Abstract
Under macromolecular crowding (MC) conditions such as cellular, extracellular, food and other environments of biotechnological interest, the thermodynamic activity of the different macromolecules present in the system is several orders of magnitude higher than in dilute solutions. In this state, the diffusion rates are affected by the volume exclusion induced by the crowders. Immiscible liquid phases, which may arise in MC by liquid–liquid phase separation, may induce a dynamic confinement of reactants, products and/or enzymes, tuning reaction rates. In cellular environments and other crowding conditions, membranes and macromolecules provide, on the whole, large surfaces that can perturb the solvent, causing its immobilisation by adsorption in the short range and also affecting the solvent viscosity in the long range. The latter phenomenon can affect the conformation of a protein and/or the degree of association of its protomers and, consequently, its activity. Changes in the water structure can also alter the enzyme–substrate interaction, and, in the case of hydrolytic enzymes, where water is one of the substrates, it also affects the reaction mechanism. Here, we review the evidence for how macromolecular crowding affects the catalysis induced by hydrolytic enzymes, focusing on the structure and dynamics of water.
Keywords: Hydrolases, Macromolecular crowding, Membrane-water interface, Mesoporous matrix, Water structure, Water spin isomers
On the macromolecular crowding concept
The natural environment for proteins and enzymes both with intracellular and extracellular location is characterised by the presence of large amounts of different types of macromolecules that can affect their activities. Instead, activity and structure studies are still usually evaluated in dilute media, at concentrations far from the media in which the enzymes are actually found. The fact that high concentrations of macromolecules can affect the behaviour of proteins was first proposed by Laurent and Ogston (1963) and recently reviewed by Cammarata et al. (2023). Lauren and Ogston (1963), observed a deviation from ideality in the osmotic pressure of albumin–hyaluronic acid solutions. They proposed that this deviation was the result of the “exclusion” of albumin from the volume of the solution occupied by hyaluronic acid. However, these concepts did not become relevant until the 1980s when Allen Minton developed the principles of molecular crowding (MC) (Minton 1981). Despite the relevance and recognition of these concepts, currently only a small percentage of research is designed to take MC into account as a necessary requirement to simulate physiological conditions.
Initially, the MC approach emphasised the effect of the excluded volume, a physical non-specific effect based on steric repulsion (Ellis 2001). Volume exclusion theory predicts that native states are favoured over unfolded states due to the smaller volume of the former; this would be important for the initial collapse of the polypeptide chain, both for those being synthesised and for those being refolded in vitro from an unfolded state. In addition to stabilising the native state, the excluded volume increases the effective concentration of macromolecules. This has thermodynamic and kinetic consequences on macromolecular properties. MC affects processes that depend on available volume, including protein aggregation, which is generally increased compared to dilute conditions (Siddiqui and Naeem 2023). However, it is important to note that protein aggregation is influenced by various factors such as the specific model protein, the crowding agent used, and the conditions inducing the partially folded state.
The diffusion coefficient is also affected in MC conditions: it is reduced by a factor of up to 10 compared to dilute media, depending on particle size and media occupancy (Nenninger et al. 2010). This effect will affect both large and small molecules, but the diffusion of large molecules will be more affected. Any diffusion-limited process such as enzyme–substrate binding or oligomerisation will be affected (Nolan et al. 2020).
MC systems consist of membranes and macromolecules that provide, on the whole, large surfaces, with which water can interact (Burgos et al. 2016, 2019; Clop et al. 2012, 2014). As a result, changes in solvent structure and solvent availability can occur, which can affect protein structure, stability and activity (Burgos et al. 2016, 2019; Eggers and Valentine 2001b; Nolan et al. 2015, 2019). The hydrophobic effect is the driving force for protein folding: the tendency of non-polar amino acids to avoid contact with water causes them to hide inside the hydrophobic protein core, releasing water molecules and reducing free energy. MC reduces the free water content and then the hydrophobic effect is less pronounced thus, the protein fold is destabilised (Némethy and Scheraga 1962). In the case of enzymes, their activity can also be modified by reducing the hydrophobic enzyme–substrate interaction. In particular, for hydrolases, where water acts as both solvent and substrate, the effect of water structure on their function is greater (Burgos et al. 2016).
In addition to the general effects of macromolecular crowding on proteins and enzymes, certain aspects of water would play a crucial role in enzymatic activity and protein folding processes. Specifically, the spin isomers of water, known as ortho and para water, have garnered interest due to their distinguishable physical and chemical properties (Bunkin et al. 2005; Tikhonov and Volkov 2002). The widely known water model (Kontogeorgis et al. 2022; Neophytou et al. 2022; Nilsson and Pettersson 2011), which proposes a mixture of two types of liquid water and has recently been validated by experimental evidence (Huang et al. 2009; Kontogeorgis et al. 2022; Nilsson and Pettersson 2011; Pershin et al. 2010), has been linked to the spin isomers of water, ortho and para (Pershin 2013). It has been proposed that the para isomer, due to its ability to not rotate, would be the most abundant water subtype that would form more rigid structures. However, to date, relatively little is known about the specific role of water spin isomers in biological processes.
In this context, the aim of this review was to compile and analyse the information existing related to the role of water, and its molecular structure, in protein structure and activity, particularly hydrolytic catalysis, in conditions of different models of MC.
Water structured by macromolecular crowding and confinement
In solution
Various macromolecules are added to solutions to simulate crowding conditions: dextran, ficoll, PEG and albumin are some examples. These crowders have different characteristics in terms of flexibility, linearity and compactness (Venturoli and Rippe 2005) and could then have different effects on solution properties such as diffusion impairment and confinement. In addition, the structure and availability of water as a solvent may be affected in the presence of a crowding agent, with short and long range effects. As an example, Clop et al. (2012), through nuclear magnetic resonance spin–lattice relaxation time (T1) measurements performed with spectral resolution in aqueous solutions of PEG6000, demonstrated the presence of two contributing components identified in each proton system, PEG and water, presenting values of T1 with very different orders of magnitude. The results were consistent with the presence of an ordered and dehydrated PEG structure (folded and/or self-assembled) (short T1). The latter was in equilibrium with a more flexible monomer structure (long T1) with the ability to influence, in a concentration dependent manner, the global hydrogen bonding network of the entire aqueous solution. On the other hand, it was shown that particle size and the ratio of 1H proportions associated with the long T1 values of water and PEG6000 (PH2O/PPEG) followed a similar trend as a function of PEG6000 concentration, both showing a maximum at 15–20% w/v PEG6000. This suggests that, on the one hand, particles grow with PEG6000 concentration and, after reaching a maximum size, higher concentrations lead to an increase in their compactness at the expense of size reduction. These particles are dispersed in a medium with an increasing amount of immobilised solvent and possibly an increasing viscosity, as can be deduced from the long T1 component of the water signal, described by Clop et al. (2012) (Fig. 1a).
Fig. 1.

Effect of PEG6000 concentration on water and PEG structures. a Size of PEG particles as a function of PEG6000 concentration (●) and ratio of 1H proportions associated with the long T1 values of water and PEG 6000. Reprinted with permission from J. Phys. Chem. B., 116:11,953 − 11,958. Clop et al. “1H and 2H NMR spin–lattice relaxation probing water: PEG molecular dynamics in solution” (2012). Copyright 2012 American Chemical Society. b At growing PEG6000 concentration increases the amount of PEG aggregates (dark ball-shaped structures) in equilibrium with PEG monomers (short curved lines) and structured water (reflected by the intensity of blue around the ball-shaped structures as well as in the bulk of the solution). Adapted with Permission from Biochemical and Biophysical Research Communications, 515:190–195. Nolan et al., “Dual substrate/solvent-roles of water and mixed reaction–diffusion control of β-Galactosidase catalysed reactions in PEG-induced macromolecular crowding conditions” (2019). Copyright 2019 Elsevier Inc
Liquid–liquid phase separation in macromolecular crowded media
Alexander Oparin proposed that life originated as coacervate drops of organic matter (Oparin 1938). However, Oparin’s coacervate idea lost support because it failed to account for the membrane barriers that all cells use to separate the inside from the outside, and that eukaryotic cells use to further compartmentalise their cellular biochemistry inside membrane-bound organelles (Gomes and Shorter 2019). The organisation of cellular components into membraneless organelles, suggests that the idea of Oparin’s coacervate deserves a second look (Hyman et al. 2014; Zhang et al. 2020). In biology, liquid–liquid phase separation (LLPS) provides a means for concentrating and segregating cellular components in a spatiotemporally defined manner for diverse functional processes. The phase-separated condensates are also referred to as aggregates, bodies, granules and membraneless compartments (Zhang et al. 2020). These intracellular liquid droplets are highly dynamic allowing rapid exchange of their components with the cellular environment. As such, they are involved in the regulation of multiple spatiotemporal cellular functions, ranging from heterochromatin reorganisation to innate immune signalling (Alberti et al. 2019; Hyman et al. 2014). The characteristics of the intermolecular interactions have been reviewed by Rodríguez et al. (2023).
The physico-chemical basis of LLPS can be summarised as follows. Multi-component systems often tend to mix spontaneously and are then found in a homogeneous mixed state. However, liquids can also demix and undergo liquid–liquid phase separation (LLPS). The thermodynamics of LLPS can be described by the Flory–Huggins polymer mixing model (Callaway et al. 2018). The free energy of mixing (ΔmixG) is negative in ideal solutions, because the mixing entropy (ΔmixS) is positive and the interaction enthalpies ΔmixH = 0. In non-ideal solutions, ΔmixH can be different from zero, and the process is endothermic enough to overcome the entropic term and favour the de-mixed state. In the case of polymeric solutes which have numerous interaction sites and therefore a much lower entropic contribution than low molecular weight solutes, non-ideality can be reached by simple coacervation. The mechanism of phase separation by nucleation-growth of dense droplets (coacervates) is schematised in Fig. 2. Points along the black line correspond to minima in the ΔmixG vs. volume fraction plot (note the use of volume fraction instead of mole fraction to account for the diversity of molecular sizes).
Fig. 2.

Phase diagram. Changes in physical parameters (temperature, pH, etc.) as a function of polymer concentration (c) may be found in one or two phases above and below the black line, respectively. Light (L) and dense (D) one phase regions are indicated with the numbers 1 and 5, respectively. Phase separation does not occur beyond the critical point. Within the two phase coexistence region (below the black line), the concentration of the polymer in each phase cL and cD, at the defined value of the parameter, can be determined from each intersection of the tie line (orange) with the black line. At point 3, the ratio between the segments (c3-cL) = D and (cD-c3) = L and the whole length T of the tie line (D/T and L/T) represent the volume fraction of each phase. Bottom panels: Sliding along the tie line from left to right, the system changes from a single L phase (1), then a D phase dispersed in L (2), L liquid droplets grow (3), the system percolates showing L droplets dispersed in D phase (4) and at c > cD the system exhibits a single D phase (5). Adapted with permission from Cell,176 (3), 419–434. Alberti et al. “Considerations and Challenges in Studying Liquid–Liquid Phase Separation and Biomolecular Condensates” (2019). Copyright 2019 Elsevier Inc
Another mechanism, spinodal decomposition, is not described in the present work for the sake of simplicity, but detailed analysis can be found elsewhere (Favvas and Mitropoulos 2008; Vweza et al. 2021). However, it is worth mentioning that in spinodal decomposition phase separation takes place homogeneously throughout the material (unlike in the case of nucleation and growth, which occur at specific positions in the material). This leads to a periodic variation of the composition with distance and involves fluctuations (non-equilibrium situations) that prevent the use of thermodynamics to study them (Bai et al. 2020; Vweza et al. 2021). We hypothesise that fluctuations in LLPS in the spinodal regime may underlie the observed water fluctuation dynamics coupled to glycolytic oscillations (Thoke et al. 2015), linking biochemistry and physics from the theoretical and experimental viewpoints.
At the membrane–water interface
It is generally accepted that water near hydrophilic interfaces is organised into ice-like water that protrudes a few nanometres from the surface (Yoo et al. 2011).
In cellular environments, macromolecular crowding within and near the membrane can be exerted by cytoplasmic and ectoplasmic soluble proteins and polysaccharides, intrinsic membrane-bound proteins, cytoskeletal proteins, some of which are covalently bound to the plasma membrane from the inner leaflet, and by the oligosaccharides covalently bound to the glycolipids and glycoproteins of the outer membrane hemilayer that face the cell from the outside (Bezanilla et al. 2015; Chighizola et al. 2022; Frantz et al. 2010).
Under crowding conditions, proteins can accumulate at the membrane interface favoured by the excluded volume in the aqueous phase. Soluble proteins and crowders that do not associate with the membrane are excluded from the interface while proteins concentrated at the interface tend to induce protein–lipid and protein–protein interactions, oligomerization and/or aggregation (r.f. (Löwe et al. 2020).
The membrane alone, or through membrane-associated proteins, can influence dynamic changes in the cytoskeleton (Bezanilla et al. 2015), and this can be expected to affect water dynamics (Lenormand et al. 2011).
When proteins contact lipid headgroups, molecular dynamics simulations show that, on average, small but statistically significant local membrane curvatures are induced (Nawrocki et al. 2019). Experimentally, using lipid bilayers and cell models interacting with nanopatterned surfaces, bulky intrinsic membrane glycoproteins have been shown to avoid positively curved internal membrane leaflets (membrane invaginations) and to accumulate on membrane protrusions with a preference reversal after oligosaccharide tail cleavage (Lu 2023). This behaviour is consistent with the well-known effects on membrane curvature induced by membrane expansion at the polar headgroup region. This has been observed for cone- or inverted cone-shaped membrane components organised in lamellar or inverted phases (Perillo et al. 1994), up to the binding of small molecules that favour the outer or inner membrane hemilayer, inducing membrane protrusions or invaginations, respectively (García et al. 2000), and with the length of the polymeric tail of lipopolymer–phospholipid mixtures that self-organise into smaller versicles or even micellar structures as the length of the tail increases (Clop et al. 2014). The surface expansion associated with increased membrane curvature affects interfacial hydration in the vicinity of hydrophobic spherical solutes (Weiß et al. 2015) and penetrates deeper into the membrane (r.f. (Disalvo et al. 2015).
Self-organised lipid molecules form the basis of a lamellar membrane structure. Phospholipid carboxyl and phosphate groups exposed to the membrane–water interface are hydration sites surrounded by a tightly bound shell of water molecules, which contribute most to the dipole potential, and another water layer that can be displaced by proteins, osmotic stress and the presence of polyhydroxylated compounds (e.g. (Disalvo et al. 2008). The surrounding solvent shells of membranes and peripherally bound proteins are structurally and dynamically coupled (Fisette et al. 2016), so this fact may provide some clues to understand the change in the kinetics of reactions catalysed by soluble hydrolases up to the binding to membrane water interfaces (Clop et al. 2008; Sánchez et al. 2013; Sanchez and Perillo 2000, 2002a, b).
In the plasma membrane of every cell, close to the membrane-water interface, there is a glycocalyx (GC), which is a dense matrix of carbohydrate residues covalently linked to glycolipids or to membrane-bound glycoproteins (Alberts et al. 2008; Chighizola et al. 2022; Möckl 2020) which can be considered a molecularly crowded environment.
A model biomembrane with a simulated glycocalyx was prepared with binary mixtures of a phospholipid (dipalmitoylphosphatidylcholine, dpPC) and a lipopolymer (polyethylene glycol, PEGn, where n refers to the molecular mass of PEG covalently linked to the polar group of dipalmitoylphosphatidylethanolamine, PE) in different proportions (Clop et al. 2014). The molecular dynamics at the different membrane levels were investigated by spectral resolution 1H spin–lattice relaxation time (T1) and 31P-NMR spectroscopy analysis, with a focus on the molecular crowding conditions at the lipid–water interface. The 1H-NMR relaxation times revealed two contributing components in each proton system (PEG, phospholipids and water) for all the mixtures studied, exhibiting two values of T1 with very different orders of magnitude. Using the water as an example, one of the populations showed a faster dynamic (T1A), and the other showed a slower molecular motion (T1B). The long T1A were between 16.8 and 20 s for water protons, around 20–30 s for PEG protons, and in the range of 8–19 s for phospholipid polar head group (PH) and hydrocarbon chains (HC) (Fig. 3a). In contrast, T1B in the range of 0.03–0.22 s were found for water, while for PEG, PH and HC the values were between 0.24 and 0.86 s. As a reference, it is important to recall that T1 of 22 s corresponding to pure bulk water had been measured previously (Schneider et al. 2002). The T1B values corresponding to water showed a decreasing tendency as a function of the size of the PEG moiety, reflecting a motion restriction that increases with n and was attributed to confined water molecules. Interestingly, the proportion of immobilised water decreases with n (Fig. 3b). This may be explained by a reduction in the size of the compartment containing the pool of confined water and is consistent both with the fact that, as explained above, high PEG concentration in solution leads to compact and dehydrated aggregates (Clop et al. 2012), and with the gelation/dehydration of the polymer layer described in PE-PEG Langmuir films with growing polymer tail and up on compression (Clop et al. 2016) (Fig. 3c).
Fig. 3.

Long and short relaxation time values in PEGylated phospholipids, as a function of PEG chain length, n, obtained by fitting a two-exponential function to the magnetization (MX(t)), where X is H2O, PEG, HC or PH: a long relaxation times, T1A; b short relaxation times,. T1B; c scheme of lipopolymers with decreasing polymer tails (from left to right) and similar hydrophobic chains, at the air–water interface and at the closest packing. (a) and (b) reprinted with permission from J. Phys. Chem., B 118:6150–6158 Clop et al. “Water and Membrane Dynamics in Suspensions of Lipid Vesicles Functionalized with Poly(ethylene glycol)s” (2014). Copyright ©2014 American Chemical Society
Water dynamics coupled to cellular metabolism
The most complete theory based on concepts from colloidal physical chemistry is the association-induction hypothesis (AIH), introduced by Gilbert N. Ling in 1962. Concerning the state of water in the cell, the AIH incorporated the so-called polarised-oriented multilayer theory of cellular water (Ling 1987, 2003) which is cooperatively modulated by metabolic activity (e.g. ATP levels).
Experimental work performed in vitro and in vivo systems has shown that most of intracellular water exists in a polarised state when cells are in a resting state (Ling 1963, 1987, 2003).
Interestingly, water polarisation and metabolic substrates can enter an oscillatory regime. Analysis of the oscillations of the 6-acyl-2-(dimethylamino) naphthalene (DAN) probes are consistent with the intracellular environment behaving as a responsive hydrogel, a view with very strong experimental support (Fels et al. 2009).
Furthermore, given that the polymerization/depolymerization of cytoskeletal structures is strongly dependent on ATP and ATPase activity (Korn et al. 1987; Leterrier 2001; Stark et al. 2011), it is reasonable to assume that ATP acts on the overall state of the cytoskeleton and that this affects the dipolar relaxation of the aqueous phase, possibly through changes in viscoelastic properties. This is supported by experiments in which actin polymerization was disrupted by the addition of latrunculin B, an inhibitor of actin polymerization, during the oscillatory glycolytic regime; in this condition the oscillations coupled between metabolite concentrations and both water dipolar relaxation and intracellular K+ oscillations were eliminated (Olsen et al. 2020; Thoke et al. 2017).
Interestingly, the analysis of the fluctuation dynamics of lipophilic probes (Nile red and LAURDAN) which partition in hydrophobic membrane environments, and hydrophilic ACDAN, which senses water polarisation, revealed that the polarised state of cellular water can be transferred from the cytoplasm to the water present at the interface of membrane structures (Bagatolli et al. 2019; Thoke et al. 2017).
These observations provide a link and an integrated view of lipid membranes and membraneless organelles.
In mesoporous matrices
Protein immobilisation by encapsulation in mesoporous glasses using sol–gel techniques is of particular interest for the synthesis of biosensors (Dave et al. 1994; Depagne et al. 2011; Gupta and Chaudhury 2007) and as an experimental model for understanding the molecular crowding phenomenon (Eggers and Valentine 2001a, b; Frenkel-Mullerad and Avnir 2005; Mittal and Best 2008; Ravindra et al. 2004). Silicate synthesis by the sol–gel process begins when an organo-metallic precursor (TMOS or TEOS) is hydrolysed in acidic or basic media, and the silanol molecules produced condense in a second reaction to form a Si–O network with a gel macroscopic state (Fig. 4) (Brinker and Scherer 2013).
Fig. 4.
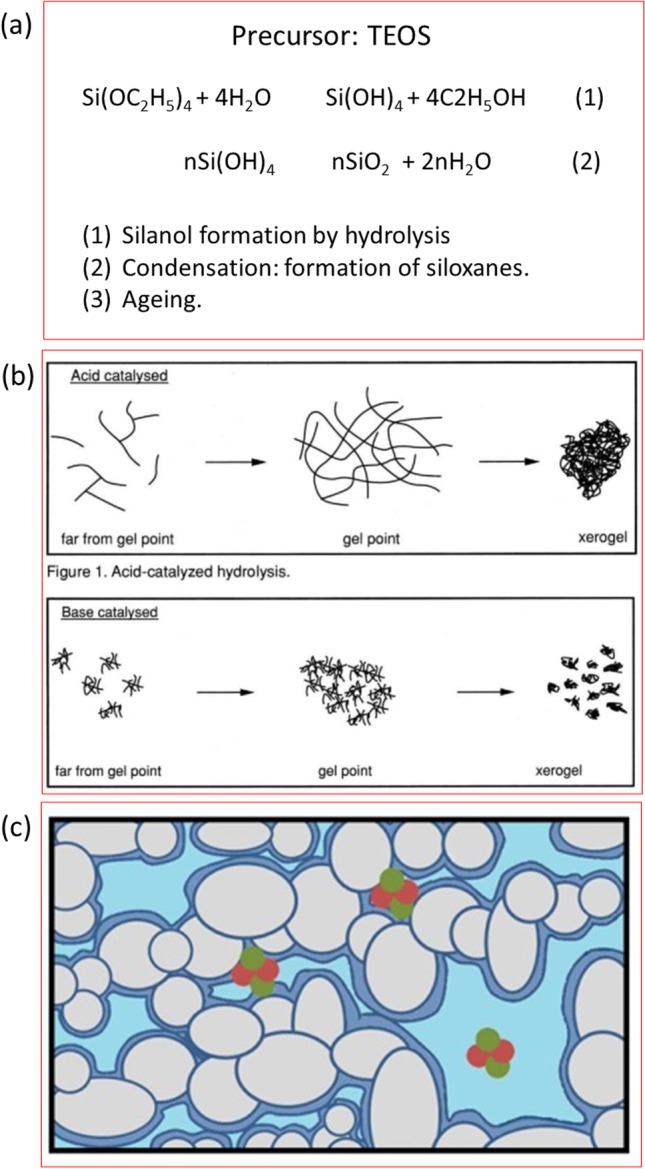
Synthesis of a silicate matrix by the sol–gel method. a The precursor is tetraethyl ortho silane (TEOS). In a first step, TEOS is hydrolysed to form a sol containing free silanol groups and in a second step silanols starts the polymerization to form the gel. The amount of covalent bonds increases over time (third step named ageing) and at the same time the mean size of the gel pores decreases. b The properties of gels produced in acid and basic media are different. c Scheme of interconnected pores. A layer of fixed solvent molecules (dark blue) and bulk solvent in the middle of the pore (light blue) are shown. If the reaction is performed in the presence of a protein it can be trapped inside the pores. Adapted with permission from (a and b) Journal of Chemical Education, 71:599. Buckley AM and Greenblatt M. “The sol–gel preparation of silica gels” (1994). Copyright ©1994 American Chemical Society and (c), Scientific Reports, 6:36,593. Burgos et al. “Environmental topology and water availability modulates the catalytic activity of β-galactosidase entrapped in a nanosporous silicate matrix” (2016). Copyright ©2016 with permission from the authors
As condensation proceeds, macromolecules such as enzymes are trapped, and small molecules and ions can move through the porous network. Previous studies have shown that encapsulated proteins retain most of their native conformation (Edmiston et al. 1994; Ellerby et al. 1992; Wu et al. 1993), their native spectroscopic properties and certain functional features (Bhatia et al. 2000; Burgos et al. 2019; Kao et al. 2014; Lloyd and Eyring 2000; Pastor et al. 2007). The entrapment of protein molecules in the sol–gel matrix appears to occur because the silicate polymerizes around the biomolecule, physically trapping it in the growing oxide network (Lan et al. 1999).
The macromolecule in this state would experience a very different environment to that found in dilute solutions. Confinement in nanopores would induce diffusion and interfacial effects, local concentration inhomogeneities of the reactants and changes in the properties of the solvent. There are several experimental and simulation studies of confined water in nanopores (Bonnaud et al. 2010; Stapf and Kimmich 1995; Takamuku et al. 1997; Zimmerman et al. 1956), which have shown that water molecules exhibit properties quite different from bulk water. At least two behaviours of water molecules can be distinguished inside the pore. One population corresponds to bound water molecules, i.e. molecules strongly adsorbed to a layer close to the surface, with reduced mobility and specific orientations towards the interface. The other population corresponds to free water molecules, i.e. molecules that are less influenced by the pore walls and have properties close to the bulk values.
The surface to volume ratio in the silicate gel is high, so most water molecules may have restricted degrees of freedom and/or be in a higher energy state compared to bulk water (Price 2009). This phenomenon could lead to different kinetics in processes where water is involved in the rate-determining step of the reaction. Investigation of hydrodynamics in silicate gels using the water proton transverse relaxation time (T2) of 1H-NMR revealed that water molecules can be categorised into three distinct mobility pools (Burgos et al. 2016). The three T2 components (T2a, T2b and T2c) were interpreted as water in the first hydration sphere of the silicon polymer (T2a; 4.8 ms), water within the gel structure (T2b; 40 ms) and water in larger pores or cracks of the gel sphere (T2c ∼ 600 ms). Similar values have been obtained for other materials (Bortolotti et al. 2012). The silicate gels were aged for 7 and 15 days. As the gel aged, the magnitude of the three T2 components decreased. T2 values measured in other polymer networks varied with changes in the matrix structure of the gel (Giussi et al. 2015). Thus, the reduction in relaxation due to increased cross-linking of the silicate matrix can be explained by limiting the movement of different groups of water molecules. These changes in water dynamics would explain the different kinetics of β-galactosidase in fresh and aged gels for the hydrolysis of ONPG and PNPG, which have different rate-limiting steps (Table 1) (Burgos et al. 2016).
Table 1.
Effect of molecular crowding and membrane bound enzymes on the catalytic behaviour of the hydrolytic reaction
| Hydrolase | Substrate | Crowding agent | Km change | kcat or Vmax change | Catalytic efficiency | Technique | Limiting step of the reaction | Reference |
|---|---|---|---|---|---|---|---|---|
| Acylphosphatase 1 humane | Benzoyl phosphate | Ficoll 200 g/L | No change | kcat /3 | (kcat/Km)/2.9 | Spectrophotometry (BP) 283 nm | Nucleophilic water molecule attack (Paoli 2003) | Vopel and Mahkatadze 2012 Plos One |
| ADP-pyrophosphatase E. coli | ADP-glucose | PEG-6000 50 g/L | Km /3.3 | Vmax *6 | (Vmax/Km)*20 | Spectrophotometry | N/D | Morán-Zorzano et al. 2007 FEBS Lett |
| Isochorismatase E. coli | Isochorismate | Ficoll 70 30% (300 g/L) | Km/1.6 | kcat*1.12 | (kcat/Km)*1.83 |
Spectrophotometry; coupled reactions |
A step involving hydrogen transfer is partially rate limiting (Rusnak 1990) |
Jiang and Guo 2007 JACS |
| Trypsin | Dextran 40,000 257 and 86 g/L | Km ↓ | N/D | Enzyme deacylation water mediated step (Bender 1962) | Laurent 1971 Eur.J.Biochem | |||
| Trypsin | Nα-benzoyl-l-arginine ethyl ester (BAEE) |
EG 100 g/L EG 200 g/L EG 300 g/L PEG 600 100 g/L PEG 600 200 g/L PEG 600 300 g/L PEG 6000 100 g/L PEG 6000 200 g/L PEG 6000 300 g/L |
No change No change Km*1.3 Km/1.2 Km*1.2 Km*2 No change Km*2.5 Km*2 |
No change No change No change No change No change No change Vmax/1.3 Vmax/1.5 Vmax/2 |
No change (kcat/Km)*1.1 (kcat/Km)*1.1 kcat/Km)*1.2 No change (kcat/Km)/1.6 (kcat/Km)/1.1 (kcat/Km)/3.7 (kcat/Km)/6.5 |
Multiple injections ITC assays | Enzyme deacylation water mediated step (Bender 1962) |
Maximova et al. 2019 Analycal Chem |
| Nα-benzoyl-DL-arginine β-naphthylamide (BANA) |
PEG 600 100 g/L PEG 600 200 g/L PEG 600 300 g/L |
Km*1.6 Km*1.6 Km*3.1 |
No change No change Vmax*1.4 |
(kcat/Km)/1.6 (kcat/Km)/40 (kcat/Km)/64 |
multiple injections ITC assays | |||
|
PEG 6000 100 g/L PEG 6000 200 g/L PEG 6000 300 g/L |
Km*6.4 N/A N/A |
Vmax*2.4 N/A N/A |
(kcat/Km)/2.7 | |||||
|
PEG 600 100 g/L PEG 600 200 g/L PEG 600 300 g/L |
Km*2.0 Km*3.7 Km*6.6 |
Vmax/1.4 Vmax/2.1 Vmax/1.9 |
(kcat/Km)/2.9 (kcat/Km)/7.9 (kcat/Km)/12 |
Fluorescence assay | ||||
|
PEG 6000 100 g/L PEG 6000 200 g/L PEG 6000 300 g/L |
Km*1.5 Km*1.5 N/A |
Vmax/2.1 Vmax/2.4 N/A |
(kcat/Km)/3.2 (kcat/Km)/3.7 |
|||||
| Protease NS3/4A Hepatitis C virus | RET S FRET peptide |
PEG 600 50 g/L 100 g/L 200 g/L PEG 6000 50 g/L 100 g/L 200 g/L Ficoll 40 50 g/L 100 g/L 200 g/L |
Km/1.8 Km/2.9 Km*1.2 Km*1.7 Km*1.9 Km/2.2 Km/2.9 Km*1.1 Km*1.3 |
kcat/1.4 kcat/1.6 kcat/1.4 kcat/1.4 kcat/1.7 kcat/4.3 kcat*6.6 kcat*7.9 kcat*2.5 |
(kcat/Km)*1.3 (kcat/Km)*1.8 (kcat/Km)/1.7 (kcat/Km)*1.2 (kcat/Km)*1.1 (kcat/Km)/1.8 (kcat/Km)*7.7 (kcat/Km)*6.0 (kcat/Km)*6.3 |
Fluorescence assay | N/D |
Popielec et al. 2020 Biochimie |
| Organophosphorus hydrolase | Paraoxon | Functionalized mesoporous silica (30 nm) confinement | 200% increase | Spectrophotometry |
Fan 2019 Water molecules are involved in the multistep mechanism from the beginning of the reaction |
Lei et al. 2008 Nanotech |
||
| Pancreatic lipase | TG C18:1 |
Ficoll400 1 g/L 100 g/L Dextran20 1 g/L 100 g/L PEG 2000 1 g/L 100 g/L |
Km/2.3 Km/1.9 Km/1.6 Km*1.5 Km/1.7 Km/2 |
kcat/1.3 kcat/1.7 kcat/2 kcat/1.1 kcat/1.3 kcat/1.7 |
(kcat/Km)*1.7 (kcat/Km)*1.1 (kcat/Km)/1.3 (kcat/Km)/1.6 (kcat/Km)*1.4 (kcat/Km)*1.2 |
Multiple injections ITC assays |
Deacylation step, water nucleophilic attack Semeriva 1974, Park 2005 (soluble substrates) Activation with the presence of surfaces |
Zhang 2009 Thermochim. Acta |
| TG C8 |
Ficoll400 1 g/L 100 g/L Dextran20 1 g/L 100 g/L PEG 2000 1 g/L 100 g/L |
Km/1.9 Km/3.8 Km*1.8 Km/1.3 Km/2.1 Km/2.1 |
kcat/1.6 kcat/1.8 kcat*1.3 kcat/1.8 kcat/1.2 kcat*1.2 |
(kcat/Km)/1.2 (kcat/Km)*2.1 (kcat/Km)/1.4 (kcat/Km)/1.4 (kcat/Km)*2.4 (kcat/Km)*1.8 |
||||
| β-Galactosidase E. coli | ONPG |
Silicate matrix confinement 0d 7d 15d |
Km (b) Km*14 Km*17 Km*8 |
Vmax (b) kcat*3 kcat*5 kcat*6 |
(kcat/Km)/3.6 (kcat/Km)/2.9 (kcat/Km)/1.2 |
Spectrophotometry |
Water nucleophilic attack to the galactosyl residue bound to the enzyme Viratelle 1969, Viratelle 1973, Tenu 1971 |
Burgos et al. 2016 Sci.Rep |
| PNPG |
Silicate matrix confinement 0d 3d 7d 15d |
No change Km*2 Km*2 Km*2 |
No change No change No change No change |
(kcat/Km)/2 (kcat/Km)/2 (kcat/Km)/2 |
Aglycone cleavage Viratelle 1969, Viratelle 1973, Tenu 1971 |
|||
| β-Galactosidase E. coli | ONPG |
PEG 6000 150 g/L 250 g/L 350 g/L |
(b) Km*3 Km*10 Km*12 |
(b) No change No change No change |
(kcat/Km)/4.4 (kcat/Km)/11 (kcat/Km)/13 |
Spectrophotometry |
Nolan et al. 2019 BBRC |
|
| PNPG |
PEG 6000 150 g/L 250 g/L |
Km/1.5 Km*2 |
Kcat/1.2 no change |
(kcat/Km)*1.2 (kcat/Km)/2.2 |
||||
|
β-Galactosidase K. lactis (same sigmoidal behaviour was found with lactose) |
ONPG |
PEG 6000 150 g/L 250 g/L 350 g/L |
Km/2 Km/2 Km*10 |
Vmax/2 Vmax*1.5 Vmax*4 |
no change (kcat/Km)*2 (kcat/Km)/3.8 |
Spectrophotometry |
Nolan et al. 2020 Foods and Colloids |
|
|
β-Galactosidase E. coli |
Lactose |
Inclusion bodies P1 |
Km*3 | Vmax*5 | (kcat/Km)*2 | Spectrophotometry |
Flores et al. 2019 Colloids and surfaces Biomembranes |
|
|
β-Galactosidase K. lactis Sigmoidal at 35 mN/m |
ONPG |
Incorporated in Langmuir-Blodget Films 15mN/m 35mN/m |
Km*1.8 Km*1.8 |
Vmax/4000 Vmax/19000 |
(kcat/Km)/7690 (kcat/Km)/38450 |
spectrophotometry |
Clop et al. 2008 Langmuir |
|
|
β-Galactosidase E. coli |
ONPG |
Membrane Interface SB PC MLVs SB PC + 5% chol SB PC + 20%chol DPPC MLVs DPPC + 5%chol DPPC + 20% chol |
no change no change no change Km*1.9 Km*1.6 Km*2 |
Vmax*1.3 Vmax*1.4 Vmax*1.1 Vmax*1.3 Vmax*1.3 Vmax*1.4 |
Spectrophotometry |
Sanchez and Perillo 2002a Colloids and Surfaces Biomembranes |
For a general comparison, the kinetic parameters as well as that of the catalytic efficiency are those obtained in the presence of a crowder or a membrane and referred to those obtained in dilute solutions. In cases marked with (b) the parameter values were obtained by fitting the data with a double hyperbola. The values presented are the ones that differ from dilute solution (named as (a) in the original paper. Parameters set to italics are the ones that are favoured in MC or heterogeneous media with respect to buffer condition (Km decreases and Vmax or kcat increases) and parameters set to bold-italics are the ones that are less favourable than the ones in buffer (Km increases and Vmax or kcat decreases)
Macromolecular crowding on the hydrolytic enzymes conformation and their catalytic properties
In solution
Macromolecular crowding (MC) affects protein folding, stability and activity through various mechanisms: exclusion volume, changes in water structure, intermolecular interactions, confinement, etc. and the observed result depends on the type of protein studied and the crowding agent used.
Macromolecular crowding was originally conceived as an effect due to molecular exclusion: under MC conditions, the available volume is reduced due to the presence of large amounts of macromolecules, and then this volume cannot be occupied by other molecules (Ellis 2001). Excluded volume theory predicts that folded state will be favoured over unfolded state, and as a consequence of that, protein stabilisation should occur. The characteristics of crowding agents such as their shape, size, and composition play an important role in the stabilisation of proteins. As an example, the flexible and rod-shaped dextran in comparison with rigid and compact ficoll of sphere-like shape resulted in more volume exclusion and hence higher stabilisation of lysozyme (Shahid et al. 2019).
Jia et al. (2017) investigated the effect of different crowding agents on protein folding and activity of alkaline phosphatase. They found that both in the absence and in the presence of crowders, the enzyme unfolds via a three-state unfolding mechanism, but under MC conditions, the protein becomes more stable. Interestingly, the free energy corresponding to the N → I process was found to increase in the presence of crowders and no changes were found in the I → U process. This could be due to the fact that the intermediate and unfolded states have similar hydrodynamic radii, and then the excluded volume effect of the crowding conditions is not affected.
In the case of oligomeric enzymes, the excluded volume effect will favour the oligomeric state over the monomeric state. Minton used glyceraldehyde-3-phosphate dehydrogenase (GADP) as an enzyme model and a mixture of unrelated proteins as a crowder to test this hypothesis. GADP consists of four identical subunits that dissociate under dilute conditions. The addition of other globular proteins was found to shift the monomer–tetramer equilibrium towards the tetramer, with no change in the specific activity of either monomer or tetramer (Minton 1981).
Using β-Gal from Kluyveromyces lactis and PEG 6000 as crowding agent, Nolan et al. (2020) found that at high concentration of crowding agent, the reaction kinetics undergoes a qualitative change in the catalytic behaviour form Michaelian, in the absence or presence of low and intermediate (0, 15 and 25%W/V) concentration of crowder, to sigmoidal at the higher concentration tested (35%W/V).This can be explained by the Monod-Wyman-Changeaux concerted model, which assumes an equilibrium between two enzyme states. Based on the results obtained, the authors propose a dimerisation reaction of the enzyme coupled to the hydrolysis of the substrate. The former reaction would only occur at very high (PEG) and is based on the excluded volume theory, which predicts that oligomerization is favoured. As described above, MC conditions also induce thermal stability; this is already important for enzymes used in industrial processes, such as β-Gal from K. lactis. When a protein is heated, it undergoes an unfolding process which, in theory, could be reversible when the temperature returns to the optimum. In practice, however, refolding is limited by another process: aggregation. Nolan et al. (2020) found that β-Gal retains its activity after pre-incubation at high temperatures in the presence of PEG, suggesting that PEG-induced MC inhibits self-aggregation of the thermally unfolded protein. This could be due to the impairment of the interaction between unfolded protein molecules induced by the crowding agent. Also, the thermal stabilisation of the enzyme demonstrated under MC conditions could be the result of the acquisition of the oligomeric state, which is known to exhibit higher thermal stability than the monomeric form (Flores et al. 2019).
Using PEG as a crowding agent, Nolan et al. (2015) found that MC altered the protein structure: both fluorescence and FT-IR spectroscopy revealed a more open protein structure in the presence of PEG compared to the conformation in its absence, with the substrate-binding domain being most affected. This could explain the almost linear decrease in catalytic efficiency observed. On the other hand, the effect of crowded systems on the thermal stability of β-Gal was noticeable, since denaturation was less cooperative and aggregation was prevented, as evidenced by the reduction in the proportion of typical bands due to the intermolecular β-sheet characteristic of aggregated proteins, which appeared in the infrared spectra in the presence of PEG compared to dilute conditions. This may be due to steric hindrance and diffusional problems that prevent unfolded molecules from meeting each other. Taking these results together, it can be concluded that β-Gal is thermodynamically trapped in a more open, flexible and stable conformation under crowded conditions than it is in dilute solutions (Nolan et al. 2015).
Crowding at the membrane water interface
Among membrane-bound proteins, there are enzymes having a transmembrane domain whose active site lies inside or outside of the lipid environment. For these, Dufrisne et al. (2017) reported a structural analysis as well as examples illustrating distinctive conformational features that provide solutions to the problem of bringing polar and hydrophobic substrates together for catalysis. In the case of hydrolytic enzymes such as phospholipase A1 which uses membrane components as substrates, the presence of a crowding agent showed a higher binding affinity which was accompanied by a decrease in the reaction rate and an early cessation of its catalytic activity (r.f. (Löwe et al. 2020).
Other types of membrane-bound enzymes are anchored not by their own transmembrane region but by the insertion of a covalently attached lipid moiety, e.g. fatty acids, isoprenoids and glycophosphatidylinositol (GPI). In particular, the GPI anchor type directs proteins to the outer hemilayer of the plasma membrane (the extracellular side of the membrane). Thus, the molecular environment of the apoprotein of a GPI-anchored protein is the cell glycocalyx (GC) ((Kinoshita and Fujita 2015) and references therein). Thus, the conformation of the protein moiety and the catalytic activity of GPI-anchored enzymes are expected to be modulated by the compaction of the GC (Clop et al. 2016) and the availability of water molecules as solvents and substrates (Clop et al. 2014, 2012; Felsztyna et al. 2020). A model membrane with glycocalyx can be built with monomolecular layers at the air–water interface, which are transferred to a solid support to form a Langmuir–Blodgett (LB) film using either a lipid mixture containing a lipopolymer whose polymeric tail facing the water phase contributes to conform a hydrophilic layer. Depending on the length of the tail and the molecular packing the hydrophilic layer acquires a thickness (Clop et al. 2016) sufficient to accommodate the protein part of the GPI-anchored protein with an expected compactness that can modulate the protein conformation and the hydration in its surroundings (Clop et al. 2014). In addition, LB can be prepared with purified membranes containing the GPI-anchored protein, which can be spread at the air–water interface and then transferred to a solid support to build LB films at a desired lateral pressure. The LB film can be used to evaluate the correlation between the molecular packing and the catalytic properties of the anchored protein as described with placental membranes (PL) (Clop and Perillo 2010) and bovine erythrocyte membranes (BEM) (Felsztyna et al. 2020) containing the GPI-anchored protein alkaline phosphatase (PLAP) and bovine erythrocyte acethylcholinesterase (BEA), respectively. When studying the activity of immobilised enzymes, diffusional, steric and electrostatic effects must be taken into account and it is important to note that, as mentioned above (r.f. Felsztyna et al. 2020), the membrane environment stabilised BEA against thermal denaturation. This observation and the observed modulation of its catalytic activity by the molecular packing may be related to the availability of water, both as a solvent due to its role in the hydrophobic effect and thus in the protein folding and, and as a substrate for the hydrolytic reaction step. This becomes relevant when considering the structure of the solvent (water) in the molecular environment of the enzyme (GC).
To study the activity of PLAP in the membrane model system with glycocalyx, monomolecular layers at the air–water interface (Langmuir films) containing dpPC-PE-PEG mixtures were used with three variants of the latter, having different molecular masses (and lengths) of the polymeric tail (350, 1000 and 5000 Da) (Fig. 5). The interfacial properties of the mixed monolayers (collapse pressure, transition pressure, mean molecular area and compressibility modulus) were influenced by the presence and size of the polymeric tail, and varied significantly with the molar fraction of PE-PEG. The dpPC/PE-PEG350 monolayers were found to be thermodynamically stable, with negative ∆Gex values, indicating attractive interactions between the components of the mixture. On the other hand, the dpPC/PE-PEG(1000–5000) monolayers showed positive ∆Gex values, indicating the occurrence of repulsive intermolecular interactions and/or steric hindrances which become more important and relevant as the molecular size of the polymer increases. For all three sizes of PE-PEG, an ideal mixing behaviour was only observed up to 10% of the lipopolymer. Beyond this percentage, the deviations from the ideality with the interactions mentioned above became apparent (Corvalan et al. 2008). It was therefore decided to incorporate purified PLAP into the dpPC/PE-PEG 9:1 mol% system. The final tertiary mixture had a composition dpPC/PLAP/dpPE-PEG (89:1:10 mol% mol/mol) regardless of the PE-PE used (Clop 2010; Clop and Perillo 2011). A binary mixture dpPC:PLAP (99:1 mol:mol) was also prepared. Once the films were formed at the air–water interface, these monolayers were transferred to silanised coverslips (Langmuir–Blodgett, LB, films) at two different lateral pressures, 10 mN/m or 35 mN/m, to also evaluate the effect of packing on the catalytic activity of PLAP. In terms of activity, we were able to conclude that under conditions of low molecular packing and thin interfacial polymer layer (PE-PEG350), the PEG tails are shorter than the thickness of the protein, but nevertheless contribute to stabilising the protein without introducing major steric restrictions on the mobility of the solutes (substrate and reaction product). As the thickness of the polymer layer increases (PEG1000 and PEG5000), the coiled structure of the PEG envelops the protein and behaves as a continuous viscous fluid. Under these conditions, the catalytic activity decreases significantly, similar to what has been observed at high concentrations of PEG in solution. In this case the degree of local molecular crowding would be even greater.
Fig. 5.
Simulation of phospholipid LB films containing anchored PLAP (black) and PE-PEG where the polymeric tail (in light blue), located close to the lipid–water interface, forms a hydrophilic environment with a thickness (indicated by the numbers) that depends on the MW of the PEG (from top to bottom 5000, 1000 and 350) and on the lateral pressure. In one of the conditions (PE-PEG5000), the thickness of the polymer layer is large enough to surround the enzyme). Clop, E. M Doctoral thesis, 2010
At high lateral pressures, the thickness of the polymer layer increases significantly, even in the case of PEG350, which increases the diffusion restrictions. As a result, the catalytic activity decreases compared to that observed at low lateral pressures, and the dependence between the activity and the length of the PEG tail is lost (Clop 2010; Clop and Perillo 2011).
In mesoporous silica matrices
In most cases described, MC induces protein stabilisation (Clop et al. 2016; Kao et al. 2014; Shin et al. 2020; Zhou and Hartmann 2013), but for some proteins, the opposite effect has been found. Eggers and Valentine (2001a), using silicate matrix as a model of molecular crowded system, found that most proteins studied showed no change on native structure at room temperature, and an increase in protein thermal stability but, for apomyoglobin, a different result was found. For this protein, the α-helical content was greatly reduced from 60% in solution to 20–25% after sol–gel encapsulation. The authors attribute this phenomenon to the water structure in the pores of the silica matrix. MC systems are characterised by large interfacial surfaces where water molecules can interact and as a consequence the amount of free water is diminished with respect to bulk water. Water structure enhancement provokes a diminution in hydrophobic effect, the driving force for protein folding. Then, apomyoglobin, a protein destabilised by the loss of the heme group, becomes partially unfolded. Using circular dichroism they demonstrated that the addition of chaotropic agents induces an enhancement in apomyoglobin folding.
Using a silicate matrix as a crowding system model, Burgos et al. (2019) found that the secondary structure of β-Gal shifts from β-sheet to α-helix upon encapsulation. In addition, silica encapsulation protected the enzyme from thermally induced secondary structure loss during thermal unfolding. On the other hand, fluorescence spectroscopy showed that the tertiary structure of the encapsulated enzyme was destabilised with respect to the soluble form. In addition, the decrease in catalytic activity on heating could not be prevented by encapsulation. This suggested that at the structural level, the tertiary structure seemed to be the most relevant in the maintenance of enzyme activity.
Natural and artificial catalytic aggregates: other molecular crowding models
The formation and properties of protein aggregates involve multiple length and time scale phenomena. Advances in analytical techniques and analysis methods allow the study of the formation of protein particles and their corresponding properties-size, structure, microstructure and rheology (Amin et al. 2014).
Among protein aggregates, bacterial inclusion bodies (IBs) are a naturally occurring phenomenon in microbial physiology. They have implications for biotechnology, medicine and basic research. These structures consist of dense, insoluble aggregates of proteins and other biomolecules that are produced by bacteria under a variety of stress conditions, such as high temperature, nutrient limitation, exposure to toxins or the expression of heterologous proteins (de Marco et al. 2019; Salinas et al. 2020).
Although the structure of IBs is complex, there are a number of features that are common to the different types of IBs. They are made up of a mixture of folded, misfolded and amyloid-like protein structures. They have a sponge-like shape with a protease-resistant amyloid backbone that provides mechanical stability, while a protease-sensitive fraction accumulates in the pores (Cano-Garrido et al. 2013).
Inclusion bodies can be thought of as a molecular crowding model: they are heterogeneous in composition and structure, and the solvation and energetic state of water molecules respond to this complexity. Amyloid fibrils, due to their morphology and molecular structures, cause highly heterogeneous water environments that are uncommon for hydration water of other biomolecular assemblies (Wang et al. 2017).
Historically, IBs have been considered one of the major obstacles in protein production, limiting the success of relevant recombinant DNA-derived proteins. Inclusion bodies were previously thought to be amorphous deposits formed by passive and unspecific precipitation of unfolded proteins. Subsequently, IBs were shown to contain a significant amount of active protein through the ease of isolation. Some of the proteins trapped in these aggregates remain functional, and these proteins are in a dynamic equilibrium with both monodisperse (native) forms and conventional (insoluble) IBs (de Marco et al. 2005; Stampolidis et al. 2009; Van der Henst et al. 2010). A hydrolytic enzyme such as β-galactosidase in IBs has special characteristics compared to the soluble version of the enzyme. The particulate enzyme was highly active against lactose at physiological or acidic pH, and retained its activity after pre-incubation at high temperature (Flores et al. 2019).
Artificial inclusion bodies were designed by Sanchez et al. (2021). They developed and thoroughly characterised an efficient, stable and reusable enzyme platform based on Escherichia coli β-galactosidase. The enzyme was assembled in vitro as stable protein microparticles with catalytic activity using divalent cations as molecular linkers. In this assembled microstructure, β-galactosidase has a specific conformation within the microparticles, sharing structural features (a high cross-parallel beta-sheet content) with the bacterial inclusion bodies and secretory amyloids from the mammalian endocrine system. This provides improved thermal stability compared to the soluble protein version and ensures high reusability in industrial processes. The complex state of water, in addition to the exclusion volume, local concentration and conformational properties of the enzyme within IBs, may contribute to the novel kinetic properties of these assemblies. However, it is still unclear whether the enzyme molecules in the core of the IB are involved in catalysing the hydrolytic reaction.
Hydration as the determinant step of the overall reaction rate
In hydrolysis reactions, water molecules play a role as reactants, and the structure of the water can influence the kinetics, depending on whether or not the hydrolysis itself takes place in the rate-limiting step of the mechanism of the reaction. The enzymes listed in Table 1 are hydrolases whose activity has been measured in heterogeneous media. Kinetic data have been obtained for enzymes in the presence of different crowding agents, at different concentrations and, in some cases, with different substrates. Different conditions have been applied, from confined enzymes in mesoporous matrices and in the presence of interfaces, providing a large number of cases for analysis. The parameter that allows the analysis of the enzyme performance is the catalytic efficiency, which is the quotient between the catalytic constant, kcat (or Vmax) and the Michaelis–Menten constant, Km. Due to the many variables, it is quite difficult to describe a trend, but in general, it can be said that the value of Km decreases at low crowding agent concentrations and increases at high concentrations of crowding agent relative to the value of the enzyme in dilute conditions. On the one hand, the effect at low crowding agent concentrations can be explained by the increase in the thermodynamic activity of solutes in crowded media (Minton 1983), which favours the binding of the enzyme to the substrate. On the other hand, at high concentrations of crowding agents, diffusional limitations are stronger and would prevent the formation of the enzyme–substrate complex. The nature of the crowding agent would influence the changes in activity, as Ficoll and PEG 600 are more compact than PEG 6000. Although Ficoll 400 has a high molecular weight, it is highly branched and has a globular shape, similar to the globular conformation of proteins. In most of the works cited, the experimental method used to monitor enzymatic activity was spectrophotometric (light absorption or fluorescence emission); however, when other methods were used, such as multiple injection ITC, the data obtained differed significantly from those obtained by spectroscopic methods. A special case seemed to be the lipase enzyme; systematically lower Km were obtained for two different substrates and three different crowding agents (in all cases at low concentration of crowding agent) (Lei et al. 2008). The authors related the changes in kinetic parameters to structural changes, but the results were not clearly correlated. Otherwise, the local concentration of the substrates enhanced by molecular crowding should be taken into account.
Dual role of water: solvent and substrate
Up to this point, the analysis is general to enzymes in crowded media, but we propose that special attention should be paid to hydrolytic enzymes in heterogeneous media and the state of water in this condition. For hydrolytic enzymes, the water molecule plays an important role as a reactant, especially when nucleophilic water attack is the rate-limiting step of the catalytic mechanism. We have therefore collected information on the role of water in the kinetic mechanisms with these substrates, where available.
As an example, Fig. 6 shows the hydrolysis of lactose catalysed by β-galactosidase. The general mechanism involves a first step in which glucose is released leaving the enzyme in a galactosylated form, and a second step with the incorporation of water, the hydrolysis of the enzyme-galactose complex, yielding free galactose and recovering the enzyme for a new catalytic cycle.
Fig. 6.
The double displacement mechanism followed by β-galactosidase from Aspergillus oryzae using lactose as a substrate and water as a glycosyl acceptor. Acid/base and nucleophile: catalytic Glu residues. Reprinted with permission from J.Dairy Science, 105:4772–4782. Shi et al. “Engineering the optimum pH of β-galactosidase from Aspergillus oryzae for efficient hydrolysis of lactose” (2022). Copyright ©2022 with permission from the authors
In the particular case of β-galactosidase from E. coli confined in the pores of a silicate gel, kcat increased with respect to the enzyme in a buffered solution when the substrate was ONPG, but not when PNPG was the substrate. This different behaviour could be explained by considering the mechanism proposed for both substrates, where the rate-limiting step for ONPG was water nucleophilic attack, whereas for PNPG it was aglycone cleavage (Burgos et al. 2016).
As explained below, we were able to determine that the water inside the nanopores of the silicate matrix is more structured and were able to establish a correlation between the increase in kcat in aged gels and the decrease in the diffusion constant of water molecules. Organophosphorus hydrolase entrapped in mesoporous silica also showed increased activity in the hydrolysis of paraoxon, and water is known to be involved in the higher activation energy steps (Lei et al. 2008). Further experiments to confirm this hypothesis would be of great interest. Overexpressed β-galactosidase from E. coli forms inclusion bodies (IBs), and the enzyme in this suprastructure was not only active, but also showed a higher catalytic efficiency for lactose hydrolysis than the soluble form (Flores et al. 2019). In this sense, the proportion of non-diffusible water among the entrapped water has been described in protein aggregates formed by high temperature and divalent cation salts (Urbonaite et al. 2015) and in amyloid fibrils (Wang et al. 2017), both of which (divalent cation salts and amyloid fibrils) are present in the IBs (Sanchez et al. 2020).
Although recombinant β-galactosidase from E. coli bears a His-tag at the N-terminus and its mechanism of action on the lactose hydrolysis has not yet been investigated, the role of structured water as a reactant could play a key role in the significant increase in Vmax observed. With the exception of data obtained for β-galactosidase encapsulated in silicate gels (Burgos et al. 2016) and β-galactosidase in PEG at different concentrations (Nolan et al. 2019), saturation curves (V0 vs. [substrate]) were analysed assuming a Michaelian behaviour. Linearisation of the data for an Eadie-Hofstee analysis showed a biphasic behaviour (Burgos et al. 2016; Nolan et al. 2019), interpreted as two populations of enzymes sensing on average, two different environments. One that resembles the catalytic activity in solution and another group that senses a more spatially confined medium. It is important to recall that the water structure, both in the silicate matrix and in MC media showed at least two populations with different mobilities as shown by 1H-NMR (Burgos et al. 2016; Clop et al. 2014).
Our experience with β-gal has clearly shown that the reaction that takes place in MC media (either with soluble polymers or in the silicate matrix) using the substrate that shows the incorporation of water in the rate-determining step is biphasic, suggesting that this is the expression of different water populations. It would be difficult to extend this study to other enzymes since, as described in Table 1, such in-depth kinetic analysis is not performed in most cases. However, to go deeper into the role of water, other physical properties can be considered such as the concept of water spin isomers.
Molecular models of water in bulk and near surfaces
The structure of pure water
The “structure” of water is a key concept in the MC phenomenon. It is described as being defined by the number and orientations of hydrogen bonds, which can be perturbed differently in the presence of charged and uncharged solutes as well as hydrophobic and hydrophilic surfaces. However, beyond the description of different water populations according to their respective mobilities, there is no analysis that considers details of molecular arrangements at a fundamental level. In this sense, we wondered whether such a deeper view of the water structure could shed light on the availability of water molecules as substrates under MC conditions. Therefore, in this section, we present ‘water models’ from other fields of science, based on the concept that the thermodynamic properties of water are strongly linked to its ability to form intermolecular hydrogen bonds.
Water models have been discussed for more than 100 years (see references in (Kontogeorgis et al. 2022; Pershin 2013)) as an attempt to explain the anomalous behaviour of many thermodynamic variables of water (e.g. increased density upon melting, decreased viscosity under pressure, density maximum at 4 °C, high surface tension and many more (Nilsson and Pettersson 2011)).
Scientists have been searching for a unified picture of the structure of water that is valid from the boiling point down to the supercooled regime. The two-liquid water model has strong experimental support and is currently the accepted model for tetrahedral liquids such as water (Gallo et al. 2016; Malenkov 2009; Neophytou et al. 2022; Nilsson and Pettersson 2011) (Fig. 7a). Liquid–liquid phase transition (LLPT) in tetrahedral networks can be described as a transition between a random tetrahedral network, “unentangled low-density liquid (LDL) and an entangled, high-density liquid (HDL)”. The latter consists of two or more disordered but locally interpenetrating networks that conform an ensemble of topologically complex motifs which leaded Neophytou et al. (2022) to coinage the concept of colloidal analogue of water, a tetrahedral network liquid self-assembled from designer triblock patchy colloidal particles via tetrahedral clusters (Fig. 7b). In the colloidal model, although the LDL and HDL are transient networks (that is, bonds are constantly breaking and forming), and so their topological state is not constant, the topological fingerprints for each phase are well defined (Fig. 7c, d).
Fig. 7.
a Schematic phase diagram of liquid water consistent with X-ray spectroscopy data. Only the liquid–liquid coexistence line, the liquid–liquid critical point (LLCP) and the Widom line are shown for simplicity. Reprinted with permission from Chemical Physics 389,1.34. Nilsson and Pettersson, Perspective on the structure of liquid water. Copyright © 2011, Elsevier. b Cluster formation via the hierarchical self-assembly of triblock patchy particles leading to a colloidal model of water. Patches A (red) and B (blue), are of different sizes and form bonds of different strengths. The A patches form stronger bonds than the B patches. c and d Representative snapshots of the low density liquid (LDL) c and high density liquid (HDL) d networks of colloidal water at a temperature below the critical temperature Tc, and pressures either side of the critical pressure Pc. Tetrahedral clusters in the HDL network forming a trefoil knot (top) and a Hopf link (bottom) are highlighted in d, visualised in a reduced representation as tetrahedral patchy particles. Adapted with permission from Nature Physics, 18, 1248–1253. Neophytou et al., “Topological nature of the liquid–liquid phase transition in tetrahedral liquids” (2022). Copyright © 2022, Springer Nature
Spin-isomers of water in bulk and in interfaces
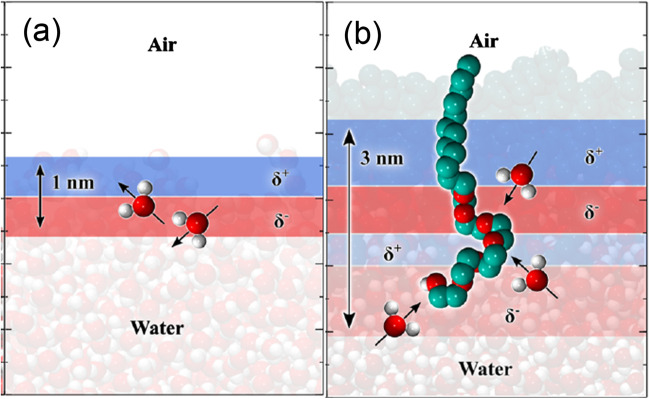
As explained above, the LLPT arose in an effort to explain the origin of the anomalous behaviour of water thermodynamic functions which are enhanced in supercooled states. At the quantum level, ortho/para H2O spin isomers are also considered as a key factor that determines water properties taking into account the ortho/para conversion and the unbalanced (1:1) ortho/para ratio in water.
Water molecules, like other symmetric molecules, exist in nature in the form of nuclear spin isomers named ortho-H2O and para-H2O which differ in the states of the spins of the identical H nuclei (Fig. 8).
Fig. 8.

Taken from https://water.lsbu.ac.uk/water/ortho_para_water.html. Martin Chaplin. Copyright © 2021 https://creativecommons.org/licenses/by-nc-nd/2.0/uk/legalcode
In gas phase, ortho-H2O always rotates, and some para-H2O do not rotate even at room temperature, so they are energetically more prone to form ice-like structures than ortho-H2O. Hence, it can be expected, as demonstrated in the case of ortho and para H2, the existence of a mix of two independent states of liquid water with a large life time and two kinds of hydrogen bond, which are determined by the spin states of H2O monomers before formation of complexes (Pershin 2013).
The ortho/para spin ratio is 1:1 in distilled water and 3:1 in gas at equilibrium at room temperature but near “specific temperatures” (Ts) of H2O — corresponding to maximum density (4 °C), maximum surface tension (19 °C), minimum heat capacity (36 °C) and maximum speed of sound (76 °C) — a strong deformations of the OH Raman band was observed and was interpreted as a manifestation of the water structure rearrangement induced by H2O ortho-para conversion since at these points the quantum energy hΩmn of closely located rotational transitions in the ortho and para spin isomers of H2O molecules coincides with the translation energy kT (Pershin 2008).
The ortho/para H2O conversion speeds up in the presence of catalysts (atoms, ions, molecules, etc.) with a magnetic moment (r.f (Pershin 2013) which can lead to an increase in the chemical reactivity of H2O due to the fact that para-water is able to attract its reactant more strongly than the ortho-form (Kilaj et al. 2018). Moreover, in the presence of an electric field of intensity ~ 103 V/cm the conversion rate increases by an order because of displacement of energy levels of ortho and para isomers (Stark effect) (r.f. Pershin 2013). In this sense, it is interesting to note that surfactant-water interfaces can exhibit strong electric fields such as that of hexaethylene glycol monododecyl ether (C12E6) which create a net interfacial electric field of ∼1 V/nm (~ 107 V/cm) pointing towards the bulk (Fig. 9), which was determined by molecular dynamic simulation and confirmed by Kelvin-probe surface potential experiments (Gera et al. 2021). Thus, it can be hypothesised that such an interface might be able to induce ortho/para transition in interfacial water and by this means also affect its reactivity.
Fig. 9.
a and b The molecular arrangement at the air–water a and surfactant–water b interfaces, responsible for the ensuing charge and electric field distributions. Adapted with permission from J. Am. Chem. Soc, 143, 37, 15,103–15112. Gera, R et al., “Emergence of Electric Fields at the Water–C12E6 Surfactant Interface” (2021) Copyright © 2021 American Chemical Society
It has also been determined a selective interaction of the para-isomer with biomolecules such as DNA, collagen and lysozyme in the gas phase (Potekhin and Khusainova 2005; Tikhonov and Volkov 2006) and a-chymotrypsin in the liquid phase (Bunkin et al. 2006), suggesting that the hydration shell of DNA and proteins should be enriched with para H2O isomer. Moreover, the preferential interaction of para water molecules with proteins has been related with changes in the cell dynamics. Pershin (2009) observed that the phenomenon in which erythrocyte water suspensions suffer a cooperative change in their permeability through a microcapillary at a temperature of 36.6 °C (Artmann et al. 1998), could be related to the energy of 313–202 transition of water para isomer at that temperature, which increases the probability for excitation of para water spin isomers in collisions and induces conversion of para isomers to ortho water (Fig. 10).
Fig. 10.

Jump in the erythrocyte penetrability (squares) and the model contour of the 215.13 cm−1 line of the 331 − 202 transition for para H2O isomers constructed with the half-width 0.4 cm−1 on the temperature scale. Adapted with permission from Pershin (2009) Conversion of Ortho-Para H2O Isomers in Water and a Jump in Erythrocyte Fluidity Through a Microcapillary at a Temperature of 36.6 ± 0.3 °C. Reprinted with permission from Physics of Wave Phenomena, 17, 241–250. Pershin, SM “Conversion of ortho-para H2O isomers in water and a jump in erythrocyte fluidity through a microcapillary at a temperature of 36.6 ± 0.3 C”. (2009). Copyright © 2009 Springer
Strategies to evidence the ortho/para water spin isomers
Studies of molecular motions in liquids allow understanding the character of intermolecular interactions and can be assessed by the spectroscopy of low-frequency (0–3 THz). The classical methods of optical spectroscopy, such as spontaneous Raman scattering and IR absorption spectroscopy, cannot effectively probe condensed media because of a low level of the useful response, the presence of parasitic signals, and significant background absorption in water and water vapour in the far IR range (r.f.(Gera et al. 2021).
By means of four-photon Rayleigh wing coherent laser spectroscopy the spectra of water reveal resonances at the frequencies corresponding to low-frequency (0–3 THz) rotational transitions involving the ground vibrational states of the ortho and para spin isomers of water. Bunkin et al. (2006) measured the band of OH stretching vibrations in free molecules in the Raman spectrum of liquid water at room temperature. This showed the decrease in the intensity of para-water signal in the presence of α-chymotrypsin in the aqueous solution as evidence of its preferential binding with respect to the ortho spin isomer.
It is interesting to note that the conversion of ortho water to para water follows a second order kinetics, indicating that it is facilitated by water–water interactions that may involve the electric dipole moments of neighbouring water molecules (Meier et al. 2018).
The fraction of para-H2O is enhanced by thermal equilibration at low temperature, and raising the temperature rapidly by dissolving the material in a warm solvent. The 1H NMR spectrum was observed while para-water converts back to ortho-water at ambient temperature (Mamone et al. 2014). These techniques may help to get deep in this physical phenomenon and provide information beyond water molecules more or less mobile.
Concluding remarks
In our laboratory, we have studied various hydrolytic enzymes in heterogeneous media. The interest in studying certain enzymes whose activity was already known in homogeneous and dilute systems, as was being done in classical biochemistry, was to understand how enzyme activity is modulated by its molecular environment “in vivo”. We began to study the activity of β-gal in the vicinity of lipid interfaces, moving away from the assumption that because an enzyme is soluble, it is incapable of interacting with a model membrane.
We also began to explore the kinetics of enzymes anchored in the membrane, embedded in a molecularly crowded environment, and with enzymes encapsulated in different inorganic and lipid matrices. Like Zeno’s paradox, in which states that it is impossible to reach a certain goal, the ultimate goal would be to be able to explain the enzymatic kinetics of an enzyme immersed in its natural environment. An example of this is the fact that molecular crowding could be assumed as a model for the cell cytoplasm. However, most of the experiments under MC conditions are performed in homogeneous media. The discovery, in cells, of membraneless organelles formed by LLPS shows that the cytoplasm is not a well-mixed environment. The actual molecular system becomes even more complex when we think about water. Normally, we tend to assume that there is an excess of water in a RE, which is not true in systems with MC and is even more important when we talk about hydrolytic enzymes where water is a substrate.
In this review, we have discussed how the different levels of complexity with which we understand the molecular environment of an enzyme within a cell can be addressed and understood by studying the structure of water.
In the words of Bagatolli et al. (2021), to which we adhere, “it is surprising that a large number of articles describing liquid immiscibility in cells —a common feature in colloidal systems— do not even mention the AIH or investigate the behaviour of key cellular components such as intracellular water and the extent and consequences of ionic association”.
Acknowledgements
This work was partially financed by the SeCyT-Universidad Nacional de Córdoba, Foncyt and CONICET from Argentina. All authors are members of the later institution. JMS is supported with a María Zambrano postdoctoral researcher contract (677904) from the Ministerio de Universidades and European Union (“Financed by European Union-Next GenerationEU”).
Author contribution
MAP conceived the study. All authors contributed to the writing and read and approved the final manuscript.
Declarations
Ethics approval
This article does not require ethics approval since it does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Does not apply to this work.
Consent for publication
Does not apply to this work.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alberti S, Gladfelter A, Mittag T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell. 2019;176:419–434. doi: 10.1016/j.cell.2018.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Lewis J, Raff M, Keith R, Watson JD (2008) Molecular Biology of the cell. Garland Publishing, Inc.
- Amin S, Barnett GV, Pathak JA, Roberts CJ, Sarangapani PS. Protein aggregation, particle formation, characterization & rheology. Curr Opin Colloid Interface Sci. 2014;19:438–449. doi: 10.1016/j.cocis.2014.10.002. [DOI] [Google Scholar]
- Artmann GM, Kelemen C, Porst D, Buldt G, Chien S. Temperature transitions of protein properties in human red blood cells. Biophys J. 1998;75:3179–3183. doi: 10.1016/S0006-3495(98)77759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagatolli LA, Stock RP, Olsen LF. Coupled response of membrane hydration with oscillating metabolism in live cells: an alternative way to modulate structural aspects of biological membranes? J Biomolecules. 2019;9:687. doi: 10.3390/biom9110687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagatolli LA, Mangiarotti A, Stock RP. Cellular metabolism and colloids: realistically linking physiology and biological physical chemistry. Prog Biophys Mol Biol. 2021;162:79–88. doi: 10.1016/j.pbiomolbio.2020.06.002. [DOI] [PubMed] [Google Scholar]
- Bai Q, Zhang Q, Jing H, Chen J, Liang D. Liquid–liquid phase separation of peptide/oligonucleotide complexes in crowded macromolecular media. J Phys Chem B. 2020;125:49–57. doi: 10.1021/acs.jpcb.0c09225. [DOI] [PubMed] [Google Scholar]
- Bezanilla M, Gladfelter AS, Kovar DR, Lee WL. Cytoskeletal dynamics: a view from the membrane. J Cell Biol. 2015;209:329–337. doi: 10.1083/jcb.201502062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia RB, Brinker CJ, Gupta AK, Singh AK. Aqueous sol− gel process for protein encapsulation. Chem Mater. 2000;12:2434–2441. doi: 10.1021/cm000260f. [DOI] [Google Scholar]
- Bonnaud PA, Coasne B, Pellenq RJ. Molecular simulation of water confined in nanoporous silica. J Phys Condensed Matter. 2010;22:284110. doi: 10.1088/0953-8984/22/28/284110. [DOI] [PubMed] [Google Scholar]
- Bortolotti V, Fantazzini P, Mongiorgi R, Sauro S, Zanna S. Hydration kinetics of cements by time-domain nuclear magnetic resonance: application to Portland-cement-derived endodontic pastes. Cem Concr Res. 2012;42:577–582. doi: 10.1016/j.cemconres.2011.12.006. [DOI] [Google Scholar]
- Brinker CJ, Scherer GW (2013) Sol-gel science: the physics and chemistry of sol-gel processing. Academic Press, Inc.
- Buckley AM, Greenblatt M. The sol-gel preparation of silica gels. J Chem Educ. 1994;71:599. doi: 10.1021/ed071p599. [DOI] [Google Scholar]
- Bunkin AF, Nurmatov AA, Pershin SM, Vigasin AA (2005) Four-photon coherent spectroscopy of orientational motion of H2O molecules in liquid water. J Raman Spectroscopy 36:145–147. 10.1002/jrs.1291
- Bunkin AF, Pershin SM, Nurmatov AA (2006) Four-photon spectroscopy of ortho/para spin-isomer H2O molecule in liquid water in sub-millimeter range. Laser Phys Lett 3:275–277. 10.1002/lapl.200610007
- Burgos MI, Ochoa A, Perillo MA. beta-sheet to alpha-helix conversion and thermal stability of beta-Galactosidase encapsulated in a nanoporous silica gel. Biochem Biophys Res Commun. 2019;508:270–274. doi: 10.1016/j.bbrc.2018.11.077. [DOI] [PubMed] [Google Scholar]
- Burgos MI, Velasco MI, Acosta RH, Perillo MA. Environmental topology and water availability modulates the catalytic activity of beta-galactosidase entrapped in a nanosporous silicate matrix. Sci Rep. 2016;6:36593. doi: 10.1038/srep36593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway CP, Hendrickson K, Bond N, Lee SM, Sood P, Jang SS. Molecular modeling approach to determine the flory-huggins interaction parameter for polymer miscibility analysis. ChemPhysChem. 2018;19:1655–1664. doi: 10.1002/cphc.201701337. [DOI] [PubMed] [Google Scholar]
- Cammarata M, Piazza F, Rivas G, Schirò G, Temussi PA, Pastore A. Revitalizing an important field in biophysics: the new frontiers of molecular crowding. Front Mol Biosci. 2023 doi: 10.3389/fmolb.2023.1153996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Garrido O, Rodríguez-Carmona E, Díez-Gil C, Vázquez E, Elizondo E, Cubarsi R, Seras-Franzoso J, Corchero JL, Rinas U, Ratera I, Ventosa N, Veciana J, Villaverde A, García-Fruitós E. Supramolecular organization of protein-releasing functional amyloids solved in bacterial inclusion bodies. Acta Biomater. 2013;9:6134–6142. doi: 10.1016/j.actbio.2012.11.033. [DOI] [PubMed] [Google Scholar]
- Chighizola M, Dini T, Marcotti S, D’Urso M, Piazzoni C, Borghi F, Previdi A, Ceriani L, Folliero C, Stramer B, Lenardi C, Milani P, Podestà A, Schulte C. The glycocalyx affects the mechanotransductive perception of the topographical microenvironment. J Nanobiotechnol. 2022;20:418. doi: 10.1186/s12951-022-01585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clop EM (2010) Modulación de la Actividad de Fosfatasa Alcalina Placentaria inducida por la organización dinámica de su entorno molecular Facultad de Ciencias Exáctas Físicas y Naturales, vol Doctor en Ciencias Biológicas. Universidad Nacional de Córdoba, Córdoba, Argentina, pp 111
- Clop EM, Perillo MA (2010) Langmuir films from human placental membranes: preparation, rheology, transfer to alkylated glasses, and sigmoidal kinetics of alkaline phosphatase in the resultant Langmuir-Blodgett film. Cell Biochem Biophys 56:91–107. 10.1007/s12013-009-9073-4 [DOI] [PubMed]
- Clop EM, Perillo MA (2011) Efecto del hacinamiento molecular en membranas modelo con glicocáliz en la cinética enzimática de fosfatasa alcalina placentaria XL Reunión Anual de la Sociedad Argentina de Biofísica. Sociedad Argentina de Biofísica, Buenos Aires, Argentina, pp 151. https://goo.su/DiCFby
- Clop EM, Clop PD, Sanchez JM, Perillo MA. Molecular packing tunes the activity of Kluyveromyces lactis beta-galactosidase incorporated in Langmuir-Blodgett films. Langmuir. 2008;24:10950–10960. doi: 10.1021/la801679m. [DOI] [PubMed] [Google Scholar]
- Clop EM, Chattah AK, Perillo MA. Water and membrane dynamics in suspensions of lipid vesicles functionalized with poly(ethylene glycol)s. J Phys Chem B. 2014;118:6150–6158. doi: 10.1021/jp410894x. [DOI] [PubMed] [Google Scholar]
- Clop EM, Corvalan NA, Perillo MA. Langmuir films of dipalmitoyl phosphatidylethanolamine grafted poly(ethylene glycol). In-situ evidence of surface aggregation at the air-water interface. Colloids Surf B. 2016;148:640–649. doi: 10.1016/j.colsurfb.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Clop EM, Perillo MA, Chattah AK. 1H and 2H NMR spin-lattice relaxation probing water: PEG molecular dynamics in solution. J Phys Chem B. 2012;116:11953–11958. doi: 10.1021/jp304569a. [DOI] [PubMed] [Google Scholar]
- Corvalan NA, Clop EM, Perillo MA (2008) Surface behaviour of dipalmitoylphosphatidylethanolamine grafted poly(ethylene glycol) alone or in mixtures with dipalmitoylphosphatidylcholine XXXVII Reunión Anual de la Sociedad Argentina de Biofísica. Sociedad Argentina de Biofísica, Buenos Aires, Argentina. https://goo.su/FA6XZod
- Dave BC, Dunn B, Valentine JS, Zink J. Sol-gel encapsulation methods for biosensors. Anal Chem. 1994;66:1120A–1127A. doi: 10.1021/ac00094a001. [DOI] [Google Scholar]
- de Marco A, Vigh L, Diamant S, Goloubinoff P. Native folding of aggregation-prone recombinant proteins in Escherichia coli by osmolytes, plasmid- or benzyl alcohol-overexpressed molecular chaperones. Cell Stress Chaperones. 2005;10:329–339. doi: 10.1379/csc-139r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marco A, Ferrer-Miralles N, Garcia-Fruitos E, Mitraki A, Peternel S, Rinas U, Trujillo-Roldan MA, Valdez-Cruz NA, Vazquez E, Villaverde A. Bacterial inclusion bodies are industrially exploitable amyloids. FEMS Microbiol Rev. 2019;43:53–72. doi: 10.1093/femsre/fuy038. [DOI] [PubMed] [Google Scholar]
- Depagne C, Roux C, Coradin T. How to design cell-based biosensors using the sol–gel process. Anal Bioanal Chem. 2011;400:965–976. doi: 10.1007/s00216-010-4351-y. [DOI] [PubMed] [Google Scholar]
- Disalvo EA, Lairion F, Martini F, Tymczyszyn E, Frías M, Almaleck H, Gordillo GJ (2008) Structural and functional properties of hydration and confined water in membrane interfaces. Biochim Biophys Acta (BBA) Biomembranes 1778:2655–2670. 10.1016/j.bbamem.2008.08.025 [DOI] [PubMed]
- Disalvo EA, Pinto OA, Martini MF, Bouchet AM, Hollmann A, Frías MA (2015) Functional role of water in membranes updated: a tribute to Träuble. Biochim Biophys Acta (BBA) Biomembranes 1848:1552–1562. 10.1016/j.bbamem.2015.03.031 [DOI] [PubMed]
- Dufrisne MB, Petrou VI, Clarke OB, Mancia F (2017) Structural basis for catalysis at the membrane-water interface. Biochim Biophys Acta (BBA) Mol Cell Biol Lipids 1862:1368–1385. 10.1016/j.bbalip.2016.11.011 [DOI] [PMC free article] [PubMed]
- Edmiston PL, ClL Wambolt, Smith MK, Saavedra SS. Spectroscopic characterization of albumin and myoglobin entrapped in bulk sol-gel glasses. J Colloid Interface Sci. 1994;163:395–406. doi: 10.1006/jcis.1994.1119. [DOI] [Google Scholar]
- Eggers DK, Valentine JS. Crowding and hydration effects on protein conformation: a study with sol-gel encapsulated proteins. J Mol Biol. 2001;314:911–922. doi: 10.1006/jmbi.2001.5166. [DOI] [PubMed] [Google Scholar]
- Eggers DK, Valentine JS. Molecular confinement influences protein structure and enhances thermal protein stability. Protein Sci. 2001;10:250–261. doi: 10.1110/ps.36201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerby LM, Nishida CR, Nishida F, Yamanaka SA, Dunn B, Valentine JS, Zink J. Encapsulation of proteins in transparent porous silicate glasses prepared by the sol-gel method. Science. 1992;255:1113–1115. doi: 10.1126/science.1312257. [DOI] [PubMed] [Google Scholar]
- Ellis RJ. Macromolecular crowding: obvious but underappreciated. Trends Biochem Sci. 2001;26:597–604. doi: 10.1016/S0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]
- Favvas EP, Mitropoulos AC. What is spinodal decomposition. J Eng Sci Technol Rev. 2008;1:25–27. doi: 10.25103/jestr.011.05. [DOI] [Google Scholar]
- Fels J, Orlov SN, Grygorczyk R. The hydrogel nature of mammalian cytoplasm contributes to osmosensing and extracellular pH sensing. Biophys J . 2009;96:4276–4285. doi: 10.1016/j.bpj.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsztyna I, Turina AV, Perillo MA, Clop EM. Sensing molecular organizational changes through the catalytic activity of acetylcholinesterase from erythrocyte membranes in Langmuir-Blodgett films. Biochim Biophys Acta Biomembr. 2020;1862:183188. doi: 10.1016/j.bbamem.2020.183188. [DOI] [PubMed] [Google Scholar]
- Fisette O, Päslack C, Barnes R, Isas JM, Langen R, Heyden M, Han S, Schäfer LV. Hydration dynamics of a peripheral membrane protein. J Am Chem Soc. 2016;138:11526–11535. doi: 10.1021/jacs.6b07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores SS, Nolan V, Perillo MA, Sánchez JM. Superactive β-galactosidase inclusion bodies. Colloids Surf B. 2019;173:769–775. doi: 10.1016/j.colsurfb.2018.10.049. [DOI] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel-Mullerad H, Avnir D. Sol−gel materials as efficient enzyme protectors: preserving the activity of phosphatases under extreme pH conditions. J Am Chem Soc. 2005;127:8077–8081. doi: 10.1021/ja0507719. [DOI] [PubMed] [Google Scholar]
- Gallo P, Amann-Winkel K, Angell CA, Anisimov MA, Caupin F, Chakravarty C, Lascaris E, Loerting T, Panagiotopoulos AZ, Russo J, Sellberg JA, Stanley HE, Tanaka H, Vega C, Xu L, Pettersson LG. Water: a tale of two liquids. Chem Rev. 2016;116:7463–7500. doi: 10.1021/acs.chemrev.5b00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García DA, Quiroga S, Perillo MA. Flunitrazepam partitioning into natural membranes increases surface curvature and alters cellular morphology. Chem-Biol Interact. 2000;129:263–277. doi: 10.1016/S0009-2797(00)00254-4. [DOI] [PubMed] [Google Scholar]
- Gera R, Bakker HJ, Franklin-Mergarejo R, Morzan UN, Falciani G, Bergamasco L, Versluis J, Sen I, Dante S, Chiavazzo E, Hassanali AA. Emergence of electric fields at the water–C12E6 surfactant interface. J Am Chem Soc. 2021;143:15103–15112. doi: 10.1021/jacs.1c05112. [DOI] [PubMed] [Google Scholar]
- Giussi JM, Velasco MI, Longo GS, Acosta RH, Azzaroni O. Unusual temperature-induced swelling of ionizable poly(N-isopropylacrylamide)-based microgels: experimental and theoretical insights into its molecular origin. Soft Matter. 2015;11:8879–8886. doi: 10.1039/c5sm01853f. [DOI] [PubMed] [Google Scholar]
- Gomes E, Shorter J. The molecular language of membraneless organelles. J Biol Chem. 2019;294:7115–7127. doi: 10.1074/jbc.TM118.001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Chaudhury NK. Entrapment of biomolecules in sol–gel matrix for applications in biosensors: problems and future prospects. Biosens Bioelectron. 2007;22:2387–2399. doi: 10.1016/j.bios.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Huang C, Wikfeldt KT, Tokushima T, Nordlund D, Harada Y, Bergmann U, Niebuhr M, Weiss T, Horikawa Y, Leetmaa M. The inhomogeneous structure of water at ambient conditions. Proc Natl Acad Sci U S A. 2009;106:15214–15218. doi: 10.1073/pnas.0904743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, Weber CA, Julicher F. Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol. 2014;30:39–58. doi: 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- Jia J, Peng X, Qi W, Su R, He Z. Effects of macromolecular crowding on alkaline phosphatase unfolding, conformation and stability. Int J Biol Macromol. 2017;101:373–382. doi: 10.1016/j.ijbiomac.2017.03.113. [DOI] [PubMed] [Google Scholar]
- Jiang M, Guo Z. Effects of macromolecular crowding on the intrinsic catalytic efficiency and structure of enterobactin-specific isochorismate synthase. J Am Chem Soc. 2007;129:730–731. doi: 10.1021/ja065064+. [DOI] [PubMed] [Google Scholar]
- Kao K-C, Lin T-S, Mou C-Y. Enhanced activity and stability of lysozyme by immobilization in the matching nanochannels of mesoporous silica nanoparticles. J Phys Chem C. 2014;118:6734–6743. doi: 10.1021/jp4112684. [DOI] [Google Scholar]
- Kilaj A, Gao H, Rösch D, Rivero U, Küpper J, Willitsch S. Observation of different reactivities of para and ortho-water towards trapped diazenylium ions. Nat Commun. 2018;9:2096. doi: 10.1038/s41467-018-04483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Fujita M. Biosynthesis of GPI-anchored proteins: special emphasis on GPI lipid remodeling. J Lipid Res. 2015;57:6–24. doi: 10.1194/jlr.R063313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontogeorgis GM, Holster A, Kottaki N, Tsochantaris E, Topsøe F, Poulsen J, Bache M, Liang X, Blom NS, Kronholm J. Water structure, properties and some applications – a review. Chem Thermodyn Therm Anal. 2022;6:100053. doi: 10.1016/j.ctta.2022.100053. [DOI] [Google Scholar]
- Korn ED, Carlier M-F, Pantaloni D. Actin polymerization and ATP hydrolysis. Science. 1987;238:638–644. doi: 10.1126/science.3672117. [DOI] [PubMed] [Google Scholar]
- Lan EH, Dave BC, Fukuto JM, Dunn B, Zink J, Valentine JS. Synthesis of sol-gel encapsulated heme proteins with chemical sensing properties. J Mater Chem. 1999;9:45–53. doi: 10.1039/A805541F. [DOI] [Google Scholar]
- Laurent TC. Enzyme reactions in polymer media. Eur J Biochem. 1971;21:498–506. doi: 10.1111/j.1432-1033.1971.tb01495.x. [DOI] [PubMed] [Google Scholar]
- Laurent TC, Ogston AG. The interaction between polysaccharides and other macromolecules. 4. The osmotic pressure of mixtures of serum albumin and hyaluronic acid. Biochem J. 1963;89:249. doi: 10.1042/bj0890249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C, Soares TA, Shin Y, Liu J, Ackerman EJ. Enzyme specific activity in functionalized nanoporous supports. Nanotechnology. 2008;19:125102. doi: 10.1088/0957-4484/19/12/125102. [DOI] [PubMed] [Google Scholar]
- Lenormand G, Millet E, Park CY, Hardin CC, Butler JP, Moldovan NI, Fredberg JJ. Dynamics of the cytoskeleton: how much does water matter? Phys Rev E. 2011;83:061918. doi: 10.1103/PhysRevE.83.061918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier JF (2001) Water and the cytoskeleton. Cell Mol Biol (Noisy-le-grand) 47:901–923 [PubMed]
- Ling GN (1962) A physical theory of the living state: the association-induction hypothesis. Blaisdell publishing Co, A division of random house, Inc., New York. https://goo.su/de5x
- Ling GN. A physical theory of the living state: the association-induction hypothesis. J Med Educ. 1963;38:530. [Google Scholar]
- Ling GN (1987) In search of the physical basis of life. Adv Physiol Res 469–492. 10.1007/978-1-4615-9492-5
- Ling GN. A new theoretical foundation for the polarized-oriented multilayer theory of cell water and for inanimate systems demonstrating long-range dynamic structuring of water molecules. Physiol Chem Phys Med NMR. 2003;35:91–130. [PubMed] [Google Scholar]
- Lloyd CR, Eyring EM. Protecting heme enzyme peroxidase activity from H2O2 inactivation by sol−gel encapsulation. Langmuir. 2000;16:9092–9094. doi: 10.1021/la000255q. [DOI] [Google Scholar]
- Löwe M, Kalacheva M, Boersma AJ, Kedrov A. The more the merrier: effects of macromolecular crowding on the structure and dynamics of biological membranes. FEBS J. 2020;287:5039–5067. doi: 10.1111/febs.15429. [DOI] [PubMed] [Google Scholar]
- Lu C-H. Membrane curvature regulates the spatial distribution of bulky glycoproteins. Biophys J . 2023;122:91a. doi: 10.1016/j.bpj.2022.11.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenkov G. Liquid water and ices: understanding the structure and physical properties. J Phys Condens Matter. 2009;21:283101. doi: 10.1088/0953-8984/21/28/283101. [DOI] [PubMed] [Google Scholar]
- Mamone S, Concistrè M, Carignani E, Meier B, Krachmalnicoff A, Johannessen OG, Lei X, Li Y, Denning M, Carravetta M, Goh K, Horsewill AJ, Whitby RJ, Levitt MH (2014) Nuclear spin conversion of water inside fullerene cages detected by low-temperature nuclear magnetic resonance. J Chem Phys 140. 10.1063/1.4873343 [DOI] [PubMed]
- Maximova K, Wojtczak J, Trylska J. Enzyme kinetics in crowded solutions from isothermal titration calorimetry. Anal Biochem. 2019;567:96–105. doi: 10.1016/j.ab.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Meier B, Kouřil K, Bengs C, Kouřilová H, Barker TC, Elliott SJ, Alom S, Whitby RJ, Levitt MH. Spin-isomer conversion of water at room temperature and quantum-rotor-induced nuclear polarization in the water-endofullerene H2O@C60. Phys Rev Lett. 2018;120:266001. doi: 10.1103/PhysRevLett.120.266001. [DOI] [PubMed] [Google Scholar]
- Minton AP. Excluded volume as a determinant of macromolecular structure and reactivity. Biopolym Orig Res Biomol. 1981;20:2093–2120. doi: 10.1002/bip.1981.360201006. [DOI] [Google Scholar]
- Minton AP. The effect of volume occupancy upon the thermodynamic activity of proteins: some biochemical consequences. Mol Cell Biochem. 1983;55:119–140. doi: 10.1007/BF00673707. [DOI] [PubMed] [Google Scholar]
- Mittal J, Best RB. Thermodynamics and kinetics of protein folding under confinement. Proc Natl Acad Sci U S A. 2008;105:20233–20238. doi: 10.1073/pnas.0807742105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möckl L. The emerging role of the mammalian glycocalyx in functional membrane organization and immune system regulation. Front Cell Dev Biol. 2020;8:253. doi: 10.3389/fcell.2020.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morán-Zorzano MT, Viale AM, Muñoz FJ, Alonso-Casajús N, Eydallín GG, Zugasti B, Baroja-Fernández E, Pozueta-Romero J. Escherichia coli AspP activity is enhanced by macromolecular crowding and by both glucose-1,6-bisphosphate and nucleotide-sugars. FEBS Lett. 2007;581:1035–1040. doi: 10.1016/j.febslet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Nawrocki G, Im W, Sugita Y, Feig M. Clustering and dynamics of crowded proteins near membranes and their influence on membrane bending. Proc Natl Acad Sci U S A. 2019;116:24562–24567. doi: 10.1073/pnas.1910771116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Némethy G, Scheraga HA. Structure of water and hydrophobic bonding in proteins. I. A model for the thermodynamic properties of liquid water. J Chem Phys. 1962;36:3382–3400. doi: 10.1063/1.1732472. [DOI] [Google Scholar]
- Nenninger A, Mastroianni G, Mullineaux CW. Size dependence of protein diffusion in the cytoplasm of Escherichia coli. J Bacteriol. 2010;192:4535–4540. doi: 10.1128/JB.00284-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neophytou A, Chakrabarti D, Sciortino F. Topological nature of the liquid–liquid phase transition in tetrahedral liquids. Nat Phys. 2022;18:1248–1253. doi: 10.1038/s41567-022-01698-6. [DOI] [Google Scholar]
- Nilsson A, Pettersson LGM. Perspective on the structure of liquid water. Chem Phys. 2011;389:1–34. doi: 10.1016/j.chemphys.2011.07.021. [DOI] [Google Scholar]
- Nolan V, Clop PD, Burgos MI, Perillo MA. Dual substrate/solvent- roles of water and mixed reaction-diffusion control of beta-Galactosidase catalyzed reactions in PEG-induced macromolecular crowding conditions. Biochem Biophys Res Commun. 2019;515:190–195. doi: 10.1016/j.bbrc.2019.05.081. [DOI] [PubMed] [Google Scholar]
- Nolan V, Collin A, Rodriguez C, Perillo MA. Effect of polyethylene glycol-induced molecular crowding on the enzymatic activity and thermal stability of beta-galactosidase from Kluyveromyces lactis. J Agric Food Chem. 2020;68:8875–8882. doi: 10.1021/acs.jafc.0c02316. [DOI] [PubMed] [Google Scholar]
- Nolan V, Sanchez JM, Perillo MA. PEG-induced molecular crowding leads to a relaxed conformation, higher thermal stability and lower catalytic efficiency of Escherichia coli beta-galactosidase. Colloids Surf B. 2015;136:1202–1206. doi: 10.1016/j.colsurfb.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Olsen LF, Stock RP, Bagatolli LA. Glycolytic oscillations and intracellular K(+) concentration are strongly coupled in the yeast Saccharomyces cerevisiae. Arch Biochem Biophys. 2020;681:108257. doi: 10.1016/j.abb.2020.108257. [DOI] [PubMed] [Google Scholar]
- Oparin AI. The origin of life. New York: McMillan; 1938. [Google Scholar]
- Pastor I, Ferrer ML, Lillo MP, Gomez J, Mateo CR. Structure and dynamics of lysozyme encapsulated in a silica sol-gel matrix. J Phys Chem B. 2007;111:11603–11610. doi: 10.1021/jp074790b. [DOI] [PubMed] [Google Scholar]
- Perillo MA, Scarsdale NJ, Yu RK, Maggio B. Modulation by gangliosides of the lamellar-inverted micelle (hexagonal II) phase transition in mixtures containing phosphatidylethanolamine and dioleoylglycerol. Proc Natl Acad Sci U S A. 1994;91:10019–10023. doi: 10.1073/pnas.91.21.10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershin SM. Coincidence of rotational energy of H2O ortho-para molecules and translation energy near specific temperatures in water and ice. Phys Wave Phenomena. 2008;16:15–25. doi: 10.1007/s11975-008-1003-x. [DOI] [Google Scholar]
- Pershin SM. Conversion of ortho-para H2O isomers in water and a jump in erythrocyte fluidity through a microcapillary at a temperature of 36.6±0.3°C. Phys Wave Phenomena. 2009;17:241–250. doi: 10.3103/S1541308X09040025. [DOI] [Google Scholar]
- Pershin SM. Effect of quantum differences of ortho and para H2O spin-isomers on water properties: biophysical aspect. Biofizika. 2013;58:910–918. [PubMed] [Google Scholar]
- Pershin SM, Bunkin AF, Luk'yanchenko VA. Evolution of the spectral component of ice in the OH band of water at temperatures from 13 to 99°C. Quantum Electron. 2010;40:1146. doi: 10.1070/QE2010v040n12ABEH014397. [DOI] [Google Scholar]
- Popielec A, Ostrowska N, Wojciechowska M, Feig M, Trylska J. Crowded environment affects the activity and inhibition of the NS3/4A protease. Biochimie. 2020;176:169–180. doi: 10.1016/j.biochi.2020.07.009. [DOI] [PubMed] [Google Scholar]
- Potekhin SA, Khusainova RS. Spin-dependent absorption of water molecules. Biophys Chem. 2005;118:84–87. doi: 10.1016/j.bpc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Price WS. NMR studies of translational motion: principles and applications. Cambridge University Press; 2009. [Google Scholar]
- Ravindra R, Zhao S, Gies H, Winter R. Protein encapsulation in mesoporous silicate: the effects of confinement on protein stability, hydration, and volumetric properties. J Am Chem Soc. 2004;126:12224–12225. doi: 10.1021/ja046900n. [DOI] [PubMed] [Google Scholar]
- Rodríguez LC, Foressi NN, Celej MS (2023) Modulation of α-synuclein phase separation by biomolecules. Biochim Biophys Acta (BBA) Proteins Proteomics 1871:140885. 10.1016/j.bbapap.2022.140885 [DOI] [PubMed]
- Salinas N, Povolotsky TL, Landau M, Kolodkin-Gal I. Emerging roles of functional bacterial amyloids in gene regulation, toxicity, and immunomodulation. Microbiol Mol Biol Rev. 2020;85:e00062–e20. doi: 10.1128/MMBR.00062-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JM, Perillo MA. α-Amylase kinetic parameters modulation by lecithin vesicles: binding versus entrapment. Colloids Surf B: Biointerfaces. 2000;18:31–40. doi: 10.1016/S0927-7765(99)00128-9. [DOI] [Google Scholar]
- Sanchez JM, Perillo MA. Membrane adsorption or penetration differentially modulates β-galactosidase activity against soluble substrates. Colloids Surf B. 2002;24:21–31. doi: 10.1016/S0927-7765(01)00216-8. [DOI] [Google Scholar]
- Sanchez JM, Perillo MA. Membrane topology modulates beta-galactosidase activity against soluble substrates. Biophys Chem. 2002;99:281–295. doi: 10.1016/s0301-4622(02)00229-6. [DOI] [PubMed] [Google Scholar]
- Sanchez JM, Lopez-Laguna H, Alamo P, Serna N, Sanchez-Chardi A, Nolan V, Cano-Garrido O, Casanova I, Unzueta U, Vazquez E, Mangues R, Villaverde A. Artificial inclusion bodies for clinical development. Adv Sci (Weinh) 2020;7:1902420. doi: 10.1002/advs.201902420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JM, López-Laguna H, Serna N, Unzueta U, Clop PD, Villaverde A, Vazquez E. Engineering the performance of artificial inclusion bodies built of catalytic β-galactosidase. ACS Sustain Chem Eng. 2021;9:2552–2558. doi: 10.1021/acssuschemeng.0c08345. [DOI] [Google Scholar]
- Sánchez JM, Nolan V, Perillo MA. β-Galactosidase at the membrane–water interface: a case of an active enzyme with non-native conformation. Colloids Surf B. 2013;108:1–7. doi: 10.1016/j.colsurfb.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Schneider MF, Lim K, Fuller GG, Tanaka M. Rheology of glycocalix model at air/water interface. Phys Chem Chem Phys. 2002;4:1949–1952. doi: 10.1039/B110631G. [DOI] [Google Scholar]
- Shahid S, Ahmad F, Hassan MI, Islam A. Mixture of macromolecular crowding agents has a non-additive effect on the stability of proteins. Appl Biochem Biotechnol. 2019;188:927–941. doi: 10.1007/s12010-019-02972-9. [DOI] [PubMed] [Google Scholar]
- Shi X, Wu D, Xu Y, Yu X. Engineering the optimum pH of beta-galactosidase from Aspergillus oryzae for efficient hydrolysis of lactose. J Dairy Sci. 2022;105:4772–4782. doi: 10.3168/jds.2021-21760. [DOI] [PubMed] [Google Scholar]
- Shin S, Kim HS, Kim MI, Lee J, Park HG, Kim J. Crowding and confinement effects on enzyme stability in mesoporous silicas. Int J Biol Macromol. 2020;144:118–126. doi: 10.1016/j.ijbiomac.2019.12.034. [DOI] [PubMed] [Google Scholar]
- Siddiqui GA, Naeem A. Connecting the dots: macromolecular crowding and protein aggregation. J Fluoresc. 2023;33:1–11. doi: 10.1007/s10895-022-03082-2. [DOI] [PubMed] [Google Scholar]
- Stampolidis P, Kaderbhai NN, Kaderbhai MA. Periplasmically-exported lupanine hydroxylase undergoes transition from soluble to functional inclusion bodies in Escherichia coli. Arch Biochem Biophys. 2009;484:8–15. doi: 10.1016/j.abb.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Stapf S, Kimmich R. Molecular dynamics in confined monomolecular layers. A field-cycling nuclear magnetic resonance relaxometry study of liquids in porous glass. J Chem Phys. 1995;103:2247–2250. doi: 10.1063/1.469700. [DOI] [Google Scholar]
- Stark BC, Wen K-K, Allingham JS, Rubenstein PA, Lord M. Functional adaptation between yeast actin and its cognate myosin motors. J Biol Chem. 2011;286:30384–30392. doi: 10.1074/jbc.M111.262899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamuku T, Yamagami M, Wakita H, Masuda Y, Yamaguchi T. Thermal property, structure, and dynamics of supercooled water in porous silica by calorimetry, neutron scattering, and NMR relaxation. J Phys Chem B. 1997;101:5730–5739. doi: 10.1021/jp9631238. [DOI] [Google Scholar]
- Thoke HS, Thorsteinsson S, Stock RP, Bagatolli LA, Olsen LF. The dynamics of intracellular water constrains glycolytic oscillations in Saccharomyces cerevisiae. Sci Rep. 2017;7:16250. doi: 10.1038/s41598-017-16442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoke HS, Tobiesen A, Brewer J, Hansen PL, Stock RP, Olsen LF, Bagatolli LA. Tight coupling of metabolic oscillations and intracellular water dynamics in Saccharomyces cerevisiae. PLoS One. 2015;10:e0117308. doi: 10.1371/journal.pone.0117308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonov VI, Volkov AA. Separation of water into its ortho and para isomers. Science. 2002;296:2363. doi: 10.1126/science.1069513. [DOI] [PubMed] [Google Scholar]
- Tikhonov VI, Volkov AA. Adsorption isotherms of ortho- and para-water. ChemPhysChem. 2006;7:1026–1027. doi: 10.1002/cphc.200500627. [DOI] [PubMed] [Google Scholar]
- Urbonaite V, de Jongh HH, van der Linden E, Pouvreau L. Protein aggregates may differ in water entrapment but are comparable in water confinement. J Agric Food Chem. 2015;63:8912–8920. doi: 10.1021/acs.jafc.5b03784. [DOI] [PubMed] [Google Scholar]
- Van der Henst C, Charlier C, Deghelt M, Wouters J, Matroule JY, Letesson JJ, De Bolle X. Overproduced Brucella abortus PdhS-mCherry forms soluble aggregates in Escherichia coli, partially associating with mobile foci of IbpA-YFP. BMC Microbiol. 2010;10:248. doi: 10.1186/1471-2180-10-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturoli D, Rippe B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: effects of molecular size, shape, charge, and deformability. Am J Physiol-Renal Physiol. 2005;288:F605–F613. doi: 10.1152/ajprenal.00171.2004. [DOI] [PubMed] [Google Scholar]
- Vöpel T, Makhatadze GI. Enzyme activity in the crowded milieu. PLoS One. 2012;7:e39418. doi: 10.1371/journal.pone.0039418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vweza A-O, Song C-G, Chong K-T. Liquid–liquid phase separation in the presence of macromolecular crowding and state-dependent kinetics. Int J Mol Sci. 2021;22:6675. doi: 10.3390/ijms22136675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Jo H, DeGrado WF, Hong M. Water distribution, dynamics, and interactions with Alzheimer’s β-amyloid fibrils investigated by solid-state NMR. J Am Chem Soc. 2017;139:6242–6252. doi: 10.1021/jacs.7b02089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiß RG, Heyden M, Dzubiella J. Curvature dependence of hydrophobic hydration dynamics. Phys Rev Lett. 2015;114:187802. doi: 10.1103/PhysRevLett.114.187802. [DOI] [PubMed] [Google Scholar]
- Wu S, Ellerby LM, Cohan JS, Dunn B, El-Sayed MA, Valentine JS, Zink JI. Bacteriorhodopsin encapsulated in transparent sol-gel glass: a new biomaterial. Chem Mater. 1993;5:115–120. doi: 10.1021/cm00025a022. [DOI] [Google Scholar]
- Yoo H, Paranji R, Pollack GH. Impact of hydrophilic surfaces on interfacial water dynamics probed with NMR spectroscopy. J Phys Chem Lett. 2011;2:532–536. doi: 10.1021/jz200057g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Luo X-A, Zhu L-J, Wang S-Z, Jia M-Q, Chen Z-X. Catalytic behavior of pancreatic lipase in crowded medium for hydrolysis of medium-chain and long-chain lipid: an isothermal titration calorimetry study. J Thermochim Acta. 2019;672:70–78. doi: 10.1016/j.tca.2018.12.015. [DOI] [Google Scholar]
- Zhang H, Ji X, Li P, Liu C, Lou J, Wang Z, Wen W, Xiao Y, Zhang M, Zhu X. Liquid-liquid phase separation in biology: mechanisms, physiological functions and human diseases. Sci China Life Sci. 2020;63:953–985. doi: 10.1007/s11427-020-1702-x. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hartmann M. Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem Soc Rev. 2013;42:3894–3912. doi: 10.1039/C3CS60059A. [DOI] [PubMed] [Google Scholar]
- Zimmerman JR, Holmes BG, Lasater JA. A study of adsorbed wateron silica gel by nuclear resonance techniques. J Phys Chem B. 1956;60:1157–1161. doi: 10.1021/j150543a002. [DOI] [Google Scholar]