Abstract
INTRODUCTION:
Plasma biomarkers – cost-effective, non-invasive indicators of Alzheimer’s disease and related disorders (ADRD) – have largely been studied in clinical research settings. Here, we examined plasma biomarker profiles and their associated factors in a population-based cohort to determine whether they could identify an at-risk group, independently of brain and CSF biomarkers.
METHODS:
We measured plasma phosphorylated-tau181(p-tau181), neurofilament light (NfL), glial fibrillary acidic protein (GFAP), and amyloid-β (Aβ)42/40 ratio in 847 participants from a population-based cohort in southwestern Pennsylvania.
RESULTS:
K-medoids clustering identified two distinct plasma Aβ42/40 modes, further categorizable into three biomarker profile groups: normal, uncertain and abnormal. In different groups, plasma p-tau181, NfL and GFAP were inversely correlated with Aβ42/40, Clinical Dementia Rating, and memory composite score, with the strongest associations in the abnormal group.
DISCUSSION:
Abnormal plasma Aβ42/40 ratio identified older-adult groups with lower memory scores, higher dementia risks and higher ADRD biomarker levels, with potential implications for population screening.
Keywords: Plasma ADRD biomarkers, Monongahela-Youghiogheny Healthy Aging Team (MYHAT), cognitive impairment, cluster modeling, aging, epidemiology
1. Introduction
Alzheimer’s disease (AD) is characterized by brain deposition of amyloid-β (Aβ) plaques and tau neurofibrillary tangles1. Additional pathophysiological features include neurodegeneration/axonal damage and glial activation2, 3. While brain Aβ, tau, neurodegeneration and glial activation are quantifiable in vivo using established neuroimaging and cerebrospinal fluid (CSF) biomarkers4–6, their prohibitive costs and limited availability hinder population-level applications7. Plasma biomarkers are accurate, more accessible, and cost-effective methods that can circumvent these limitations7. Multiple independent studies have demonstrated that plasma Aβ42/40 and p-tau181 are associated with brain Aβ and tau8 9–14. Furthermore, plasma neurofilament light (NfL) and glial fibrillary acidic protein (GFAP) associate with brain degeneration and glial activation, respectively, which are found in both AD and related neurodegenerative disorders (ADRDs)3, 12, 15, 16. Nonetheless, previous investigations were limited mostly to clinical research cohorts with CSF/neuroimaging biomarkers categorization and also lacked diversity/heterogeneity in terms of social, economic and geographic origins7, 17. It is essential to assess plasma biomarker performance in population-based cohorts to: 1) verify their utility in less homogeneous groups of older adults18, 2) understand biomarker associations with cognitive impairment and demographic characteristics, and 3) ascertain the potential generalizability of results documented in earlier studies.
Plasma biomarkers will be pivotal in community screening to identify at-risk individuals7. There is general agreement that plasma biomarkers will be pivotal in community screening to identify at-risk individuals. However, there is as yet no identified strategy for doing so. In this study, we investigated plasma Aβ42/Aβ40 ratio, p-tau181, GFAP, and NfL in a population-based cohort of older adults from medically under-served small towns of relatively low socio-economic status. We subsequently applied a novel clustering approach to categorize the participants into groups of distinct plasma Aβ42/Aβ40 profiles. We hypothesized that associations between plasma biomarkers and memory will enable the identification of individuals at risk of ADRD in the community.
2. Methods
2.1. Study Setting and Participants
The Monongahela-Youghiogheny Health Aging Team (MYHAT) is an ongoing population-based study cohort drawn from a Rust Belt region of southwestern Pennsylvania, USA. These are formerly vibrant steel-manufacturing towns that never recovered from the economic blows of the steel industry’s collapse in the 1970s. MYHAT participants are followed annually for the development of mild cognitive impairment (MCI) and dementia. Participants were selected by age-stratified random sampling from the publicly available voter registration lists over two time periods: 2006–2008 and 2016–2019. Inclusion criteria at study entry included: 1) 65+ years old, 2) living in a designated town, 3) not residing in long-term care settings, 4) having sufficient hearing and vision to complete neuropsychological testing, and 5) having decisional capacity. Recruitment procedures in 2016 for the new cohort were identical to those of 2006, except that participants were limited to the 65–74 age group, so as to replenish the cohort with participants ten years younger than the youngest members of the initial cohort. At initial recruitment, n=2,036 and n=708 participants provided written informed consent in the first and second recruitment phases respectively. All participants were briefly assessed using the Mini Mental State Examination (MMSE)19. Since the study investigates the epidemiology of MCI, we screened out those who already showed substantial cognitive impairment by scoring <21/30 on the age-education-corrected MMSE20. The full assessment was then administered to n=1,982 and n=703 participants in the original and second recruitment cohorts respectively. All study procedures were approved by the University of Pittsburgh Institutional Review Board and all participants provided written informed consent.
2.2. Study Assessments
Detailed assessment interviews included:
Demographics:
age, sex, education (less than eighth grade or eighth to eleventh grade [<HS]; graduated from high school or GED [=HS]; graduated from college, 4-year college program or graduate school [>HS]), and self-identified race/ethnicity (White; Black or African American, more than one race, unknown or not reported [non-White]).
Clinical Dementia Rating:
At each annual assessment, certified research interviewers rated participants based on independence in cognitively-driven everyday activities using the Clinical Dementia Rating (CDR)®21. CDR was categorized into three groups: 0=normal, 0.5=MCI, ≥1=dementia.
Neuropsychological tests:
At each visit, participants were administered a battery of neuropsychological tests tapping five cognitive domains: memory, attention, language, executive function, and visuospatial ability. Here, we focus on the memory domain. A composite score for the memory domain was generated by first standardizing each individual test score (Fuld Object Memory Evaluation22, Wechsler Memory Scale-Revised Logical Memory and Visual Reproduction23, and modified 12-item Face Name Associative Memory Exam24) and then calculating the mean of all the standardized scores in the memory domain.
Blood collection.
For those recruited in the initial 2006–2008 cohort, blood samples were collected during the annual assessments in 2014 or later. For the new cohort participants, blood was collected at visits in 2016 or later. Venous blood was collected in the morning following overnight fasting into purple-top ethylenediaminetetraacetic acid tubes. Samples were incubated at room temperature for 30–45 minutes, then centrifuged at 2,000 g for 10 min, 4°C. The plasma was collected into polypropylene tubes and stored at −80°C until use. Less than 10% of the participants (n=87) self-reported that they did not follow the overnight fasting procedure. However, we have shown that this does not significantly affect plasma biomarkers25, and we confirmed that the results for these 87 participants did not differ from the rest of the cohort.
Apolipoprotein E (APOE) genotyping
Genotyping was performed using blood or saliva specimens. Genotypes for the APOE/rs429358 (APOE*4) and APOE/rs7412 (APOE*2) single-nucleotide polymorphisms (SNPs) were determined using TaqMan genotyping assays. Because of the strong linkage disequilibrium between the two SNPs, this is also treated as a three-allele APOE polymorphism: APOE*2, APOE*3, and APOE*4, resulting in six genotypes (2/2, 2/3, 2/4, 3/3, 3/4, 4/4)25. Individuals with any ε4 allele (2/4,3/4,4/4) were classified as APOE*4 carriers and those without an ε4 allele as non-carriers.
2.3. Plasma biomarker measurements
Plasma biomarker concentrations were measured in singlicates using Single molecule array (SIMOA) methods on an HD-X instrument (Quanterix, Billerica, MA, USA) at the Department of Psychiatry, University of Pittsburgh School of Medicine, USA. All frozen samples underwent a single thawing cycle. Plasma p-tau181 was measured with the p-tau181 V2 Advantage (#103714) while NfL, GFAP, Aβ42 and Aβ40 concentrations were measured with the Neurology 4-Plex E (#103670) commercial assays from Quanterix (Billerica, MA, USA). For each assay, two or three quality control samples of different concentrations were analyzed in duplicates both at the start and the end of each technical run to estimate reproducibility. The pooled quality control data showed that the within-run (p-tau181=4.6–8.9%, NfL=10.9–17.7%, GFAP=6.6–13.2%, Aβ42=5.0–12.7% and Aβ40=5.8–13.5%) and between-run (p-tau181=10.8–13.5%, NfL=17.1–19.5%, GFAP=12.4–23.2%, Aβ42=9.0–17.0% and Aβ40=12.1–17.1%) variations in signal were mostly <20%.
2.5. Statistical analyses
Statistical analyses were performed using R 4.1.326. We first compared the demographics for participants whose plsma samples were available versus not available. We then examined descriptive statistics of biomarkers and baseline demographics overall and by CDR group or a binary CDR variable (CDR=0 normal vs CDR≥0.5, MCI/dementia). Medians and interquartile ranges were calculated for each continuous variable; frequencies and percentages were calculated for categorical variables. We performed Kruskal-Wallis tests for continuous variables and Fisher’s exact tests for categorical variables to compare biomarker distributions among CDR groups.
We further performed Kruskal-Wallis tests to compare biomarker distributions among age (65–74, 75–84, and 85+ years old) and education (<HS: less than high school, =HS: high school, >HS: higher than high school) groups, and Wilcoxon rank-sum tests to compare biomarker distributions between sex and APOE*4 carrier and non-carrier groups.
To classify individuals into homogeneous plasma Aβ42/40 and Aβ42 groups, we applied an unsupervised clustering method: K-medoids27, a more robust version of K-means which minimizes the distance between points labeled as being in the same cluster. All biomarker values were given as natural log-transformed; the distance matrix was calculated using the Euclidean distance, and the number of clusters was fixed to 2 since we aimed to find the threshold of two one-directional groups. We tested the difference in charasteristics (demographics and biomarkers) among groups. For each cluster, we further examined the correlations between pairs of biomarkers and between the memory composite and biomarkers using Spearman’s correlation28. We further tested the above-mentioned associations by stratifying according to age, sex, education, or APOE*4 allele individually using Spearman’s correlation. To find the directions and magnitude of the above-mentioned associations overall or by strata, we fit robust linear regression, alternatives to least squares regressions when data are contaminated with outliers or influential observations, for each pair of variables of which we examined correlations. The associations of the memory composite score and biomarkers were similarly examined by CDR group.
3. Results
3.1. Participant and plasma biomarker characteristics
Among total 2685 participants in MYHAT study, plasma samples were available from 920 participants. The distributions of age at study entrance, sex, race and education were all significantly different between people who gave plasma samples versus those who did not. The demographic characteristics of the participants who consented to blood collection and were thus included in this study agreed with previous reports29, 30; younger, more females, more self-identified White, and more highly educated (Supp Table. 1 and Supp Fig. 1). After further excluding 19 participants missing one or more biomarker values, we had data from 901 participants. Biomarker levels below the assays’ quantification limits were assigned the manufacturer-provided lower limits of detection values (Aβ40=0.384, Aβ42=0.136, NfL=0.09, GFAP=0.441, and p-tau181=0.028). In this way, we reassigned 2 values for Aβ40, 5 values for Aβ42 and 2 values for p-tau181.
No biomarker presented a normal distribution without transformation (Supp Fig. 2). While Aβ42/40 ratio, GFAP, NfL, and p-tau181 were unimodal and right-skewed, Aβ40 and Aβ42 showed bimodal distributions. After natural log transformations (Supp Fig. 3), Aβ42/40 was still slightly right-skewed; p-tau181, NfL, and GFAP were normally distributed. We removed n=54 outliers (red rectangles; Supp Fig. 3) which were out of the outer fence (Q1 – 3*IQR, Q3 + 3*IQR) and separated from the bulk values in the histograms; Aβ42/40 (n=2), p-tau181 (n=31), NfL and GFAP (identical n=21), leaving n=847 participants for the final analyses.
The characteristics of the 847 MYHAT participants by CDR groups are presented in Table 1. There were 125(14.8%) participants with MCI and 10(1.2%) with dementia, with the rest being cognitively normal individuals (~85%). The median(Q1, Q3) age of the cohort was 74.0(69.0, 83.0) years; 465 (54.9%) were aged 65–74 years, 216 (25.5%) aged 75–84 years, and the rest above 85 years. 306(36.1%) were male; 809(95.5%) were White; and 179 (21.1%) were APOE*4 carriers. Sex and race distributions were similar among the three CDR groups. The participants in the CDR≥0.5 group were significantly older, less educated, and had a higher proportion of APOE*4 carriers than the CDR=0 group. The median Aβ42/40 for the CDR=0 was slightly higher versus the CDR≥0.5 group. Median Aβ40 was significantly lower in the CDR >=1 group. Plasma p-tau181, NfL, and GFAP were each higher in the CDR≥0.5 group (Table. 1).
Table 1.
Participant characteristics, median (Q1, Q3) or N (%), by CDR scores.
| Normal (N=712) (CDR=0) |
MCI (N=125) (CDR=0.5) |
Dementia (N=10) (CDR≥1) |
Total (N=847) | p-value* | |
|---|---|---|---|---|---|
| Age, years | < 0.001 | ||||
| Median | 73.00 | 80.00 | 89.50 | 74.00 | |
| Q1, Q3 | 69.00, 81.00 | 70.00, 87.00 | 88.00, 91.00 | 69.00, 83.00 | |
| Age group, N (%) | < 0.001 | ||||
| 65–74-year-olds | 414 (58.1%) | 50 (40.0%) | 1 (10.0%) | 465 (54.9%) | |
| 75–84-year-olds | 186 (26.1%) | 30 (24.0%) | 0 (0.0%) | 216 (25.5%) | |
| 85+-year-olds | 112 (15.7%) | 45 (36.0%) | 9 (90.0%) | 166 (19.6%) | |
| Sex, N (%) | 0.829 | ||||
| Male | 260 (36.5%) | 43 (34.4%) | 3 (30.0%) | 306 (36.1%) | |
| Female | 452 (63.5%) | 82 (65.6%) | 7 (70.0%) | 541 (63.9%) | |
| Race, N (%) | 0.272 | ||||
| White | 683 (95.9%) | 116 (92.8%) | 10 (100.0%) | 809 (95.5%) | |
| Non-White | 29 (4.1%) | 9 (7.2%) | 0 (0.0%) | 38 (4.5%) | |
| Education, N (%) | < 0.001 | ||||
| < High School | 26 (3.7%) | 16 (12.8%) | 2 (20.0%) | 44 (5.2%) | |
| = High School | 250 (35.1%) | 51 (40.8%) | 4 (40.0%) | 305 (36.0%) | |
| > High School | 436 (61.2%) | 58 (46.4%) | 4 (40.0%) | 498 (58.8%) | |
| APOE4, N (%) | < 0.001 | ||||
| Non-carriers | 577 (81.0%) | 88 (70.4%) | 3 (30.0%) | 668 (78.9%) | |
| Carriers | 135 (19.0%) | 37 (29.6%) | 7 (70.0%) | 179 (21.1%) | |
| Aβ40, pg/mL | 0.069 † | ||||
| Median | 4.60 | 4.63 | 2.89 | 4.60 | |
| Q1, Q3 | 3.93, 4.77 | 4.37, 4.86 | 0.75, 4.89 | 4.04, 4.78 | |
| Aβ42, pg/mL | 0.323 † | ||||
| Median | 1.86 | 1.86 | 1.22 | 1.86 | |
| Q1, Q3 | 1.18, 2.07 | 1.55, 2.06 | −0.77, 2.02 | 1.28, 2.07 | |
| Aβ42/Aβ40 ratio | 0.023 † | ||||
| Median | −2.66 | −2.72 | −2.69 | −2.67 | |
| Q1, Q3 | −2.80, −2.51 | −2.87, −2.59 | −2.85, −2.16 | −2.81, −2.52 | |
| p-tau181, pg/mL | < 0.001† | ||||
| Median | 0.45 | 0.77 | 0.86 | 0.49 | |
| Q1, Q3 | 0.11, 0.83 | 0.40, 1.11 | 0.45, 1.14 | 0.14, 0.89 | |
| NfL, pg/mL | < 0.001† | ||||
| Median | 3.11 | 3.41 | 3.96 | 3.15 | |
| Q1, Q3 | 2.81, 3.51 | 2.94, 3.73 | 3.49, 4.24 | 2.83, 3.57 | |
| GFAP, pg/mL | < 0.001† | ||||
| Median | 4.85 | 5.07 | 5.51 | 4.88 | |
| Q1, Q3 | 4.48, 5.23 | 4.68, 5.48 | 5.21, 5.66 | 4.50, 5.29 |
: Kruskal-Wallis tests were used for continuous variables with non-parametric distributions, whereas Fisher Exact tests were used for categorical variables.
: Plasma biomarkers natural log transformed to better approximate normality and variance homogeneity. MCI: mild cognitively impaired. <HS : less than eighth grade or eighth to eleventh grade; =HS: graduated from high school or GED; >HS: graduated from college, 4-year college program or graduate school. Non-White: White; Black or African American, more than one race, unknown or not reported. Aβ: amyloid beta, p-tau181: phosphorylated-tau 181, GFAP: glial fibrillary acidic protein, NfL: neurofilament light chain.
3.2. Distributions of plasma biomarkers according to demographics
As shown in Supp Fig. 4A, Aβ40 and Aβ42 were each significantly higher and Aβ42/40 ratio significantly lower in the 65–74-year-olds compared with the older age groups, whereas the levels were comparable between the 75–84 and 85+ age groups. Conversely, plasma p-tau181, GFAP, and NfL were each higher in 75–84 and 85+ age groups versus the 65–74-year-olds. Aβ42/40, GFAP, and NfL were significantly higher, whereas p-tau181 was lower, in females versus males (Supp. Fig. 4B). When stratified according to education (Supp. Fig. 4C), there was no significant difference in Aβ42/40. However, NfL, GFAP, and p-tau181 were each lower in the =HS and >HS groups compared with the <HS group . After adjusting for age, there were no differences in the distributions of plasma biomarkers among education levels except that NfL was still significantly lower in the ≥HS education group compared to the <HS education group among people aged 65–74. APOE*4 carriers had significantly lower Aβ42, Aβ42/40, and NfL values, but non-significantly higher GFAP and p-tau181 levels (Supp. Fig. 4D).
3.3. Clustering according to plasma Aβ42/40 ratio and p-tau181 identifies two separate modes that reveal an intermediate group
The clustering result for Aβ42/40 (Fig. 1A) identified a bimodal distribution for both Aβ42 and Aβ40 (Fig. 1E–F), in agreement with previously reported CSF Aβ results31, 32. The optimal Aβ42/40 threshold to differentiate the two clusters was −2, resulting in 2 Aβ42/40 modes: normal (N=730) and abnormal (N=117). Plotting plasma Aβ42/40 ratio against p-tau181 also identified an abnormal and a normal group which showed pathophysiological AD and biomarker-negative profiles, respectively (since p-tau181 is specifically higher according to AD pathology10, 33–36), according to associations between the plasma biomarkers. The boundary of the new clusters became a diagonal line with a positive slope instead of a vertical line at the −2 threshold for Aβ42/40 ratio alone (Fig. 1B), suggesting that the clustering result of the Aβ42/40 ratio depended on p-tau181. Some participants with slight Aβ42/40 suprathreshold and subthreshold values might still be in the normal versus abnormal cluster respectively. This allowed the definition of a third, “uncertain”, group (blue rectangle in Fig. 1C) to include these individuals. As a result, the participants were clustered into three groups based on the log-transformed Aβ42/40: normal (−4, −2.3), uncertain (−2.3, −1.7), and abnormal (−1.7, 1). The uncertain group was established as +/−0.3 units around the original −2 threshold. Table. 2 shows the characteristics of participants by those 3 groups. There were 40 participants in the uncertain group, 97 in the abnormal group, and 710 in the normal group. Participants in the non-normal groups were older and less educated and included more females when compared with those in the normal group. Fig. 1B–C illustrate that data points in the uncertain group were more spread out (similar to those in the abnormal group and contrary to the closely packed normal group), suggesting that individuals in the uncertain group were in an intermediate state. Fig. 1D–G show the histograms of Aβ42/40, Aβ40, Aβ42, and p-tau181 respectively color-filled according to the different group of Aβ42/40. Whilst the color-coded Aβ40 and Aβ42 distributions showed overlaps between groups, a clear separation of all three groups was observed when using the Aβ42/40.
Figure 1. Top: Clustering results and modes/groups for plasma Aβ42/40 ratio.
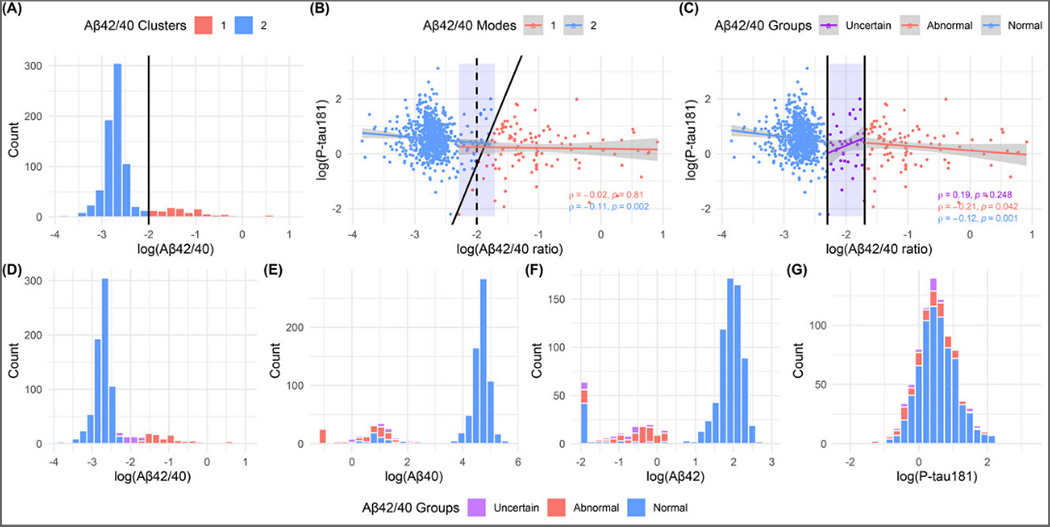
The figure A shows the distribution for Aβ42/40 ratio filled by its K-medoids clustering result; the cutoff point is −2. Figure B is the scatterplot for plasma Aβ42/40 ratio and p-tau181 colored by their K-medoids clustering result. The black dash line is the cutoff point for Aβ42/40 ratio mapped to 2-dimension. The scatterplot in figure C shows the association between Aβ42/40 ratio and p-tau181 colored by three groups. The groups (Normal: < −2.3, Uncertain: [−2.3, −1.7], and Abnormal: > −1.7) are defined based on the plasma Aβ42/40 ratio values. The black solid lines are the boundaries of modes or groups. The blue rectangles are the uncertain group. Bottom: Distributions of biomarkers. The histograms of plasma (D) Aβ42/40 ratio, (E) Aβ40, (F) Aβ42, and (G) p-tau181 filled by Aβ42/40 ratio groups. Aβ = amyloid β, p-tau181 = tau phosphorylated at threonine 181, GFAP = glial fibrillary acidic protein, NfL = neurofilament light.
Table 2.
Participant characteristics, median (Q1, Q3) or N (%), by 3 Aβ42/Aβ40 ratio groups.
| Normal (N=710) (−4, −2.3) |
Uncertain (N=40) (−2.3, −1.7) |
Abnormal (N=97) (−1.7, 1) |
p-value* | |
|---|---|---|---|---|
| Age, years | < 0.001 | |||
| Median | 73.00 | 82.00 | 83.00 | |
| Q1, Q3 | 69.00, 80.00 | 77.00, 87.00 | 77.00, 87.00 | |
| Age group, N (%) | < 0.001 | |||
| 65–74-year-olds | 445 (62.7%) | 8 (20.0%) | 12 (12.4%) | |
| 75–84-year-olds | 155 (21.8%) | 16 (40.0%) | 45 (46.4%) | |
| 85+-year-olds | 110 (15.5%) | 16 (40.0%) | 40 (41.2%) | |
| Sex, N (%) | 0.036 | |||
| Male | 269 (37.9%) | 13 (32.5%) | 24 (24.7%) | |
| Female | 441 (62.1%) | 27 (67.5%) | 73 (75.3%) | |
| Race, N (%) | 0.363 | |||
| White | 675 (95.1%) | 39 (97.5%) | 95 (97.9%) | |
| Non-White | 35 (4.9%) | 1 (2.5%) | 2 (2.1%) | |
| Education, N (%) | 0.044 | |||
| < High School | 34 (4.8%) | 2 (5.0%) | 8 (8.2%) | |
| = High School | 243 (34.2%) | 17 (42.5%) | 45 (46.4%) | |
| > High School | 433 (61.0%) | 21 (52.5%) | 44 (45.4%) | |
| APOE * 4, N (%) | 0.333 | |||
| Non-carriers | 554 (78.0%) | 32 (80.0%) | 82 (84.5%) | |
| Carriers | 156 (22.0%) | 8 (20.0%) | 15 (15.5%) | |
| Aβ40, pg/mL | < 0.001† | |||
| Median | 4.66 | 1.24 | 0.64 | |
| Q1, Q3 | 4.46, 4.83 | 0.55, 1.95 | −0.96, 1.11 | |
| Aβ42, pg/mL | < 0.001† | |||
| Median | 1.93 | −0.66 | −0.43 | |
| Q1, Q3 | 1.71, 2.11 | −1.42, −0.11 | −0.97, −0.12 | |
| Aβ42/Aβ40 ratio | < 0.001† | |||
| Median | −2.71 | −2.00 | −1.06 | |
| Q1, Q3 | −2.83, −2.61 | −2.11, −1.83 | −1.42, −0.63 | |
| p-tau181, pg/mL | < 0.001† | |||
| Median | 0.52 | 0.36 | 0.34 | |
| Q1, Q3 | 0.19, 0.91 | −0.27, 0.73 | −0.22, 0.74 | |
| NfL, pg/mL | < 0.001† | |||
| Median | 4.80 | 5.21 | 5.40 | |
| Q1, Q3 | 4.44, 5.16 | 4.98, 5.58 | 4.94, 5.75 | |
| GFAP, pg/mL | < 0.001† | |||
| Median | 3.06 | 3.62 | 3.70 | |
| Q1, Q3 | 2.78, 3.44 | 3.15, 3.92 | 3.35, 3.97 |
: Kruskal-Wallis tests were used for continuous variables with non-parametric distributions, whereas Chi-square tests were used for categorical variables.
: Plasma biomarkers natural log transformed to better approximate normality and variance homogeneity. Aβ: amyloid beta, p-tau181: phosphorylated-tau 181, GFAP: glial fibrillary acidic protein, NfL: neurofilament light chain.
3.4. Associations between plasma Aβ42/40 modes and other plasma biomarkers
After determining the distinct modes of plasma Aβ42/40, we tested associations between plasma biomarker values for each Aβ42/40 mode (Fig. 1C and Fig. 2). When considering the two modes, there were significant inverse associations between Aβ42/40 and each of p-tau181 (ρ= −0.11, P=0.002; Fig. 1B), NfL (ρ= −0.11, P=0.002) and GFAP (ρ= −0.17, P<0.001; Fig 2C–D) in the normal mode. When split into three groups, the significant negative correlations between Aβ42/Aβ40 and each of p-tau181 (normal: ρ= −0.12, P=0.001; abnormal: ρ = −0.21, P=0.042) and NfL (normal: ρ= −0.18, P<0.001; abnormal: ρ= −0.26, P=0.011) was strongest in the abnormal group whilst the association with GFAP (normal: ρ= −0.24, P<0.001; abnormal: ρ= −0.21, P=0.043) was similar in the normal versus abnormal groups. No significant associations between plasma Aβ42/Aβ40 and other biomarkers were recorded in the uncertain group, potentially because of its comparatively small size.
Figure 2. Associations between plasma biomarkers by Aβ42/40 ratio modes and groups.
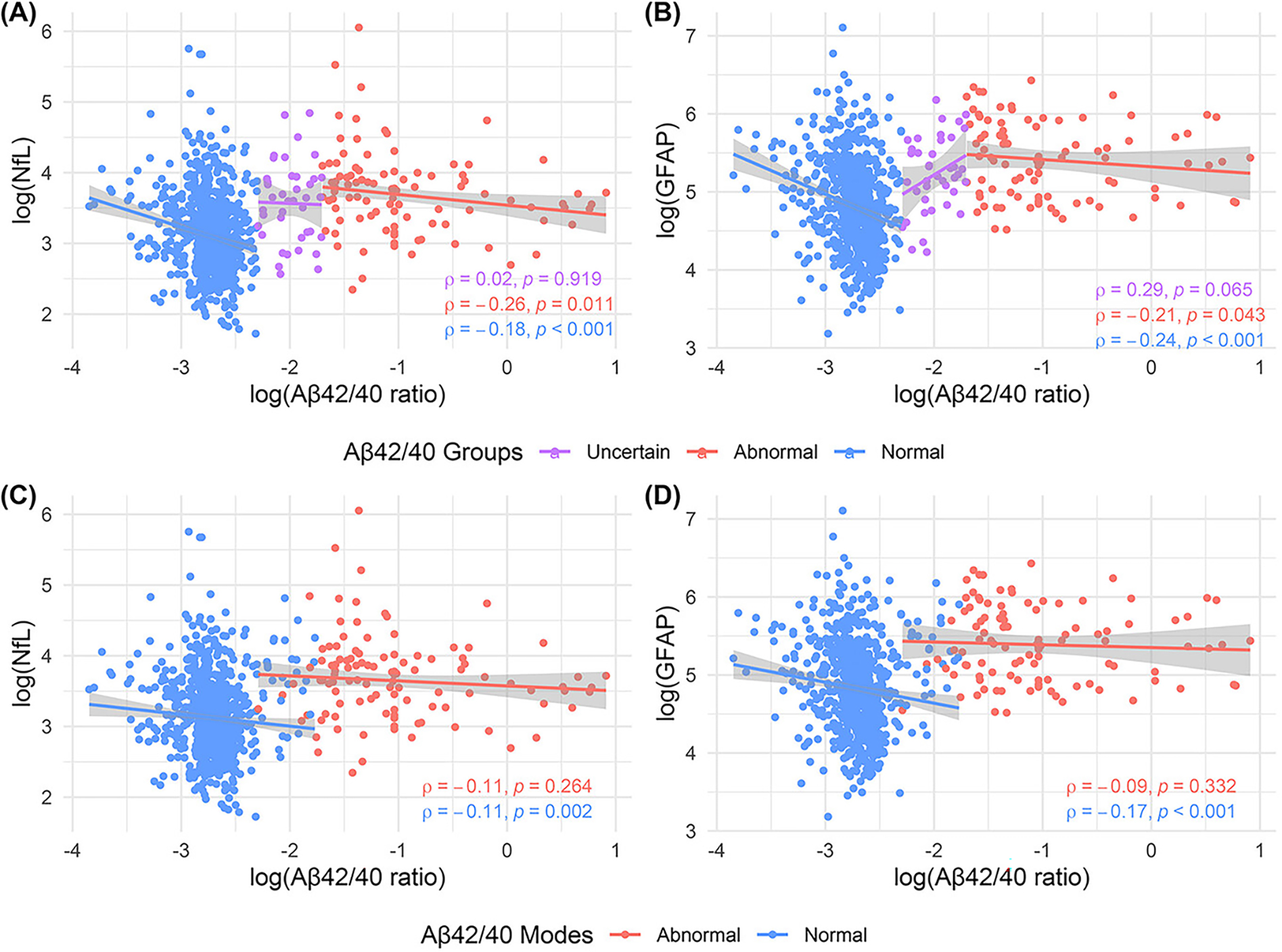
The upper panel shows the association between plasma Aβ42/40 ratio and (A) NfL and (B) GFAP by Aβ42/40 ratio groups (Normal, Uncertain, and Abnormal; defined using pre-defined cutoffs). The lower panel shows the association between plasma Aβ42/40 ratio and (C) NfL and (D) GFAP by Aβ42/40 ratio modes (Normal, and Abnormal; defined based on previous clustering results). All statistical associations were tested using Spearman’s correlation. Each individual point is colored based on plasma Aβ42/40 ratio modes or groups. All statistical tests were two-sided with no adjustment for multiple comparisons. Shaded areas represent 95% confidence intervals of the robust linear regression lines. Aβ = amyloid β, p-tau181 = tau phosphorylated at threonine 181, GFAP = glial fibrillary acidic protein, NfL = neurofilament light. The side-by-side presentation of plasma Aβ42/40 ratio associations with the other plasma biomarkers in the three- versus two-group clusters allows for a demonstration of how consideration of the intermediate zone affects these associations.
3.5. Associations between plasma biomarkers and memory composite score by plasma Aβ42/40 mode groups
Among the five cognitive domains (attention, executive, language, memory, and visuospatial), memory deficit defines “amnestic” MCI 37. We therefore focused on the association between the memory composite score and plasma biomarkers. When grouped by Aβ42/40 groups (Fig. 3A–D), the memory composite score showed a stronger negative correlation with p-tau181 in the abnormal (ρ= −0.33, P<0.001) versus the normal mode (ρ= 0.09, P=0.042). For NfL, the inverse association was stronger in the uncertain versus abnormal group (abnormal: ρ= −0.23, P=0.026; uncertain: ρ= −0.40, P=0.017) but non-existent in the normal group. Association between memory and GFAP was limited to the abnormal group (ρ= −0.22, P=0.036). Similar results were obtained when considering the two modes only (without the uncertain group; Fig. 3E–H). There were no significant associations between the memory composite score and plasma Aβ42/40 after adjusting for age, sex, education, or APOE*4 (Supp Fig. 5A). However, there was a significantly negative association between memory and p-tau181 (Supp Fig. 5B) in the Aβ42/40 abnormal group in females, non-APOE*4 carriers, and people above high school education. The memory composite score of Aβ42/40-abnormal females, >HS educated people, and non- APOE*4 carriers was inversely associated with NfL after the adjustments (Supp Fig. 5C). The negative association was also present among 85+-year-olds with normal Aβ42/40 profiles. When controlled for all covariates except age, >75-year-olds and with a normal Aβ42/40 profile showed inverse association between GFAP and memory; additionally, females or highly educated individuals within the abnormal mode showed associations between memory composite score and GFAP (Supp Fig. 5D).
Figure 3. Associations between memory composite score and plasma biomarkers by Aβ42/40 ratio modes and groups.
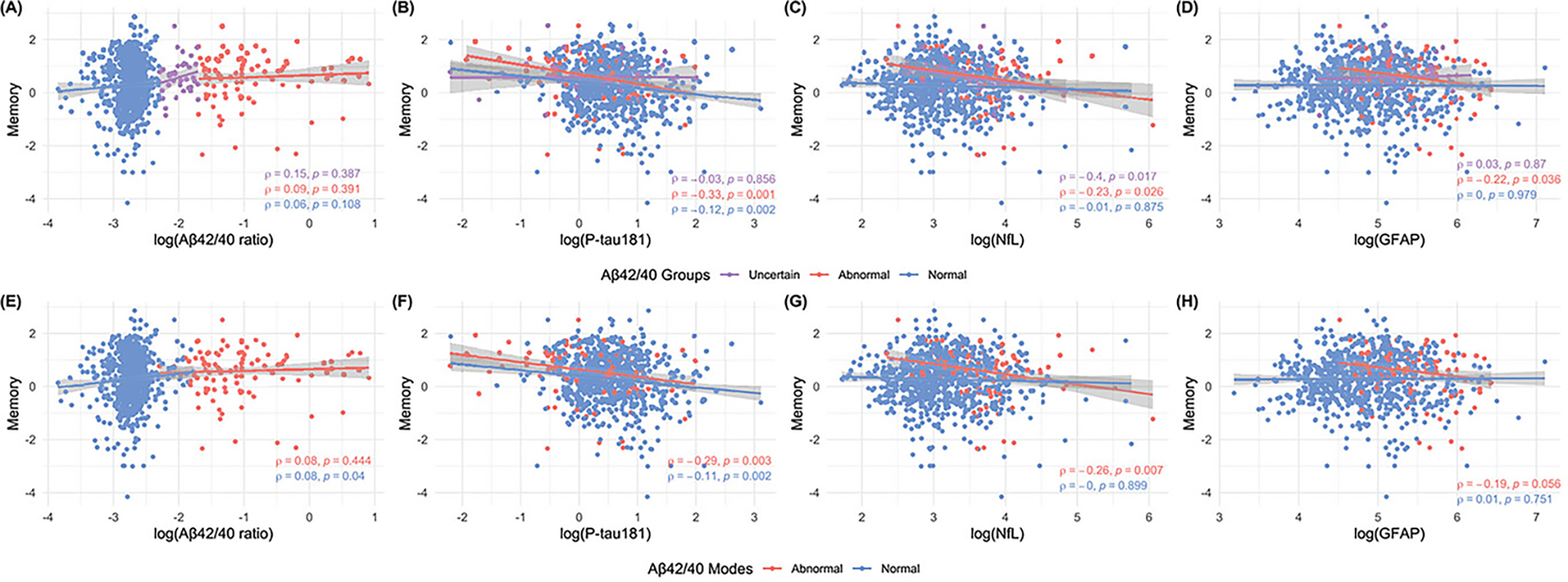
The upper panel shows the association between memory composite score with plasma (A) Aβ42/40 ratio, (B) p-tau181, (C) NfL, and (D) GFAP by Aβ42/40 ratio groups (Normal, Uncertain, and Abnormal; defined using pre-defined cutoffs). The lower panel shows the association between memory composite score with plasma (E) Aβ42/40 ratio, (F) p-tau181, (G) NfL, and (H) GFAP by Aβ42/40 ratio modes (Normal, and Abnormal; defined based on previous clustering results). All figures are annotated with Spearman’s rho rank correlations and corresponding unadjusted two-sided p-values. Points are colored by plasma Aβ42/40 groups or modes. The regression lines are fitted by robust linear regression and shaded areas represent the 95% confidence intervals. Aβ = amyloid β, p-tau181 = tau phosphorylated at threonine 181, GFAP = glial fibrillary acidic protein, NfL = neurofilament light. The side-by-side presentation of plasma Aβ42/40 ratio associations with composite meomory scores in the three- versus two-group clusters enabled evaluation of how the intermediate zone alters the relationships.
3.6. Associations between plasma biomarkers and memory composite score in CDR groups by plasma Aβ42/40 mode
In CDR=0 individuals, plasma NfL and GFAP were each positively associated with the memory composite score in the normal Aβ42/40 group (Supp Fig. 6A). Plasma p-tau181 showed an inverse association with memory composite score in the abnormal Aβ42/40 group (Supp Fig. 5B). Among CDR≥0.5 individuals with normal Aβ42/40 profiles, p-tau181 was negatively correlated with memory composite score (Supp Fig. 6A–B).
4.0. Discussion
We have described the profiles of ADRD plasma biomarkers in the population-based MYHAT cohort. Plasma p-tau181, GFAP, and NfL levels were higher in older individuals. The bimodal Aβ42/40 (or Aβ42) profiles (in agreement with CSF results31, 32) separated the population into two modes; participants with an abnormal Aβ42/40 profile had stronger associations with plasma NfL, p-tau181 and GFAP compared to those with a normal profile. Furthermore, plasma p-tau181 was associated with composite memory score, pointing to its utility to identify potentially at-risk individuals with or without cognitive impairment. Additionally, combining plasma Aβ42/40 with p-tau181 allowed us to apply the model more specifically to detect probable biomarker evidence of AD. Associations of NfL and GFAP with the memory composite in the normal Aβ42/40 modes/groups provide some validation to the notion that a negative Aβ42/40 profile might also be a potential marker for non-AD neurodegenerative diseases, and thus might help identify older adults at risk of those conditions. Future work, expanding our investigations into additional cognitive domains might help further clarify this issue.
The age-, cognition- and APOE*4 carriership-associated higher levels in plasma p-tau181, GFAP, and NfL and lower levels in Aβ42/40 are in line with recent CSF/neuroimaging studies10, 12, 38. Notably, females showed higher GFAP and NfL levels, also corroborating recent findings39. Lower plasma p-tau181 in females has also been reported40. While Aβ42/40 ratio was not affected by education, NfL, GFAP, and p-tau181 were lower in the more-educated groups. Education can increase brain reserve, i.e., resistance to brain pathology, and could be reflected in plasma biomarker abnormalities41. Since the older participants were from a less-educated generation, this could be simply age-driven. However, after adjusting for age, the difference among different education levels mostly disappeared, suggesting that age has minimal effects on the results.
One of the most promising potentials and achievable goals of plasma biomarkers is population screening to identify at-risk older adults for further clinical and/or research evaluations 7, 42. However, despite dozens of reports that plasma biomarkers associate strongly with CSF/neuroimaging biomarkers9, 10, 43 and can even predict neuropathologic diagnosis33, 44, studies examining their utility at the population level are lacking. Plasma Aβ42/40 were bimodally distributed, just as in CSF and Aβ-PET7, enabling identification of two Aβ42/40-dependent modes. Associations with memory composite and other biomarkers suggested that the abnormal mode was enriched for individuals at risk for AD irrespective of cognitive status whilst the normal mode included CDR=0 and CDR≥0.5 participants potentially affected by non-AD neurodegenerative diseases. Clustering jointly with Aβ42/40 and p-tau181 allowed for validation, given the specificity of p-tau181 to AD10, 33–36. The strength of associations of the modes/groups with NfL, GFAP and p-tau181 were higher according to Aβ42/40 abnormality. Similarly, Giudici et al. showed that plasma Aβ42/40 classifies older adults into low, intermediate and high risk groups of Aβ-PET abnormalities45. Our clustering efficiency and interdependence with p-tau181 were stronger with Aβ42/40 versus Aβ42 alone in line with CSF results46, 47.
In plasma, Aβ40, Aβ42 and the Aβ42/40 ratio have inverse relationships with their equivalent levels in the brain as measured with Aβ-PET 7, 8, 48. This suggests that individuals with higher levels of these plasma Aβ peptide levels have lower brain amyloidosis whilst those with lower plasma Aβ levels have higher brain amyloidosis 7, 8, 48. In agreement with previous reports49, 50, our findings suggest that cognitively impaired groups demonstrated lower plasma Aβ42/40 suggesting higher likelihood of brain amyloidosis. Furthermore, plasma NfL, GFAP and p-tau181 showed stronger associations with memory performance in the abnormal compared with the normal and intermediate mode/groups, also indicating that participants with an abnormal Aβ42/40 have higher odds for neurodegeneration, glial actication and AD pathophysiology. These results persisted in the CDR=0 participants, suggesting that plasma biomarker changes occur before cognitive symptoms appear38, and thus demonstrating potential effectiveness to identify at-risk community-dwelling individuals without cognitive concerns.
In AD, being the leading cause of cognitive impairment51, one of the earliest pathophysiological changes is a decrease of Aβ42/40 ratio levels in plasma8, 48, 50, 52. Other biological changes including abnormal tau phosphorylation, neurodegeneration and inflammatory alterations tend to be evident after Aβ42/40 reduction38, 53, 54. This explains why Aβ42/40 ratio clustering identified groups of individuals with different plasma biomarker and cognitive profile associations. The results corroborate what has been shown for CSF Aβ42/40 and Aβ PET54, 55. The results indicate that plasma Aβ42/40 has a high screening value in identifying both symptomatic and asymptomatic individuals at significant risk of AD, to be eventually confirmed by CSF/neuroimaging tests. Confirmatory tests on this enriched sub-population would significantly reduce the number of individuals and the associated time and costs compared to assessing the entire cohort with CSF/neuroimaging tests.
An additional strength is that we used Aβ42 and Aβ40 immunoassay methods from Quanterix, that are more widely available, cost-effective and easier-to-implement alternatives to the immunoprecipitation-mass spectrometry (IP-MS) methods only accessible in a few research and clinical laboratories. Although previous studies suggested that immunoassay Aβ methods perform less favorably than IP-MS Aβ assays, we used improved immunoassays with superior antibody performances38. The Aβ42 and Aβ40 assays were shown in a recent study to perform superior to plasma p-tau181, GFAP and NfL to identify abnormal brain Aβ status in a cohort of cognitively normal older adults38. Furthermore, our clustering method is independent of age and APOE*4 genotype, making it more practical compared with other approaches like the Amyloid Probability Score developed using a IP-MS plasma Aβ method45.
The clustering method may also be useful for the differential prognosis of AD from other neurodegenerative diseases; older adults with abnormal Aβ42/40 or Aβ42 profiles and high p-tau181 should be at higher odds for AD whilst the normal profiles may include participants with non-AD neurodegenerative diseases in addition to unaffected individuals. Associations between plasma NfL and GFAP in the normal Aβ42/40 mode/group will be important to evaluate neurodegeneration and glial activation independent of AD.
The key novelty of this report is the data-driven approach that separates participants into plasma Aβ42/40- or Aβ42-dependent clusters with distinct p-tau181, NfL and GFAP association profiles according to the cohort characteristics. This approach can be applied to other cohorts to accelerate threshold generation for plasma biomarkers, just as it was done for CSF biomarkers. Our findings should be replicated in other population-based studies, particularly those with greater racial/ethnic diversity.
The study’s main strength is the use of a well-characterized community based cohort. The three-group approach described also has an advantage of identifying individuals with incipient disease compared with the two-group approach that only classifies individuals as positive and negative. Moreover, as the study sample was randomly sampled from the voter registration list, it is not subject to the selection bias typical of studies conducted in clinical settings. However, since the sample is of largely European ancestry, our findings should be replicated in population samples with greater racial and ethnic diversity56.
In conclusion, we have shown, in the population-based MYHAT cohort, that plasma biomarkers associate with cognitive impairment, APOE*4 carriership and older age. Additionally, we demonstrate a clustering model to identify individuals at risk of AD pathophysiology. Once replicated in other population-based cohorts, these results will be important to screen for biomarker evidence of AD in older adults with or without cognitive concerns. This strategy will help enrich for individuals with biological evidence of disease for inclusion in intervention trials, early detection and longitudinal monitoring campaigns.
Supplementary Material
Systematic review:
We searched PubMed for plasma biomarkers of Alzheimer’s disease and related disorders (ADRDs). Dozens of studies have shown that: plasma Aβ42/40, GFAP and p-tau181 associate with brain Aβ pathology; p-tau181 correlates with brain tau pathology; and NfL is a strong indicator of neurodegeneration. Consequently, we sought to apply these tools to identify community-dwelling older adults with at-risk biomarker and clinical profiles.
Interpretation:
Bimodal distribution of plasma Aβ42/40 ratio allowed classification of n=847 population-based participants into three main groups. Plasma NfL, GFAP and p-tau181 correlated strongest with Aβ42/40 ratio and memory composite in the abnormal group. Furthermore, significant associations were observed in the normal and uncertain Aβ42/40 groups, suggesting sensitivity to identify individuals with emerging ADRD pathophysiology.
Future directions:
Future studies are needed to validate these results in other population-based cohorts and examine the capacity of biomarkers to identify people with incipient ADRD for clinical monitoring and/or inclusion in therapeutic trials.
Highlights.
Population-based plasma biomarker studies are lacking, particularly in cohorts without CSF or neuroimaging data;
In the MYHAT study (n=847), plasma biomarkers associated with worse memory and Clinical Dementia Rating, APOEε4, and greater age;
Plasma Aβ42/40 ratio levels allowed clustering participants into abnormal, uncertain and normal groups;
Plasma Aβ42/40 correlated differently with NfL, GFAP, p-tau181, memory composite and Clinical Dementia Rating in each group;
Plasma biomarkers will enable relatively affordable and non-invasive community screening for evidence of ADRD pathophysiology
Funding
The authors thank all MYHAT study personnel for their efforts and all MYHAT participants for their time, data, and specimens. M.G is supported by the NIH (R37 AG023651). PCLF is supported by Alzheimer’s Association (AARFD-22-923814). BB is supported by CAPES [88887.336490/2019-00] and Alzheimer’s Association (AARFD-22-974627). BS is supported by the National Institute of Aging (R01 AG023651). TAP is supported by the National Institute of Aging (R01AG075336, R01AG073267) and the Alzheimer’s Association (AACSF-20-648075). HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme – Neurodegenerative Disease Research (JPND2021-00694), and the UK Dementia Research Institute at UCL (UKDRI-1003). TKK was funded by the Swedish Research Council (Vetenskapsrådet #2021-03244), the Alzheimer’s Association (#AARF-21-850325), the Swedish Alzheimer Foundation (Alzheimerfonden), the Aina (Ann) Wallströms and Mary-Ann Sjöbloms stiftelsen, and the Emil och Wera Cornells stiftelsen.
Abbreviations:
- Aβ
Amyloid-β
- ADRD
Alzheimer’s disease and related disorders
- AD
Alzheimer’s disease
- APOE
Apolipoprotein E
- CDR
Clinical Dementia Rating
- CSF
Cerebrospinal fluid
- GFAP
Glial Fibrillary Acidic Protein
- HS
High School
- IP-MS
Immuno-Precipitation Mass Spectrometry
- MCI
Mild Cognitive Impairment
- MMSE
Mini Mental State Examination
- MYHAT
Monongahela-Youghiogheny Health Aging Team
- NfL
Neurofilament Light
- PET
Positron Emission Tomography
- P-tau181
Phosphorylated-tau181
- SIMOA
Single Molecule Array
Footnotes
Conflicts of interest and Disclosure Statement
HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Passage Bio, Pinteon Therapeutics, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Prothena, Roche Diagnostics, and Siemens Healthineers. MG has given lectures at University of Connecticut and is Associate Editor honorarium at Journal of the American Geriatrics Society. PCLF, YZ, BS, C-CHC, BB, EJ, MIK, TAP, VLV, TKK reports no disclosures.
References
- 1.Jack CR Jr., Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nature Communications 2020;11:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedet AL, Milà-Alomà M, Vrillon A, et al. Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum. JAMA Neurol 2021;78:1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molinuevo JL, Ayton S, Batrla R, et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol 2018;136:821–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004;55:306–319. [DOI] [PubMed] [Google Scholar]
- 6.Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC. Tau imaging: early progress and future directions. Lancet Neurol 2015;14:114–124. [DOI] [PubMed] [Google Scholar]
- 7.Karikari TK, Ashton NJ, Brinkmalm G, et al. Blood phospho-tau in Alzheimer disease: analysis, interpretation, and clinical utility. Nat Rev Neurol 2022;18:400–418. [DOI] [PubMed] [Google Scholar]
- 8.Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019;93:e1647–e1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashton NJ, Pascoal TA, Karikari TK, et al. Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol 2021;141:709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 2020;19:422–433. [DOI] [PubMed] [Google Scholar]
- 11.Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. Jama 2020;324:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashton NJ, Janelidze S, Al Khleifat A, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nature Communications 2021;12:3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson JE, Ince PG, Lace G, et al. Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol Aging 2010;31:578–590. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros R, LaFerla FM. Astrocytes: conductors of the Alzheimer disease neuroinflammatory symphony. Exp Neurol 2013;239:133–138. [DOI] [PubMed] [Google Scholar]
- 15.Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol 2019;76:791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira JB, Janelidze S, Smith R, et al. Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer’s disease. Brain 2021;144:3505–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karikari TK. Blood Tests for Alzheimer’s Disease: Increasing Efforts to Expand and Diversify Research Participation Is Critical for Widespread Validation and Acceptance. J Alzheimers Dis 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mielke MM, Dage JL, Frank RD, et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nature Medicine 2022;28:1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 20.Mungas D, Marshall SC, Weldon M, Haan M, Reed BR. Age and education correction of Mini-Mental State Examination for English- and Spanish-speaking elderly. Neurology 1996;46:700–706. [DOI] [PubMed] [Google Scholar]
- 21.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 22.Fuld PA. Fuld object-memory evaluation: Stoelting Company, 1977.
- 23.Wechsler D Wechsler memory scale-revised. Psychological Corporation 1987.
- 24.Papp KV, Amariglio RE, Dekhtyar M, et al. Development of a psychometrically equivalent short form of the Face-Name Associative Memory Exam for use along the early Alzheimer’s disease trajectory. Clin Neuropsychol 2014;28:771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer PF, Ashton NJ, Karikari TK, et al. Plasma p-tau231, p-tau181, PET Biomarkers, and Cognitive Change in Older Adults. Ann Neurol 2022;91:548–560. [DOI] [PubMed] [Google Scholar]
- 26.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. [Google Scholar]
- 27.Kaufman LR, Peter J. Partitioning Around Medoids (Program PAM). Finding Groups in Data1990: 68–125. [Google Scholar]
- 28.Spearman C The Proof and Measurement of Association between Two Things. The American Journal of Psychology 1904;15:72–101. [PubMed] [Google Scholar]
- 29.Milani SA, Swain M, Otufowora A, Cottler LB, Striley CW. Willingness to Participate in Health Research Among Community-Dwelling Middle-Aged and Older Adults: Does Race/Ethnicity Matter? J Racial Ethn Health Disparities 2021;8:773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashford MT, Eichenbaum J, Williams T, et al. Effects of sex, race, ethnicity, and education on online aging research participation. Alzheimers Dement (N Y) 2020;6:e12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of β-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry 2012;69:98–106. [DOI] [PubMed] [Google Scholar]
- 32.Gobom J, Parnetti L, Rosa-Neto P, et al. Validation of the LUMIPULSE automated immunoassay for the measurement of core AD biomarkers in cerebrospinal fluid. Clin Chem Lab Med 2022;60:207–219. [DOI] [PubMed] [Google Scholar]
- 33.Lantero Rodriguez J, Karikari TK, Suárez-Calvet M, et al. Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol 2020;140:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med 2020;26:379–386. [DOI] [PubMed] [Google Scholar]
- 35.Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med 2020;26:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol 2021;20:739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and Management of Dementia: Review. Jama 2019;322:1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milà-Alomà M, Ashton NJ, Shekari M, et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer’s disease. Nature Medicine 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kivisäkk P, Sweeney T, Carlyle BC, et al. Plasma biomarkers for diagnosis of Alzheimer’s disease and prediction of cognitive decline in individuals with mild cognitive impairment. medRxiv 2022:2022.2004.2018.22272912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsiknia AA, Edland SD, Sundermann EE, et al. Sex differences in plasma p-tau181 associations with Alzheimer’s disease biomarkers, cognitive decline, and clinical progression. Mol Psychiatry 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosselli M, Uribe IV, Ahne E, Shihadeh L. Culture, Ethnicity, and Level of Education in Alzheimer’s Disease. Neurotherapeutics 2022;19:26–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashton NJ, Hye A, Rajkumar AP, et al. An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat Rev Neurol 2020;16:265–284. [DOI] [PubMed] [Google Scholar]
- 43.Teunissen CE, Chiu MJ, Yang CC, et al. Plasma Amyloid-β (Aβ42) Correlates with Cerebrospinal Fluid Aβ42 in Alzheimer’s Disease. J Alzheimers Dis 2018;62:1857–1863. [DOI] [PubMed] [Google Scholar]
- 44.Smirnov DS, Ashton NJ, Blennow K, et al. Plasma biomarkers for Alzheimer’s Disease in relation to neuropathology and cognitive change. Acta Neuropathologica 2022;143:487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu Y, Kirmess KM, Meyer MR, et al. Assessment of a Plasma Amyloid Probability Score to Estimate Amyloid Positron Emission Tomography Findings Among Adults With Cognitive Impairment. JAMA Network Open 2022;5:e228392–e228392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janelidze S, Zetterberg H, Mattsson N, et al. CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol 2016;3:154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewczuk P, Matzen A, Blennow K, et al. Cerebrospinal Fluid Aβ42/40 Corresponds Better than Aβ42 to Amyloid PET in Alzheimer’s Disease. J Alzheimers Dis 2017;55:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018;554:249–254. [DOI] [PubMed] [Google Scholar]
- 49.Giudici KV, de Souto Barreto P, Guyonnet S, Li Y, Bateman RJ, Vellas B. Assessment of Plasma Amyloid-β42/40 and Cognitive Decline Among Community-Dwelling Older Adults. JAMA Netw Open 2020;3:e2028634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Wolf F, Ghanbari M, Licher S, et al. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain 2020;143:1220–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gauthier S, Webster C, Servaes S, Morais JA, Rosa-Neto P. World Alzheimer Report 2022: Life after diagnosis: Navigating treatment, care and support. London, England: 2022. [Google Scholar]
- 52.Schindler SE, Karikari TK, Ashton NJ, et al. Effect of Race on Prediction of Brain Amyloidosis by Plasma Aβ42/Aβ40, Phosphorylated Tau, and Neurofilament Light. Neurology 2022;99:e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashton NJ, Janelidze S, Mattsson-Carlgren N, et al. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nature Medicine 2022;28:2555–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmqvist S, Insel PS, Stomrud E, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer’s disease. EMBO Molecular Medicine 2019;11:e11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young AL, Oxtoby NP, Daga P, et al. A data-driven model of biomarker changes in sporadic Alzheimer’s disease. Brain 2014;137:2564–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kukull WA, Ganguli M. Generalizability: the trees, the forest, and the low-hanging fruit. Neurology 2012;78:1886–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


