Abstract
The recently discovered human lncRNA NORAD is induced after DNA damage in a p53-dependent manner. It plays a critical role in the maintenance of genomic stability through interaction with Pumilio proteins, limiting the repression of their target mRNAs. Therefore, NORAD inactivation causes chromosomal instability and aneuploidy, which contributes to the accumulation of genetic abnormalities and tumorigenesis. NORAD has been detected in several types of cancer, including breast cancer, which is the most frequently diagnosed and the second-leading cause of cancer death in women. In the present study, we confirmed upregulated NORAD expression levels in a set of human epithelial breast cancer cell lines (MDA-MB-231, MDA-MB-436, and MDA-MB-468), which belong to the most aggressive subtypes (triple-negative breast cancer). These results are in line with previous data showing that high NORAD expression levels in basal-like tumors were associated with poor prognosis. Here, we demonstrate that NORAD downregulation sensitizes triple-negative breast cancer cells to chemotherapy, through a potential accumulation of genomic aberrations and an impaired capacity to signal DNA damage. These results show that NORAD may represent an unexploited neoadjuvant therapeutic target for chemotherapy-unresponsive breast cancer.
Keywords: MT: Non-coding RNAs, NORAD, DNA damage, breast cancer, chemotherapy, H2Ax, triple-negative breast cancer
Graphical abstract
Bernardes de Jesus and colleagues uncover how NORAD downregulation sensitizes triple-negative breast cancer cells to chemotherapy, through an impaired DNA damage response, highlighting how NORAD may represent an unexploited therapeutic target for breast cancer.
Introduction
The Human Genome Project provided scientists and society with transformational insights into the intriguing complexity of the transcriptome of human cells.1 Long non-coding RNAs (lncRNAs) constitute the broadest class of non-coding RNAs, displaying a tissue-specific spatiotemporal expression profile,2 with numerous biological roles identified, spanning from development to aging, in both normal and pathological conditions, such as age-related diseases.3,4,5 The study of differential gene expression in cancer has led to the identification of thousands of associated lncRNAs2 involved in several cancer hallmarks including genomic instability, tumor-promoting inflammation, and evasion of immune detection.6,7 LncRNA NORAD8 is a 5.3 kb transcript, annotated as LINC00657, localized on chromosome 20 (20q11.23).9 NORAD shows strong evolutionary conservation and is widely expressed in human tissues and cell lines.9,10 NORAD seems to play a crucial role in the maintenance of genomic stability: its inactivation triggers chromosomal instability in previously karyotypically stable cell lines, and expression levels of this lncRNA seem to increase after inducing DNA damage with doxorubicin.9 One of the possible mechanisms involves NORAD sequestering PUMILIO-1 and PUMILIO-2 RNA-binding proteins that target mRNAs and reduce their stability.9,11,12,13 PUMILIO interaction seems to be mediated by SAM68, an abundant and multifunctional cell-cycle-regulated RNA-binding protein.14 Therefore, NORAD levels directly influence the availability of PUMILIO to downregulate a set of factors involved in mitosis, DNA repair, and DNA replication.9 Nonetheless, many genes regulated by NORAD are not PUMILIO targets, suggesting that other mechanistic events are involved, such as miRNA sponging. Considering the complexity of the NORAD network, NORAD appears to have a dual effect depending on the tumor type.9,15,16 Among those interactors, there was shown to be enrichment of DNA damage response (DDR)-associated proteins, mitotic cell cycle and minichromosome maintenance (MCM) complex.17 Some previously identified NORAD interactors are nucleosome assembly protein 1-like 4 (NAP1L4),17 a histone chaperone18 involved in the chromatin assembly step related to DNA replication and repair.19 The nucleosome assembly protein 1 is an H2A-H2B chaperone,20 preventing excessive accumulation of these chromatin marks.21 NORAD also binds to the RNA binding motif protein X-linked (RBMX), which participates in the DDR, inducing the assembly of the NORAD-activated ribonucleoprotein complex 1 nucleic complex, through RBMX, promoting genomic and chromosomal stability.22
The intrinsic resistance of neoplastic disorders to chemotherapy and targeted therapy represents a major clinical concern.23 The underlying causes of resistance can be attributed to intratumor heterogeneity,23,24 in part due to genomic instability.25 Chromosomal instability, a hallmark of cancer, is often associated with cancer progression, correlating with poor breast cancer prognosis.26 Paradoxically, by affecting cancer cell fitness, chromosomal instability may be exploited and have beneficial roles against cancer, namely in estrogen receptor (ER)-negative tumors, which were found to be associated with a better long-term survival when extreme levels of chromosome instability were present.27
Considering the correlation between NORAD and genome instability, as well as the contradictory effect of chromosomal instability in tumor progression, we investigate whether targeting NORAD could act synergistically with cytotoxic agents.27,28 Here, we demonstrate that downregulation of NORAD sensitizes human breast cancer cells to doxorubicin. NORAD expression was shown to be needed to signal the DNA damage after doxorubicin treatment. Our results underline the potential contribution of NORAD in chemotherapy-resistant cancer cells.
Results
NORAD is highly expressed in triple-negative breast cancer
Breast cancer is a heterogeneous disease and four main clinicopathological groups (luminal A-like, luminal B-like, HER2-positive (non-luminal), and triple-negative) are defined based on the expression of ERs, progesterone receptors (PRs), human epidermal growth factor receptor 2 (ERBB2/HER2), and Ki67.29
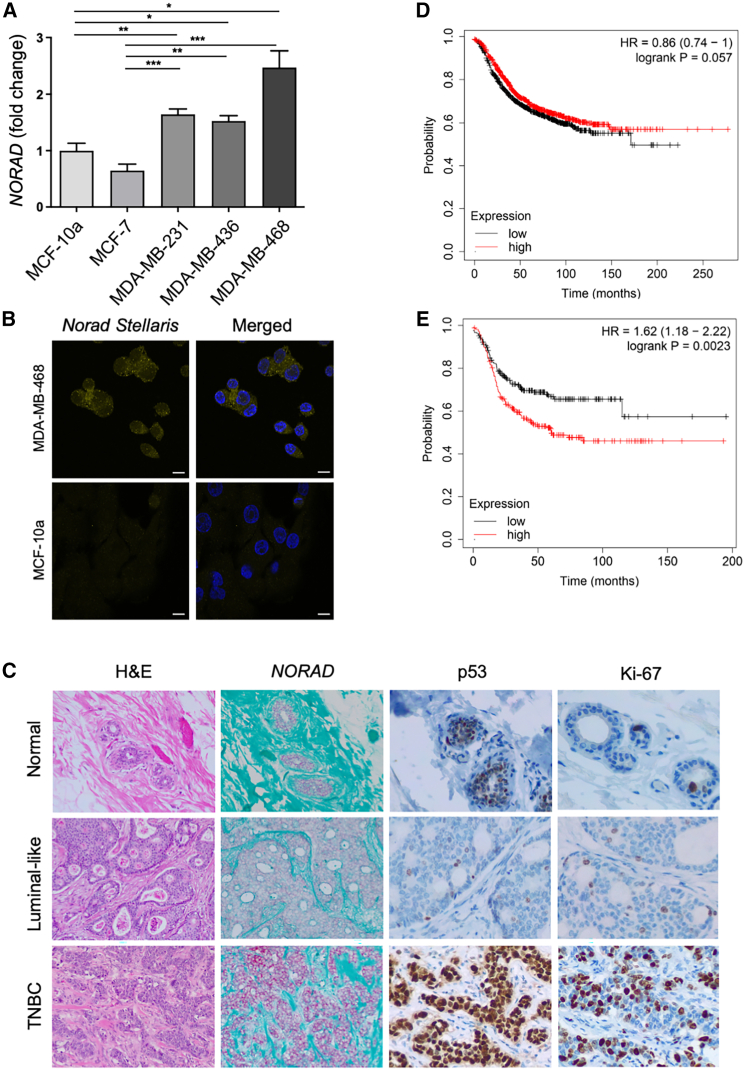
Initially, we determined the basal mRNA levels of NORAD in a set of human breast cancer cell lines (MCF-7, MDA-MB-231, -436, and -468) and in a non-malignant human mammary epithelial cell line (MCF-10A) by real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). We observed that MDA-MB-231, -436, and -468 cell lines, corresponding to triple-negative breast cancer (TNBC), express higher levels of NORAD. On the other hand, the luminal A-like subtype MCF-7 cell line expresses NORAD at comparable levels with control MCF-10A cell line (Figure 1A). The same pattern could be detected when we compared NORAD levels through fluorescence in situ hybridization (FISH) using Stellaris-specific probes (Figure 1B).
Figure 1.
NORAD characterization
(A) NORAD mRNA basal levels in human epithelial breast cancer cell lines (qRT-PCR) (n = 3). (B) NORAD subcellular localization in the MDA-MB-468 and MCF10a cell lines (smRNA FISH). Scale bar, 10 μm). (C) Higher NORAD expression (by RNA in situ hybridization, RNAscope) in triple-negative breast invasive carcinoma (TNBC) (lower) compared with luminal-like invasive (cribriform) carcinoma (middle) and human normal mammary tissue (upper) (n = 1, each); strong and diffuse p53 immunostaining and higher proliferative index (Ki67) in TNBC; hematoxylin and eosin (H&E) staining and NORADin situ hybridization at 100×; p53 and Ki67 immunostaining at 200× magnification. (D and E) Correlation between NORAD expression and prognosis of breast cancer patients (Kaplan-Meier Plotter): relapse-free survival irrespective of breast cancer subtype (A) and for basal-like breast cancer (E) (curves show the probability of survival over time and are colored based on the NORAD levels, the x axis represents time in months, and the y axis represents the proportion of patients who are still alive without relapse). For statistical analysis we used one-way ANOVA with a control condition for multiple comparisons. No symbol, p > 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
TNBC, considered the most aggressive breast cancer subtype, is defined by the absence of ER, PR, and HER2, detected through immunohistochemical staining, which limits targeted hormonal therapeutic options.30 On the contrary, luminal-like breast cancer is characterized by the expression of hormonal receptors (ER and/or PR) and a more indolent clinical behavior. Therefore, our findings suggest that high expression of NORAD may be indicative of a more aggressive form of breast cancer. These results align with those obtained by analyzing formalin-fixed paraffin-embedded (FFPE) human breast samples using single-molecule RNA in situ hybridization (RNAscope), which revealed higher NORAD expression in TNBC compared with the luminal-like tumor and normal mammary epithelium (Figure 1C). Neither patient had received previous chemotherapy. Despite the low level of evidence to recommend p53 immunohistochemical assessment for routine use, some studies suggest that abnormal staining correlates with aggressiveness features.31 The p53 expression pattern strongly differed between the two neoplasms, being heterogeneous (“wild-type” pattern) in the luminal-like tumor, with strong and diffuse staining (overexpression/accumulation, “mutated-type” pattern) in TNBC. This observation is concordant with the higher frequency of TP53 mutations in tumors classified as basal-like, which are a subtype of TNBC defined by specific gene expression patterns (Figure 1C).
Next, we asked how NORAD expression levels correlated with cancer patients’ outcome. We used the Kaplan-Meier Plotter Tool to correlate NORAD levels with prognosis of breast cancer patients. The KMPlotter database incorporates several gene expression profiles; breast cancer samples are stratified into high- and low-expression groups using the median gene expression level as a cutoff.32 Considering all breast cancer subtypes as a group, NORAD levels do not correlate with relapse-free survival (n = 2032, p = 0.057) (Figure 1D) nor overall survival (n = 943, p = 0.25) (Figures S1A and S1B). However, there is a statistically significant negative correlation between NORAD levels and relapse-free survival (n = 953, p = 0.002) when considering, in isolation, basal-like tumors (defined by PAM50 genetic profiling) (Figure 1E). Even though high NORAD levels are correlated with a lower relapse-free survival in poorly differentiated (grade 3) tumors (n = 417, p = 0.026), no association between NORAD expression and survival for the remaining tumor subtypes (luminal or HER2+) was found (Figures S1C–S1F). Therefore, high NORAD levels seem to be a survival prognostic factor specifically for patients with TNBC.
NORAD knockdown affects relevant tumor-specific phenotypes and sensitizes TNBC cells to chemotherapy
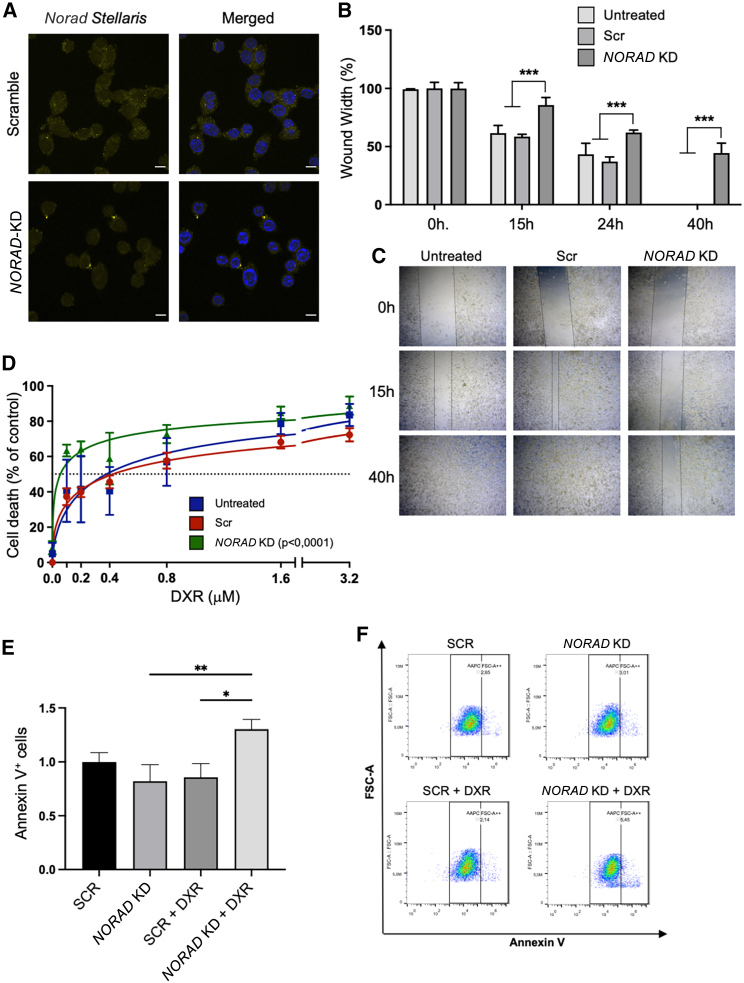
To unveil the role of NORAD in breast cancer, we used LNA GapmeRs and siRNAs targeting both the nuclear and cytoplasmic fractions of NORAD. Two LNA GapmeRs that target different regions of NORAD were tested individually and in combination in the MDA-MB-231 and MDA-MB-468 (Figures S2A–S2C) at final concentrations of 25 and 50 nM. We observed the most significant and consistent reduction of NORAD, confirmed by smRNA FISH in MDA-MB-468, using LNA GapmeRs in combination with siRNAs, at a final concentration of 25 nM, with an interval of 24 h between transfections (Figure 2A).
Figure 2.
NORAD KD effects on tumor-relevant phenotypes
(A) NORAD levels in the MDA-MB-468 cell line (smRNA FISH) treated with control siRNA + LNA or NORAD-specific siRNA + LNA. (B and C) NORAD KD effect on cell migration in the MDA-MB-231 cell line (wound healing assay); (B) is the gap quantification at the indicated time points (n = 3), (C) is a representative image of the wound healing. (D) NORAD KD sensitizes cells to doxorubicin (DXR), measured through the alamarBlue reduction assay (n = 3). (E and F) NORAD KD and doxorubicin effects on cell apoptosis in the MDA-MB-231 cell line (n = 3) as measured through the increase in Annexin V+ cells and as visualized in the representative plots (F). No symbol, p > 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Characterization of breast cancer cells was performed 48 h after NORAD downregulation, the time at which we detected lower NORAD levels. In addition, it has been demonstrated previously that 24 h after NORAD knockdown (KD) is not sufficient to affect PUMILIO-targeted mRNAs related to cell cycle and mitosis.12 We first tested whether NORAD affected the capacity of TNBC cells to migrate through the wound healing assay. After NORAD KD, a reduction in the migration rates of cells was evident, in comparison with the controls (Figures 2B, 2C, and S3). This result supports the role of NORAD in tumorigenesis, since invasiveness is one of the hallmarks of cancer.6,7 Considering the higher expression levels of NORAD in aggressive tumors, we addressed whether this transcript might be associated with chemoresistance. We tested the chemotherapeutic agent doxorubicin, an anthracycline commonly used in breast cancer treatment,33,34 which disrupts topoisomerase II-dependent DNA repair and mitochondrial function.35 Initially, we determined the half-maximal inhibitory concentration (IC50) for MCF-10A (Figure S4A) and MDA-MB-231 (Figure S4B) cell lines, confirming the higher values for non-malignant human mammary epithelial cells and demonstrating the chemotherapy selectivity toward cancer cells with higher proliferation rates. Then, we evaluated the effects of combining NORAD KD with chemotherapy. We observed a reduction in doxorubicin IC50 upon NORAD KD through alamarBlue cellular viability assay (Figures 2D and S5, IC50 shifted from 0.3779 to 0.05680 μM), indicating that NORAD KD sensitizes breast cancer cells to chemotherapy. This was accompanied by an increase in Annexin V+ cells (Figures 2E and 2F), something previously observed after doxorubicin treatment.36
NORAD dowregulation impairs DNA damage pathways
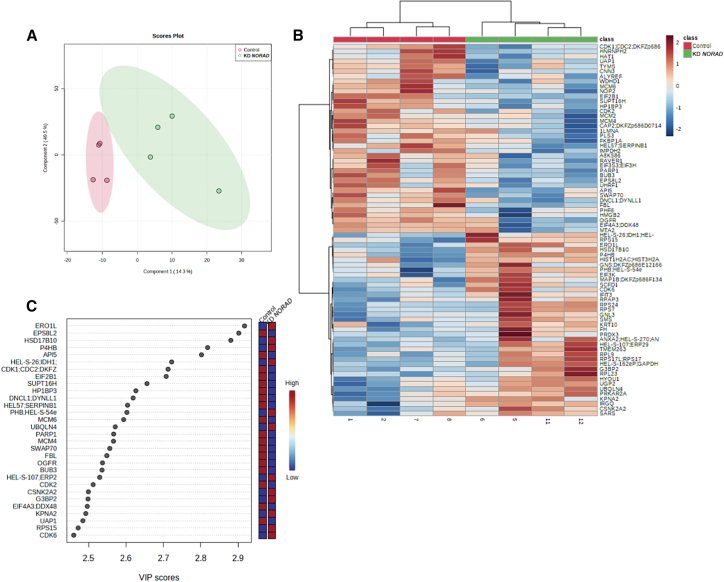
To identify NORAD-associated proteins that could be mediating the increased sensitivity to doxorubicin we performed liquid chromatography-tandem mass spectrometry (LC-MS/MS) for quantitative comparison between groups (NORAD wild-type vs. NORAD KD) in the MDA-MB-231 cell line. Four independent conditions were individually analyzed. Whole proteome analysis by LC-MS/MS retrieved 4,167 unique proteins with at least two unique peptides. Of all proteins detected, 1,464 were common to all the conditions studied, leading to 35% of common proteins. Partial least-squares discriminant analysis (PLS-DA) was used to visualize group separation based on proteome datasets by means of dimensionality reduction. PLS-DA showed a clear separation of the experimental groups (Figure 3A). NORAD KD appeared, however, to increase the heterogeneity of the proteome, which was probably related with the KD efficiency (Figure 3A). To find quantitative patterns between the experimental groups, comparative and grouped analysis was performed (Figure 3B). When looking at experimental groups, two main clusters were apparent with a different profile of proteins being either overexpressed or repressed in the different experimental conditions (adjusted p < 0.05). NORAD KD downregulated proteins that were predominantly involved in biological processes related with G1/S transition of mitotic cell cycle and DDR (Figure 3C). The analysis revealed a preponderant altered modulation of proteins involved in the regulation of DNA repair, chromatin remodeling, and epigenetic regulation (Figure S6), suggesting that NORAD KD could affect the sensitivity of the MDA-MB-231 cells to doxorubicin by modulating the activity of proteins involved in DNA repair and epigenetic regulation. One example is MCM protein 6 (MCM6), the levels of which strongly decrease after NORAD KD. MCM6 is involved in the initiation of DNA replication and is a strong predictor of survival in cancer patients,37 or ALYREF, a known interactor of NORAD,22 a factor associated with poor survival in breast cancer patients.38 The lower expression of some detected proteins could be moderately confirmed by qPCR, demonstrating that NORAD may regulate the level of these proteins by other pathways, not only at the transcriptional level (Figures S6B–S6D).
Figure 3.
NORAD KD alters the proteome balance toward genetic instability
(A) Partial least-squares discriminant analysis (PLS-DA) of the different variables tested (control vs. NORAD KD). (B) Heatmap showing the hierarchical clustering of the top 100 hits contributing to the separation of the variables control vs. NORAD KD. (C) Variable importance in projection (VIP) scores of the top 30 genes between the conditions control vs. NORAD KD.
NORAD in the response to DNA damage
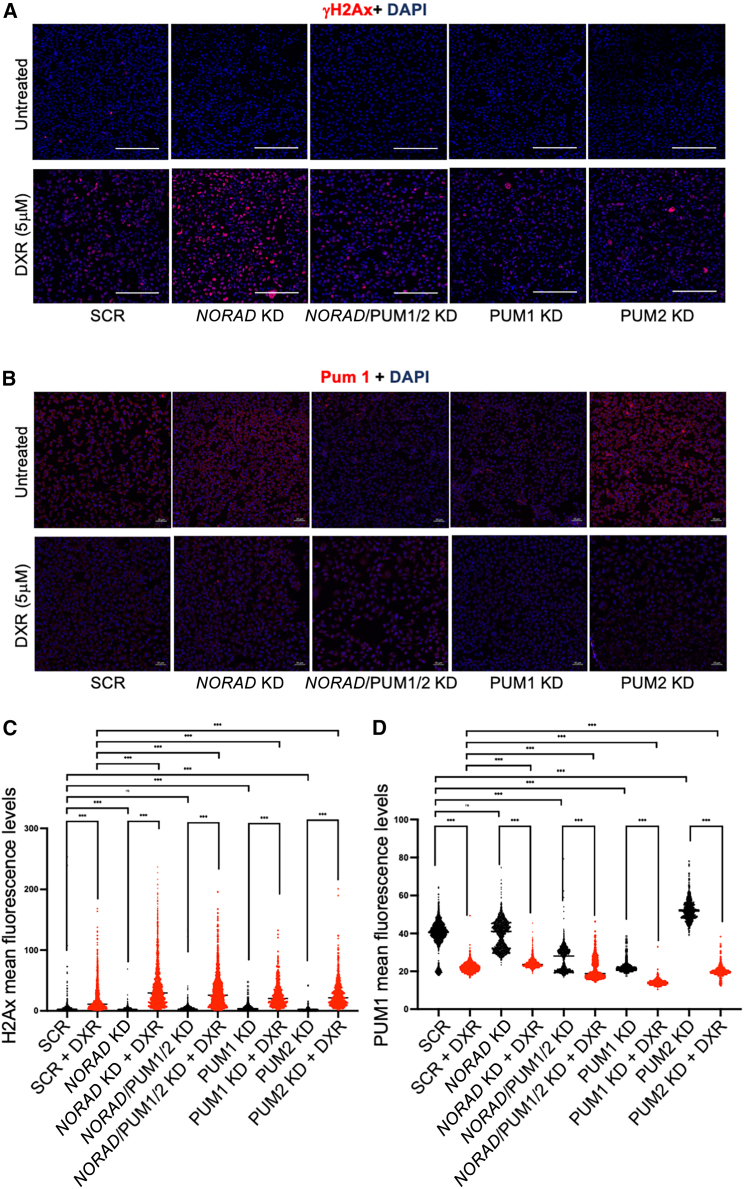
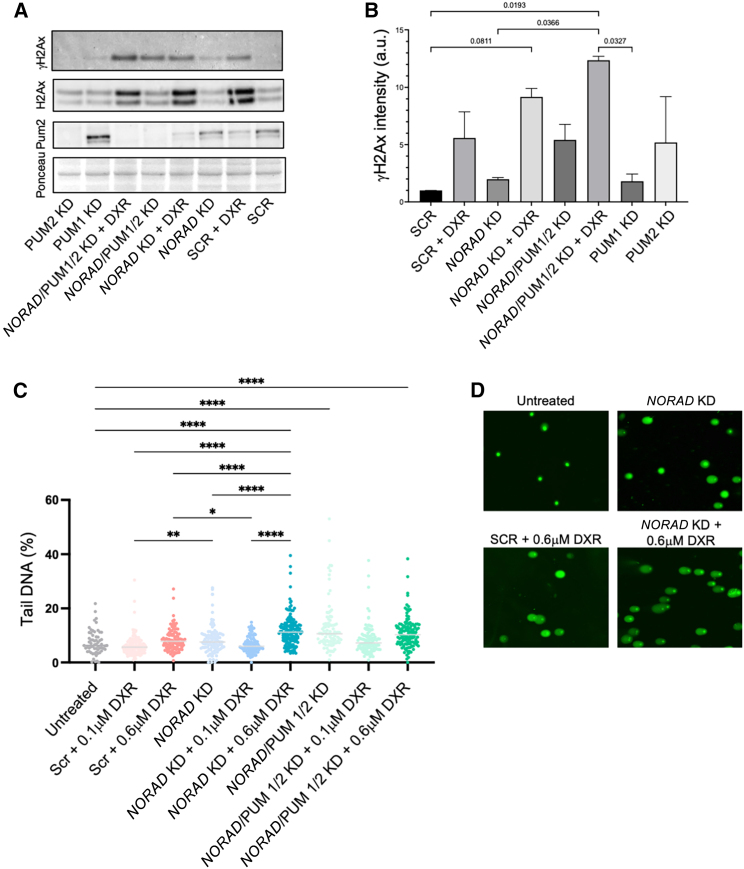
In line with previous observations on the role of NORAD in DDR, and our own results supporting the sensitivity of NORAD KD cells to doxorubicin, we wondered how MDA-MB-231 or MDA-MB-468 cells with silenced NORAD would recognize and repair DNA lesions. Immediately after DNA double-strand breaks (DSBs) occur, histone H2AX is phosphorylated (γH2AX) mainly by ATM at C-terminal Ser136 and Ser139 residues,39 leading to signal amplification that ends with chromatin remodeling and recruitment of DNA repair proteins, such as BRCA1 and 53BP1. Using immunofluorescence (IF) we observed that NORAD KD resulted in an exacerbated accumulation of γH2AX in the MDA-MB-231 cells exposed to different concentrations of doxorubicin (Figures 4A, 4C, S7, and S8). This effect was not exacerbated when the Pumilio 1 and 2 proteins were concomitantly targeted with NORAD. Targeting Pumilio proteins separately (Figure S9) also resulted in some accumulation of γH2AX, although not equivalent to NORAD KD (Figures 4A and 4C), demonstrating a multifaceted role for Pumilio family of proteins in the setting of doxorubicin-induced DNA damage in breast cancer cells. Pumilio proteins have crucial roles in several cellular pathways, spanning mitosis and DNA repair. Whether DNA damage may induce Pumilio expression was also assessed by IF (Figures 4B and 4D). The presence of doxorubicin was shown to significantly decrease the presence of Pumilio 1 proteins independently of NORAD in cancer cells. Interestingly, in the absence of Pumilio 2 there is a compensatory expression of Pumilio 1, previously observed in the context of stemness and embryogenesis.40 Still, this increased expression does not impact on the signaling of DNA damage (Figures 4A and 4C). To further explore these results we evaluated γH2AX, H2AX, and Pum1 expression by western blot (WB) (Figures 5A and 5B). Similarly to IF, we could detect an increased expression of γH2AX in the conditions where NORAD was absent. Interestingly, the same compensatory role of Pumilio could be observed, since Pum2 levels greatly increased when Pum1 was targeted (Figures 5A and 5B). Of note, NORAD/PUM1/2 KD has a comparable level of yH2AX levels as SCR + DXR alone by WB, showing an impact of these proteins on DDR.
Figure 4.
NORAD KD alters γH2Ax accumulation after DNA damage
(A and B) Immunofluorescence (A) for γH2Ax and (B) for Pum1 in the depicted experimental conditions in the MDA-MB-231 cell line. Scale bars, 200 μm (A) and 50 μm (B). (C and D) Quantification of the signal corresponding to γH2Ax (C) and Pum1 (D) in the depicted experimental conditions in the MDA-MB-231 cell line (see materials and methods). No symbol, p > 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Figure 5.
NORAD KD alters the DDR
(A) Western blot analysis of γH2Ax, H2Ax, and Pum2 in the depicted experimental conditions in the MDA-MB-231 cell line. Ponceau was used as loading control. (B) Quantification of the western blot bands for the depicted proteins and conditions using the Ponceau band as loading control (n = 3). (C and D) DNA damage detection in the MDA-MB-231 cell line through comet assay (see materials and methods) in the depicted conditions. Quantification (C) and representative images (D) of the Comet assay is depicted. No symbol, p > 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
To understand whether this increase in DNA damage signaling correlated with an accumulation of DNA breaks, Comet assay was performed to explore different cellular conditions. DNA damage is measured by the presence of DNA in the comet tail, indicative of DNA break intensity.41,42 As expected, DNA breaks have been detected in the presence of doxorubicin. KD of NORAD increased the amount of DNA damage at 0.6 μM of DXR (Figures 5C and 5D), supporting previous results showing an increased accumulation of γH2Ax or cell death in the NORAD KD condition. Again, the condition NORAD/PUM1/2 KD had an increased amount of DNA damage even in the absence of DXR.
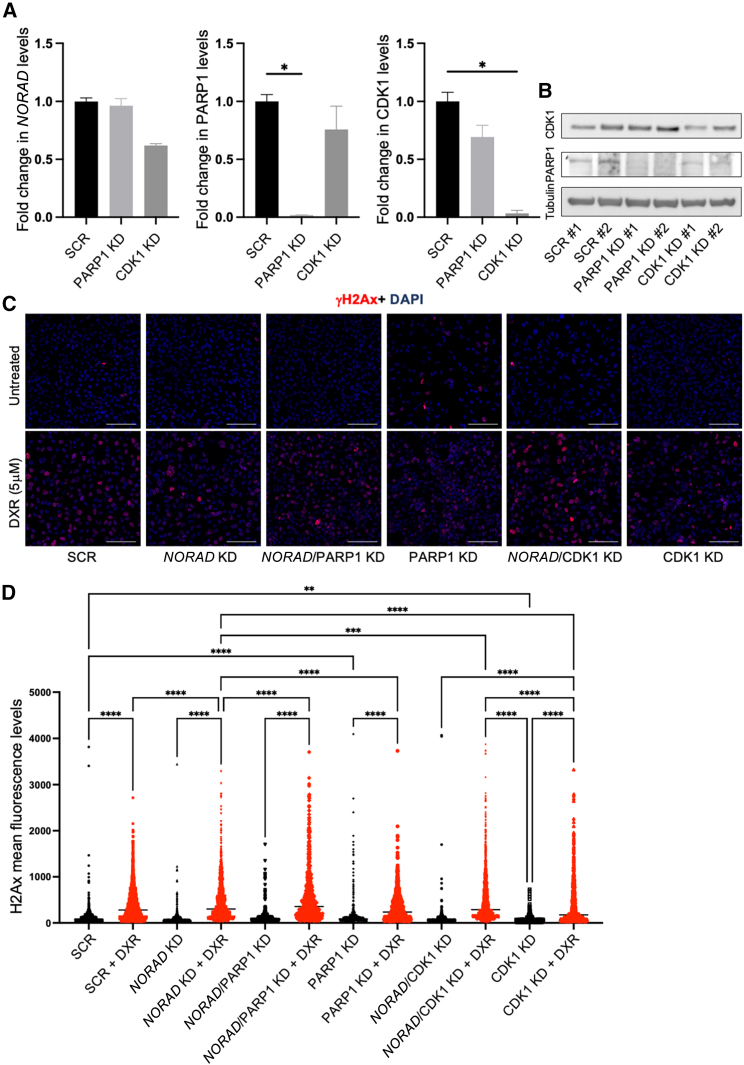
To further explore the involvement of the DDR proteins in the NORAD-mediated accumulation of γH2Ax we used siRNAs to KD (Figures 6A and 6B) two of the hits identified in the NORAD-associated proteome (LC-MS/MS experiment, Figure 3), namely PARP1 and CDK1, both proteins being extensively linked to cancer and DXR response.43,44,45,46,47 Although NORAD KD already impacted on the levels of PARP1 and CDK1 (Figure 3), siRNA-mediated KD showed a more consistent and severe reduction of their levels. Using IF we observed that NORAD/PARP1 KD, in the presence of DXR, resulted in a higher level of accumulation of γH2Ax, demonstrating the synergistic role of these two factors (Figures 6C and 6D). PARP1 alone, in the absence of DXR, increased the mean γH2Ax intensity (SCR, 105, to PARP1 KD, 189). Although CDK1-KD increased the 75th percentile of mean γH2Ax intensity (NORAD KD + DXR, 614; NORAD/PARP1 KD + DXR, 692; and NORAD/CDK1 KD + DXR, 692), the average intensity was not altered, probably due to an incomplete reduction of CDK1 levels, as depicted by WB (Figure 6B).
Figure 6.
NORAD KD synergizes with PARP1 in the DDR
(A) NORAD, PARP1, and CDK1 mRNA levels after KD of the conditions represented, using siRNAs (n = 3). (B) Western blot analysis of PARP1 and CDK1 in the depicted experimental conditions in the MDA-MB-231 cell line. Tubulin was used as loading control. (C) Immunofluorescence for γH2Ax in the depicted experimental conditions in the MDA-MB-231 cell line. Scale bar, 100 μm. (D) Quantification of the signal corresponding to γH2Ax in the experimental conditions represented in the MDA-MB-231 cell line (see materials and methods). No symbol, p > 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Discussion
In this study, we reported that NORAD is overexpressed in breast cancer, conferring resistance to chemotherapy, and interfering with γH2AX signaling upon DNA damage.
It was described previously in esophageal, breast, lung, pancreatic, bladder, and colorectal cancers that NORAD functions as a potential oncogenic factor, suggesting that it may constitute a tumor biomarker, defining patient prognosis, predicting therapy response, and/or be used as a therapeutic target.16,28,48,49,50 Our results support this scenario where higher NORAD levels are associated with an aggressive breast cancer subtype (TNBC) and poor relapse-free survival of patients, while NORAD KD inhibits cancer cell viability and migration. Despite this association, it was also described previously in liver cancer that NORAD functions as a potential tumor suppressor.51 These opposing results may be explained by the distinctive interacting partners of NORAD, as it is known to sponge a myriad of miRNAs, albeit binding preferentially to PUMILIO proteins known to repress mRNAs involved in mitosis, DNA repair, and replication (e.g., PRC1, PARP1, and WDHD1),9,12 but also repress mRNAs involved in various cancer pathways (e.g., E2F3).52 Alternatively, SAM68 binds to conserved secondary structures immediately downstream of the PUMILIO response elements in NORAD.12 Of note, SAM68 is upregulated in several cancer types including breast cancer and could be involved in the deregulation of the AKT pathway.53 Interestingly, SAM68 also presents tumor suppressor-like activities as a transcriptional coactivator of p53.14 Nevertheless, it is still debated whether NORAD action is solely mediated through PUMILIO proteins. One example is the role of RBMX, a component of the DDR that may be mediating NORAD function in genomic (in)stability, whose role has been discussed by different authors.13,22 Given the complexity of NORAD and the different molecules that can associate with it, one would expect that different binding molecules may cooperate to different responses.10,54 Here, we demonstrate that the absence of PUMILIO did not synergize with NORAD in the intensity of γH2Ax signal or the levels of DNA damage after DXR. NORAD KD is somehow destabilizing the DDR complex, as supported by the LC-MS/MS data where we see, for instance, lower levels of PARP1, MCM6, or ALYREF (previously shown to be in a complex with NORAD22 and involved in carcinogenesis38). Whether PUMILIO may have a compensatory role in the response to DXR, in the NORAD KD scenario, later in time, or whether DXR may induce changes in the transcriptional program evading some of the PUMILIO regulated genes, is still unknown. It is known, however, that PUMILIO proteins need to be tightly regulated to maintain genome stability in human cells.9 The need for such a tight regulation of PUMILIO activity might be also the case for the NORAD-PUMILIO axis in DDR and could explain why the NORAD/PUM1/PUM2 triple KD does not rescue the phenotypes observed in NORAD KD cells.
Breast cancer is the most frequently diagnosed malignancy in women, accounting for about one-third of female cancers. Systemic therapies have been shown to be successful in treating early breast cancer; however, once the disease recurs, it tends to be more aggressive and resistant to therapy. The combination of conventional chemotherapeutic agents with novel molecular-targeted agents is a promising therapeutic approach. First, since the targets and mechanisms of action of these agents are different, there is no cross-resistance. Second, in combinatorial approaches, lower concentrations of chemotherapeutic agents may be considered, reducing both their side effects and off-target effects. Third, alterations in expression and/or activity of genes that regulate mitogenic signals caused by molecular-targeted agents may not only disturb cell growth, but also sensitize cancer cells to chemotherapeutic agents.55,56,57 For example, lncRNA HOTAIR contributes to colorectal cancer and 5-FU resistance through the recruitment of EZH2 and subsequent silencing of miR-218, upregulation of VOPP1 expression and subsequent activation of the NF-κB/TS pathway.58 Similarly, H19 lncRNA plays a leading role in breast cancer chemoresistance, mediated mainly through a H19-CUL4A-ABCB1/MDR1 pathway. H19 expression was greatly upregulated in doxorubicin-resistant breast cancer cells (MCF-7) and its KD sensitizes them to chemotherapy.59
Our results show that the DNA damage induced by doxorubicin is enduringly signaled by γH2AX in the absence of NORAD. Faulty DNA damage signaling may be caused by an error downstream or upstream of NORAD. Upon DNA damage, SAM68 is recruited and stimulates the catalytic activity of PARP1.60 Defective ATM activity and reduced γH2AX foci formation in response to γ-irradiation were observed in PARP1-deficient cells. In a NORAD KD background as presented here, PARP1 inhibition leads to an increase in γH2Ax deposition, demonstrating a synergistic role of NORAD and PARP1 in DDR. In addition, PARP1 is thought to recruit Nbs1 and Mre11 to DSBs in a γH2AX- and MDC1-independent manner.61 It is important to know exactly at which point the DNA damage signaling is compromised, especially considering that PARP inhibitors are currently used in patients with advanced-stage breast cancer, in the context of germline mutations in BRCA1 or BRCA2 genes, which frequently belong to the triple-negative subtype.62
In summary, we demonstrated that NORAD confers resistance of breast cancer cells to a chemotherapeutic agent. Therefore, NORAD may represent an actionable molecular target and could be used in a combinatorial approach.
Materials and methods
Cell lines and culture conditions
The following human breast cell lines and control were used in this study: MCF-10A (non-tumoral, mammary epithelial cell line), MCF-7 (breast carcinoma cell line, luminal A subtype), and MDA-MB-231, MDA-MB-436, and MDA-MB-468 (breast carcinoma cell lines, triple-negative subtype). The MCF-10A cell line was cultured in DMEM/F-12 (Dulbecco’s modified Eagle’s medium/nutrient mixture F-12, Gibco by Life Technologies), supplemented with 5% (v/v) horse serum, epidermal growth factor (20 ng/mL), hydrocortisone (0.5 μg/mL), cholera toxin (100 ng/mL), insulin (10 μg/mL), and 1% (v/v) penicillin-streptomycin. The MCF-7, MDA-MB-231, MDA-MB-436, and MDA-MB-468 cell lines were cultured in DMEM (Gibco by Life Technologies), supplemented with 10% (v/v) heat-inactivated fetal bovine serum and 1% (v/v) penicillin-streptomycin. All cell lines were grown under adherent conditions at 37°C in a humidified incubator with 5% CO2. MCF-10A and MCF-7 cell lines were a kind gift from Dr. Sérgio de Almeida (Instituto de Medicina Molecular João Lobo Antunes, Lisbon, Portugal), while MDA-MB-231, MDA-MB-436, and MDA-MB-468 were generously offered by Dr. Sérgio Dias (Instituto de Medicina Molecular João Lobo Antunes, Lisbon, Portugal).
Obtaining patient tissue samples
After study approval (Project “RefaCE – JMS/is – Estudo 64”) by the Ethics Committee of CUF Descobertas Hospital (Lisbon, Portugal), a retrospective analysis of breast cancer cases diagnosed between 2019 and 2021 at the Pathology Department (CUF Descobertas Hospital) was performed. Clinicopathological information was retrieved, and both hematoxylin and eosin-stained and immunohistochemistry (IHC) slides from selected cases were reviewed by a pathologist with experience in breast pathology. After validating tissue quality and confirming diagnosis, representative tumor and control sections were obtained from FFPE samples.
RNAscope in tissue samples
Selected FFPE samples were cut to 3 μm sections on positively charged slides. For the RNAscope of the clinical tissue samples, the manufacturer protocol for RNAscope 2.5 Assay was followed, starting with FFPE drying in an oven at 60°C for 1 h. Sequential incubations in xylene and 100% alcohol, accompanied by air drying, were used to deparaffinize the sections. Next, RNAscope Hydrogen Peroxide was applied for 10 min at room temperature (RT), and the slides were rinsed. The RNAscope 1X Target Retrieval Reagent was used for target retrieval for 15 min at 100°C, following standard instructions. After rinsing, the slides were incubated in 100% alcohol for 3 min, and dried at RT, and the tissue area delimited using an Immedge hydrophobic barrier pen to delimit the tissue section. In the HybEz Humidity Control Tray, the slides were incubated with RNAscope Protease Plus at 40°C for the standard time of 30 min. At this point, the tissues were incubated with the probes that could be hybridized to the negative (dapB) or positive (PPIB) controls, or NORAD itself. This took place in a HybEz Oven for 2 h at 40°C. The kit contained probes for six sequential amplifications to amplify the hybridization signal. The signal was then ready to be detected after incubating with the Fast RED solutions mix for 10 min at RT. Fast Green Stain Solution (Thermo Scientific, 88024) was applied to the slides. To mount the samples, 1–2 drops of VectaMount Permanent Mounting Medium (Vector Laboratories, H-5000-60) were placed on the slides and the coverslips were dipped in xylene and placed over the sections carefully. Once dry, the samples were evaluated in Nikon ecliplse Ti-U, an inverted wide-field microscope with a CCD color digital camera, at 10× magnification (Achno ADL objective).
IHC in tissue samples
Tissue sections with a thickness of 3 μm were cut from FFPE samples to positively charged slides for IHC with p53 (clone DO-7, Roche, Switzerland, cat. no. 800–2912) and Ki67 (clone 30-9, Roche, cat. no. 790–4286) antibodies. All IHC was performed on the Ventana BenchMark ULTRA automated staining platform. Antibodies were pre-diluted and run using the OptiView DAB IHC Detection Kit (Roche, cat. no. 760-700) with ULTRA CC1 antigen retrieval (Roche, cat. no. 950-224), and slides evaluated using the optical microscope Leica DM1000 Led, at 200× magnification.
LNA GapmeR and siRNA transfection
NORAD downregulation was performed using RNase H-activating LNA GapmeRs (Exiqon), consisting of chimeric antisense oligonucleotides that contain a central block of DNA, which activates RNase H-dependent cleavage of complementary RNA targets, and are flanked by modified nucleotides (hence LNA [locked nucleic acid]) to offer higher protection of the oligonucleotides against nuclease degradation63,64 and siRNA (Table 1).
Table 1.
List of siRNAs and Gapmers
| siRNAs | |||
|---|---|---|---|
| Target mRNA | Sequence (5′–3′) | Reference | |
| NORAD | CUGUGUAUAUAGCGGACAA | siRNA N038095-17 | Lincode SMARTpool Human LOC647979 038095-00-0010 (Dharmacon) |
| CAUCUAAGCUUUACGAAUG | siRNA N038095-18 | ||
| AGUGCACAAUGUAGGUUAA | siRNA N038095-19 | ||
| CGACCCAAGCCUCGACGAA | siRNA N038095-20 | ||
| PUM1 | GGUCAGAGUUUCCAUGUGA | siRNA J-014179-05 | ON-TARGETplus SMARTpool L-014179-00-0005 (Dharmacon) |
| GGAGGAGGCGGCUAUAAUA | siRNA J-014179-06 | ||
| GGAGAUAAGCUAGGAGAUU | siRNA J-014179-07 | ||
| CGGAAGAUCGUCAUGCAUA | siRNA J-014179-08 | ||
| PUM2 | CUGAAGUAGUUGAGCGCUU | siRNA J-014031-17 | ON-TARGETplus SMARTpool L-014031-02-0005 (Dharmacon) |
| GCAGAGUAAUUCAGCGCAU | siRNA J-014031-18 | ||
| GACAAAUGGUAGUGGUCGA | siRNA J-014031-19 | ||
| AGACAUAACAGUAACACGA | siRNA J-014031-20 | ||
Cells were transfected with either control (non-specific) LNA GapmeRs or LNA GapmeRs directed against NORAD, using Lipofectamine RNAiMAX transfection reagent (Invitrogen), with 24 or 48 h between the two transfections, following standard procedures (final concentration of 25 nM). Two different GapmeRs were designed using the Antisense LNA GapmeR design tool, with the following central sequences: 5′-CTAGACGTAAATTAGG-3’ (human NORAD GapmeR 1) and 5′- ACTTTACTAAAAACGC-3’ (human NORAD GapmeR 2). siRNAs used for NORAD, PUM1 and PUM2 were the same as in Tichon et al.12 KD efficiency was assessed by qRT-PCR. siRNAs used for PARP1 (Santa Cruz, sc-29437) and CDK1 (Thermo Fisher Scientific, AM16704 103821) were used at 25 nM. KD efficiency was assessed by qRT-PCR and WB. A combination of unspecific siRNA + scrambled LNA Gapmer was used as a control at the same concentrations.
Single-molecule RNA FISH
Stellaris FISH probes recognizing NORAD and labeled with Quasar 570 dye were purchased from Biosearch Technologies. The probe set sequences utilized in the experiments had been described previously and each set comprises 48 different oligonucleotides (20 nucleotides in length).9
Cells were seeded on gelatin-coated glass coverslips in flat-bottom 24-well cell culture plates (TPP), washed in phosphate-buffered saline (PBS), and fixed with 3.7% paraformaldehyde for 10 min at RT. Fixed cells were then washed in PBS, permeabilized in 70% ethanol for 1 h at RT, and washed with a solution containing 20× saline sodium citrate (SSC), deionized formamide, and nuclease-free water. Within a humidified chamber, coverslips were transferred onto drops of hybridization buffer (containing probe, 50% dextran sulfate, 20× SSC, deionized formamide, 100% formaldehyde, and nuclease-free water), and hybridized overnight at 37°C. Coverslips were washed with the previsouly detailed buffer and with 2× SSC, following which they were mounted with VECTASHIELD and 4′,6-diamidino-2-phenylindole mounting medium. Images were acquired using a laser scanning confocal inverted microscope (LSM710, Carl Zeiss).
Cellular viability assay
Cells were seeded in 48-well plates (TPP), at a density of 20,000–60,000 cells/well and incubated at 37°C in a humidified incubator with 5% CO2. When applicable, NORAD downregulation was performed at the time of seeding and 24 h later, as mentioned above or in the figure legend.
Seventy-two hours after plating, cells were incubated with doxorubicin (Sigma-Aldrich, D2975000) for 24 h, in a range of concentrations. Specific culture medium containing 10% (v/v) alamarBlue Cell Viability Reagent (Thermo Fisher Scientific) was then added to the cells and resazurin assay performed. Plates were incubated for 2 h at 37°C protected from light and the fluorescence intensity was then quantified using a plate-reading fluorometer (Microplate Reader Infinite M200, Tecan) with excitation wavelength at 560 nm and emission wavelength at 590 nm. The relative viable cell number was standardized to untreated cells and the IC50 for each drug determined from dose-response curves using GraphPad Prism software.
Cell apoptosis analysis
Analysis of apoptosis was performed using the Annexin V Apoptosis Detection Conjugate (Thermo Fisher Scientific, A35110). Cells were trypsinized, centrifuged at 1,200 rpm for 5 min, washed with 1× PBS and resuspended in 1× Binding Buffer Solution at a final concentration of 1 × 106 cells/mL. To each 100 μL of cell suspension were added 2.5 μL of Annexin V-CF blue conjugate and 5 μL of 7-AAD staining solution. After incubation at RT for 15 min in the dark, 400 μL of 1× binding buffer solution was added, cells were transferred to FACS tubes and analyzed in a BD LSRFortessaTM X-20 cytometer. Results were analyzed using the FlowJo software.
qPCR analysis of gene expression
Total RNA was isolated using NZYol following manufacturer’s instructions (NZYTech). RNA quality was verified using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific). cDNA was synthesized with random primers using the Roche Transcriptor High Fidelity cDNA Synthesis Kit. qRT-PCR analysis was performed in the ViiA 7 Real-Time PCR System (Thermo Fisher Scientific) using SYBR Green PCR master mix (Thermo Fisher Scientific). Gene-specific primer pairs (Sigma) were used as follows:
NORAD forward 5′-TGTTTGTGCAGTGGTTCAGG-3′
reverse: 5′-TCTTGCCTCGCTGTAAACAG-3′
p53 forward: 5′-CCCCTCCTGGCCCCTGTCATCTTC-3′
reverse: 5′-GCAGCGCCTCACAACCTCCGTCAT-3′
18s forward: 5′-GGATGTAAAGGATGGAAAATACA-3′
reverse: 5′-TCCAGGTCTTCACGGAGCTTGTT-3′
GAPDH forward: 5′-GACAGTCAGCCGCATCTTCT-3′
reverse: 5′-TTAAAAGCAGCCCTGGTGAC-3′
PUM1 forward: 5′- CCGGGCGATTCCTGTCTAA-3′
reverse: 5′- CCTTTGTCGTTTTCATCACTGTCT-3′
PUM2 forward: 5′- GGGAGCTTCTCACCATTCA-3′
reverse: 5′- CCATGAAAACCCTGTCCAGATC-3′
MCM6 forward: 5′- GAGGAACTGATTCGTCCTGAGA
reverse: 5′- CAAGGCCCGACACAGGTAAG
PARP1 forward: 5′-GCAGAGTATGCCAAGTCCAACAG-3′
reverse: 5′-ATCCACCTCATCGCCTTTTC-3′
BUB3. forward: 5′- GGTTCTAACGAGTTCAAGCTGA
reverse: 5′- GGCACATCGTAGAGACGCAC
Relative fold changes in gene expression were calculated based on the threshold cycle (Ct), using the 2−ΔΔCt method, considering GAPDH exclusively or in combination with 18S ribosomal RNA as endogenous controls.
Correlation analysis between NORAD expression and survival: KM Plotter Online
The open access KM Plotter Online Tool was used to explore the association between NORAD expression and the clinical outcome for breast cancer patients, namely overall survival and relapse-free survival.32,65 This platform integrates information available at Gene Expression Omnibus, European Genome-phenome Archive and The Cancer Genome Atlas, incorporating high-throughput data with clinical information.32,65 After selecting the genes of interest and the characteristics of the study sample, a Kaplan-Meier survival curve, the hazard ratio with 95% confidence intervals, and log rank p values are displayed for each combination.
Sample preparation for spectrometric analysis
Samples (10 μg) were reduced with dithiothreitol (30 nmol, 37°C, 60 min) and alkylated in the dark with iodoacetamide (60 nmol, 25°C, 30 min). The resulting protein extract was diluted to 2 M urea with 200 mM ammonium bicarbonate for digestion with endoproteinase LysC (1:10 w:w, 37°C, 6 h, Wako, cat. no. 129–02541), and then diluted 2-fold with 200 mM ammonium bicarbonate for trypsin digestion (1:10 w:w, 37°C, o/n, Promega cat. mo. V5113).
After digestion, peptide mix was acidified with formic acid and desalted using a MicroSpin C18 column (The Nest Group) prior to LC-MS/MS analysis.
LC-MS
Samples were analyzed using an LTQ-Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific, San Jose, CA) coupled to an EASY-nLC 1200 (Thermo Fisher Scientific [Proxeon], Odense, Denmark). Peptides were loaded directly onto the analytical column and were separated by reversed-phase chromatography using a 50 cm column with an inner diameter of 75 μm, packed with 2 μm C18 particles (Thermo Scientific).
Chromatographic gradients started at 95% buffer A and 5% buffer B with a flow rate of 300 nL/min for 5 min and gradually increased to 25% buffer B and 75% A in 79 min and then to 40% buffer B and 60% A in 11 min. After each analysis, the column was washed for 10 min with 10% buffer A and 90% buffer B. Buffer A: 0.1% formic acid in water. Buffer B: 0.1% formic acid in 80% acetonitrile.
The mass spectrometer was operated in positive ionization mode with nanospray voltage set at 2.4 kV and source temperature at 305°C. The acquisition was performed in data-dependent acquisition mode and full MS scans with one micro scan at resolutions of 120,000 were used over a mass range of m/z 350–1,400 with detection in the Orbitrap mass analyzer. Auto gain control (AGC) was set to “standard” and injection time to “auto.” In each cycle of data-dependent acquisition analysis, following each survey scan, the most intense ions above a threshold ion count of 10,000 were selected for fragmentation. The number of selected precursor ions for fragmentation was determined by the “Top Speed” acquisition algorithm and a dynamic exclusion of 60 s. Fragment ion spectra were produced via high-energy collision dissociation at a normalized collision energy of 28% and acquired in the ion trap mass analyzer. AGC was set to 2E4, and an isolation window of 0.7 m/z and a maximum injection time of 12 ms were used.
Digested bovine serum albumin (New England Biolabs, cat. no. P8108S) was analyzed between each sample to avoid sample carryover and to assure stability of the instrument, and QCloud66 was used to control instrument longitudinal performance during the project.
Proteomic data analysis
The LC-MS/MS raw files were elaborated using MaxQuant (v.1.6.17.0) for the processes of protein identification and quantification according to the LFQ algorithm.67,68 Runs were analyzed using the Andromeda search engine against the freely available reference proteome of Homo sapiens downloaded from the UniProtKB database (January 2021). The allowable tolerance for precursor mass and fragment mass was set at 4.5 and 20 ppm, respectively. The minimum peptide length was set at seven amino acids and trypsin and LysC were selected as the proteolytic enzyme allowing up to two missing cleavage sites. Carbamidomethylation (Cys) was set as the fixed modification, while oxidation (Met), deamidation (ND), and N-terminal protein acetylation were the variable modifications. The false discovery rate was set at 1% at both the protein and peptide levels. In this analysis, the inter-run agreement option was selected. According to the MaxLFQ algorithm, proteins were quantified based on the extracted ion currents of the precursor ion peptides. The results of this analysis were first imported into Perseus (v.1.6.14.0) and then into MetaboAnalyst 5.0 for univariate and multivariate statistical data analysis and visualization. In brief, proteins identified as site only, reverse, and contaminants were removed. Expression values were transformed to a logarithmic scale with base 2. Samples were annotated according to their respective groups. Abundance of proteins between two groups were compared using a two-tailed t test, with the adjusted p value set at <0.05. Principal-component analysis (PCA) was performed on the matrix before logarithmic transformation; after filtering valid values, a multistream plot and histogram were generated; after the two-sample t test, a volcano plot was generated. PLSDA and variable importance in projection from the previous analysis were extracted. A heatmap was performed from the top 100 proteins, the 2 clusters (downregulated and upregulated) were extracted and filtered according to the PCA values (>2). Resulting clusters were run on STRING and g:PROFILER to explore the biological functions of the proteins.
The MS proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE69 partner repository with the dataset identifier PXD039920.
WB analysis
Proteins were extracted on ice after cell washing in PBS (Fisher Bioreagents, BP399-1), with RIPA buffer containing protease and phosphatase inhibitors (RIPA, Thermo Scientific, 89901; EDTA 100×, Thermo Scientific, 1861275; Cocktail protease inhibitor 100×, Thermo Scientific, 1861278; Cocktail phosphatase inhibitor 100×, Thermo Scientific, 1861277). Cell lysates were incubated on ice for 30 min and centrifuged at 15,000 × g at 4°C for 15 min. Protein levels were evaluated using the BCA assay according to the manufacturer’s protocol (Pierce BCA Protein Assay Kit, Thermo Scientific, 23227). Thirty micrograms of protein from each sample was prepared for loading in NuPAGE LDS Sample Buffer (Invitrogen, NP0004) and separated in a precast gel (Bolt 4%–12%, Bis-Tris, 1.0 mm, Mini Protean Gels, NW04120Box, Thermo Scientific; Running Buffer: 20× Bolt MES SDS Running Buffer, B0002, Thermo Scientific). After wet transfer, the nitrocellulose membranes were stained with Ponceau S (0.1%, w/v) for 15 min to assess gel loading. Prior to immunoblotting, membranes were blocked with 5% bovine serum albumin (BSA), prepared in TBS-T, and then incubated with the indicated antibodies. Membranes were visualized in a chemiluminescence-based system (ChemiDoc Touch [Bio-Rad]), and protein levels were calculated using the Ponceau S staining for normalization. For a list of the antibodies used see Table 2.
Table 2.
– List of antibodies
| Antibody | Type | Reference | Dilution |
|---|---|---|---|
| γH2AX | primary antibody | ab2893 (Abcam) | 1:1,000 |
| PUM2 | primary antibody | ab92390 (Abcam) | 1:10,000 |
| H2AX | primary antibody | 10856-1-AP (Proteintech) | 1:2,000 |
| PARP1 | primary antibody | MA3-950 (Thermo Fisher Scientific) | 1:500 |
| CDK1 | primary antibody | Ab131450 (Abcam) | 1:500 |
| Anti-mouse | cross-adsorbed secondary antibody, HRP | G21040 (Invitrogen) | 1:10,000 |
| Anti-rabbit | cross-adsorbed secondary antibody, HRP | G21234 (Invitrogen) | 1:10,000 |
Immunofluorescence
Cells previously seeded on 24-well plates in coverslips with gelatin coating were prepared according to the different experimental conditions (see figure legends) and fixed with 4% formaldehyde for 20–25 min, washed with PBS, and permeabilized (0.2% Triton X-100 in PBS) for 10 min. Samples were then washed three times with PBS and blocked for 1 h in PBS containing 1% BSA, incubated overnight at 4°C with primary antibodies diluted in PBS containing 1% BSA, washed with PBS containing 0.05% Triton X, and incubated for 2 h at RT with secondary antibodies conjugated with Alexa 647 (diluted in PBS with 1% BSA), and finally washed again with 0.01% Triton X. Images were acquired using a Zeiss confocal microscope (Zeiss LSM 880).
Wound healing assay
MDA-MB-231 and MDA-MB-468 cells were seeded into 24-well plates and grown to sub-confluence. Cell proliferation was blocked by a 2 h pre-treatment with mitomycin C (100 ng/mL) in serum-free medium. A scratch was made in each well using a 1,000 μL pipette tip and the wounded monolayers washed twice with PBS to remove cell debris and floating cells. Wound width was monitored over time (see corresponding images and figure legends) under an inverted microscope with a digital camera. Percentage wound recovery was expressed compared with the width of the wound at t = 0 (100%).
Comet assay
MDA-MB-231 cells were seeded and exposed to the different experimental conditions as depicted in the corresponding pictures. For the Comet assay we follow the manufacturer’s protocol (Fischer Scientific, 13464434). Tail DNA analysis was processed with the Cometscore 2.0 software.
Statistical analysis
Results are presented by the mean along with the standard deviation, and respective p value. Kruskal-Wallis (medians of three or more independent groups) and Mann-Whitney (comparison of two groups) tests were used to calculate statistical significance. A log rank test was used to calculate the statistical differences in the survival curves (KM Plotter Online Tool). Statistical power is detailed in the corresponding figure legends.
Acknowledgments
We thank members of the Carmo-Fonseca, Bernardes de Jesus, and Nóbrega-Pereira laboratories for insightful discussions and advice. We are also grateful to José Rino and Mariana Alves for help with the confocal microscope (a node of PPBI, Portuguese Platform of BioImaging: POCI-01-0145-FEDER-022122). We further acknowledge Sérgio Almeida and Sérgio Dias (IMM-JLA, Lisbon) for providing human cell lines. This work was supported by Fundação para a Ciência e Tecnologia (FCT), and FEDER (EXPL/BIA-CEL/0358/2021, 2022.01199.PTDC and LISBOA-01-0145-FEDER-007391, project cofunded by FEDER, through POR Lisboa 2020 – Programa Operacional Regional de Lisboa, PORTUGAL 2020, and Fundação para a Ciência e a Tecnologia), EPIC-XS (project no. 0000309), and Bolsa de Investigação em Oncologia Dr. Dário Cruz, do Núcleo Regional do Centro da Liga Portuguesa Contra o Cancro 2023. C.A.-V. received funding from Faculdade de Medicina da Universidade de Lisboa (20th “Educação pela Ciência” Program – project no. 20170029). C.T.M. received funding from the Verão com Ciência_iBiMED BII/UI98/10332/2022 program. F.S. is funded by an FCT individual scholarship (SFRH/BD/146204/2019). S.N.-P. received funding from FCT CEECIND Program (2020.00355.CEECIND). B.P. acknowledges FCT for individual support (CEECIND/03235/2017) and project funding (PTDC/BIA-CEL/0456/2021) used during this work. A.M.C. received a “Summer with Science 2022” FCT Research Initiation Grant. This project has also received funding from the European Union's Horizon 2020 research and innovation program under grant agreement no. 823839 (EPIC-XS). The CRG/UPF Proteomics Unit is part of the Spanish Infrastructure for Omics Technologies (ICTS OmicsTech) and it is supported by “Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement de la Generalitat de Catalunya” (2017SGR595). We also acknowledge support of the Spanish Ministry of Science and Innovation to the EMBL partnership, the Centro de Excelencia Severo Ochoa and the CERCA Programme/Generalitat de Catalunya. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
C.A.-V., A.M.C., and C.T.M. carried out most of the molecular and cell assays. B.P. carried out the tissue RNA-FISH assays. F.S., B.D.-S., and C.P.G. participated in some of the assays represented, namely in Figure 3 and Figure 1. A.M.C., G.E., R.V., and E.S. participated in the acquisition and analysis of mass spectrometry data. S.N.-P. and B.B.d.J. conceived the study, coordinated, and drafted the manuscript. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2023.08.019.
Supplemental information
Data and code availability
Data available on request from the authors.
References
- 1.Hood L., Rowen L. The human genome project: Big science transforms biology and medicine. Genome Med. 2013;5:79–88. doi: 10.1186/GM483/METRICS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huarte M. The emerging role of lncRNAs in cancer. Nat. Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 3.Sousa-Franco A., Rebelo K., da Rocha S.T., Bernardes de Jesus B. LncRNAs regulating stemness in aging. Aging Cell. 2019;18:e12870. doi: 10.1111/acel.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomes C.P., Nóbrega-Pereira S., Domingues-Silva B., Rebelo K., Alves-Vale C., Marinho S.P., Carvalho T., Dias S., Bernardes de Jesus B. An antisense transcript mediates MALAT1 response in human breast cancer. BMC Cancer. 2019;19:1–11. doi: 10.1186/s12885-019-5962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Jesus B.B., Marinho S.P., Barros S., Sousa-Franco A., Alves-Vale C., Carvalho T., Carmo-Fonseca M. Silencing of the lncRNA Zeb2-NAT facilitates reprogramming of aged fibroblasts and safeguards stem cell pluripotency. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-017-01921-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 8.Hart T., Komori H.K., LaMere S., Podshivalova K., Salomon D.R. Finding the active genes in deep RNA-seq gene expression studies. BMC Genom. 2013;14:778–1471. doi: 10.1186/1471-2164-14-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S., Kopp F., Chang T.C., Sataluri A., Chen B., Sivakumar S., Yu H., Xie Y., Mendell J.T. Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell. 2016;164:69–80. doi: 10.1016/j.cell.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghafouri-Fard S., Azimi T., Hussen B.M., Abak A., Taheri M., Dilmaghani N.A. Non-coding RNA Activated by DNA Damage: Review of Its Roles in the Carcinogenesis. Front. Cell Dev. Biol. 2021;9:714787. doi: 10.3389/fcell.2021.714787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elguindy M.M., Mendell J.T. NORAD-induced Pumilio phase separation is required for genome stability. Nature. 2021;595:303–308. doi: 10.1038/S41586-021-03633-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tichon A., Gil N., Lubelsky Y., Havkin Solomon T., Lemze D., Itzkovitz S., Stern-Ginossar N., Ulitsky I. A conserved abundant cytoplasmic long noncoding RNA modulates repression by Pumilio proteins in human cells. Nat. Commun. 2016;7:12209. doi: 10.1038/ncomms12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elguindy M.M., Kopp F., Goodarzi M., Rehfeld F., Thomas A., Chang T.-C., Mendell J.T. PUMILIO, but not RBMX, binding is required for regulation of genomic stability by noncoding RNA NORAD. Elife. 2019;8:e48625. doi: 10.7554/eLife.48625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tichon A., Perry R.B.T., Stojic L., Ulitsky I. SAM68 is required for regulation of pumilio by the NORAD long noncoding RNA. Genes Dev. 2018;32:70–78. doi: 10.1101/GAD.309138.117/-/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soghli N., Yousefi T., Abolghasemi M., Qujeq D. NORAD, a critical long non-coding RNA in human cancers. Life Sci. 2021;264:118665. doi: 10.1016/J.LFS.2020.118665. [DOI] [PubMed] [Google Scholar]
- 16.Liu W., Zhou X., Li Y., Jiang H., Chen A. Long Non-Coding RNA NORAD Inhibits Breast Cancer Cell Proliferation and Metastasis by Regulating miR-155-5p/SOCS1 Axis. J. Breast Cancer. 2021;24:330–343. doi: 10.4048/JBC.2021.24.E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiniello M., Knoener R.A., Steinbrink M.I., Yang B., Cesnik A.J., Buxton K.E., Scalf M., Jarrard D.F., Smith L.M. HyPR-MS for Multiplexed Discovery of MALAT1, NEAT1, and NORAD lncRNA Protein Interactomes. J. Proteome Res. 2018;17:3022–3038. doi: 10.1021/acs.jproteome.8b00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez P., Munroe D., Prawitt D., Chu L.L., Bric E., Kim J., Reid L.H., Davies C., Nakagama H., Loebbert R., et al. Functional Characterization of Human Nucleosome Assembly Protein-2 (NAP1L4) Suggests a Role as a Histone Chaperone. Genomics. 1997;44:253–265. doi: 10.1006/geno.1997.4868. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez P., Pelletier J., Price G.B., Zannis-Hadjopoulos M. NAP-2: histone chaperone function and phosphorylation state through the cell cycle. J. Mol. Biol. 2000;298:225–238. doi: 10.1006/jmbi.2000.3674. [DOI] [PubMed] [Google Scholar]
- 20.Kimura H., Takizawa N., Allemand E., Hori T., Iborra F.J., Nozaki N., Muraki M., Hagiwara M., Krainer A.R., Fukagawa T., Okawa K. A novel histone exchange factor, protein phosphatase 2Cγ, mediates the exchange and dephosphorylation of H2A–H2B. J. Cell Biol. 2006;175:389–400. doi: 10.1083/jcb.200608001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X., D’Arcy S., Radebaugh C.A., Krzizike D.D., Giebler H.A., Huang L., Nyborg J.K., Luger K., Stargell L.A. Histone Chaperone Nap1 Is a Major Regulator of Histone H2A-H2B Dynamics at the Inducible GAL Locus. Mol. Cell Biol. 2016;36:1287–1296. doi: 10.1128/MCB.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munschauer M., Nguyen C.T., Sirokman K., Hartigan C.R., Hogstrom L., Engreitz J.M., Ulirsch J.C., Fulco C.P., Subramanian V., Chen J., et al. The NORAD lncRNA assembles a topoisomerase complex critical for genome stability. Naturen. 2018;561:132–136. doi: 10.1038/s41586-018-0453-z. [DOI] [PubMed] [Google Scholar]
- 23.Holohan C., Van Schaeybroeck S., Longley D.B., Johnston P.G. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer. 2013;13:714–726. doi: 10.1038/NRC3599. [DOI] [PubMed] [Google Scholar]
- 24.Meacham C.E., Morrison S.J. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher R., Pusztai L., Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br. J. Cancer. 2013;108:479–485. doi: 10.1038/bjc.2012.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter S.L., Eklund A.C., Kohane I.S., Harris L.N., Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat. Genet. 2006;38:1043–1048. doi: 10.1038/NG1861. [DOI] [PubMed] [Google Scholar]
- 27.Roylance R., Endesfelder D., Gorman P., Burrell R.A., Sander J., Tomlinson I., Hanby A.M., Speirs V., Richardson A.L., Birkbak N.J., et al. Relationship of extreme chromosomal instability with long-term survival in a retrospective analysis of primary breast cancer. Cancer Epidemiol. Biomarkers Prev. 2011;20:2183–2194. doi: 10.1158/1055-9965.EPI-11-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H., Li J., Koirala P., Ding X., Chen B., Wang Y., Wang Z., Wang C., Zhang X., Mo Y.Y. Long non-coding RNAs as prognostic markers in human breast cancer. Oncotarget. 2016;7:20584–20596. doi: 10.18632/oncotarget.7828. 7828 [pii]10.18632/oncotarget.7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schettini F., Brasó-Maristany F., Kuderer N.M., Prat A. A perspective on the development and lack of interchangeability of the breast cancer intrinsic subtypes. npj Breast Cancer. 2022;81:1–4. doi: 10.1038/s41523-022-00451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sørlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M.B., Van De Rijn M., Jeffrey S.S., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gasco M., Shami S., Crook T. The p53 pathway in breast cancer. Breast Cancer Res. 2002;4:70–76. doi: 10.1186/BCR426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Györffy B., Lanczky A., Eklund A.C., Denkert C., Budczies J., Li Q., Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat. 2010;123:725–731. doi: 10.1007/S10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 33.Lankelma J., Dekker H., Luque F.R., Luykx S., Hoekman K., van der Valk P., van Diest P.J., Pinedo H.M. Doxorubicin gradients in human breast cancer. Clin. Cancer Res. 1999;5:1703–1707. [PubMed] [Google Scholar]
- 34.Perez E.A. Doxorubicin and paclitaxel in the treatment of advanced breast cancer: Efficacy and cardiac considerations. Cancer Invest. 2001;19:155–164. doi: 10.1081/CNV-100000150. [DOI] [PubMed] [Google Scholar]
- 35.Nitiss J.L. DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer. 2009;9:327–337. doi: 10.1038/NRC2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S., Konorev E.A., Kotamraju S., Joseph J., Kalivendi S., Kalyanaraman B. Doxorubicin Induces Apoptosis in Normal and Tumor Cells via Distinctly Different Mechanisms: INTERMEDIACY OF H2O2- AND p53-DEPENDENT PATHWAYS. J. Biol. Chem. 2004;279:25535–25543. doi: 10.1074/JBC.M400944200. [DOI] [PubMed] [Google Scholar]
- 37.Schrader C., Janssen D., Klapper W., Siebmann J.U., Meusers P., Brittinger G., Kneba M., Tiemann M., Parwaresch R. Minichromosome maintenance protein 6, a proliferation marker superior to Ki67 and independent predictor of survival in patients with mantle cell lymphoma. Br. J. Cancer. 2005;93:939–945. doi: 10.1038/SJ.BJC.6602795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klec C., Knutsen E., Schwarzenbacher D., Jonas K., Pasculli B., Heitzer E., Rinner B., Krajina K., Prinz F., Gottschalk B., et al. ALYREF, a novel factor involved in breast carcinogenesis, acts through transcriptional and post-transcriptional mechanisms selectively regulating the short NEAT1 isoform. Cell. Mol. Life Sci. 2022;79:391. doi: 10.1007/s00018-022-04402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burma S., Chen B.P., Murphy M., Kurimasa A., Chen D.J. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 2001;276:42462–42467. doi: 10.1074/JBC.C100466200. [DOI] [PubMed] [Google Scholar]
- 40.Uyhazi K.E., Yang Y., Liu N., Qi H., Huang X.A., Mak W., Weatherbee S.D., de Prisco N., Gennarino V.A., Song X., et al. Pumilio proteins utilize distinct regulatory mechanisms to achieve complementary functions required for pluripotency and embryogenesis. Proc. Natl. Acad. Sci. USA. 2020;117:7851–7862. doi: 10.1073/PNAS.1916471117/SUPPL_FILE/PNAS.1916471117.SD03.XLSX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowden R.D., Buckwalter M.R., McBride J.F., Johnson D.A., Murray B.K., O’Neill K.L. Tail profile: a more accurate system for analyzing DNA damage using the Comet assay. Mutat. Res. 2003;537:1–9. doi: 10.1016/S1383-5718(03)00056-1. [DOI] [PubMed] [Google Scholar]
- 42.Lu Y., Liu Y., Yang C. Evaluating In Vitro DNA Damage Using Comet Assay. J. Vis. Exp. 2017 doi: 10.3791/56450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiewer M.J., Knudsen K.E. Transcriptional Roles of PARP1 in Cancer. Mol. Cancer Res. 2014;12:1069–1080. doi: 10.1158/1541-7786.MCR-13-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaremba T., Thomas H., Cole M., Plummer E.R., Curtin N.J. Doxorubicin-induced suppression of poly(ADP-ribose) polymerase-1 (PARP-1) activity and expression and its implication for PARP inhibitors in clinical trials. Cancer Chemother. Pharmacologist. 2010;66:807–812. doi: 10.1007/s00280-010-1359-0. [DOI] [PubMed] [Google Scholar]
- 45.Spiegel J.O., Van Houten B., Durrant J.D. PARP1: Structural insights and pharmacological targets for inhibition. DNA Repair. 2021;103:103125. doi: 10.1016/j.dnarep.2021.103125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jabbour-Leung N.A., Chen X., Bui T., Jiang Y., Yang D., Vijayaraghavan S., McArthur M.J., Hunt K.K., Keyomarsi K. Sequential Combination Therapy of CDK Inhibition and Doxorubicin Is Synthetically Lethal in p53-Mutant Triple-Negative Breast Cancer. Mol. Cancer Therapeut. 2016;15:593–607. doi: 10.1158/1535-7163.MCT-15-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sofi S., Mehraj U., Qayoom H., Aisha S., Almilaibary A., Alkhanani M., Mir M.A. Targeting cyclin-dependent kinase 1 (CDK1) in cancer: molecular docking and dynamic simulations of potential CDK1 inhibitors. Med. Oncol. 2022;39:133. doi: 10.1007/s12032-022-01748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y., Li Y. Long non-coding RNA NORAD contributes to the proliferation, invasion and EMT progression of prostate cancer via the miR-30a-5p/RAB11A/WNT/β-catenin pathway. Cancer Cell Int. 2020;20:571. doi: 10.1186/S12935-020-01665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawasaki N., Miwa T., Hokari S., Sakurai T., Ohmori K., Miyauchi K., Miyazono K., Koinuma D. Long noncoding RNA NORAD regulates transforming growth factor-β signaling and epithelial-to-mesenchymal transition-like phenotype. Cancer Sci. 2018;109:2211–2220. doi: 10.1111/CAS.13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J., Li X.Y., Hu P., Ding Y.S. LncRNA NORAD contributes to colorectal cancer progression by inhibition of miR-202-5p. Oncol. Res. 2018;26:1411–1418. doi: 10.3727/096504018X15190844870055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X., Cai J.B., Peng R., Wei C.Y., Lu J.C., Gao C., Shen Z.Z., Zhang P.F., Huang X.Y., Ke A.W., et al. The long noncoding RNA NORAD enhances the TGF-β pathway to promote hepatocellular carcinoma progression by targeting miR-202-5p. J. Cell. Physiol. 2019;234:12051–12060. doi: 10.1002/JCP.27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miles W.O., Tschöp K., Herr A., Ji J.Y., Dyson N.J. Pumilio facilitates miRNA regulation of the E2F3 oncogene. Genes Dev. 2012;26:356–368. doi: 10.1101/GAD.182568.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frisone P., Pradella D., Di Matteo A., Belloni E., Ghigna C., Paronetto M.P. SAM68: Signal Transduction and RNA Metabolism in Human Cancer. BioMed Res. Int. 2015;2015:528954. doi: 10.1155/2015/528954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chorostecki U., Saus E., Gabaldón T. Structural characterization of NORAD reveals a stabilizing role of spacers and two new repeat units. Comput. Struct. Biotechnol. J. 2021;19:3245–3254. doi: 10.1016/j.csbj.2021.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhong L., Li Y., Xiong L., Wang W., Wu M., Yuan T., Yang W., Tian C., Miao Z., Wang T., Yang S. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct. Targeted Ther. 2021;61:201–248. doi: 10.1038/s41392-021-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dias M.H., Bernards R. Playing cancer at its own game: activating mitogenic signaling as a paradoxical intervention. Mol. Oncol. 2021;15:1975–1985. doi: 10.1002/1878-0261.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ricci M.S., Zong W.-X. Chemotherapeutic Approaches for Targeting Cell Death Pathways. Oncol. 2006;11:342–357. doi: 10.1634/THEONCOLOGIST.11-4-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li P., Zhang X., Wang L., Du L., Yang Y., Liu T., Li C., Wang C. Vol. 8. 2017. (lncRNA HOTAIR contributes to 5FU resistance through suppressing miR-218 and activating NF-Κb/TS signaling in colorectal cancer). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kallen A.N., Zhou X.B., Xu J., Qiao C., Ma J., Yan L., Lu L., Liu C., Yi J.S., Zhang H., et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell. 2013;52:101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun X., Fu K., Hodgson A., Wier E.M., Wen M.G., Kamenyeva O., Xia X., Koo L.Y., Wan F. Sam68 Is Required for DNA Damage Responses via Regulating Poly(ADP-ribosyl)ation. PLoS Biol. 2016;14:1002543. doi: 10.1371/JOURNAL.PBIO.1002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z., Wang F., Tang T., Guo C. The role of PARP1 in the DNA damage response and its application in tumor therapy. Front. Med. 2012;6:156–164. doi: 10.1007/S11684-012-0197-3. [DOI] [PubMed] [Google Scholar]
- 62.Tutt A.N.J., Garber J.E., Kaufman B., Viale G., Fumagalli D., Rastogi P., Gelber R.D., de Azambuja E., Fielding A., Balmaña J., et al. Adjuvant Olaparib for Patients with BRCA1 - or BRCA2 -Mutated Breast Cancer. N. Engl. J. Med. 2021;384:2394–2405. doi: 10.1056/NEJMOA2105215/SUPPL_FILE/NEJMOA2105215_DATA-SHARING.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kauppinen S., Vester B., Wengel J. Locked nucleic acid: High-affinity targeting of complementary RNA for RNomics. Handb. Exp. Pharmacol. 2006;173:405–422. doi: 10.1007/3-540-27262-3_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kauppinen S., Vester B., Wengel J. Locked nucleic acid (LNA): High affinity targeting of RNA for diagnostics and therapeutics. Drug Discov. Today Technol. 2005;2:287–290. doi: 10.1016/J.DDTEC.2005.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lánczky A., Győrffy B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021;23:e27633. doi: 10.2196/27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiva C., Olivella R., Borràs E., Espadas G., Pastor O., Solé A., Sabidó E. QCloud: A cloud-based quality control system for mass spectrometry-based proteomics laboratories. PLoS One. 2018;13:e0189209. doi: 10.1371/JOURNAL.PONE.0189209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Costanzo M., Fiocchetti M., Ascenzi P., Marino M., Caterino M., Ruoppolo M. Proteomic and bioinformatic investigation of altered pathways in neuroglobin-deficient breast cancer cells. Molecules. 2021;26:2397. doi: 10.3390/MOLECULES26082397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Costanzo M., Caterino M., Cevenini A., Jung V., Chhuon C., Lipecka J., Fedele R., Guerrera I.C., Ruoppolo M. Proteomics reveals that methylmalonyl-coa mutase modulates cell architecture and increases susceptibility to stress. Int. J. Mol. Sci. 2020;21:4998–5029. doi: 10.3390/IJMS21144998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perez-Riverol Y., Bai J., Bandla C., García-Seisdedos D., Hewapathirana S., Kamatchinathan S., Kundu D.J., Prakash A., Frericks-Zipper A., Eisenacher M., et al. The Pride database resources in 2022: a hub for mass spectrometry-based proteomics evidences. 50. 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request from the authors.