Abstract
Vascular prosthesis replacement and thoracic endovascular repair (TEVAR) are used to treat patients with enlarged chronic type B aortic dissection. A case in which thrombosis of the false lumen was achieved by the staged combination of these two methods is presented. A 41-year-old woman with a thoracoabdominal aortic aneurysm (maximum short diameter 44 mm) identified 5 years earlier was being monitored as an outpatient in our department when she presented with back pain. Computed tomography (CT) showed acute type B aortic dissection (DeBakey type IIIa), which was managed conservatively. When CT showed an aortic dissection with a patent false lumen immediately below the left subclavian artery bifurcation, one-debranching TEVAR was performed to close the entry, along with right axillary artery to left axillary artery bypass surgery. Outpatient CT at 3 months postoperatively showed rapid enlargement in the vicinity of the celiac artery. Thoracoabdominal aortic replacement to prevent rupture was performed, and the patient was then monitored as an outpatient. CT at age 43 years showed enlargement of the residual false lumen. Additional TEVAR was successfully performed. Thus, three-stage treatment was conducted to enlarge the residual false lumen, causing successful thrombosis of the false lumen.
Keywords: staged hybrid repair, chronic type B aortic dissection, TEVAR
Introduction
Vascular prosthesis replacement and thoracic endovascular repair (TEVAR) are being used to treat patients with enlarged chronic type B aortic dissection.1-3) The case of a patient in whom thrombosis of the false lumen was achieved by the staged combination of these two methods is presented along with a discussion of noteworthy points.
Case report
A 41-year-old woman with a thoracoabdominal aortic aneurysm (maximum short diameter 44 mm) identified 5 years earlier was being monitored as an outpatient in our department. Seven months earlier, she presented to the emergency department with back pain. Computed tomography (CT) showed acute type B aortic dissection (DeBakey type IIIa), which was managed conservatively. Surgical treatment was considered after the dissection of the thoracic descending aorta started to enlarge. She had a history of hypertension. Her family history was unremarkable. Marfan’s syndrome, Bechet’s disease, and other aortitis syndromes were not identified among her blood relatives. Respiratory function tests were within normal limits, and blood tests showed normal liver and kidney functions. CT showed an aortic dissection with a patent false lumen immediately below the left subclavian artery bifurcation (Figure 1). The entry was observed to be in the thoracic descending aorta approximately 10 mm distal to the left subclavian bifurcation. All the visceral arteries were perfused via the true lumen. The maximum aortic diameter was 58.3 mm. There was an Adamkiewicz artery originating from left L1. One-debranching TEVAR (VALIANT Captivia; VAMF2424C150TJ, VAMF2424C150TJ, Medtronic Inc., Santa Rosa, CA, USA) was performed to close the entry. Right axillary artery to left axillary artery bypass surgery (Gelsoft ringed 8 mm, Terumo, Tokyo, Japan) was also performed (Figure 2, left). No remarkable fragility of the subclavian arteries was seen as a surgical finding. Although CT at 3 months postoperatively (Figure 2, middle) confirmed thrombosis and shrinkage of the false lumen close to the thoracic stent graft placement site, rapid enlargement had occurred in the vicinity of the celiac artery. The decision was made to conduct thoracoabdominal aortic replacement to prevent rupture.
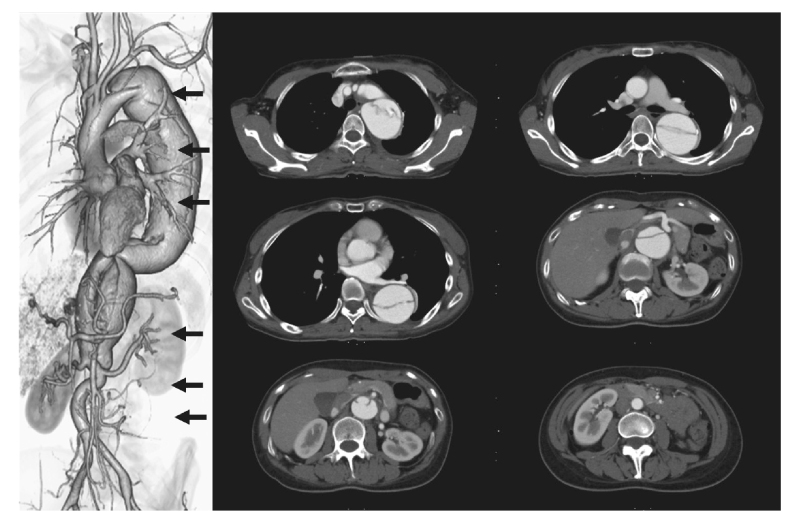
Fig. 1.
Thoracoabdominal contrast-enhanced CT before 1-debranching TEVAR. A DeBakey type IIIb patent false lumen is present. The aortic diameter is 47.6 mm at the distal arch, 51.3 mm× 58.3 mm at the pulmonary artery bifurcation, 53.2 mm× 54.1 mm at the left pulmonary vein bifurcation, 40.6 mm× 45.0 mm at the celiac artery bifurcation, and 29.5 mm× 34.6 mm above the renal artery.
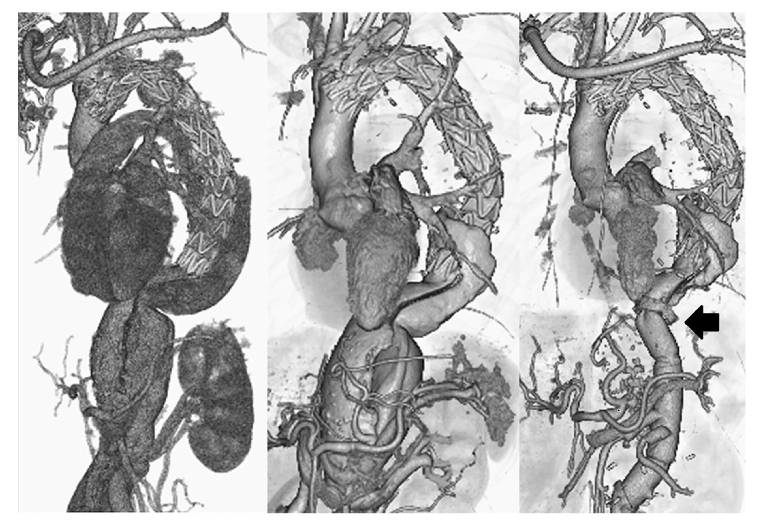
Fig. 2.
Contrast-enhanced CT images (three-dimensional reconstructed images). Left: Contrast-enhanced CT immediately after 1-debranching TEVAR. Blood flow from the entry immediately below the left subclavian artery has disappeared.
Middle: Contrast-enhanced CT before thoracoabdominal aortic replacement 3 months after the initial surgery. Although blood flow into the false lumen near the stent graft placement site has visibly reduced, the residual false lumen of the thoracoabdominal aorta has enlarged. The aortic diameter was 46.9 mm on the distal side of the left subclavian artery bifurcation, 51.2 mm× 64.0 mm at the pulmonary artery bifurcation, 48.6 mm× 56.2 mm at the left pulmonary vein bifurcation, 45.4 mm× 51.4 mm at the celiac artery bifurcation, 35.7 mm× 38.7 mm above the renal artery, and 14.3 mm× 16.0 mm below the renal artery.
Right: Contrast-enhanced CT after thoracoabdominal aortic replacement. Contrast agent inflow into the false lumen from the proximal anastomosis (Black Marker) of the thoracoabdominal replacement is evident.
A spinal drain was inserted the day before surgery. The procedure was started with the patient under general anesthesia in the right semi-lateral decubitus position. A Stony incision was made, and left 6th intercostal thoracotomy was performed. Under systemic heparinization, the abdominal aorta was clamped. A single-branch vascular prosthesis (J graft Vascular Graft Prosthesis; JNT-2009 20/9 mm × 40 cm, 1-branched, Japan Lifeline, Tokyo, Japan) was anastomosed to the abdominal aorta, and its branch was used as the antegrade blood supply channel for extracorporeal circulation. A blood drainage tube was inserted in the right femoral vein, and partial extracorporeal circulation was started. The intention had been to clamp the thoracic aorta at the stent graft interpolation site, but given the presence of atherothrombosis of the false lumen, the fact that its diameter exceeded 50 mm, which is too large for stump formation, and the risk of clamping-induced stent graft migration, clamping was actually conducted at Th11, on the distal side of the stent graft interpolation site. First, a J-graft vascular prosthesis with part of the 9-mm-diameter branch cut off was joined by the inclusion technique with an end-to-end anastomosis to the L1 lumbar artery believed to be perfusing the Adamkiewicz artery, and extracorporeal blood supply was added. The proximal-side anastomosis in the thoracoabdominal aortic replacement was formed by making stumps from the true lumen and false lumen of the native vessels and anastomosing them to a Coselli graft (Gelweave Coselli 20 mm, Terumo, Tokyo, Japan). The visceral arteries were reconstructed, and an anastomosis was finally formed between the Coselli graft and the abdominal aortic vascular prosthesis. The operating time was 7 hours 49 minutes. There was no postoperative paraplegia. Postoperative contrast-enhanced CT (Figure 2, right) showed that, although there was blood flow from the proximal anastomosis to the false lumen, the amount was small, the false lumen had not enlarged, and the patient was subsequently monitored as an outpatient. CT at age 43 years, approximately 2 years after thoracoabdominal replacement, showed enlargement of the residual false lumen (49.0 mm× 58.3 mm at the pulmonary vein bifurcation, 50.5 mm× 52.0 mm near the distal end of the stent graft) and a distal stent-graft-induced endoleak (dSINE); further treatment was therefore scheduled. Additional TEVAR via a left femoral artery approach was performed under general anesthesia (VALIANT Navion; VNMF2525C185TJ, Medtronic Inc., Santa Rosa, CA, USA). The proximal end of the stent graft was placed inside the previously placed thoracic stent graft, and the distal end inside the vascular prosthesis used to replace the thoracoabdominal aorta in the previous operation. There was no postoperative paraplegia, and no endoleak was evident on postoperative contrast-enhanced CT (Figure 3). Two years after the final operation, thrombosis of the false lumen of the thoracic descending aorta has been achieved, and there has been no enlargement of the aortic diameter.
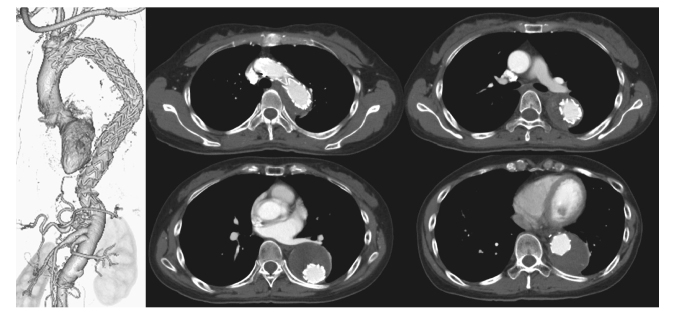
Fig. 3.
Contrast-enhanced CT after additional TEVAR 2 years after the initial surgery. The contrast agent inflow into the false lumen has disappeared.
Discussion
The early treatment of uncomplicated acute type B aortic dissections by stent graft insertion to prevent potential vascular events is increasingly being reported,1) although rest and hypotensive management basically remain the most widely used initial treatments.2) In either case, regular CT to monitor the aortic diameter during the chronic phase is required to prevent rupture, but the residual false lumen may become enlarged during this time, in which case therapeutic intervention must be considered.2)
From the perspective of preventing major blood vessel rupture, vascular prosthesis replacement3) or TEVAR4) is performed to treat enlargement of the residual false lumen of chronic type B aortic dissection. In particular, the nature of TEVAR as a minimally invasive treatment means that reports of its use have been increasing in recent years.5-7)
The main objectives of TEVAR for chronic type B aortic dissection are to reduce blood flow into the false lumen by means of entry closure and to stabilize the true lumen by fixing the dissection flap. During the late phase, however, enlargement of the residual false lumen distal to the stent graft placement site has also been reported.6,8) Although endovascular therapy for thoracoabdominal aortic lesions has been attempted,5) a standard procedure has yet to be established, and since the present patient was young, thoracoabdominal replacement was performed. With this use of staged treatment, the hope was to reduce the surgical invasiveness of each procedure, decreasing the possibility of complications such as paraplegia.9,10)
One issue in this treatment is the thoracoabdominal proximal anastomosis. There are four options: diect anastomosis to the stent graft alone (Figure 4, upper left); stump formation using the distal end of the stent graft (Figure 4, upper right); stump formation using the native vessel distal to the stent graft (Figure 4, lower left); and double-barreled anastomosis (Figure 4, lower right). Direct anastomosis to the stent graft is a simple procedure, but bleeding from the needle holes and problems with hemostasis from the intercostal arteries of the false lumen can be anticipated (Figure 4, upper left), and stump formation using the distal end of the stent graft provides a more reliable proximal anastomosis9) (Figure 4, upper right). Choosing a proximal anastomosis site at which stump formation is easy for both the true lumen and the false lumen may be also effective, because it will also encourage thrombosis of the false lumen (Figure 4, lower left). In the present case, the preoperative plan had been to form a stump at the distal end of the stent graft, but given the large diameter of the false lumen, the presence of adhesions with the surrounding tissue making it difficult to dissect, and the fact that the tissue was thickened with inflammation and hardened, the intraoperative decision was made that stump formation at this site was infeasible. Stump formation using the thoracic descending aorta in the vicinity of the diaphragm was judged to be possible, and the native vessel was used to form the stump. However, the present patient did develop blood flow into the false lumen via a crack and dSINE, and additional TEVAR was required.
Fig. 4.
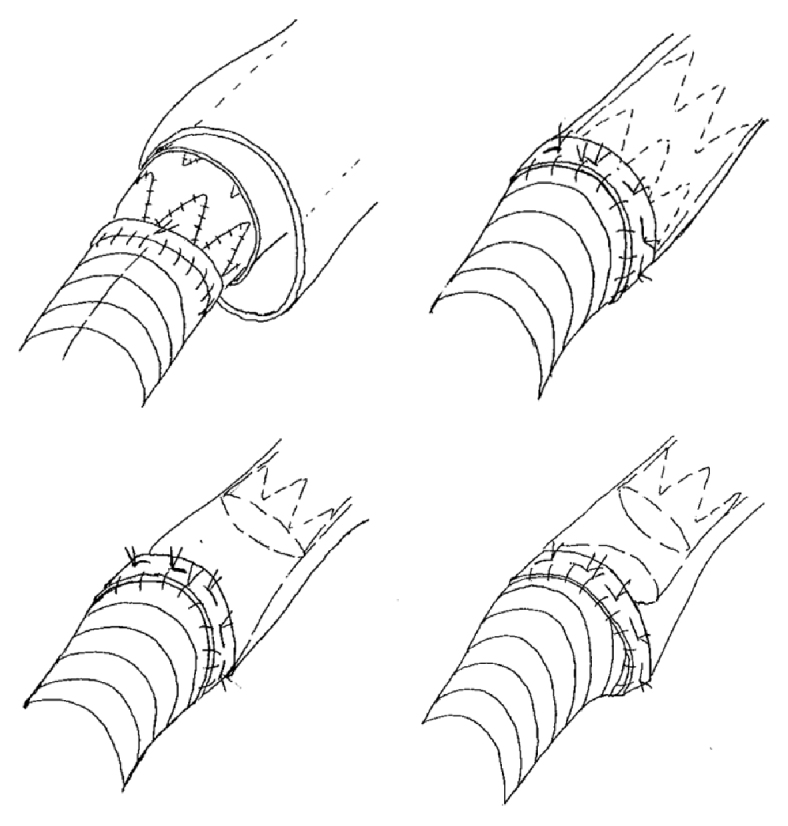
The schema of the variation for the proximal anastomosis. Upper left: direct anastomosis to the stent graft alone. Upper right: stump formation using the distal end of the stent graft. Lower left: stump formation using the native vessel distal to the stent graft. Lower right: double-barreled anastomosis.
If the true lumen cannot be sutured to a narrower diameter, double-barreled anastomosis formation is another option (Figure 4, lower right). It may be possible to preserve postoperative blood flow into the intercostal arteries that originate from the false lumen, which has the advantage of decreasing the risk of perioperative paraplegia. However, fresh blood flow into the residual false lumen may increase the pressure within the false lumen or cause it to enlarge, and the possibility of additional TEVAR must be kept in mind.10)
Whichever anastomosis method is used, additional treatment with TEVAR is a possibility. Accordingly, consideration of the graft design of the vascular prosthesis in thoracoabdominal aortic replacement so as to ensure a distal landing zone of adequate length for an additional stent graft is equally as important as the choice of proximal anastomosis technique. In our department, we do this so that the distance from the proximal anastomosis of the vascular prosthesis used to replace the thoracoabdominal aorta to the celiac artery bifurcation is 5 cm.
In conclusion, three-stage treatment was conducted to enlarge the residual false lumen in a young patient with chronic type B aortic dissection, which resulted in successful thrombosis of the false lumen. Dividing the treatment into stages may reduce the risk of perioperative complications associated with each procedure, but choices related to the proximal anastomosis in thoracoabdominal vascular prosthesis replacement must be made in accordance with the vascular lesions concerned.
References
- 1.Xiea E, Yang F, Liua Y, et al. Timing and outcome of endovascular repair for uncomplicated Type B aortic dissection. Eur J Vasc Endovasc Surg, 61: 788-797, 2021. [DOI] [PubMed] [Google Scholar]
- 2.Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of Type B aortic dissection. Longterm results of the Randomized Investigation of Stent Grafts in Aortic Dissection Trial. Circ Cardiovasc Interv, 6: 407-416, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Coselli JS, LeMaire SA, Preventza O, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg, 151: 1323-1338, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Andersen ND, Keenan JE, Ganapathi AM, Gaca JG, McCann RL, Hughes GC. Current management and outcome of chronic type B aortic dissection ; results with open and endovascular repair since the advent of thoracic endografting. Ann Cardiothorac Surg, 3: 264-274, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoynezhad A, Toluie S, Al-Atassi T. Treatment of chronic Type B aortic dissection: the pro-endovascular argument. Semin Thoracic Surg, 29: 131-136, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Kang WC, Greenberg R, Mastracci T, et al. Endovascular repair of complicated chronic distal aortic dissections: Intermediate outcomes and complications. J Thorac Cardiovasc Surg, 142: 1074-1083, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Lombardi JV, Cambria RP, Nienaber CA, et al. Aortic remodeling after endovascular treatment of complicated type B aortic dissection with the use of a composite device design. J Vasc Surg, 59: 1544-1554, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Hellgren T, Kuzniar M, Wanhainen A, Steuer J, Mani K. Clinical and morphologic outcome of endovascular repair for subacute and chronic Type B aortic dissection. Ann Vasc Surg, 72: 390-399, 2021. [DOI] [PubMed] [Google Scholar]
- 9.Jain A, Flohr T, Johnston W, et al. Staged hybrid repair of extensive thoracoabdominal aortic aneurysms secondary to chronic aortic dissection. J Vasc Surg, 63: 62-69, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Yamane Y, Uchida N, Mochizuki S, Furukawa T, Yamada K. Endovascular repair by graft-to-graft bridging in a patient with Marfan syndrome. Ann Vascular Diseases, 9: 111-113, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]