Abstract
Female adolescents have a greatly increased risk of depression starting at puberty, which continues throughout the reproductive lifespan. Sex hormone fluctuation has been highlighted as a key proximal precipitating factor in the development of mood disorders tied to reproductive events; however, hormone-induced affective state change is poorly understood in the pubertal transition. The present study investigated the impact of recent stressful life events on the relationship between sex hormone change and affective symptoms in peripubertal female participants. 35 peripubertal participants (ages 11–14, premenarchal, or within 1 year of menarche) completed an assessment of stressful life events and provided weekly salivary hormone collections (estrone, testosterone, dehydroepiandrosterone (DHEA)) and mood assessments for eight weeks. Linear mixed models tested whether stressful life events provided a context in which within-person changes in hormones predicted weekly affective symptoms. Results indicated that exposure to stressful life events proximal to the pubertal transition influenced the directional effects of hormone change on affective symptoms. Specifically, greater affective symptoms were associated with increases in hormones in a high stress context and decreases in hormones in a low stress context. These findings provide support for stress-related hormone sensitivity as a diathesis for precipitating affective symptoms in the presence of pronounced peripubertal hormone flux.
Keywords: puberty, female, depression, sex hormones, stress
1. INTRODUCTION
The pubertal transition (peripuberty) is characterized by nearly a 3-fold greater risk of a depressive disorder in female adolescents (SAMHSA, 2020). Among other factors, this increased risk may be a result of simultaneous increases in sex hormone fluctuations and interpersonal stressors in female adolescents (Balzer et al. 2015). The sex disparity first emerges at mid-puberty (Tanner stage 3 or 4) and continues throughout the female reproductive lifespan, suggesting a role of ovarian hormone fluctuations in vulnerability to psychopathology (Angold, 1993; Angold et al., 1999; Schiller et al., 2016). An abnormal neurobiological sensitivity to normal reproductive hormone flux can trigger affective symptoms in hormone-sensitive individuals. Furthermore, stress exposure interferes with the maturation of neurodevelopmental trajectories (Walker et al., 2004) and can predict who is at risk for developing reproductive mood disorders (Bromberger et al., 2011; Namavar Jahromi et al., 2011). However, the biological mechanisms by which life stress exposure interacts with the peripubertal reproductive endocrine environment to promote depression risk are poorly understood. The present study examines how within-person deviations in sex hormones may predict depressive symptoms in peripubertal female adolescents, particularly in the context of recent stressful life events.
1.1. Mechanisms of depression risk during the pubertal transition
Reproductive hormone environment
The pubertal transition is accompanied by extensive changes in ovarian and adrenal hormones (Andersen et al. 2022; Apter 1980; Apter et al. 1989), heightened interpersonal stress exposure and a critical window in which neurodevelopmental trajectories are established (Blakemore, 2008). Adrenarche, increased activity of the hypothalamic-pituitary-adrenal (HPA) axis, initiates the developmental hormone cascade with the production of androgens, including dehydroepiandrosterone (DHEA). This is followed by reactivation of the hypothalamic-pituitary-gonadal (HPG) axis (i.e., gonadarche) and stimulation of sex steroid production, for example estradiol (E2), estrone (E1), and testosterone (T). Moreover, not only absolute concentrations but also the degree of hormone fluctuation increases with the onset of the first menstrual cycle (menarche)(Andersen et al. 2022; Janfaza et al. 2006). The first gynecological year (first year after menarche) is characterized by frequent anovulatory (up to 66%) and irregular cycles that further increase exposure to hormone fluctuation (Carlson & Shaw, 2019; Gunn et al., 2018). This dramatic reproductive endocrine instability may contribute to the abrupt emergence of affective illness during the pubertal transition in female adolescents (Angold, 1993; Patton et al., 2008).
Given the significant regulatory effect of sex hormones on mood and behavior, women are more likely to experience affective dysregulation during reproductive transition events characterized by significant hormonal flux, including premenstrual, postpartum, and perimenopausal periods (Balzer et al. 2015; Schiller et al. 2016). Hormone manipulation studies simulating reproductive transition events indicate that a vulnerability to normal changes in sex hormones, rather than the absolute level, precipitate the emergence of depressive symptoms in women with a history of reproductive hormone-related mood impairment (Bloch et al. 2000; Gordon et al. 2015, 2016; Schmidt et al. 1998, 2017). Accordingly, fluctuations in sex hormones such as estrogens (e.g., estradiol, estrone), progesterone and its derivatives, and testosterone have been shown to trigger the development of affective symptoms in a subset of hormone-sensitive individuals across the lifespan (premenstrual: Schmidt et al. 1998; perinatally: (Bloch et al., 2000); perimenopausal: (Sander et al., 2021; Schmidt et al., 2015); in males: (Schmidt et al., 2004). We recently demonstrated that a large proportion of peripubertal female adolescents showed mood sensitivity to sex hormone flux. Specifically, 57% of participants exhibited increased dysphoric mood with weekly changes (i.e., increase, decrease or large change in either direction from the previous week) in estrone and 37% of participants were mood sensitive to weekly changes in testosterone (Andersen et al. 2022). Our previous findings suggest that peripubertal female adolescents’ mood sensitivity to sex-hormone flux can be categorized into four different hormone sensitivity profiles: 1) insensitive (no increase in mood symptoms with weekly change in hormone); 2) withdrawal sensitive (increased mood symptom severity with decreases in hormone change from the previous week); 3) increase sensitive (increased mood severity with increased hormone change); and 4) change sensitive (increased mood severity with large changes in hormone, regardless of direction) (Andersen et al. 2022). The identification of these different hormone sensitivity profiles highlights the importance of assessing within-person hormone variability in determining mood and hormone relationships.
Life stress exposure and HPA axis dysfunction
Along with profound physical developmental changes, the pubertal transition is characterized by elevated life stress exposure (Spear, 2009). The impact of life stress is especially relevant during critical developmental windows characterized by dramatic neurodevelopment, including childhood and adolescence (Blakemore, 2008). Exposure to life stress during these early developmental periods has been shown to modify biological stress response systems (i.e., HPA axis), and is associated with sustained alterations in brain function, behavior and cognition (Lupien et al., 2009). As such, dysregulation of the HPA axis can have long-term consequences and increase the risk for psychological disorders (De Carvalho Tofoli et al., 2011). The deleterious effect of stress may be even more pronounced for adolescents who experience earlier pubertal maturation relative to their peers (i.e., early pubertal timing)(Hamlat et al., 2014). African American or Black and Latino/Latina adolescents, youth from lower socioeconomic contexts (James-Todd et al., 2010), and those exposed to early life adversity (Colich & McLaughlin, 2022) are disproportionately more likely to experience early pubertal timing (Colich and McLaughlin 2022). Therefore, social context and diversity may converge with the unique reproductive endocrine features of puberty to promote greater risk of psychopathology during the pubertal transition (Mendle et al., 2019).
Although null findings exist (Meadows et al., 2006), a number of studies report increased stress exposure for female compared to male adolescents (Ge et al., 1994, 2001). This applies particularly to interpersonal stress (Hankin et al., 2007), possibly due to social gender roles (McLeod & Kessler, 1990; Turner et al., 1995). Female adolescents also experience increased rates of severe stressful life events, including physical and sexual abuse, which also contributes to long-term modifications in stress reactivity (LeMoult et al., 2015, 2020). Exposure to stressful life events is strongly associated with an increased risk of affective disorders, including depression (Hammen, 2005; Monroe & Reid, 2008; Paykel, 2003). The association of stress exposure and depression has been shown to be stronger in female adolescents compared to male adolescents (Ge et al., 1994; Shih et al., 2006). Further, stressful life events proximal to reproductive transition events have been shown to be strong predictors of the emergence of reproductive mood disorders (Bromberger et al., 2011). As such, in initially euthymic perimenopausal women, the number of recent stressful life events in combination with greater estradiol variability over the menopause transition predicted the emergence of depression (Gordon et al. 2016). However, this relationship between hormone fluctuation and affective state change is poorly understood during the pubertal transition, which shares many parallels with the menopause transition and is similarly characterized by substantial reproductive hormone flux and heightened life stress.
1.2. Aims of the current study
The present study employed a longitudinal, dimensional approach to examine within-person relationships between life stress, weekly changes in sex hormones (estrone, testosterone, DHEA) and affective state change in peripubertal female adolescents. The present study tests a novel diathesis-stress model of adolescent depression in which we propose that life stress modifies the brain’s sensitivity to hormone fluctuation and precipitates vulnerability (i.e., a diathesis) to depressive symptoms in the presence of normal peripubertal hormone flux (i.e., an acute hormonal stressor). The rationale for examining stress-related hormone sensitivity as a diathesis for affective symptoms is twofold. First, sensitivity to hormonal flux during specific reproductive events has been shown to trigger affective symptoms in women who are more susceptible to stress and, second, sex hormones are powerful neuroregulators of frontal and limbic neural networks (Ottowitz et al., 2008) and the HPA stress axis, each of which is implicated in depression (Pandya et al., 2012). Specifically, the present analysis examines recent life stress as a moderator of the relationship between weekly hormone deviations and specific affective symptom constructs (e.g., interpersonal depressive symptoms, low positive affect, somatic symptoms) (Figure 1). The initial investigation of peripubertal hormone and mood relationships was restricted to general dysphoric mood and did not examine hormone-induced changes in distinct symptom domains (i.e., anxiety, irritability, etc.). This additional decomposition of affective symptoms is warranted given that previously reported hormone-related mood disturbances are symptom and hormone specific (Eisenlohr-Moul et al. 2016; Schiller et al. 2022; Schmidt et al. 1998). Consistent with hormone-mood relationships observed in other female reproductive transitions (i.e., premenstrual phase, perimenopause), weekly deviations in hormones (estrone, testosterone, DHEA) in the context of high life stress were expected to be associated with greater depressive symptoms and distress.
Figure 1. Diathesis-Stress Model of Hormone-Related Mood Dysregulation and Affective Illness.
Illustration of the proposed model in which life stress modifies the brain’s sensitivity to hormone fluctuation and precipitates vulnerability (i.e., a diathesis) to depressive symptoms in the presence of normal peripubertal hormone flux (i.e., an acute hormonal stressor).
2. METHODS
2.1. Participants
35 participants assigned female at birth, between the ages of 11 and 14, and who were undergoing a healthy pubertal transition were recruited from the local community using flyers, mass emails to university staff and students, word-of-mouth, and online posts on middle school parent sites. The protocol was approved by the local Institutional Review Board and was conducted in accordance with the Declaration of Helsinki. Parents provided written consent and adolescent participants provided written assent to be involved in the study. Participants received a $150.00 Visa gift card for full compliance.
2.2. Procedure
The study was conducted between 2018 and 2020, and consisted of three phases, including 1) screening, 2) enrollment, and 3) weekly hormone and mood measurements, described in more detail below and in Andersen et al. (2022). Participants included in the present analysis were a subsample of a larger study (n=52) who all had weekly (vs. daily) mood assessments and morning (vs. afternoon) salivary hormone collections. The present analysis with this subsample (n=35) permitted the investigation of life stress and its effect on hormone-related changes in distinct symptom constructs of depression with refined collection parameters.
2.2.1. Screening
Parents of interested participants completed an online screening form to assess eligibility. Participants were recruited based on parental and self-reported pubertal stage (Tanner Stage 3 or 4, breast development and pubic hair growth started, but not complete - as assessed by the Pubertal Development Scale (PDS) at screening and enrollment) and were premenarchal or within one-year post-menarche. Non-English speakers and participants with psychotic or bipolar disorders, active suicidal ideation, or severe symptoms that would interfere with participation were excluded. To obtain a wide range and adequate variance of depressive symptoms, current self-reported clinical diagnoses of depression or anxiety were permitted if no suicidal ideation, and no symptoms of psychosis or mania were reported at enrollment. Participants did not use hormonal agents or contraceptives, or mood-altering medications (e.g., antidepressants). If eligible, the screening was followed by a phone call with the parent to discuss the specifics of the study procedure and confirm eligibility before scheduling the enrollment session.
2.2.2. Enrollment
At enrollment, participants and their parents completed questionnaires assessing personal and family medical history, including self-reported clinical diagnoses, demographic characteristics, mood, stressful life events, and pubertal stage. An abbreviated Structured Clinical Interview for DSM-V (SCID) was administered to screen for psychosis symptoms or bipolar disorder. Height and weight measurements were collected to calculate age-corrected BMI according to Centers for Disease Control and Prevention guidelines, as BMI consistently increases with pubertal maturation (Bini et al., 2000). Parental reports of highest educational attainment and total household income were collected and assessed as indicators of socioeconomic status. Participants and their parents were instructed on best practices for collecting saliva samples and were given clearly labeled collection vials.
2.2.3. Weekly hormone and mood assessments
Following the enrollment session, participants completed eight consecutive weeks of saliva and mood assessments. Using the unstimulated passive drool technique, participants provided 3mL of saliva into cryovials immediately upon awakening to capture the expected peak hormone levels for 8 consecutive weeks (Hucklebridge et al., 2005; Janfaza et al., 2006; Kuzawa et al., 2016). To prevent contamination and dilution, participants were instructed not to visit the dentist within two days before the next collection, refrain from drinking (water included) and brushing their teeth thirty minutes prior to their collection, and refrain from eating one hour before the collection. Saliva samples were immediately placed in the participant’s home freezer before being transferred to a −80°C laboratory freezer every 1–3 weeks. Weekly compliance checks were completed along with the surveys to determine if there were any changes in activity level, diet, sleep, or medication use prior to the saliva collection.
2.3. Measures
2.3.1. Enrollment assessments
Pubertal Development was self-reported using a combination of the Pubertal Development Scale (PDS; Petersen et al., 1988) and Tanner line staging, in which participants selected line drawings of breast development and pubic hair growth that best matched their own (Taylor et al., 2001). Line drawings corresponded to Tanner stage criteria, with 1 indicating no development (“breasts are flat”; “no hair”) and 5 indicating complete development (“only the nipple sticks out beyond the breast”; “hair has spread over the thighs”). Participants rated menarche status, height or growth spurt, breast development, pubic hair growth and appearance of acne on the PDS using a 4-point scale ranging from 1) no development/no menses, 2) barely begun, 3) definitely underway, to 4) completed/menses. The PDS score was calculated as the mean of the five responses. The category PDS score corresponds to the sum of the items pertaining to pubic hair growth, breast development and menarche status, as described previously (Carskadon & Acebo, 1993). Category scores range from 3- prepubertal to 12 post-pubertal. PDS and tanner line staging scores were averaged for breast development and pubic hair growth. Self-reported PDS for female breast and pubic hair development has shown excellent agreement with a physical examination (85% within 1 Tanner stage) (Schmitz et al., 2004) and good reliability (Cronbach’s alpha for PDS in the present sample was 0.70).
Recent Stressful Life Events were assessed using a modified version of the Coddington Life Events Scale (CLES) (Coddington, 1972). Participants were asked to indicate whether or not they had experienced any of the 25 stressful experiences listed in the measure in the past year by checking ‘yes’ or ‘no’ for each item. Samples items include “death of a family member,” “end of romantic relationship,” “serious personal injury/illness,” “change in acceptance by peers,” “move or change in residence,” and “parents’ divorce.” A total score was calculated from the aggregated number of stressful life events that the participant reported. The number of stressful life events (continuous) was standardized using a z-score.
Mood and Feelings Questionnaire (MFQ; Costello and Angold 1988) is a 33-item self-report inventory to assess adolescent depressive symptoms. Participants rate how they have been feeling or acting over the past 2 weeks by indicating whether each statement is not true (0), somewhat true (1) or mostly true (2) for them. The total score reflects the sum of the 33 items and ranges from 0 to 68, with larger scores reflecting greater depressive symptoms. Cronbach’s alpha for MFQ in the present sample was 0.96.
2.3.2. Weekly mood assessments
The Center for Epidemiological Studies Depression Scale for Children (CES-DC) is a 20- item assessment for child and adolescent depressive symptoms (Roberts et al., 1990). Items are scored 0 (not at all) to 3 (a lot) with 4 items reverse coded for a range between 0–60. Cronbach’s alpha for all 20 items in the present sample was 0.95. Previous studies on reproductive mood disorders have reported that the psychological relevance of hormone change may be specific to certain symptom domains (Eisenlohr-Moul et al., 2016; Gordon et al., 2019). As such, the current study employed a dimensional approach to elucidate specific mood and hormone relationships. To refine the investigation of depressive symptom domains, four predefined subscales were computed (reviewed in Shafer, 2006), including interpersonal (2 items, α (present sample)=.862), low positive affect (4 items, α (present sample)= .852), depressed affect (7 items, α (present sample)=.932) and somatic symptoms (7 items, α (present sample) =.855).
The Perceived Stress Scale (for children) is a 10-item self-report assessment to evaluate perceived coping and distress associated with stressful situations (Cohen et al., 1983). Items are rated on a 5-point Likert scale from 0 (never) to 4 (very often). Summary scores were calculated after reversing 4 items. Higher scores represent higher subjective stress. Consistent with previous reports, two factors were confirmed in the current sample: a positive-worded factor associated with perceived coping and a negative-worded factor indexing perceived distress (Ezzati et al., 2014; Hewitt et al., 1992) both of which have been found to predict depression symptoms. Correlations among scale items ranged from r=.210 to r=.742, with Cronbach’s α=.896 for all 10 items, α=.780 for the coping factor, and α=.889 for the distress factor.
Weekly Subjective Stress Rating. Each week, participants were asked to indicate whether they experienced “any problems” over the last week by checking a box next to relevant items or filling in their own response. The seven items included in the list were: 1) performed poorly on an exam or assignment, 2) received a bad report card, 3) felt overbooked with extracurricular activities, 4) got in a fight with a friend(s), 5) didn’t get along with parents, 6) experienced bullying, 7) didn’t get along with siblings, and 8) other. They were also asked to enter the most stressful event that happened each week and how stressed it made them feel on a sliding bar from 1 (not very stressful) to 10 (extremely stressful).
2.4. Salivary hormone collection and analysis
Salivary samples were assayed at ZRT Laboratory (Beaverton, OR) using liquid chromatography-tandem mass spectrometry (LC-MS/MS) to achieve the most sensitive and accurate quantification of salivary hormones. Average inter-assay precision was 7.27% for estrone, 9.77% for testosterone, and 7.03% for DHEA, with the average intra-assay coefficients of variance as follows: 9.47% for estrone, 4.20% for testosterone, and 7.27% for DHEA. Minimum detection limits were 0.4 pg/mL for estrone, 3.2 pg/mL for testosterone, and 17.1 pg/mL for DHEA. Measurements that fell below assay sensitivity were assigned a value that was one-half the limit of detection. Estrone was selected over estradiol (E2) for the present analyses because estrone rises before E2 during the pubertal transition (Biro et al., 2014) allowing for easier detection, and has been shown to be highly correlated with E2 in previous reports (r=0.80, p<.0001) (Andersen et al., 2022). Lower concentrations of E2 are expected in peripubertal females – especially premenarche (Janfaza et al., 2006).
2.5. Analytic Plan
2.5.1. Data coding and preparation
Analyses were performed in SAS On Demand for Academics. Hormone measurements were centered within-person (CWP) to extract within-person hormone variance (i.e., [hormone level at this sample] – [participant’s average hormone level across all samples]). As such, greater values of hormone CWP reflect increased levels relative to a participant’s individual mean (Eisenlohr-Moul et al., 2015).
2.5.2. Weekly hormone and mood relationships
Separate multilevel models were performed to predict each affective symptom outcome (CES-DC-depressed, CES-DC-interpersonal, CES-DC-low positive affect, CES-DC-somatic, PSS-coping, PSS-distress) from weekly deviations from an individual’s average hormone level (DHEA, testosterone, estrone), number of stressful life events (SLE, sample standardized), and all associated interactions. “Week” was specified as a repeated statement to account for serial correlation of weekly hormone and mood assessments, and a random slope within-person was applied with a variance component structure. Null multilevel models (without predictors) were used to calculate the intraclass correlation coefficient (ICC) for each predictor and outcome variable (see Table 1). Given the small sample size, restricted maximum likelihood estimation (REML) was implemented to account for potential biases in random-effect variances and the Kenward-Roger correction was used to address biases in standard error estimation (McNeish & Matta, 2018). Age, race, parental education (as a proxy for socioeconomic status) and BMI did not significantly contribute to the models and were therefore excluded from final analyses.
Table 1.
Demographic and Sample Characteristics
| N | Range (min - max) | Mean ± SD | |
|---|---|---|---|
|
| |||
| Age (months) | 132 – 176 | 149.77 ± 12.11 | |
| Race | |||
| White | 26 (74.3%) | ||
| Black or African American | 1 (2.9 %) | ||
| Multiracial | 4 (11.4%) | ||
| Hispanic/Latino/Spanish | 4 (11.4%) | ||
| BMI (z-score) | −1.73 – 3.31 | −0.021 ± 1.09 | |
| Female Gender Identity | 34 (97.1%) | ||
| Identify as LGBTQIA+ | |||
| No | 29 (82.8%) | ||
| Yes | 3 (8.6%) | ||
| No response | 3 (8.6%) | ||
| Pubertal Development Score | 1.8 – 3.8 | 2.66 ± 0.63 | |
| PDS (avg) category score | 5 – 12.5 | 8.33 ± 2.30 | |
| Menarche status | 17 (48.6%) | ||
| Breast (pictorial score) | 2 – 5 | 3.23 ± 0.69 | |
| Pubic hair (pictorial score) | 1 – 5 | 2.94 ± 0.87 | |
| Stressful Life Events | 2 – 11 | 5.03 ± 2.55 | |
| Mood & Feelings | 0 – 53 | 14.43 ± 13.32 | |
| Parental Education (%) | 4.5 – 7 | 6.26 ± 0.68 | |
| High School Diploma | 0 | ||
| >4 Years of College | 5 (14.28%) | ||
| 4-Year College Degree | 15 (42.86%) | ||
| Professional Degree | 15 (42.86%) | ||
| Household Income | |||
| <$30,000 | 1 (2.86%) | ||
| $30,000 – $70,000 | 1 (2.86%) | ||
| $70,000 – $100,000 | 6 (17.14%) | ||
| $101,000 – $150,000 | 12 (34.29%) | ||
| $150,000+ | 15 (42.86%) | ||
| ICC | Range (min-max) | Mean ± SD | |
| Weekly Self Report | |||
| CES-DC-interpersonal | 0.704 | 0 – 6 | 1.28 ± 1.73 |
| CES-DC-depressed | 0.760 | 0 – 18 | 4.14 ± 4.68 |
| CES-DC-somatic | 0.774 | 0 – 24 | 6.00 ± 5.62 |
| CES-DC-low positive affect | 0.770 | 0 – 12 | 3.81 ± 3.06 |
| PSS-coping | 0.581 | 0 – 14 | 6.02 ± 2.86 |
| PSS-distress | 0.652 | 0 – 22 | 9.38 ± 5.34 |
| Hormones | |||
| Estrone | 0.364 | 0.20 ± 4.20 | 1.10 ± 0.68 |
| Testosterone | 0.638 | 1.50 ± 30.00 | 6.89 ± 4.28 |
| DHEA | 0.537 | 8.50 ± 319.00 | 77.68 ± 47.12 |
Note. Means are presented ± standard deviation. BMI: body mass index, LGBTQIA+: lesbian, gay, bisexual, transgender, queer, intersex, asexual and more, PDS: Pubertal Development Scale average category score (averaged PDS and tanner line staging score for breast development and pubic hair growth), Primary parent completed education level, CES-DC: Center for Epidemiological Studies Depression Scale for Children, PSS: Perceived Stress Scale, DHEA: dehydroepiandrosterone.
The effect of stress was further probed by calculating the simple slopes of hormone (CWP) at various levels of stress for each combination of symptom and hormone pair. The simple slopes were calculated from three levels of SLE: mean SLE = 5.029, mean SLE + standard deviation (sd) = 7.578, and mean SLE − sd = 2.479.
2.5.3. Power Analysis
With 35 participants that completed CES-DC ratings and hormone collections, the current study had 86% power with α=0.05 to detect small effects (f=0.15) of within-person weekly hormone and mood associations (8 repeated measures with 0.50 correlation).
3. RESULTS
3.1. Descriptive information
Demographic and sample characteristics are presented in Table 1. 35 participants (mean age =12.48, SD=1.01) provided weekly mood ratings and salivary hormone samples. The majority of participants identified as White (74.3%), with 11.4% identifying as multiracial, 11.4% as Hispanic/Latino or Spanish, and 2.9% Black. Participants’ parents were highly educated (graduated or postgraduate) and were affluent middle to upper class, representative of the local community. Participants were self-reported mid-puberty (PDS category score: mean = 8.33, SD = 2.30), with 51.4% (18/35) of the sample pre-menarche and 48.6% (17/35) of participants post-menarche within one year. Pictorial rating of breast (mean = 3.23, SD = 0.69) and pubic hair development (mean = 2.94, SD = 0.87) subjectively confirmed peripubertal status. One participant identified as male. Pubertal status, BMI, MFQ, number of stressful life events and average estrone, testosterone and DHEA levels did not differ by race or ethnicity, nor were these variables associated with parental education (as a proxy for socioeconomic status). Pubertal status was correlated with BMI (rs=.484, p=.004), and associated with greater estrone (rs=.535, p=.001), testosterone (rs=.477, p=.004) and DHEA (rs=.398, p=.018) levels.
The frequency of stressful life events ranged from 2 to 11 events, with an average of 5.03 (SD = 2.55). As expected, there was a wide range of depressive symptom severity reported on the mood and feelings questionnaire, ranging from 0 to 53 (mean=14.43, SD=13.31, a score of 29 or above is considered clinically significant (Burleson Daviss et al., 2006)), with increased symptom severity associated with a greater number of stressful life events (rs=.385, p=.022). Further, more stressful life events were related to higher DHEA levels (rs=.380, p=.024). The most frequently experienced weekly stress was associated with academic performance (26.84%), followed by conflicts with parents (16.67%) and “other” (16.67%). Descriptions of “other” included being excluded from a sleepover, feeling jealous, not getting enough sleep, getting braces, a sick pet, etc. There were no major life events reported during the 8-week collection period.
Study adherence was high, with only 13 weekly surveys missed (4.6%, 267/280) and 5 saliva samples missed (1.8%, 275/280) across all participants. Estrone and testosterone were below the limit of detection in 4% (E1:12/275; T:10/275) of the samples, and DHEA was undetectable in 2% (6/275) of the samples collected.
3.2. Intraclass correlations of repeated hormones and outcomes
Intraclass correlations indicated that the majority of the variance in symptoms could be attributed to stable between-person differences (trait-like; ICCs between 0.58 and 0.77). Variability in estrone was primarily at the within-person level (ICC=0.36), whereas 64% of testosterone and 54% of DHEA variance was trait-like (Table 1).
3.3. Interactive effects of stressful life events and hormone change on symptoms
Results of models examining the moderating role of stressful life events on the association between within-person deviations in hormones (CWP) and symptom expression for all outcomes are reported in Table 2. P-values presented in Table 2 are FDR corrected and Cohen’s f2 was calculated for mixed-effects models using recommendations proposed by Selya et al. 2012.
Table 2.
Fixed effects of stressful life events, within-person hormone deviations, and their interactions on symptoms.
| Weekly CES-DC score | |||||||||||||||||||
| Parameter | Somatic | Low positive affect | Depressed affect | Interpersonal | |||||||||||||||
|
| |||||||||||||||||||
| Model: E1 × SLE | Est. | (SE) | p | f 2 | Est. | (SE) | p | f 2 | Est. | (SE) | p | f 2 | Est. | (SE) | p | f 2 | |||
| Intercept | 5.454 | 0.686 | <.0001 | 3.838 | 0.445 | <.0001 | 4.783 | 0.777 | <.0001 | 1.277 | 0.246 | <.0001 | |||||||
| zSLE | 1.725 | 0.701 | 0.019 | 1.033 | 0.454 | 0.030 | 1.816 | 0.794 | 0.029 | 0.498 | 0.251 | 0.055 | |||||||
| E1CWP | 0.396 | 0.290 | 0.172 | 0.190 | 0.179 | 0.289 | −0.115 | 0.304 | 0.706 | 0.076 | 0.113 | 0.499 | |||||||
| zSLE*E1CWP | 0.848 | 0.337 | 0.028* | 0.119 | 0.155 | 0.208 | 0.482* | 0.091 | 0.838 | 0.354 | 0.036* | 0.103 | 0.342 | 0.132 | 0.026* | 0.069 | |||
| Model: T × SLE | |||||||||||||||||||
| Intercept | 5.456 | 0.686 | <.0001 | 3.839 | 0.445 | <.0001 | 4.786 | 0.777 | <.0001 | 1.277 | 0.246 | <.0001 | |||||||
| zSLE | 1.721 | 0.701 | 0.019 | 1.031 | 0.454 | 0.030 | 1.812 | 0.793 | 0.029 | 0.498 | 0.251 | 0.055 | |||||||
| TCWP | 0.005 | 0.060 | 0.932 | 0.005 | 0.037 | 0.888 | −0.171 | 0.062 | 0.007 | 0.022 | 0.024 | 0.360 | |||||||
| zSLE*TCWP | 0.226 | 0.075 | 0.014* | 0.122 | 0.086 | 0.046 | 0.091* | 0.094 | 0.264 | 0.077 | 0.006* | 0.111 | 0.054 | 0.029 | 0.091* | 0.065 | |||
| Model: DHEA × SLE | |||||||||||||||||||
| Intercept | 5.446 | 0.686 | <.0001 | 3.835 | 0.445 | <.0001 | 4.774 | 0.777 | <.0001 | 1.274 | 0.245 | <.0001 | |||||||
| zSLE | 1.701 | 0.700 | 0.021 | 1.023 | 0.454 | 0.031 | 1.801 | 0.794 | 0.030 | 0.492 | 0.250 | 0.058 | |||||||
| DHEACWP | 0.014 | 0.005 | 0.005 | 0.002 | 0.003 | 0.449 | −0.001 | 0.005 | 0.864 | 0.003 | 0.002 | 0.193 | |||||||
| zSLE* DHEACWP | 0.015 | 0.005 | 0.014* | 0.121 | 0.007 | 0.003 | 0.042* | 0.095 | 0.015 | 0.005 | 0.019* | 0.106 | 0.005 | 0.002 | 0.026* | 0.072 | |||
| Weekly PSS Score | |||||||||||||||||||
| Parameter | Coping | Distress | |||||||||||||||||
|
| |||||||||||||||||||
| Model: E1 × SLE | Est. | (SE) | p | f 2 | Est. | (SE) | p | f 2 | |||||||||||
| Intercept | 6.024 | 0.386 | <.0001 | 9.453 | 0.707 | <.0001 | |||||||||||||
| zSLE | 0.449 | 0.395 | 0.263 | 1.777 | 0.722 | 0.019 | |||||||||||||
| E1CWP | −0.010 | 0.223 | 0.966 | 0.690 | 0.375 | 0.067 | |||||||||||||
| zSLE*E1CWP | −0.253 | 0.259 | 0.395* | 0.013 | 0.775 | 0.437 | 0.099* | 0.110 | |||||||||||
| Model: T × SLE | |||||||||||||||||||
| Intercept | 6.026 | 0.386 | <.0001 | 9.458 | 0.707 | <.0001 | |||||||||||||
| zSLE | 0.450 | 0.395 | 0.262 | 1.767 | 0.722 | 0.002 | |||||||||||||
| TCWP | −0.081 | 0.046 | 0.083 | −0.040 | 0.077 | 0.609 | |||||||||||||
| zSLE*TCWP | −0.030 | 0.057 | 0.595* | 0.013 | 0.377 | 0.095 | .0002* | 0.130 | |||||||||||
| Model: DHEA × SLE | |||||||||||||||||||
| Intercept | 6.023 | 0.386 | <.0001 | 9.448 | 0.708 | <.0001 | |||||||||||||
| zSLE | 0.448 | 0.394 | 0.264 | 1.756 | 0.724 | 0.021 | |||||||||||||
| DHEACWP | −0.006 | 0.004 | 0.109 | 0.008 | 0.006 | 0.215 | |||||||||||||
| zSLE* DHEACWP | 0.003 | 0.004 | 0.482* | 0.014 | 0.016 | 0.007 | 0.030* | 0.112 | |||||||||||
Note: Estimates are presented with standard error (SE) and p value. SLE: stressful life events, zSLE: standardized stressful life events score, CWP: Centered Within Person, E1: estrone, T: testosterone, DHEA: dehydroepiandrosterone, CES-DC: Center for Epidemiological Studies Depression Scale for Children, PSS: perceived stress scale.
FDR adjusted p-values
3.3.1. Stressful life events and within-person weekly hormone deviations
Estrone.
Stressful life events interacted with estrone CWP to predict somatic (F(1,230)=6.32, p=.013), interpersonal (F(1,230)=6.78, p=.01), and depressed affect (F(1,230)=5.62, p=.019) symptoms. Specifically, within-person elevations of estrone predicted greater somatic (t(230.2) = 2.67, p=0.008) and interpersonal (t(230.1) = 2.31, p=0.022) symptoms in the context of high stress (SLE+sd), and lower-than-usual estrone predicted greater depressed affect (t(230.1) = −2.14, p=0.033) at low stress (SLE-sd).
Testosterone.
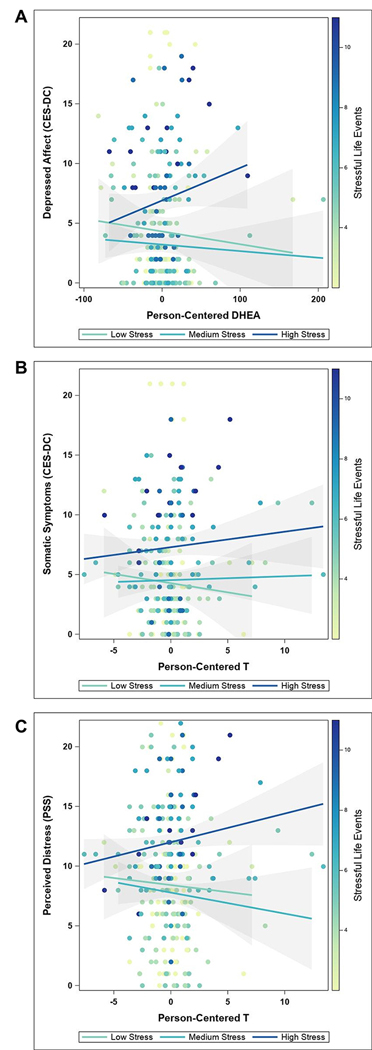
Within-person deviations in testosterone interacted with stressful life events to predict somatic (F(1,230)=9.16, p=.003), depressed affect (F(1,230)=11.81, p=.001), and perceived distress (F(1,230)=15.74, p<.001) symptoms. Probing the impact of stress revealed differential effects of within-person deviations in testosterone on somatic symptoms and perceived distress, with higher-than-usual testosterone predicting greater symptoms at high stress (somatic: t(230.2) = 2.65, p=0.009; distress: t(230.2) = 3.04, p=0.003), and lower-than-usual testosterone predicting greater symptoms at low stress (somatic: t(230.1) = −2.11, p=0.036; distress: t(230.2) = −3.12, p=0.002) (Figure 2b, 2c). Additionally, low (t(230.1) = −4.05, p<0.001) to average (t(230.1) = −2.72, p=0.007) stress was associated with greater depressed affect following lower-than-usual testosterone.
Figure 2. Stress unmasks differential effects of within-person hormone deviations on affective symptoms.
The stress context created a divergence in DHEA-related depressed affect (a) and testosterone-related somatic symptoms (b) and perceived distress (c). Stressful life events are presented as a gradient (with a low number of events indicated by yellow and a high number of events represented by dark blue colors). The regression lines represent tertile groups with 90% confidence intervals. DHEA: dehydroepiandrosterone, CES-DC: Center for Epidemiological Studies Depression Scale for Children, PSS: perceived stress scale.
DHEA.
DHEA CWP interacted with stressful life events to predict somatic (F(1,230)=8.88, p=.003), low positive affect (F(1,230)=5.05, p=.026), depressed affect (F(1,230)=7.90, p=.005), interpersonal symptoms (F(1,230)=6.75, p=.010), and perceived distress (F(1,230)=6.02, p=.015). A similar pattern of stress-hormone interactions was found for DHEA, with higher-than-usual DHEA predicting greater depressed affect symptoms at high stress (t(230.3) = 2.04, p=0.042), and lower-than-usual DHEA predicting greater symptoms at low stress (t(230.1) = −1.98, p=0.049) (Figure 2a). At high stress, within-person elevations in DHEA predicted increased symptom ratings for low positive affect (t(230.3) = 2.31, p=0.022), interpersonal symptoms (t(230.3) = 3.00, p=0.003), perceived distress (t(230.5) = 2.84, p=0.005), and somatic symptoms (t(230.3) = 4.43, p<0.001; average: t(230.1) = 2.83, p=0.005).
4. DISCUSSION
4.1. Summary of results
The present study used a dimensional approach with weekly measures of sex hormones and depressive symptom ratings to determine the role of recent stressful life events in modifying the relationship between hormone flux and depressive symptoms in peripubertal females. Weekly hormone (estrone, testosterone, and DHEA) deviations predicted differential changes in key constructs of adolescent depression and perceived stress. Results indicated that stress exposure provides a context that unmasks directional effects of hormone flux on affect state change in peripubertal females. Specifically, a consistent pattern emerged with within-person elevations in hormones predicting greater affective symptoms in high stress and decreases in hormones predicting greater symptom severity in low stress contexts. Stress-related hormone effects on affective symptoms appear to be hormone-specific. Namely, DHEA interacted with stressful life events to broadly impact symptom expression, whereas estrone and testosterone had more restricted effects on affective symptoms. The present study supports a diathesis-stress model of adolescent depression in which exposure to life stress proximal to the pubertal transition modifies vulnerable neural networks, making them more sensitive to normal peripubertal hormone flux, which in turn, promotes the emergence of adolescent mood dysregulation and affective illness.
4.2. DHEA deviations predicted a range of depressive symptoms
DHEA interacted with stressful life events to predict all symptom outcomes except perceived coping ability, and higher levels of DHEA were associated with a greater number of stressful life events. DHEA and cortisol are both products of the HPA stress axis that begin to rise with adrenarche and progressively increase during the pubertal transition, and exert opposing physiological actions (Kamin & Kertes, 2017). Because of DHEA’s reported antagonistic effects on glucocorticoids (i.e., cortisol), the preferential production of DHEA over cortisol is thought to buffer against stress-related mood impairment (Karishma & Herbert, 2002). At low stress, elevated DHEA in the present study predicted less severe symptom ratings, particularly depressed affect. This is consistent with previous reports that DHEA has antidepressant-like effects, with administration of DHEA improving depressive symptoms by over 50% in patients with midlife-onset depression (Schmidt et al., 2005). More recently, DHEA administration was found to reduce amygdala and hippocampus engagement and enhance limbic connectivity during an emotion regulation paradigm, which in turn, was associated with improved negative effect (Sripada et al., 2013). However, at high stress in the present analyses, higher-than-usual DHEA predicted greater symptom expression, including somatic and interpersonal symptoms, depressed and low positive affect, and perceived distress. The greater symptom expression with DHEA elevations in the high stress context resembles the higher DHEA levels in response to acute stress (Dutheil et al., 2021), also observed among adolescents with affective symptoms (Shirtcliff et al., 2007). Given DHEA’s antiglucocorticoid properties, DHEA may be protective against the deleterious effects of cortisol during the pubertal transition, which is characterized by heightened stress exposure (Flinn et al. 2011). DHEA relative to other hormones, including cortisol, could be investigated in future studies to advance our understanding of stress-related mood sensitivity to peripubertal DHEA changes.
4.3. Estrone deviations predicted depressed affect, somatic and interpersonal symptoms of depression
The interaction of estrone and stressful life events predicted somatic symptoms, depressed affect, and interpersonal depression symptoms. When probing the effects of different levels of stress, estrone surges were associated with increased interpersonal and somatic symptoms at high stress and decreased depressed affect at low stress. The relevance of estrogen fluctuations and interpersonal symptoms has also been reported in the menopausal transition (Gordon et al. 2016), similarly characterized by substantial ovarian hormone flux and heightened stress exposure. In fact, estradiol variability was found to predict greater sensitivity to rejection during psychosocial stress in perimenopausal women. Moreover, in women who had experienced a greater number of recent stressful life events, rejection sensitivity predicted the emergence of depression symptoms one year later (Gordon et al. 2016). Importantly, rejection sensitivity is a proximal risk factor for clinical depression and transdiagnostic factor shared across psychopathologies (Kupferberg, Bicks, and Hasler 2016; Owens et al. 2018; Slavich and Irwin 2014, Slavich et al. 2010). The results of the present study further support the pathophysiologic relevance of estrogens, specifically estrone, in the interpersonal symptoms of depression, and for the first time, demonstrated a relationship between estrone change and increased interpersonal depressive symptoms during the pubertal transition. The specificity of symptoms highlights the importance of conducting dimensional, symptom-specific research.
4.4. Divergent effect of testosterone-related affect dysregulation in the context of stress
The stress context created a divergence in testosterone-related affective symptoms, particularly for somatic symptoms and perceived distress. Participants exhibited increased symptoms with lower-than-usual testosterone levels at low stress, yet testosterone elevations were associated with increased symptoms at high stress. The role of testosterone in depression has been investigated particularly in regard to pharmacological use (with testosterone treatment showing antidepressant effects) and primarily in male samples (Zarrouf et al., 2009). In adolescents, and especially female adolescents, the relationship between depression and testosterone is understudied. One previous longitudinal study investigated 630 female participants annually through adolescence for up to seven years (Copeland et al., 2019). Higher serum testosterone concentrations predicted diagnoses of depressive disorders significantly, even after controlling for factors such as pubertal status or age. Moreover, testosterone has been shown to have puberty-unique influences on neurofunction and neuroarchitecture in brain regions associated with the development of affective disorders (Bramen et al., 2012). We recently demonstrated that a significant proportion of female adolescents showed meaningful associations between fluctuations of testosterone and mood (Andersen et al. 2022). The results reported here take these investigations one step further and highlight the essential role of stressful life events on the testosterone-mood relationship. However, the biological mechanisms contributing to the interaction of life stress and mood sensitivity to testosterone flux have yet to be identified. Research on other sex steroids, such as progesterone, suggest that dysregulated receptor functioning can cause hormone sensitivity and HPA axis dysregulation in some individuals (as reviewed for progesterone by Gordon et al. 2015). Future research could explore this as a potential mechanism underlying sensitivity to testosterone flux.
4.5. The impact of stressful life events on hormone-induced affective symptoms
The results not only support the interaction of sex hormones and mood in the pubertal transition, but they also suggest that life stress exposure has an essential moderating effect on this interaction. One theory suggests that this moderation is biologically underpinned by dysregulation of the HPA axis. Early and chronic life stress can alter HPA axis functioning with long-term effects on mental health (Ho & King, 2021). These HPA axis alterations have been specifically demonstrated for severe forms of life stress in the form of trauma exposure (Steudte-Schmiedgen et al., 2016) and for life stress experienced early in life, which is especially relevant to adolescents (Young et al., 2021). The pubertal transition is a unique window of vulnerability to the impact of stress-related mood impairment and contributes to differential stress responses following early life stress (Flannery et al., 2017; King et al., 2017; Nelson & Gabard-Durnam, 2020; Sisk & Gee, 2022). The HPA and HPG axes interact on various levels to regulate sex steroids, and in turn, sex steroids modulate HPA function (Oyola & Handa, 2017). Changes in HPA activity during adolescence [possibly through estrogen modifications of the GABAergic system in the amygdala (Walker et al., 2004)] may contribute to hormonal sensitivity and precipitate depressive symptoms in susceptible individuals. Results from the present study suggest that experiencing a greater number of stressful life events proximal to the pubertal transition can modify hormone-mood relationships. However, the positive relationship between stressful life events and depressive symptoms at enrollment in the present study suggest that greater depressive symptoms at enrollment could have influenced the report of stressful life events, particularly stressful events that are more subjective (e.g., change in acceptance by peers). Consequently, it remains unclear whether the type or severity of stressor within the context of baseline depressive symptoms differentially impacts hormone-related mood dysregulation.
In summary, early life stress, via modifications of developing neural circuitry, may give rise to hormone sensitivity, thus enhancing vulnerability for affective symptoms in the context of normal peripubertal hormone fluctuations. Stress related alterations of HPA functioning and the strong interaction of HPA and HPG axes may underlie the relationship between sex hormone fluctuation and mood. However, the neurophysiological mechanisms supporting this relationship are poorly understood and remains a promising pursuit of future research.
4.6. Limitations
Despite the strengths of the longitudinal, dimensional approach employed in the current study, there are several limitations to address. While serum hormone measurement is typically preferred to saliva methods because of its greater detection sensitivity, salivary hormone collections offer a non-invasive alternative with superior feasibility and compliance in adolescent participants. Given the current study’s objective to determine the relationship between hormone change and mood does not require the ability to detect the absolute lowest hormone level, the trade-off between ultra-low sensitivity and feasibility is appropriate. To account for potential confounds in saliva hormone measurement (time of day, food and water intake, etc.), we provided participants and their parents with detailed instructions and required sample collection to occur first thing upon awakening. Furthermore, steroid concentrations measured in saliva provide integrative information on free, unbound estradiol and testosterone and thereby measure the biologically active component of the hormone (Gao et al., 2015; Yasuda et al., 2008). The present study is also limited by the observation interval, which occurred weekly, and the relatively small sample size to examine moderation effects. While weekly observations may be sufficient to demonstrate how hormone flux can influence depressive symptoms from week to week (Gordon et al. 2016, 2020; Lozza-Fiacco et al. 2022), daily measures may more precisely asses hormone flux and should be considered in future studies.
For the same reasons that make the pubertal transition a critical developmental window for examining the impact of hormones on mental illness trajectories, the peripubertal endocrine environment is associated with unique study design challenges. It is difficult to study specific hormones of interest, particularly progesterone and its metabolites, because of the limited sensitivity of the available analysis methods for the relatively low levels expected during the first gynecological year. While valuable guidelines exist for determining ovulation status and cycle phasing in adult participants (Schmalenberger et al., 2021), these recommendations have only limited utility for identifying menstrual cycle phase and ovulation status in peripubertal participants, in which anovulatory and irregular cycles are common (Carlson and Shaw 2019; Sharma, Deuja, and Saha 2016). Typical hormone thresholds (i.e., progesterone rise, LH peak) for determining cycle phase in adults are not applicable because of overall lower peripubertal hormone concentrations, and counting methods are not relevant when shorter luteal phases are expected (Carlson and Shaw 2019; Sun et al. 2019). Currently, there are no clear guidelines for determining ovulation status and cycle phasing during the first gynecological year, and thus, the impact of the menstrual cycle on hormone-mood relationships during the pubertal transition remains an important area of investigation.
4.7. Future Directions
Future research is needed to examine the neurobiological mechanisms underlying the relationship between hormone change, stress and depressive symptoms. Because sex hormones (e.g., estradiol, testosterone) influence the regulation of the HPA stress system and the abundance of sex hormone receptors in limbic brain regions engaged during emotion, the probability exists that the HPA stress response may mediate the hormone-mood relationship. Moreover, the biological mechanisms supporting the relationship between psychopathology and sex-hormones (i.e., DHEA and testosterone) and their fluctuation are poorly understood – not only in adolescents but across the female reproductive lifespan and should be a focus of future studies to close these existing research gaps.
The results from the present study confirm and further highlight the need to include stress exposure in studies linking sex hormones and affective symptoms. As shown in trauma research (Forbes et al., 2013; López-Martínez et al., 2018), not only the absolute number of stressful life events, but also the type of stressor is a promising factor to investigate (McLaughlin et al., 2021). Results also highlight the importance of using a dimensional approach. Limiting the investigation to total depression symptom scores could dilute or mask significant effects only apparent on the symptom level. Accordingly, we recommend future studies similarly employ a dimensional and symptom-specific approach to investigate key constructs of depression in adolescents. This dimensional approach will also allow more specific exploration of the neurobiological mechanisms underlying the etiology of affective illness. Finally, the current results provide a foundation for understanding the impact of life stress on hormone-mood relationships in a low-risk sample reflecting the socioeconomic, racial and ethnic composition of the local community. A larger and more diverse sample of adolescents could, for instance, be employed to elucidate unique psychosocial and neuroendocrine features of the pubertal transition experienced by participants who identify differently from their sex assigned at birth. Including a wide range of depressive symptoms was not only important to achieve adequate variability, but it also allowed for greater generalizability of a developmental window in which elevated stress and depression are common. That said, the diathesis model tested in the present analyses could inform future investigations of the biological, social, environmental, and cultural factors that converge to make the pubertal transition a unique window of vulnerability for psychopathology.
4.8. Conclusion
The present study contributes to our understanding of peripubertal hormone-mood relationships by demonstrating that life stress differentially impacts hormone-related changes in distinct symptom constructs of depression. This study reveals how life stress predisposes sensitivity to hormone fluctuation in peripubertal female adolescents, ultimately resulting in depressive symptoms in response to normative hormone flux. Results indicated that sensitivity to within-person deviations in DHEA, testosterone and estrone predict affective symptoms, and that mood-sensitivity to weekly hormone deviations is stress context specific. Given the role of sex hormones in regulating neural networks involved in cognitive and emotional processing, the combination of increased sex hormones and their variability during the pubertal transition and more frequent stress exposure may promote heightened emotional reactivity and arousal and thus, promote greater risk for psychopathology. The impact of stressful life events on the unique peripubertal neuroendocrine environment should continue to be investigated to inform advancements in the early detection and intervention of affective illness.
Acknowledgments and Funding Sources
This study was supported by the National Institute of Mental Health Grant K01MH121575 (to EA), the National Institute of Health T32 postdoctoral fellowship MH093315 (to EA), the NIH Clinical Translational Science Award pilot grant UL1TR002489 (to EA), and a Foundation of Hope for Research and Treatment of Mental Illness grant (to EA). The “Stiftung der Deutschen Wirtschaft” provided doctoral funding for HK.
Footnotes
Declarations of Interest The authors have no conflicts of interest to disclose.
References
- Andersen E, Fiacco S, Gordon J, Kozik R, Baresich K, Rubinow D, & Girdler S (2022). Methods for characterizing ovarian and adrenal hormone variability and mood relationships in peripubertal females. Psychoneuroendocrinology, 105747. 10.1016/j.psyneuen.2022.105747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A (1993). Puberty onset of gender differences in rates of depression: A developmental, epidemiologic and neuroendocrine perspective. Journal of Affective Disorders, 29(2–3), 145–158. 10.1016/0165-0327(93)90029-J [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, & Worthman CM (1999). Pubertal changes in hormone levels and depression in girls. Psychological Medicine, 29(5), 1043–1053. [DOI] [PubMed] [Google Scholar]
- Apter D (1980). Serum steroids and pituitary hormones in female puberty: A partly longitudinal study. Clinical Endocrinology, 12(2), 107–120. 10.1111/j.1365-2265.1980.tb02125.x [DOI] [PubMed] [Google Scholar]
- Apter D, Cacciatore B, Alfthan H, & Stenman U (1989). Serum luteinizing hormone concentrations increase 100-fold in females from 7 years of age to adulthood, as measured by time-resolved immunofluorometric assay. The Journal of Clinical Endocrinology & Metabolism, 68(1), 53–57. 10.1210/jcem-68-1-53 [DOI] [PubMed] [Google Scholar]
- Balzer BWR, Duke SA, Hawke CI, & Steinbeck KS (2015). The effects of estradiol on mood and behavior in human female adolescents: A systematic review. European Journal of Pediatrics, 174(3), 289–298. 10.1007/s00431-014-2475-3 [DOI] [PubMed] [Google Scholar]
- Bini V, Celi F, Berioli MG, Bacosi ML, Stella P, Giglio P, Tosti L, & Falorni A (2000). Body mass index in children and adolescents according to age and pubertal stage. European Journal of Clinical Nutrition, 54(3). 10.1038/sj.ejcn.1600922 [DOI] [PubMed] [Google Scholar]
- Biro FM, Pinney SM, Huang B, Baker ER, Walt Chandler D, & Dorn LD (2014). Hormone changes in peripubertal girls. The Journal of Clinical Endocrinology & Metabolism, 99(10), 3829–3835. 10.1210/jc.2013-4528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9(4), 267–277. 10.1038/nrn2353 [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau MA, Murphy J, Nieman LK, & Rubinow DR (2000). Effects of gonadal steroids in women with a history of postpartum depression. The American Journal of Psychiatry, 157(6), 924–930. [DOI] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, Dinov ID, Worthman CM, & Sowell ER (2012). Sex matters during adolescence: Testosterone-related cortical thickness maturation differs between boys and girls. PLoS ONE, 7(3), e33850. 10.1371/journal.pone.0033850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberger JT, Kravitz HM, Chang YF, Cyranowski JM, Brown C, & Matthews KA (2011). Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychological Medicine, Psychological Medicine, 41, 41(9, 9), 1879, 1879–1888. 10.1017/S003329171100016X, 10.1017/S003329171100016X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleson Daviss W, Birmaher B, Melhem NA, Axelson DA, Michaels SM, & Brent DA (2006). Criterion validity of the Mood and Feelings Questionnaire for depressive episodes in clinic and non-clinic subjects: Criterion validity of Mood and Feelings Questionnaire. Journal of Child Psychology and Psychiatry, 47(9), 927–934. 10.1111/j.1469-7610.2006.01646.x [DOI] [PubMed] [Google Scholar]
- Carlson LJ, & Shaw ND (2019). Development of ovulatory menstrual cycles in adolescent girls. Journal of Pediatric and Adolescent Gynecology, 32(3), 249–253. 10.1016/j.jpag.2019.02.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, & Acebo C (1993). A self-administered rating scale for pubertal development. Journal of Adolescent Health, 14(3), 190–195. 10.1016/1054-139X(93)90004-9 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(386–396). [PubMed] [Google Scholar]
- Colich NL, & McLaughlin KA (2022). Accelerated pubertal development as a mechanism linking trauma exposure with depression and anxiety in adolescence. Current Opinion in Psychology, 46, 101338. 10.1016/j.copsyc.2022.101338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Worthman C, Shanahan L, Costello EJ, & Angold A (2019). Early pubertal timing and testosterone associated with higher levels of adolescent depression in girls. Journal of the American Academy of Child & Adolescent Psychiatry, 58(12), 1197–1206. 10.1016/j.jaac.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, & Angold A (1988). Scales to assess child and adolescent depression: Checklists, screens, and nets. Journal of the American Academy of Child & Adolescent Psychiatry, 27(6), 726–737. 10.1097/00004583-198811000-00011 [DOI] [PubMed] [Google Scholar]
- De Carvalho Tofoli SM, Von Werne Baes C, Martins CMS, & Juruena M (2011). Early life stress, HPA axis, and depression. Psychology & Neuroscience, 4(2), 229–234. 10.3922/j.psns.2011.2.008 [DOI] [Google Scholar]
- Dutheil F, de Saint Vincent S, Pereira B, Schmidt J, Moustafa F, Charkhabi M, Bouillon-Minois JB, & Clinchamps M (2021). DHEA as a biomarker of stress: A systematic review and meta-analysis. Frontiers in Psychiatry, 12, 688367. 10.3389/fpsyt.2021.688367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, DeWall CN, Girdler SS, & Segerstrom SC (2015). Ovarian hormones and borderline personality disorder features: Preliminary evidence for interactive effects of estradiol and progesterone. Biological Psychology, 109, 37–52. 10.1016/j.biopsycho.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, Rubinow DR, Schiller CE, Johnson JL, Leserman J, & Girdler SS (2016). Histories of abuse predict stronger within-person covariation of ovarian steroids and mood symptoms in women with menstrually related mood disorder. Psychoneuroendocrinology, 67, 142–152. 10.1016/j.psyneuen.2016.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati A, Jiang J, Katz MJ, Sliwinski MJ, Zimmerman ME, & Lipton RB (2014). Validation of the Perceived Stress Scale in a community sample of older adults. International Journal of Geriatric Psychiatry, 29(6), 645–652. 10.1002/gps.4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery JE, Gabard-Durnam LJ, Shapiro M, Goff B, Caldera C, Louie J, Gee DG, Telzer EH, Humphreys KL, Lumian DS, & Tottenham N (2017). Diurnal cortisol after early institutional care—Age matters. Developmental Cognitive Neuroscience, 25, 160–166. 10.1016/j.dcn.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn MV, Nepomnaschy PA, Muehlenbein MP, & Ponzi D (2011). Evolutionary functions of early social modulation of hypothalamic-pituitary-adrenal axis development in humans. Neuroscience & Biobehavioral Reviews, 35(7), 1611–1629. 10.1016/j.neubiorev.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Forbes D, Lockwood E, Phelps A, Wade D, Creamer M, Bryant RA, McFarlane A, Silove D, Rees S, Chapman C, Slade T, Mills K, Teesson M, & O†Donnell M (2013). Trauma at the hands of another: Distinguishing PTSD patterns following intimate and nonintimate interpersonal and noninterpersonal trauma in a nationally representative sample. The Journal of Clinical Psychiatry, 74(2), 21205. 10.4088/JCP.13m08374 [DOI] [PubMed] [Google Scholar]
- Gao W, Stalder T, & Kirschbaum C (2015). Quantitative analysis of estradiol and six other steroid hormones in human saliva using a high throughput liquid chromatography–tandem mass spectrometry assay. Talanta, 143, 353–358. 10.1016/j.talanta.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, & Elder GH (2001). Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology, 37(3), 404–417. 10.1037/0012-1649.37.3.404 [DOI] [PubMed] [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, & Simons RL (1994). Trajectories of stressful life events and depressive symptoms during adolescence. Developmental Psychology, 30(4), 467–483. https://doi-org.libproxy.lib.unc.edu/10.1037/0012-1649.30.4.467 [Google Scholar]
- Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, Prairie BA, Moses-Kolko E, Joffe H, & Wisner KL (2015). Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: A novel heuristic model. American Journal of Psychiatry, 172(3), 227–236. 10.1176/appi.ajp.2014.14070918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Peltier A, Grummisch JA, & Sykes Tottenham L (2019). Estradiol fluctuation, sensitivity to stress, and depressive symptoms in the menopause transition: A pilot study. Frontiers in Psychology, 10, 1319. 10.3389/fpsyg.2019.01319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Leserman J, & Girdler SS (2016). Estradiol variability, stressful life events, and the emergence of depressive symptomatology during the menopausal transition. Menopause, 23(3), 257–266. 10.1097/GME.0000000000000528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Sander B, Eisenlohr-Moul TA, & Sykes Tottenham L (2020). Mood sensitivity to estradiol predicts depressive symptoms in the menopause transition. Psychological Medicine, 1–9. 10.1017/S0033291720000483 [DOI] [PubMed] [Google Scholar]
- Gunn HM, Tsai MC, McRae A, & Steinbeck KS (2018). Menstrual patterns in the first gynecological year: A systematic review. Journal of Pediatric and Adolescent Gynecology, 31(6), 557–565.e6. 10.1016/j.jpag.2018.07.009 [DOI] [PubMed] [Google Scholar]
- Hamlat EJ, Stange JP, Abramson LY, & Alloy LB (2014). Early pubertal timing as a vulnerability to depression symptoms: Differential effects of race and sex. Journal of Abnormal Child Psychology, 42(4), 527–538. 10.1007/s10802-013-9798-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C (2005). Stress and depression. Annual Review of Clinical Psychology, 1(1), 293–319. 10.1146/annurev.clinpsy.1.102803.143938 [DOI] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, & Roesch L (2007). Sex differences in adolescent depression: Stress exposure and reactivity models. Child Development, 78(1), 279–295. 10.1111/j.1467-8624.2007.00997.x [DOI] [PubMed] [Google Scholar]
- Hewitt P, Flett G, & Mosher SW (1992). The Perceived Stress Scale: Factor structure and relation to depression symptoms in a psychiatric sample. Journal of Psychopathology and Behavioral Assessment, 14(3). 10.1007/BF00962631 [DOI] [Google Scholar]
- Ho TC, & King LS (2021). Mechanisms of neuroplasticity linking early adversity to depression: Developmental considerations. Translational Psychiatry, 11(1), 517. 10.1038/s41398-021-01639-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucklebridge F, Hussain T, Evans P, & Clow A (2005). The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology, 30(1), 51–57. 10.1016/j.psyneuen.2004.04.007 [DOI] [PubMed] [Google Scholar]
- James-Todd T, Tehranifar P, Rich-Edwards J, Titievsky L, & Terry MB (2010). The impact of socioeconomic status across early life on age at menarche among a racially diverse population of girls. Annals of Epidemiology, 20(11), 836–842. 10.1016/j.annepidem.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janfaza M, Sherman TI, Larmore KA, Brown-Dawson J, & Klein KO (2006). Estradiol levels and secretory dynamics in normal girls and boys as determined by an ultrasensitive bioassay: A 10 year experience. Journal of Pediatric Endocrinology and Metabolism, 19(7), 901–910. [DOI] [PubMed] [Google Scholar]
- Kamin HS, & Kertes DA (2017). Cortisol and DHEA in development and psychopathology. Hormones and Behavior, 89, 69–85. 10.1016/j.yhbeh.2016.11.018 [DOI] [PubMed] [Google Scholar]
- Karishma KK, & Herbert J (2002). Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression: DHEA in hippocampus. European Journal of Neuroscience, 16(3), 445–453. 10.1046/j.1460-9568.2002.02099.x [DOI] [PubMed] [Google Scholar]
- King LS, Colich NL, LeMoult J, Humphreys KL, Ordaz SJ, Price AN, & Gotlib IH (2017). The impact of the severity of early life stress on diurnal cortisol: The role of puberty. Psychoneuroendocrinology, 77, 68–74. 10.1016/j.psyneuen.2016.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferberg A, Bicks L, & Hasler G (2016). Social functioning in major depressive disorder. Neuroscience & Biobehavioral Reviews, 69, 313–332. 10.1016/j.neubiorev.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Georgiev AV, McDade TW, Bechayda SA, & Gettler LT (2016). Is there a testosterone awakening response in humans? Adaptive Human Behavior and Physiology, 2(2), 166–183. 10.1007/s40750-015-0038-0 [DOI] [Google Scholar]
- LeMoult J, Humphreys KL, Tracy A, Hoffmeister JA, Ip E, & Gotlib IH (2020). Meta-analysis: Exposure to early life stress and risk for depression in childhood and adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 59(7), 842–855. 10.1016/j.jaac.2019.10.011 [DOI] [PubMed] [Google Scholar]
- LeMoult J, Ordaz SJ, Kircanski K, Singh MK, & Gotlib IH (2015). Predicting first onset of depression in young girls: Interaction of diurnal cortisol and negative life events. Journal of Abnormal Psychology, 124(4), 850–859. 10.1037/abn0000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Martínez AE, Serrano-Ibáñez ER, Ruiz-Párraga GT, Gómez-Pérez L, Ramírez-Maestre C, & Esteve R (2018). Physical health consequences of interpersonal trauma: A systematic review of the role of psychological variables. Trauma, Violence, & Abuse, 19(3), 305–322. 10.1177/1524838016659488 [DOI] [PubMed] [Google Scholar]
- Lozza-Fiacco S, Gordon JL, Andersen EH, Kozik RG, Neely O, Schiller C, Munoz M, Rubinow DR, & Girdler SS (2022). Baseline anxiety-sensitivity to estradiol fluctuations predicts anxiety symptom response to transdermal estradiol treatment in perimenopausal women – A randomized clinical trial. Psychoneuroendocrinology, 143, 105851. 10.1016/j.psyneuen.2022.105851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Humphreys KL, Belsky J, & Ellis BJ (2021). The value of dimensional models of early experience: Thinking clearly about concepts and categories. Perspectives on Psychological Science, 16(6), 1463–1472. 10.1177/1745691621992346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod JD, & Kessler RC (1990). Socioeconomic status differences in vulnerability to undesirable life events. Journal of Health and Social Behavior, 31(2), 162. 10.2307/2137170 [DOI] [PubMed] [Google Scholar]
- McNeish D, & Matta T (2018). Differentiating between mixed-effects and latent-curve approaches to growth modeling. Behavior Research Methods, 50(4), 1398–1414. 10.3758/s13428-017-0976-5 [DOI] [PubMed] [Google Scholar]
- Mendle J, Beltz AM, Carter R, & Dorn LD (2019). Understanding puberty and its measurement: Ideas for research in a new generation. Journal of Research on Adolescence, 29(1), 82–95. 10.1111/jora.12371 [DOI] [PubMed] [Google Scholar]
- Monroe SM, & Reid MW (2008). Gene-environment interactions in depression research: Genetic polymorphisms and life-stress polyprocedures. Psychological Science, 19(10), 947–956. 10.1111/j.1467-9280.2008.02181.x [DOI] [PubMed] [Google Scholar]
- Namavar Jahromi B, Pakmehr S, & Hagh-Shenas H (2011). Work stress, premenstrual syndrome and dysphoric disorder: Are There Any Associations? Iranian Red Crescent Medical Journal, 13(3), 199–202. [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, & Gabard-Durnam LJ (2020). Early adversity and critical periods: Neurodevelopmental consequences of violating the expectable environment. Trends in Neurosciences, 43(3), 133–143. 10.1016/j.tins.2020.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottowitz WE, Derro D, Dougherty DD, Lindquist MA, Fischman AJ, & Hall JE (2008). Analysis of amygdalar-cortical network covariance during pre- versus post-menopausal estrogen levels: Potential relevance to resting state networks, mood, and cognition. Neuro Endocrinology Letters, 29(4), 467–474. [PMC free article] [PubMed] [Google Scholar]
- Owens SA, Helms SW, Rudolph KD, Hastings PD, Nock MK, & Prinstein MJ (2018). Interpersonal stress severity longitudinally predicts adolescent girls’ depressive symptoms: The moderating role of subjective and HPA axis stress responses. Journal of Abnormal Child Psychology, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyola MG, & Handa RJ (2017). Hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes: Sex differences in regulation of stress responsivity. Stress, 20(5), 476–494. 10.1080/10253890.2017.1369523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M, Altinay M, Malone DA, & Anand A (2012). Where in the brain Is depression? Current Psychiatry Reports, 14(6), 634–642. 10.1007/s11920-012-0322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Olsson C, Bond L, Toumbourou JW, Carlin JB, Hemphill SA, & Catalano RF (2008). Predicting female depression across puberty: A two-nation longitudinal study. Journal of the American Academy of Child & Adolescent Psychiatry, 47(12), 1424–1432. 10.1097/CHI.0b013e3181886ebe [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paykel ES (2003). Life events and affective disorders. Acta Psychiatrica Scandinavica, 108, 61–66. 10.1034/j.1600-0447.108.s418.13.x [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Andrews JA, Lewinsohn PM, & Hops H (1990). Assessment of depression in adolescents using the Center for Epidemiologic Studies Depression Scale. Psychological Assessment: A Journal of Consulting and Clinical Psychology, 2(2), 122–128. 10.1037/1040-3590.2.2.122 [DOI] [Google Scholar]
- SAMHSA, 2020. NSDUH Detailed Tables | CBHSQ Data. Retrieved November 1, 2021, from https://www.samhsa.gov/data/report/2020-nsduh-detailed-tables
- Sander B, Muftah A, Sykes Tottenham L, Grummisch JA, & Gordon JL (2021). Testosterone and depressive symptoms during the late menopause transition. Biology of Sex Differences, 12(1), 44. 10.1186/s13293-021-00388-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Johnson SL, Abate AC, Schmidt PJ, & Rubinow DR (2016). Reproductive steroid regulation of mood and behavior, Comprehensive Physiology (pp. 1135–1160). John Wiley & Sons, Inc. 10.1002/cphy.c150014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Walsh E, Eisenlohr-Moul TA, Prim J, Dichter GS, Schiff L, Bizzell J, Slightom SL, Richardson EC, Belger A, Schmidt P, & Rubinow DR (2022). Effects of gonadal steroids on reward circuitry function and anhedonia in women with a history of postpartum depression. Journal of Affective Disorders, 314, 176–184. 10.1016/j.jad.2022.06.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalenberger KM, Tauseef HA, Barone JC, Owens SA, Lieberman L, Jarczok MN, Girdler SS, Kiesner J, Ditzen B, & Eisenlohr-Moul TA (2021). How to study the menstrual cycle: Practical tools and recommendations. Psychoneuroendocrinology, 123, 104895. 10.1016/j.psyneuen.2020.104895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Berlin KL, Danaceau MA, Neeren A, Haq NA, Roca CA, & Rubinow DR (2004). The effects of pharmacologically induced hypogonadism on mood in healthy men. Archives of General Psychiatry, 61(10), 997–1004. 10.1001/archpsyc.61.10.997 [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, Simpson St. Clair L, Murphy JH, Haq N, & Rubinow DR (2005). Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Archives of General Psychiatry, 62(2), 154–162. 10.1001/archpsyc.62.2.154 [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Dor RB, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, Koziol DE, Nieman LK, & Rubinow DR (2015). Effects of estradiol withdrawal on mood in women with past perimenopausal depression: A randomized clinical trial. JAMA Psychiatry, 72(7), 714–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, Wakim PG, & Rubinow DR (2017). Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. The American Journal of Psychiatry. 10.1176/appi.ajp.2017.16101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, & Rubinow DR (1998). Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. New England Journal of Medicine, 338(4), 209–216. [DOI] [PubMed] [Google Scholar]
- Selya A, Rose J, Dierker L, Hedeker D, & Mermelstein R (2012). A practical guide to calculating cohen’s f2, a measure of local effect size, from PROC MIXED. Frontiers in Psychology, 3. https://www.frontiersin.org/articles/10.3389/fpsyg.2012.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer AB (2006). Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. Journal of Clinical Psychology, 62(1), 123–146. 10.1002/jclp.20213 [DOI] [PubMed] [Google Scholar]
- Sharma S, Deuja S, & Saha CG (2016). Menstrual pattern among adolescent girls of Pokhara Valley: A cross sectional study. BMC Women’s Health, 16(1), 74. 10.1186/s12905-016-0354-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JH, Eberhart NK, Hammen CL, & Brennan PA (2006). Differential exposure and reactivity to interpersonal stress predict sex differences in adolescent depression. Journal of Clinical Child & Adolescent Psychology, 35(1), 103–115. 10.1207/s15374424jccp3501_9 [DOI] [PubMed] [Google Scholar]
- Shirtcliff E, Zahn-Waxler C, Klimes-Dougan B, & Slattery M (2007). Salivary dehydroepiandrosterone responsiveness to social challenge in adolescents with internalizing problems. Journal of Child Psychology and Psychiatry, 48(6), 580–591. [DOI] [PubMed] [Google Scholar]
- Sisk LM, & Gee DG (2022). Stress and adolescence: Vulnerability and opportunity during a sensitive window of development. Current Opinion in Psychology, 44, 286–292. 10.1016/j.copsyc.2021.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Irwin MR (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin, 140(3), 774–815. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, O’donovan A, Epel ES, & Kemeny ME (2010). Black sheep get the blues: A psychobiological model of social rejection and depression. Neuroscience & Biobehavioral Reviews, 35(1), 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2009). Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology, 21(01), 87. 10.1017/S0954579409000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rajaram N, Garfinkel SN, Abelson JL, & Liberzon I (2013). DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 38(9), 1798–1807. 10.1038/npp.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S, Kirschbaum C, Alexander N, & Stalder T (2016). An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: Insight from recent hair cortisol findings. Neuroscience & Biobehavioral Reviews, 69, 124–135. 10.1016/j.neubiorev.2016.07.015 [DOI] [PubMed] [Google Scholar]
- Sun BZ, Kangarloo T, Adams JM, Sluss PM, Welt CK, Chandler DW, Zava DT, McGrath JA, Umbach DM, Hall JE, & Shaw ND (2019). Healthy post-menarchal adolescent girls demonstrate multi-level reproductive axis immaturity. The Journal of Clinical Endocrinology & Metabolism, 104(2), 613–623. 10.1210/jc.2018-00595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, & Cook DG (2001). Performance of a new pubertal self-assessment questionnaire: A preliminary study. Paediatric and Perinatal Epidemiology, 15(1), 88–94. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Wheaton B, & Lloyd DA (1995). The epidemiology of social stress. American Sociological Review, 60(1), 104. 10.2307/2096348 [DOI] [Google Scholar]
- Walker EF, Sabuwalla Z, & Huot R (2004). Pubertal neuromaturation, stress sensitivity, and psychopathology. Development and Psychopathology, 16(04). 10.1017/S0954579404040027 [DOI] [PubMed] [Google Scholar]
- Yasuda M, Honma S, Furuya K, Yoshii T, Kamiyama Y, Ide H, Muto S, & Horie S (2008). Diagnostic significance of salivary testosterone measurement revisited: Using liquid chromatography/mass spectrometry and enzyme-linked immunosorbent assay. Journal of Men’s Health, 5(1), 56–63. 10.1016/j.jomh.2007.12.004 [DOI] [Google Scholar]
- Young ES, Doom JR, Farrell AK, Carlson EA, Englund MM, Miller GE, Gunnar MR, Roisman GI, & Simpson JA (2021). Life stress and cortisol reactivity: An exploratory analysis of the effects of stress exposure across life on HPA-axis functioning. Development and Psychopathology, 33(1), 301–312. 10.1017/S0954579419001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrouf FA, Artz S, Griffith J, Sirbu C, & Kommor M (2009). Testosterone and depression: Systematic review and meta-analysis. Journal of Psychiatric Practice, 15(4), 289–305. 10.1097/01.pra.0000358315.88931.fc [DOI] [PubMed] [Google Scholar]