Abstract
Background
A significant proportion of cardiac surgery intensive care unit (CSICU) patients require long-term ventilation, necessitating tracheostomy placement. The goal of this study was to evaluate the long-term postoperative outcomes and complications associated with percutaneous dilatational tracheostomy (PDT) in CSICU patients.
Methods
All patients undergoing PDT after cardiac, thoracic, or vascular operations in the CSICU between January 1, 2013 and January 1, 2021 were identified. They were evaluated for mortality, decannulation time, and complications including bleeding, infection, and need for surgical intervention. Multivariable regression models were used to identify predictors of early decannulation and the complication rate.
Results
Ninety-three patients were identified for this study (70 [75.3%] male and 23 [24.7%] female). Furthermore, 18.3% of patients had chronic obstructive pulmonary disease (COPD), 21.5% had history of stroke, 7.5% had end-stage renal disease, 33.3% had diabetes, and 59.1% were current smokers. The mean time from PDT to decannulation was 39 days. Roughly one-fifth (20.4%) of patients were on dual antiplatelet therapy and 81.7% had anticoagulation restarted 8 hours post-tracheostomy. Eight complications were noted, including 5 instances of bleeding requiring packing and 1 case of mediastinitis. There were no significant predictors of decannulation prior to discharge. Only COPD was identified as a negative predictor of decannulation at any point in time (hazard ratio, 0.28; 95% confidence interval, 0.08–0.95; p=0.04).
Conclusion
Percutaneous tracheostomy is a safe and viable alternative to surgical tracheostomy in cardiac surgery ICU patients. Patients who undergo PDT have a relatively short duration of tracheostomy and do not have major post-procedural complications.
Keywords: Tracheostomy, Cardiac surgery, Intensive care unit
Introduction
Critically ill patients in surgical intensive care units (ICUs) tend to have prolonged respiratory distress, necessitating long-term ventilatory support. In such cases, tracheostomy is a common procedure, proven to reduce the incidence of pneumonia, decrease the duration of ventilatory support, minimize the need for sedation, and enhance pulmonary hygiene [1-3]. Approximately 10% of patients undergoing cardiac surgery require prolonged mechanical ventilation and, subsequently, a tracheostomy [4]. However, in this demographic, tracheostomy often signifies a poor prognosis, with a mortality rate exceeding 60% within the first year and a mere 16% survival rate at 5 years [4,5].
In recent years, bedside percutaneous dilatational tracheostomy (PDT) has gained popularity in surgical ICUs due to its reduced incidence of wound infection, clinically relevant bleeding, and lower costs compared to open tracheostomies [1-4,6]. However, there are concerns about performing PDT on patients in the cardiac surgery ICU (CSICU), particularly those requiring therapeutic anticoagulation, such as patients on extracorporeal membrane oxygenation (ECMO) or those with support devices like a left ventricular assist device (LVAD) [7-9]. In this retrospective study conducted at a single quaternary referral center and safety net hospital, we aimed to assess the long-term postoperative outcomes and complications associated with PDT in CSICU patients, including those who had undergone cardiac, thoracic, and vascular surgery. Additionally, we describe the PDT technique and our strategies for optimizing clinical success.
Methods
Study design
This retrospective cohort study examined all patients who underwent bedside PDT in the CSICU. The patients were identified from a single institution, the Virginia Commonwealth University Medical Center, during the period from January 1, 2013 to January 1, 2021. The inclusion criteria encompassed all patients who underwent bedside PDT in the CSICU. Patient information was obtained from electronic records, which included demographics, comorbidities, perioperative lab values, anticoagulation status, decannulation status, mortality, and complications. The time of decannulation was determined based on the earliest documentation or radiologic evidence of the patient being free from tracheostomy. Clinically significant bleeding was defined as bleeding that necessitated packing around the tracheostomy site. The presence of a surgical site infection (SSI) was determined based on whether provider notes explicitly mentioned erythema or fluctuance around the tracheostomy site, or whether antibiotics were initiated for a suspected SSI within 10 days of PDT. The Institutional Review Board at the Virginia Commonwealth University Health System (Protocol ID: HM20021658) granted an exemption for this study protocol and approved a waiver of consent due to the retrospective nature of the study.
Statistical analysis
Continuous variables were expressed as a mean and standard deviation. Categorical variables were presented as percentages and absolute numbers. To evaluate predictor variables for mortality and decannulation, we generated multivariable logistic or linear regression models using backward elimination methods. These models took into account multiple comorbidities and perioperative values. We reported parameter estimates for all tested variables, complete with 95% confidence intervals. We deemed all p-values less than 0.05 as statistically significant. We conducted all statistical analyses using IBM SPSS ver. 28.0 (IBM Corp., Armonk, NY, USA).
Tracheostomy technique
Preparation
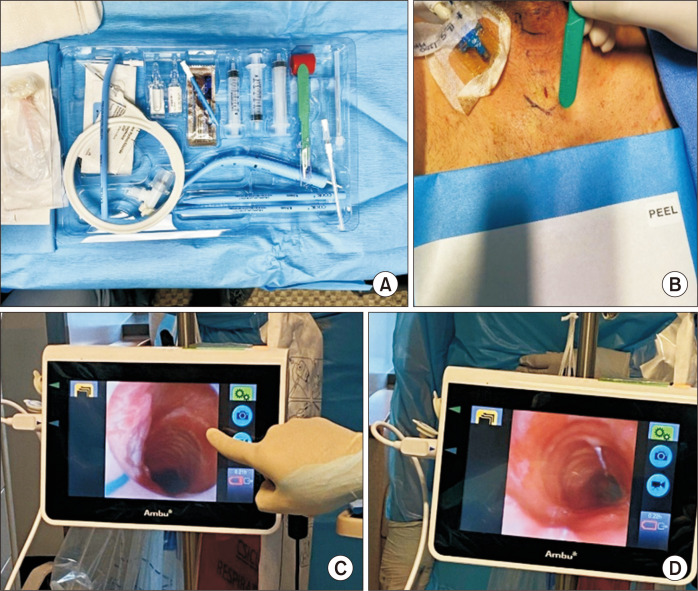
All patients who underwent PDT required prolonged mechanical ventilation secondary to either primary or postoperative respiratory distress. Before the operation, the necessary equipment was gathered at the bedside. This included a flexible video bronchoscope, medications for sedation and paralysis, a bronchoscope attachment for the ventilator, a bag mask, surgical lubricant, a tracheostomy tube (usually a 7.5- to 8.5-French cuffed Shiley), and a percutaneous tracheostomy kit. We typically start with an 8-French cuffed Shiley tracheostomy tube, but smaller sizes (6 or 7) can be used if needed. The kit (Fig. 1A) used at our institution is the Ciaglia G2 Blue Rhino set (Cook Medical Inc., Bloomington, IN, USA). These kits contain an introducer needle and dilator, guidewire, tracheal dilator, protective sheath, #15 scalpel, curved hemostat, and a tracheostomy tube with an inner cannula. To ensure the procedure was conducted safely, all personnel in the room were required to wear personal protective equipment suitable for an aerosol-generating procedure. The procedure required 3 providers: the operating physician, a respiratory therapist, and a bronchoscopist, who was typically the anesthesiologist overseeing the ICU.
Fig. 1.
(A) The G2 Blue Rhino set from Cook Medical. (B) Once the patient was appropriately positioned and prepped, landmarks were identified and an 8-mm vertical incision was made over the cricoid cartilage. (C) Once the operator had dissected down to the trachea, the bronchoscope and endotracheal tube were withdrawn until the first three tracheal rings were visualized by confirming the triangulation of the subglottic trachea. (D) Once proper visualization was obtained, the introducer needle was placed, ideally anywhere between the first and third tracheal rings.
Technique
The patient was positioned supine with their neck extended. A shoulder roll was utilized to facilitate exposure of the anterior neck. The ICU bed was set to its maximum inflation setting to provide a firm surface that would not yield under pressure when the tracheostomy tube was pushed through using the guidewire. The patient was then administered 100% FiO2 for 5 minutes for pre-oxygenation, and a bronchoscopy was performed to clear all secretions. Once appropriate sedation and paralysis had been administered, the anterior neck was sterilized and draped, ensuring easy access to the endotracheal tube. The endotracheal tube circuit was modified to accommodate the bronchoscope. An 8 mm vertical incision was made in the neck, overlying the trachea and cricoid cartilage (Fig. 1B). The pretracheal tissue was bluntly dissected until the trachea could be palpated. This dissection was kept to a minimum to prevent the creation of a large subcutaneous space where bleeding could occur. The bronchoscope was then advanced into the airway and aligned with the end of the tube, ensuring that secretions were cleared along the way. At this point, the respiratory therapist deflated the tube cuff. The tube and the bronchoscope were then slowly withdrawn until the first 3 tracheal rings were identified, along with the triangulation of the subglottic trachea. This confirmed that the endotracheal tube had been retracted sufficiently to allow accurate counting of the tracheal rings (Fig. 1C). External palpation of the trachea can assist in identification.
The tracheostomy tube was inserted using the Seldinger technique. The introducer needle was positioned through the anterior tracheal wall under direct bronchoscopic visualization. The needle should be oriented bevel-down, perpendicular to the trachea, and ideally inserted between the first and third tracheal rings (Fig. 1D). It is crucial that the endotracheal tube is located proximal to the needle insertion point to ensure the balloon remains intact throughout the procedure. If the patient’s oxygen saturation levels drop, the endotracheal tube can be advanced and the balloon re-inflated. Next, the guidewire was fed through the needle and visualized as it advanced distally toward the carina. The bevel-down orientation of the needle should guide this directionality. The needle was then removed over the wire while the wire’s position was confirmed via the bronchoscope. The small tracheal dilator was advanced over the wire to widen the tract. Following this, a progressive dilator with a sheath was advanced over the wire. The dilator was then removed and an appropriately sized tracheostomy tube was inserted over the wire and sheath under direct visualization. A small skin incision was made, and the tracheostomy tube should meet with slight resistance as it passed through the skin. This resistance created a snug seal between the tube and skin, which is a crucial technical point that helps minimize post-procedure bleeding in patients on systemic anticoagulation or dual antiplatelet therapy. Once the tube was in place, the sheath was removed, the tracheostomy cuff was inflated, and the circuit was connected to the tube to resume ventilation.
Initial confirmation was achieved by detecting the presence of end-tidal carbon dioxide in the circuit. Visual confirmation should be secured by inserting the bronchoscope through the tube and verifying that the tube is positioned above the carina, rather than lying in a pretracheal plane. Once the tube was satisfactorily placed, it was secured with 2 Prolene stitches on each side and a tracheostomy collar. Subsequently, the oral endotracheal tube was removed. The stitches were typically removed on postoperative day 7.
Results
Ninety-three patients were identified for this study (70 [75.3%] male and 23 [24.7%] female). The mean age was 57.8 years, and majority of the patients were either overweight or obese (67.4%). Furthermore, 18.3% of patients had chronic obstructive pulmonary disease (COPD), 21.5% had a history of stroke, 7.5% had end-stage renal disease (ESRD), 33.3% had diabetes, and 59.1% were current smokers. The majority of patients in this group underwent cardiac surgery, with small minority undergoing thoracic and vascular procedures (Table 1). At the time of operation, the mean hemoglobin level, white blood cell count, platelet count, international normalized ratio, and partial prothrombin time, were 8.1 g/dL, 19.5 (109)/L, 191.6 (109)/L, 1.5, and 55.2 seconds, respectively. In total, 22.6% of patients were either on ECMO or had an LVAD prior to or at the time of operation. The average post-oxygenator O2 was 267.2 mm Hg for patients on ECMO during the procedure (Table 2).
Table 1.
Patient demographics/characteristics
| Characteristic | Value |
|---|---|
| Sex | |
| Male | 70 (75.3) |
| Female | 23 (24.7) |
| Age (yr) | 57.8±15.6 (17.0–84.0) |
| COPD | 17 (18.3) |
| Stroke | 20 (21.5) |
| End-stage renal disease | 7 (7.5) |
| Diabetes | 31 (33.3) |
| Current smoking | 55 (59.1) |
| Body mass index | |
| Underweight | 4 (4.3) |
| Normal | 26 (28.3) |
| Overweight | 18 (19.6) |
| Obese | 44 (47.8) |
| Surgery type | |
| Valve | 19 (20.7) |
| CABG | 11 (12.0) |
| ECMO | 11 (12.0) |
| Transplant | 8 (8.7) |
| CABG+ | 8 (8.7) |
| Aortic replacement | 9 (9.8) |
| Impella/LVAD | 7 (7.6) |
| Lung resection | 5 (5.4) |
| Vascular | 4 (4.3) |
| Esophagectomy | 3 (3.3) |
| Total artificial heart | 1 (1.1) |
| Esophageal stent | 1 (1.1) |
| Embolectomy | 1 (1.1) |
| Aorto-enteric fistula | 1 (1.1) |
| Other thoracic | 3 (3.3) |
Values are presented as number (%) or mean±standard deviation (range).
COPD, chronic obstructive pulmonary disease; CABG, coronary artery bypass graft; ECMO, extracorporeal membrane oxygenation; LVAD, left ventricular assist device.
Table 2.
Perioperative factors for patients undergoing percutaneous dilatational tracheostomy
| Perioperative factors | Value |
|---|---|
| Hemoglobin (g/dL) | 8.1±1.2 (6.2–13.9) |
| White blood cells (×109/L) | 19.5±20.8 (4.6–149.0) |
| Platelet count (×109/L) | 191.6±128.6 (8.7–750.0) |
| International normalized ratio | 1.5±0.3 (0.9–2.6) |
| Partial thromboplastin time (sec) | 55.2±19.6 (4.2–130.0) |
| Chronic kidney disease status | |
| Stage 1 | 16 (17.4) |
| Stage 2 | 21 (22.8) |
| Stage 3 | 38 (41.3) |
| Stage 4 | 14 (15.2) |
| Stage 5 | 3 (3.3) |
| Ejection fraction (%) | 47.7 (10.0–75.0) |
| Dual antiplatelet therapy | 19 (20.4) |
| Anticoagulation after 8 hours | 76 (81.7) |
| ECMO/LVAD | 21 (22.6) |
| ECMO O2 (mm Hg) (n=10) | 267.2±114.9 (120.0–471.0) |
Values are presented as mean±standard deviation (range), number (%), or mean (range). Laboratory values closest in time prior to the procedure were utilized.
ECMO, extracorporeal membrane oxygenation; LVAD, left ventricular assist device.
The average duration from the index surgery to tracheostomy was 12.5 days. The average time from the placement of the tracheostomy to the first downsize was 18.9 days. The mean duration from the procedure to decannulation was 39 days. Prior to discharge, 30 patients (32.3%) were decannulated. The average length of stay in the hospital and the ICU was 56.4 days and 40.2 days, respectively. Of the patients, 28 (30.1%) died while receiving inpatient care, 9 (9.7%) were discharged to their homes, and the remaining 56 (60.2%) were transferred to rehabilitation, a skilled nursing facility, or a long-term care facility (Table 3).
Table 3.
Postoperative outcomes in patients receiving percutaneous dilatational tracheostomy
| Outcome variable | Value |
|---|---|
| Time to tracheostomy (day) | 12.5 (1.0–33.0) |
| Time to first downsize (day) (n=57) | 18.9 (3.0–95.0) |
| Time to decannulation (day) (n=44) | 39.0 (9.0–110.0) |
| Decannulation prior to discharge | 30 (32.3) |
| Intensive care unit time (day) | 40.2 (8.0–174.0) |
| Ventilation time (day) | 37.4 (2.0–122.0) |
| Total length of stay (day) | 56.4 (13.0–292.0) |
| 6-Month survival (n=78) | 48 (61.5) |
| 1-Year survival (n=69) | 39 (56.5) |
| 2-Year survival (n=65) | 33 (50.8) |
| Disposition | |
| Death | 28 (30.1) |
| Home | 9 (9.7) |
| Inpatient transfer | 4 (4.3) |
| Long-term acute care | 20 (21.5) |
| Rehab | 23 (24.7) |
| Skilled nursing facility | 6 (6.5) |
| Outside hospital transfer | 3 (3.2) |
Values are presented as mean (range) or number (%). Patients were dropped in 6-month, 1-year, and 2-year survival due to lack of follow-up.
Eight complications were noted. Clinically significant bleeding necessitating bedside packing was experienced by 5 patients (5.4%). Two patients (2.2%) required transfusions of packed red blood cells. None of these bleeding incidents necessitated a visit to the operating room or a bronchoscopy to remove clots or clear the airway. One patient (1.1%) developed mediastinitis during their hospital stay. There were no instances of skin site infections or conversions to open tracheostomy. One patient needed a bedside revision due to an accidental dislodgement of the tracheostomy (Table 4).
Table 4.
Complication rates associated with PDT
| Complication | No. (%) |
|---|---|
| Site infection | 0 |
| Bleeding | 5 (5.4) |
| Transfusion | 2 (2.2) |
| Need for surgical intervention | 0 |
| Mediastinitis | 1 (1.1) |
Complication rates associated with PDT are noted as percentages. Bleeding was considered significant if any bedside packing or operative intervention was required. Infection was noted if documentation explicitly mentioned erythema/fluctuance or antibiotic initiation in association with the PDT site.
PDT, percutaneous dilatational tracheostomy.
A logistic regression model was employed to determine the influence of various comorbidities and perioperative factors on decannulation before discharge and postoperative complications. However, none of the parameters emerged as significant predictors of either decannulation prior to discharge or postoperative complications. Another logistic regression was conducted to identify predictors of decannulation at any given time. By employing backward elimination to discard insignificant variables, a suitable model was created (c2=4.73, N-R2=0.07, p=0.03) that demonstrated COPD as a significant predictor of eventual decannulation (hazard ratio, 0.28; 95% confidence interval, 0.08–0.95; p=0.04). Attempts were made to generate multivariable linear regression models for tracheostomy exchange and ventilatory time. However, these models failed to achieve statistical significance.
Discussion
Patients in surgical ICUs, especially those undergoing cardiothoracic surgery, are increasingly presenting with a higher number of comorbidities and are more critically ill than in previous years. This trend has amplified the demand for long-term respiratory support. PDT is a proven method for providing this respiratory support. It has been shown to be quicker and safer than open tracheostomy in the surgical ICU environment, even for coagulopathic populations such as cirrhotic and liver transplant patients. However, there are currently limited data on the use of PDT in CSICU patients and the predictive factors associated with its effectiveness.
The characteristics and comorbidities of our patients, which included age, sex, COPD, ESRD, diabetes, stroke, and ejection fraction, align with those found in other single-center studies. The only exception is smoking status, as the majority of our cohort were current smokers upon admission. The rate of complications was also minimal, a finding that is consistent with other studies that have investigated tracheostomy in cardiac surgery or ECMO/LVAD patients [4,7-11]. The survival rate observed in this study is higher than that reported in other studies that have examined tracheostomies in either cardiac surgery or mixed ICUs. While other retrospective studies typically report a 1-year survival rate of 30%–40% [4,10,11], our cohort demonstrated a 2-year survival rate of 50%. This discrepancy could potentially be attributed to our smaller sample size and younger population.
Our average time to decannulation was 39 days, with 44 (67.7%) surviving patients achieving decannulation upon follow-up. This duration is slightly longer than the average 2-week period typically observed in general surgical ICUs. This aligns with a recent study by Krebs et al. [5], which examined tracheostomy outcomes (both open and percutaneous) in a single-center CSICU. Their data revealed a longer decannulation time of 60 days, but a high decannulation rate of 80% at 1 year. The differences between these 2 datasets are likely due to our smaller sample size and higher 1-year survival rate. In terms of decannulation before discharge, our models did not identify any significant predictors. When assessing decannulation at any given time, COPD was the only statistically significant negative predictor. Several studies have identified various predictors of early versus late decannulation in ICU patients, including absence of coma, cardiothoracic surgery status, and chronic pulmonary disease [12,13]. Furthermore, in their study, Krebs et al. [5] found that younger age, lack of COPD, and the absence of dialysis were independently associated with early decannulation. The influence of COPD on decannulation can be reasonably attributed to the fact that obstructive pulmonary processes can negatively affect secretion management and capping tolerance, thereby limiting readiness for decannulation. However, this factor is not associated with the tracheostomy insertion technique and could be applicable to both percutaneous and open tracheostomy.
Previous studies have highlighted that PDT is both time and cost-efficient when compared to open tracheostomy. Although there are no documented times for bedside procedures, the authors’ experience suggests that most tracheostomies in this series typically take around 5–15 minutes. In contrast, open tracheostomy at our institution usually takes 15–20 minutes, excluding the time for setup and patient transport to the operating room. This not only presents an additional risk to critically ill patients but also necessitates the involvement of more healthcare professionals. Moreover, the cost of PDT is generally $900–$1,600 less than that of traditional open tracheostomy. This is because the standard approach includes the cost of the operating room and additional personnel [14,15].
As previously discussed, this study has several significant limitations. It included only 93 patients, which, while larger than most other studies examining PDT in CSICU patients, still constitutes a low-power study. The patient population was highly heterogeneous, having undergone a range of index operations, including routine coronary artery bypass grafting, aortic arch replacement, heart transplant, esophagectomy, lobectomy, and peripheral vascular reconstruction. A crucial variable associated with tracheostomy placement is the initial indication. For the majority of patients, the need for tracheostomy was due to prolonged ventilatory requirements, which restricts our ability to evaluate the predictive value of this variable. These factors undermine the reliability and robustness of the regression models. Although a study has examined these patients over a longer period [4], a 2-year follow-up is inadequate for determining long-term outcomes. Moreover, the data were not collected as part of a prospective trial, resulting in a significant number of patients lost to follow-up at each time point. The findings of this study may not be applicable to other hospitals, thus highlighting the need for a multicenter, prospective design.
In this study, we explored our institution’s experience with the use of percutaneous tracheostomy within the context of CSICU patients at our institution. The PDT procedure exhibits minimal postoperative complications, and the 1-year survival rate of these patients aligns with those reported in other studies. Although larger prospective studies are necessary to identify factors that reduce postoperative complications and decannulation time, our research underscores the safety and viability of PDT as an alternative to surgical tracheostomy in this group of patients.
Funding Statement
Funding This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Article information
Author contributions
Conceptualization: VV, RDS. Data curation: GI, TJ, KA, VV. Formal analysis: VV, YA. Methodology: VV, YA, RDS. Project administration: VV, RDS. Visualization: VV, RDS. Writing–original draft: VV, YA, GI, TJ, KA. Writing–review & editing: all authors. Final approval of the manuscript: all authors.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Murthy SC, Arroliga AC, Walts PA, et al. Ventilatory dependency after cardiovascular surgery. J Thorac Cardiovasc Surg. 2007;134:484–90. doi: 10.1016/j.jtcvs.2007.03.006. https://doi.org/10.1016/j.jtcvs.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Sareh S, Toppen W, Ugarte R, et al. Impact of early tracheostomy on outcomes after cardiac surgery: a national analysis. Ann Thorac Surg. 2021;111:1537–44. doi: 10.1016/j.athoracsur.2020.07.027. https://doi.org/10.1016/j.athoracsur.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Freeman BD, Morris PE. Tracheostomy practice in adults with acute respiratory failure. Crit Care Med. 2012;40:2890–6. doi: 10.1097/CCM.0b013e31825bc948. https://doi.org/10.1097/CCM.0b013e31825bc948. [DOI] [PubMed] [Google Scholar]
- 4.Terragni PP, Antonelli M, Fumagalli R, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA. 2010;303:1483–9. doi: 10.1001/jama.2010.447. https://doi.org/10.1001/jama.2010.447. [DOI] [PubMed] [Google Scholar]
- 5.Krebs ED, Chancellor WZ, Beller JP, et al. Long-term implications of tracheostomy in cardiac surgery patients: decannulation and mortality. Ann Thorac Surg. 2021;111:594–9. doi: 10.1016/j.athoracsur.2020.05.052. https://doi.org/10.1016/j.athoracsur.2020.05.052. [DOI] [PubMed] [Google Scholar]
- 6.Smith MC, Evans PT, Prendergast KM, et al. Surgical outcomes and complications of bedside tracheostomy in the ICU for patients on ECMO. Perfusion. 2022;37:26–30. doi: 10.1177/0267659120979564. https://doi.org/10.1177/0267659120979564. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer A, Schneeberger Y, Reichart D, et al. Percutaneous dilatation tracheostomy in patients with left ventricular assist device and established phenprocoumon therapy. ASAIO J. 2016;62:715–8. doi: 10.1097/MAT.0000000000000426. https://doi.org/10.1097/MAT.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 8.Bektas S, Cavus M, Turan S. Percutaneous dilatational tracheostomy in patients with mechanical circulatory support: Is the procedure safe? Turk Gogus Kalp Damar Cerrahisi Derg. 2020;28:435–41. doi: 10.5606/tgkdc.dergisi.2020.19642. https://doi.org/10.5606/tgkdc.dergisi.2020.19642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braune S, Kienast S, Hadem J, et al. Safety of percutaneous dilatational tracheostomy in patients on extracorporeal lung support. Intensive Care Med. 2013;39:1792–9. doi: 10.1007/s00134-013-3023-8. https://doi.org/10.1007/s00134-013-3023-8. [DOI] [PubMed] [Google Scholar]
- 10.Ballotta A, Kandil H, Generali T, et al. Tracheostomy after cardiac operations: in-hospital and long-term survival. Ann Thorac Surg. 2011;92:528–33. doi: 10.1016/j.athoracsur.2011.02.002. https://doi.org/10.1016/j.athoracsur.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Walts PA, Murthy SC, Arroliga AC, et al. Tracheostomy after cardiovascular surgery: an assessment of long-term outcome. J Thorac Cardiovasc Surg. 2006;131:830–7. doi: 10.1016/j.jtcvs.2005.09.038. https://doi.org/10.1016/j.jtcvs.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 12.Leung R, MacGregor L, Campbell D, Berkowitz RG. Decannulation and survival following tracheostomy in an intensive care unit. Ann Otol Rhinol Laryngol. 2003;112:853–8. doi: 10.1177/000348940311201005. https://doi.org/10.1177/000348940311201005. [DOI] [PubMed] [Google Scholar]
- 13.Tobin AE, Santamaria JD. An intensivist-led tracheostomy review team is associated with shorter decannulation time and length of stay: a prospective cohort study. Crit Care. 2008;12:R48. doi: 10.1186/cc6864. https://doi.org/10.1186/cc6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman BD, Isabella K, Cobb JP, et al. A prospective, randomized study comparing percutaneous with surgical tracheostomy in critically ill patients. Crit Care Med. 2001;29:926–30. doi: 10.1097/00003246-200105000-00002. https://doi.org/10.1097/00003246-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Bowen CP, Whitney LR, Truwit JD, Durbin CG, Moore MM. Comparison of safety and cost of percutaneous versus surgical tracheostomy. Am Surg. 2001;67:54–60. doi: 10.1177/000313480106700113. https://doi.org/10.1177/000313480106700113. [DOI] [PubMed] [Google Scholar]