Abstract
Objectives
HIV disease progression has been well documented in Western populations. This study aimed to estimate the short-term risk of AIDS and death from the TREAT Asia HIV Observational Database (TAHOD), a prospective, multicentre cohort study in Asia and the Pacific region.
Methods
Prospective data were analysed to estimate short-term disease progression. Endpoints were defined as the time from study entry to diagnosis with AIDS or death. Antiretroviral treatment was fitted as a time-dependent variable. Predictors of disease progression were assessed using Cox proportional hazards models, and prognostic models were developed using Weibull models.
Results
A total of 1260 patients with prospective follow-up data contributed 477 person-years of follow-up, during which 18 patients died and 34 were diagnosed with AIDS, a combined rate of 10.1 per 100 person-years. Compared with patients receiving antiretroviral treatment, patients not on treatment had a higher rate of disease progression (17.6 vs. 8.1 per 100 person-years, respectively). Baseline CD4 count was the strongest predictor of disease progression. Prognostic models, using either a baseline CD4 count as the sole marker or markers including baseline haemoglobin, AIDS-related symptoms and previous or current antiretroviral treatment, were successful at identifying patients at high risk of short-term disease progression.
Conclusions
Similar to the situation in Western countries, baseline CD4 count was the strongest predictor of short-term disease progression. Prognostic models based on readily available clinical data and haemoglobin level should be useful in estimating short-term clinical risk in HIV-infected patients in Asia and the Pacific region.
Introduction
Rates of disease progression in HIV disease, in terms of survival and newly diagnosed AIDS-defining illnesses, are relatively well documented in Western populations [1–6]. However, these aspects of HIV disease are less well described in Asian populations.
Estimates of the probability of disease progression in HIV-infected patients are important for understanding the natural history of HIV disease. This information is also important for developing guidelines of treatment and care, and prioritizing and allocating resources for health services, especially in developing countries. CD4 counts and HIV viral load measurements are important predictors for assessing disease progression and informing treatment and care [6–8]. However, in resource-poor settings these laboratory tests are not always in place. An algorithm using less sophisticated markers, such as haemoglobin [6,10,11], is useful in such settings.
The TREAT Asia HIV Observational Database (TAHOD) is the first collaborative study by the TREAT Asia network, a co-operative network of clinicians throughout Asia and the Pacific, which is funded by the American Foundation of AIDS Research (amfAR). Using the prospective data in TAHOD over the first 6 months of follow up, this study aimed:
to estimate the short-term risk of AIDS and death in patients receiving or not receiving antiretroviral therapy; and
to develop algorithms to predict the risk of AIDS and death in individual patients using prognostic markers including CD4 counts, HIV viral loads and other markers with fewer resource requirements, such as haemoglobin level and body mass index.
Methods
TAHOD is a collaborative observational cohort study involving 11 sites in the Asia and Pacific region (see Appendix). Detailed methods were published elsewhere [9], but briefly, each site recruited 200 patients, both treated and untreated with antiretroviral drugs. Recruitment was based on a consecutive series of patients regularly attending a given site from a particular start-up time.
The following data were collected: (1) patient demographics: date of the clinical visit, age, gender, ethnicity, exposure category, date of first positive HIV test, HIV-1 subtype, and date and result of hepatitis B virus, hepatitis C virus and syphilis serology; (2) stage of disease: CD4 and CD8 counts, HIV viral load estimation, prior AIDS-defining illness, and date and cause of death; (3) treatment history: prior and current prescribed antiretroviral treatments, reason for treatment changes (e.g. treatment failure, disease progression and adverse events) and prophylactic treatments for opportunistic infection.
A modified version of the 1993 Centers for Disease Control and Prevention (CDC) AIDS case definition [12] was adopted, in which a presumptive diagnosis was available for most illnesses. All data were entirely observational, with test or intervention performed only according to the clinical guideline at each site. It was also noted that the laboratory methods varied across the sites. For example, HIV viral load estimations were carried out using Roche Amplicor monitor (Roche Molecular Systems Inc., Branchburg, NJ, USA) or Quantiplex bDNA assay (Chiron Diagnostics, East Walpole, MA, USA).
Data were combined via standardized formats in Microsoft Excel and transferred electronically (compressed with password-protection) to the National Centre in HIV Epidemiology and Clinical Research (NCHECR) for central aggregation. The first data transfer was in September 2003, with updated data in March and September each year.
Ethical approval for the study was obtained from the University of New South Wales Ethics Committee. Each site also approached a local ethics committee for approval.
A combined endpoint of progression to a newly diagnosed AIDS-defining illness or death since entering TAHOD was used in this study, because of the short period of follow-up and thus limited events in the early phase of the TAHOD cohort. Deaths from all causes were included. AIDS cases were defined using the 1993 CDC revision of the AIDS case definition [12].
TAHOD started patient recruitment in 2003 and continued recruiting patients until each site reached 200 patients. Analyses in this study were based on all patients with baseline data and at least one appropriate prospective follow-up visit. Follow-up was censored at date of AIDS diagnosis or death, date of the most recent visit, or the date the patient was last known to be alive.
Predictors for progression to AIDS or death were assessed by univariate and multivariate analyses using Cox proportional hazards models. Antiretroviral treatment was fitted as a time-dependent variable. Multivariate models were built using forward stepwise techniques. Two models, with and without baseline CD4 count and HIV viral load measurement, were developed to assess the predictors of progression to AIDS or death. Statistical significance was taken as P<0.05.
Prognostic models were developed using parametric survival models. Several parametric forms were considered, including Weibull, log-logistic, lognormal, exponential, generalized gamma, and gompertz distributions, with the best-fitting form chosen on likelihood criteria [2]. Independent predictors from the two multivariate Cox models were used in developing models. In the model not including baseline CD4 measurements, prior and current antiretroviral treatments both independently showed significance in the model but lost significance when put together. Since previous and current treatments were highly correlated in this cohort, patients having prior treatment being most likely to remain on treatment after entering TAHOD, two models were developed using prior and current treatments separately as prognostic variables and yielded almost identical results.
Results
From September 2003 to May 2004, 1887 patients were recruited to TAHOD. Of the 1279 patients recruited to TAHOD at the first data transfer in September 2003, 1112 had follow-up data at the second data transfer, an overall follow-up rate of 87%. A further 148 patients recruited to TAHOD after the first data exchange also had follow-up data available and were included in the analysis. Table 1 summarizes the characteristics of the 1260 patients who were included in this study. The majority of the patients were male, with a median age of 36 years [interquartile range (IQR) 32–42 years] at recruitment to TAHOD. Chinese, Indian and Thai were the main ethnic groups. Most patients (80%) were infected through heterosexual contact. At time of recruitment to TAHOD, 9% of patients were diagnosed with CDC category B disease while not having AIDS, and 44% were diagnosed with AIDS. The median CD4 count was 280 cells/μL (IQR 147–416 cells/μL); the median viral load was <400 HIV-1 RNA copies/mL (IQR <400–6541 copies/mL). Most patients (75%), when entering TAHOD, were receiving antiretroviral treatment, of whom the majority (73%) were on a combination of three drugs or more [or highly active antiretroviral treatment (HAART)]; in particular, 70% of them were treated with a combination of three or more drugs which includes at least one nonnucleoside reverse transcriptase inhibitor (NNRTI), but excludes protease inhibitors (PIs). Among patients who had baseline haemoglobin tested at time of entry to TAHOD, 56% had normal levels of haemoglobin (greater than 13 g/dL for male patients and 12 g/dL for female patients), 41% had mild anaemia (haemoglobin 8–13 g/dL for male and 8–12 g/dL for female patients) and 3% had severe anaemia (haemoglobin less than 8 g/dL for both male and female patients).
Table 1.
Baseline patient characteristics
| Age at entry to TAHOD (years) | |
| Median (IQR) | 36 (32–42) |
| <20 | 1 (<1) |
| 20–29 | 198 (16) |
| 30–39 | 636 (50) |
| 40–49 | 288 (23) |
| 50+ | 135 (11) |
| Missing | 2 (<1) |
| Gender | |
| Male | 902 (72) |
| Female | 357 (28) |
| Transgender | 1 (<1) |
| Ethnicity | |
| Chinese | 520 (41) |
| Thai | 357 (28) |
| Indian | 266 (21) |
| Philippine | 76 (6) |
| Malay | 27 (2) |
| Caucasian | 5 (<1) |
| Other | 9 (1) |
| Exposure category (29 missing) | |
| Heterosexual contact | 987 (80) |
| Homosexual contact | 129 (10) |
| Reception of blood/product | 38 (3) |
| Heterosexual contact and IDU | 16 (1) |
| IDU only | 8 (1) |
| Other | 53 (5) |
| First year diagnosed with HIV (83 missing) | |
| Median (IQR) | 2001 (1998–2002) |
| Before 1997 | 152 (13) |
| 1997–1999 | 262 (22) |
| 2000–2002 | 550 (47) |
| 2003–2004 | 213 (18) |
| CDC clinical classification for HIV infection* | |
| Category A | 588 (47) |
| Category B | 114 (9) |
| Category C | 558 (44) |
| Baseline CD4 count† (cells/μL) (103 not tested) | |
| Median (IQR) | 280 (147–416) |
| <50 | 106 (9) |
| 50–199 | 289 (25) |
| 200–499 | 548 (48) |
| 500+ | 202 (18) |
| Baseline HIV viral load† | |
| (HIV-1 RNA copies/mL) (541 not tested) | |
| Median (IQR) | 399 (<400–6541) |
| Not detectable (<400 copies/mL) | 516 (65) |
| 400–10000 | 87 (11) |
| 10000+ | 189 (24) |
| Antiretroviral treatment at entry of TAHOD | |
| Not on treatment | 321 (25) |
| Mono/double therapy | 61 (5) |
| 3+(NRTI+/−PI−NNRTI)‡ | 165 (13) |
| 3+(NRTI+NNRTI−PI)‡ | 662 (53) |
| 3+(NNRTI+PI+/−NRTI)‡ | 51 (4) |
IQR, interquartile range; IDU, injecting drug use;
Centers for Disease Control and Prevention (CDC) 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS among Adolescents and Adults [12].
CD4 count and HIV viral load measured at time of entry to TAHOD (CD4 within 180 days and HIV viral load within 365 days).
Highly active antiretroviral treatment (HAART): 3+(NRTI+/−PI−NNRTI), combination of three or more drugs including NRTI and/or PI, but excluding NNRTI; 3+(NRTI+NNRTI−PI), combination of three or more drugs including at least one NNRTI, but excluding PI; 3+(NNRTI+PI+/−NRTI), combination of three or more drugs including NNRTI, and PI and/or NRTI.
Median follow-up time was 129.5 days (IQR 91–182 days). During a total of 477 person-years, 48 patients (3.8% of all patients) experienced clinical events since entering TAHOD. Of the patients experiencing these events, 18 (37.5%) died and 34 (62.5%) had a newly diagnosed AIDS-defining illness. Cause of death was directly related to HIV/AIDS in 15 patients; the remaining three died as a result of peritoneal sepsis, lactic acidosis and head injury. Among the 34 patients who were newly diagnosed with AIDS, the most common illness was tuberculosis (n=16, 44%), followed by oesophageal candidiasis (n=4), toxoplasmosis (n=4), non-tuberculosis mycobacterial diseases (n=3), extrapulmonary cryptococcosis (n=2), herpes simplex infection (n=2), Salmonella septicaemia (n=2), immunoblastic lymphoma (n=1), primary lymphoma of the brain (n=1), and recurrent pneumonia (n=1). There were two patients diagnosed with two AIDS-defining illnesses on the same day.
The rate of progression to AIDS or death was 10.1 per 100 person-years [95% confidence interval (CI) 7.6–13.3; Table 2]. Patients who were not on antiretroviral treatment had a higher rate of disease progression than patients who were on treatment (17.6 vs. 8.1 per 100 person-years, respectively). Baseline CD4 count, HIV viral load, haemoglobin level, CDC clinical classification of HIV infection, and prior and current antiretroviral treatments were all significantly predictive of disease progression in the univariate analysis. Baseline body mass index (BMI) was not significant, possibly because of the large proportion of patients who did not have either weight or height recorded.
Table 2.
Univariate analysis of predictors for progression to AIDS or death using the Cox proportional hazards model
| Gender | ||||||
| Male | 902 | 338.4 | 35 | 10.3 | ||
| Female | 357 | 138.6 | 13 | 9.4 | 0.88 | 0.697 |
| Transgender | 1 | 0.3 | 0 | 0.0 | – | – |
| Age at entry to TAHOD (years) | ||||||
| <30 | 252 | 101.8 | 11 | 10.8 | ||
| 31–40 | 628 | 232.6 | 26 | 11.2 | 1.06 | 0.872 |
| 41+ | 378 | 141.8 | 11 | 7.8 | 0.71 | 0.417 |
| Missing | 2 | 1.1 | 0 | 0.0 | – | – |
| Exposure | ||||||
| Heterosexual | 1003 | 387.7 | 43 | 11.1 | ||
| Homosexual | 129 | 47.0 | 0 | 0.0 | – | – |
| Other | 128 | 42.6 | 5 | 11.7 | 1.03 | 0.944 |
| HBV status | ||||||
| Negative/not tested | 1126 | 429.3 | 45 | 10.5 | ||
| Positive | 134 | 48.0 | 3 | 6.2 | 0.61 | 0.412 |
| HCV status | ||||||
| Negative/not tested | 1223 | 465.3 | 45 | 9.7 | ||
| Positive | 37 | 12.0 | 3 | 25.0 | 2.62 | 0.107 |
| Baseline CD4 (cells/μL) | ||||||
| <50 | 106 | 40.8 | 13 | 31.8 | ||
| 50–199 | 289 | 116.3 | 17 | 14.6 | 0.46 | 0.034 |
| 200+ | 750 | 279.1 | 8 | 2.9 | 0.09 | <0.001 |
| Not tested | 115 | 41.1 | 10 | 24.3 | 0.74 | 0.478 |
| Baseline HIV viral load | ||||||
| (HIV-1 RNA copies/mL) | ||||||
| <400 | 516 | 194.8 | 7 | 3.6 | ||
| 400+ | 276 | 106.3 | 10 | 9.4 | 2.60 | 0.053 |
| Not tested | 468 | 176.2 | 31 | 17.6 | 4.74 | <0.001 |
| Baseline BMI* | ||||||
| Underweight (< 18.5) | 137 | 49.0 | 7 | 14.3 | ||
| Normal (18.5–24.9) | 479 | 177.1 | 14 | 7.9 | 0.55 | 0.195 |
| Overweight (25.0+) | 104 | 38.6 | 4 | 10.4 | 0.72 | 0.600 |
| Missing | 540 | 212.6 | 23 | 10.8 | 0.72 | 0.454 |
| Baseline haemoglobin† | ||||||
| Normal | 661 | 242.7 | 17 | 7.0 | ||
| Anaemia | 476 | 195.7 | 30 | 15.3 | 2.21 | 0.009 |
| Severe anaemia | 35 | 10.6 | 4 | 37.6 | – | – |
| Not tested | 88 | 39.0 | 1 | 2.6 | 0.37 | 0.334 |
| Baseline AIDS-related symptoms | ||||||
| Asymptomatic | 588 | 218.0 | 12 | 5.5 | ||
| Symptomatic | 672 | 259.3 | 36 | 10.0 | 2.56 | 0.005 |
| CDC category B | 114 | 41.4 | 9 | 21.7 | 4.00 | 0.002 |
| CDC category C | 558 | 217.8 | 27 | 12.4 | 2.29 | 0.017 |
| Antiretroviral treatment | ||||||
| No | 321 | 96.7 | 17 | 17.6 | ||
| Yes | 939 | 380.6 | 31 | 8.1 | 0.50 | 0.020 |
| Prior antiretroviral treatment | ||||||
| No | 270 | 99.5 | 19 | 19.1 | ||
| Yes | 990 | 377.8 | 29 | 7.7 | 0.41 | 0.002 |
HBV, hepatitis B virus; HCV, hepatitis C virus; CDC, Centers for Disease Control and Prevention.
BMI, body mass index. BMI=weight (kg)/[height (m)]2); underweight, BMI<18.5; normal, BMI 18.5–24.9; overweight, BMI>25.0.
Definition of anaemia: normal, haemoglobin>13 g/dL for male and 12 g/dL for female patients; anaemia, 8–13 g/dL for male and 8–12 g/dL for female patients; severe anaemia,<8 g/dL for both male and female patients.
A Weibull proportional hazards model was chosen from the parametric survival models considered in developing prognostic models, based on likelihood criteria and the literature [2]. Three sets of variables were included from the multivariate Cox model (Table 3). There was a trend for decreased risk of disease progression with an increased baseline CD4 count. Compared with a baseline CD4 count of <50 cells/μL, the risk of progress to AIDS or death was 46% when CD4 counts were between 50 and 199 cells/μL and 9% when CD4 counts were≥200 cells/μL. In the second and third models, because of the correlation between previous and current treatments, the results were similar in terms of hazard ratio of baseline haemoglobin (diagnosis with anaemia carried an 88 to 91% increased risk of progress to AIDS or death), baseline AIDS-related symptoms (being symptomatic carried a 125 to 133% increased risk), and having previous or current antiretroviral treatment (being untreated carried a 170 to 188% increased risk).
Table 3.
Hazard ratios of progression to AIDS or death, from Weibull models
| Baseline CD4 count (cells/μL) | |||
| <50 | 1.00 | ||
| 50–199 | 0.46 | 0.22–0.95 | 0.036 |
| 200+ | 0.09 | 0.04–0.22 | <0.001 |
| Model 2 (n=1172) | |||
| Baseline haemoglobin | |||
| Normal | 1.00 | ||
| Anaemia | 1.91 | 1.05–3.50 | 0.035 |
| Baseline AIDS-related symptoms | |||
| Asymptomatic | 1.00 | ||
| Symptomatic | 2.33 | 1.19–4.57 | 0.014 |
| Antiretroviral treatment | |||
| Yes | 1.00 | ||
| No | 2.70 | 1.48–4.93 | 0.001 |
| Model 3 (n=1172) | |||
| Baseline haemoglobin | |||
| Normal | 1.00 | ||
| Anaemia | 1.88 | 1.03–3.45 | 0.041 |
| Baseline AIDS-related symptoms | |||
| Asymptomatic | 1.00 | ||
| Symptomatic | 2.25 | 1.15–4.39 | 0.018 |
| Prior antiretroviral treatment | |||
| Yes | 1.00 | ||
| No | 2.88 | 1.60–5.17 | <0.001 |
We assessed the fitness of the first two models by calculating the expected number of events at 3 months after entry to TAHOD and comparing this with the observed events, stratified by different risk category (Table 4). In the first model, which involved only baseline CD4 count, a total of 23 events were observed at 3 months after entry to TAHOD, with 24.3 events estimated from the model. The observed and model-predicted numbers of events appeared to agree reasonably well according to the different categories of baseline CD4 counts, i.e. 8 observed vs. 8.2 predicted in patients with baseline CD4 counts <50 cells/μL, 11 vs. 10.7, respectively, in patients with CD4 counts between 50 and 199 cells/μL and 4 vs. 5.4, respectively, in patients with CD4 counts≥200 cells/μL. In the second model, which included baseline haemoglobin, baseline AIDS-related symptoms and current treatment, a total of 32 events were observed at 3 months, with 31.1 events estimated from the model. Patients were grouped into three risk categories: the most favourable situation (normal haemoglobin, no AIDS symptoms and treated); the least favourable situation (anaemic, with symptoms and without treatment); and other patients in between those two extreme situations. In each of these situations, the number of observed events was close to the number estimated from the model.
Table 4.
Observed and model-predicted events according to risk categories at 3 months
| CD4 count (cells/μL) | |||
| <50 | 106 | 8 | 8.2 |
| 50–199 | 289 | 11 | 10.7 |
| 200+ | 750 | 4 | 5.4 |
| Total | 1145 | 23 | 24.3 |
| Model 2 | |||
| Anaemic, symptomatic and no treatment | 72 | 6 | 5.7 |
| Not anaemic, asymptomatic and treated | 246 | 3 | 2.1 |
| Other | 854 | 23 | 23.3 |
| Total | 1172 | 32 | 31.1 |
The fitted survival functions are compared with observed Kaplan–Meier survival estimates in Fig. 1. In the model with baseline CD4 count (Fig. 1a), patients with lower baseline CD4 count progressed more quickly to AIDS or death than patients with higher CD4 counts. In the model with baseline haemoglobin, AIDS-related symptoms and prior antiretroviral treatment (Fig. 1b), patients were grouped into the same three risk categories as above: the most favourable situation, the least favourable situation and situations in between. Patients with the least favourite situation progressed more quickly to AIDS or death than the others. Both models fitted the data reasonably well, especially in the first 6 months after entering TAHOD.
Fig. 1.
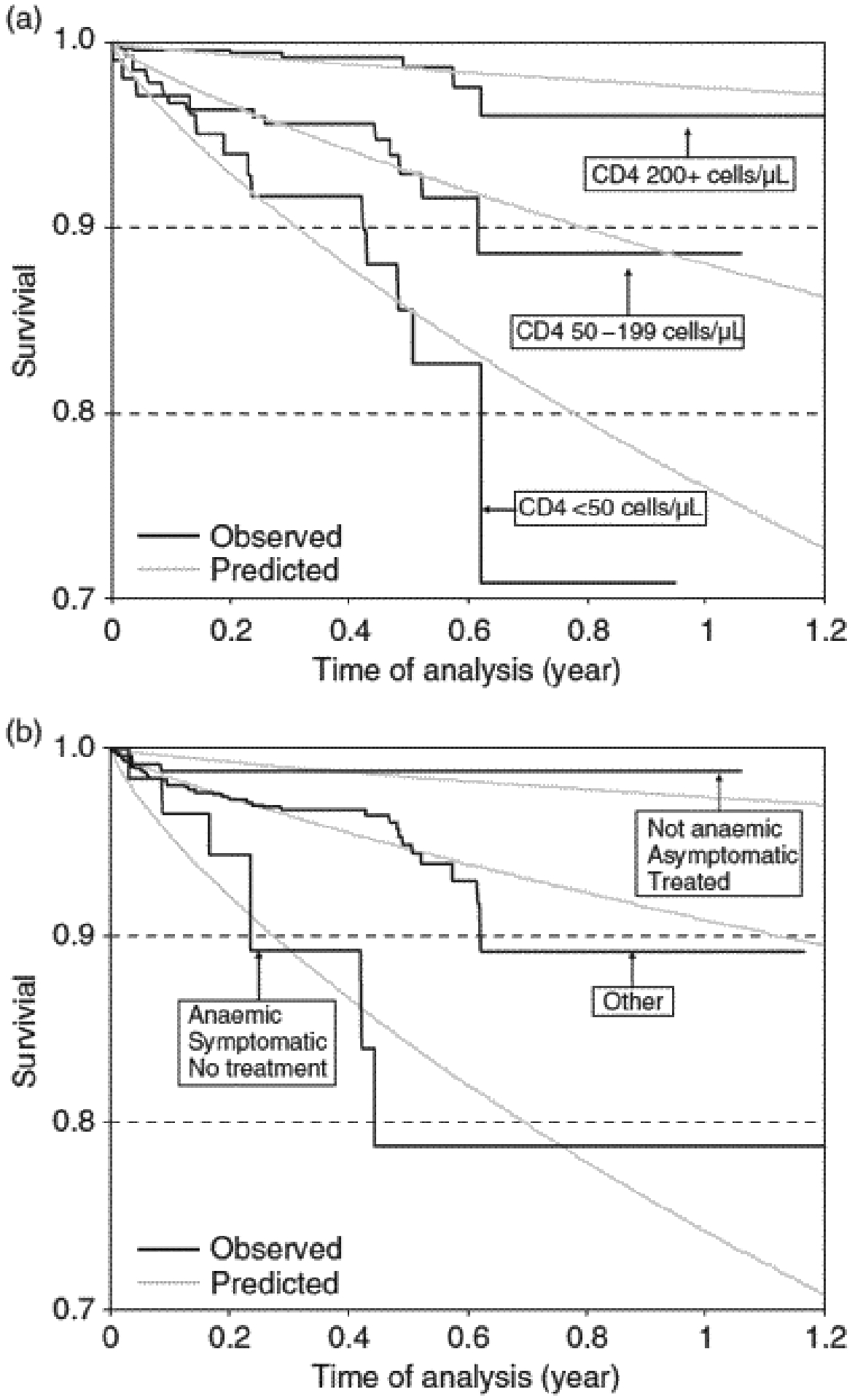
Observed and predicted survival according to (a) baseline CD4 count and (b) baseline haemoglobin level, baseline AIDS-related symptoms and prior antiretroviral treatment (grouped into three categories).
Discussion
Analyses using the prospective data from TAHOD found a short-term risk of combined newly diagnosed AIDS and death of 10.1 per 100 person-years. Compared with patients who were receiving antiretroviral treatment, patients not on treatment had a higher rate of disease progression. Baseline CD4 count was the strongest predictor of disease progression. Prognostic models were developed, using either a baseline CD4 count as the sole marker or markers including baseline haemoglobin level, AIDS-related symptoms and previous or current antiretroviral treatment, and appeared to be reasonably successful at identifying patients at high risk of short-term disease progression.
The introduction of HAART in 1996–1997 led to a marked reduction of mortality and morbidity which was well documented in Western countries [1,4,13]. In the EuroSIDA study [8], before the HAART era, the combined rate of AIDS and death was over 40 per 100 person-years in 1994–1995. This rate continued to decrease to around 10 per 100 person-years in 1996–1997 and to <5 per 100 person-years in 1998. The combined rate of AIDS and death found in this study is similar to those found in Western countries in 1996–1997, when HAART had just been introduced, but is generally higher than those seen recently in similar observational studies in Western countries [1,3,7,8,13], where HAART was largely available. However, similar rates of disease progression were found in studies conducted in Asian patients [14]. Whether this higher rate reflected higher rates of treatment interruption, poorer patient compliance, more frequent treatment failure or higher rates of AIDS illnesses diagnosed at higher CD4 counts, such as tuberculosis, is as yet unclear.
Studies have shown that CD4 count, rather than HIV viral load, is the most important predictor of short-term risk of HIV disease progression [1,3,7,8,13,15,16]. Consistent with these findings, in our analysis, baseline CD4 count accounted for most of the difference in relative risk for AIDS and death. There was a trend for decreased risk of disease progression with higher baseline CD4 count. Compared with a baseline CD4 count of < 50 cells/μL, the risk of progression to AIDS or death was 46% when CD4 counts were between 50 and 199 cells/μL and 9% when CD4 counts were ≥200 cells/μL. This trend was similar in both Western cohorts [2,8] and Asian cohorts [14,17].
Anaemia has been previously shown to be an independent predictor of HIV disease progression [6,10,11]. In this study, baseline haemoglobin measurement was a significant predictor of disease progression only when baseline CD4 count was excluded from the prognostic model. This is probably a result of the short follow-up time, the relatively low statistical power and the baseline CD4 count being a stronger predictor for short-term disease progression. In due course, with longer follow-up, haemoglobin may emerge as an independent factor in TAHOD.
Similar to the findings of other studies conducted in Asia, the most common AIDS-defining illness was tuberculosis [14,17]. In Western cohorts, while tuberculosis is one of the more frequent AIDS-defining illnesses, oesophageal candidiasis and Kaposi’s sarcoma are more frequently diagnosed [2,6]. The modified version of the CDC 1993 criteria which we adopted for defining AIDS cases, in which a presumptive diagnosis was available for most illnesses, limited the necessity for extensive laboratory and technical diagnostic facilities. However, the effect of this on rates of illnesses is not quantifiable.
There are a number of limitations to be considered in relation to the findings. First, TAHOD is a young cohort with relatively little prospective follow-up. Statistical analyses therefore have limited power, and hence our simple models may not be able to optimally identify patients at high risk of disease progression. Analyses will be repeated based on longer follow-up when this is available, and this may result in more accurate predictive models. Secondly, the TAHOD patients, recruited based on clinicians’ judgement of good follow up, cannot be seen as entirely representative of HIV patients in Asia and the Pacific region. However, information on the natural history of HIV disease and responses to antiretroviral treatment can still be derived from a cohort of TAHOD patients with good follow up, albeit with some limitations on the extent to which the findings can be generalized. Thirdly, although the follow-up rate is satisfactory, patients lost to follow-up must be considered when interpreting the results. AIDS may be underreported if a patient does not regularly visit a clinic or if a patient dies. Efforts have been made to maximize follow up, with each site developing its own mechanism for contacting patients whenever possible. Finally, in resource-poor settings, certain laboratory tests routinely available in Western countries, such as the virological tests, are simply not always available for all patients at TAHOD participating sites; a large proportion of patients did not have a baseline HIV viral load estimation at time of entry to TAHOD. This may have led to an underestimate or overestimate of the observed rate of detected HIV viral load.
The cost of antiretroviral treatment has decreased, and treatment is increasingly available in countries in Asia and the Pacific region [18,19]. However, routine CD4 counts and HIV viral load measurements remain comparatively expensive and complex for resource-poor settings [20]. Proper monitoring of disease progression, as well as the side effects of antiretroviral treatment and drug resistance, is important to achieve a satisfactory treatment response [18]. Our prognostic model based on readily available clinical data and haemoglobin level should be useful in providing estimates of short-term clinical risk in patients with HIV infection in Asia and the Pacific region.
Acknowledgements
TREAT Asia and TAHOD are funded by a grant from the American Foundation for AIDS Research (amfAR). The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales.
Appendix: the TREAT Asia HIV Observational Database
F. Zhang,* H. Zhao and N. Han, Beijing Ditan Hospital, Beijing, China; P. Li* and M. P. Lee, Queen Elizabeth Hospital, Hong Kong, China; Y. M. A. Chen,* W. W. Wong and D. C. C. Wang, Taipei Veterans General Hospital and AIDS Prevention and Research Centre, National Yang-Ming University, Taipei, Taiwan; N. Kumarasamy,*w S. Anand and J. A. Cecelia, YRG Centre for AIDS Research and Education, Chennai, India; S. Pujari* and K. Joshi, HIV Project, Ruby Hall Clinic, Pune, India; C. K. C. Lee* and S. Kaur, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; A. Kamarulzaman* and S. Kaur, University of Malaya,
Kuala Lumpur, Malaysia; R. Ditangco* and R. Capistrano, Research Institute for Tropical Medicine, Manila, Philippine; N. I. Paton* and M. Yap, Tan Tock Seng Hospital, Singapore; P. Phanuphak,* U. Siangphe and M. Khongphattanayothing, HIV-NAT/The Thai Red Cross AIDS Research Centre, Bangkok, Thailand; A. Vibhagool,* S. Kiertiburanakul and W. Kiatatchasai, Ramathibodi Hospital, Bangkok, Thailand; J. Chuah,* W. Fankhauser and B. Dickson, Gold Coast Sexual Health Clinic, Miami, Queensland, Australia; K. Frost* and S. Wong, American Foundation for AIDS Research, New York, USA; D. A. Cooper,* M. G. Law,* K. Petoumenos and J. Zhou,* National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, Australia.
(Symbols: *Steering Committee member; wcurrent Steering Committee chair).
References
- 1.Egger M, Hirschel B, Francioli P et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. Swiss HIV Cohort Study. Br Med J 1997; 315: 1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egger M, May M, Chene G et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 2002; 360: 119–129. [DOI] [PubMed] [Google Scholar]
- 3.Hogg RS, Heath KV, Yip B et al. Improved survival among HIVinfected individuals following initiation of antiretroviral therapy. J Am Med Assoc 1998; 279: 450–454. [DOI] [PubMed] [Google Scholar]
- 4.Mocroft A, Vella S, Benfield TL et al. Changing patterns of mortality across Europe in patients infected with HIV-1. Eurosida Study Group. Lancet 1998; 352: 1725–1730. [DOI] [PubMed] [Google Scholar]
- 5.Mocroft A, Phillips AN, Friis-Moller N et al. Response to antiretroviral therapy among patients exposed to three classes of antiretrovirals: results from the EuroSIDA study. Antivir Ther 2002; 7: 21–30. [PubMed] [Google Scholar]
- 6.Miller V, Phillips AN, Clotet B et al. Association of virus load, CD4 cell count, and treatment with clinical progression in human immunodeficiency virus-infected patients with very low CD4 cell counts. J Infect Dis 2002; 186: 189–197. [DOI] [PubMed] [Google Scholar]
- 7.Lundgren JD, Mocroft A, Gatell JM et al. A clinically prognostic scoring system for patients receiving highly active antiretroviral therapy: results from the EuroSIDA study. J Infect Dis 2002; 185: 178–187. [DOI] [PubMed] [Google Scholar]
- 8.Mocroft A, Ledergerber B, Katlama C et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet 2003; 362: 22–29. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, Kumarasamy N, Ditangco R et al. The TREAT Asia HIV Observational Database: Baseline and Retrospective Data. J Acquir Immune Defic Syndr 2005; 38: 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mocroft A, Kirk O, Barton SE et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. Eurosida Study Group. AIDS 1999; 13: 943–950. [DOI] [PubMed] [Google Scholar]
- 11.Lundgren JD, Mocroft A. Anemia and survival in human immunodeficiency virus. Clin Infect Dis 2003; 37 (Suppl. 4): S297–S303. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbidity and Mortality weekly Report (MMWR) 1992; 41: 1–19. [PubMed] [Google Scholar]
- 13.Palella FJ Jr, Delaney KM, Moorman AC et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338: 853–860. [DOI] [PubMed] [Google Scholar]
- 14.Wannamethee SG, Sirivichayakul S, Phillips AN et al. Clinical and immunological features of human immunodeficiency virus infection in patients from Bangkok. Thailand Int J Epidemiol 1998; 27: 289–295. [DOI] [PubMed] [Google Scholar]
- 15.Coakley EP, Samore MH, Gillis JM et al. The values of quantitative serum HIV-1 RNA levels and CD4 cell counts for predicting survival time among HIV-positive individuals with CD4 counts of less than or equal to 50 10(6) cells/l. AIDS 2000; 14: 1147–1153. [DOI] [PubMed] [Google Scholar]
- 16.Sterling TR, Chaisson RE, Moore RD. HIV-1 RNA, CD4 Tlymphocytes, and clinical response to highly active antiretroviral therapy. AIDS 2001; 15: 2251–2257. [DOI] [PubMed] [Google Scholar]
- 17.Kumarasamy N, Solomon S, Flanigan TP et al. Natural history of human immunodeficiency virus disease in southern India. Clin Infect Dis 2003; 36: 79–85. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organisation (WHO). Scaling up Antiretroviral Therapy in Resource-Limited Settings. Guidelines for a Public Health Approach. 2003 Revision. Geneva, World Health Organisation, 2004. [Google Scholar]
- 19.Kumarasamy N, Flanigan TP, Mahajan AP et al. Monitoring HIV treatment in the developing world. Lancet Infect Dis 2002; 2: 656–657. [DOI] [PubMed] [Google Scholar]
- 20.Stephenson J Cheaper HIV drugs for poor nations bring a new challenge: monitoring treatment. J Am Med Assoc 2002; 288: 151–153. [DOI] [PubMed] [Google Scholar]


