Summary
Drug-target interactions (DTIs) prediction is an important step in drug discovery. As traditional biological experiments or high-throughput screening are high cost and time-consuming, many deep learning models have been developed. Overfitting must be avoided when training deep learning models. We propose a simple framework, called OverfitDTI, for DTI prediction. In OverfitDTI, a deep neural network (DNN) model is overfit to sufficiently learn the features of the chemical space of drugs and the biological space of targets. The weights of trained DNN model form an implicit representation of the nonlinear relationship between drugs and targets. Performance of OverfitDTI on three public datasets showed that the overfit DNN models fit the nonlinear relationship with high accuracy. We identified fifteen compounds that interacted with TEK, a receptor tyrosine kinase contributing to vascular homeostasis, and the predicted AT9283 and dorsomorphin were experimentally demonstrated as inhibitors of TEK in human umbilical vein endothelial cells (HUVECs).
Subject areas: Biological sciences, Biochemistry, Structural biology, Mathematical biosciences
Graphical abstract

Highlights
-
•
A simple framework called OverfitDTI was proposed for DTIs prediction
-
•
Overfit model fit nonlinear relationship between drug and target with high accuracy
-
•
We turned a limitation of deep neural network into a beneficial feature
-
•
AT9283 and dorsomorphin were predicted and validated as inhibitors of TEK in HUVECs
Biological sciences; Biochemistry; Structural biology; Mathematical biosciences
Introduction
Predicting drug-target interactions (DTIs, also referred to as compound-protein interactions) is the early step in drug discovery pipeline, and corresponding methods have been studied and developed for many years.1,2,3,4,5 Researchers generally use in vitro or in vivo experiments to identify DTIs at an early stage of research, and high-throughput screening is then performed in a later stage to develop new drugs.6,7,8 Although great progress has been made, these methods suffer in practice from high cost and long delays. To alleviate these concerns, the computational (in silico) prediction of DTIs has been developed in recent years. Methods of in silico DTI prediction generally fall into two categories: docking simulations and machine learning methods.9 Because machine learning does not require knowledge of the three-dimensional structure of a protein, and due to the rapid development of computational chemistry, many studies using machine learning, especially chemogenomics methods, such as TransformerCPI,10 DeepDTA,11 GraphDTA,12 and graph neural network (GNN13), have reported high accuracy.
The interaction between drugs and targets in a DTI dataset is a complex nonlinear relationship that involves many factors.9 When predicting DTIs using the chemogenomics approach, the representations (features) of drugs and targets are first learned by a feature learning method (encoder), and the concatenated features are then input into a downstream classifier to predict binding scores.14 Because of the complexity of the drugs (compounds) and targets space, no methods can comprehensively mine the nonlinear relationship between drugs and targets. Therefore, various methods attempt to learn the representations of the dataset to find a nonlinear mapping from these encoded representations to binding scores that performs satisfactorily on this dataset.11,13,15,16,17,18,19
A well-known feature of neural network is that it can effectively fit any nonlinear relationship with high accuracy.20 Several studies21,22,23 focus on the powerful nonlinear fitting ability of neural network recently, and these researches demonstrate that a purposefully overfit deep neural network (DNN) can serve as a solid shape representation for a variety of tasks in computer graphics. Using sampled points of an object’s signed distance fields (SDFs), the authors23 trained a DNN model to predict the signed distances of each input point. The relationship between sampled points of the SDFs induced by a geometric dataset is a complex nonlinear relationship, and the interaction between drugs and targets is also a complex nonlinear relationship. When predicting the signed distances, the neural implicit representing outputs a number indicating whether the given query point is inside, outside, or on a surface, which is similar to the process of DTI prediction (predict a value to determine whether the drug and target interact). The feature learning methods used in chemogenomics methods are various types of DNN (e.g., convolutional neural network [CNN], GNN). When predicting DTI using these state-of-the-art (SOTA) DNN-based methods, it is common to divide the dataset into training set, validation set, and test set. The test set is used to test the model’s performance and to avoid overfitting. After splitting the dataset, not all data are used to train the models; i.e., not all data are used to adequately fit the nonlinear relationship between drugs and targets. Besides, overfitting must be avoided when training these models, but overfitting is not all bad.
Inspired by these studies,21,22,23 here we focus on the nonlinear fitting ability of DNN in representing a DTI dataset. We propose a simple yet effective framework for DTI prediction called OverfitDTI and thus identify potential drugs from DTI datasets. In OverfitDTI, all data are used to train a DNN model. Once overfit, the DNN model “memorizes” the features of the dataset and can reconstruct the dataset. For the unseen drugs and targets (without labels, i.e., binding affinities between drugs and target are not given), in order to use all data for overfitting training, a variational autoencoder (VAE)24 model was used to obtain the features of unseen data. VAE is an unsupervised learning method that can be used to reconstruct datasets to obtain the features and generate new data; here we use its reconstruct function. We tested OverfitDTI on three benchmark datasets, and the results showed that OverfitDTI reconstructed these datasets well and could be used for DTI prediction. OverfitDTI outperformed SOTA baselines and improved the generalization ability of the model even in the scenario of the cold start.
TEK, a receptor tyrosine kinase, plays critical roles in vascular development and angiogenesis.25 Activation of TEK by angiopoietin-1 (Ang1) stimulates a number of downstream effectors, notably Akt, which promotes the survival, migration, and tube formation of endothelial cells.26,27 Ang/TEK signaling is also involved in pathological angiogenesis, which occurs under ischemia, and in diabetic retinopathy, venous malformations, inflammation, and cancer development.28,29 Many strategies, including genetic model, soluble receptor, and small interfering RNA (siRNA) that interfere with TEK, have been used to study the role of Ang/TEK signaling in physiological and pathological angiogenesis.25,30,31 Nevertheless, the limited small-molecule inhibitors of TEK hamper further study on its role in disease pathophysiology. Given these facts, we took the screening of potential drugs for TEK as a case study and identified several compounds that interacted with TEK in three DTI datasets. Furthermore, two predicted compounds (i.e., AT9283 and dorsomorphin) were experimentally demonstrated as inhibitors of TEK in human umbilical vein endothelial cells (HUVECs). All these results indicate that OverfitDTI is a practically useful tool to predict DTIs.
Results
Overview of OverfitDTI
As shown in Figure 1, OverfitDTI architecture consists of two components: supervised learning and unsupervised learning. For the drugs and targets in DTI dataset, the chemical space of the drugs and the biological space of the targets are combined to form an integrated space; then the features of drugs and targets are learned by drug and target encoders (DNN), respectively. Next, the concatenated features are fed into DNN (a feedforward neural network, FNN) to learn the implicit representation of the nonlinear relationship. All data are used to overfit the DNN model. Once the DNN model is overfit, the implicit representation function can be used to predict the binding scores. When OverfitDTI is used for unseen drugs and targets, all data are first fed into the VAE model for unsupervised training to obtain the latent features of the data; then these latent features are concatenated with the features obtained from the aforementioned encoders and sent to DNN for overfitting training. Different encoders can learn different features of the drugs and targets, and different combinations of encoders form different models; the DTIs predicted by them may also differ. It is difficult to determine which predictions should be selected in this situation. To cope with the issue, we trained multiple DNN models to predict DTIs, and the DTIs that can be predicted by all DNN models are selected as the potential predictions.
Figure 1.
Schematic of OverfitDTI
(A) OverfitDTI first performs features learning for drugs and targets; the learned features of drugs Xdrug and targets Ytarget are concatenated and form the features of the integrated space. Then the concatenated features are fed into DNN to perform the overfitting training. After the model is overfit, the implicit representation f is obtained, which can be used to approximate the nonlinear relationship Z between drugs and targets. For a drug Xi and a target Yi in this dataset, the nonlinear relationship between them Zi can be calculated using the implicit representation f, which can then be used to predict the binding scores and determine whether Xi and Yi interact.
(B) If Xi and Yi are not in this dataset, a VAE model is used to obtain the features of these unseen data.
Recently, various types of encoders of drug and protein are developed; we select several representative encoders to evaluate our method, and these encoders form eight different models, including Morgan-CNN, Message Passing Neural Network (MPNN)32-CNN, Daylight-AAC, CNN-Transforme,33 CNN-CNN (DeepDTA11), GNN-CNN(GNN13), Graph Convolutional Network (GCN)-CNN (GraphDTA12), and NeuralFP34-CNN, where the former is drug encoder and the latter is protein encoder.35 More details of the OverfitDTI pipeline, all the parameters of these DNN models, the VAE models, and the training procedure can be found in STAR methods and Tables S1–S12. The selection of batch size depended on the memory size of the used graphics processing unit (GPU) (NVidia RTX 3090, 24GB), and we found that the model performance was not sensitive to the batch size.
Performance of OverfitDTI on DTI dataset in two scenarios
OverfitDTI is evaluated on three DTI datasets in the scenario of warm start and cold start. The scenario of the warm start is relatively simple as the data from the test set are present in the training set, and we focus on the model’s ability to fit the dataset rather than its generalization ability. The cold start scenario is more realistic and can evaluate the model’s ability of generalizing to unseen data. Performance of OverfitDTI under these two scenarios is analyzed in detail as follows.
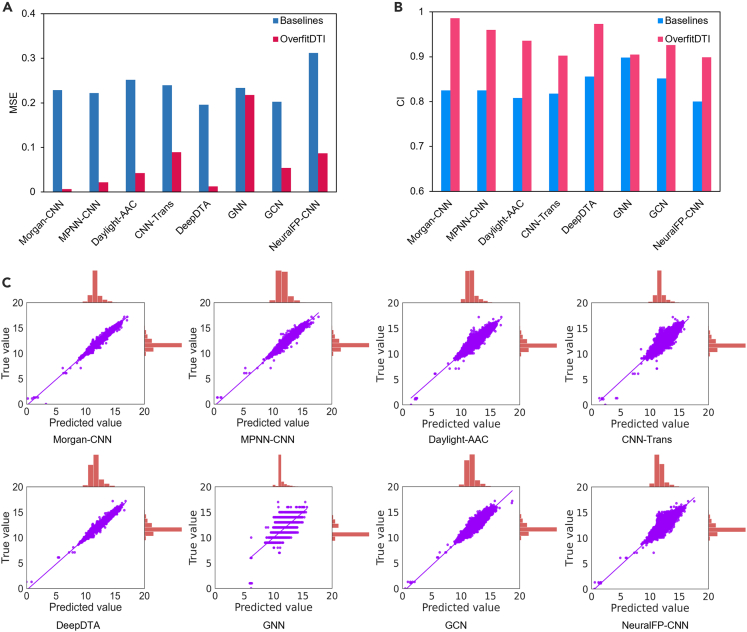
We first evaluated the performance of OverfitDTI on the Kinase Inhibitor BioActivity (KIBA) dataset (see STAR methods) in the scenario of warm start. We used the same training/validation/testing data splits as in DeepPurpose35 (i.e., 70% used for training, 10% for validation, and 10% for testing) to train the eight aforementioned models and used them as benchmarks to compare with OverfitDTI. It was predictable that OverfitDTI outperformed the baselines as we used all the data to overfit the DNN models while only part of the data were used in the baselines. As shown in Figure 2, OverfitDTI significantly outperformed all baselines across the mean square error (MSE) and concordance index36 (CI) metrics, confirming its powerful performance. Morgan-CNN achieved the most obvious performance improvement (MSE dropped by about two orders of magnitude) while GNN had a small performance improvement (0.77% in terms of MSE). The low MSE values of the eight DNN models in OverfitDTI indicated that these DNN models overfit the KIBA dataset well; that is, these DNN models had learned the nonlinear relationship between drugs and targets and could reconstruct the feature space of this dataset. The high CI values of DNN models in OverfitDTI indicated that the prediction accuracy of these models on the KIBA dataset was high; that is, these DNN models could be used to predict DTIs within the KIBA dataset. The predicted binding scores were highly correlated with the true values with Pearson correlation37 (PC) values ranging from 0.8161 to 0.9954, indicating the reliable predictions of OverfitDTI (Figure 2). It should be noted that for the GNN model, because we used the data processing method as in the original paper, we deleted part of the data that did not conform to the format used, which made the label a discrete value rather than a continuous value.
Figure 2.
Comparison results of OverfitDTI and baselines on the KIBA dataset in the scenario of warm start
(A–C) The predictive performance were evaluated with MSE (A) and CI (B) values. (C) Scatterplots of binding scores between the predicted values and the true values. OverfitDTI were trained with all data while the baselines were trained with 70% of all data.
We also conducted experiments with two other datasets (the Drug Target Commons [DTC] and BindingDB datasets, see STAR methods). For the DTC dataset, except for the GNN model, the MSE values of DNN models in OverfitDTI were not greater than 0.0844, and the CI values were not less than 0.9480 (Figure S1). The baselines had relative lower performance with MSE ranging from 0.1594 to 0.5075 and CI ranging from 0.8808 to 0.9263 (Figure S1). The predicted binding scores of DNN models in OverfitDTI (Figure S1) were more correlated with the true values than the baselines, which was consistent with the results shown in Figure 2. The performance of the GNN model was significantly worse than that of other models, which might be due to the fact that the model was unsuitable for this dataset. As for the BindingDB dataset, the MSE values of most of the models (Figure S2) in OverfitDTI were greater than those in Figures 2 and S1. This is probably due to the fact that the BindingDB dataset contained more noises than other two datasets, which can influence the fitting effect of the nonlinear relationship and lead to the increase of MSE values. The CI values of models in OverfitDTI (Figure S2) were less than values in Figure 2 and Figure S1, but the difference was small, indicating that the prediction accuracy of these models on the BindingDB dataset was also high. The PC values were greater than 0.8466, indicating the high-quality prediction for the BindingDB dataset (Figure S2).
In the scenario of the cold start, we considered the following two scenarios: the cold start for drugs and the cold start for both drugs and proteins. We evaluated the performance of OverfitDTI on the DTC and BindingDB datasets (see STAR methods). We first trained OverfitDTI on the BindingDB dataset and tested on the DTC dataset, and then we trained OverfitDTI on the DTC dataset and tested on the BindingDB dataset. All models in OverfitDTI and baselines were same as models in the warm start. Since we want to maximize the amount of training data and there was no need for the test dataset, we used a 90%/10% training/validation random split for the baselines.
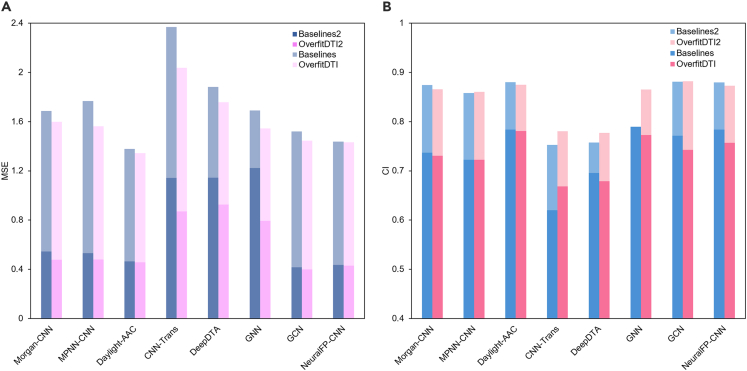
When the training set is BindingDB and the drugs in test dataset were not observed (unseen), the experimental results are shown in Figure 3. The comparative results showed that our approach performed well across the MSE metric and achieved competitive performance across the CI metric. GCN model in OverfitDTI performed best in terms of MSE and CI. Compared to the scenario of the warm start, the performance of baselines and OverfitDTI dropped with different degrees, indicating that the generalization ability of these models got worse. This phenomenon was in line with our estimation as the drug-related information was missing. However, due to the fact that the OverfitDTI contained latent features produced by VAE models, partial features of the DTC dataset (test dataset) were used during the overfitting training, thus achieving relatively higher predictive performance. The improvements of generalization ability of different models in OverfitDTI were different, with the drop of MSE ranging from 1.19% (NeuralFP-CNN) to 35.09% (GCN). The latent space of drugs and proteins generated by VAE models was shown in Figures 3C and 3D. The feature space of drugs from the BindingDB dataset had some overlap with the feature space of drugs from the DTC dataset, which allowed the training set to acquire partial features of the test set, thus improving the generalization ability of models. Additionally, we designed the DTI prediction as a regression problem and avoided the creation of negative samples, which might also contribute to the improvement of model performance. As for the scenario of the cold start for both drugs and proteins, all the models in OverfitDTI and baselines dropped significantly in all metrics compared to the scenario of the cold start for drugs, suggesting that the protein-related information was important for the DTI prediction (Figure 4). In comparison between OverfitDTI and baselines, benefitting from the latent features derived from VAE models, OverfitDTI achieved better performance in terms of MSE. As for the CI metric, OverfitDTI got close or higher CI values, indicating that the information extracted by VAE models was effective for DTI prediction in the scenario of the cold start.
Figure 3.
Comparison results of OverfitDTI and baselines on the BindingDB dataset in the scenario of cold start for drugs
(A and B) Comparison of MSE (A) and CI (B) across different methods that were trained on the BindingDB dataset and tested on the DTC dataset.
(C) The latent space of drugs for VAE, where X and Y are the first and second axis in the space, respectively.
(D) The latent space of proteins for VAE, where X and Y are the first and second axis in the space, respectively.
Figure 4.
Comparison results of OverfitDTI and baselines on the BindingDB dataset in the scenario of cold start for both drugs and proteins
(A and B) Two metrics are reported: MSE (A) and CI (B). Results under the scenario of cold start for drugs are added for comparison. Baselines and OverfitDTI indicate that the results are from cold start for both drugs and proteins while Baselines2 and OverfitDTI2 indicate that the results are from cold start for drugs.
The evaluation performance under two scenarios behaved slightly differently when the training set was the DTC dataset (Figure S3). Morgan-CNN model in OverfitDTI performed best in terms of MSE and CI in the scenario of the cold start for drugs while NeuralFP-CNN in OverfitDTI performed best in terms of MSE in the scenario of the cold start for both drugs and proteins. The overall performance of baselines and OverfitDTI was worse than that when the training set was the BindingDB dataset, which was probably due to the fact that the BindingDB dataset contained more unique number of drugs and targets. The performance of DNN model behaved differently on these two datasets under two scenarios, indicating that the features learned by various encoders in different scenarios were different and the performance improvement methods such as overfitting training played a different role. In the two scenarios, all models in OverfitDTI consistently outperformed the baselines across the MSE metrics, indicating that OverfitDTI had better generalization ability than baselines. Taken together, these results on three datasets under two scenarios demonstrated that OverfitDTI could reconstruct the dataset well and served as a useful DTI prediction tool.
Screening of potential drugs using overfitDTI
In this section, we describe the application of OverfitDTI in screening potential drugs for TEK receptor tyrosine kinase. Drugs that interact with TEK are present in these three datasets, so this is the warm start scenario. Here we overfit ten models to screen drugs (Tables S4–S6). We first screened potential drugs in the KIBA dataset. Following the pipeline described in the STAR methods section, we completed the screen and obtained 27 compounds that interacted with TEK (Table1).
Table 1.
Compounds that were predicted to interact with TEK in the KIBA dataset
| CHEMBL ID or PubChem CID | Synonym/Chemical Name | Molecular Formula | Reference |
|---|---|---|---|
| CHEMBL520144 | 4-Hydroxyalternariol 9-methyl ether | C15H12O6 | a; Aly et al.38 |
| CHEMBL1784637 | Compound 8h | C30H33F3N6O3 | a; Thomas et al.39 |
| CHEMBL463054 | Macrosporin | C16H12O5 | a; Debbab et al.40 |
| CHEMBL483526 | Alternariol Monomethyl ether | C15H12O5 | a; Aly et al.38 |
| CHEMBL554993 | Alterporriol G/H | C32H26O13 | a; Debbab et al.40 |
| CHEMBL483532 | Desmethylaltenusin | C14H12O6 | a; Aly et al.38 |
| CHEMBL556684 | 6-O-methylalaternin | C16H12O6 | a; Debbab et al.40 |
| CHEMBL483525 | Alternariol 5-O-sulfate | C14H10O8S | a; Aly et al.38 |
| CHEMBL512054 | Altersolanol A | C16H16O8 | a; Debbab et al.40 |
| CHEMBL519982 | Alternariol | C14H10O5 | a; Aly et al.38 |
| CHEMBL1684800 | Unavailable | C27H24ClF3N6O3 | Unavailable |
| CHEMBL521470 | Altertoxin I | C20H16O6 | a; Aly et al.38 |
| CHEMBL483531 | Alutenusin | C15H14O6 | a; Aly et al.38 |
| CHEMBL507058 | Alterlactone | C15H12O6 | a; Aly et al.38 |
| CHEMBL519395 | Alternarienonic acid | C14H14O6 | a; Aly et al.38 |
| CHEMBL495727 | AT9283 | C19H23N7O2 | Unavailable |
| CHEMBL408995 | Talaroflavone | C14H12O6 | a |
| CHEMBL453737 | AMG-51 | C34H33F2N5O5 | a |
| CHEMBL2010872 | Cep-11981 | C28H27N7O | a; Hudkins et al.41 |
| CHEMBL1967116 | Kinome_478 | C25H22N6OS | Unavailable |
| CHEMBL262433 | Kinome_541 | C19H15FN6OS | a; Ji et al.42 |
| CHEMBL381207 | Thienopyrimidine deriv. 28 | C21H15F4N5OS | a; Cao et al.43 |
| CHEMBL478629 | Dorsomorphin | C24H25N5O | Unavailable |
| CHEMBL2151321 | Compound 2c | C19H20FN7O | Unavailable |
| CHEMBL373882 | 2-MT-63 | C29H30F4N8O2 | a; Hodous et al.44 |
| CHEMBL246356 | Unavailable | C25H20F4N6O3 | a; Hodous et al.45 |
| 90954131 | Unavailable | C27H26F4N6O | a |
Indicates that the drug-TEK interaction is included in the KIBA dataset. We also conduct a literature search to find evidence on the predicted compounds.
As shown in Table 1, 22 compounds had been described in the KIBA dataset that could interact with TEK. As for the remaining five predicted compounds (Figure S4; Table S13), information in the KIBA dataset did not indicate whether they interacted with TEK, and no studies about the interactions between these compounds and TEK were found in the literature, namely, new compounds that interacted with TEK. Among these five compounds, AT9283 (a multitarget kinase inhibitor) shows therapeutic potential in hematological malignancies and various solid tumors,46,47 and dorsomorphin (also termed compound C) is a small-molecule inhibitor of adenosine monophosphate-activated protein kinase (AMPK) that has been widely used to explore the function of AMPK,48 while the studies about other three compounds have been rarely reported.
Three compounds in the DTC dataset and seven in the BindingDB dataset were predicted to interact with TEK (Tables 2, 3, S14, and S15; Figures S5 and S6). At present, these compounds were not described in the DTC and BindingDB dataset whether they interacted with TEK, and their effects on TEK had not been discussed in the published literature. The new compounds predicted to interact with TEK in the DTC and BindingDB datasets were different from those in the KIBA dataset. This was mainly because the contents of the three datasets were different, and therefore the compounds that were predicted to interact with TEK also differed. The KIBA dataset was constructed specifically for the study of kinase-targeting compounds, and all of the targets in the dataset were related to kinases; however, the targets in the other two datasets had various functions and were not all kinases, and therefore the corresponding compounds were not all concerned with kinases and were different from those in KIBA dataset. In view of availability in the market, AT9283 and dorsomorphin were selected to validate their interactions with TEK in the next sections.
Table 2.
Compounds that were predicted to interact with TEK in the DTC dataset
| CHEMBL ID | Synonym | Molecular Formula |
|---|---|---|
| CHEMBL2443026 | Unavailable | C31H38N4O7 |
| CHEMBL3696218 | Unavailable | C34H44N4O7 |
| CHEMBL3696220 | Unavailable | C32H40N4O7 |
Table 3.
Compounds that were predicted to interact with TEK in the BindingDB dataset
| CHEMBL ID/BINDINGDB ID | Synonym | Molecular Formula |
|---|---|---|
| CHEMBL3982108 | Unavailable | C27H30F4N6O2 |
| BDBM205432 | (6S+2S)-PEG2 | C45H46Cl2N10O6S2 |
| BDBM205433 | (6S+2S)-PEG3 | C47H50Cl2N10O7S2 |
| BDBM205434 | (6S+2S)-PEG4 | C49H54Cl2N10O8S2 |
| BDBM205435 | (6S+2S)-PEG7 | C55H66Cl2N10O11S2 |
| BDBM10884 | dorzolamide | C10H16N2O4S3 |
| CHEMBL4108876 | Unavailable | C28H33N7O |
Molecular docking suggests the binding modes
In this section, we carried out molecular docking to explore the possible binding modes of two compounds (i.e., AT9283 and dorsomorphin) with TEK. The docking results showed that both AT9283 and dorsomorphin docked to the structure of TEK (PDB ID: 6mwe)49 and displayed high affinity (Figure 5). When binding to TEK, AT9283 formed hydrogen bonds with both residues A905 and P906, and the main interaction was hydrogen bond; dorsomorphin formed hydrogen bond with residue E872, and the main interaction was hydrophobic interaction. In addition, there existed water bridges between AT9283 and TEK, while there existed salt bridge between dorsomorphin and TEK. When binding to TEK, AT9283 and dorsomorphin formed different hydrogen bond network in the binding pocket, and the key residues involved in the interaction and main types of interactions were different. The results of docking analysis may help uncover the molecular mechanisms of the action of these two compounds.
Figure 5.
Molecular docking results between two compounds (i.e., AT9283, dorsomorphin) and TEK
(A and B) docked pose for AT9283; (B) docked pose for dorsomorphin. TEK is displayed in gray color. AT9283 is represented by sticks and colored in cyan, and dorsomorphin is represented by sticks and colored in yellow. The residues of the pocket that interact with these two compounds are colored in magentas and red in (A) and (B), respectively. Hydrogen bonds are represented by the blue dashed lines. Hydrophobic interactions are represented by the gray dashed lines. Water bridges are represented by the orange dashed lines. Salt bridge is represented by the yellow dashed line.
In vitro validation of predicted potential drugs
We tested the inhibitory activities of AT9283 and dorsomorphin against the kinase TEK to see whether the predicted interactions between these two compounds and TEK could be further validated. Ang1 binds to and induces TEK autophosphorylation, leading to the activation of downstream pathways.50 Hence, we used Ang1 as a chemoattractant for endothelial cells in this study. First, the inhibitory activities of these two compounds against human TEK activity were assessed using an enzyme-linked immunosorbent assay (ELISA) kit in HUVECs, which express high levels of TEK.51 AT9283 and dorsomorphin inhibited Ang1-stimulated TEK kinase activity with half-maximal inhibitory concentration (IC50) values of 0.591 μM and 4.719 μM, respectively (Figures 6A and 6B). Akt is a major downstream target of the Ang1/TEK signaling pathway. Upon TEK stimulation, Akt is phosphorylated, and phosphorylated Akt (p-Akt) acts as an important mediator of endothelial cell motility and sprouting. We then tested the effects of AT9283 and dorsomorphin on the phosphorylation of TEK and its downstream effector protein Akt by western blotting. As shown in Figures 6C–6H, both AT9283 and dorsomorphin decreased the protein levels of Ang1-induced p-TEK and p-Akt in a concentration-dependent manner. In addition, the inhibitory effect of AT9283 and dorsomorphin was statistically significant at 0.5 μM and 5 μM, respectively.
Figure 6.
AT9283 and dorsomorphin inhibits the receptor tyrosine kinase TEK
HUVECs were treated with Ang1 (200 ng/mL) in the presence or absence of AT9283 (or Dorsomorphin) for 24 h.
(A–B) The inhibition of TEK kinase activity by AT9283 and dorsomorphin was measured by an ELISA kit for human phospho-TEK. n = 6 in each group.
(C–E) DM, Dorsomorphin (C–E) Representative western blot images and protein quantification results showing changes in the protein phosphorylation levels of TEK and the downstream protein Akt after dorsomorphin treatment. β-Actin was used as loading control. n = 5 in each group. ∗p < 0.05 vs. control, #p < 0.05 vs. Ang1. Data are shown as mean ± SEM. DM, Dorsomorphin.
(F–H) Representative western blot images and protein quantification results showing changes in the protein phosphorylation levels of TEK and the downstream protein Akt after AT9283 treatment. β-Actin was used as loading control. n = 5 in each group. ∗p < 0.05 vs. control, #p < 0.05 vs. Ang1. Data are shown as mean ± SEM.
It is a well-known fact that Ang1-driven TEK activation contributes to migration and sprouting of endothelial cells. Thus several functional assays were conducted to further validate the interactions of these two predicted compounds with TEK. In chemotaxis assays, AT9283 and dorsomorphin significantly inhibited the Ang1-mediated migration of HUVECs (Figures 7A–7F). In addition, a tube-formation assay indicated that Ang1 enhanced the ability of HUVECs to form tubes, but this effect was reversed by AT9283 and dorsomorphin treatment (Figures 7G–7J). Collectively, our data indicated that both AT9283 and dorsomorphin inhibited TEK kinase activity and blocked the tube formation of endothelial cells.
Figure 7.
AT9283 and dorsomorphin inhibits HUVEC migration and tube formation
HUVECs were treated with Ang1 (200 ng/mL) in the presence or absence of AT9283 (or dorsomorphin) for 24 h.
(A–B) Representative images showing the healing of HUVECs treated with AT9283 and dorsomorphin at 0 h and 24 h after wounding. Bar, 200 μm.
(C–D) Quantification of the migration of HUVECs evaluated by wound healing assays. n = 6 in each group. ∗p < 0.05 vs. control, #p < 0.05 vs. Ang1.
(E–F) Transmigrated cells were measured using Boyden chamber assays. Bar, 50 μm.
(G–H) Representative images showing tube formation from HUVECs at 3 h after AT9283 and dorsomorphin treatment. Bar, 100 μm.
(I–J) Quantification of total length measured by tube-formation assays. n = 6 in each group. ∗p < 0.05 vs. control, #p < 0.05 vs. Ang1. Data are shown as mean ± SEM. DM, Dorsomorphin.
Discussion
Identifying new DTIs plays a key role in the drug discovery process, traditionally accomplishing in vivo or biochemical experiments. Due to the vast costs and labor involved in biological experiments, computational approaches of DTI prediction have therefore been developed to alleviate these concerns recently. With the emergence of large-scale chemical and pharmacological data and the rapid growth of computing resources, various SOTA methods for DTI prediction based on DNN have been proposed. In order to ensure the generalization ability of these models, overfitting must be avoided during training. There are few studies about whether the overfit models can be used for DTI prediction. In this work, we propose OverfitDTI as a framework for DTI prediction. Compared with other DTI prediction methods, OverfitDTI is simple to use and extensible. Any DNN models that are suitable for the dataset can be contained in OverfitDTI depending on the research goal. If we wish to screen potential drugs within a dataset, we can simply use all data of the dataset to overfit DNN models contained in OverfitDTI; if OverfitDTI is applied to unseen dataset, we need to use VAE model for unsupervised training to obtain the features of unseen dataset, so as to achieve the purpose of overfitting using all data indirectly. Thus, we have turned a limitation (namely, overfitting) of neural networks into a beneficial feature and applied it to DTI prediction.
In theory,20 the neural networks can effectively approximate any nonlinear relationship. Therefore, the DNN models in OverfitDTI can learn the complex nonlinear relationship between drugs and targets with infinite precision. However, some studies have observed that the total error of a DNN model can be decomposed into aleatoric and epistemic contributions,52,53 among which the former is irreducible and inherent to the data and makes a greater contribution to the total variance. It is obvious that due to the aleatoric contribution in the KIBA dataset, the MSE values of the eight DNN models will not continue to decline even if we increase the complexity of the models or number of training epochs.
Most methods used one DNN model for drug and target feature learning and then used it for DIT prediction. It could be observed from the results on three datasets under two scenarios that the performance of each DNN model (e.g., Morgan-CNN) was different for different datasets. Therefore, in order to improve the accuracy of DTI prediction, it was necessary to select the suitable feature learning method for the used DTI dataset. Furthermore, because different feature learning methods could learn different features of the drugs and targets, it was better to consider the prediction results of different DNN models to effectively screen out potential drugs and improve the confidence of the screening results (e.g., the method used in OverfitDTI).
Ang/TEK signaling has become a promising target for angiogenesis-related diseases, while the mechanism is still less studied. Discovery of available small-molecule inhibitor of TEK for more detailed studies is highly desirable. We identified five compounds that interacted with TEK in the KIBA dataset, three in the DTC dataset, and seven in the BindingDB dataset. Two compounds, AT9283 and dorsomorphin, were verified through molecular docking and in vitro experiments. AT9283, which exhibited high efficacy against Aurora kinase, had been under evaluation in clinical trials for the treatment of solid tumors.47 Our findings indicated that AT9283 could also be the inhibitor of TEK. Its anti-tumor effects may partially be due to its inhibitory activities against TEK and angiogenesis. Dorsomorphin had been reported to inhibit colorectal cancer by inhibiting the activity of Akt,54 while its effect on TEK had not been investigated. Our results probably added new insights into its anti-tumor effects as the inhibitor of TEK. Besides providing an alternative to discover small-molecule inhibitor of TEK, the predicted results are helpful for identifying the potential new applications of existing compounds. Overall, screening results on three datasets and laboratory experimentation indicate that OverfitDTI is a useful tool to predict DTIs and thus contribute to the drug discovery or repositioning.
Limitations of the study
When selecting a model with high performance for a certain DTI dataset, many preliminary experiments are required, which also increase the demand for computing resources. HUVECs from the American Type Culture Collection (ATCC) were used as in vitro human vascular models. As no sex information of HUVECs was available for reference, sex differences were not under consideration in the study. Subsequent studies in the future should focus on these aspects, which we leave for future work.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-p-Akt (Ser473) | Cell Signaling Technology | Cat#9271; RRID: AB_329825 |
| Rabbit polyclonal anti-Akt | Cell Signaling Technology | Cat#9272; RRID: AB_329827 |
| Rabbit polyclonal anti-p-TEK (Tyr992) | Cell Signaling Technology | Cat# 4221; RRID: AB_2203198 |
| Mouse monoclonal anti-TEK | Cell Signaling Technology | Cat#4224; RRID: AB_2203197 |
| Rabbit monoclonal anti-β-actin | Cell Signaling Technology | Cat# 8457; RRID: AB_10950489 |
| Chemicals, peptides, and recombinant proteins | ||
| AT9283 | Target Molecule Corp | T3068; CAS: 896466-04-9 |
| Dorsomorphin | MedChemExpress | HY-13418A; CAS: 866405-64-3 |
| Human Ang1 | PeproTech | 130–06 |
| Critical commercial assays | ||
| Human phospho-TEK ELISA kit | R&D Systems | DYC2720 |
| Deposited data | ||
| BindingDB dataset | The Binding Database | https://www.bindingdb.org/bind/index_original.jsp |
| KIBA dataset | He et al.55 | https://zenodo.org/record/164436 |
| DTC dataset | DTC website | https://drugtargetcommons.fimm.fi/ |
| Source code for constructing OverfitDTI models and analyzing of corresponding result. | This paper | https://github.com/believemetoo/OverfitDTI |
| Experimental models: Cell lines | ||
| HUVECs | ATCC | CRL-1730 |
| Software and algorithms | ||
| ImagePro Plus | Media Cybernetics | RRID: SCR_007369 |
| Prism 7 | GraphPad Software | https://www.graphpad.com/features |
| Python 3.8 | Python Software Foundation | https://www.python.org/ |
| Pytorch 1.4.0 | GitHub | https://pytorch.org/ |
| DeepPurpose | Huang et al.35 | https://github.com/kexinhuang12345/DeepPurpose |
| AutoDock Vina | The Scripps Research Institute, CCSB | https://vina.scripps.edu/ |
| Pymol | The PyMOL Molecular Graphics System, Version 2.2 Schrödinger, LLC. | https://pymol.org/2/ |
| ChemDraw 20 | PerkinElmer | https://www.perkinelmer.com/category/chemdraw |
| RDKit | Open-Source Software | https://www.rdkit.org/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Du Xinping (xpdu2021@sina.com).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Cell culture
HUVECs (ATCC, VA, USA) were maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin in a humidified incubator at 37°C and 5% CO2. Unless otherwise stated, cells were subjected to 12 h of starvation prior to experiments. AT9283 (Target Molecule Corp, MA, USA) and dorsomorphin (MedChemExpress, NJ, USA) were examined in the follow-up experiments.
Method details
OverfitDTI as a pipeline for predicting DTIs
The data in DTI dataset are drug-target pairs and their corresponding labels (values indicating the strength of binding affinity). For a DTI dataset, OverfitDTI first performs features learning for drugs and targets respectively. There are various DNNs (feature learning methods) currently, the DeepPurpose toolkit summarizes these methods well35 and is used for drugs and targets feature learning here. The learned features of drugs and targets are concatenated and form the features of the integrated space of the DTI dataset (Figure 1A). We achieve overfit training as in the following way: first, all data are used as the training set to sufficiently learn the features of the integrated space; second, we use a relatively large epoch to excessively train the model until the error of training set and validation set will not decrease. As a comparison, the baselines use an early stop policy to prevent overfitting. Ten percent of the dataset are used as the validation set. To evaluate the fitting performance of the DNN model, all data are used as the test set. After the DNN model is overfit, the implicit representation of the nonlinear relation in the integrated space is obtained, represented by implicit function f (Figure 1A); We can then use f (more specifically, the trained neural network weights) to calculate the binding affinity between a drug and a target in the dataset, that is, predict the DTI.
VAE is an unsupervised method that is widely used as generative model. The latent representation or latent space z produced by VAE can be used to generate new data or as latent features of the dataset.24 When OverfitDTI is applied to unseen data, the DNN model cannot be overfit trained as the unseen data have no label, this is where VAE comes into play. We constructed VAE model with a CNN encoder and a CNN decoder (Figure 1B). Considering the differences in the features of drugs and proteins, we constructed and trained VAE models for drugs and proteins respectively. For the VAE model of drugs, the simplified molecular-input line-entry system (SMILES) strings of drugs were first embedded into a 63-dimensional vector and went through three convolutional layers with kernel size [5, 5, 5], each with leaky rectified linear unit (Leaky ReLU) activation. A max-pooling layer which took the maximum value along the sequence was performed and output the final encoded features,
| (Equation 1) |
Where xemb is the embedded input characters, BN(·) is the batch normalization layer, and σ(·) is the Leaky ReLU layer. Convolutional layer Conv1d1, Conv1d2, and Conv1d3 transforms the hidden feature dimensions from 63 to 128, 128 to 256, and 256 to 512, respectively. All convolutional layers have a zero-padding length of 2 on both edges.
Then two dense layers are used to calculate the mean and log-variance of the latent representation, which transforms the features dimensions from 512 to 10. The decoder neural networks reconstruct the input data from latent representation. The decoder connects directly to the latent dense layers and consists of three transposed convolutional layers with kernel size [5, 5, 5], which is the opposite of the encoder portion,
| (Equation 2) |
Where z is the sampled features and FC(·) is the fully connected layer. Reshape(·) reshapes the hidden features. Transposed convolutional layer ConvTranspose1d1, ConvTranspose1d2, and ConvTranspose1d3 transforms the hidden feature dimensions from 512 to 256, 256 to 128, and 128 to 63, respectively. All transposed convolutional layers have the same kernel size of 5 and the same padding length of 2.
Except for the model parameters (Tables S1–S12), the structure of VAE model of protein is similar to that of drug. We set a maximum length of 100 characters for drug and 1000 characters for protein; shorter strings are padded with spaces to this same length.
The VAE model is trained via an objective function that minimizes binary cross entropy (Equation 3) between the reconstructed characters and one-hot-encoded targets, namely the reconstruction error, where n represents the number of samples, and ti and pi, the i-th target and predicted variables, respectively. In order to ensure normally distributed noise is added to the latent representation during training, a Kullback−Leibler divergence (Equation 4) is added to the objective function. DKL(q(z|x)||p(z)) is the Kullback−Leibler divergence between generative distributions p and posterior distributions q over variables x and latent variables z. σ is the standard deviation of the distribution, and μ is the mean of the distribution.
| (Equation 3) |
| (Equation 4) |
During model training, we first combined the labeled datasets with unlabeled datasets to train VAE model; after the training, the VAE model obtained the ability to reconstruct the merged dataset. Then we sent the labeled dataset back into the VAE model for encoding and got its latent features. We generated 10-dimensional drug features and protein features using the VAE models. Since the VAE model was trained by labeled and unlabeled data, these latent features produced by the VAE model carried some information about the unlabeled data. In this way, we incorporated partial features of the unlabeled dataset into the labeled dataset. The features were concatenate to the features obtained by supervised learning and sent into DNN for overfitting training.
Screening of potential drugs
To screen potential drugs for a desired target using OverfitDTI, we can calculate the binding affinities between the desired target and all drugs in the DTI dataset using the overfit models, then drugs are ranked based on their binding affinities, and the top listed drugs are more likely to interact with the desired target. Specifically, when screening potential drugs for the desired target, the top 2% of the prediction results with each model are selected first, and the drugs that are common to all these sets of selected prediction results are then selected as the final list of potential drugs. The 2% threshold is only a suggested value, and other values (for example, 5%) can also be used,56 depending on the research goal. In our study, due to the different number of drugs contained in each dataset, for the KIBA dataset, the top 100 results with each model were selected; for the DTC and BindingDB datasets, the top 200 results with each model were selected.
Datasets
We evaluated OverfitDTI on three public DTI benchmark datasets: KIBA dataset, DTC dataset and BindingDB dataset. These datasets had been exploited in various SOTA methods10,11,19,35 recently. For these three datasets here, the data type of drug is SMILES string and the target is amino acid sequence. More details about the data preprocessing can be found in Supplemental information (Figures S7–S9) and the original code we report.
KIBA is a model-based integration approach that integrates the information of the IC50, inhibition constant (Ki), and dissociation constant (Kd) measurements into a single bioactivity score, and the KIBA dataset is collected based on this approach.57 He et al.55 processed the dataset57 by removing all drugs and targets with less than ten interactions, resulting in a dataset, called the KIBA dataset, that includes 2,068 unique drugs, 229 unique targets, and 118,254 drug–target pairs; the binding affinities in this dataset were given as KIBA values. We used the processed dataset here.55
DTC is a web-based community platform to standardize the collection, management and annotation of the heterogeneous drug-target bioactivity measurements.58 We download the original data and build a dataset suitable for this study on this basis. After several filtering and preprocessing steps on the original data, the dataset used in this study, which we named the DTC dataset, included 7,626 unique drugs, 895 unique targets, and 61,479 drug-target pairs.
BindingDB is a widely used public dataset that focuses mainly on the binding affinities between small molecules and target proteins.59 In the original BindingDB dataset, the binding affinity is measured by Kd, IC50, Ki, and half maximal effective concentration (EC50) values. For the sake of calculation, we used only the Kd values, which were transformed to log space pKd, facilitating training when using the OverfitDTI. After data transformation and several filters, the BindingDB dataset that we used included 10,661 unique drugs, 1,413 unique target proteins, and 66,434 drug-target pairs.
For the experimental setting of the warm start, all data in the dataset are used for training and testing, and no additional processing is required on the dataset. The binding affinity is measured by Kd in DTC and BindingDB dataset which is different from the KIBA value used in KIBA dataset, so we use the DTC and BindingDB dataset to evaluate the performance of the OverfitDTI in the cold start scenario. We preprocess the datasets as follow: when the training set is BindingDB dataset, the common drugs in DTC dataset (test set) are removed for the cold start for drugs while both the common drugs and targets are removed for the cold start for both drugs and proteins; when the training set is DTC dataset, a similar process is performed on BindingDB dataset. As for GNN model, the authors excluded the data containing ‘.’ in the SMILES format.13 For fair comparison, we used the same data preprocessing method as in the original paper.
Evaluation
When implementing DTI prediction using the three aforementioned datasets, DTI prediction was designed as a regression problem: the result of the predicted interaction (binding score or affinity) was a continuous value, rather than a binary 0 or 1. This is a more realistic formulation, as the DTI prediction is not a simple as a binary classification problem. Therefore, we did not have to construct negative samples in these three datasets.CI, MSE and PC are used to evaluate the performance of the DNN model. The CI measures whether the predicted binding affinity of two random drug-target pairs is predicted in the same order as their true values are11:
| (Equation 5) |
Where pi is the predicted value for the larger binding affinity δi, pj is the predicted value for the smaller binding affinity δj, Z is a normalization constant, h is the step function.11 The CI value of a model reflects its prediction accuracy, and a larger CI value means the better prediction performance of the model.
The MSE value of a model reflects its fitting precision to the nonlinear relationship here:
| (Equation 6) |
Where pi is the predicted value and ti corresponds to the label for i-th drug-traget pair. n is the number of samples.
The PC value of a model reflects the correlation between the true and predicted binding scores37:
| (Equation 7) |
Where mt is mean of labels and mp is mean of the predicted values.
Molecular docking
The AutoDock Vina60 was used to conduct docking experiment. The crystal structure of TEK was downloaded from the Protein Data Bank49 (PDB ID: 6mwe). The three-dimensional structure files (i.e., AT9283 and dorsomorphin) were obtained from the ChEMBL database.61 Visualization of results was implemented by Pymol.62
In vitro validation of predicted compounds as new inhibitors of TEK
ELISA
Kinase activity was assessed with a commercially available ELISA kit for human phospho-TEK (R&D Systems, MN, USA). Briefly, cells were seeded in 6-well plates at 4 × 105 cells per well and treated with Ang1 to activate TEK. Cell lysates were collected and then subjected to ELISA with a kit according to the manufacturer’s instructions. The absorbance at 450 nm was measured using a microtiter-plate reader (Bio Tek Instrument, VT, USA).
Boyden chamber assays
Boyden chamber assays were performed as described in a previous study.63 In brief, Boyden chamber (Corning Inc., NY, USA) was comprised of upper chamber and lower chamber, which were separated by a polycarbonate membrane of 8-μm-pore-size. HUVECs were collected and seeded in the upper chamber at 1 × 105 cells in 200 μL of medium. Ang1 (PeproTech, NJ, USA) was added to the lower chamber in 600 μL of medium as a chemoattractant. After being allowed to migrate for 24 h at 37°C, cells on the upper side of the filter that had not migrated were removed. The migrated cells were fixed in 4% paraformaldehyde and stained with crystal violet. Images were collected under a light microscope (x200).
Wound healing assays
Cell migration was measured as previously described.63 Briefly, cells that reached 90% confluence were seeded in 6-well plates and wounded using a 200-μL pipette tip. Images were obtained at 0 h and 24 h using a light microscope (x40). The wound area was measured using ImagePro Plus software (Media Cybernetics Inc. MD, USA)
Western blot analysis
Western blotting was conducted as described in our previous study.64 Briefly, cells were lysed with RIPA buffer containing 1% protease inhibitor and 10% phosphatase inhibitor and centrifuged at 12 000 g for 15 min at 4°C. Protein concentrations were determined using a BCA Protein Assay Kit (Solarbio, Beijing, China). Proteins were fractionated on 8–12% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. The membranes were incubated with primary antibodies against the following: p-TEK (Tyr992) (1:1000, 4221, Cell Signaling Technology, MA, USA), TEK (1:1000, 4224, Cell Signaling Technology, MA, USA), p-Akt (Ser473) (1:1000, 9271, Cell Signaling Technology, MA, USA), Akt (1:1000, 9272, Cell Signaling Technology, MA, USA) and β-actin (1:1000, 8457, Cell Signaling Technology, MA, USA), and subsequently probed with horseradish peroxidase-conjugated secondary antibodies. Bands were visualized using ECL reagents and analyzed using ImagePro Plus software (Media Cybernetics Inc. MD, USA).
Tube formation assays
HUVECs were starved prior to the experiment and seeded at a density of 1.5 × 104 per well into 96-well plates precoated with Matrigel. Images were collected using a light microscope (x100) after 3 h of stimulation with Ang1. The total sprout length was measured with Angiogenesis Analyzer, a plug-in of ImagePro Plus software (Media Cybernetics Inc. MD, USA).
Quantification and statistical analysis
The experimental data are presented as the mean ± SEM. Statistical analyses were conducted with Prism 7 software (GraphPad Software, CA, USA). Comparisons between two groups were performed using Student’s t test. Comparisons of three or more groups were performed using one-way ANOVA followed by Tukey’s test. p < 0.05 was used to indicate statistical significance.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81900407, 81901526), Tianjin Natural Science Foundation (Grant No. 21JCZDJC01270), Tianjin Science and Technology Plan Project (Grant No. 22ZYQYSY00030), Tianjin Municipal Health Commission (Grant No. ZC20033, TJWJ2022XK043), Tianjin Binhai New Area Health Commission (Grant No. 2019BWKQ029, 2022BWKY004), and Tianjin Municipal Education Commission Scientific Research Project (Grant No. 2022KJ262).
We acknowledge XianYu Technology Company for its helpful suggestions on the computational docking studies. We thank the authors of the DeepPurpose as part of our models are implemented using their open source library.
Author contributions
Conceptualization: X. X. L., D. X. P., and L. X. Z.; Methodology: X. X. L.; Software: T. Z.; Investigation and Formal Analysis: H. G. P., L. H. W., G. J. K., B. X. Y., and M. X. F.; Writing – Original Draft: X. X. L. and T. Z.; Writing – Review & Editing: L. Y. X., X. N., Z. C. Y., and G. R.; Supervision: W. K., Z. C., W. C. C., and L. M. Y.
Declaration of interests
The authors declare no competing interests.
Published: August 15, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107646.
Supplemental information
Data and code availability
-
•
This paper analyzes existing, publicly available datasets (KIBA, DTC, BindingDB). Accession URL for these datasets are listed in the key resources table.
-
•
Source code, details of data processing and tutorials for implementing the OverfitDTI model are publicly available online at https://github.com/believemetoo/OverfitDTI.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Hopkins A.L. Drug discovery: Predicting promiscuity. Nature. 2009;462:167–168. doi: 10.1038/462167a. [DOI] [PubMed] [Google Scholar]
- 2.Keiser M.J., Setola V., Irwin J.J., Laggner C., Abbas A.I., Hufeisen S.J., Jensen N.H., Kuijer M.B., Matos R.C., Tran T.B., et al. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayr A., Klambauer G., Unterthiner T., Steijaert M., Wegner J.K., Ceulemans H., Clevert D.A., Hochreiter S. Large-scale comparison of machine learning methods for drug target prediction on. Chem. Sci. 2018;9:5441–5451. doi: 10.1039/c8sc00148k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivanenkov Y.A., Zagribelnyy B.A., Aladinskiy V.A. Are We Opening the Door to a New Era of Medicinal Chemistry or Being Collapsed to a Chemical Singularity? J. Med. Chem. 2019;62:10026–10043. doi: 10.1021/acs.jmedchem.9b00004. [DOI] [PubMed] [Google Scholar]
- 5.Sakai M., Nagayasu K., Shibui N., Andoh C., Takayama K., Shirakawa H., Kaneko S. Prediction of pharmacological activities from chemical structures with graph convolutional neural networks. Sci. Rep. 2021;11:525. doi: 10.1038/s41598-020-80113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L., Sedykh A., Tripathi A., Zhu H., Afantitis A., Mouchlis V.D., Melagraki G., Rusyn I., Tropsha A. Identification of putative estrogen receptor-mediated endocrine disrupting chemicals using QSAR- and structure-based virtual screening approaches. Toxicol. Appl. Pharmacol. 2013;272:67–76. doi: 10.1016/j.taap.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riniker S., Wang Y., Jenkins J.L., Landrum G.A. Using information from historical high-throughput screens to predict active compounds. J. Chem. Inf. Model. 2014;54:1880–1891. doi: 10.1021/ci500190p. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter K.A., Cohen D.S., Jarrell J.T., Huang X. Deep learning and virtual drug screening. Future Med. Chem. 2018;10:2557–2567. doi: 10.4155/fmc-2018-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagherian M., Sabeti E., Wang K., Sartor M.A., Nikolovska-Coleska Z., Najarian K. Machine learning approaches and databases for prediction of drug-target interaction: a survey paper. Brief. Bioinform. 2021;22:247–269. doi: 10.1093/bib/bbz157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L., Tan X., Wang D., Zhong F., Liu X., Yang T., Luo X., Chen K., Jiang H., Zheng M. TransformerCPI: improving compound-protein interaction prediction by sequence-based deep learning with self-attention mechanism and label reversal experiments. Bioinformatics. 2020;36:4406–4414. doi: 10.1093/bioinformatics/btaa524. [DOI] [PubMed] [Google Scholar]
- 11.Öztürk H., Özgür A., Ozkirimli E. DeepDTA: deep drug-target binding affinity prediction. Bioinformatics. 2018;34:i821–i829. doi: 10.1093/bioinformatics/bty593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen T., Le H., Quinn T.P., Nguyen T., Le T.D., Venkatesh S. GraphDTA: predicting drug-target binding affinity with graph neural networks. Bioinformatics. 2021;37:1140–1147. doi: 10.1093/bioinformatics/btaa921. [DOI] [PubMed] [Google Scholar]
- 13.Tsubaki M., Tomii K., Sese J. Compound-protein interaction prediction with end-to-end learning of neural networks for graphs and sequences. Bioinformatics. 2019;35:309–318. doi: 10.1093/bioinformatics/bty535. [DOI] [PubMed] [Google Scholar]
- 14.Wan F., Zhu Y., Hu H., Dai A., Cai X., Chen L., Gong H., Xia T., Yang D., Wang M.W., Zeng J. DeepCPI: A Deep Learning-based Framework for Large-scale in silico Drug Screening. Dev. Reprod. Biol. 2019;17:478–495. doi: 10.1016/j.gpb.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H., Sun J., Guan J., Zheng J., Zhou S. Improving compound-protein interaction prediction by building up highly credible negative samples. Bioinformatics. 2015;31:i221–i229. doi: 10.1093/bioinformatics/btv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Y., Zhao X., Zhou J., Yang J., Zhang Y., Kuang W., Peng J., Chen L., Zeng J. A network integration approach for drug-target interaction prediction and computational drug repositioning from heterogeneous information. Nat. Commun. 2017;8:573. doi: 10.1038/s41467-017-00680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Souza S., Prema K.V., Balaji S. Machine learning models for drug-target interactions: current knowledge and future directions. Drug Discov. Today. 2020;25:748–756. doi: 10.1016/j.drudis.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Rifaioglu A.S., Nalbat E., Atalay V., Martin M.J., Cetin-Atalay R., Doğan T. DEEPScreen: high performance drug-target interaction prediction with convolutional neural networks using 2-D structural compound representations. Chem. Sci. 2020;11:2531–2557. doi: 10.1039/c9sc03414e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng S., Li Y., Chen S., Xu J., Yang Y. Predicting drug–protein interaction using quasi-visual question answering system. Nat. Mach. Intell. 2020;2:134–140. doi: 10.1038/s42256-020-0152-y. [DOI] [Google Scholar]
- 20.Hornik K., Stinchcombe M., White H. Multilayer feedforward networks are universal approximators. Neural Network. 1989;2:359–366. doi: 10.1016/0893-6080(89)90020-8. [DOI] [Google Scholar]
- 21.Chen Z., Zhang H. 2019 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR) 2019. Learning Implicit Fields for Generative Shape Modeling; pp. 5932–5941. [DOI] [Google Scholar]
- 22.Park J.J., Florence P., Straub J., Newcombe R., Lovegrove S. 2019 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR) 2019. DeepSDF: Learning Continuous Signed Distance Functions for Shape Representation; pp. 165–174. [DOI] [Google Scholar]
- 23.Davies T., Nowrouzezahrai D., Jacobson A. Overfit Neural Networks as a Compact Shape Representation. arXiv. 2020 Preprint at. [Google Scholar]
- 24.Kingma D.P., Welling M. Auto-Encoding Variational Bayes. arXiv. 2013 Preprint at. [Google Scholar]
- 25.Sato T.N., Tozawa Y., Deutsch U., Wolburg-Buchholz K., Fujiwara Y., Gendron-Maguire M., Gridley T., Wolburg H., Risau W., Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 26.Koblizek T.I., Weiss C., Yancopoulos G.D., Deutsch U., Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr. Biol. 1998;8:529–532. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- 27.Papapetropoulos A., Fulton D., Mahboubi K., Kalb R.G., O'Connor D.S., Li F., Altieri D.C., Sessa W.C. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J. Biol. Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 28.Moss A. The angiopoietin:Tie 2 interaction: a potential target for future therapies in human vascular disease. Cytokine Growth Factor Rev. 2013;24:579–592. doi: 10.1016/j.cytogfr.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Boscolo E., Limaye N., Huang L., Kang K.T., Soblet J., Uebelhoer M., Mendola A., Natynki M., Seront E., Dupont S., et al. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J. Clin. Invest. 2015;125:3491–3504. doi: 10.1172/JCI76004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin P., Polverini P., Dewhirst M., Shan S., Rao P.S., Peters K. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J. Clin. Invest. 1997;100:2072–2078. doi: 10.1172/JCI119740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parikh S.M., Mammoto T., Schultz A., Yuan H.T., Christiani D., Karumanchi S.A., Sukhatme V.P. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilmer J., Schoenholz S.S., Riley P.F., Vinyals O., Dahl G.E. Proceedings of the 34th International Conference on Machine Learning. 2017. Neural Message Passing for Quantum Chemistry; pp. 1263–1272. [DOI] [Google Scholar]
- 33.Vaswani A., Shazeer N.M., Parmar N., Uszkoreit J., Jones L., Gomez A.N., Kaiser L., Polosukhin I. Proceedings of the 31st International Conference on Neural Information Processing Systems. 2017. Attention Is All You Need; pp. 6000–6010. [DOI] [Google Scholar]
- 34.Duvenaud D., Maclaurin D., Aguilera-Iparraguirre J., Gómez-Bombarelli R., Hirzel T., Aspuru-Guzik A., Adams R.P. Proceedings of the 28th International Conference on Neural Information Processing Systems. 2015. Convolutional Networks on Graphs for Learning Molecular Fingerprints; pp. 2224–2232. [DOI] [Google Scholar]
- 35.Huang K., Fu T., Glass L.M., Zitnik M., Xiao C., Sun J. DeepPurpose: a deep learning library for drug-target interaction prediction. Bioinformatics. 2021;36:5545–5547. doi: 10.1093/bioinformatics/btaa1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gönen M., Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92:965–970. doi: 10.1093/biomet/92.4.965. [DOI] [Google Scholar]
- 37.Roy K., Chakraborty P., Mitra I., Ojha P.K., Kar S., Das R.N. Some case studies on application of "rm2" metrics for judging quality of quantitative structure–activity relationship predictions: Emphasis on scaling of response data. J. Comput. Chem. 2013;34:1071–1082. doi: 10.1002/jcc.23231. [DOI] [PubMed] [Google Scholar]
- 38.Aly A.H., Edrada-Ebel R., Indriani I.D., Wray V., Müller W.E.G., Totzke F., Zirrgiebel U., Schächtele C., Kubbutat M.H.G., Lin W.H., et al. Cytotoxic metabolites from the fungal endophyte Alternaria sp. and their subsequent detection in its host plant Polygonum senegalense. J. Nat. Prod. 2008;71:972–980. doi: 10.1021/np070447m. [DOI] [PubMed] [Google Scholar]
- 39.Thomas M., Huang W.S., Wen D., Zhu X., Wang Y., Metcalf C.A., Liu S., Chen I., Romero J., Zou D., et al. Discovery of 5-(arenethynyl) hetero-monocyclic derivatives as potent inhibitors of BCR-ABL including the T315I gatekeeper mutant. Bioorg. Med. Chem. Lett. 2011;21:3743–3748. doi: 10.1016/j.bmcl.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 40.Debbab A., Aly A.H., Edrada-Ebel R., Wray V., Müller W.E.G., Totzke F., Zirrgiebel U., Schächtele C., Kubbutat M.H.G., Lin W.H., et al. Bioactive metabolites from the endophytic fungus Stemphylium globuliferum isolated from Mentha pulegium. J. Nat. Prod. 2009;72:626–631. doi: 10.1021/np8004997. [DOI] [PubMed] [Google Scholar]
- 41.Hudkins R.L., Becknell N.C., Zulli A.L., Underiner T.L., Angeles T.S., Aimone L.D., Albom M.S., Chang H., Miknyoczki S.J., Hunter K., et al. Synthesis and biological profile of the pan-vascular endothelial growth factor receptor/tyrosine kinase with immunoglobulin and epidermal growth factor-like homology domains 2 (VEGF-R/TIE-2) inhibitor 11-(2-methylpropyl)-12,13-dihydro-2-methyl-8-(pyrimidin-2-ylamino)-4H-indazolo[5, 4-a]pyrrolo[3,4-c]carbazol-4-one (CEP-11981): a novel oncology therapeutic agent. J. Med. Chem. 2012;55:903–913. doi: 10.1021/jm201449n. [DOI] [PubMed] [Google Scholar]
- 42.Ji Z., Ahmed A.A., Albert D.H., Bouska J.J., Bousquet P.F., Cunha G.A., Glaser K.B., Guo J., Li J., Marcotte P.A., et al. Isothiazolopyrimidines and isoxazolopyrimidines as novel multi-targeted inhibitors of receptor tyrosine kinases. Bioorg. Med. Chem. Lett. 2006;16:4326–4330. doi: 10.1016/j.bmcl.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 43.Cao H., Zhang H., Zheng X., Gao D. 3D QSAR studies on a series of potent and high selective inhibitors for three kinases of RTK family. J. Mol. Graph. Model. 2007;26:236–245. doi: 10.1016/j.jmgm.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Hodous B.L., Geuns-Meyer S.D., Hughes P.E., Albrecht B.K., Bellon S., Bready J., Caenepeel S., Cee V.J., Chaffee S.C., Coxon A., et al. Evolution of a highly selective and potent 2-(pyridin-2-yl)-1,3,5-triazine Tie-2 kinase inhibitor. J. Med. Chem. 2007;50:611–626. doi: 10.1021/jm061107l. [DOI] [PubMed] [Google Scholar]
- 45.Hodous B.L., Geuns-Meyer S.D., Hughes P.E., Albrecht B.K., Bellon S., Caenepeel S., Cee V.J., Chaffee S.C., Emery M., Fretland J., et al. Synthesis, structural analysis, and SAR studies of triazine derivatives as potent, selective Tie-2 inhibitors. Bioorg. Med. Chem. Lett. 2007;17:2886–2889. doi: 10.1016/j.bmcl.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 46.Santo L., Hideshima T., Cirstea D., Bandi M., Nelson E.A., Gorgun G., Rodig S., Vallet S., Pozzi S., Patel K., et al. Antimyeloma activity of a multitargeted kinase inhibitor, AT9283, via potent Aurora kinase and STAT3 inhibition either alone or in combination with lenalidomide. Clin. Cancer Res. 2011;17:3259–3271. doi: 10.1158/1078-0432.CCR-10-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno L., Marshall L.V., Pearson A.D.J., Morland B., Elliott M., Campbell-Hewson Q., Makin G., Halford S.E.R., Acton G., Ross P., et al. A phase I trial of AT9283 (a selective inhibitor of aurora kinases) in children and adolescents with solid tumors: a Cancer Research UK study. Clin. Cancer Res. 2015;21:267–273. doi: 10.1158/1078-0432.CCR-14-1592. [DOI] [PubMed] [Google Scholar]
- 48.Lee H., Zandkarimi F., Zhang Y., Meena J.K., Kim J., Zhuang L., Tyagi S., Ma L., Westbrook T.F., Steinberg G.R., et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020;22:225–234. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H., Shindyalov I.N., Bourne P.E. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witzenbichler B., Maisonpierre P.C., Jones P., Yancopoulos G.D., Isner J.M. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J. Biol. Chem. 1998;273:18514–18521. doi: 10.1074/jbc.273.29.18514. [DOI] [PubMed] [Google Scholar]
- 51.Bogdanovic E., Nguyen V.P.K.H., Dumont D.J. Activation of Tie2 by angiopoietin-1 and angiopoietin-2 results in their release and receptor internalization. J. Cell Sci. 2006;119:3551–3560. doi: 10.1242/jcs.03077. [DOI] [PubMed] [Google Scholar]
- 52.Beker W., Wołos A., Szymkuć S., Grzybowski B.A. Minimal-uncertainty prediction of general drug-likeness based on Bayesian neural networks. Nat. Mach. Intell. 2020;2:457–465. doi: 10.1038/s42256-020-0209-y. [DOI] [Google Scholar]
- 53.Hie B., Bryson B.D., Berger B. Leveraging Uncertainty in Machine Learning Accelerates Biological Discovery and Design. Cell Syst. 2020;11:461–477.e9. doi: 10.1016/j.cels.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Ghanaatgar-Kasbi S., Amerizadeh F., Rahmani F., Hassanian S.M., Khazaei M., Ferns G.A., Avan A. AMP-kinase inhibitor dorsomorphin reduces the proliferation and migration behavior of colorectal cancer cells by targeting the AKT/mTOR pathway. IUBMB Life. 2019;71:1929–1936. doi: 10.1002/iub.2136. [DOI] [PubMed] [Google Scholar]
- 55.He T., Heidemeyer M., Ban F., Cherkasov A., Ester M. SimBoost: a read-across approach for predicting drug-target binding affinities using gradient boosting machines. J. Cheminform. 2017;9:24. doi: 10.1186/s13321-017-0209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S., Sun Q., Xu Y., Pei J., Lai L. A transferable deep learning approach to fast screen potential antiviral drugs against SARS-CoV-2. Brief. Bioinform. 2021 doi: 10.1093/bib/bbab211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang J., Szwajda A., Shakyawar S., Xu T., Hintsanen P., Wennerberg K., Aittokallio T. Making sense of large-scale kinase inhibitor bioactivity data sets: a comparative and integrative analysis. J. Chem. Inf. Model. 2014;54:735–743. doi: 10.1021/ci400709d. [DOI] [PubMed] [Google Scholar]
- 58.Tanoli Z., Alam Z., Vaha-Koskela M., Ravikumar B., Malyutina A., Jaiswal A., Tang J., Wennerberg K., Aittokallio T. Drug Target Commons 2.0: a community platform for systematic analysis of drug-target interaction profiles. Database. 2018;2018:1–13. doi: 10.1093/database/bay083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu T., Lin Y., Wen X., Jorissen R.N., Gilson M.K. BindingDB: a web-accessible database of experimentally determined protein-ligand binding affinities. Nucleic Acids Res. 2007;35:D198–D201. doi: 10.1093/nar/gkl999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mendez D., Gaulton A., Bento A.P., Chambers J., De Veij M., Félix E., Magariños M.P., Mosquera J.F., Mutowo P., Nowotka M., et al. ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res. 2019;47:D930–D940. doi: 10.1093/nar/gky1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schrödinger, LLC The PyMOL Molecular Graphics System, Version 2.0. https://pymol.org/2/
- 63.Xiao X.L., Hu N., Zhang X.Z., Jiang M., Chen C., Ma R., Ma Z.G., Gao J.L., Xuan X.C., Sun Z.J., Dong D.L. Niclosamide inhibits vascular smooth muscle cell proliferation and migration and attenuates neointimal hyperplasia in injured rat carotid arteries. Br. J. Pharmacol. 2018;175:1707–1718. doi: 10.1111/bph.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y.C., Xiao X.L., Li N., Yang D., Xing Y., Huo R., Liu M.Y., Zhang Y.Q., Dong D.L. Oestrogen inhibits BMP4-induced BMP4 expression in cardiomyocytes: a potential mechanism of oestrogen-mediated protection against cardiac hypertrophy. Br. J. Pharmacol. 2015;172:5586–5595. doi: 10.1111/bph.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This paper analyzes existing, publicly available datasets (KIBA, DTC, BindingDB). Accession URL for these datasets are listed in the key resources table.
-
•
Source code, details of data processing and tutorials for implementing the OverfitDTI model are publicly available online at https://github.com/believemetoo/OverfitDTI.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.