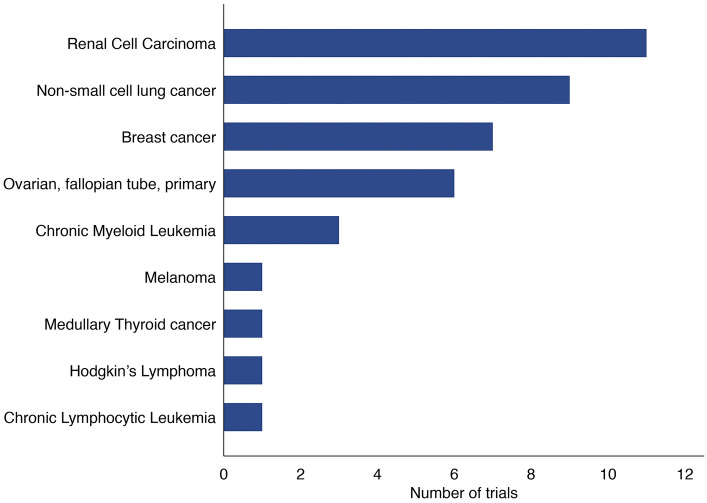
In total, 40 randomized controlled trials were identified in the review (Fig. 1), with available evidence for 16 of 22 drug indications. The majority of trials concerned an advanced disease stage (73%) and mainly targeted renal cell carcinoma, non-small cell lung cancer (NSCLC), breast cancer, and ovarian cancer (Fig. 2). The trials were initiated from 2006 to 2017 and had an average sample size of 499 patients (range 43–5761). Most trials were funded by the pharmaceutical industry (83%) and consisted of phase III trials with an active control treatment (Fig. S2 of the ESM). The average duration from trial initiation to data cut-off was 4.3 years (range 1.8–12 years), and 33% of trials were at least single-blinded.
Fig. 1.
Flow diagram over the search and selection process of reports and trials for all drug-indications
Fig. 2.
Diseases targeted in the included trials
Of the 40 trials, a majority reported results on OS and of these, 18% reported statistically significant improvements in OS (n = 7), while 5% showed negative results on OS for the drug of interest (n = 2). A lack of reporting OS data was found for 13% of the trials, primarily due to data immaturity (n = 5). Results on QoL were reported for 58% of the included trials (n = 23); out of these, around 17% reported a statistically significant improvement of the impact on QoL (n = 4), and the remaining found non-statistically significant results of both negative and positive effects on QoL (n = 19). Both disease-specific and generic instruments were used in the trials (Table S22 of the ESM). A pre-defined threshold for clinically meaningful changes in QoL was reported in around half of all the trials with a reported QoL analysis (n = 12). The threshold was reached in one trial.
Post-Reimbursement Evidence of Improvements in OS and QoL
Of the 22 drug indications, 68% (n = 15) continued to have limited evidence of improvements in both OS and QoL after a mean follow-up period of 6.6 (range 2–12) years (Table 1; Table S22 of the ESM). For six of these drug indications, no evidence from an RCT was found (Table S22 of the ESM).
Table 1.
Number of drug indications with improvement in final outcomes, n (%)
| Overall survival | Quality of life | |
|---|---|---|
| No evidence of improvement | Evidence of improvement | |
| No evidence of improvement | 15 (68) | 2 (9) |
| Evidence of improvement | 4 (18) | 1 (5) |
Note: Overview of the share of reimbursed drug indications with subsequent evidence of improvements in overall survival and quality of life (n = 22)
In total, seven drug indications (32%) showed evidence of improvements in OS or QoL in the post-reimbursement period (Table 2). Out of these, alectinib, everolimus, olaparib, and ribociclib were shown to improve OS for their indications as: first-line treatment for advanced ALK-positive NSCLC, advanced renal cell carcinoma, maintenance therapy for BRCA-mutated ovarian cancer, and initial endocrine therapy for advanced breast cancer, respectively. Likewise, ceritinib and palbociclib were found to improve QoL for indications of advanced ALK-positive NSCLC and breast cancer. One drug, osimertinib, was found to improve both OS and QoL for the indication of advanced NSCLC with EGFR T790M mutations (Table 2). The improvements in median OS for these ranged from 2 to 13 months (n = 5).
Table 2.
Summary of findings: drug indications with evidence of improvements in OS or QoL (in at least one trial) in the post-reimbursement period
| Trial | Setting | Start | Cut-off | Sample | Drugs | HR (95% CI) |
P-value | Intervention MOS | Control MOS |
QoL Analysis |
Instrument | QoL Result | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Alectinib As first-line treatment for patients with ALK+ advanced, non-small cell lung cancer | |||||||||||||
| J-ALEX [28] JapicCTI-132316 | Japan | 11/2013 | NR/2020 | 207 | Alectinib vs crizotinib |
1.03 (0.67–1.58) |
0.910 | NE | NE | NR | NR | NR | NR |
| ALEX [29, 30] NCT02075840 | NR | 08/2014 | 11/2019c | 303 | Alectinib vs crizotinib |
0.67 (0.46–0.98) |
0.037 | NE |
57.4 (34.6–NE) |
Yes | EORTC QLQ-C30, LC13 | No difference | NR |
| ALESIA [31] NCT02838420 | 4 countries | 08/2016 | 05/2018 | 187 | Alectinib vs crizotinib |
0.28 (0.12–0.68) |
0.002 | NE |
NE (19.8–NE) |
NR | NR | NR | NR |
| NR [32] | China | 01/2017 | NR | 120 | Alectinib vs crizotinib | NR | NR |
12.66 (8.08–17.24) |
10.15 (6.74–13.56) |
NR | Karnofsky Performance Status Scale | Positive difference | NR |
|
Ceritinib Patients with ALK+ non-small cell lung cancer who previously have been treated with crizotinib | |||||||||||||
|
ASCEND-5 [33] |
20 countries | 06/2013 | 01/2016 | 231 | Ceritinib vs chemotherapy |
1.00 (0.67–1.49) |
0.500 |
18.1 (13.4–23.9) |
20.1 (11.9–25.1) |
Yes |
LCSS EORTC QLQ-C30, LC13 EQ-5D-5L |
Positive difference | <0.001 |
|
Everolimus Patients with advanced RCC, following VEGFR-targeted therapy treatment | |||||||||||||
| RECORD-1 [34, 35] NCT00410124 | 5 countries | 12/2006 | 11/2008 | 416 | Everolimus vs placebo |
0.87 (0.65–1.15) |
0.162 |
14.8 (NR) |
14.4 (NR) |
Yes |
FKSI-DRS EORTC QLQ-C30 |
Negative difference | 0.228 |
| ROVER [36] NCT01442090a | 5 countries | 10/2011 | 01/2014 | 85 | Everolimus vs apitolisib |
0.56 (0.31–1.03) |
0.060 |
22.8 (12.4–NE) |
16.5 (10.8–21.3) |
NR | NR | NR | NR |
| NCT01136733 [37]a | 5 countries | 03/2012 | 12/2014 | 153 | Everolimus vs levnatinib |
1.47 (0.88–2.44) |
0.12 |
15.4 (11.8–19.6) |
19.1 (13.6–26.2) |
NR | NR | NR | NR |
|
CHECKMATE02 [38] NCT0166878a |
24 countries | 10/2012 | 08/2019 | 821 | Everolimus vs nivolumab |
1.37 (1.18–1.61) |
<0.001 |
19.7 (17.6–22.1) |
25.8 (22.2–29.8) |
NR | NR | NR | NR |
| METEOR [39, 40]a NCT01865747 | 26 countries | 08/2013 | 10/2016c | 658 |
Everolimus vs cabozantinib |
1.43 (1.18–1.72) |
<0.001 |
17.4 (NR) |
21.4 (NR) |
Yes |
FKSI-19 FKSI-DRS EQ-5D-5L |
No difference | NR |
|
ZEBRA [41]a EudraCT 2008-005614-44 |
UK | 02/2013 | 01/2015 | 49 | Everolimus vs AZD2014 |
0.32 (0.12–0.91) |
0.02 |
16.7 (6–NE) |
6.2 (4.5–14.0) |
NR | NR | NR | NR |
| NCT02724020 [42]a | 35 countries | 06/2016 | 02/2020 | 96 |
Everolimus vs sapanisertib ± TAK-117 |
0.57 (0.29–1.12) |
0.212 |
22.4 (8.2–NE) |
22.49 (9.0–19.9) |
Yes |
EORTC QLQ-C30 FKSI-DRS |
No difference | NR |
| NCT01239342 [43]a | NR | NR | NR | 43 |
Everolimus vs MK-2206 |
NR | NR |
15.7 (6.5–NE) |
23.5 (10.7–37.4) |
NR | NR | NR | NR |
|
Olaparib Maintenance treatment of patients with platinum-sensitive relapsed high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer with BRCA mutations who are in response to platinum-based chemotherapy | |||||||||||||
|
Study 19 [44] |
16 countries | 08/2008 | 05/2016 | 265 | Olaparib vs placebo |
0.73 (0.55–0.95) |
0.02 |
29.8 (26.9–35.7) |
27.8 (24.9–33.7) |
NR | NR | NR | NR |
| SOLO 2 [45] NCT01874353 | 16 countries | 09/2013 | 02/2020c | 295 | Olaparib vs placebo |
0.74 (0.54–1.0) |
0.054 |
51.7 (41.5– 59.1) |
38.8 (31.4–48.6) |
Yes |
FACT-O EQ-5D-5L |
No difference | 0.980 |
| SOLO 3 [46] NCT02282020 | 13 countries | 02/2015 | 10/2018 | 266 | Olaparib vs chemotherapy | NR | NR | NR | NR | Yes | FACT-O | Positive difference | 0.108 |
| NCT02446600 [47] | 4 countries | 02/2016 | 02/2020 | 565 | Olaparib vs chemotherapy | NR | NR |
29.3 (23.9–32.7) |
31.3 (26.4–34.4) |
Yes | NFOSI | Positive difference | 0.7 |
|
Osimertinib Patients with locally advanced or metastatic non-small cell lung cancer with an EGFR T790M mutation | |||||||||||||
| AURA3 [48, 49] NCT02151981 | 18 countries | 08/2014 | 02/2019 | 419 | Osimertinib vs pemetrexed + carboplatin, cisplatin |
0.87 (0.67–1.12) |
0.277 |
26.8 (23.5–31.5) |
22.5 (20.2–28.8) |
Yes |
EORTC QLQ-C30, LC13 |
Positive difference | 0.007 |
| FLAURA [50] NCT02296125 | 29 countries | 12/2014 | 06/2019c | 556 | Osimertinib vs gefitinib, erlotinib |
0.80 (0.64–1.0) |
0.046 |
38.6 (34.5–41.8) |
31.8 (26.6–36.0) |
Yes |
EORTC QLQ-C30, LC13 |
Positive difference | 0.165 |
| NCT02959749 [51] | China | 04/2015 | 02/2017 | 147 | Osimertinib vs docetaxel + bevacizumab |
0.79 (0.38–1.61) |
0.551 | NE | NE | Yes | NR | No difference | NR |
|
Palbociclib Patients with locally advanced or metastatic HR+, HER2− breast cancer (in combination with an aromatase inhibitor) | |||||||||||||
| PALOMA-2 [52] NCT01740427 | 17 countries | 02/2013 | 05/2017 | 666 | Palbociclib + letrozole vs placebo + letrozole | NR | NR | NR | NR | Yes | FACT-B | Negative difference | 0.629 |
| PENELOPE-B [53] NCT01864746 | 11 countries | 02/2014 | 08/2020 | 1250 | Palbociclib + ET vs placebo + ET |
0.87 (0.61–1.22) |
0.420 | NR | NR | NR | NR | NR | NR |
| PEARL [54] NCT02028507 | 4 countries | 03/2014 | 01/2019 | 296 | Palbociclib + examestane vs capaceitabine | NR | NR | NR | NR | Yes |
EORTC QLQ C30, BR23 EQ-5D-3L |
Positive difference | 0.001 |
|
PALOMA-4 [55]b |
5 countries | 03/2015 | 02/2020 | 340 | Palbociclib + letrozole vs placebo + letrozole |
0.95 (0.70–1.29) |
0.365 |
51.7 (43.0–NE) |
51.5 (41.0–NE) |
Yes |
EQ-5D-5L EQ-VAS FACT-B |
Positive difference | 0.007 |
| PALLAS [56] NCT02513394 | 21 countries | 09/2015 | 11/2020 | 5761 | Palbociclib + ET vs ET |
1.32 (0.98–1.78) |
NR | NR | NR | NR | NR | NR | NR |
|
Ribociclib In combination with an aromatase inhibitor as initial endocrine-based therapy for postmenopausal women with locally advanced or metastatic HR+, HER− breast cancer | |||||||||||||
| 29 countries | 01/2014 | 06/2021 | 668 | Ribociclib vs placebo |
0.76 (0.63–0.93) |
0.008 |
63.9 (52.4–71.0) |
51.4 (47.2–59.70) |
Yes | EORTC QLQ-C30 | No difference | NR | |
Overview of all trials included for the drug indications with shown evidence of improvement (in at least one trial) during the 6.6 years of follow-up from reimbursement decision. Trial characteristics are presented along with hazard ratio estimations for the comparative effect on OS with 95% CI and reported p-values. MOS presented for both control and intervention (the drug of interest)
BPI Brief Pain Inventory, CI confidence interval, DRS disease-related symptoms, EORTC QLQ-C30, - LC13 European Organization for the Research and Treatment of Cancer Core Quality of Life Questionnaire, EQ-5D, -3L European Quality of Life-5 Dimension Scale Questionnaire, -3 level, EQ-VAS European Quality of Life Visual Analogue Scale, FACT-O, -B Functional Assessment of Cancer Therapy - Ovarian Cancer, - Breast Cancer, FKSI Functional Assessment of Cancer Therapy Kidney Symptom Index, LCSS Lung Cancer Symptom Scale, MOS median overall survival, NA no article, NE not estimable (outcome not reached), NR not reported, OS overall survival, QoL quality of life, RCC renal cell carcinoma, VEGFR vascular endothelial growth factor
aDrug of interest was tested against a newer agent. Hazard ratios were inverted to facilitate interpretation and presentation of the results
bComplementary information for the trial were retrieved from ClinicalTrial.gov information
cDifferent data cut-off for the quality-of-life analysis
For two drug indications, the drugs of interest (everolimus and axitinib) were also included as controls against newer agents, and mixed results of both negative and positive effects on OS were observed (Table 2; Table S22 of the ESM). Likewise, for most drug indications with evidence of an improvement in QoL (n = 3), additional trials indicated mixed effects on the outcome (Table 2). A clinically relevant improvement in QoL according to the pre-defined thresholds in the trials was found for one drug, osimertinib. Considerations of cross-over adjustments, different comparators, and alternative doses within trials did not change the conclusions of the available evidence for the drug indications (Table S23 of the ESM). Pooled HRs from the meta-analyses generally confirmed the conclusion of improved OS for the drug indications (Table S24 of the ESM). However, for one drug, everolimus, the pooled effect estimate showed a significant negative impact on OS.
Discussion
In this study, we reviewed the post-reimbursement evidence of the effects on OS and QoL for cancer drugs with limited clinical evidence of added improvements at the time of reimbursement in 2010–20, Sweden. Despite subsequent published RCT reports for a majority of the reviewed drug indications, we found that a majority of the reviewed drug indications continued to have limited evidence of comparative effectiveness on both OS and QoL. After a mean follow-up of 6.6 years after reimbursement, only 32% of the 22 drug indications had available evidence supporting improvements in OS or QoL.
Our findings are in line with previous studies assessing cancer drugs and the evidence of the effects on OS and QoL in the post-approval period [7, 21, 59–61]. For instance, Grössman et al. [7, 59] found that evidence of improvements in OS and QoL increased over time but continued to be limited for a substantial share of approvals by the European Medicines Agency. In contrast to previous research, which has focused on market authorizations, our study focused on a context of reimbursement decisions where additional considerations of drugs’ cost effectiveness — based on the clinical evidence of the effects on OS and QoL — are of fundamental importance to inform third-party payers within healthcare systems. Considering the high costs of cancer drugs, the lack of subsequent data fulfilling the assumptions of improvement in outcomes is troubling, implying substantial uncertainty in the economic evaluations of a considerable share of reimbursed cancer drugs.
It is usually emphasized that considerable time is needed to ensure mature survival data in cancer settings [62, 63]. While intermediate outcomes such as progression-free survival are relevant for clinicians’ recommendations for a patient, improvements in OS and QoL are the main relevant endpoints for health policymakers considering what treatments should be available or not [14, 64]. Most drug indications in the review concerned advanced stages of cancer, where improvements in OS and QoL are the main treatment objectives and could be expected [12]. Our findings of continuous uncertainties of the comparative effectiveness in OS and QoL — despite demonstrated benefits in surrogate outcomes — likewise highlight a need to review acceptable outcomes and the assumptions made for these in reimbursement decisions. Previous studies analyzing cancer drugs approved on surrogate endpoints have also found that a high share of market approvals have shown limited evidence of OS improvement despite a median follow-up of 4–5 years [21, 65]. As cancer trials increasingly rely on intermediate outcomes to inform clinical benefits, possibilities to enhance data on OS may be limited owing to a lack of power in the studies or use of for example, cross-overs. Given the lack of validation of surrogacy for many intermediate outcomes and OS or QoL, more research must validate surrogate outcomes and their usefulness in informing reimbursement decisions.
We also observed that only 2 out of 22 drug indications had subsequent evidence of improvements in QoL, and 42% of the identified trials did not report an analysis of QoL. The lack of subsequent data on QoL and reporting poses a challenge in assessing the relative QoL benefits of new drugs. In this paper, QoL results were regarded irrespective of the type of instrument used. However, different types of instruments and analytical methods convey varying sensitivity to changes in QoL, which can influence the possibility of establishing evidence of effects. Given the importance of QoL for patients and the difficulties of ensuring OS benefits in certain settings, our findings suggest that health technology assessment requirements should emphasize QoL. Future research is needed on the implications of instruments as well as the justification and rationale of methods used to assess QoL in cancer drug trials.
It is important to acknowledge that additional factors may influence the possibility of acquiring evidence of the effect on OS and QoL, such as differences in the time of follow-up, disease stage, and trial characteristics. No difference in the time of follow-up (p = 0.86 according to a t-test) nor metastatic/advanced setting (p = 0.6 according to Fisher’s exact test) was however found for drugs with or without post-reimbursement evidence in this study. For three drug indications with an orphan designation, no evidence from RCTs was identified in our review. The severity and rarity of diseases can limit the possibilities of conducting a well-designed RCT, and different requirements and value assessments may be regarded. While more consideration must be given to these settings, it is further essential to address under what circumstances uncertainties can be acceptable. Given the increasing market approvals using accelerated pathways, the possibilities (or lack thereof) to enhance updated evidence to inform the implementation need additional consideration at regulatory levels.
Our study is limited by using only two databases to identify studies. However, PubMed and ClinicalTrial.gov are among the largest medical research databases/trial registries, which should ensure high RCT coverage [66, 67]. Furthermore, we considered data from the main study population and primary analyses of the included trials. Some trials showed improvements in specific subgroups, which could be used to inform restricted reimbursement decisions; a list of all included reports can be found in Table S25 of the ESM.
Another limitation is that we relied on a fixed threshold for statistical significance in our classification of limited evidence. While p-value thresholds indicate the uncertainty of study results, the widths of the confidence intervals and directions of estimates are also important to consider. We note that many studies in our review showed imprecise (non-significant) improvements in OS and QoL (n = 19). However, data showing imprecise detrimental effects compared to the comparator were also observed in some cases (n = 6). An additional concern is that we classified drug indications as having evidence of improvement based on improvements in OS or QoL from at least one study. While we conducted meta-analyses for drug indications to assess the sensitivity of our results to this approach (whenever possible), the risk of bias, heterogeneity assessments, and publication bias of the trials could give additional insights into the reliability of the available evidence.
Several additional factors regarding the uncertainty of cancer drugs have been raised, which were not addressed in this study. For instance, concerns have been raised regarding the inappropriate use of cross-over, suboptimal control arms, and unrepresentative samples in cancer trials [20, 68, 69], which can impact the validity and applicability of the results. Finally, clinical relevance should also be recognized. Previous studies have shown that many drugs with OS or QoL data offer non-substantial clinical benefits according to value frameworks established by EMSO and ASCO, where outcomes such as progression-free survival, OS, and QoL are regarded [61, 70–72]. In our review, only one trial reported a clinically relevant improvement in QoL compared to the alternative for the reimbursed indication [49]. Future research and assessments of minimally important clinical differences and the effects of cancer drugs would be informative for future evaluations and decision making.
Conclusions
With the challenges of limited resources within health systems and the increasing disease burden of cancer, ensuring value for money is an essential public health goal. Implementing expensive treatments with uncertain effectiveness affects not only patients with cancer but may impose high opportunity costs in terms of displaced benefits for other patients. Our results suggest a need to discuss the requirements informing reimbursement approvals and continue monitoring reimbursed cancer drugs.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Open access funding provided by University of Gothenburg. This work was supported by a research grant from Jan Wallanders and Tom Hedelius stiftelse and Tore Browaldhs stiftelse [#P21-0018]. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of Interest
Gabriella Chauca Strand, Naimi Johansson, Niklas Jakobsson, Carl Bonander, and Mikael Svensson have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
This study was based on publicly accessible literature and did not involve individual patient information. Therefore, no institutional review was required.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
All data generated or analyzed during this study were publicly available and are included in this published article and its supplementary information files.
Code Availability
Not applicable.
Authors’ Contributions
Concept and design: GCS, NJo, NJa, CB, MS. Acquisition of data: GCS, NJo, NJa, CB, MS. Analysis and interpretation of data: GCS. Drafting of the manuscript: GCS. Critical revision of the paper for important intellectual content: GCS, NJo, NJa, CB, MS. Funding acquisition: MS. Administrative, technical, or logistic support: GCS. Supervision: CB, MS.
References
- 1.Prasad V, De Jesús K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14(6):381–390. doi: 10.1038/nrclinonc.2017.31. [DOI] [PubMed] [Google Scholar]
- 2.Howard DH, Bach PB, Berndt ER, Conti RM. Pricing in the market for anticancer drugs. J Econ Perspect. 2015;29(1):139–162. doi: 10.1257/jep.29.1.139. [DOI] [PubMed] [Google Scholar]
- 3.Hofmarcher T, Lindgren P, Wilking N, Jönsson B. The cost of cancer in Europe 2018. Eur J Cancer. 2020;29:41–49. doi: 10.1016/j.ejca.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 4.IQVIA. Global oncology trends 2018: innovation, expansion and disruption. IQVIA Institute USA; 2018.
- 5.Prasad V. Do cancer drugs improve survival or quality of life? BMJ. 2017;359:J4528. doi: 10.1136/bmj.j4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherny N. An appraisal of FDA approvals for adult solid tumours in 2017–2021: has the eagle landed? Nat Rev Clin Oncol. 2022;19(7):486–492. doi: 10.1038/s41571-022-00636-y. [DOI] [PubMed] [Google Scholar]
- 7.Grössmann N, Robausch M, Rosian K, Wild C, Simon J. Monitoring evidence on overall survival benefits of anticancer drugs approved by the European Medicines Agency between 2009 and 2015. Eur J Cancer. 2019;110:1–7. doi: 10.1016/j.ejca.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Vokinger KN, Hwang TJ, Daniore P, Lee CC, Tibau A, Grischott T, et al. Analysis of launch and postapproval cancer drug pricing, clinical benefit, and policy implications in the US and Europe. JAMA Oncol. 2021;7(9):e212026. doi: 10.1001/jamaoncol.2021.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vokinger KN, Hwang TJ, Grischott T, Reichert S, Tibau A, Rosemann T, et al. Prices and clinical benefit of cancer drugs in the USA and Europe: a cost-benefit analysis. Lancet Oncol. 2020;21(5):664–670. doi: 10.1016/S1470-2045(20)30139-X. [DOI] [PubMed] [Google Scholar]
- 10.Del Paggio JCMD, Sullivan RP, Schrag DP, Hopman WMMA, Azariah BM, Pramesh CSP. Delivery of meaningful cancer care: a retrospective cohort study assessing cost and benefit with the ASCO and ESMO frameworks. Lancet Oncol. 2017;18(7):887–894. doi: 10.1016/S1470-2045(17)30415-1. [DOI] [PubMed] [Google Scholar]
- 11.OECD . Fiscal sustainability of health systems: bridging health and finance perspectives. Paris: OECD; 2015. [Google Scholar]
- 12.Peppercorn JM, Smith TJ, Helft PR, DeBono DJ, Berry SR, Wollins DS, et al. American Society of Clinical Oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol. 2011;29(6):755–760. doi: 10.1200/JCO.2010.33.1744. [DOI] [PubMed] [Google Scholar]
- 13.Davis C. Drugs, cancer and end-of-life care: a case study of pharmaceuticalization? Soc Sci Med. 2015;131:207–214. doi: 10.1016/j.socscimed.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma D, Aggarwal AK, Downey LE, Prinja S. National healthcare economic evaluation guidelines: a cross-country comparison. Pharmacoeconomics. 2021;5(3):349–354. doi: 10.1007/s41669-020-00250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemp R, Prasad V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 2017;15(1):134. doi: 10.1186/s12916-017-0902-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haslam A, Hey SP, Gill J, Prasad V. A systematic review of trial-level meta-analyses measuring the strength of association between surrogate end-points and overall survival in oncology. Eur J Cancer. 2019;106:196–211. doi: 10.1016/j.ejca.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Kovic B, Jin X, Kennedy SA, Hylands M, Pędziwiatr M, Kuriyama A, et al. Evaluating progression-free survival as a surrogate outcome for health-related quality of life in oncology: a systematic review and quantitative analysis. JAMA Intern Med. 2018;178(12):1586–1596. doi: 10.1001/jamainternmed.2018.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naci H, Davis C, Savović J, Higgins JPT, Sterne JAC, Gyawali B, et al. Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014–16: cross sectional analysis. BMJ. 2019;366:l5221. doi: 10.1136/bmj.l5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnog JB, Samson MJ, Gans ROB, Duits AJ, et al. An urgent call to raise the bar in oncology. Br J Cancer. 2021;125(11):1477–1485. doi: 10.1038/s41416-021-01495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilal T, Gonzalez-Velez M, Prasad V. Limitations in clinical trials leading to anticancer drug approvals by the US Food and Drug Administration. JAMA Intern Med. 2020;180(8):1108–1115. doi: 10.1001/jamainternmed.2020.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and drug administration approvals. JAMA Intern Med. 2015;175(12):1–2. doi: 10.1001/jamainternmed.2015.5868. [DOI] [PubMed] [Google Scholar]
- 22.Chauca Strand G, Bonander C, Jakobsson N, Johansson N, Svensson M. Assessment of the clinical and cost-effectiveness evidence in the reimbursement decisions of new cancer drugs. ESMO Open. 2022;7(5):100569. doi: 10.1016/j.esmoop.2022.100569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyers DE, Jenei K, Chisamore TM, Gyawali B. Evaluation of the clinical benefit of cancer drugs submitted for reimbursement recommendation decisions in Canada. JAMA Intern Med. 2021;181(4):499–508. doi: 10.1001/jamainternmed.2020.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raymakers AJN, Regier DA, Peacock SJ. Health-related quality of life in oncology drug reimbursement submissions in Canada: a review of submissions to the pan-Canadian Oncology Drug Review. Cancer. 2020;126(1):148–155. doi: 10.1002/cncr.32455. [DOI] [PubMed] [Google Scholar]
- 25.Gyawali B, Hey SP, Kesselheim AS. Assessment of the clinical benefit of cancer drugs receiving accelerated approval. JAMA Intern Med. 2019;179(7):906–913. doi: 10.1001/jamainternmed.2019.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Naci H, Wagner AK, Xu Z, Yang Y, Zhu J, et al. Overall survival benefits of cancer drugs approved in China From 2005 to 2020. JAMA Netw Open. 2022;5(8):e2225973. doi: 10.1001/jamanetworkopen.2022.25973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.TLV. Handbok för företag vid ansökan om subvention och pris för läkemedel 2020. Available from: https://www.tlv.se/download/18.3cea0a4c179ef9d61368a5d8/1624006663114/handbok_version3.9_foretag_vid_ansokan_om_subvention_och_pris.pdf. [Accessed 23 Mar 2023].
- 28.Hotta K, Hida T, Nokihara H, Morise M, Kim YH, Azuma K, et al. Final overall survival analysis from the phase III J-ALEX study of alectinib versus crizotinib in ALK inhibitor-naïve Japanese patients with ALK-positive non-small-cell lung cancer. ESMO Open. 2022;7(4):100527. doi: 10.1016/j.esmoop.2022.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31(8):1056–1064. doi: 10.1016/j.annonc.2020.04.478. [DOI] [PubMed] [Google Scholar]
- 30.Pérol M, Pavlakis N, Levchenko E, Platania M, Oliveira J, Novello S, et al. Patient-reported outcomes from the randomized phase III ALEX study of alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer. Lung Cancer. 2019;138:79–87. doi: 10.1016/j.lungcan.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Zhou C, Kim SW, Reungwetwattana T, Zhou J, Zhang Y, He J, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019;7(5):437–446. doi: 10.1016/S2213-2600(19)30053-0. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Zhao J. Clinical efficacy and safety of crizotinib and alectinib in ALK-positive non-small cell lung cancer treatment and predictive value of CEA and CA125 for treatment efficacy. Am J Transl Res. 2021;13(11):13108–13116. [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw AT, Kim TM, Crinò L, Gridelli C, Kiura K, Liu G, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18(7):874–886. doi: 10.1016/S1470-2045(17)30339-X. [DOI] [PubMed] [Google Scholar]
- 34.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 35.Beaumont JL, Butt Z, Baladi J, Motzer RJ, Haas T, Hollaender N, et al. Patient-reported outcomes in a phase iii study of everolimus versus placebo in patients with metastatic carcinoma of the kidney that has progressed on vascular endothelial growth factor receptor tyrosine kinase inhibitor therapy. Oncologist. 2011;16(5):632–640. doi: 10.1634/theoncologist.2010-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powles T, Lackner MR, Oudard S, Escudier B, Ralph C, Brown JE, et al. Randomized ppen-label phase II trial of apitolisib (GDC-0980), a novel inhibitor of the PI3K/mammalian target of rapamycin pathway, versus everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. 2016;34(14):1660–1668. doi: 10.1200/JCO.2015.64.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473–1482. doi: 10.1016/S1470-2045(15)00290-9. [DOI] [PubMed] [Google Scholar]
- 38.Motzer RJ, Escudier B, George S, Hammers HJ, Srinivas S, Tykodi SS, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126(18):4156–4167. doi: 10.1002/cncr.33033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motzer RJ, Escudier B, Powles T, Scheffold C, Choueiri TK. Long-term follow-up of overall survival for cabozantinib versus everolimus in advanced renal cell carcinoma. Br J Cancer. 2018;118(9):1176–1178. doi: 10.1038/s41416-018-0061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cella D, Escudier B, Tannir NM, Powles T, Donskov F, Peltola K, et al. Quality of life outcomes for cabozantinib versus everolimus in patients with metastatic renal cell carcinoma: METEOR phase III randomized trial. J Clin Oncol. 2018;36(8):757–764. doi: 10.1200/JCO.2017.75.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powles T, Wheater M, Din O, Geldart T, Boleti E, Stockdale A, et al. A randomised phase 2 study of AZD2014 versus everolimus in patients with VEGF-refractory metastatic clear cell renal cancer. Eur Urol. 2016;69(3):450–456. doi: 10.1016/j.eururo.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 42.Choueiri T, Porta C, Suárez C, Hainsworth J, Voog E, Duran I, et al. Randomized phase II trial of sapanisertib ± TAK-117 vs. everolimus in patients with advanced renal cell carcinoma after VEGF-targeted therapy. Oncologist. 2022 doi: 10.1093/oncolo/oyac192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonasch E, Hasanov E, Corn PG, Moss T, Shaw KR, Stovall S, et al. A randomized phase 2 study of MK-2206 versus everolimus in refractory renal cell carcinoma. Ann Oncol. 2017;28(4):804–808. doi: 10.1093/annonc/mdw676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedlander M, Matulonis U, Gourley C, du Bois A, Vergote I, et al. Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy. Br J Cancer. 2018;119(9):1075–1085. doi: 10.1038/s41416-018-0271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poveda A, Floquet A, Ledermann JA, Asher R, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22(5):620–631. doi: 10.1016/S1470-2045(21)00073-5. [DOI] [PubMed] [Google Scholar]
- 46.Penson RT, Valencia RV, Cibula D, Colombo N, Leath CA, Bidziński M, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol. 2020;38(11):1164–1174. doi: 10.1200/JCO.19.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu JF, Brady MF, Matulonis UA, Miller A, Kohn EC, Swisher EM, et al. Olaparib with or without cediranib versus platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer (NRG-GY004): a randomized, open-label, phase III trial. J Clin Oncol. 2022;40(19):2138–2147. doi: 10.1200/jco.21.02011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papadimitrakopoulou VA, Mok TS, Han JY, Ahn MJ, Delmonte A, Ramalingam SS, et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol. 2020;31(11):1536–1544. doi: 10.1016/j.annonc.2020.08.2100. [DOI] [PubMed] [Google Scholar]
- 49.Lee CK, Novello S, Rydén A, Mann H, Mok T. Patient-reported symptoms and impact of treatment with osimertinib versus chemotherapy in advanced non-small-cell lung cancer: the AURA3 Trial. J Clin Oncol. 2018;36(18):1853–1860. doi: 10.1200/JCO.2017.77.2293. [DOI] [PubMed] [Google Scholar]
- 50.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 51.Nie K, Zhang Z, Zhang C, Geng C, Zhang L, Xu X, et al. Osimertinib compared docetaxel-bevacizumab as third-line treatment in EGFR T790M mutated non-small-cell lung cancer. Lung Cancer. 2018;121:5–11. doi: 10.1016/j.lungcan.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Rugo HS, Finn RS, Diéras V, Ettl J, Lipatov O, Joy AA, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loibl S, Marmé F, Martin M, Untch M, Bonnefoi H, Kim SB, et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer: the Penelope-B Trial. J Clin Oncol. 2021;39(14):1518–1530. doi: 10.1200/JCO.20.03639. [DOI] [PubMed] [Google Scholar]
- 54.Martin M, Zielinski C, Ruiz-Borrego M, Carrasco E, Turner N, Ciruelos EM, et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: a phase III randomised controlled trial-PEARL. Ann Oncol. 2021;32(4):488–499. doi: 10.1016/j.annonc.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Xu B, Hu X, Li W, Sun T, Shen K, Wang S, et al. Palbociclib plus letrozole versus placebo plus letrozole in Asian postmenopausal women with oestrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: primary results from PALOMA-4. Eur J Cancer. 2022;175:236–245. doi: 10.1016/j.ejca.2022.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Gnant M, Dueck AC, Frantal S, Martin M, Burstein HJ, Greil R, et al. Adjuvant palbociclib for early breast cancer: the PALLAS trial results (ABCSG-42/AFT-05/BIG-14-03) J Clin Oncol. 2022;40(3):282–293. doi: 10.1200/JCO.21.02554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386(10):942–950. doi: 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 58.Janni W, Alba E, Bachelot T, Diab S, Gil-Gil M, Beck TJ, et al. First-line ribociclib plus letrozole in postmenopausal women with HR+, HER2− advanced breast cancer: tumor response and pain reduction in the phase 3 MONALEESA-2 trial. Breast Cancer Res Treat. 2018;169(3):469–479. doi: 10.1007/s10549-017-4658-x. [DOI] [PubMed] [Google Scholar]
- 59.Grössmann N, Robausch M, Rothschedl E, Wild C, Simon J. Publicly accessible evidence of health-related quality of life benefits associated with cancer drugs approved by the European Medicines Agency between 2009 and 2015. Eur J Cancer. 2020;129:23–31. doi: 10.1016/j.ejca.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 60.Davis C, Naci H, Gurpinar E, Poplavska E, Pinto A, Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009–13. BMJ. 2017;359:j4530. doi: 10.1136/bmj.j4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arciero V, Delos Santos S, Koshy L, Rahmadian A, Saluja R, Everest L, et al. Assessment of Food and Drug Administration- and European Medicines Agency-approved systemic oncology therapies and clinically meaningful improvements in quality of life: a systematic review. JAMA Netw Open. 2021;4(2):e2033004. doi: 10.1001/jamanetworkopen.2020.33004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lux MP, Ciani O, Dunlop WCN, Ferris A, Friedlander M. The impasse on overall survival in oncology reimbursement decision-making: how can we resolve this? Cancer Manag Res. 2021;13:8457–8471. doi: 10.2147/CMAR.S328058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson MK, Collyar DB, Chingos D, Friedlander M, Ho T, Karakasis K, et al. Outcomes and endpoints in cancer trials: bridging the divide. Lancet Oncol. 2015;16(1):e43–52. doi: 10.1016/S1470-2045(14)70380-8. [DOI] [PubMed] [Google Scholar]
- 64.Angelis A, Lange A, Kanavos P. Using health technology assessment to assess the value of new medicines: results of a systematic review and expert consultation across eight European countries. Eur J Health Econ. 2018;19(1):123–132. doi: 10.1007/s10198-017-0871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zettler M, Basch E, Nabhan C. Surrogate end points and patient-reported outcomes for novel oncology drugs approved between 2011 and 2017. JAMA Oncol. 2019;5(9):1358–1359. doi: 10.1001/jamaoncol.2019.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tse T, Williams RJ, Zarin DA. Update on registration of clinical trials in ClinicalTrials.gov. Chest. 2009;136(1):304–305. doi: 10.1378/chest.09-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karolinska Institute. Seraching for information: useful databases and websites. 2022. Available from: https://kib.ki.se/en/search-evaluate/searching-information/useful-databases-and-websites. [Accessed 17 Oct 2022].
- 68.Hilal T, Sonbol MB, Prasad V. Analysis of control arm quality in randomized clinical trials leading to anticancer drug approval by the US Food and Drug Administration. JAMA Oncol. 2019;5(6):887–892. doi: 10.1001/jamaoncol.2019.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16(1):495. doi: 10.1186/s13063-015-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grössmann N, Wolf S, Rothschedl E, Wild C. Twelve years of European cancer drug approval: a systematic investigation of the ‘magnitude of clinical benefit’. ESMO Open. 2021;6(3):100166. doi: 10.1016/j.esmoop.2021.100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bujosa A, Moltó C, Hwang TJ, Tapia JC, Vokinger KN, Templeton AJ, et al. Associations with definitive outcomes and clinical benefit of cancer drugs at the time of marketing approval and in the postmarketing period. J Natl Compr Canc Netw. 2021 doi: 10.6004/jnccn.2021.7003. [DOI] [PubMed] [Google Scholar]
- 72.Thomson S, Witzke N, Gyawali B, Delos Santos S, Udayakumar S, Cardone C, et al. Assessing the benefit of cancer drugs approved by the European Medicines Agency using the European Society for Medical Oncology Magnitude of Clinical Benefit Scale over time. Eur J Cancer. 2021;150:203–210. doi: 10.1016/j.ejca.2021.03.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.