Abstract
Cellular systems must deal with mechanical forces to satisfy their physiological functions. In this context, proteins with mechanosensitive properties play a crucial role in sensing and responding to environmental changes. The discovery of aquaporins (AQPs) marked a significant breakthrough in the study of water transport. Their transport capacity and regulation features make them key players in cellular processes. To date, few AQPs have been reported to be mechanosensitive. Like mechanosensitive ion channels, AQPs respond to tension changes in the same range. However, unlike ion channels, the aquaporin’s transport rate decreases as tension increases, and the molecular features of the mechanism are unknown. Nevertheless, some clues from mechanosensitive ion channels shed light on the AQP-membrane interaction. The GxxxG motif may play a critical role in the water permeation process associated with structural features in AQPs. Consequently, a possible gating mechanism triggered by membrane tension changes would involve a conformational change in the cytoplasmic extreme of the single file region of the water pathway, where glycine and histidine residues from loop B play a key role. In view of their transport capacity and their involvement in relevant processes related to mechanical forces, mechanosensitive AQPs are a fundamental piece of the puzzle for understanding cellular responses.
Keywords: Aquaporins, Water transport, Water channel, Osmosis, Swelling, Membrane stretch
Introduction
The mechanome refers to the complete mechanical state of a biological system, encompassing the distribution of forces from tissues to cells to molecules, and how they interact with ongoing biological processes. It plays a critical role in various biological processes, such as tissue development, wound healing, and disease (Kamm and Mofrad, 2009). The mechanome is a complex and interconnected system of essential components that work together to enable biological systems to sense and respond to mechanical stimuli. These components include extracellular matrix (ECM) molecules (e.g., fibronectin), transmembrane proteins (e.g., integrins, cadherins), mechanosensitive ion channels (e.g., Piezo and TRP channels) and water channels (e.g., orthodox aquaporins), cytoskeletal proteins (e.g., actin filaments and microtubules), adaptor and scaffolding proteins (e.g., zyxin, vinculin, and talin), phosphatases and kinases (e.g., focal adhesion kinase, FAK), nuclei, and the lipid bilayer (Janmey and McCulloch, 2007; Jansen et al., 2017).
Cells possess a remarkable ability to respond to both internally generated and externally applied forces, and to sense and respond to changes in several surface parameters, including extracellular matrix (ECM) specificity, nanoscale surface topographies, geometric confinement, membrane curvature, and rigidity (Vogel and Sheetz, 2006). Physical signals can elicit a local response, directly affecting adhesion sites, or a global response, activating signaling pathways that regulate various cellular processes such as proliferation, cell growth, differentiation, and programmed cell death (Geiger et al., 2009). The cellular response to mechanical stimuli is influenced by multiple factors, including the nature of the mechanical signal, duration, magnitude, and frequency.
The attribute of mechanosensation can be ascribed to an old trait inherited from the evolution of the primal organisms present on Earth (Booth et al., 2015). Primordial living beings were subjected to potentially harmful mechanical and osmotic stresses. It is hypothesized that primitive life evolved in aqueous environments (Lazcano et al., 1983), rendering osmotic pressure as one of the main mechanical forces present when life consisted only of unicellular systems. Thus, mechanotransduction functions evolved to safeguard life against hazardous alterations in the environment’s solute concentrations (Balleza, 2011).
The early presence of mechano-transductive features in evolution resulted in the vast assortment of force-gated proteins that we know today. These proteins are expressed in various species, supporting a diverse array of physiological functions, ranging from cell tone regulation to hearing. Moreover, it has been suggested that all channel proteins have the potential to be mechanosensitive (Schmidt et al., 2012), indicating that evolutionary changes were directed towards suppressing this ancestral trait rather than its selection (Booth et al., 2015).
Upon their discovery, aquaporins (AQPs) were declared as the master water channels (Preston et al., 1992; Agre et al., 1993), with a high-water transport capacity (up to 1 × 109 water molecules per second). However, this view of AQPs exclusively as water channels is a limited perspective that fails to appreciate their full capabilities. AQPs are ubiquitous and widely expressed in cells from all living organisms, from bacteria and archaea to plants and animals. Interestingly, they are present where cell mechanics is compromised, for example, in tissues subjected to shear forces (blood vessels and airway conduits in animals), in cells subjected to cyclic events (heart contraction and relaxation), or even in cell migration and tumor development (Verkman, 2011). In plant cells, AQPs are involved in stomata regulation in leaves, as well as in other circadian rhythm-associated processes (Sutka et al., 2017).
AQPs belong to the membrane intrinsic proteins (MIP) family and have both highly conserved sequence and structure (Ozu et al., 2022). However, their transport function is highly diversified (Soto et al., 2012; Perez Di Giorgio et al., 2014). While some AQPs only transport water (the so-called orthodox aquaporins), others can also transport small solutes (such as urea, glycerol, ammonia, boric acid, H2O2), metalloids (such as arsenite), gasses (such as CO2), or even ions (in a non-selective manner) (Ozu et al., 2018).
Variations in AQPs’ function can be attributed to the single file region of the channel contained in each monomer and possibly the central pore formed by the four monomers in the tetramer (Ozu et al., 2018). The functional diversity together with results from knock-out experiments casts doubts on the relevance of AQPs being only a main gateway to facilitate water transport (Hill et al., 2004). The osmosensor hypothesis was then proposed. According to this hypothesis, the tetrameric structure of the AQP serves as a cooperative membrane sensor, which responds to changes in osmolarity by undergoing conformational changes and then transmitting a signal to the cell interior (Hill et al., 2004). Evidence in support of this hypothesis came from the discovery of mechanosensitive AQPs (Hill and Shachar-Hill, 2015).
To date, few members of the aquaporin family have been reported to be mechanosensitive (Ozu et al., 2018). To understand how membrane tension modulates the AQPs’ water transport capability, two factors must be considered. The first is how mechanical forces are transduced from the cell membrane to the protein. The second consists of the molecular features that determine water transport, i.e., the water-water and the water-protein interactions that drive the concerted movement of water molecules in a single file.
In the following sections, we delve into the mechanome towards mechanosensitivity in aquaporins. First, some examples of tissues and organs in which cell mechanics is compromised and AQP members perform important physiological functions are introduced. Then, the evidence that demonstrates mechanosensation in AQPs is revisited. The next section discusses three points that can contribute to consider a mechanosensitive mechanism in AQPs: (i) key points of mechanosensitivity in ion channels; (ii) the relationship of AQPs with the lipidic environment; and (iii) the role of GxxxG sequences in mechanotransduction and in the water transport properties of AQPs. Afterwards, a possible mechanism is discussed. Finally, those questions and issues that are still open in the field are presented in a perspectives section.
AQPs in mechanical processes
Efficiently responding to mechanical forces is a fundamental requirement for living and to adapt to different environments. In vivo, cells are capable of detecting and responding to a wide range of physical and chemical signals that interact and modulate cellular responses (Haselwandter and Phillips, 2013). Therefore, cells can respond to externally applied forces and sense features such as the topography, rigidity, and geometric confinement of the underlying substrate. Shear flow, tensile stretch, mechanical compression, and hydrostatic pressure are among the most common types of active mechanical stimuli (Wang J. et al., 2014a). Some examples of these stimuli in animals, where some AQPs are involved, are mentioned below.
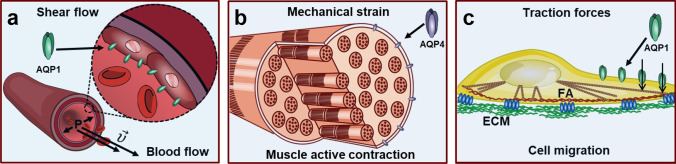
Shear flow can induce various changes in protein expression, translocation, and degradation in live cells. Animal aquaporins are expressed in many fluid-transporting tissues, such as kidney tubules, and vascular and respiratory systems (Verkman, 2011), which are physiologically exposed to shear stress due to fluid flow (Fig. 1a). For example, laminar shear stress due to blood flow can upregulate the expression of AQP1 in human umbilical vein endothelial cells, promoting endothelial cell migration and wound repair (Mun et al., 2013). Similarly, luminal fluid shear stress can trigger AQP2 translocation to the apical plasma membrane in collecting duct cells, leading to actin cytoskeletal reorganization (Jang et al., 2011). However, in human bronchial epithelial cells exposed to luminal shear stress—generated by airflow—AQP5 abundance decreases due to protein internalization and degradation (Sidhaye et al., 2008).
Fig. 1.
AQPs play important physiological functions in processes where cell mechanics are compromised. a AQPs expressed in vascular endothelial cells are exposed to mechanical forces exerted by the laminar shear stress due to blood flow. Arrows indicated by P and indicate blood pressure and velocity, respectively. Red blood cells are represented by ovoidal forms. For simplicity, AQPs are represented only in endothelial cells. b Muscle cells can exert active contraction, modifying the mechanical strain and the mechanical context of membrane proteins such as AQPs. Sarcomeres are schematically represented. c Migrating cells generate traction forces, in a process that impacts on focal adhesion (FA) dynamics and that is dependent on the AQPs function. Microtubules, cortical cytoskeleton, and the extracellular matrix (ECM) are schematically represented. Water flux through AQPs is indicated by arrows. All drawings are original and were created by the authors by using Inkscape 1.2.2 free software
Mechanical strain can have a profound impact on tissues and organs, triggering significant changes in cellular responses and tissue remodeling at both molecular and cellular levels (Tamura et al., 2001; Zhou et al., 2005; Richard et al., 2007). Cells in mechanically active organs, such as the heart and muscles, are stretched continuously during daily activities. In addition, lung cells are constantly subjected to stretching in regular cycles due to breathing movements, and bladder cells are exposed to mechanical stretching due to urine accumulation, although this occurs in a non-cyclic manner. Recent research has shown that sustained mechanical strain, which resembles the physiological mechanical stimulus caused by milk accumulation during lactation, can alter the focal adhesion dynamics in mammary epithelial cells. Focal adhesions are dynamic structures that not only provide a mechanical link between cytoskeleton components and the ECM, via integrin membrane receptors, but also exhibit mechanosensitive properties, acting as signaling organelles in the cell mechanotransduction process (Vogel and Sheetz, 2006). Mechanical strain stabilizes the focal adhesions of mammary epithelial cells, enhancing their persistence and preventing their disassembly (Sigaut et al., 2018). Furthermore, at the molecular level, mechanical strain alters the mechanoresponses of two focal adhesion proteins, zyxin and vinculin, by increasing the molecular tension across the vinculin molecule and slowing zyxin dissociation dynamics from the adhesive site.
The trabecular meshwork of the mammalian eye is part of a dynamic environment subjected to multiple forms of stress, including mechanical strain. Previous studies have shown that the AQP1 expression increases in trabecular meshwork cells in response to sustained but not to cyclic mechanical stretch (Baetz et al., 2009). Hence, AQP1 may play a protective role, by facilitating rapid changes in cell shape during sustained mechanical strain to maintain trabecular meshwork homeostasis.
In skeletal muscle (Fig. 1b), AQP4 can be regulated by changes in muscle use by increasing its expression during the transition from slow- to fast-twitch fibers (Frigeri et al., 2001). During increased physical activity, i.e., increased mechanical strain, AQP4 facilitates higher water fluxes between muscle tissue and blood (Frigeri et al., 2001; Frigeri et al., 2004).
In addition, it has been reported that mechanical forces enhance the metastatic features of cancer cells. For instance, mechanical compression of breast cancer cells activates the mechanosensitive Piezo1 ion channels to mediate enhanced cell invasion, which involves both cellular events and matrix degradation (Luo et al., 2022). Also, shear stress regulates prostate cancer metastasis through Piezo1 mediated signaling (Kim et al., 2022).
Cells can not only detect and respond to external mechanical forces but can also actively generate them through actomyosin contractility and actin branching, which are involved in morphological changes, adhesion processes, and motility. For example, cells use generated traction forces to test mechanical properties of the microenvironment, such as the stiffness of the extracellular matrix. Cellular traction forces are increased in cells cultivated at increasing substrate stiffness (Califano and Reinhart-King, 2010; Han et al., 2012; Scott et al., 2015; Sigaut et al., 2018). At a molecular level, a correlation has been established between traction force generation and the molecular dynamics of certain adhesive proteins (Zhou et al., 2017; Sigaut et al., 2021). In particular, in adhesion sites that generate greater forces, zyxin protein is less likely to dissociate from the adhesion site (Sigaut et al., 2021).
Cell migration is based on the precise spatiotemporal coordination of different components of the mechanome, including ECM, mechanoreceptors, and cytoskeleton (Fig. 1c). AQP1 localizes to the leading-edge during migration and is associated with the increased turnover of cell membrane protrusions, as it facilitates water entry and increases hydrostatic pressure causing membrane protrusion (Papadopoulos et al., 2008). Cells use integrin-mediated adhesion sites to apply traction forces on the ECM to move during cell migration. In fact, AQP2 interacts with integrins to promote renal epithelial cell migration, contributing to the structural and functional integrity of the mammalian kidney. AQP2 expression modulates integrin trafficking and internalization, facilitating its turnover at focal adhesions (Chen et al., 2012). Furthermore, both focal-adhesion area and lamellipodia volume are smaller in AQP2-expressing cells than in cells without expression of AQP2 in the plasma membrane, which is in agreement with an increase in cell migration (Di Giusto et al., 2020). AQP1 plays a crucial role during neural crest migration in chick embryos. Like AQP2, AQP1 is involved in promoting integrin turnover and in stabilizing neural crest cell filopodia. Therefore, its overexpression increases neural crest cell speed and invasion (McLennan et al., 2020).
These examples show that AQPs are involved in processes where the mechanical state of the system is compromised. To date, the knowledge about the participation of AQPs in these processes is limited to changes in expression levels or volume changes. However, AQPs are highly regulated channels. One regulatory mechanism is based on mechanosensitive properties. The next section presents the evidence that demonstrates that some AQPs are mechanosensitive channels.
Mechanical regulation in AQPs
The function of AQPs as water channels is closely related to cell volume changes. Several works in plants and animals evidenced relationships between transmembrane water fluxes and cell volume or intracellular pressure (Alexandre and Lassalles, 1991; Niemietz and Tyerman, 1997; Meinild et al., 1998; Ohshima et al., 2001; Vandeleur et al., 2005; Ozu et al., 2011). This leads to the suggestion of the existence of a mechanical regulation in AQPs, and some groups reported calculations or simulation results with models that considered a membrane tension effect on the water transport capacity of AQPs from yeast (Soveral et al., 2008), plants (Wan et al., 2004; Ye et al., 2004; Leitão et al., 2014), and mammals (Soveral et al., 1997; Ozu et al., 2013). Then, other studies tested the direct effect of membrane mechanics on the water transport capacity of aquaporins from animals and plants (Tong et al., 2012; Tong et al., 2016; Goldman et al., 2017). To the best of our knowledge, few aquaporins are known to be regulated by membrane tension. The list includes yeast AQY1 (Soveral et al., 2008; Fischer et al., 2009), rat AQP4 (Tong et al., 2012), human AQP1 (Ozu et al., 2013), and two tonoplast intrinsic proteins, VvTIP2;1 from gravepine (Leitão et al., 2014) and BvTIP1;2 from red beet (Goldman et al., 2017), while the plasma membrane intrinsic protein BvPIP2;1 does not respond to mechanical stress (Goldman et al., 2017).
The most typical experiments to functionally characterize AQPs are based on recordings of cell volume changes driven by osmotic gradients (Alleva et al., 2009; Alleva et al., 2012; Ozu et al., 2018). Usually, these experiments are performed by testing only one gradient magnitude, and the result indicates whether the AQP has a high or low water transport capacity, characterized by the (surface-area normalized) osmotic permeability coefficient (Pf). However, the function of the channel can be studied akin ion channels, i.e., by applying driving forces of different magnitude. By doing this, the relationship between the water flux and the applied osmotic gradient (Jw-Δosm curve) is analogous to the current-voltage relationship (I-V curve) typically employed in ion channel studies (Ozu et al., 2011). For example, it is accepted that the water transport through AQPs is bidirectional and that most of the AQPs are constitutively open (Meinild et al., 1998; Ozu et al., 2013). The Jw-Δosm relationship shows this but also provides more information about the function of the channel. Since the osmotic law establishes the linear dependence of the water flux on the applied osmotic gradient, then the experimental Jw-Δosm curve is expected to be linear, with a positive slope, i.e., the Pf. If this is the case, then, Pf determined with a low gradient must be equal to that determined with a larger gradient; any non-linearity in the Jw-Δosm relationship points towards changes along the water permeation pathway as the osmotic gradient increases.
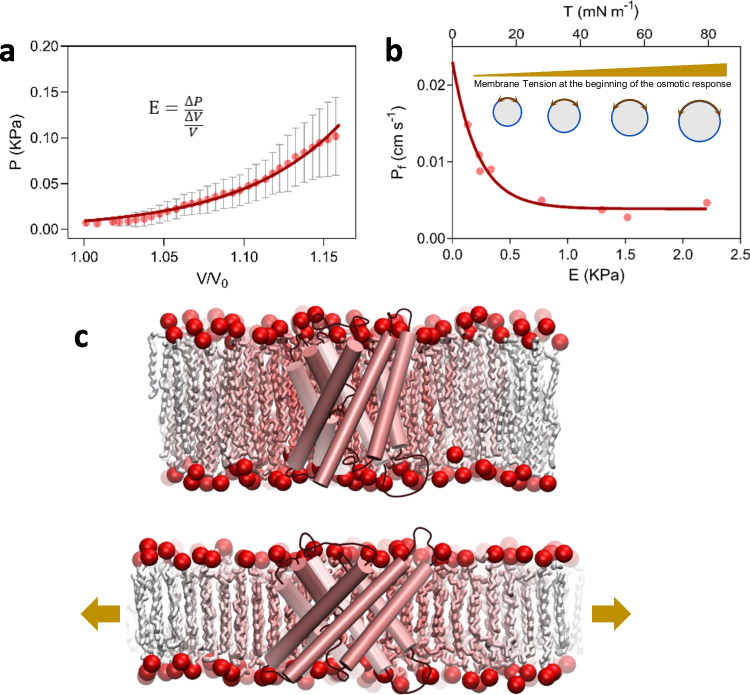
The Xenopus oocyte is well-employed in the literature as a system to study AQPs allowing the analysis of the Jw-Δosm relationship. The assumptions which this method is based on are valid only in a short time interval at the beginning of the osmotic response (Ozu et al., 2012; Ozu et al., 2013). By simultaneous measurement of cell volume and pressure, it was observed that both mechanical parameters, such as the elastic modulus or the membrane tension, increase with higher gradients (Ozu et al., 2013; Goldman et al., 2017) (Fig. 2a). Under controlled experimental conditions, and in the absence of other stimuli, non-linear Jw-Δosm relationships have demonstrated the mechanical regulation of human AQP1 and BvTIP1;2 (Ozu et al., 2013; Goldman et al., 2017). Additionally, the Pf-Δosm curve revealed the cooperative nature of the mechanism in human AQP1 (Ozu et al., 2013). These findings have been similarly observed in vesicles from rabbit renal brush border membranes, which also contain AQPs (Soveral et al., 1997), and in VvTIP1;2 from grape vine expressed in yeast (Leitão et al., 2014). Furthermore, experimental Pf determinations under different membrane tension conditions showed that the sensitivities of AQP1 and BvTIP1;2 are around 3 mN m−1 (Ozu et al., 2013) and 10 mN m−1 (Goldman et al., 2017), respectively. These values are in the sensitivity range observed for the well-known large and small conductance mechanosensitive ion channels (MscL and MscS, respectively). However, unlike MS ion channels, AQPs show a decrease in their water transport capacity as membrane tension increases (Fig. 2b). This finding suggests that membrane tension may trigger a conformational change in the water pathway of these AQPs, leading to an open-closed transition (Fig. 2c).
Fig. 2.
Experimental evidence demonstrates mechanosensitivity in AQPs. a Cell mechanics can be studied in Xenopus oocytes by the simultaneous measurement of pressure and volume during the osmotic response. The pressure-volume relationship evidences that the volumetric elastic module (E) rises as volume increases (Ozu et al., 2013). Dots represents the mean ± sem registered in BvTIP1;2-expressing oocytes during the osmotic response driven by a 100 mOsmol Kgw−1 gradient. Continuous line is illustrative, just to emphasize the nonlinearity of the response. Similar results were reported in Goldman et al. (2017). b By setting different membrane tension states at the beginning of the osmotic response (driven by the same osmotic gradient) demonstrates that the water permeability coefficient (Pf) decreases as the initial membrane tension increases. Dots represent independent experiments performed with BvTIP1;2-expressing oocytes. The osmotic gradient was the same in all the experiments. Figure modified from Goldman et al. (2017) under license permission from the publisher. The continuous line is illustrative, just to emphasize the non-linear decreasing relationship. c The lipid-protein interaction and the ordering of lipids around aquaporins were studied by both experimental and simulation approaches. Reported data suggest that there is not a fixed shell of lipids around AQPs. Instead, a dynamic interchanging occurs between this annular shell and bulky lipids (Stansfeld et al., 2013). This is represented by the lipid color gradient. The membrane tension increment (arrows in lower panel) produces thinning of the membrane. Therefore, hydrophobic regions of the protein tend to be exposed to the aqueous solution. Then, as occurs in MS ion channels, the hydrophobic mismatch is solved by a protein conformational change. Both the protein and the membrane are schematic representations with general purposes only
To date, information on mechanical gating at a molecular level in AQPs has been provided only by a handful of molecular dynamics (MD) simulations reports. The structure of the yeast aquaporin AQY1, resolved at 1.1 5Å, reveals a closed state mediated by the bending of the N-terminal segment towards the cytoplasmic entrance of the pore and its stabilization by multiple H-bonds among Tyr residues, water molecules, and Gly residues from loop B (Fischer et al., 2009). Since the configuration of the N-terminal segment is similar to the one found in MscL from Mycobacterium tuberculosis (Chang et al., 1998), the authors tested the effect of membrane tension by simulating lateral pressure increments or by bending the membrane towards the intracellular side. The results showed that the water transport rate of AQY1 increases as membrane tension decreases (Fischer et al., 2009). Fitting simulations to AQP1 experiments showed the same response after inducing the closure of the channel, indicating that the membrane tension effect is reversible (Ozu et al., 2013).
A recent report investigated the effects of shockwaves on the function of AQP4. The simulations reveal that rotations of Arg216 in the selectivity filter and H95 in the cytoplasmic end of the single file region trigger the closure of the channel during membrane compression (Wei et al., 2022). Intriguingly, the same study shows that the shock-induced bubble collapse opens the channel, increasing the water permeability while decreasing the number of H-bonds between key lining residues and water molecules.
Towards a possible membrane tension-dependent mechanism
Mechanosensitive clues in ion channels
The structural determinants of mechanosensitivity have not been studied in AQPs. On the contrary, much is known about MS ion channels. This section provides a summary of information regarding MS ion channels that may be useful in considering the mechanosensitive mechanism in AQPs.
It is possible that the bacterial and archaeal MS channels retain a great number of structural features from those primitive mechano-electrical transducers (Pohorille and Deamer., 2009). Since their discovery and cloning (Chang et al., 1998; Bass et al., 2002), MscL and MscS have been the most studied MS ion channels and are considered paradigmatic in the field. Consequently and for historical reasons, researchers have made useful comparisons and adapted multiple techniques from those studies to understand the gating mechanism of other mechanosensitive structures. Moreover, the simplicity of these proteins compared to mammalian MS channels provides an excellent system for understanding the basis of mechanotransduction mechanisms (Persat, 2017; Cox et al., 2018).
MscL and MscS refer to the large and small conductance mechanosensitive ion channels originally identified in E. coli giant spheroplasts (Martinac et al., 1987; Blount and Iscla, 2020). Among all known mechanically gated channels, MscL possesses the lowest tension sensitivity. For example, its activation threshold is around 12 mN m−1 (Nomura et al., 2012), about one order of magnitude higher than the mammalian MS channel TRAAK, which is around 0.5 mN m−1 (Brohawn et al., 2014).
The MscL architecture is characterized by the formation of pentamers. Each monomer is composed of two alpha helices (TM1 and TM2), the N-terminus that forms an alpha-helix-structured segment (S1), and the C-terminus that forms a cytoplasmic helix (CP) (Perozo et al., 2002). The cylindrical pore is formed by the five subunits. Close to the intracellular side, there is a sequence of hydrophobic residues that stabilizes the channel in its closed state and is known as hydrophobic lock (Yoshimura et al., 1999, Yoshimura et al., 2001, Birkner et al., 2012). The configuration of the channel changes when it is subjected to a mechanical stimulus (Yoshimura et al., 1999, Yoshimura et al., 2001, Birkner et al., 2012). The channel activates with the tension increase and in its full open conformation has a pore diameter of 28–36 Å (Wang Y. et al., 2014b), ten times wider than the pores in AQPs. The gating mechanism is intimately linked to lipid-protein interactions (Nomura et al., 2012). The participation of two glycine residues (G22 and G26) is crucial for the transition to the open state (Sawada and Sokabe., 2015). Particularly, the polarity of G22 is essential for hydrophobic interactions that maintain the closed state, as well as is responsible for allowing the breaking of the lock and the stabilization of the full open state when membrane tension increases (Yoshimura et al., 1999; Shukarev et al., 2001).
In MscS, each monomer possesses three transmembrane segments that form a homoheptamer. Although the structure of the E. coli MscS differs from that of MscL, both channels share some functional similarities. The surface of the pore is uniformly hydrophobic along its entire length, and the permeation pathway presents two constriction zones that form an 8 Å-length hydrophobic lock at the narrowest site of the pore (Naismith and Booth., 2012). In MscS, a complex relationship between the channel and the lipidic environment is observed. The MscS channels possess several types of lipid-protein regions that can affect both gate and pore conformational states. Pore lipids, gatekeeper lipids, and pocket lipids are essential in channel function (Reddy et al., 2019, Zhang et al., 2021), of which gatekeeper lipids are critical elements in tension sensitivity (Flegler et al., 2021). Both TM1 and TM2 appear to be essential in the channel mechanosensitivity, especially near their ends (Malcolm et al., 2011). This region has been coined as the tension sensor. In the closed state, the membrane-exposed residues interact tightly with the lipid tail groups. The aperture process of MscS channels occurs by movements of the periplasmic region of the pore (Vásquez et al., 2008). Two possible gating modes of MscS channels can be proposed, involving the interactions of lipids with two different channel regions exerting a release of the pore from lipids, and the unblocking of the permeation pathway (Rasmussen et al., 2019).
About the AQP-lipids interaction
Reports on both MscL and MscS channels provide evidence of the interaction between transmembrane segments and the membrane. While evidence of lipid-protein interaction in different types of mechanosensitive channels suggests a straightforward modulation of ion conductivity by diverse mechanisms, such effects are not explicitly established in water permeation by AQPs. To elucidate this point, it is necessary to understand how aquaporins interact with the membrane. This section summarizes this issue.
In a so-called “lipid moves first” model, it is assumed that membrane tension creates a vacuum between protein TM alpha helices and the surrounding lipid molecules. In ion channels, such a vacuum would in turn pull it to its open state (Pliotas et al., 2015). When the membrane is mechanically stressed, the applied force causes thinning of the lipid membrane (Rawicz et al., 2000), resulting in the exposure of hydrophobic areas from protein TM segments to the aqueous solution (Lee, 2004). The mismatch between the lipid bilayer and hydrophobic areas of TM helices is solved by the protein conformational change, which minimizes the global free energy and keeps the alpha helices embedded in the lipid membrane (Yoshimura and Sokabe, 2010).
Furthermore, the lipid membrane is disordered at the interface between TM segments and phospholipids (Rao et al., 2017). This allows the channel, during the gating mechanism induced by the hydrophobic mismatch, to stabilize the neighboring lipids in their open state (Wiggins and Phillips, 2005; Yoshimura and Sokabe, 2010).
Theoretically, if a membrane has a uniform lipidic composition, the mechanical tension should be distributed evenly throughout the bilayer, without local variations in tension. Nevertheless, a wide variety of lipids are present in biological membranes. In fact, eukaryotic membranes can contain over 1000 distinct lipid molecules (Sud et al., 2007). Furthermore, biological membranes possess fatty acids of 14 to 24 carbons in length and an asymmetric lipid distribution between both layers (van Meer et al., 2008). This produces heterogeneity in the membrane composition, as well as different biophysical and mechanical properties. For example, gramicidin A reconstituted in phosphatidylcholine (PC) with a chain length of 18 carbon atoms (PC18-vesicles) behaves as a stretch-inactivated channels, meanwhile the same protein reconstituted in PC20-vesicles behaves as a stretch-activated channel (Martinac and Hamill, 2002). This effect was also observed in prokaryotic channels (Perozo et al., 2002; Nomura et al., 2012).
In AQPs, the effects of lipid composition are still under debate. For example, the addition of phosphatidylserine (PS), phosphatidylcholine (PC), and cholesterol did not affect the AQP1 water permeability (Zeidel et al., 1994). However, the modification of the lipid composition reduced water transport in AQP4 M1 and M23 isoforms (Tong et al., 2012).
Molecular dynamics simulations performed to analyze the organization of lipids around 10 different transmembrane proteins indicate that the lipid shell is unique for each protein, suggesting that different proteins impact on the lipid organization in distinct ways, resulting in a specific “fingerprint” for each protein-lipid interaction (Corradi et al., 2018). However, MD simulations performed on 40 AQP structures showed dynamical lipids relocalization between annular shells and bulky lipids. Therefore, a dynamic instead of a fixed annular shell of lipids was proposed for AQPs (Stansfeld et al., 2013). This could explain why the interaction of AQP0 with the lipid membrane resembles the interaction of the tension sensor described in MscL and MscS channels (Malcolm et al., 2011).
The bilayer composition also influences the incorporation or depletion of different lipid classes in the annular lipid shell, as was observed in AQP1 (Corradi et al., 2018). In addition, AQP0 interacting with two different lipidic environments also showed lipid positional rearrangements on the annular shell, although no protein structural modifications were observed (Hite et al., 2010).
An intimate crosstalk between annular lipids accommodation on specific protein sites depends on the degree of protein mobility in the lipid membrane (Briones et al., 2017). Molecular dynamics approaches and crystallographic refinements revealed how preferred positions of lipids surrounding AQP0 are established, indicating a priority on the local movements of the protein but a secondary role of specific H-bond formation (Aponte Santamaría et al., 2012).
Lipid shells are proposed to adapt its shape to the surface protein irregularities, exerting interactions through the long acyl chains instead of polar head groups (Aponte Santamaría et al., 2012). However, the previously mentioned simulations performed with 40 AQP structures showed two different protein regions with a crucial role in lipid interaction. These locking regions occur in the intracellular and extracellular sides of the protein, by contacting the head groups of the lipid membrane, and were proposed to fix the protein to the lipid membrane. These functional regions show no classical conserved sequences, but a pattern of conservation on amino acid composition with numerous basic (K or R) or aromatic (W or Y) residues (Stansfeld et al., 2013). In line with this, other MD simulations showed that when AQP4 is in a thin membrane the extracellular region of the channel maintains a more conserved structure than the cytoplasmic region, this may be due to the asymmetric distribution of Try residues, which usually locate at membrane-water interfaces and are more abundant in the extracellular than in the intracellular side of AQP4 (Tong et al., 2016).
Molecular features of AQPs water transport
Aquaporins are highly symmetrical. Each monomer is ~ 40 Å across and ~ 60 Å long (Sui et al., 2001) and has a right-handed barrel structure (Cheng et al., 1997; Walz et al., 1997). This causes the transmembrane segments (TM) to be highly “twisted” (Fujiyoshi et al., 2002). In addition, each transmembrane segment is constituted by a left-handed alpha helix. The special disposition in the tetramer left TM2 and TM5 facing to the inner side of the tetramer, and TM3 and TM6 facing to the outer side, while TM1 and TM4 have an intermediate position (Heymann and Engel, 2000; de Groot et al., 2000). In this scenario, the structure of each monomer is stabilized by forces between highly conserved glycine residues that form contact sites between transmembrane segments (G104 for TM1-TM3, G219 for TM4-TM6, G57 and G173 for TM2-TM5, G82 for HB-TM6, and G198 for HE-TM3) (Murata et al., 2000; Fushiyoshi et al., 2002). In addition, the tetrameric arrangement is supported by large intermolecular forces between adjacent monomers. These forces are developed by the contact of TM1 and TM2 from one monomer with TM4 and TM5 from the adjacent monomer (Möller et al., 2003). Moreover, transmembrane segments are stabilized by the position of the two short alpha helices of loop B and E through ion pairs and hydrogen bonds. In human AQP1, H74 (loop B) forms an ion pair with Glu17 from H1, A195 (HE) is connected by a salt bridge to Glu142 (H4), and polar residues (Thr80, Gln101, S196) stabilize these ion pairs (Murata et al., 2000). At the same time, loops B and E are stabilized by highly conserved threonine residues (Murata et al., 2000).
The shape of the water channel resembles an hourglass model (Jung et al., 1994), allowing the passage of a single file of water molecules along a region located in the center of the membrane (Fig. 3a) (Murata et al., 2000; de Groot & Grubmüller, 2001). This region is ~ 30 Å long and is well-defined between the selectivity filter (SF, facing to the extracellular side of the membrane and also known as the ar/R filter) and the cytoplasmic extreme of the single file region. In the middle, the NPA filter is located. This filter is formed by the encounter of the two (signature motif) NPA repetitions (asparagine, proline, alanine). These three sites in the single file region are keys to enable and regulate the water flux (for a detailed revision, see Ozu et al. (2022)). While one wall of the water pathway is hydrophobic, the lining residues in the opposite wall offer H-bond donors to the water molecules. Thus, water molecules are confined, forming a water wire in a narrow pore. Then, the water molecules pass from one side to the other by forming and breaking H-bonds with the protein and adjacent water molecules. Therefore, water transport through AQPs is not a frictionless process (Horner and Pohl, 2018) and is guaranteed by the concerted movement of the molecules inside the single file region. These general features are well-documented by models derived from very high-resolution structural data and MD simulations.
Fig. 3.
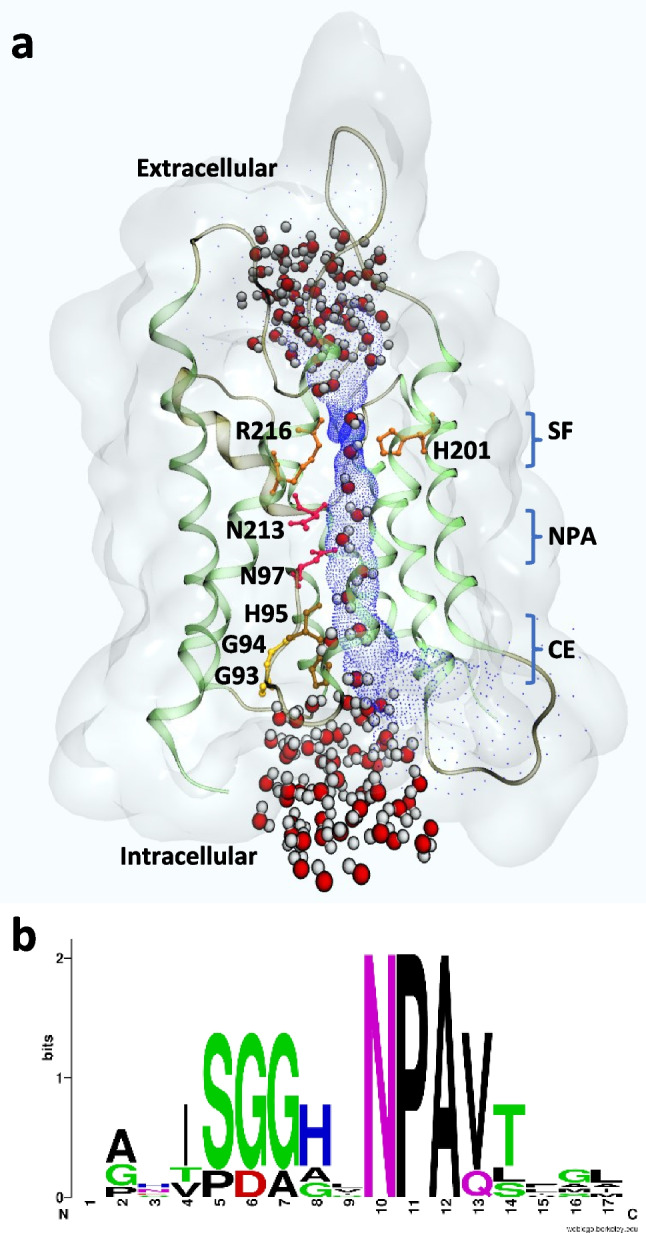
The key of mechanosensitive AQPs could be in the cytoplasmic extreme of the single file region of the water pathway. a Model of the AQP4 monomer (PDB 3GD8) showing the water pathway in blue. The key regions of the single file path are indicated on the right (SF, selectivity filter; NPA, NPA filter; CE, cytoplasmic extreme of the single file region). Transmembrane segments and loops are shown in light and dark green, respectively. The channel (blue) allows the passage of waters in single file between the SF and the CE. The SF or Ar/R filter (His201 from TM5 and Arg216 from loop E, shown in orange) restricts the transit to only two water molecules at a time by a geometric hinderance and offers the possibility to form H-bonds with the protein, ordering the water molecules. Both Asn97 from loop B and Asn213 from loop E are shown in coral at the center of the membrane. This is the NPA filter, where the passage of protons is prevented and the water dipole moment reversal prompted. Both Gly93 and Gly94 from the GxxxG motif of loop B (shown in yellow) are located at the CE of the single file region (also called the cytoplasmic mouth) and contribute with H-bonds to the ordering of water molecules. Next, His95 (shown in ochre) can occlude the pore by a gating mechanism mediated by cytosolic acidification. Visualization made by means of VMD (Humphrey et al., 1996). Far and near clipping planes have been defined on behalf of clarity. b Logo of the loop B sequence alignment from mechanosensitive aquaporins AQY1, AQP1, AQP4, BvTIP1;2, and VvTIP2;1. Arbitrary positions 6, 7, and 8 show the key Gly and His residues that play a key role in ordering water molecules at the cytoplasmic extreme of the single file, just before the NPA. The image was created with WebLogo (Schneider and Stephens, 1990; Crooks et al., 2004)
The NPA filter is involved in two important aspects of water transport, which occur at the center of the membrane. The first one is that protons passage is prevented (Zeidel et al., 1992) by an electrostatic barrier that is maximal at this site (de Groot et al., 2003) and arises from the distribution of charged and polar groups of the protein, essentially forming a positive charge density (Chakrabarti et al., 2004). The second one is that the water wire is interrupted at the NPA, and the water molecules experience the reversal of the dipole moment; i.e., the water molecule suffers a 180° rotation during the passage through the channel (Fig. 3a), thus impeding proton passage via water hopping (de Groot and Grubmüller, 2001).
About 4 to 8 Å from the NPA towards the extracellular side is the SF. The key residues are a highly conserved His from TM5 and a highly conserved Arg from loop E. The mutation of one of these residues, or both at the same time, changes the selectivity properties of the channel (Beitz et al., 2006). Both residues provide water molecules the possibility to form H-bonds with the protein. The structure resolution of bovine AQP1 at 2.2 Å resolution (Sui et al., 2001) and AQY1 from Pichia pastoris at 0.88 Å resolution (Kosinska-Eriksson et al., 2013) showed that four water positions exist in the SF. However, water molecules can only move in pairs through this region because of geometric conditioning. So, the spatial disposition of His and Arg is crucial for the correct ordering of water molecules in the extracellular extreme of the single file region (Sui et al., 2001; Kosinska-Eriksson et al., 2013).
Below the NPA filter is the cytoplasmic extreme of the single file region. Just before the first NPA is the GxxxG motif. This tandem of GxxxG and NPA motifs in loop B is highly conserved in the AQPs family (Murata et al., 2000; Sui et al., 2001; Fujiyoshi et al., 2002; Ho et al., 2009; Kosinska-Eriksson et al., 2013). Due to its key location, this GxxxG motif could play a key role in the mechanosensitive response of AQPs.
The GxxxG motifs are commonly found in transmembrane helices and are believed to promote helix-helix interactions within the membrane (Russ and Engelman, 2000). Specifically, these motifs facilitate the oligomerization of transmembrane proteins by stabilizing their structure through hydrophobic interactions between glycine residues of adjacent helices (Xiao et al., 2020; Mueller., et al., 2014). Furthermore, they may also play a role in signal transduction events (Javadpour et al., 1999; Teese and Langosch, 2015). For example, G22 from E. coli MscL (Yoshimura et al., 1999) and G104 or G108 from E. coli MscS (Edwards et al., 2005) are crucial in the sensitivity to membrane tension changes for pore opening transitions. This motif was termed “glycine zipper”—by the analogy with the known leucine zipper motif (Kim et al., 2005). Glycine zippers were also detected in other non-related ion channels, such as KscA from Streptomyces lividans, where the motif lies in a pore lining highly hydrophobic region, crucial for the pore formation (Shealy et al., 2003). Besides this, the G104 from KscA was proposed to be crucial in the TM bending upon channel gating (Cuello et al., 2010). Although KscA is not a MS channel, the MS properties of voltage gated channels are well-known (Morris, 2011).
Furthermore, the GxxxG motif is not always found in transmembrane proteins that form helix dimers or oligomers. In some cases, the motif may play a role in stabilizing the transmembrane helix itself by limiting the conformational flexibility of the glycine residues and preventing kinks or bends in the helix (Högel et al., 2018). In other cases, the motif may be involved in mediating protein-lipid interactions, such as the binding of cholesterol or other lipids to transmembrane domains (Yano et al., 2022).
The role of the GxxxG motif in mechanosensitivity (Balleza et al., 2014, Perozo et al., 2002) may be related to its effects on membrane organization and protein-lipid interactions (Yano et al., 2017). For example, this motif can promote the formation of lipid rafts, specialized regions of the membrane enriched in certain lipids and cholesterol that serve as platforms for signaling and trafficking events (Fernandez Muñoz et al., 2011; Lorent and Levental, 2015). They are also thought to be important in mechanosensitivity by modulating the mechanical properties of the membrane (Nickels et al., 2019).
In AQPs, the GxxxG motifs are repeated several times. For example, in AQP1, there are 5 GxxxG motifs; three in TM1, TM4, and TM6; and other two in loops B and D. This distribution is similar in other AQPs, but it is not conserved. The mechanosensitive aquaporins AQP1 and BvTIP1;2 present a similar amount and distribution of GxxxG motifs. Moreover, BvPIP2;1, which is not mechanosensitive, presents a different distribution of GxxxG motifs which is in agreement with our working hypothesis (Goldman et al., 2017).
The GxxxG motif in loop B contributes one or two Gly residues to the cytoplasmic extreme of the single file region (Fig. 3b). These glycine residues, together with a highly conserved His, are involved in H-bonds formed with the water molecules passing through the channel (Ho et al., 2009; Kosinska-Eriksson et al., 2013; Horner et al., 2015). This conserved His, as well as other residues in this region, has been suggested to play a key role in water transport.
In AQP0, His66 and Val155 seem to act as a valve that controls the passage of water molecules. According to MD simulations, the carbonyl group of His66 forms an H-bond to water and its side chain swings between the wall and the lumen of the water pathway (Smolin et al., 2008). In AQP4, the homolog His95 mediates the pH gating triggered by intracellular acidification. In the closed state, the singly protonated His95 swings towards the water pathway, while the double protonated H95 swings to the channel’s wall, resulting in higher permeability values (Kaptan et al., 2015). In addition, H95 and C178 were proposed to constitute a cytoplasmic gate that together with the key residues of the selectivity filter (His201 and Arg216) give rise to a four-state gating mechanism mediated by electric fields (English and Garate, 2016).
In human AQP10, the pH-dependent gating mechanism relies heavily on the homolog H80, which acts as the pH sensor and triggers the promotion of glycerol flux and the shutting down of water flux upon acidification (Gotfryd et al., 2018). The protonation of H80 induces a conformational change in loop B, resulting in a cytoplasmic pore that is often water-depleted and hydrophobic (Truelsen et al., 2022). This conformational change leads to a reorganization of water molecules that could lower the energy barrier for folding along multiple dihedral angles of loop B residues, ultimately resulting in a glycerol permeable pore (Truelsen et al., 2022). These results suggest that the loss of water coordination plays a critical role in destabilizing the closed conformation at low pH.
A possible mechanism for mechanical gating in AQPs
Although there is a detailed description about the passage of water molecules through the single file region, information about the molecular events involved in the mechanical regulation of water transport through aquaporins is lacking. Nevertheless, a possible mechanism for mechanical regulation would lie on the cytoplasmic extreme of the single file (Ozu et al., 2018). Briefly, as membrane tension increases, it is expected that membrane thickness diminishes. In experiments with AQP4 reconstituted in vesicles, it was found that water permeability decreases with decreasing lipid bilayer thickness (Tong et al., 2016). The same study reported MD simulation results that showed no significant structural changes in the pore at the selectivity filter, the NPA filter, and the extracellular entrance of the pore as membrane thickness decreases. However, structural changes become apparent at the cytoplasmic aperture of the water pathway. These simulations revealed that lower membrane thickness produces a narrower and longer cytoplasmic extreme at the single file region of the water pathway (Tong et al., 2016). Other simulations demonstrated that shape modifications at the entrance to a single file water pathway alter the transport rate, highlighting the importance of the conical shape of the AQPs channel at the extremes of the single file region (Gravelle et al., 2013). Therefore, changes triggered by increments in membrane tension could potentially impact the optimal geometry for H-bond formation between water molecules and pore lining residues. Since the water transport capacity of channels is dependent on the number of H-bonds formed between water molecules and pore lining residues (Horner et al., 2015), thickening of the membrane could lead to lower permeability values, in accordance with experimental observations (Soveral et al., 2008; Tong et al., 2012; Ozu et al., 2013; Leitao et al., 2014; Goldman et al., 2017).
Perspectives
To date, only a handful of studies have reported mechanosensitivity in aquaporins. Few members from yeast, plant, and animals were proved to be mechanosensitive (AQY1, AQP1, AQP4, VvTIP2;1, BvTIP1;2), and only one (BvPIP2;1) was reported as a not mechanosensitive aquaporin (Soveral et al., 2008; Fischer et al., 2009; Tong et al., 2012; Ozu et al., 2013; Leitão et al., 2014; Goldman et al., 2017). This latter is a PIP-type member, evolutionarily related to AQP1 and AQP4 from animals (Soto et al., 2012). However, BvPIP2;1 is a PIP2-type aquaporin that does not show the typical behavior of PIP2 members (Bellati et al., 2010; Jozefkowicz et al., 2013). So, it is not discarded that other PIP2 members might also be mechanosensitive.
The sensitivity of AQPs towards membrane tension changes is in the range of 3 to 10 mN m−1, like that of MscL and MscS. In spite of that, unlike MS ion channels, the transport rate of AQPs diminishes with membrane tension increments. Although it seems to be a rule that mechanosensitive aquaporins partially close with an increase in membrane tension, further studies are required to fully understand these processes and address unresolved questions.
To date, there is no information about the molecular features of the gating mechanism triggered by membrane tension. Nonetheless, knowledge about the structure and function of aquaporins allows us to hypothesize that a conformational change occurs in the cytoplasmic extreme of the single file region of the water pathway. One possibility is that this region changes its shape, in a similar way to the one proposed for AQP4 when the membrane thickness diminishes (Tong et al., 2016). Another possibility is that the functional group of a single amino acid would suffer a torsional movement occluding the channel, in a similar way to that described for H95 of human AQP4 under cytoplasmic acidification (Kaptan et al., 2015). Between those, several options are possible, including a combination of both. It is important to emphasize, that given the dimensions of both the transported species and the single file nature of the permeation process, small changes, of a couple of angstroms, can lead to macroscopic repercussions. Consequently, models that combine carefully designed experiments and molecular resolution simulations are required to fully understand this phenomenon.
The study of mechanosensitive AQPs is more than the study of the gating mechanism and its physiological implications. It points to the study of how water molecules pass through the water pathway, and this is more than the study of the single file or the water dipole orientation. Another channel in which water is structurally confined in a single file in the permeation pathway is the Hv1 proton channel. Hv1 is present as a homodimer, but, like AQPs, each subunit can function independently (Tombola et al., 2008; Koch et al., 2008). Hv1 is a well-known voltage-dependent channel (Tombola et al., 2010; Gonzalez et al., 2013; Carmona et al., 2018). Its activation is also dependent on pH, and the increase in water permeation events may be involved in a mechanism that would facilitate the movement of protons in the active state (Carmona et al., 2021). In addition, like many gating mechanisms in AQPs, the Hv1 voltage dependance is cooperative (Gonzalez et al., 2010; Tombola et al., 2010; Fujiwara et al., 2012). Recently Hv1 was reported to be mechanosensitive, and like MS ion channels, it activates by increasing membrane stretch (Pathak et al., 2016). According to experimental and simulation evidence, the passage of protons through the permeation pathway of Hv1 seems not to occur by protons jumping along the water wire. Instead, the water wire seems to be interrupted and proton permeation be mediated by H-bonds with key residues at a particular site of the permeation pathway (Boytsov et al., 2023). This evidence constitutes Hv1 in an ideal partner of AQPs in the study of permeation processes in single file water-confined channels.
All the available information regarding mechanosensitivity in AQPs has been obtained by studying homotetramers. However, it is well-documented that the regulation of membrane water permeability in plants can be achieved by modulating the expression of heterotetramers (Yaneff et al., 2015). Type 1 and 2 subunits from the PIP subfamily can combine to form heterotetramers with different stoichiometry. This has two consequences: (i) the water permeability can be tuned in a wide range (Bellati et al., 2010) and (ii) the cooperative pH-regulation is modified, with homo- and heterotetramers showing different sensitivity (Yaneff et al., 2014). Therefore, PIP1-PIP2 heterotetramers are a good system to study the cooperative clues of mechanosensitivity in AQPs.
Regarding the relevance of mechanosensitive aquaporins in physiology, there are several processes in which aquaporins are important players and cell mechanics is compromised. Since the mechanical regulation of AQPs is reversible (Ozu et al., 2013), it raises the question of how the function of these channels can affect cellular systems in which the mechanical membrane properties undergo cyclic changes.
In summary, cells have developed different strategies to respond to mechanical stimuli, and there are various non-conserved proteins that are mechanosensitive. The literature provides extremely useful information on the structure-function relationship of some of these proteins. The discovery of mechanosensitive aquaporins expands the panorama, not only in terms of the cell’s ability to respond with an additional element of mechanical modulation, but also to identify the structural characteristics that confer this property to water channels, and the true impact of the lipid environment. Given the high-water transport capacity of orthodox AQPs and the fundamental role of osmosis in cellular metabolism, there is no doubt that a new fundamental piece is introduced into the game for understanding cellular responses. The fact that aquaporins are so ancestral and ubiquitous and that mechanosensitivity has been reported in yeast, plant, and animal, AQPs allow complementary approaches to identify the most conserved mechanisms that have diversified.
Acknowledgements
This research was supported by Fondo para la Investigación Científica y Tecnológica (FONCYT), grants Préstamo BID PICT2020-1438 (to GA), Préstamo BID PICT-2018-02026 (to SPD - LIP), and Préstamo BID PICT2017-0368 (to MO); Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), grant PIP2021-2023 N° 11220200100610CO (to MO); Universidad de Buenos Aires, grant UBACyT2020 N° 20020190200141BA (to MO); Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), Regular Grant No. 1221260 (to JAG). The Millennium Nucleus in NanoBioPhysics (NNBP) is supported by the Iniciativa Científica Milenio-Agencia Nacional de Investigación y Desarrollo (ICM-ANID), Project NCN2021, NNBP. The Centro Científico y Tecnológico de Excelencia Ciencia y Vida is supported by Financiamiento Basal para Centros Científicos y Tecnológicos de Excelencia de ANID Project FB210008.
Author contributions
MO had the idea for the article. MO, LG, JJAA, MF, AC, LS, and LIP performed the literature search and wrote the article. MO, JJAA, AC, and RZ performed the figures. All the authors critically revised the work and participated in the writing process.
Declarations
Ethics approval
Does not correspond.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marcelo Ozu, Email: mozu@bg.fcen.uba.ar, Email: ozu.marcelo@gmail.com.
José Antonio Garate, Email: jgarate@dlab.cl.
References
- Agre P, Sasaki S, Chrispeels MJ. Aquaporins: a family of water channel proteins. Am J Phys. 1993;265(3 Pt 2):F461. doi: 10.1152/ajprenal.1993.265.3.F461. [DOI] [PubMed] [Google Scholar]
- Alexandre J, Lassalles J-P. Hydrostatic and osmotic pressure activated channel in plant vacuole. Biophys J. 1991;60(6):1326–1336. doi: 10.1016/S0006-3495(91)82170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva K, Chara O, Sutka MR, Amodeo G. Analysis of the source of heterogeneity in the osmotic response of plant membrane vesicles. Eur Biophys J. 2009;38(2):175–184. doi: 10.1007/s00249-008-0365-1. [DOI] [PubMed] [Google Scholar]
- Alleva K, Chara O, Amodeo G. Aquaporins: Another piece in the osmotic puzzle. FEBS Lett. 2012;586(19):2991–2999. doi: 10.1016/j.febslet.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Aponte Santamaría C, Briones R, Schenk AD, Walz T, de Groot BL. Molecular driving forces defining lipid positions around aquaporin-0. Proc Natl Acad Sci U S A. 2012;109(25):9887–9892. doi: 10.1073/pnas.1121054109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetz NW, Hoffman EA, Yool AJ, Stamer WD. Role of aquaporin-1 in trabecular meshwork cell homeostasis during mechanical strain. Exp Eye Res. 2009;89(1):95–100. doi: 10.1016/j.exer.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleza D. Toward Understanding Protocell Mechanosensation. Orig Life Evol Biosph. 2011;41:281–304. doi: 10.1007/s11084-010-9225-y. [DOI] [PubMed] [Google Scholar]
- Balleza D, Carrillo E, Gómez-Lagunas F. Conservation analysis of residues in the S4–S5 linker and the terminal part of the S5-P-S6 pore modulus in Kv and HCN channels: flexible determinants for the electromechanical coupling. Eur J Phys. 2014;467(10):2069–2079. doi: 10.1007/s00424-014-1647-3. [DOI] [PubMed] [Google Scholar]
- Bass RB, Strop P, Barclay M, Rees DC. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298(5598):1582–1587. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T. Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc Natl Acad Sci U S A. 2006;103(2):269–274. doi: 10.1073/pnas.0507225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellati J, Alleva K, Soto G, Vitali V, Jozefkowicz C, Amodeo G. Intracellular pH sensing is altered by plasma membrane PIP aquaporin co-expression. Plant Mol Biol. 2010;74(1):105–118. doi: 10.1007/s11103-010-9658-8. [DOI] [PubMed] [Google Scholar]
- Birkner JP, Poolman B, Koçer A. Hydrophobic gating of mechanosensitive channel of large conductance evidenced by single-subunit resolution. Proc Natl Acad Sci U S A. 2012;109(32):12944–12949. doi: 10.1073/pnas.1205270109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount P, Iscla I. Life with Bacterial Mechanosensitive Channels, from Discovery to Physiology to Pharmacological Target. Microbiol Mol Biol Rev. 2020;84(1):10–1128. doi: 10.1128/mmbr.00055-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth IR, Miller S, Müller A, Lehtovirta-Morley L. The evolution of bacterial mechanosensitive channels. Cell Calcium. 2015;57(3):140–150. doi: 10.1016/j.ceca.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Boytsov D, Brescia S, Chaves G, Koefler S, Hannesschlaeger C, Siligan C, Goessweiner-Mohr N, Musset B, Pohl P. Trapped Pore Waters in the Open Proton Channel HV1. Small. 2023;2023:2205968. doi: 10.1002/smll.202205968. [DOI] [PubMed] [Google Scholar]
- Briones R, Aponte-Santamaría C, de Groot BL. Localization and ordering of lipids around aquaporin-0: Protein and lipid mobility effects. Front Physiol. 2017;8:124. doi: 10.3389/fphys.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, Campbell EB, MacKinnon R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K1 channel. Nature. 2014;516(7529):126–141. doi: 10.1038/nature14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Califano JP, Reinhart-King CA. Substrate stiffness and cell area predict cellular traction stresses in single cells and cells in contact. Cell Mol Bioeng. 2010;3(1):68–75. doi: 10.1007/s12195-010-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona EM, Larsson HP, Neely A, Alvarez O, Latorre R, Gonzalez C. Gating charge displacement in a monomeric voltage-gated proton (Hv1) channel. Proc Natl Acad Sci U S A. 2018;115(37):9240–9245. doi: 10.1073/pnas.1809705115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona EM, Fernandez M, Alvear-Arias JJ, Neely A, Larsson HP, Alvarez O, Garate JA, Latorre R, Gonzalez C. The voltage sensor is responsible for ∆pH dependence in Hv1 channels. Proc Natl Acad Sci U S A. 2021;118(19):e2025556118. doi: 10.1073/pnas.2025556118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti N, Roux B, Pomès R. Structural determinants of proton blockage in aquaporins. J Mol Biol. 2004;343(2):493–510. doi: 10.1016/j.jmb.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 1998;282:2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- Chen Y, Rice W, Gu Z, Li J, Huang J, Brenner MB, Van Hoek A, Xiong J, Gundersen GG, Norman JC, Hsu VW, Fenton RA, Brown D, Lu HA. Aquaporin 2 promotes cell migration and epithelial morphogenesis. J Am Soc Nephrol. 2012;23(9):1506–1517. doi: 10.1681/ASN.2012010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, van Hoek AN, Yeager M, Verkman AS, Mitra AK. Three-dimensional organization of a human water channel. Nature. 1997;387(6633):627–630. doi: 10.1038/42517. [DOI] [PubMed] [Google Scholar]
- Corradi V, Mendez-Villuendas E, Ingólfsson HI, Gu RX, Siuda I, Melo MN, Moussatova A, Degagné LJ, Sejdiu BI, Singh G, Wassenaar TA, Delgado Magnero K, Marrink SJ, Tieleman DP. Lipid-protein interactions are unique fingerprints for membrane proteins. ACS Central Sci. 2018;4(6):709–717. doi: 10.1021/acscentsci.8b00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CD, Bavi N, Martinac B. Bacterial mechanosensors. Annu Rev Physiol. 2018;80:71–93. doi: 10.1146/annurev-physiol-021317-121351. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14(6):1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello LG, Jogini V, Cortes DM, Perozo E. Structural mechanism of C-type inactivation in K+ channels. Nature. 2010;466(7303):203–208. doi: 10.1038/nature09153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot BL, Grubmüller H. Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science. 2001;294(5550):2353–2357. doi: 10.1126/science.1062459. [DOI] [PubMed] [Google Scholar]
- de Groot BL, Heymann JB, Engel A, Mitsuoka K, Fujiyoshi Y, Grubmüller H. The fold of human aquaporin 1. J Mol Biol. 2000;300(4):987–994. doi: 10.1006/jmbi.2000.3913. [DOI] [PubMed] [Google Scholar]
- de Groot BL, Frigato T, Volkhard H, Grubmüller H. The mechanism of proton exclusion in the aquaporin-1 water channel. J Mol Biol. 2003;333(2):279–293. doi: 10.1016/j.jmb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Di Giusto G, Pizzoni A, Rivarola V, Beltramone N, White A, Ford P, Capurro C. Aquaporin-2 and Na+/H+ exchanger isoform 1 modulate the efficiency of renal cell migration. J Cell Physiol. 2020;235(5):4443–4454. doi: 10.1002/jcp.29320. [DOI] [PubMed] [Google Scholar]
- Edwards MD, Li Y, Kim S, Miller S, Bartlett W, Black S, Dennison S, Iscla I, Blount P, Bowie JU, Booth IR. Pivotal role of the glycine-rich TM3 helix in gating the MscS mechanosensitive channel. Nat Struct Mol Biol. 2005;12(2):113–119. doi: 10.1038/nsmb895. [DOI] [PubMed] [Google Scholar]
- English NJ, Garate JA. Near-microsecond human aquaporin 4 gating dynamics in static and alternating external electric fields: Non-equilibrium molecular dynamics. J Chem Phys. 2016;145(8):085102. doi: 10.1063/1.4961072. [DOI] [PubMed] [Google Scholar]
- Fernández-Muñoz B, Yurrita MM, Martín-Villar E, Carrasco-Ramírez P, Megías D, Renart J, Quintanilla M. The transmembrane domain of podoplanin is required for its association with lipid rafts and the induction of epithelial-mesenchymal transition. Int J Biochem Cell Biol. 2011;43(6):886–896. doi: 10.1016/j.biocel.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Fischer G, Kosinska-Eriksson U, Aponte-Santamaría C, Palmgren M, Geijer C. Crystal structure of a yeast aquaporin at 1.15 Å reveals a novel gating mechanism. PLoS Biol. 2009;7(6):1000130. doi: 10.1371/journal.pbio.1000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegler VJ, Rasmussen A, Borbil K, Boten L, Chen HA, Deinlein H, Halang J, Hellmanzik K, Löffler J, Schmidt V, Makbul C, Kraft C, Hedrich R, Rasmussen T, Böttcher B. Mechanosensitive channel gating by delipidation. Proc Natl Acad Sci U S A. 2021;118(33):e2107095118. doi: 10.1073/pnas.2107095118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri A, Nicchia GP, Desaphy JF, Pierno S, De Luca A, Camerino DC, Svelto M. Muscle loading modulates aquaporin-4 expression in skeletal muscle. FASEB J. 2001;15(7):1282–1284. doi: 10.1096/fj.00-0525fje. [DOI] [PubMed] [Google Scholar]
- Frigeri A, Nicchia GP, Balena R, Nico B, Svelto M. Aquaporins in skeletal muscle: reassessment of the functional role of aquaporin-4. FASEB J. 2004;18(7):905–907. doi: 10.1096/fj.03-0987fje. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Kurokawa T, Takeshita K, Kobayashi M, Okochi Y, Nakagawa A, Okamura Y. The cytoplasmic coiled-coil mediates cooperative gating temperature sensitivity in the voltage-gated H(+) channel Hv1. Nat Commun. 2012;3:816. doi: 10.1038/ncomms1823. [DOI] [PubMed] [Google Scholar]
- Fujiyoshi Y, Mitsuoka K, de Groot BL, Philippsen A, Grubmüller H, Agre P, Engel A. Structure and function of water channels. Curr Opin Struct Biol. 2002;12(4):509–515. doi: 10.1016/s0959-440x(02)00355-x. [DOI] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10(1):21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Goldman RP, Jozefkowicz C, Canessa Fortuna A, Sutka M, Alleva K, Ozu M. Tonoplast (BvTIP1;2) and plasma membrane (BvPIP2;1) aquaporins show different mechanosensitive properties. FEBS Lett. 2017;591(11):1555–1565. doi: 10.1002/1873-3468.12671. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Koch HP, Drum BM, Larsson HP. Strong cooperativity between subunits in voltage-gated proton channels. Nat Struct Mol Biol. 2010;17:51–56. doi: 10.1038/nsmb.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Rebolledo S, Perez ME, Larsson HP. Molecular mechanism of voltage sensing in voltage-gated proton channels. J Gen Physiol. 2013;141(3):275–285. doi: 10.1085/jgp.201210857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotfryd K, Mósca AF, Missel JW, Truelsen SF, Wang K, Spulber M, Krabbe S, Hélix-Nielsen C, Laforenza U, Soveral G, Pedersen PA, Gourdon P. Human adipose glycerol flux is regulated by a pH gate in AQP10. Nat Commun. 2018;9(1):4749. doi: 10.1038/s41467-018-07176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravelle S, Joly L, Detcheverry F, Ybert C, Cottin-Bizonne C, Bocquet L. Optimizing water permeability through the hourglass shape of aquaporins. Proc Natl Acad Sci U S A. 2013;110(41):16367–16372. doi: 10.1073/pnas.1306447110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Bielawski KS, Ting LH, Rodriguez ML, Sniadecki NJ. Decoupling substrate stiffness, spread area, and micropost density: a close spatial relationship between traction forces and focal adhesions. Biophys J. 2012;103(4):640–648. doi: 10.1016/j.bpj.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselwandter CA, Phillips R. Connection between oligomeric state and gating characteristics of mechanosensitive ion channels. PLoS Comput Biol. 2013;9(5):e1003055. doi: 10.1371/journal.pcbi.1003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann JB, Engel A. Structural clues in the sequences of the aquaporins. J Mol Biol. 2000;295(4):1039–1053. doi: 10.1006/jmbi.1999.3413. [DOI] [PubMed] [Google Scholar]
- Hill AE, Shachar-Hill Y. Are aquaporins the missing transmembrane osmosensors? J Membr Biol. 2015;248(4):753–765. doi: 10.1007/s00232-015-9790-0. [DOI] [PubMed] [Google Scholar]
- Hill AE, Shachar-Hill B, Shachar-Hill Y. What are aquaporins for? J Membr Biol. 2004;197(1):1–32. doi: 10.1007/s00232-003-0639-6. [DOI] [PubMed] [Google Scholar]
- Hite RK, Li Z, Walz T. Principles of membrane protein interactions with annular lipids deduced from aquaporin-0 2D crystals. EMBO J. 2010;29(10):1652–1658. doi: 10.1038/emboj.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JD, Yeh R, Sandstrom A, Chorny I, Harries WEC, Robbins RA, Miercke LJW, Stroud RM. Crystal structure of human aquaporin 4 at 1.8 A and its mechanism of conductance. Proc Natl Acad Sci U S A. 2009;106(18):7437–7442. doi: 10.1073/pnas.0902725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högel P, Götz A, Kuhne F, Ebert M, Stelzer W, Rand KD, Scharnagl C, Langosch D. Glycine perturbs local and global conformational flexibility of a transmembrane helix. Biochemistry. 2018;57(8):1326–1337. doi: 10.1021/acs.biochem.7b01197. [DOI] [PubMed] [Google Scholar]
- Horner A, Pohl P. Single-file transport of water through membrane channels. Faraday Discuss. 2018;157(207890):243. doi: 10.1039/C8FD00122G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner A, Zocher F, Preiner J, Ollinger N, Siligan C, Akimov SA, Pohl P. The mobility of single-file water molecules is governed by the number of H-bonds they may form with channel-lining residues. Sci Adv. 2015;1(2):e1400083. doi: 10.1126/sciadv.1400083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graph. 1996;14(1):33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Jang KJ, Cho HS, Kang DH, Bae WG, Kwon TH, Suh KY. Fluid-shear-stress-induced translocation of aquaporin-2 and reorganization of actin cytoskeleton in renal tubular epithelial cells. Integr Biol. 2011;3(2):134–141. doi: 10.1039/c0ib00018c. [DOI] [PubMed] [Google Scholar]
- Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- Jansen KA, Atherton P, Ballestrem C. Mechanotransduction at the cell-matrix interface. Semin Cell Dev Biol. 2017;71:75–83. doi: 10.1016/j.semcdb.2017.07.027. [DOI] [PubMed] [Google Scholar]
- Javadpour MM, Eilers M, Groesbeek M, Smith SO. Helix packing in polytopic membrane proteins: Role of glycine in transmembrane helix association. Biophys J. 1999;77(3):1609–1618. doi: 10.1016/S0006-3495(99)77009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozefkowicz C, Rosi P, Sigaut L, Soto G, Pietrasanta LI, Amodeo G, Alleva K. Loop A is critical for the functional interaction of two Beta vulgaris PIP aquaporins. PLoS One. 2013;8(3):e57993. doi: 10.1371/journal.pone.0057993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JS, Preston GM, Smith BL, Guggino WB, Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994;269(20):14648–14654. doi: 10.1016/s0021-9258(17)36674-7. [DOI] [PubMed] [Google Scholar]
- Kamm RD, Mofrad MRK. In: Cellular mechanotransduction: diverse perspectives from molecules to tissues. 1. Mofrad MRK, Kamm RD, editors. Cambridge University Press; 2009. pp. 1–19. [Google Scholar]
- Kaptan S, Assentoft M, Schneider HP, Fenton RA, Deitmer JW, MacAulay N, de Groot BL. H95 Is a pH-dependent gate in aquaporin 4. Structure. 2015;23(12):2309–2318. doi: 10.1016/j.str.2015.08.020. [DOI] [PubMed] [Google Scholar]
- Kim S, Jeon T-J, Oberai A, Yang D, Schmidt JJ, Bowie JU. Transmembrane glycine zippers: physiological and pathological roles in membrane proteins. Proc Natl Acad Sci U S A. 2005;102(40):14278–14283. doi: 10.1073/pnas.0501234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim OH, Choi YW, Park JH, Hong SA, Hong M, Chang IH, Lee HJ. Fluid shear stress facilitates prostate cancer metastasis through Piezo1-Src-YAP axis. Life Sci. 2022;308:120936. doi: 10.1016/j.lfs.2022.120936. [DOI] [PubMed] [Google Scholar]
- Koch HP, Kurokawa T, Okochi Y, Sasaki M, Okamura Y, Larsson HP. Multimeric nature of voltage-gated proton channels. Proc Natl Acad Sci U S A. 2008;105(26):9111–9116. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinska Eriksson U, Fischer G, Friemann R, Enkavi G, Tajkhorshid E, Neutze R. Subangstrom resolution X-ray structure details aquaporin-water interactions. Science. 2013;340(6138):1346–1349. doi: 10.1126/science.1234306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazcano A, Oró J, Miller SL. Primitive Earth environments: organic syntheses and the origin and early evolution of life. Precambrian Res. 1983;20(2–4):259–282. doi: 10.1016/0301-9268(83)90076-1. [DOI] [Google Scholar]
- Lee AG. How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta Biomembr. 2004;1666(1–2):62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Leitão L, Prista C, Loureiro-Dias MC, Moura TF, Soveral G. The grapevine tonoplast aquaporin TIP2;1 is a pressure gated water channel. Biochem Biophys Res Commun. 2014;450(1):289–294. doi: 10.1016/j.bbrc.2014.05.121. [DOI] [PubMed] [Google Scholar]
- Lorent JH, Levental I. Structural determinants of protein partitioning into ordered membrane domains and lipid rafts. Chem Phys Lipids. 2015;192:23–32. doi: 10.1016/j.chemphyslip.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Luo M, Cai G, Ho KKY, Wen K, Tong Z, Deng L, Liu AP. Compression enhances invasive phenotype and matrix degradation of breast cancer cells via Piezo1 activation. BMC Mol Cell Biol. 2022;23(1):1. doi: 10.1186/s12860-021-00401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm HR, Heo YY, Elmore DE, Maurer JA. Defining the role of the tension sensor in the mechanosensitive channel of small conductance. Biophys J. 2011;101(2):345–352. doi: 10.1016/j.bpj.2011.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B, Hamill OP. Gramicidin A channels switch between stretch activation and stretch inactivation depending on bilayer thickness. Proc Natl Acad Sci U S A. 2002;99(7):4308–4312. doi: 10.1073/pnas.072632899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987;84(8):2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan R, McKinney MC, Teddy JM, Morrison JA, Kasemeier-Kulesa JC, Ridenour DA, Manthe CA, Giniunaite R, Robinson M, Baker RE, Maini PK, Kulesa PM. Neural crest cells bulldoze through the microenvironment using aquaporin 1 to stabilize filopodia. Development. 2020;147(1):dev185231. doi: 10.1242/dev.185231. [DOI] [PubMed] [Google Scholar]
- Meinild A-K, Klaerke DA, Zeuthen T. Bidirectional water fluxes and specificity for small hydrophilic molecules in aquaporins 0–5. J Biol Chem. 1998;273(49):32446–32451. doi: 10.1074/jbc.273.49.32446. [DOI] [PubMed] [Google Scholar]
- Möller C, Fotiadis D, Suda K, Engel A, Kessler M, Müller DJ. Determining molecular forces that stabilize human aquaporin-1. J Struct Biol. 2003;142(3):369–378. doi: 10.1016/S1047-8477(03)00066-2. [DOI] [PubMed] [Google Scholar]
- Morris CE. Voltage-gated channel mechanosensitivity: fact or friction? Front Physiol. 2011;2(25):25. doi: 10.3389/fphys.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BK, Subramaniam S, Senes A. A frequent, GxxxG-mediated, transmembrane association motif is optimized for the formation of interhelical Cα-H hydrogen bonds. Proc Natl Acad Sci U S A. 2014;111(10):E888–E895. doi: 10.1073/pnas.1319944111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun GI, Jang SI, Boo YC. Laminar shear stress induces the expression of aquaporin 1 in endothelial cells involved in wound healing. Biochem Biophys Res Commun. 2013;430(2):554–559. doi: 10.1016/j.bbrc.2012.11.114. [DOI] [PubMed] [Google Scholar]
- Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407(6804):599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- Naismith JH, Booth IR. Bacterial mechanosensitive channels-MscS: evolution’s solution to creating sensitivity in function. Annu Rev Biophys. 2012;41(1):157–177. doi: 10.1146/annurev-biophys-101211-113227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels JD, Smith MD, Alsop RJ, Himbert S, Yahya A, Cordner D, Zolnierczuk P, Stanley CB, Katsaras J, Cheng X, Rheinstädter MC. Lipid rafts: buffers of cell membrane physical properties. J Phys Chem B. 2019;123(9):2050–2056. doi: 10.1021/acs.jpcb.8b12126. [DOI] [PubMed] [Google Scholar]
- Niemietz CM, Tyerman SD. Characterization of water channels in wheat root membrane vesicles. Plant Physiol. 1997;115(2):561–567. doi: 10.1104/pp.115.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Cranfield CG, Deplazes E, Owen DM, Macmillan A, Battle AR, Maryrose C, Sokabe M, Martinac B. Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc Natl Acad Sci U S A. 2012;109(22):8770–8775. doi: 10.1073/pnas.1200051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y, Iwasaki I, Suga S, Murakami M, Inoue K, Maeshima M. Low aquaporin content and low osmotic water permeability of the plasma and vacuolar membranes of a CAM plant Graptopetalum paraguayense: comparison with radish. Plant Cell Physiol. 2001;42(10):1119–1129. doi: 10.1093/pcp/pce141. [DOI] [PubMed] [Google Scholar]
- Ozu M, Dorr RA, Politi MT, Parisi M, Toriano R. Water flux through human aquaporin 1: inhibition by intracellular furosemide and maximal response with high osmotic gradients. Eur Biophys J. 2011;40(6):737–746. doi: 10.1007/s00249-011-0687-2. [DOI] [PubMed] [Google Scholar]
- Ozu M, Dorr RA, Gutiérrez F, Politi MT, Toriano R. A counterpoint between computer simulations and biological experiments to train new members of a laboratory of physiological sciences. Adv Physiol Educ. 2012;36(4):345–351. doi: 10.1152/advan.00069.2012. [DOI] [PubMed] [Google Scholar]
- Ozu M, Dorr RA, Gutiérrez F, Politi MT, Toriano R. Human AQP1 is a constitutively open channel that closes by a membrane-tension-mediated mechanism. Biophys J. 2013;104(1):85–95. doi: 10.1016/j.bpj.2012.11.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozu M, Galizia L, Acuña C, Amodeo G. Aquaporins: more than functional monomers in a tetrameric arrangement. Cells. 2018;7(11):209. doi: 10.3390/cells7110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozu M, Alvear-Arias JJ, Fernandez M, Caviglia A, Peña-Pichicoi A, Carrillo C, Carmona E, Otero-Gonzalez A, Garate JA, Amodeo G, Gonzalez C. Aquaporin gating: a new twist to unravel permeation through water channels. Int J Mol Sci. 2022;23(20):12317. doi: 10.3390/ijms232012317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch. 2008;456(4):693–700. doi: 10.1007/s00424-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak MM, Tran T, Hong L, Joós B, Morris CE, Tombola F. The Hv1 proton channel responds to mechanical stimuli. J Gen Physiol. 2016;148(5):405–418. doi: 10.1085/jgp.201611672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Di Giorgio J, Soto G, Alleva K, Jozefkowicz C, Amodeo G, Muschietti JP, Ayub ND. Prediction of aquaporin function by integrating evolutionary and functional analyses. J Membr Biol. 2014;247(2):107–125. doi: 10.1007/s00232-013-9618-8. [DOI] [PubMed] [Google Scholar]
- Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 2002;418(6901):942–948. doi: 10.1038/nature00992. [DOI] [PubMed] [Google Scholar]
- Persat A. Bacterial mechanotransduction. Curr Opin Microbiol. 2017;36:1–6. doi: 10.1016/j.mib.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Pliotas C, Dahl ACE, Rasmussen T, Mahendran KR, Smith TK, Marius P, Gault J, Banda T, Rasmussen A, Miller S, Robinson CV, Bayley H, Sansom MSP, Booth IR, Naismith JH. The role of lipids in mechanosensation. Nat Struct Mol Biol. 2015;22(12):991–998. doi: 10.1038/nsmb.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorille A, Deamer D. Self-assembly and function of primitive cell membranes. Res Microbiol. 2009;160(7):449–456. doi: 10.1016/j.resmic.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Rao BD, Shrivastava S, Chattopadhyay A. Hydrophobic mismatch in membranes: when the tail matters. In: Chattopadhyay A, editor. Membrane Organization and Dynamics. Springer Series in Biophysics. Cham: Springer; 2017. [Google Scholar]
- Rasmussen T, Flegler VJ, Rasmussen A, Böttcher B. Structure of the mechanosensitive channel MscS embedded in the membrane bilayer. J Mol Biol. 2019;431(17):3081–3090. doi: 10.1016/j.jmb.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Rawicz W, Olbrich KC, McIntosh T, Needham D, Evans E. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys J. 2000;79:328–339. doi: 10.1016/S0006-3495(00)76295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy B, Bavi N, Lu A, Park Y, Perozo E. (2019) Molecular basis of force-from-lipids gating in the mechanosensitive channel mscs. ELife. 2019;8:e50486. doi: 10.7554/eLife.50486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard MN, Deniset JF, Kneesh AL, Blackwood D, Pierce GN. Mechanical stretching stimulates smooth muscle cell growth, nuclear protein import, and nuclear pore expression through mitogen-activated protein kinase activation. J Biol Chem. 2007;282(32):23081–23088. doi: 10.1074/jbc.M703602200. [DOI] [PubMed] [Google Scholar]
- Russ WP, Engelman DM. The GxxxG motif: A framework for transmembrane helix-helix association. J Mol Biol. 2000;296(3):911–919. doi: 10.1006/jmbi.1999.3489. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Sokabe M. Molecular dynamics study on protein–water interplay in the mechanogating of the bacterial mechanosensitive channel MscL. Eur Biophys J. 2015;44(7):531–543. doi: 10.1007/s00249-015-1065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Del Maŕmol J, MacKinnon R. Mechanistic basis for low threshold mechanosensitivity in voltage-dependent K+ channels. Proc Natl Acad Sci U S A. 2012;109(26):10352–10357. doi: 10.1073/pnas.1204700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider TD, Stephens RM. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 1990;18(20):6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LE, Mair DB, Narang JD, Feleke K, Lemmon CA. Fibronectin fibrillogenesis facilitates mechano-dependent cell spreading, force generation, and nuclear size in human embryonic fibroblasts. Integr Biol. 2015;7(11):1454–1465. doi: 10.1039/c5ib00217f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shealy RT, Murphy AD, Ramarathnam R, Jakobsson E, Subramaniam S. Sequence-function analysis of the K 1-selective family of ion channels using a comprehensive alignment and the KcsA channel structure. Biophys J. 2003;84(5):2929–2942. doi: 10.1016/S0006-3495(03)70020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhaye VK, Schweitzer KS, Caterina MJ, Shimoda L, King LS. Shear stress regulates aquaporin-5 and airway epithelial barrier function. Proc Natl Acad Sci U S A. 2008;105(9):3345–3350. doi: 10.1073/pnas.0712287105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigaut L, von Bilderling C, Bianchi M, Burdisso JE, Gastaldi L, Pietrasanta LI. Live cell imaging reveals focal adhesions mechanoresponses in mammary epithelial cells under sustained equibiaxial stress. Sci Rep. 2018;8(1):9788. doi: 10.1038/s41598-018-27948-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigaut L, Bianchi M, von Bilderling C, Pietrasanta LI. Correlation of cellular traction forces and dissociation kinetics of adhesive protein zyxin revealed by multi-parametric live cell microscopy. PLoS One. 2021;16(5):e0251411. doi: 10.1371/journal.pone.0251411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolin N, Li B, Beck DAC, Daggett V. Side-chain dynamics are critical for water permeation through aquaporin-1. Biophys J. 2008;95(3):1089–1098. doi: 10.1529/biophysj.107.125187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto G, Alleva K, Amodeo G, Muschietti J, Ayub ND. New insight into the evolution of aquaporins from flowering plants and vertebrates: orthologous identification and functional transfer is possible. Gene. 2012;503(1):165–176. doi: 10.1016/j.gene.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Soveral G, Macey RI, Moura TF. Membrane stress causes inhibition of water channels in brush border membrane vesicles from kidney proximal tubule. Biol Cell. 1997;89(5–6):275–282. doi: 10.1111/j.1768-322X.1997.tb01023.x. [DOI] [PubMed] [Google Scholar]
- Soveral G, Madeira A, Loureiro-Dias MC, Moura TF. Membrane tension regulates water transport in yeast. Biochim Biophys Acta Biomembr. 2008;1778(11):2573–2579. doi: 10.1016/j.bbamem.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Stansfeld PJ, Jefferys EE, Sansom MSP. Multiscale simulations reveal conserved patterns of lipid interactions with aquaporins. Structure. 2013;21(5):810–819. doi: 10.1016/j.str.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud M, Fahy E, Cotter D, Brown A, Dennis EA, Glass CK, Merrill AH, Murphy RC, Raetz CRH, Russell DW, Subramaniam S. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007;35(SUPPL. 1):D527–D532. doi: 10.1093/nar/gkl838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui H, Han BG, Lee JK, Walian P, Jap BK. Structural basis of water-specific transport through the AQP1 water channel. Nature. 2001;414(6866):872–878. doi: 10.1038/414872a. [DOI] [PubMed] [Google Scholar]
- Sukharev S, Durell SR, Guy HR. Structural models of the MscL gating mechanism. Biophys J. 2001;81(2):917–936. doi: 10.1016/S0006-3495(01)75751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutka M, Amodeo G, Ozu M. Plant and animal aquaporins crosstalk: what can be revealed from distinct perspectives. Biophys Rev. 2017;9(5):545–562. doi: 10.1007/s12551-017-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura I, Rosenbloom J, Macarak E, Chaqour B. Regulation of Cyr61 gene expression by mechanical stretch through multiple signaling pathways. Am J Physiol Cell Physiol. 2001;281(5):C1524–C1532. doi: 10.1152/ajpcell.2001.281.5.C1524. [DOI] [PubMed] [Google Scholar]
- Teese MG, Langosch D. Role of GxxxG motifs in transmembrane domain interactions. Biochemistry. 2015;54(33):5125–5135. doi: 10.1021/acs.biochem.5b00495. [DOI] [PubMed] [Google Scholar]
- Tombola F, Ulbrich MH, Isacoff EY. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 2008;58(4):546–556. doi: 10.1016/j.neuron.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombola F, Ulbrich MH, Kohout SC, Isacoff EY. The opening of the two pores of the Hv1 voltage-gated proton channel is tuned by cooperativity. Nat Struct Mol Biol. 2010;17:44–50. doi: 10.1038/nsmb.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Briggs MM, McIntosh TJ. Water permeability of aquaporin-4 channel depends on bilayer composition, thickness, and elasticity. Biophys J. 2012;103(9):1899–1908. doi: 10.1016/j.bpj.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Wu Z, Briggs MM, Schulten K, McIntosh TJ. The water permeability and pore entrance structure of aquaporin-4 depend on lipid bilayer thickness. Biophys J. 2016;111(1):90–99. doi: 10.1016/j.bpj.2016.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truelsen SF, Missel JW, Gotfryd K, Pedersen PA, Gourdon P, Lindorff-Larsen K, Hélix-Nielsen C. The role of water coordination in the pH-dependent gating of hAQP10. Biochim Biophys Acta Biomembr. 2022;1864(1):183809. doi: 10.1016/j.bbamem.2021.183809. [DOI] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeleur R, Niemietz C, Tilbrook J, Tyerman SD. Roles of aquaporins in root responses to irrigation. Plant Soil. 2005;274:141–161. doi: 10.1007/s11104-004-8070-z. [DOI] [Google Scholar]
- Vásquez V, Sotomayor M, Cordero-Morales J, Schulten K, Perozo E. A structural mechanism for MscS gating in lipid bilayers. Science. 2008;321(5893):1210–1214. doi: 10.1126/science.1159674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS. Aquaporins at a glance. J Cell Sci. 2011;124(13):2107–2112. doi: 10.1242/jcs.079467. [DOI] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7(4):265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Walz T, Hirai T, Murata K, Heymann JB, Mitsuoka K, Fujiyoshi Y, Smith BL, Agre P, Engel A. The tree-dimensional structure of aquaporin-1. Nature. 1997;387(6633):624–627. doi: 10.1038/42512. [DOI] [PubMed] [Google Scholar]
- Wan X, Steudle E, Hartung W. Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl2. J Exp Bot. 2004;55(396):411–422. doi: 10.1093/jxb/erh051. [DOI] [PubMed] [Google Scholar]
- Wang J, Lü D, Mao D, Long M. Mechanomics: an emerging field between biology and biomechanics. Protein Cell. 2014;5(7):518–531. doi: 10.1007/s13238-014-0057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu Y, DeBerg HA, Nomura T, Hoffman MT, Rohde PR, Schulten K, Martinac B. Selvin PR (2014) Single molecule FRET reveals pore size and opening mechanism of a mechano-sensitive ion channel. Elife. 2014;3:e01834. doi: 10.7554/eLife.01834.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Zhou M, Gu L, Yang H, Zhou Y, Li M. A novel gating mechanism of aquaporin-4 water channel mediated by blast shockwaves for brain edema. J Phys Chem Lett. 2022;13(11):2486–2492. doi: 10.1021/acs.jpclett.2c00321. [DOI] [PubMed] [Google Scholar]
- Wiggins P, Phillips R. Membrane-protein interactions in mechanosensitive channels. Biophys J. 2005;88(2):880–902. doi: 10.1529/biophysj.104.047431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Zeng B, Berner N, Frishman D, Langosch D, Teese MG. Experimental determination and data-driven prediction of homotypic transmembrane domain interfaces. Comput Struct Biotechnol J. 2020;18:3230–3242. doi: 10.1016/j.csbj.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaneff A, Sigaut L, Marquez M, Alleva K, Pietrasanta LI, Amodeo G. Heteromerization of PIP aquaporins affects their intrinsic permeability. Proc Natl Acad Sci U S A. 2014;111(1):231–236. doi: 10.1073/pnas.1316537111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaneff A, Vitali V, Amodeo G. PIP1 aquaporins: intrinsic water channels or PIP2 aquaporin modulators? FEBS Lett. 2015;589(23):3508–3515. doi: 10.1016/j.febslet.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Yano Y, Kondo K, Watanabe Y, Zhang TO. Ho JJ, Oishi S, Fujii N, Zanni MT, Matsuzaki K. GXXXG-mediated parallel and antiparallel dimerization of transmembrane helices and its inhibition by cholesterol: single-pair FRET and 2D IR studies. Angew Chem. 2017;56(7):1756–1759. doi: 10.1002/anie.201609708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano Y, Morise T, Matsuzaki K. Effects of Gly residue and cholesterol on the GXXXG-mediated parallel association of transmembrane helices: a single-pair FRET study. ChemBioChem. 2022;23(23):e202200160. doi: 10.1002/cbic.202200160. [DOI] [PubMed] [Google Scholar]
- Ye Q, Wiera B, Steudle E. A cohesion/tension mechanism explains the gating of water channels (aquaporins) in Chara internodes by high concentration. J Exp Bot. 2004;55(396):449–461. doi: 10.1093/jxb/erh040. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Sokabe M. Mechanosensitivity of ion channels based on protein-lipid interactions. J Roy Soc Int. 2010;7(SUPPL. 3):S307–S320. doi: 10.1098/rsif.2010.0095.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Batiza A, Schroeder M, Blount P, Kung C. Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophys J. 1999;77(4):1960–1972. doi: 10.1016/S0006-3495(99)77037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Batiza A, Kung C. Chemically charging the pore constriction opens the mechanosensitive channel MscL. Biophys J. 2001;80(5):2198–2206. doi: 10.1016/S0006-3495(01)76192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidel ML, Ambudkar SV, Smith BL, Agre P. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry. 1992;31(33):7436–7440. doi: 10.1021/bi00148a002. [DOI] [PubMed] [Google Scholar]
- Zeidel ML, Nielsen S, Smith BL, Ambudkar SV, Maunsbach AB, Agre P. Ultrastructure, pharmacologic inhibition, and transport selectivity of aquaporin channel-forming integral protein in proteoliposomest. Biochemistry. 1994;33(6):1606–1615. doi: 10.1021/bi00172a042. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Daday C, Gu RX, Cox CD, Martinac B, de Groot BL, Walz T. Visualization of the mechanosensitive ion channel MscS under membrane tension. Nature. 2021;590(7846):509–514. doi: 10.1038/s41586-021-03196-w. [DOI] [PubMed] [Google Scholar]
- Zhou D, Lee HS, Villarreal F, Teng A, Lu E, Reynolds S, Qin C, Smith J, Sung KL. Differential MMP-2 activity of ligament cells under mechanical stretch injury: an in vitro study on human ACL and MCL fibroblasts. J Orthop Res. 2005;23(4):949–957. doi: 10.1016/j.orthres.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Zhou DW, Lee TT, Weng S, Fu J, García AJ. Effects of substrate stiffness and actomyosin contractility on coupling between force transmission and vinculin-paxillin recruitment at single focal adhesions. Mol Biol Cell. 2017;28(14):1901–1911. doi: 10.1091/mbc.E17-02-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]