Abstract
The gastrointestinal pathogen Helicobacter pylori requires supplementation with either fetal calf serum (FCS), bovine serum albumin (BSA), or (2,6-dimethyl)-β-cyclodextrin (CD) for growth in a complex or defined medium. Because the availability of medium in which all components were chemically defined would facilitate metabolic studies of H. pylori, growth of the type strain, ATCC 43504, was compared in a defined medium with different growth additives. The dependency of H. pylori growth on FCS or BSA in a defined medium could partially be replaced by dependency on CD and cholesterol when the last two components were both added to the defined medium. Growth and cell yield were not affected by the addition of glucose, but the culture viability (numbers of CFU per milliliter was extended. Because therapeutic antifoams are used to relieve gastrointestinal symptoms we studied whether the unique susceptibility of H. pylori to the emulsifier polyoxyethylene-20-stearylether (Brij 78) was growth dependent or medium specific. The bactericidal activity exerted in buffer at pH 5 was independent of the preculture medium, and a 5-h exposure of the bacteria to 1.28 to 2.56 μg of Brij 78 per ml reduced the numbers of viable bacteria by >5 log10. The MICs (0.16 to 0.32 μg/ml) were lower than the corresponding minimal bactericidal concentrations in different growth media and were affected by FCS or BSA. In conclusion, CD plus cholesterol promotes the growth of H. pylori in a serum-free defined medium in which glucose enhances cell viability. The antibacterial activity exerted by Brij 78 is neither growth dependent nor medium specific.
Cultivation of the gastrointestinal pathogen Helicobacter pylori (2, 15, 17, 24) in complex medium for routine laboratory procedures and for large-scale fermentations (5) requires supplementation of the growth medium with blood factors (27). However, (2,6-dimethyl)-β-cyclodextrin (CD) also supports the growth of H. pylori (23). This has important implications for the production of antigens and enzymes, since H. pylori appears to maintain its biochemical characteristics and antigenic properties in a serum-free medium.
Bovine serum albumin (BSA) reduces the toxic effects of fatty acids, which inhibit the growth of H. pylori (11, 14, 21, 25), and CD probably acts in the same way by binding the toxic metabolites produced by the bacteria. However, blood and serum may contain other growth-stimulatory factors required by helicobacters.
Defined media for H. pylori have been described by Nedenskov (20) and Reynolds and Penn (26). These media support the growth of different strains in the presence of amino acids and in the presence of amino acids and BSA, respectively. However, very little is known about the nutritional requirements of this pathogen, and its capability to ferment saccharides has been discussed previously. Recent evidence indicates that H. pylori can catabolize glucose (18, 19). The complete genome sequence of H. pylori, which has been published previously (28), further increases our ability to understand the biology and evolution of this bacterium, and studies of its growth in a defined medium may replace or complement nuclear magnetic resonance studies (3).
The purpose of the study described here was to evaluate the growth of H. pylori in a medium in which all components were chemically defined and to determine the effect of an oxidizable substrate on growth yield and cell viability. Furthermore, we investigated whether the antibacterial activity exerted by polyoxyethylene-20-stearylether (Brij 78) (13) was medium dependent because antifoams with similar properties are used as alimentary therapeutic agents.
(Part of this study was presented at the IXth International Workshop on Gastroduodenal Pathology and Helicobacter pylori, Copenhagen, Denmark, 16 to 19 October 1996.)
MATERIALS AND METHODS
Bacteria and growth conditions.
The H. pylori type strain, ATCC 43504, was used in this study and was stored at −70°C in brucella broth (BB; pH 7.0; Difco, Detroit, Mich.) with 10% fetal calf serum (FCS; Gibco BRL, Life Technologies, Paisley, Scotland) supplemented with 20% glycerol (Prolabo, Paris, France). The solid medium was Columbia blood agar, which consisted of 42.5 g of Columbia Agar Base II (Becton Dickinson, Cockesville, Md.) per liter, 15 g of Bacto Agar (Difco) per liter, 7% horse blood, and 1.0% IsoVitaleX (pH 7.3 ± 0.2); Beckton-Dickinson). The defined medium was prepared as described by Reynolds and Penn (26), with all the inorganic chemicals, carbon sources, and supplemented amino acids being from Sigma (St. Louis, Mo.), since strain ATCC 43504 is auxotrophic for Met, Ala, Arg, His, Ile, Leu, Phe, and Val. The medium supplements studied were 10% FCS, 0.1% CD (Sigma), 0.5% BSA (fraction V powder; minimum, 98%; Sigma), or 2.0 μg of cholesterol (99+%; catalogue no. C 3045; Sigma) per ml. The organisms growing on agar plates and in liquid cultures in flasks on a platform shaker (Unimax 2010; Heidolph, Kelheim, Germany) at 150 rpm at 37°C were incubated in an automatic CO2-O2 incubator (Forma Scientific, Inc. Marietta, Ohio) under microaerophilic conditions (85% N2, 10% CO2, 5% O2). The growth in liquid medium was monitored at 560 nm, and viable counts (numbers of CFU per milliliter) were determined on Columbia blood agar plates after dilution in phosphate-buffered saline (pH 7.2) and 3 days of incubation at 37°C. Unless stated otherwise, the results presented here are representative of the results of three experiments.
Determination of MICs and MBCs.
Bacteria grown for 2 days on Columbia blood agar were washed twice in phosphate-buffered saline by centrifugation at 17,000 × g for 10 min at room temperature and were resuspended in BB supplemented with 10% FCS or defined medium with different supplements. MICs and minimal bactericidal concentrations (MBCs) were then determined by twofold serial dilution of Brij 78 in the respective medium in the presence of approximately 106 CFU/ml. After 72 h of incubation, the MIC was determined by reading the optical density at 560 nm, and 10 μl from each well was applied with a replicator to large Columbia blood agar plates (120 by 120 by 17 mm). The plates were incubated for 72 h before determination of the MBCs. Brij 78 was tested over a concentration range of 0.01 to 82 μg/ml. The MIC and the MBC were defined as the lowest concentrations of the compound giving complete inhibition of growth and <10 colonies per spot, respectively (28).
Determination of bactericidal activity in buffer at pH 5.
Bacteria grown on Columbia blood agar or on defined agar medium with different additives were washed as described above. The bacteria were then resuspended in sterile 100 mM citrate-phosphate buffer (pH 5). Twofold serial dilutions of the compound in buffer with approximately 106 CFU/ml were incubated in microtiter plates for 5 h, and the lowest concentration of the compound reducing the viable counts by >5 log10 was determined by dilution and replica plating onto large Columbia blood agar plates, which were read after 72 h of incubation.
RESULTS
Growth of H. pylori in complex and defined media supplemented with FCS or CD.
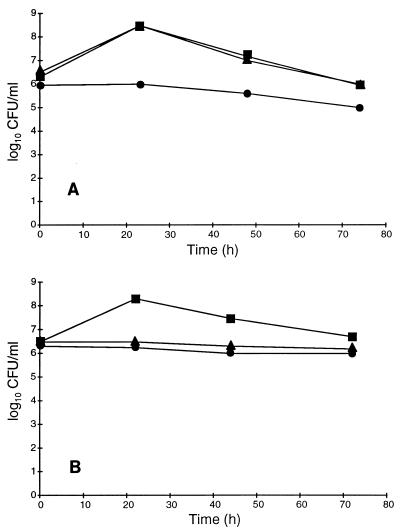
In order to investigate the requirements for growth factors and the compounds involved in the binding of toxic metabolites, BB and defined medium were supplemented with FCS or CD. The results in Fig. 1A indicate that there was no growth of H. pylori in BB alone. The enhancement of H. pylori growth was similar in BB in the presence of 10% FCS or BB in the presence of 0.1% CD, resulting in about 4 × 108 CFU/ml and the same loss of viability on further incubation. In defined medium (Fig. 1B), CD was not able to promote growth. The growth yield in the presence of 10% FCS was good and almost comparable to that obtained in the complex medium with the same additive.
FIG. 1.
Growth of H. pylori ATCC 43504 in BB (A) and defined medium (B) supplemented with different additives: no additive (•), 10% FCS (▪), and 0.1% CD (▾). Growth was determined by estimation of viable counts (numbers of CFU per milliliter.
Growth patterns of H. pylori in a defined medium supplemented with different growth additives.
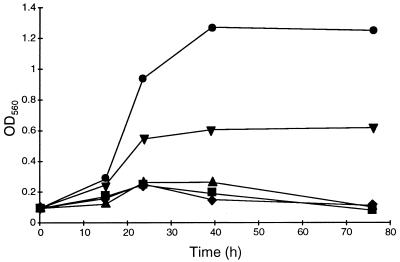
In order to find a medium for helicobacters in which all the constituents are chemically defined, the growth of H. pylori was studied in a defined medium with 0.2% glucose and different growth additives. Figure 2 shows the growth patterns in a defined medium supplemented with BSA (0.5%), CD (0.1%), or cholesterol (2.0 μg/ml). In this medium, neither CD nor cholesterol alone promoted growth, but the addition of both compounds to the defined medium resulted in growth that was about half of that obtained in the defined medium with BSA. The growth was initiated after a short lag period but ceased after about 24 h of incubation. The optical density was not changed when incubation was prolonged to 72 h.
FIG. 2.
Growth patterns of H. pylori ATCC 43504 in a defined medium with 0.2% glucose supplemented with different additives. ⧫, defined medium; ▪, defined medium plus 2.0 μg of cholesterol per ml; ▴, defined medium plus 0.1% CD; ▾, defined medium plus 2.0 μg of cholesterol per ml plus 0.1% CD; •, defined medium plus 0.5% BSA. Growth was determined by reading the optical density at 560 nm (OD560).
Effects of different carbon sources on growth and viability of H. pylori in a defined medium.
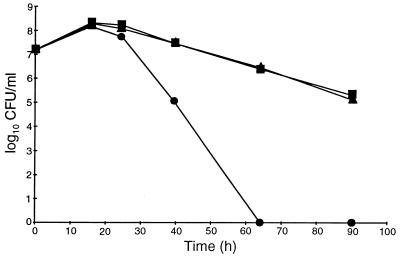
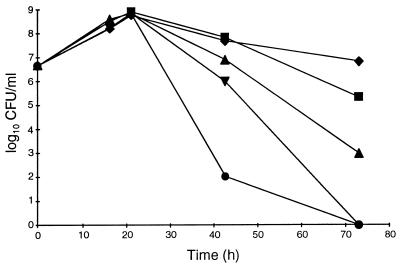
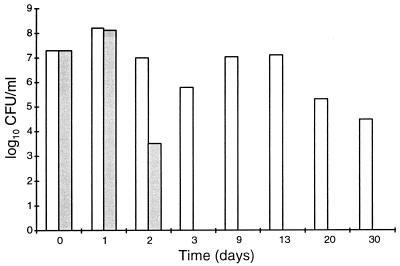
We studied the effects of an additional carbon source on the growth and yield of H. pylori cells by adding 0.2 or 1.0% glucose to defined medium supplemented with 0.5% BSA. No differences in growth or cell yield (numbers of CFU per milliliter) were observed (Fig. 3). Furthermore, the stimulatory effects of glucose, pyruvate, succinate, or citrate on growth, cell yield, and viability were compared. At a concentration of 0.2%, none of these carbon sources supported growth or enhanced the cell yield in batch culture, and the cell numbers after about 22 h of incubation were approximately 6 × 108 CFU/ml in all cultures (Fig. 4). However, after prolonged incubation, there were marked differences in the levels of reduction of the numbers of viable bacteria in the cultures. All carbon sources improved viability, but glucose had the most notable effect; after 3 days of incubation the viability was not reduced by more than about 1 log10, whereas the reduction was about 8 log10 in the culture without an additional carbon source. In another study (n = 1), the presence of glucose retained a high level of cell viability (>4 log10 CFU/ml) for more than 4 weeks (Fig. 5). Together these results indicate that in a defined medium, the presence of an oxidizable substrate markedly enhanced the viability of H. pylori but not the cell number.
FIG. 3.
Effect of glucose on growth of H. pylori ATCC 43504 in a defined medium plus BSA. •, no glucose; ▪, 0.2% glucose; ▴, 1% glucose.
FIG. 4.
Growth and viability of H. pylori ATCC 43504 in a defined medium plus BSA in the presence of different carbon sources: 0.2% glucose (⧫), 0.2% pyruvate (▪), 0.2% succinate (▴), 0.2% citrate (▾), or no additional carbon source (•).
FIG. 5.
Viability after prolonged incubation of H. pylori ATCC 43504 in a defined medium plus BSA without glucose (░⃞) and with 0.2% glucose (□).
MIC and MBCs of Brij 78 for H. pylori in different media.
The MICs and MBCs of Brij 78 for H. pylori were read in complex and defined media with different supplements. The results in Table 1 indicate that the MICs of Brij 78 were 0.16 to 0.32 μg/ml in BB or the defined medium supplemented with FCS or BSA. However, in the defined medium supplemented with CD and cholesterol, the susceptibility to Brij 78 was increased and the MIC was 0.04 μg/ml. MBCs in all media were four- to eightfold higher than the MICs.
TABLE 1.
MIC and MBC of Brij 78 for H. pylori ATCC 43504 in different media and bactericidal activity in buffer pH 5
| Medium | MIC (μg/ml) | MBC (μg/ml)a | Lowest concn (μg/ml) reducing viable counts by >5 log10b |
|---|---|---|---|
| BB + 10% DEFc + 10% FCS | 0.32 | 2.30 | 2.56 |
| 0.16 | 1.15 | 2.56 | |
| DEF + 0.5% BSA | 0.32 | 2.30 | 2.56 |
| DEF + 0.1% CD + 2 μg of cholesterol per ml | 0.04 | 0.29 | 1.28 |
MBCs were determined in different media after 72 h of exposure of the bacteria to Brij 78.
The lowest concentration of Brij 78 reducing viable counts by >5 log10 CFU/ml after a 5-h exposure of the bacteria in buffer at pH 5. The bacteria were precultured in different media. No reductions in viable counts were noted in the absence of Brij 78.
DEF, defined medium.
Susceptibility of H. pylori precultured in different media to Brij 78 in phosphate buffer at pH 5.
The antibacterial activity of Brij 78 against H. pylori precultured in different media was determined in 100 mM citrate-phosphate buffer at pH 5. The results presented in Table 1 indicate that the antibacterial activity is bactericidal at a pH possible in the habitat of H. pylori and that the killing mechanism is not growth dependent. Furthermore, there was no difference in susceptibility between bacteria precultured in different media, and the lowest concentration of Brij 78 reducing the number of survival bacteria (numbers of CFU per milliliter) by 5 log10 after an exposure time of 5 h was 1.28 to 2.56 μg/ml.
DISCUSSION
Although H. pylori is a fastidious organism requiring special culture conditions in vitro (29), good growth is now achieved in many complex media (27) and in the defined medium for helicobacters described by Reynolds and Penn (26). The growth rates and cell yields in the defined medium in the presence of 0.2% glucose and 0.5% BSA and in a complex medium with 5% fetal bovine serum are comparable (26). However, conversion to the nonculturable coccoid form was observed to be slower in the defined medium than in the complex medium.
Because the availability of a medium in which all the components were chemically defined would further facilitate biological studies of H. pylori, we compared different growth additives in BB and a defined medium. The level of growth supported by FCS or CD in BB was about the same. However, the inability of CD to support growth in the defined medium indicates that some factor(s) supporting the growth of H. pylori is limiting. The growth-limiting factor could be cholesterol, which was seen to stimulate growth in the presence of CD. However, growth in the presence of cholesterol was not fully comparable to that in the presence of BSA, and the lag phase obtained in the defined medium may depend on enzyme induction.
The inability of CD to support growth in the defined medium shows that BB contains growth-stimulating factors that are present in serum and that CD probably acts by binding toxic metabolites and is not a real growth factor. One growth-stimulatory effect of cholesterol may be the reversal of fatty acid inhibition (6).
Ansorg et al. (1) have shown earlier that H. pylori cells grown on cholesterol-free medium adsorb cholesterol from serum, and the difficulty of detaching cholesterol by washing the cells may indicate the intensive binding of cholesterol to the cell surface or even the uptake of cholesterol.
The fact that many Helicobacter spp. have been found to have unique cholesterol glucosides (9, 10, 12) and that most of the bacteria having cholesterol glucosides require cholesterol or serum for growth may explain the growth response obtained by the addition of cholesterol to the defined medium. Despite the results of nuclear magnetic resonance studies (3) and the availability of the genome sequence of H. pylori (30), which indicate that H. pylori can catabolize glucose (18, 19), we could not obtain growth enhancement in the presence of glucose in the defined medium as described by Reynolds and Penn (26). We have no explanation for this discrepancy, but it may depend on strain variations similar to the variations in the requirements for amino acids that have been found. However, if growth is measured only as an increase in optical density, differences in the optical density may be obtained, depending on the enhanced viability (optical density at 560 nm) in the presence of glucose, even though there is no increase in cell number.
The extension of the viability of H. pylori for weeks after the cessation of growth in the presence of glucose indicates a slower conversion to the nonculturable form. This may depend on an oxidative metabolism, demonstrated by substrate-enhanced tetrazolium reduction in nonculturable H. pylori (8). It may also explain the enhanced cell yield and cell viability obtained from the glucose in BB supplemented with CD, as shown by Marchini et al. (16). Conversion of H. pylori to the nonculturable form in vitro is induced by different agents (22), and higher amounts of toxic metabolites in complex media probably accelerate this transfer.
The clinical significance of the nonculturable form found in vivo (4) and whether the failure of antibiotic treatment of H. pylori may partially depend on the fact that the nongrowing form is present are being debated.
The bactericidal activity exerted by Brij 78 in buffer at a low pH mimicking the in vivo environment of H. pylori is rapid, and the killing mechanism is neither growth nor medium dependent. These properties make it possible to make the nonculturable form of helicobacters insusceptible to growth-requiring antibiotics (29). MICs and MBCs are not medium dependent, and a possible difference in the constitution of the cell membrane among H. pylori strains (7) does not affect the susceptibilities of the strains. However, the higher MICs obtained in serum-supplemented media probably depend more on the binding of Brij 78 to serum than on the biochemical characteristics of the bacteria, because the addition of serum to buffer also reduced the bactericidal activity (data not shown).
In conclusion, our results indicate that studies of the growth of H. pylori in a medium in which all constituents are chemically defined are possible in the presence of CD and cholesterol, in which the addition of glucose enhances cell viability. Furthermore, the antibacterial properties of Brij 78 indicate the potential of using this type of compound in the eradication of both growing and nonculturable forms of H. pylori.
ACKNOWLEDGMENTS
We thank Håkan Larsson for valuable discussions, M.-L. Berglund for skillful technical assistance, and Lena Ruehl for typing the manuscript.
REFERENCES
- 1.Ansorg R, Müller K-D, von Recklinghausen G, Nalik H P. Cholesterol binding of Helicobacter pylori. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1992;276:323–329. doi: 10.1016/s0934-8840(11)80538-4. [DOI] [PubMed] [Google Scholar]
- 2.Blaser M J. Helicobacter pyloriand the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- 3.Chalk P A, Roberts A D, Blows W M. Metabolism of pyruvate and glucose by intact cells of Helicobacter pylori studied by 13C NMR spectroscopy. Microbiology. 1994;140:2085–2092. doi: 10.1099/13500872-140-8-2085. [DOI] [PubMed] [Google Scholar]
- 4.Chan W Y, Hui P K, Chow J, Kwoki F, Ng C S. Coccoid forms of Helicobacter pyloriin the human stomach. Am J Clin Pathol. 1994;102:503–507. doi: 10.1093/ajcp/102.4.503. [DOI] [PubMed] [Google Scholar]
- 5.Deshpande M, Calenoff E, Daniels L. Rapid large-scale growth of Helicobacter pyloriin flasks and fermentors. Appl Environ Microbiol. 1995;61:2431–2435. doi: 10.1128/aem.61.6.2431-2435.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galbraith H, Miller T B, Paton A M, Thompson J K. Antibacterial activity of long chain fatty acids and the reversal with calcium, magnesium, ergocalciferol and cholesterol. J Appl Bacteriol. 1995;34:803–813. doi: 10.1111/j.1365-2672.1971.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 7.Geis G, Leying H, Suerbaum S, Opferkuch W. Unusual fatty acid substitution in lipids and lipopolysaccharides of Helicobacter pylori. J Clin Microbiol. 1990;28:930–932. doi: 10.1128/jcm.28.5.930-932.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gribbon L T, Barer M R. Oxidative metabolism in nonculturable Helicobacter pylori and Vibrio vulnificanscells studied by substrate-enhanced tetrazolium reduction and digital image processing. Appl Environ Microbiol. 1995;61:3379–3384. doi: 10.1128/aem.61.9.3379-3384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haque M, Hirai Y, Yokota K, Oguma K. Lipid profiles of Helicobacter pylori and Helicobacter mustelaegrown in serum-supplemented and serum-free media. Acta Med Okayama. 1995;49:205–211. doi: 10.18926/AMO/30380. [DOI] [PubMed] [Google Scholar]
- 10.Hague M, Hirai Y, Yokota K, Oguma K. Steryl glycosides: a characteristic feature of Helicobacter spp.? J Bacteriol. 1995;177:5334–5337. doi: 10.1128/jb.177.18.5334-5337.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazell S L, Graham D Y. Unsaturated fatty acids and viability of Helicobacter (Campylobacter) pylori. J Clin Microbiol. 1990;28:1060–1061. doi: 10.1128/jcm.28.5.1060-1061.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirai Y, Haque M, Yoshida T, Yokota K, Yasuda T, Oguma K. Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J Bacteriol. 1995;177:5327–5333. doi: 10.1128/jb.177.18.5327-5333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane A V, Plaut A G. Unique susceptibility of Helicobacter pylorito simethicone emulsifiers in alimentary therapeutic agents. Antimicrob Agents Chemother. 1996;40:500–502. doi: 10.1128/aac.40.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khulusi S, Ahmed H A, Patel P, Mendall M A, Northfield T C. The effects of unsaturated fatty acids on Helicobacter pylori in vitro. J Med Microbiol. 1995;42:276–282. doi: 10.1099/00222615-42-4-276. [DOI] [PubMed] [Google Scholar]
- 15.Lee A, Fox J, Hazell S. Pathogenicity of Helicobacter pylori: a perspective. Infect Immun. 1993;16:1601–1610. doi: 10.1128/iai.61.5.1601-1610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchini A, Massari P, Manetti R, Olivieri R. Optimized conditions for the fermentation of Helicobacter pyloriand production of vacuolating cytotoxin. FEMS Microbiol Lett. 1994;124:55–60. doi: 10.1111/j.1574-6968.1994.tb07261.x. [DOI] [PubMed] [Google Scholar]
- 17.Marshall B J, Royce H, Annear D I, Goodwin C S, Pearman J W, Warren J R, Armstrong J A. Original isolation of Campylobacter pyloridisfrom human gastric mucosa. Microbios Lett. 1984;25:83–88. [Google Scholar]
- 18.Mendz G L, Hazell S L. Evidence for a pentose phosphate pathway in Helicobacter pylori. FEMS Microbiol Lett. 1991;84:331–336. [Google Scholar]
- 19.Mendz G L, Hazell S L. Glucose phosphorylation in Helicobacter pylori. Arch Biochem Biophys. 1993;300:522–525. doi: 10.1006/abbi.1993.1071. [DOI] [PubMed] [Google Scholar]
- 20.Nedenskov P. Nutritional requirements for growth of Helicobacter pylori. Appl Environment Microbiol. 1994;60:3450–3453. doi: 10.1128/aem.60.9.3450-3453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieman C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev. 1954;18:147–163. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilius M, Ströhle A, Bode G, Malfertheiner P. Coccoid-like form (CLF) of Helicobacter pylori. Enzyme activity and antigenicity. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1993;280:259–272. doi: 10.1016/s0934-8840(11)80964-3. [DOI] [PubMed] [Google Scholar]
- 23.Olivieri R, Bugnoli M, Armellini D, Bianciardi S, Rappuoli R, Bayeli P F, Abate L, Esposito E, de Gregorio L, Aziz J, Basagni C, Figura N. Growth of Helicobacter pyloriin media containing cyclodextrins. J Clin Microbiol. 1993;31:160–162. doi: 10.1128/jcm.31.1.160-162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pyloriinfection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 25.Petschow B W, Batema R P, Ford L L. Susceptibility of Helicobacter pylorito bactericidal properties of medium-chain monoglycerides and free fatty acids. Antimocrob Agents Chemother. 1996;40:302–306. doi: 10.1128/aac.40.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds D J, Penn C W. Characteristics of Helicobacter pylorigrowth in a defined medium and determination of its amino acid requirements. Microbiology. 1994;140:2649–2656. doi: 10.1099/00221287-140-10-2649. [DOI] [PubMed] [Google Scholar]
- 27.Shahamat M, Mai U E H, Paszko-Kolva C, Yamamoto H, Colwell R R. Evaluation of liquid media for growth of Helicobacter pylori. J Clin Microbiol. 1991;29:2835–2837. doi: 10.1128/jcm.29.12.2835-2837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sjöström J E, Fryklund J, Kühler T, Larsson H. In vitro antibacterial activity of omeprazole and its selectivity for Helicobacter pylorispp. are dependent on incubation conditions. Antimicrob Agents Chemother. 1996;40:621–626. doi: 10.1128/aac.40.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sjöström J E, Larsson H. Factors affecting growth and antibiotic susceptibility of Helicobacter pylori: effect of pH and urea on the survival of a wild-type strain and a urease-deficient mutant. J Med Microbiol. 1996;44:425–433. doi: 10.1099/00222615-44-6-425. [DOI] [PubMed] [Google Scholar]
- 30.Tomb J-F, White O, Kerlavge A R, Clayton R A, Sutton G G, Fleischman R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khakak H G, Glodek A, McKenney K, Fizegerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Haynes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]