Abstract
Introduction
Idiopathic rapid eye movement (REM) sleep behavior disorder (iRBD) is linked to Parkinson’s disease and other alpha-synucleinopathies, but various subsets of iRBD may not carry equal risk (i.e., those with depression are at higher risk than those without). Here, we prospectively focus on neurologic and psychiatric aspects of subjects with iRBD, in an attempt to determine what factors are prominent in those who undergo phenoconversion as opposed to those who do not.
Methods
We analyzed data from the “REM Sleep Behavior Disorder Associations with Parkinson’s Disease Study (RAPiDS)” cohort both at baseline and then at follow-up evaluations (1 to 3 years later) utilizing several neurologic batteries, including the Movement Disorder Society’s Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), the Montreal Cognitive Assessment (MoCA), the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP), the 10-M Walk Test (10MWT), and the Epworth Sleepiness Scale. Determination of phenoconversion was ascertained from physical examination and medical chart review from the initial evaluation onward.
Results
Of those who completed both evaluations, there were 33 subjects with iRBD, with an average age of 63.1 ± 12.8 years, with 9 women and 24 men. Of these, 8 (24%) iRBD subjects developed neurodegenerative illness, and demonstrated multiple areas of neurologic and psychiatric signs and symptoms, such as speech and movement problems as well as anxiety and depression.
Conclusions
Our data adds to the literature regarding risk of phenoconversion in those with iRBD. Further study will be needed, but it is clear that not all subjects with iRBD present the same risk for neurodegeneration.
Keywords: RBD, MDS-UPDRS, MoCA: QUIP, Epworth, Neurodegeneration, Phenoconversion, Parkinsonism
1. Introduction
Rapid eye movement (REM) sleep behavior disorder (RBD) consists of abnormally increased muscle tone during REM sleep (noted during polysomnogram [PSG] testing) combined with a history of recurrent nocturnal dream enactment behavior [1]. Idiopathic RBD (iRBD) occurs in the absence of conditions known to cause secondary RBD (i.e. autoimmune or inflammatory disorders), and when other causes of possible abnormal nocturnal behavior have been ruled out (i.e. nocturnal seizures) [2], [3]. It is well-known that the development of iRBD is linked to Parkinson’s disease (PD) and other alpha-synucleinopathies, but there seems to exist a differential risk across various subsets of iRBD (i.e. those with depression are at higher risk than those without) [4].
Previously, we had reported (Fig. 1.) on the psychiatric, autonomic, and sleep impact of iRBD in a cross-sectional fashion [5]; here, we prospectively evaluate (Table 1) neurologic and psychiatric aspects of patients with iRBD, in an attempt to determine what factors are prominent in those who undergo phenoconversion as opposed to those who do not. The aim is to add to the current literature, and to establish a more robust understanding of what signs and symptoms may be particularly important in both clinical and research settings.
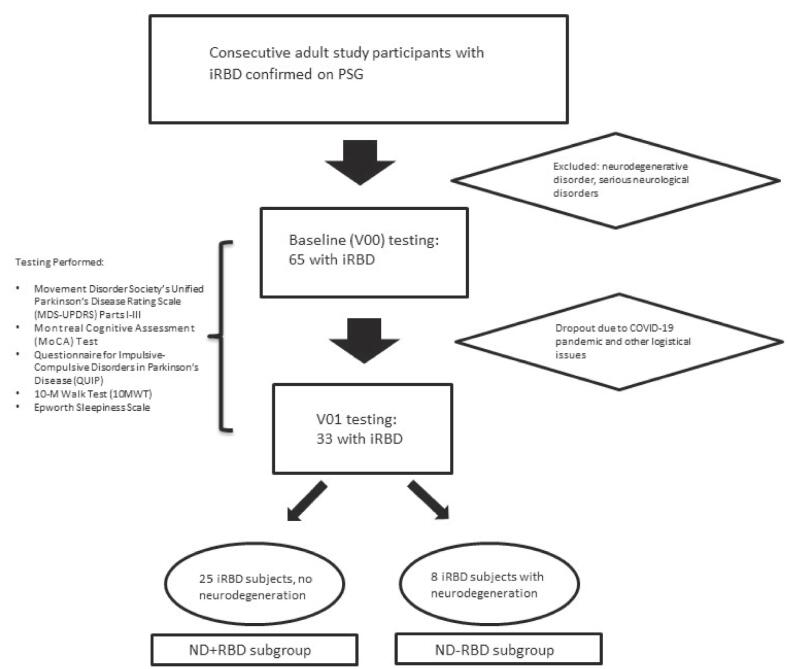
Fig. 1.
Flowchart of subjects analyzed. iRBD, Idiopathic REM behavior; ND, neurodegenerative disease; PSG, polysomnogram; V00, visit 00 (baseline); V01, follow-up visit 01.
Table 1.
Demographics.
| Subjects with iRBD Completing both Baseline and V01 Evaluations, n = 33 | ||
|---|---|---|
| ND + RBD, n = 8 | ND-RBD, n = 25 | |
| Avg age (years) | 72.0 ± 6.6 | 60.3 13.1 |
| Sex (# of women) | 0 | 9 |
| Antidepressant use (#) | 2 | 14 |
| Race | Hispanic (1), White (7), | Hispanic (1), Black (2), White (22) |
| Education | Master (6) or Bachelor (2) | Doctoral (1), Master (15), Bachelor (7), Some college (1), High school (1) |
| Caffeinated drinks per day (#) | 3.6 | 4.3 |
| Smoking status | former smoker (3), never (5) | former smoke (9), never (16) |
| Alcohol use | some (7), excessive (1) | excessive (2), some (20), none (3) |
| Development of PD and Other Neurodegenerative disease (all subjects were formally diagnosed within the 1 to 3 year follow up window of the study) | 1.Early PD with cognitive impairment or DLB -------------------------------------------------- 2. Multidomain MCI that may represent prodromal DLB -------------------------------------------------- 3. Amnestic MCI -------------------------------------------------- 4. PD -------------------------------------------------- 5. MCI (likely AD) -------------------------------------------------- 6. DLB -------------------------------------------------- 7. Strongly suggestive of a Parkinsonian syndrome -------------------------------------------------- 8. PD, MCI |
N/A |
iRBD, Idiopathic REM behavior; ND, neurodegenerative disease PD: Parkinson’s disease; DLB: dementia with Lewy bodies; MCI: mild cognitive impairment; AD: Alzheimer’s disease.
We analyzed data from the “REM Sleep Behavior Disorder Associations with Parkinson’s Disease Study (RAPiDS)” cohort (described previously [5]) both at baseline and then at follow-up evaluations utilizing multiple neurologic batteries. Our report complements the current literature by confirming the utility of said batteries, examining the usage of additional, inexpensive testing, and demonstrating the unique differences among subjects with iRBD.
2. Methods
The institutional review board at Weill Cornell Medical College approved this protocol, and informed consent was provided by all participants. Each author completed a Conflict-of-Interest Disclosure form. This study was funded through a private grant.
Consecutive adult study participants with iRBD confirmed on polysomnogram (PSG) according to American Academy of Sleep Medicine Criteria [6] were prospectively recruited from the Weill Cornell Center for Sleep Medicine. Individuals with an existing diagnosis of any neurodegenerative disorder were excluded. Those with other serious neurological disorders including stroke, epilepsy, and a history of brain tumor, hydrocephalus, encephalitis and other disorders were excluded. Additional exclusion criteria were existence of conditions that would confound autonomic and other neurological testing, such as cardiac disease, uncontrolled thyroid disease or diabetes, autoimmune conditions, and specific medications.
Following confirmation of iRBD and recruitment into the study, all participants were evaluated with the battery of assessments, both at baseline and then at follow-up visits (1 to 3 years). The assessments included the Movement Disorder Society’s Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [7], The Montreal Cognitive Assessment (MoCA) [8], the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) [9], the 10-M Walk Test (10MWT) [10], and the Epworth Sleepiness Scale [11] This battery was chosen as there is a precedent of their usage in the literature for subjects with RBD and/or PD. Although the MDS-UPDRS, MoCA, and Epworth Sleepiness Scale have been well-studied in those with iRBD, this is the first time, to our knowledge, that QUIP [9], [12] and 10MWT [13], [14] have been utilized in these subjects. Determination of phenoconversion to a neurodegenerative illness was ascertained through physical examination and medical chart review of subjects.
The Linear Mixed Effects Models [15] was used to obtain associations/trends going from baseline (V00) to V01 in any parameter. There are two outputs: coefficient value and p-value. The coefficient value implies the increase (Positive coefficient) or decrease (Negative coefficient) relationship from baseline to visit 1 (V01). The p-value roughly indicates whether the positive or negative correlation is statistically significant (p value less than 0.05) or not. During computing, the median value was used to fill in missing values. In addition, the p-value (T-test/ Mann–Whitney U test) was provided, which is used to test whether there is the difference between baseline and V01 in any parameter. The p-value (T-test/ Mann–Whitney U test) is obtained by t-test or Mann–Whitney U test that is nonparametric test. The Bonferroni correction was utilized in the ND + RBD versus ND-RBD group comparison regarding data from the MDS-UPDRS Parts I-III and QUIP.
Adjustment for age, sex, race, education, antidepressant use, caffeine, alcohol, and smoking usage was performed for each variable, Race included self-identification as Asian, Black, Hispanic, White, or Mixed; education was defined as either high school, some college, bachelor’s degree, Master’s degree, or Doctoral degree; antidepressant use was defined as positive or negative, regardless of whether the usage was current or former; smoking status included never, former, or current; and alcohol use was defined as either none, some (less than 3 drinks per day).or excessive (3 or more drinks per day).
3. Results
For baseline testing, 65 subjects with iRBD were recruited; of those who completed both baseline and V01 evaluations, 33 remained and were analyzed (see Figure). During the time frame of this study, various factors played into the large attrition rate, most notable being the COVID-19 pandemic and the resultant lack of subject’s ability and willingness to participate. The average age was 63.1 ± 12.8 years, with 9 women and 24 men. Based on physical examination and electronic medical record data, 8 iRBD subjects, which were all men, developed neurodegenerative illness, which included PD, parkinsonism, Lewy body dementia, and other forms of dementia and/or cognitive impairment; these were labeled the “ND + RBD” group (i.e., neurodegeneration + RBD). The 25 iRBD subjects who did not phenoconvert were labeled the “ND-RBD” group (see Table). The raw data and full results for the following sections can be found in Supplementary Data.
4. Neurologic factors
4.1. MDS-UPDRS part II
There were no significant differences between the groups at baseline. For the ND + RBD group there was a significant increase in speech problems over time (p = 0.003); however, following adjustment for age, sex, race, education, antidepressant use, caffeine, alcohol, and smoking usage, this change was not significant. Additionally, the ND + RBD group reported significantly more drooling at V01 as compared to the ND-RBD group (p = 0.013); following adjustment, this comparison remained significant (p = 0.027).
4.2. MDS-UPDRS part III
At baseline, there was more rigidity in the right upper extremity (p = 0.013), more difficulty with pronation-supination movement of the left hand (p = 0.05), and more problems with posture (p = 0.012) in the ND + RBD group as compared to the ND-RBD group. There were several significant differences in the ND + RBD group compared to the ND-RBD group both at V01 and over time. However, following adjustment, only finger tapping at baseline (right finger) was worse for ND + RBD group as compared to the ND-RBD group (p = 0.028).
4.3. MoCA
There were no significant differences between the groups at baseline. At V01, the scores in the ND + RBD group were significantly lower than ND-RBD group (p = 0.024). Following adjustment, this difference remained significant (p = 0.038). Additionally, the scores of ND + RBD group did not change over time, whereas the scores of the ND-RBD improved significantly (p = 0.002). This improvement remained significant following adjustment for age, sex, race, antidepressant use, caffeine, alcohol, and smoking usage.
4.4. Quip
There were no significant differences between the groups at baseline, nor were there any significant differences at V01.
4.5. 10MWT
There were no significant differences between the groups at baseline. The ND + RBD group was slower than the ND-RBD group, which was significant at V01. Following adjustment, this finding approached significance (p = 0.067).
4.6. Epworth Sleepiness Scale
There were no significant differences between the groups at baseline. At V01 the ND-RBD group was sleepier than the ND + RBD group, and this maintained following adjustment (p = 0.049). Additionally, the ND + RBD group became significantly less sleepy over time, which remained significant following adjustment.
5. Psychological factors
5.1. MDS-UPDRS part I
There were no significant differences between the groups at baseline, nor were there any significant differences at V01. There was a significant increase in both depressed mood and anxious mood over time in the ND + RBD group. And these changes remained significant (p = 0.018 for depressed mood, and p = 0.048 for anxious mood) following adjustment.
6. Discussion
Variables predicting phenoconversion to neurogenerative disease in those with iRBD have been previously studied and categorized [4]; our present analysis validates those findings and may allow for other potential signs and symptoms to become relevant in future work. Of import, the ND + RBD group was significant older than the ND-RBD group, which is a factor which cannot be overlooked and may play a major role in the data that follows. It has been shown that age predicts phenoconversion [4].
7. Neurologic factors
7.1. MDS-UPDRS part II
A recent meta-analysis demonstrated that dysphagia occurs in more than one-third of PD patients and was associated with decreased quality of life and more severe motor symptoms [16]. Additionally, it has been shown that PD patients with dysphagia were significantly more depressed than patients without dysphagia [17]. In our ND + RBD group, there was a significant increase in speech problems over time; and as compared to the ND-RBD group, there was a significant increase in drooling at V01. While not dysphagia per se, these symptoms point to a bulbar dysfunction that is in agreement with these prior data. Thus, perhaps screening for dysphagia and drooling in iRBD patients might be useful both clinically and in future studies.
7.2. MDS-UPDRS part III
It has been found that in those with PD and RBD, the MDS-UPDRS part III score progressed faster than in those with PD alone. [7] In our cohort, there were many significant differences in the ND + RBD group compared to the ND-RBD group. For example, it is known that motor functions are diminished in PD with probable RBD, particularly gait and pronation–supination movements [18], and our ND + RBD group demonstrated more difficulty with pronation/supination movement L hand at baseline as compared to the ND-RBD group.
Previous reports have revealed that falls and gait freezing were more frequent in PD with than without RBD. [19] Notably, were several instances of increased rigidity in our ND + RBD group as compared to the ND-RBD group, bolstering the hypothesis that increased rigidity in the context of iRBD is associated with risk of phenoconversion.
Other findings include postural changes, in which the ND + RBD group reported worse postural problems at both baseline and V01 as compared to the ND-RBD group. The worse posture in the ND + RBD group is not surprising, as those with RBD may exhibit unrefined sway, probably due to rigidity [20]; those with RBD may also demonstrate alternations in forward trunk acceleration [21], which also may affect posture.
While the above data is in agreement with prior work, following adjustment only finger tapping at baseline was worse for ND + RBD group as compared to the ND-RBD group. Literature has shown that finger tapping can be used to show differences between healthy controls and patients with PD and between medication states (on/off).[22] This is in alignment with ND + RBD group demonstrating a worse performance at baseline, but it is not clear why this effect did not persist upon re-evaluation at V01.
7.3. MoCA
Patients with PD plus RBD exhibit mild cognitive impairment mainly in the areas of visual space construction, executive function and word memory impairment [23]. Other studies have found that the decision-making ability and feedback learning ability of RBD patients are significantly reduced in a complex environment, and other cognitive areas are relatively late affected [24]. RBD is considered a risk factor for dementia in PD patients, but whether RBD predisposes nondemented PD patients to cognitive impairment remains unclear [25], with some studies suggesting greater susceptibility to severe cognitive impairment and other studies contradicting this view. [26], [27], [28] Surprisingly, in our cohort, both the ND + RBD and ND-RBD groups demonstrated increased MoCA scores over time; however, the values at V01 were significantly lower in the ND + RBD group than those in the ND-RBD group, reflecting more profound cognitive impairment.
7.4. Quip
Previously, a global screening instrument titled the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) was developed and validated to assess impulse control disorders (ICD) and related disorders (punding, hobbyism, and dopamine dysregulation syndrome) in PD [9]. Impulse control behaviors have been found to be common in the early stages of PD; however, iRBD, in the absence of PD or another neurodegenerative disorder, was not in itself associated with a greater risk of ICD. Although the mechanisms for ICD are not yet fully understood, the primary driver is thought be dopaminergic medication [12]; as this type of medication was not used in our cohort, the lack of symptoms of ICD is not unexpected.
7.5. 10MWt
The 10MWT is widely used and recommended as a measure of gait speed in those with PD, and it can be used to identify changes in gait speed in response to therapeutic interventions.[10] In another study of those with PD, it was found that increased level of RSWA (determined via PSG analysis) were correlated with worse gait impairment. [13] Prior work has demonstrated balance and gait having significant correlations with some cognitive features in parkinsonian patients [14]; and exercise is among the potential protective lifestyle factors identified in delaying progression of cognitive deficits [29]. Unsurprisingly, the ND + RBD group was slower than the ND-RBD group, which was significant at V01. It is known that in patients with PD, the 10MWT and similar tests will show impairment, but perhaps all iRBD patients should be tested in this regard, both for quality of life impact as well as monitoring for potential phenoconversion.
7.6. Epworth Sleepiness Scale
Prior work has demonstrated that those with iRBD and symptomatic RBD were sleepier than controls, and that sleepiness in iRBD predicted earlier conversion to Parkinson disease; this suggests that sleepiness, as measured by the Epworth Sleepiness Score, may be an early marker of neuronal loss in brainstem arousal systems. [30] Whereas the ND-RBD group actually became sleepier over time, the ND + RBD group became less sleepy. These results do not fit with prior work as above; thus, it is possible that fatigue, not sleepiness, is the main factor in both quality of life and risk for phenoconversion in those with iRBD. Future work should attempt to parcel out the differences between fatigue and sleepiness in those with iRBD.
8. Psychiatric factors
8.1. MDS-UPDRS part I
Phenoconversion to neurodegenerative disease includes the potential prodromal markers such as autonomic abnormalities, olfactory loss, cognitive changes, depression, and anxiety [31]; however the 2019 study by the International RBD Study Group did not find any predictive value in daytime somnolence, insomnia, restless legs syndrome, sleep apnea, urinary dysfunction, orthostatic symptoms, depression, or anxiety.[4] In our cohort, there was a significant increase in depressed mood and anxious mood over time in the ND + RBD group. A recent meta-analysis demonstrated that subjects with depression subjects had a 2.06-fold higher risk of developing RBD compared to those without depression [32], and a previous study has shown that depression may be associated with longer disease duration, more severe symptoms, and more severe illness in patients with PD and probable RBD. [33].
The most extensively studied neuropsychiatric prodromal symptoms of PD are depression and anxiety [34]; these are very common symptoms in the general population aged over 55 years of age as 14% suffers from depression [35]. In one meta-analysis, a diagnosis of depression at baseline was associated with an increased the risk of later developing PD [36].
In a recent study [34] almost 9% of subjects had signs of an anxiety disorder, corresponding to the prevalence reported by Fung et al [37]; similar to depression, anxiety disorders are also associated with the risk of developing PD [38]. Furthermore, it has been demonstrated in those with anxiety disorder an odds ratio of 2.2 for developing PD, of 1.9 for depression and even higher for the combination of both (odds ratio 2.4) [39]. While there are conflicting data in the literature, our study seems to point in favor of mood disorders as a major factor in phenoconversion in those with iRBD.
9. Limitations
There were some important limitations in our study, including pandemic-related lack of follow-up and possible bias in terms of which subjects presented for evaluation. Small sample size may have affected the impact of our findings, as did the relatively short period from baseline to follow-up, although the follow-up period of 1 to 3 years is typical for this type of project. Ideally, continued testing of the recruited subjects over a longer timeframe, as well as re-recruitment of those lost to follow-up would garner further important data; to this point, the ND + RBD group was significantly older than the ND-RBD group. Additionally, there was no control group, as even subjects in the ND-RBD group did have iRBD. Finally, subjects were enrolled from a single sleep center, presenting the possibility of referral bias, and the cohort is derived from an urban setting thus limiting its generalizability.
10. Conclusions
In summary, it is clear that iRBD is a risk for phenoconversion to neurodegenerative illness, but how and why this occurs is still a mystery. With our data, and those of other groups, it is hopeful that we will discover the answers to these questions. While the MDS-UPDRS, MoCA, and Epworth Sleepiness Scale have been studied in those with iRBD [4], this is the first time, to our knowledge, that QUIP and 10MWT have been utilized in these subjects. The findings of our cohort lend credence to the notion that subjects with iRBD are not all the same; the presence of additional signs and symptoms (i.e. “RBD+” as was previously described [5]) may aid in future efforts to determine which of these subjects might benefit the most from potential neuroprotection.
CRediT authorship contribution statement
Daniel A. Barone: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Harini Sarva: Data curation, Validation, Writing – review & editing. Natalie Hellmers: Data curation. Fei Wang: Formal analysis, Resources, Software. Zhenxing Wu: Formal analysis, Resources, Software. Ana C. Krieger: Validation, Writing – review & editing. Claire Henchcliffe: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prdoa.2023.100216.
Contributor Information
Daniel A. Barone, Email: dab9129@med.cornell.edu.
Harini Sarva, Email: has9059@med.cornell.edu.
Natalie Hellmers, Email: nah9011@med.cornell.edu.
Fei Wang, Email: few2001@med.cornell.edu.
Zhenxing Wu, Email: zhx2005@med.cornell.edu.
Ana C. Krieger, Email: ack2003@med.cornell.edu.
Claire Henchcliffe, Email: chenchcl@hs.uci.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Schenck C.H., Bundlie S.R., Ettinger M.G., Mahowald M.W. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9(2):293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 2.Iranzo A., Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28:203–206. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- 3.Peever J., Luppi P.-H., Montplaisir J. Breakdown in REM sleep circuitry underlies REM sleep behavior disorder. Trends Neurosci. 2014;37(5):279–288. doi: 10.1016/j.tins.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142:744-759. [DOI] [PMC free article] [PubMed]
- 5.Barone D.A., Wang F., Ravdin L., Vo M., Lee A., Sarva H., Hellmers N., Krieger A.C., Henchcliffe C. Comorbid neuropsychiatric and autonomic features in REM sleep behavior disorder. Clinical Parkinson. Related Disord. 2020;3 doi: 10.1016/j.prdoa.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Classification of Sleep Disorders (3rd ed.), American Academy of Sleep Medicine, Darien, IL (2014). [DOI] [PMC free article] [PubMed]
- 7.Barasa A., Wang J., Dewey R.B. Probable REM Sleep Behavior Disorder Is a Risk Factor for Symptom Progression in Parkinson Disease. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.651157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 9.Weintraub D., Hoops S., Shea J.A., Lyons K.E., Pahwa R., Driver-Dunckley E.D., Adler C.H., Potenza M.N., Miyasaki J., Siderowf A.D., Duda J.E., Hurtig H.I., Colcher A., Horn S.S., Stern M.B., Voon V. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson's disease. Mov. Disord. 2009;24(10):1461–1467. doi: 10.1002/mds.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloem B.R., Marinus J., Almeida Q., et al. Measurement instruments to assess posture, gait, and balance in Parkinson's disease: Critique and recommendations. Mov. Disord. 2016;31:1342–1355. doi: 10.1002/mds.26572. [DOI] [PubMed] [Google Scholar]
- 11.Johns M.W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 12.Baig F, Kelly MJ, Lawton MA, et al. Impulse control disorders in Parkinson disease and RBD: A longitudinal study of severity. Neurology. 2019;93:e675-e687. [DOI] [PMC free article] [PubMed]
- 13.Amundsen-Huffmaster S.L., Petrucci M.N., Linn-Evans M.E., Chung J.W., Howell M.J., Videnovic A., Tuite P.J., Cooper S.E., MacKinnon C.D. REM Sleep Without Atonia and Gait Impairment in People with Mild-to-Moderate Parkinson's Disease. J. Parkinsons Dis. 2021;11(2):767–778. doi: 10.3233/JPD-202098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varalta V, Picelli A, Fonte C, et al. Relationship between Cognitive Performance and Motor Dysfunction in Patients with Parkinson’s Disease: A Pilot Cross-Sectional Study. BioMed Res. Int.. 2015;2015:365959. [DOI] [PMC free article] [PubMed]
- 15.https://www.statsmodels.org/stable/mixed_linear.html.
- 16.Gong S., Gao Y., Liu J., Li J., Tang X., Ran Q., Tang R., Liao C. The prevalence and associated factors of dysphagia in Parkinson's disease: A systematic review and meta-analysis. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang E.J., Kim K.W., Lim J.-Y., Paik N.-J. Relationship between dysphagia and mild cognitive impairment in a community-based elderly cohort: the Korean longitudinal study on health and aging. J. Am. Geriatr. Soc. 2014;62(1):40–46. doi: 10.1111/jgs.12606. [DOI] [PubMed] [Google Scholar]
- 18.Iijima M, Okuma Y, Suzuki K, et al. Associations between probable REM sleep behavior disorder, olfactory disturbance, and clinical symptoms in Parkinson's disease: A multicenter cross-sectional study. PLoS One. 2021;16:e0247443. [DOI] [PMC free article] [PubMed]
- 19.Bargiotas P., Ntafouli M., Lachenmayer M.L., Krack P., Schüpbach W.M.M., Bassetti C.L.A. Apathy in Parkinson's disease with REM sleep behavior disorder. J. Neurol. Sci. 2019;399:194–198. doi: 10.1016/j.jns.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 20.Chen T.Z., Xu G.J., Zhou G.A., Wang J.R., Chan P., Du Y.F. Postural sway in idiopathic rapid eye movement sleep behavior disorder: a potential marker of prodromal Parkinson's disease. Brain Res. 2014;1559:26–32. doi: 10.1016/j.brainres.2014.02.040. [DOI] [PubMed] [Google Scholar]
- 21.Nobleza C.M.N., Siddiqui M., Shah P.V., Balani P., Lopez A.R., Khan S. The Relationship of Rapid Eye Movement Sleep Behavior Disorder and Freezing of Gait in Parkinson's Disease. Cureus. 2020;12:e12385. doi: 10.7759/cureus.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thijssen E., Makai‐Bölöni S., van Brummelen E., den Heijer J., Yavuz Y., Doll R.-J., Groeneveld G.J. A Placebo-Controlled Study to Assess the Sensitivity of Finger Tapping to Medication Effects in Parkinson's Disease. Mov Disord Clin Pract. 2022;9(8):1074–1084. doi: 10.1002/mdc3.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong-Wen W, Sha-Sha L, Xu E. Predictors of rapid eye movement sleep behavior disorder in patients with Parkinson's disease based on random forest and decision tree. PLoS One. 2022;17:e0269392. [DOI] [PMC free article] [PubMed]
- 24.Delazer M, Högl B, Zamarian L, et al. Decision making and executive functions in REM sleep behavior disorder. Sleep. 2012;35:667-673. [DOI] [PMC free article] [PubMed]
- 25.Yan Y.-Y., Lei K.e., Li Y.-Y., Liu X.-F., Chang Y. The correlation between possible RBD and cognitive function in Parkinson's disease patients in China. Ann. Clin. Transl. Neurol. 2019;6(5):848–853. doi: 10.1002/acn3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plomhause L., Dujardin K., Duhamel A., Delliaux M., Derambure P., Defebvre L., Monaca Charley C. Rapid eye movement sleep behavior disorder in treatment-naïve Parkinson disease patients. Sleep Med. 2013;14(10):1035–1037. doi: 10.1016/j.sleep.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Bugalho P., Silva J.A., Neto B. Clinical features associated with REM sleep behavior disorder symptoms in the early stages of Parkinson's disease. J. Neurol. 2011;258(1):50–55. doi: 10.1007/s00415-010-5679-0. [DOI] [PubMed] [Google Scholar]
- 28.Lavault S., Leu-Semenescu S., Tezenas du Montcel S., Cochen de Cock V., Vidailhet M., Arnulf I. Does clinical rapid eye movement behavior disorder predict worse outcomes in Parkinson's disease? J. Neurol. 2010;257(7):1154–1159. doi: 10.1007/s00415-010-5482-y. [DOI] [PubMed] [Google Scholar]
- 29.Lautenschlager N.T., Cox K., Kurz A.F. Physical activity and mild cognitive impairment and Alzheimer's disease. Curr. Neurol. Neurosci. Rep. 2010;10(5):352–358. doi: 10.1007/s11910-010-0121-7. [DOI] [PubMed] [Google Scholar]
- 30.Arnulf I, Neutel D, Herlin B, et al. Sleepiness in Idiopathic REM Sleep Behavior Disorder and Parkinson Disease. Sleep. 2015;38:1529-1535. [DOI] [PMC free article] [PubMed]
- 31.Goldman J.G., Postuma R. Premotor and nonmotor features of Parkinson's disease. Curr. Opin. Neurol. 2014;27:434–441. doi: 10.1097/WCO.0000000000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Z., Wu J., Xie C., Wang L., Li H., Zhang M., Fu Z., Lin Y., Qian B., Zhu L., Yu X., He J., Qi W., Wang H. Risk factors for rapid eye-movement sleep-related behavioral disorders (RBDs): A systematic review and a meta-analysis. Gen. Hosp. Psychiatry. 2022;79:118–127. doi: 10.1016/j.genhosppsych.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Long K., Wan C., Xiang Y., Liu J., Xu Q., Sun Q., Wang Z., Tian Y., Fang L., Yang Y., Yan X., Tang B., Guo J. Study on the Clinical Features of Parkinson's Disease With Probable Rapid Eye Movement Sleep Behavior Disorder. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roos D.S., Klein M., Deeg D.J.H., Doty R.L., Berendse H.W. Prevalence of Prodromal Symptoms of Parkinson's Disease in the Late Middle-Aged Population. J. Parkinsons Dis. 2022;12(3):967–974. doi: 10.3233/JPD-213007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kok R.M., Reynolds C.F., 3rd. Management of Depression in Older Adults: A Review. J. Am. Med. Assoc. 2017;317:2114–2122. doi: 10.1001/jama.2017.5706. [DOI] [PubMed] [Google Scholar]
- 36.Wang S., Mao S., Xiang D., Fang C. Association between depression and the subsequent risk of Parkinson's disease: A meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;86:186–192. doi: 10.1016/j.pnpbp.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Fung A.W.T., Chan W.-C., Wong C.-M., Chen E.-H., Ng R.-K., Lee E.-M., Chang W.-C., Hung S.-F., Cheung E.-C., Sham P.-C., Chiu H.-K., Lam M., Chiang T.-P., van Os J., Lau J.-F., Lewis G., Bebbington P., Lam L.C.W. Prevalence of anxiety disorders in community dwelling older adults in Hong Kong. Int. Psychogeriatr. 2017;29(2):259–267. doi: 10.1017/S1041610216001617. [DOI] [PubMed] [Google Scholar]
- 38.Schrag A., Horsfall L., Walters K., Noyce A., Petersen I. Prediagnostic presentations of Parkinson's disease in primary care: a case-control study. Lancet Neurol. 2015;14(1):57–64. doi: 10.1016/S1474-4422(14)70287-X. [DOI] [PubMed] [Google Scholar]
- 39.Shiba M., Bower J.H., Maraganore D.M., McDonnell S.K., Peterson B.J., Ahlskog J.E., Schaid D.J., Rocca W.A. Anxiety disorders and depressive disorders preceding Parkinson's disease: a case-control study. Mov. Disord. 2000;15(4):669–677. doi: 10.1002/1531-8257(200007)15:4<669::aid-mds1011>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.