Abstract
Background and purpose:
In radiotherapy, dose calculations based on 4D cone beam CTs (4DCBCTs) require image intensity corrections. This retrospective study compared the dose calculation accuracy of a deep learning, projection-based scatter correction workflow (ScatterNet), to slower workflows: conventional 4D projection-based scatter correction (CBCTcor) and a deformable image registration (DIR)-based method (4DvCT).
Materials and methods:
For 26 lung cancer patients, planning CTs (pCTs), 4DCTs and CBCT projections were available. ScatterNet was trained with pairs of raw and corrected CBCT projections. Corrected projections from ScatterNet and the conventional workflow were reconstructed using MA-ROOSTER, yielding 4DCBCTSN and 4DCBCTcor. The 4DvCT was generated by 4DCT to 4DCBCT DIR, as part of the 4DCBCTcor workflow. Robust intensity modulated proton therapy treatment plans were created on free-breathing pCTs. 4DCBCTSN was compared to 4DCBCTcor and the 4DvCT in terms of image quality and dose calculation accuracy (dose-volume-histogram parameters and / gamma analysis).
Results:
4DCBCTSN resulted in an average mean absolute error of and when compared to 4DCBCTcor and 4DvCT respectively. High agreement was observed in targets with median dose differences of (4DCBCTSN-4DCBCTcor) and (4DCBCTSN-4DvCT). The gamma analysis showed high average / pass rates of for both 4DCBCTSN vs. 4DCBCTcor and 4DCBCTSN vs. 4DvCT.
Conclusions:
Accurate 4D dose calculations are feasible for lung cancer patients using ScatterNet for 4DCBCT correction. Average scatter correction times could be reduced from (4DCBCTcor) to , showing the clinical suitability of the proposed deep learning-based method.
Keywords: Proton therapy, 4DCBCT, Projection-based, Deep learning, Lung cancer, Scatter correction
1. Introduction
Cone-beam computed tomography (CBCT) scanners have been widely adopted in radiation therapy clinics. However, due to a relatively low image quality their use is mostly limited to patient positioning. Especially in particle therapy, the implementation and advancement of CBCT technology has lagged behind photon therapy systems for the last decade [1]. In photon therapy, recent commercial developments have led to CBCTs with sufficient image quality to also perform dose calculations and adaptive radiotherapy procedures [2], [3]. However, in particle therapy, range uncertainties increase the need for accurate image guidance and proton dose calculations have higher requirements on CT-number accuracy than their photon counterpart. Hence, accurate CBCT scatter correction for proton therapy is still an active field of research [4], [5], [6].
Scatter-corrected CBCT images can provide clinically relevant insights on the impact of interfractional changes of the patient’s anatomy and could thus be used to indicate the necessity of plan adaptations [7], [8]. Various scatter correction techniques have been investigated in the literature, including, among others, image-based methods relying on deformable image registration (DIR) between CTs and CBCTs, generating so-called virtual CTs (vCTs) [4], [9], and projection-based approaches using forward projection of vCTs to correct CBCT projections prior to image reconstruction (CBCTcor) [4], [5], [10]. However, these conventional scatter correction workflows often rely on computationally expensive deformable image registrations and filtering operations [4], [5], [6], [10], [11], which leads to long runtimes and hampers their clinical implementation, especially in online adaptive proton therapy workflows.
Dose calculations on CBCTs, which are routinely acquired for patient positioning in many clinics, would avoid additional dose burden for the patient from repeat CT imaging. Most scatter correction studies focused on 3D images, yet there were a few exceptions also investigating 4D applications [5], [6], [12], [13], [14]. The use of 4DCBCTs and 4D dose calculations could address uncertainties introduced by respiratory motion [7], [15], [16], [17]. However, due to sparse data, a 4D scatter correction of lung patient data is challenging and can result in poorly reconstructed images [18].
Deep convolutional neural networks (CNNs) [19], powerful tools for image feature extraction, which combine accurate scatter-corrected CBCT images with a clinically acceptable runtime, have shown promising results. In 2018, Kida et al. showed a conversion of CBCTs into vCTs [20] for 20 prostate cancer patients with a Unet [21]. Maier et al. trained a Unet for scatter prediction [22], [23], [24], which similarly to Kida et al. showed image quality improvements without, however, providing any dose calculations [25].
Hansen et al. used the U-shaped ScatterNet [26], which was also used in this study, with paired raw and corrected projections of 30 prostate patients. They evaluated their intensity correction with calculations of volumetric modulated arc therapy (VMAT) and intensity-modulated proton therapy (IMPT) plans. Landry et al. [27] investigated the same network to check whether it is optimal to train it with images or projections by using three different paired data sets (raw and corrected CBCT projections, CBCT and vCT, and CBCT and projection-corrected CBCT). More recently, Thummerer et al. [28] compared their output of a Unet trained with CBCT and CT images of 33 head and neck (H&N) patients with an analytical and a DIR correction approach. In their proton dose evaluation they showed accurate results for the network as well as for the DIR method, while the analytical method was worse. In a follow-up study, they trained separate networks with 4DCBCT and 4DCT pairs of axial, coronal, and sagittal slices of the 0% breathing phase [14]. Applying the result to all breathing phases, a complete scatter-corrected 4DCBCT could be generated. A high agreement in clinical target volume dose between generated corrected 4DCBCTs and registered 4DCTs, acquired on the same day, could be shown. Dong et al. [29] used 4DCBCT and CT slices of 20 non-small cell lung cancer (NSCLC) patients to train an unpaired network, called contrastive unpaired translation (CUT). Their network then generates from a poor-quality 4DCBCT a high-quality 4DCBCT. They showed improved image quality without any dose calculations.
This study’s main aim was speeding up a projection-based scatter correction worklfow for the generation of daily scatter-corrected 4DCBCT images, which are suitable for proton dose calculations within adaptive proton therapy workflows. We used the previously proposed ScatterNet architecture and extended it to 4DCBCT datasets of lung cancer patients. Training of ScatterNet was adapted to a 4D scenario by generating ground-truth corrected CBCT projections with a dedicated 4D scatter correction workflow [5], [6]. To evaluate the accuracy of ScatterNet-corrected 4DCBCTs, an image quality and dose calculation analysis was performed.
2. Materials and methods
2.1. Patient data
This retrospective study employed data from 26 lung cancer patients treated with photon therapy at the LMU University Hospital. For each patient, a free-breathing 3D planning CT (pCT), contoured by a trained radiation oncologist, a 4DCT, and corresponding measured CBCT projections of one arbitrary treatment fraction were collected. A Toshiba Aquilion LB (Canon Medical Systems, Japan) CT scanner was used to acquire pCTs and 4DCTs with a reconstruction grid of 1.074 × 1.074 × 3 mm3. 4DCT and pCT were acquired sequentially at the same appointment. For the CBCT acquisition, the on-board imaging system of an Elekta Synergy or VersaHD linac (XVI 4.5.1, Elekta, Sweden) with a shifted detector was used (collimator = M20, M position, 360° rotation, tube current = 40 mA, tube voltage = 120 kVp, exposure time = 40 ms, acquisition time = 2 min). By the time the data was retrospectively collected, several acquisition protocols had been in clinical use. We focused on acquisitions with more than 600 projections. Detailed patient characteristics are shown in the Supplementary materials in Table S1.
This retrospective study was exempt from requiring ethics approval. Bavarian state law (Bayrisches Krankenhausgesetz/Bavarian Hospital Law §27 Absatz 4 Datenschutz) allows the use of patient data for research, provided that any person‘s related data are kept anonymous. German radiation protection laws request a regular analysis of outcomes in the sense of quality control and assurance, thus in the case of purely retrospective studies no additional ethical approval is needed under German law.
2.2. CBCT correction approaches
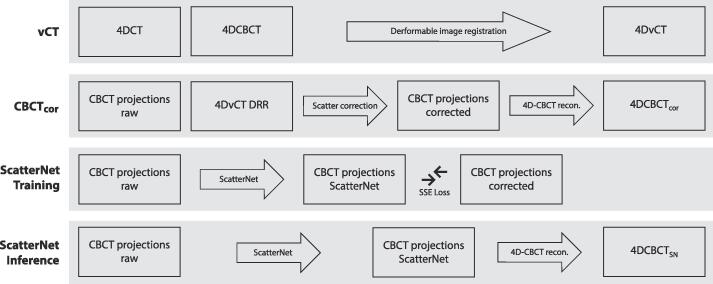
Three different CBCT correction approaches were used in this study: 1) A DIR-based workflow that generated the so-called 4DvCT. In this workflow a midposition image of the 4DCT was deformably registered to a midposition image of the 4DCBCT, yielding vCTmidp. The 4DvCT was then generated by applying the inverted deformable vector fields, calculated for the 4DCBCT midposition image, to vCTmidp [5], [6]. 2) A 4D variant of a projection-based scatter correction algorithm that was used to generate 4DCBCTcor [5], [6]. This scatter correction algorithm used the vCT and forward projected it, using the corresponding CBCT geometry, to yield CBCT projections which were assumed to be scatter free. Then the scatter contribution of the original projections was estimated by calculating the difference between scatter free and measured projections and applying a smoothing filter. Finally, scatter-corrected projections were obtained by subtracting the scatter contribution from the original projections. These projections were then used to reconstruct the scatter-corrected CBCTcor. 3) ScatterNet, a deep learning method, which is explained in detail in the Supplementary material (Fig. S1). It consisted of a 2D Unet with a single input layer and 8 channels followed by multiple resolution levels with 8, 16, 32, 64, 128, and 256 channels to model the low-frequency differences. ScatterNet generated the scatter-corrected projections which were reconstructed as the 4DCBCTSN using MA-ROOSTER [30]. The same reconstruction algorithm was also used for the scatter-corrected projections of 4DCBCTcor. Fig. 1 provides an overview of these three correction approaches and the involved datasets. A diffeomorphic Morphons DIR algorithm was used for all deformable image registrations in this study [31].
Fig. 1.
Block diagram showing the study design including the 4DvCT, 4DCBCTcor, 4DCBCTSN training and 4DCBCTSN inference workflows. The MA-ROOSTER method was used for 4DCBCT reconstruction. Abbreviations: SSE = sum of squared errors, DRR = digitally reconstructed radiograph.
For ScatterNet training, paired raw and corrected CBCT projections, the latter generated within the 4D scatter correction workflow described above [6], were considered for each patient. A total of 17,564 pairs of 2D projections from 26 patients were split 60%/20%/20%, which resulted in 15 training, 6 validation, and 5 testing patient datasets. All projections were zero-padded to a size of 512 × 512 pixels from 504 × 504 pixels. Data augmentation was performed by creating new projections through linear combination of two existing unrelated projections, also known as mixup [32] (details see Supplementary materials S1).
The field-of-view (FOV) of the CBCTcor projections was smaller in superior-inferior (SI) direction (4DvCT was smaller than the initial 4DCBCT due to the shorter 4DCT FOV in SI) than the FOV of the CBCTraw projections. Thus for training we only considered 128 rows in SI around the panel centre. When applying the network at test time, the FOV of the CBCTSN projections had the same size as the measured projections due to the fully convolutional architecture of the network.
2.3. Treatment planning
RayStation research version 8.99 (RaySearch Laboratories, Stockholm, Sweden) with a geneneric beam model resembling clinical proton therapy centres (energy range: 70 MeV to 230 MeV, nominal spot size (1 σ) at isocenter (70/230 MeV): 7 mm/2.7 mm, Bragg peak width at 80% dose level (70/230 MeV): 1.7 mm/8.5 mm) was used as treatment planning system (TPS). The pCT as well as all phases of the 4DCBCTSN, 4DvCT, and 4DCBCTcor were transferred to the TPS. For all images, the same CT-to-density calibration curve was used. For each patient an IMPT plan was generated on the pCT. A density override with muscle tissue was performed on the ITV to minimise plan degradation to mobile lung tumours [33], [34]. 3D pencil beam scanning plans, administering 60 Gy in 8 fractions were robustly optimised using the minimax optimisation method [35]. Optimisation was done individually for each beam. The Monte Carlo dose engine used a statistical error of 1%. Robustness settings were set to 3% range and 6 mm setup uncertainty [33]. A 2 mm × 2 mm × 2 mm dose grid was used and a range shifter of 7.5 cm was employed. All doses were calculated with a constant relative biological effectiveness (RBE) of 1.1 [36]. The objectives for the ITV were a minimum dose of 60 Gy, a maximum dose of 70 Gy, and a uniform dose of 60 Gy. The objectives for the considered organs-at-risk (OARs) oesophagus, heart, and bronchi were a maximum dose of 43 Gy, 65 Gy, and 46 Gy, respectively.
The proton dose distributions were recomputed without ITV density override on all phases of 4DCBCTSN, 4DvCT, and 4DCBCTcor.
2.4. Data analysis
For the analysis, 4DCBCTSN was compared with a corresponding 4DvCT and a scatter-corrected 4DCBCTcor, as generated for this cohort in Schmitz et al. [6]. Mean absolute error (MAE) and mean error (ME) in CT numbers were used to evaluate the image similarity within the union of the CBCT FOV with the CT body outline. Dose distributions were compared using DVH parameter ITV D98% and calculating global gamma pass-rates (PR) using (2%, 2 mm) and (3%, 3 mm) criteria with a fixed dose threshold of 10% of the prescribed dose.
3. Results
3.1. Network training
After 126 iterations the network training was stopped, as the validation loss no longer improved. For the final model, iteration 115 was used. Network training took around 12 h. After training, on average 3.9 s (5.7 ms per projection) were needed for the network to correct an entire projection set (up to 732 projections).
3.2. Image quality analysis
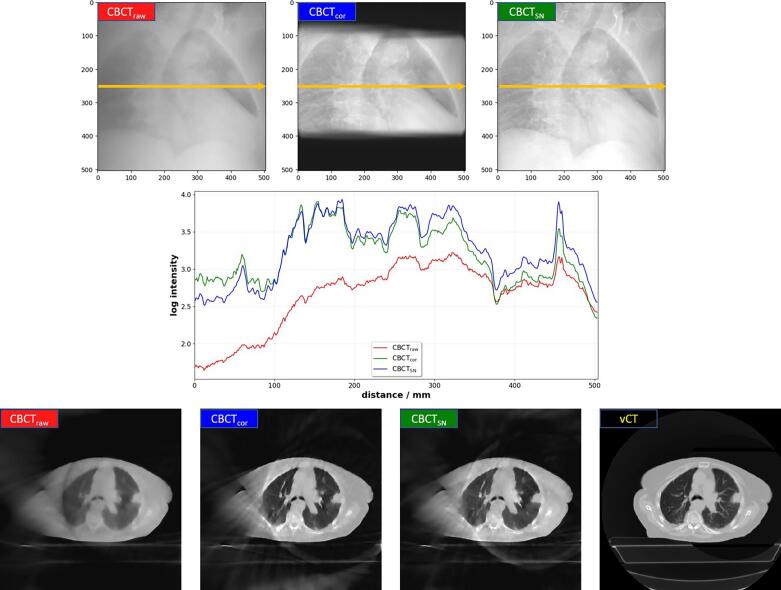
In Fig. 2 we observed that projections corrected by ScatterNet (CBCTSN) showed less blurring and enhanced structures when compared to the original projections (CBCTraw). This makes their appearance similar to the projections from the CBCTcor correction approach. The additionally presented line profiles in Fig. 2 show that CBCTcor and CBCTSN resulted in similar intensity values whereas for CBCTraw a larger difference can be observed.
Fig. 2.
(top row) Overview of the different CBCT projections shown for patient C. (middle row) Line profiles in log intensity are shown versus distance along the yellow line. The bottom row shows an axial view of phase 0 (inhale) of the reconstructed projections as well as the vCT. In each case projections and images are displayed with the same window and level.
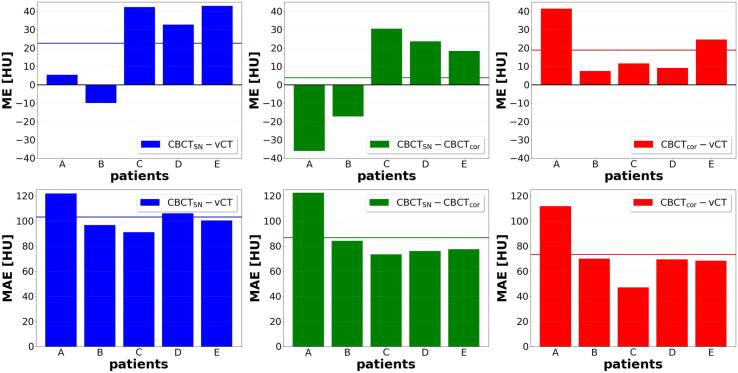
The comparison of 4DCBCTSN vs. 4DvCT, 4DCBCTSN vs. 4DCBCTcor, and 4DCBCTcor vs. 4DvCT resulted in average ME and MAE values of 23 HU, 4 HU, and 19 HU for ME and 102 HU, 87 HU, and 73 HU for MAE (averaged over the ten breathing phases) and are presented for each patient individually in Fig. 3.
Fig. 3.
For all testing patients averaged over the 10 breathing phases ME (top) and MAE (bottom) are shown for the comparisons 4DCBCTSN-4DvCT, 4DCBCTSN-4DCBCTcor, and 4DCBCTcor-4DvCT. For each case, the mean value over all test patients is indicated with a horizontal line.
3.3. Dose distribution analysis
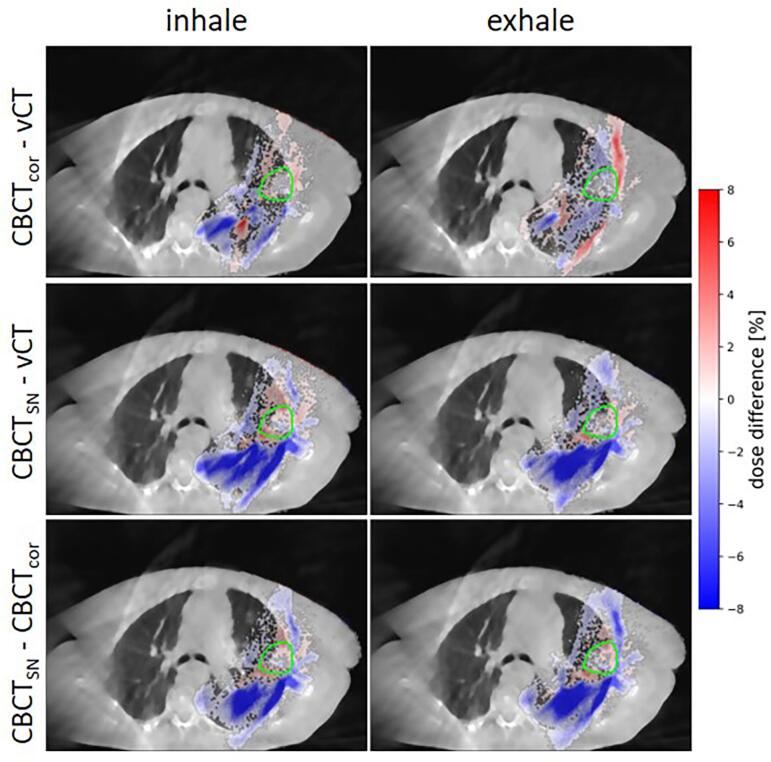
Comparing the dose distributions of the two extreme breathing phases between 4DCBCTcor and 4DvCT, 4DCBCTSN and 4DvCT, and 4DCBCTSN and 4DCBCTcor for test patient C, revealed small dose deviations of a few percent in and around the target structure (see Fig. 4). However, larger dose deviations were detected in the lung tissue when comparing 4DCBCTSN to either 4DvCT or 4DCBCTcor. This could also be observed in difference plots for an exemplary patient, presented in Supplementary Fig. S2 which is accompanied by the corresponding HU-error histogram showing the HU-error distribution (Supplementary Fig. S3).
Fig. 4.
Differences in percent of the prescribed dose of for the recalculated proton dose distribution of 4DvCT, 4DCBCTcor, and 4DCBCTSN from patient C. Dose deviations smaller than 0.4% are masked to improve the readability of the plot. The dose is overlayed on the 4DCBCTcor (top row) and 4DCBCTSN (middle and bottom row).
Table 1, which presents gamma PRs for all test patients for the comparisons CBCTSN-vCT, CBCTSN-CBCTcor, and CBCTcor-vCT, revealed lower median values for CBCTSN-vCT and CBCTSN-CBCTcor than for CBCTcor-vCT. For a 3%, 3 mm criteria, pass rates exceeded 90% for all test patients.
Table 1.
On a single patient level, gamma-index PRs in percent for a global criterion of 2%/2 mm and 3%/3 mm with a fixed dose threshold of 10% of the prescribed dose are shown. For the 10 breathing phases the median, minimum, and maximum values are shown.
| CBCTSN-vCT |
CBCTSN-CBCTcor |
CBCTcor-vCT |
||
|---|---|---|---|---|
| patient | median [min, max] | median [min, max] | median [min, max] | |
| 2%/2 mm | A | 88 [87, 89] | 90 [89, 92] | 98 [97, 99] |
| B | 85 [83, 87] | 86 [85, 87] | 99 [99, 99] | |
| C | 90 [87, 91] | 91 [89, 92] | 98 [96, 99] | |
| D | 92 [92, 93] | 93 [93, 93] | 98 [97, 98] | |
| E | 87 [81, 92] | 85 [78, 89] | 97 [96, 98] | |
| median | 88 [87, 91] | 90 [89, 92] | 98 [97, 99] | |
| 3%/3 mm | A | 97 [96, 98] | 97 [96, 98] | 100 [100, 100] |
| B | 92 [91, 93] | 93 [92, 94] | 100 [100, 100] | |
| C | 95 [93, 96] | 96 [95, 97] | 100 [99, 100] | |
| D | 96 [96, 97] | 97 [97, 97] | 99 [99, 100] | |
| E | 96 [91, 97] | 95 [90, 96] | 99 [99, 99] | |
| median | 96 [93, 97] | 96 [95, 97] | 100 [99, 100] | |
Median, minimum, and maximum ITV D98% values showed a high degree of agreement between 4DvCT, 4DCBCTcor, and 4DCBCTSN and are presented for the five test patients in Table 2. The largest median differences per patient were observed for patient B with 0.6 Gy, 0.7 Gy, and 1.3 Gy between 4DCBCTcor-4DvCT, 4DCBCTSN-vCT, and 4DCBCTSN-4DCBCTcor, respectively.
Table 2.
Median, minimum, and maximum ITV D98% values of the ten breathing phases of 4DvCT, 4DCBCTcor, and 4DCBCTSN in Gy for the five test patients.
| IMPT |
4DvCT |
4DCBCTcor |
4DCBCTSN |
|---|---|---|---|
| patient | median [min, max] | median [min, max] | median [min, max] |
| A | 61.2 [60.2, 61.7] | 60.8 [60.2, 61.2] | 60.5 [60.2, 60.9] |
| B | 64.7 [64.1, 65.3] | 64.1 [62.7, 65.3] | 63.4 [60.5, 66.5] |
| C | 60.9 [60.5, 61.1] | 60.7 [60.6, 61.1] | 60.9 [60.7, 61.1] |
| D | 60.9 [60.8, 61.0] | 60.2 [59.8, 60.5] | 60.7 [60.5, 60.8] |
| E | 63.5 [63.1, 63.8] | 63.8 [63.4, 64.4] | 63.6 [63.2, 64.4] |
| median | 62.2 [61.7, 62.6] | 61.9 [61.3, 62.5] | 61.8 [61.0, 62.7] |
4. Discussion
For the first time, deep learning was used for projection-based 4DCBCT scatter correction and evaluated in the context of proton dose calculations for adaptive proton therapy. ScatterNet addressed low frequency discrepancies, which otherwise lead to scatter and beam hardening artifacts [37], and resulted in a substantial image quality improvement over the initial 4DCBCT. A similar observation was made for the conventional 4DCBCT scatter correction workflow, yielding 4DCBCTcor [5], [6].
In the target region the dose difference plots showed a high agreement among the three investigated modalities 4DvCT, 4DCBCTcor, and 4DCBCTSN. Across the 5 testing patients, small median ITV D98% differences of less than 0.4 Gy were observed. 4DCBCTcor and 4DvCT [5], [6], similar to the methods applied in 3D [4], [10], have been previously found sufficient for proton dose calculation. Similar average 3%/3 mm gamma pass rates were observed when comparing 4DCBCTSN to 4DCBCTcor and 4DvCT (96%). Larger dose differences were observed in the lung tissue between 4DCBCTSN-4DvCT and 4DCBCTSN-4DCBCTcor. These differences were less prominent when comparing 4DCBCTcor to 4DvCT. 3%/3 mm pass rates of 4DCBCTSN were comparable or better than previously reported results [14]. This study mainly focused on target doses (ITV D98%). Future studies are required to also investigate the dose differences in organs at risk and their clinical relevance.
Compared to a previous study that utilized ScatterNet to correct 3DCBCTs in the pelvis region [26], a higher average MAE was observed for scatter-corrected 4DCBCTs in the thorax (46 HU vs. 87 HU, CBCTcor as reference). This can partially be attributed to the significantly lower image quality of 4D-CBCTs when compared to 3D-CBCTs, which made 4D-scatter correction a more challenging task. The neural network architecture of ScatterNet for 3D and 4D scatter correction was the same. The main difference between this study and previous 3D studies using ScatterNet was the use of lung cancer patient images. In 4D, the training of ScatterNet required data from a previously investigated 4D version of a conventional scatter correction workflow [6]. 3D scatter-corrected projections would not be sufficient since corrected projections would get blurred because of forward projection of a 3DCT image without motion information.
The network could potentially be improved by using a larger patient data set, using more fractions per patient or more patients overall. Interesting future research directions could be employing reconstructed images as training data similar to the CUT architecture of Dong et al. [29] or the Unet of Landry et al. [27]. For the latter 2.5D or 3D approaches such as shown by Thummerer et al. [14] or by Neppl et al. [38] could also be considered.
For clinical implementation, stability and time consumption of the method are as important as performance. Stability, which could be tested with data sets acquired from different machines or centres, needs to be investigated in future studies. In comparison to the 4D scatter correction workflow and the 4DvCT approach, which were all conducted on the same computer, scatter correction times for all phases per patient decreased from approximately 10 min (4DCBCTcor) and 30 min (4DvCT) to 3.9 s. This does not include the 4DCBCT reconstruction with MA-ROOSTER [30] and dose calculations, which required approximately 10 min and 4 min respectively. Thummerer et al., who also used MA-ROOSTER for reconstruction, even reported an average of 45 min using an NVIDIA GTX 1080Ti GPU for their six-phase 4DCBCT reconstruction [14]. A combination of an AI-based reconstruction method [39] with ScatterNet could in the future bring down the total time to clinically acceptable levels. Consequently, ScatterNet is a potentially interesting technique for daily clinical decisions such as the necessity of replanning.
The possibility of accurate proton dose calculations on scatter-corrected 4DCBCTs can enable future studies investigating clinically relevant aspects of proton therapy for moving targets, such as inter- and intra-fractional plan robustness and interplay effects [7], [16], [17], on a daily basis. Beam parameters such as spot size or the use of a range shifter, can influence plan robustness and hence might also have an influence on the observed dose differences between the various scatter correction methods. In this study, a generic beam model resembling clinical proton centres was used to generate clinically meaningful results.
In conclusion, ScatterNet corrects intensities of 4DCBCTs of lung cancer patients and enables phase-dependent proton dose calculations. In target volumes, the dose calculation accuracy was comparable to a conventional, non-AI 4D scatter correction workflow. Low computational times of the presented CNN technique suggest its suitability for daily 4D dose reconstructions and its potential value in adaptive proton therapy workflows.
CRediT authorship contribution statement
Henning Schmitz: Conceptualization, Methodology, Software. Adrian Thummerer: Writing - review & editing, Visualization. Maria Kawula: Software, Writing - review & editing. Elia Lombardo: Software, Writing - review & editing. Katia Parodi: Writing - review & editing. Claus Belka: Writing - review & editing. Florian Kamp: Conceptualization, Methodology, Writing - review & editing. Christopher Kurz: Conceptualization, Methodology, Software, Writing - review & editing. Guillaume Landry: Conceptualization, Methodology, Software, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the German Research Foundation (DFG) project number 399148265 and Research Training Group GRK 2274.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.phro.2023.100482.
Supplementary data
The following are the Supplementary data to this article:
References
- 1.Landry G., Hua C. Current state and future applications of radiological image guidance for particle therapy. Med Phys. 2018;45:11. doi: 10.1002/mp.12744. [DOI] [PubMed] [Google Scholar]
- 2.Hu Y., Arnesen M., Aland T. Characterization of an advanced cone beam CT (CBCT) reconstruction algorithm used for dose calculation on Varian Halcyon linear accelerators. Biomed Phys Eng Express. 2022;8(2) doi: 10.1088/2057-1976/ac536b. [DOI] [PubMed] [Google Scholar]
- 3.Giacometti V., Hounsell A.R., McGarry CK. A review of dose calculation approaches with cone beam CT in photon and proton therapy. Phys Med. 2020;76:243–276. doi: 10.1016/j.ejmp.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Kurz C., Kamp F., Park Y.K., Zöllner C., Rit S., Hansen D., et al. Investigating deformable image registration and scatter correction for CBCT-based dose calculation in adaptive IMPT. Med Phys. 2016;43(10):5635–5646. doi: 10.1118/1.4962933. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz H., Rabe M., Janssens G., Bondesson D., Rit S., Parodi K., et al. Validation of proton dose calculation on scatter corrected 4D cone beam computed tomography using a porcine lung phantom. Phys Med Biol. 2021;66(17) doi: 10.1088/1361-6560/ac16e9. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz H., Rabe M., Janssens G., Rit S., Parodi K., Belka C., et al. Scatter correction of 4D cone beam computed tomography to detect dosimetric effects due to anatomical changes in proton therapy for lung cancer. Med Phys. 2023;50(8):4981–4992. doi: 10.1002/mp.16335. [DOI] [PubMed] [Google Scholar]
- 7.Trnkova P., Zhang Y., Toshito T., Heijmen B., Richter C., Aznar M.C., et al. A survey of practice patterns for adaptive particle therapy for interfractional changes. Phys Imaging Radiat Oncol. 2023;26 doi: 10.1016/j.phro.2023.100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piperdi H., Portal D., Neibart S.S., Yue N.J., Jabbour S.K., Reyhan M. Adaptive Radiation Therapy in the Treatment of Lung Cancer: An Overview of the Current State of the Field. Front Oncol. 2021:11. doi: 10.3389/fonc.2021.770382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veiga C., Janssens G., Teng C.L., Baudier T., Hotoiu L., McClelland J.R., et al. First Clinical Investigation of Cone Beam Computed Tomography and Deformable Registration for Adaptive Proton Therapy for Lung Cancer. Int J Radiat Oncol Biol Phys. 2016;95(1):549–559. doi: 10.1016/j.ijrobp.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 10.Park Y.K., Sharp G.C., Phillips J., Winey B.A. Proton dose calculation on scatter-corrected CBCT image: Feasibility study for adaptive proton therapy. Med Phys. 2015;42(8):4449–4459. doi: 10.1118/1.4923179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niu T., Sun M., Star-Lack J., Gao H., Fan Q., Zhu L. Shading correction for on-board cone-beam CT in radiation therapy using planning MDCT images. Med Phys. 2010;37(10):5395–5406. doi: 10.1118/1.3483260. [DOI] [PubMed] [Google Scholar]
- 12.Bondesson D., Meijers A., Janssens G., Rit S., Rabe M., Kamp F., et al. Anthropomorphic lung phantom based validation of in-room proton therapy 4D-CBCT image correction for dose calculation. Z Med Phys. 2022;32(1):74–84. doi: 10.1016/j.zemedi.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niepel K., Kamp F., Kurz C., Hansen D., Rit S., Neppl S., et al. Feasibility of 4DCBCT-based proton dose calculation: An ex vivo porcine lung phantom study. Z Med Phys. 2019;29(3):249–261. doi: 10.1016/j.zemedi.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Thummerer A., Oria C.S., Zaffino P., Visser S., Meijers A., Marmitt G.G., et al. Deep learning–based 4D-synthetic CTs from sparse-view CBCTs for dose calculations in adaptive proton therapy. Med Phys. 2022;49(11):6824–6839. doi: 10.1002/mp.15930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bert C., Durante M. Motion in radiotherapy: particle therapy. Phys Med Biol. 2011;56(16):R113–R144. doi: 10.1088/0031-155/56/16/r01. Last edited Date: August 3, 2023 REFERENCES ScatterNet for 4DCBCT: Printed August 3, 2023 page 12. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Trnkova P., Toshito T., Heijmen B., Richter C., Aznar M., et al. A survey of practice pat terns for real-time intrafractional motion-management in particle therapy. Phys Imaging Radiat Oncol. 2023;26 doi: 10.1016/j.phro.2023.100439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebbink F., Stocchiero S., Fossati P., Engwall E., Georg D., Stock M., et al. Parameter based 4D dose calculations for proton therapy. Phys Imaging Radiat Oncol. 2023;27 doi: 10.1016/j.phro.2023.100473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shieh C.C., Gonzalez Y., Li B., Jia X., Rit S., Mory C., et al. SPARE: Sparse-view reconstruction challenge for 4D cone-beam CT from a 1-min scan. Med Phys. 2019;46(9):3799–3811. doi: 10.1002/mp.13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecun Y., Bottou L., Bengio Y., Haffner P. Gradient-based learning applied to document recognition. Proc. IEEE. 1998;86(11):2278–2324. doi: 10.1109/5.726791. [DOI] [Google Scholar]
- 20.Peroni M., Ciardo D., Spadea M.F., Riboldi M., Comi S., Alterio D., et al. Automatic Segmentation and Online virtualCT in Head-and-Neck Adaptive Radiation Therapy. Int J Radiat Oncol Biol Phys. 2012;84(3):e427–e433. doi: 10.1016/j.ijrobp.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Ronneberger O., Fischer P., Brox T. Lect. Notes Comput. Sci. Springer International Publishing; 2015. U-Net: Convolutional Networks for Biomedical Image Segmentation; pp. 234–241. [DOI] [Google Scholar]
- 22.Maier J, Sawall S, Kachelriess M, and Berker Y. Deep scatter estimation (DSE): feasibility of using a deep convolutional neural network for real-time x-ray scatter prediction in cone-beam CT. In: Medical Imaging 2018: Physics of Medical Imaging. Ed. by GH Chen, JY Lo, and TG Schmidt. SPIE, 2018. doi: 10.1117/12.2292919.
- 23.Maier J., Eulig E., Vöth T., Knaup M., Kuntz J., Sawall S., et al. Real-time scatter estimation for medical CT using the deep scatter estimation: Method and robustness analysis with respect to different anatomies, dose levels, tube voltages, and data truncation. Med Phys. 2018;46(1):238–249. doi: 10.1002/mp.13274. [DOI] [PubMed] [Google Scholar]
- 24.Maier J., Sawall S., Knaup M., Kachelrieß M. Deep Scatter Estimation (DSE): Accurate Real-Time Scatter Estimation for X-Ray CT Using a Deep Convolutional Neural Network. J. Nondestruct Evaluat. 2018;37:3. doi: 10.1007/s10921-018-0507-z. [DOI] [Google Scholar]
- 25.Rusanov B., Hassan G.M., Reynolds M., Sabet M., Kendrick J., Rowshanfarzad P., et al. Deep learning methods for enhancing cone-beam CT image quality toward adaptive radiation therapy: A systematic review. Med Phys. 2022;49(9):6019–6054. doi: 10.1002/mp.15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen D.C., Landry G., Kamp F., Li M., Belka C., Parodi K., et al. ScatterNet: A convolutional neural network for cone-beam CT intensity correction. Med Phys. 2018;45(11):4916–4926. doi: 10.1002/mp.13175. [DOI] [PubMed] [Google Scholar]
- 27.Landry G., Hansen D., Kamp F., Li M., Hoyle B., Weller J., et al. Comparing Unet training with three different datasets to correct CBCT images for prostate radiotherapy dose calculations. Phys Med Biol. 2019;64(3) doi: 10.1088/1361-6560/aaf496. [DOI] [PubMed] [Google Scholar]
- 28.Thummerer A., Zaffino P., Meijers A., Marmitt G.G., Seco J., Steenbakkers R.J.H.M., et al. Comparison of CBCT based synthetic CT methods suitable for proton dose calculations in adaptive proton therapy. Phys Med Biol. 2020;65(9) doi: 10.1088/1361-6560/ab7d54. [DOI] [PubMed] [Google Scholar]
- 29.Dong G., Zhang C., Deng L., Zhu Y., Dai J., Song L., et al. A deep unsupervised learning framework for the 4D CBCT artifact correction. Phys Med Biol. 2022;67(5) doi: 10.1088/1361-6560/ac55a5. [DOI] [PubMed] [Google Scholar]
- 30.Mory C., Janssens G., Rit S. Motion-aware temporal regularization for improved 4D cone-beam computed tomography. Phys Med Biol. 2016;61(18):6856–6877. doi: 10.1088/0031-9155/61/18/6856. [DOI] [PubMed] [Google Scholar]
- 31.Janssens G., Jacques L., de Xivry J.O., Geets X., Macq B. Diffeomorphic Registration of Images with Variable Contrast Enhancement. Int J Biomed Imaging. 2011;2011:1–16. doi: 10.1155/2011/891585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Cisse M, Dauphin YN, and Lopez-Paz D. Mixup: Beyond Empirical Risk Minimization. 2017. https://arxiv.org/abs/1710.09412.
- 33.Meijers A., Knopf A.C., Crijns A.P., Ubbels J.F., Niezink A.G., Langendijk J.A., et al. Evaluation of interplay and organ motion effects by means of 4D dose reconstruction and accumulation. Radiother Oncol. 2020;150:268–274. doi: 10.1016/j.radonc.2020.07.055. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro C.O., Visser S., Korevaar E.W., Sijtsema N.M., Anakotta R.M., Dieters M., et al. Towards the clinical implementation of intensity-modulated proton therapy for thoracic indications with moderate motion: Robust optimised plan evaluation by means of patient and machine specific information. Radiother Oncol. 2021;157:210–218. doi: 10.1016/j.radonc.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Fredriksson A., Forsgren A., Hårdemark B. Minimax optimization for handling range and setup uncertainties in proton therapy. Med Phys. 2011;38(3):1672–1684. doi: 10.1118/1.3556559. [DOI] [PubMed] [Google Scholar]
- 36.Paganetti H., Niemierko A., Ancukiewicz M., Gerweck L.E., Goitein M., Loeffler J.S., et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53(2):407–421. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 37.Zöllner C., Rit S., Kurz C., Vilches-Freixas G., Kamp F., Dedes G., et al. Decomposing a prior-CT-based cone-beam CT projection correction algorithm into scatter and beam hardening components. Phys Imaging Radiat Oncol. 2017;3:49–52. doi: 10.1016/j.phro.2017.09.002. [DOI] [Google Scholar]
- 38.Neppl S., Kurz C., Köpl D., Yohannes I., Schneider M., Bondesson D., et al. Measurement-based range evaluation for quality assurance of CBCT-based dose calculations in adaptive proton therapy. Med Phys. 2021;48(8):4148–4159. doi: 10.1002/mp.14995. [DOI] [PubMed] [Google Scholar]
- 39.Madesta F., Sentker T., Gauer T., Werner R. Self-contained deep learning-based boosting of 4D cone-beam CT reconstruction. Med Phys. 2020;47(11):5619–5631. doi: 10.1002/mp.14441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.